Summary
Inflammasomes are multiprotein complexes that include members of the NLR (nucleotide-binding domain leucine-rich repeat containing) family and caspase-1. Once bacterial molecules are sensed within the macrophage, the inflammasome is assembled mediating the activation of caspase-1. Caspase-11 mediates caspase-1 activation in response to lipopolysaccharide and bacterial toxins. Yet, its role during bacterial infection is unknown. Here, we demonstrated that caspase-11 was dispensable for caspase-1 activation in response to Legionella, Salmonella, Francisella and Listeria. We also determined that active mouse caspase-11 was required for restriction of L. pneumophila infection. Similarly, human caspase-4 and 5, homologs of mouse caspase-11, cooperated to restrict L. pneumophila infection in human macrophages. Caspase-11 promoted the fusion of the L. pneumophila- vacuole with lysosomes by modulating actin polymerization through cofilin. However, caspase-11 was dispensable for the fusion of lysosomes with phagosomes containing non-pathogenic bacteria, uncovering a fundamental difference in the trafficking of phagosomes according to their cargo.
Introduction
The inflammasome complex includes members of the NLR (nucleotide-binding domain leucine-rich repeat containing) family, the adaptor molecule apoptosis-associated speck-like protein containing a caspase recruitment domain (Asc), and caspase-1 (Martinon et al., 2002). The inflammasome is assembled when microbial molecules or danger signals are sensed by members of the NLR within the macrophage cytosol. Once assembled, the inflammasome mediates the cleavage and activation of caspase-1 with the subsequent processing and secretion of interleukin-1β (IL-1β) and IL-18 (Martinon et al., 2002). Murine caspase-11 contributes to caspase-1 activation in response to lipopolysaccharide (LPS) and bacterial toxins (Kayagaki et al., 2011). Mice lacking caspase-11 (Casp4−/−) fail to produce mature IL-1β or active caspase-1 and are resistant to endotoxic shock induced by bacterial toxins (Wang et al., 1998). Caspase-11 interacts with Aip1 to promote cofilin-mediated actin depolymerization (Li et al., 2007). However, role of caspase-11 during intracellular infection remains to be elucidated. Based on expression profiles, caspase-4 and caspase-5 are the human homologs of mouse caspase-11 (Mariathasan and Monack, 2007; Martinon et al., 2002). Human caspase-5 is also a component of the NLRP1 inflammasome, suggesting that caspase-5 activates caspase-1 (Martinon et al., 2002). Yet, the roles of human caspase-4 and -5 during bacterial infection are unknown.
Caspases are a family of cysteine proteases that play a distinct role in apoptosis and inflammation (Salvesen and Ashkenazi, 2011; Siegel, 2006; Stennicke and Salvesen, 1998). Caspases are synthesized as inactive single-chain zymogens and typically activated by cleavage. However, this cleavage appears to have a modest effect on the catalytic activity of initiator caspases, such as caspase-8, caspase-9 and caspase-11 (Srinivasula et al., 1999; Stennicke and Salvesen, 2000). Legionella pneumophila (L. pneumophila) is the causative agent of Legionnaires’ pneumonia, a severe disease in the elderly and immuno-compromised patients (Horwitz and Silverstein, 1980, 1981). Replication of L. pneumophila within human macrophages is critical for the disease and requires a functional bacterial type IV secretion (Dot) system (Vogel and Isberg, 1999). In wild-type (WT) murine macrophages, L. pneumophila flagellin leaks through the Dot system and is recognized by the NLR Nlrc4 leading to caspase-1 then caspase-7 activation that restricts L. pneumophila infection by promoting the fusion of the L. pneumophila containing vacuole with the lysosome (Akhter et al., 2009; Amer et al., 2006; Case et al., 2009). Naip5 (nucleotide oligomerization domain-like receptor family apoptosis inhibitory protein) is another NLR that restricts L. pneumophila infection. Therefore, host Nlrc4, caspase-1, caspase-7, Naip5 and bacterial flagellin are required for restriction of L. pneumophila infection in WT murine macrophages. Consequently, macrophages lacking Nlrc4 (Nlrc4−/−), caspase-1 (Casp1−/−), caspase-7 (Casp7−/−), functional Naip5 (A/J) are permissive to infection. Likewise, the isogenic L. pneumophila mutants lacking flagellin (Fla) replicate readily in WT murine macrophages (Amer et al., 2006; Ren et al., 2006). On the other hand, L. pneumophila mutants lacking a functional Dot system (dotA−/−) fail to secrete essential virulence factors, thus they traffic to the lysosome in WT and in permissive macrophages as well (Amer et al., 2006; Ren et al., 2006).
In this report, we have shown that caspase-11 was a component of the Nlrc4 inflammasome, yet non-essential for the activation of caspase-1 in response to L. pneumophila, Salmonella typhimurium (Salmonella), Francisella novicida (Francisella), or Listeria monocytogenes (Listeria) infection. In addition, caspase-11 controlled the fusion of L. pneumophila-containing phagosome with the lysosome independently of caspase-1. Caspase-11 promoted this fusion event by mediating actin remodeling whereby the assembly of F-actin facilitated phagosome-lysosome fusion and mediated the clearance of L. pneumophila. In addition, caspase-11 was dispensable for the delivery of the non-pathogenic dotA−/− L. pneumophila mutant to the lysosomes. Likewise, caspase-11 was not required for the clearance of non-pathogenic Escherichia coli (E. coli), thus uncoupling these two fundamental fusion modes at the molecular level. On the other hand, human macrophages, which are permissive to L. pneumophila infection, do not activate caspase-1 in response to this pathogen. However, we demonstrated in this study that ectopic expression of both caspase-4 and -5 in human macrophages restricted L. pneumophila infection and was accompanied by caspase-1 activation.
Caspase-11 protein was undetected in uninfected murine macrophages but its expression was induced during L. pneumophila infection independently of the host Nlrc4, Asc, Naip5 and of bacterial flagellin. However, caspase-11 interaction with the Nlrc4 inflammasome members and its activation required bacterial flagellin. Therefore, these findings provide a molecular framework to understand the complexity of the inflammasome and the role of caspase-11 in the innate immune response to bacterial infection.
RESULTS
Caspase-1 is activated in the absence of caspase-11 within macrophages infected with L. pneumophila, Salmonella, Francisella and Listeria
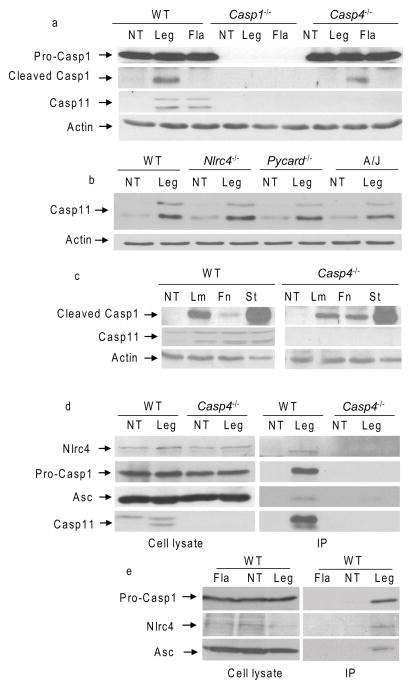
To evaluate the contribution of murine caspase-11 to caspase-1 activation upon L. pneumophila infection, we compared caspase-1 cleavage in WT, caspase-11-deficient (Casp4−/−) and caspase-1-deficient (Casp1−/−) bone marrow-derived macrophages (BMDMs) infected with L. pneumophila for 2 hrs. In WT macrophages, the bacterium induced proteolytic activation of pro-caspase-1 as determined by the detection of the mature 20-kDa subunit in cell extracts by Western blots (Figure 1a). Proteolytic processing of pro-caspase-1 in response to L. pneumophila was also detected in caspase-11-deficient macrophages (Figure 1a). Infection of WT macrophages with the L. pneumophila mutant lacking flagellin (Fla) did not lead to proteolytic activation of pro-caspase-1 (Figure 1a). These data demonstrated that caspase-11 was dispensable for caspase-1 activation in response to L. pneumophila. Quantitative polymerase chain reaction with reverse transcription (RT-PCR) showed that Casp4 (which encodes for mouse caspase-11) was induced in WT macrophages in response to L. pneumophila infection (Supplementary Figure 1a). Notably, Casp4 expression was induced independently of bacterial flagellin, the host Nlrc4 and the adaptor molecule Asc (encoded by Pycard). A functional Naip5 (A/J mice express a nonfunctional Naip5) (Wright et al., 2003) was also dispensable for caspase-11 induction in response to L. pneumophila (Figures 1a, b and Supplementary Figure 1a, b and c). Caspase-11 was also induced in response to Salmonella, Francisella and Listeria (Figure 1c).
Figure 1. Caspase-11 is dispensable for caspase-1 activation and interacts with members of the Nlrc4 inflammasome.
(a) Wild-type (WT), caspase-1-deficient (Casp1−/−) and Caspase-11-deficient (Casp4−/−) BMDMs were infected with L. pneumophila (Leg), its corresponding flagellin mutant (Fla) for 2 hrs, or left untreated (NT). Cell lysates were immunoblotted for pro-, cleaved (caspase-1) Casp1, (caspase-11) Casp11 and actin. (b) WT, Nlrc4−/−, Pycard−/− (Asc-deficient) and A/J (express mutant Naip5) BMDMs were uninfected (NT) or infected with Leg and the expression of caspase-11 was examined by Western blot. (c) WT and Casp4−/− BMDMs were infected with Listeria monocytogenes (Lm), Francisella novicida (Fn), and Salmonella Typhimurium (St) for 2 hrs or left untreated (NT). (a, b, and c) Cell lysates were immunoblotted for pro-, and cleaved Casp1, and Casp11 and actin. (d) WT and Casp4−/− BMDMs were untreated (NT) or infected with Leg for 4 hrs. (e) WT macrophages were untreated (NT) or infected with Leg, Fla for 4 hrs. (d and e) Casp11 was then immunoprecipitated from cell lysates. The Western blots of cell lysates and of immune-complexes (IP) were probed with Nlrc4, Casp1, Asc and Casp11 antibodies. Blots are representative of three independent experiments.
Then, to discern if caspase-11 is dispensable for caspase-1 activation with other inflammasome-engaging intracellular organisms, we examined the activation of caspase-1 in caspase-11-deficient macrophages infected with Listeria, Francisella, and Salmonella. Infection of WT macrophages and macrophages lacking caspase-11 with any of these organisms led to the cleavage of caspase-1 (Figure 1c). Together, these data indicate that caspase-11 is not required for the activation of caspase-1 in response to L. pneumophila, Salmonella, Francisella, and Listeria.
Given that L. pneumophila is sensed by Nlrc4 inflammasome suggests that caspase-11 is a member of the Nlrc4 inflammasome assembled during L. pneumophila infection. To test for this possibility, we next examined if caspase-11 interacted with components of the Nlrc4 inflammasome (Poyet et al., 2001; Sutterwala and Flavell, 2009). WT and caspase-11-deficient macrophages were infected with L. pneumophila, then endogenous caspase-11 was immuno-precipitated with specific caspase-11 antibodies attached to magnetic beads. Caspase-11 precipitated with endogenous pro-caspase-1, Nlrc4 and Asc exclusively in WT macrophages and only in the presence of L. pneumophila (Figure 1d). Therefore, caspase-11 interacts with members of the Nlrc4 inflammasome in the presence of L. pneumophila infection and this interaction is specific because members of the inflammasome did not precipitate in caspase-11-deficient macrophages (Figure 1d). It is possible that the lack of precipitation of the inflammasome members in uninfected macrophages is merely due to the lack of caspase-11 expression. To test this possibility, we next examined if caspase-11 interacted with members of the Nlrc4 inflammasome during infection with the L. pneumophila Fla mutant which induces caspase-11 expression but does not activate caspase-1 (Amer et al., 2006; Case et al., 2009). Despite its induction by the Fla mutant, caspase-11 did not interact with members of the Nlrc4 inflammasome (Figure 1 e). Therefore, bacterial flagellin was necessary of the interaction of caspase-11 with Nlrc4 inflammasome.
Caspase-11-deficient mice and their derived macrophages are permissive to L. pneumophila
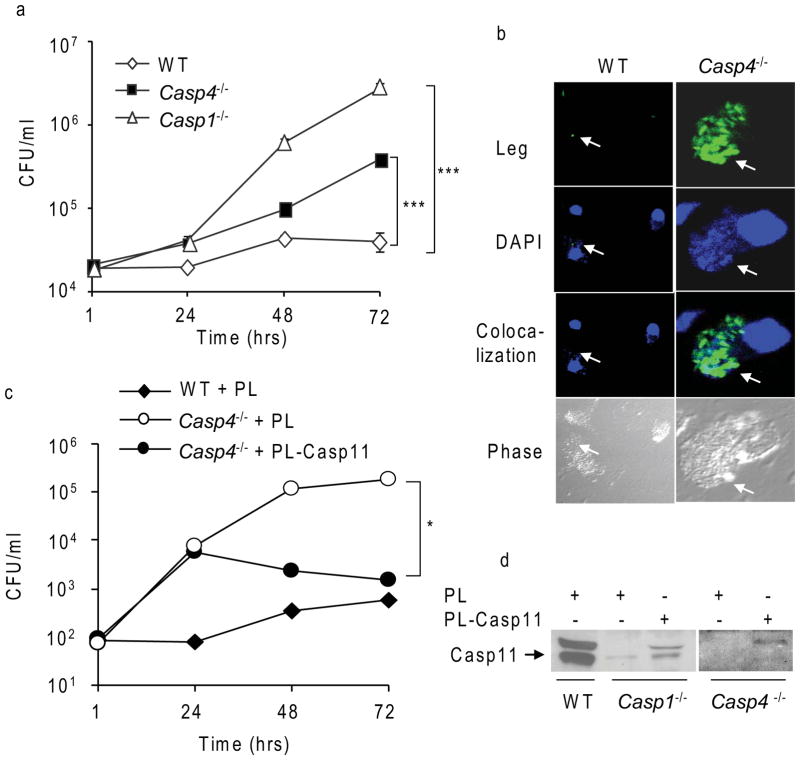
Macrophages from the great majority of mouse strains restrict L. pneumophila replication (Brieland et al., 1994; Derre and Isberg, 2004; Yamamoto et al., 1988). However, Nlrc4−/−, Casp1−/− and Casp7−/− mice and their derived macrophages are permissive to this bacterium (Akhter et al., 2009; Case et al., 2009). To determine if caspase-11 modulates the growth of L. pneumophila, we tested the ability of caspase-11-deficient (Casp4−/−) macrophages to support bacterial replication in comparison to restrictive WT macrophages. Macrophages from caspase-1 or caspase-11-deficient mice supported significant L. pneumophila replication over 72 hrs of infection (Figure 2a). Notably, caspase-11-deficient macrophages allowed L. pneumophila growth, but less than Casp1−/− macrophages (Figure 2a). In contrast, as expected the bacterial growth in WT macrophages was controlled (Figure 2a). The difference in intracellular bacterial replication between WT and caspase-11-deficient cells was not due to differential uptake of L. pneumophila because at 1 hr post infection, the number of L. pneumophila associated with different macrophages was comparable (Figure 2a). To visualize the pathogenic organism inside the cells, WT, and caspase-11-deficient macrophages were infected with L. pneumophila that constitutively express green fluorescent protein (GFP). The number of bacteria associated with macrophages was monitored by confocal laser scanning fluorescence microscopy (Figure 2b). At 24 hr post infection, only a few individual bacteria were identified inside WT macrophages, whereas expanded compartments packed with L. pneumophila were observed within caspase-11-deficient macrophages (Figure 2b). Both WT and caspase-11-deficient macrophages restricted the replication of L. pneumophila dotA−/− mutant (Supplementary Figure 4). Therefore, intracellular L. pneumophila replication which requires a functional Dot system is modulated by caspase-11.
Figure 2. Caspase-11-deficient (Casp4−/−) macrophages allow L. pneumophila intracellular replication.
(a) Wild-type (WT), caspase-11-deficient (Casp4−/−) and caspase-1-deficient (Casp1−/−) murine BMDMs were infected with L. pneumophila (Leg) and colony forming units (CFUs) were enumerated at 1, 24, 48 and 72 hrs. Data are representative of three independent experiments and presented as means ± S.D. Asterisks indicate significant differences from WT macrophages (***P<0.001). (b) Confocal microscopy of Leg-infected WT or Casp4−/− BMDMs after 24 hrs. Nuclei are stained blue with DAPI and Leg express green florescent protein (GFP). White arrows indicate the sites of Leg. (c and d) WT, Casp1−/−, and Casp4−/− BMDMs were nucleofected with plasmid harboring Casp4 (PL- Casp11) or empty vector (PL) for 24 hrs. (c) BMDMs were infected with L. pneumophila and CFUs were enumerated at 1, 24, 48 and 72 hrs. (d) Samples were lysed and immunoblotted for caspase-11 expression.
To confirm the role of caspase-11 in L. pneumophila restriction, caspase-11-deficient macrophages were complemented with a plasmid carrying the Casp4 which encodes caspase-11 (PL- Casp11) and the correlation between caspase-11 expression and bacterial replication was examined (Figure 2c and d). Ectopic expression of caspase-11 was sufficient to restore the ability of caspase-11-deficient murine macrophages to restrict L. pneumophila growth (Figure 2c and d). The expression of the control vector by the same technique did not alter the permissiveness of the caspase-11-deficient macrophages to L. pneumophila (Figure 2c).
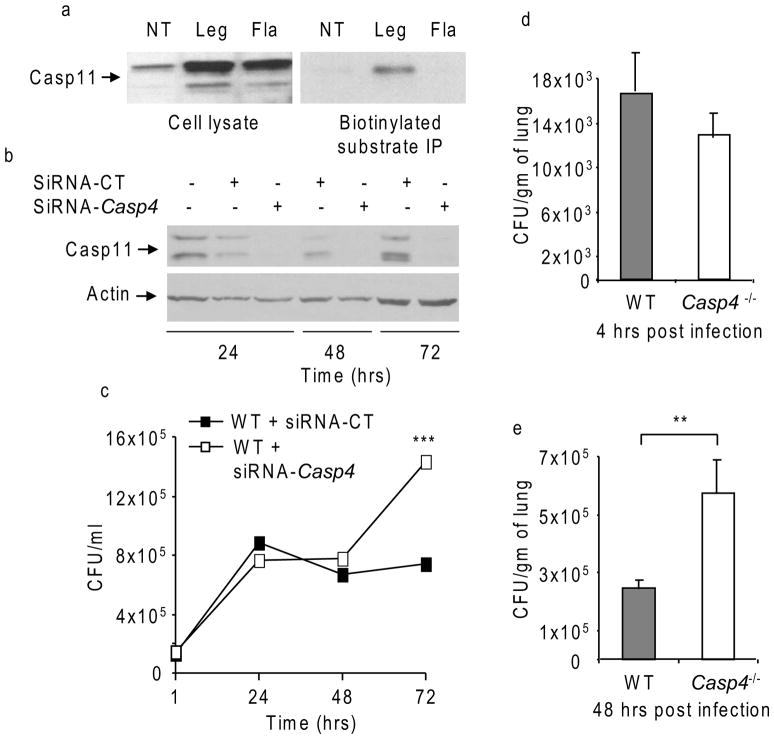
To determine if caspase-11 is activated during L. pneumophila infection, macrophages were infected with native L. pneumophila or its corresponding mutant lacking flagellin. Then macrophage lysates were mixed with biotinylated-YVAD-CMK and the presence of caspase-11 within the precipitated complex was determined by Western blots. Caspase-11 precipitated with the YVAD-biotin-coated beads only during L. pneumophila infection (Figure 3a). The interaction of caspase-11 with the substrate required bacterial flagellin (Figure 3a). Therefore, caspase-11 is activated during L. pneumophila infection and such activation requires flagellin.
Figure 3. Caspase-11 is activated during L. pneumophila infection and restricts infection in vivo.
(a) Wild-type (WT) BMDMs were infected with L. pneumophila (Leg) or its isogenic flagellin mutant (Fla), lysed (cell lysates), then mixed with biotinylated-YVAD-CMK, immunoprecipitated (IP), processed for Western blot with caspase-11 antibodies. (b) WT macrophages were nucleofected with siRNA specific to Casp4 (siRNA-Casp4) or siRNA control (siRNA-CT) then lysed and processed for Western blots to detect the expression of caspase-11 (Casp11) protein. (c) Macrophages were treated as in (b) then, infected with L. pneumophila and colony forming units (CFUs) were enumerated at 1, 24, 48 and 72 hrs. Data are representative of three independent experiments ± S.D. (d and e) WT and caspase-11-deficient (Casp4−/−) mice were infected intratracheally with L. pneumophila then CFUs recovered from homogenized lungs were enumerated and expressed as CFU per gram of lung tissue at 4 hrs (d) and 48 hrs (e). Data are represented as the means of data obtained from 4 mice ±S.D. Asterisks indicate significant differences (**P<0.01; ***P<0.001).
Next, to test whether the enzymatic activity of caspase-11 is required for the restriction of L. pneumophila infection, caspase-11-deficient macrophages were transfected with a plasmid (pCAGGS-Casp4m2) carrying a catalytically inactive mutant of caspase-11 (PL-inactive Casp11). The mutant caspase-11 failed to control L. pneumophila infection despite its comparable expression to native caspase-11 (Supplementary Figure 3a and b). Together, these results indicate that caspase-11 activity is required for the restriction of L. pneumophila growth within macrophages.
To ascertain the role of caspase-11 in restriction of L. pneumophila, caspase-11 was depleted from WT macrophages by siRNA specific to Casp4 (which encodes for murine caspase-11) (Figure 3b). The intracellular growth of L. pneumophila was evaluated (Figure 3c). WT macrophages treated with siRNA specific to Casp4 but not siRNA control allowed more L. pneumophila growth (Figure 3c).
Since Legionnaires’ disease is caused by the replication of L. pneumophila in the lungs (Horwitz, 1983b; Horwitz and Silverstein, 1980), we investigated if caspase-11 regulates bacterial growth within murine lungs in vivo. WT and caspase-11-deficient (Casp4−/−) mice were infected intratracheally with L. pneumophila and the bacterial load in the lungs was determined. Bacterial counts after 4 hrs of infection reflect the initial bacterial load in the lungs (Figure 3d), whereas bacterial counts at 48 hrs denote bacterial growth (Figure 3e). After 48 hrs of infection, significantly more L. pneumophila were recovered from the lungs of caspase-11-deficient mice compared with counts obtained from WT mice (Figure 3e). Together, these results indicate that caspase-11 restricts L. pneumophila replication in vitro and in vivo.
Because human caspase-4 and -5 are homologs of murine caspase-11, we determined if they contribute to L. pneumophila restriction in permissive human macrophages. The THP-1 macrophage cell line which is permissive to L. pneumophila, was transfected with caspase-4 (CASP4) and -5 (CASP5) plasmids individually and in combination. Then, macrophages were infected with L. pneumophila. The ectopic expression of each caspase alone partially restricted bacterial growth (Supplementary Figure 2a and b). Ectopic expression of both caspases together restricted L. pneumophila growth (Supplementary Figure 2 a and b). Notably, expression of both caspase-4 and -5 provoked caspase-1 activation upon L. pneumophila infection (Supplementary Figure 2 c). Thus, like murine caspase-11, human caspase-4 and -5 can together restrict L. pneumophila infection in human macrophages.
Ectopic expression of caspase-11 in Casp1−/− macrophages partially restricts the growth of L. pneumophila
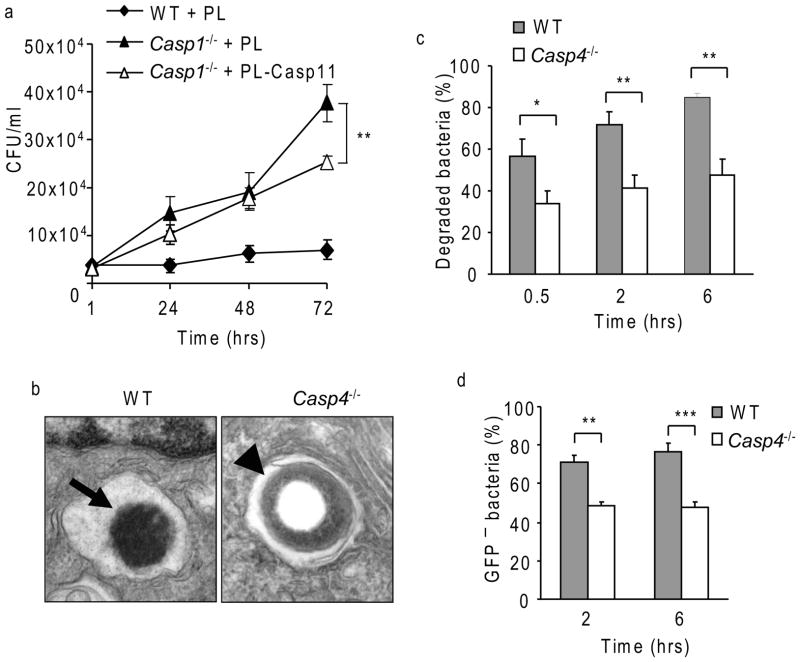
Given that both caspase-1 and caspase-11-deficient macrophages allow L. pneumophila replication, we tested if caspase-11 is expressed in Casp1−/− macrophages. As reported previously (Kayagaki et al., 2011; Li et al., 2007; Wang et al., 1998), caspase-11 protein was undetectable in Casp1−/− macrophages (Figure 1a). In addition, Casp4 mRNA was undetectable by quantitative RT PCR in Casp1−/− macrophages (Supplementary Figure 1a). Therefore, both caspase-11- and caspase-1-deficient macrophages lack caspase-11 expression (Figure 1a). Thus, we examined if the lack of caspase-11 in Casp1−/− macrophages contributed to their permissiveness to L. pneumophila growth. Distinctly, the re-establishment of caspase-11 protein with an exogenous plasmid restored caspase-11 expression in Casp1−/− macrophages and was accompanied by partial restriction of L. pneumophila infection (Figure 2d and 4a). These data suggest that the absence of caspase-11 contributes to the permissiveness of Casp1−/− macrophages to L. pneumophila.
Figure 4. Caspase-11 promotes L. pneumophila degradation in macrophages.
(a) Wild-type (WT) and caspase-1-deficient (Casp1−/−) BMDMs were nucleofected with plasmid carrying Casp4 gene (PL- Casp11) or vector alone (PL), and infected with L. pneumophila and colony forming units (CFUs) were enumerated at 1, 24, 48, and 72 hrs. (b) WT and caspase-11-deficient (Casp4−/−) macrophages were infected with L. pneumophila for 4 hrs and processed for electron microscopy. Black arrow indicates internalized L. pneumophila showing irregular contour, and black arrowhead indicates intact L. pneumophila (magnification 80,000). (c) The percent of degraded bacteria in WT and Casp4−/− BMDMs were quantified by confocal microscopy using specific L. pneumophila antibody. (d) WT and Casp4−/− macrophages were infected with the SSK strain of L. pneumophila that responds to IPTG by expressing GFP. The percentage of L. pneumophila not responding to IPTG (GFP−/−) was quantified by confocal microscopy. Data in a, c and d are representative of three independent experiments ±S.D. Asterisks indicate significant differences (*P< 0.05; **P<0.01; ***P<0.001).
Caspase-11 mediates the fusion of the L. pneumophila-containing phagosome with the lysosome in restrictive WT macrophages
To visualize L. pneumophila within WT and caspase-11-deficient macrophages, we examined L. pneumophila-infected macrophages by transmission electron microscopy. Only a few bacteria were detected within WT macrophages with signs of degradation such as irregular edges (Figure 4b arrow). In caspase-11-deficient macrophages, many L. pneumophila were identified and did not show signs of degradation (Figure 4b arrow head). We quantified the percentage of L. pneumophila showing signs of degradation by confocal microscopy using specific antibodies against L. pneumophila. Around 60–80% of L. pneumophila were degraded in WT macrophages at 30 min and 6 hrs after infection, respectively (Figure 4c). The number of degraded L. pneumophila within caspase-11-deficient macrophages did not surpass 45% at any time point (Figure 4c). To corroborate these results, WT and caspase-11-deficient macrophages were infected with the L. pneumophila strain (SSK) that expressed GFP in the presence of IPTG. The GFP-expressing L. pneumophila were considered to be live (Sturgill-Koszycki and Swanson, 2000). In WT macrophages, up to 80% of L. pneumophila failed to express GFP, whereas, in caspase-11-deficient macrophages 45% L. pneumophila lacked GFP expression (Figure 4d and Supplementary Figure 4a). These results indicate that more L. pneumophila survive and respond to IPTG within macrophages lacking caspase-11 compared to WT macrophages.
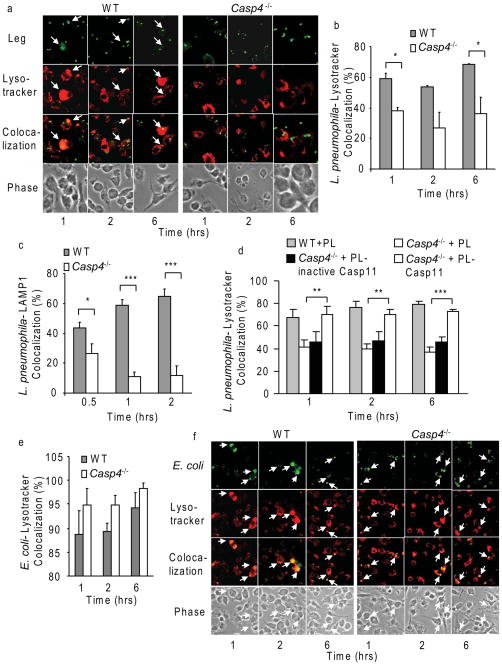
Because caspase-11 is required for the proper function of the cytoskeleton machinery (Li et al., 2007; Wang et al., 1998), we next examined if the fusion of the L. pneumophila-containing phagosome with the lysosome is defective in the absence of caspase-11, thus allowing the pathogen to avoid degradation within macrophages. The incidence of lysosome fusion was scored by following the number of L. pneumophila-containing phagosomes acquiring lysotracker red, a dye that traffics to acidic vacuoles (Figure 5a and b). In WT macrophages, the majority (55–65%) of phagocytosed L. pneumophila were contained inside phagosomes that efficiently fused with lysosomes (Figure 5a and b). In caspase-11-deficient macrophages, only 40% of the L. pneumophila containing vacuoles acquired the lysotracker within 6 hrs of infection (Figure 5a and b). Similar results were obtained using LAMP-1, a marker for late endosomes (Figure 5c). Therefore, these data indicate that caspase-11 is required for the proper fusion of L. pneumophila-containing phagosomes with the lysosomes but does not address if caspase-11 activity is required for this function. Thus, we examined the acquisition of the lysotracker by the L. pneumophila-phagosomes in caspase-11-deficient macrophages after their transfection with plasmids expressing either the native caspase-11 or mutant (inactive) caspase-11. Only macrophages expressing a functional caspase-11 delivered L. pneumophila to the lysosomes (Figure 5d).
Figure 5. Caspase-11 activity is required to promote the fusion of the lysosome with phagosomes harboring L. pneumophila but not those harboring E. coli.
(a) WT and caspase-11-deficient (Casp4−/−) BMDMs were infected with L. pneumophila (Leg) constitutively expressing GFP. Fixed samples were processed for confocal microscopy. (b and e) The colocalization of the bacteria with lysotracker red was enumerated. The sites of colocalization are indicated with white arrows (a and f). (c) Cells treated as in (a) were fixed and colocalization of Leg with the endocytic marker LAMP-1 was quantified. (d) WT and Casp4−/− BMDMs were nucleofected with vector alone (PL), plasmid carrying native Casp4 gene (PL- Casp11), or plasmid carrying mutant Casp4 gene (PL-inactive Casp11), and infected with Leg. (e and f) WT and Casp4−/− BMDMs were infected with GFP constitutively expressing Escherichia coli (E. coli). Data are representative of three independent experiments and presented as the means ± S.D. Asterisks indicate significant differences (*P<0.05; **P<0.01; ***P<0.001).
To examine if these results reflect an inherent defect in phagosome-lysosome fusion in caspase-11-deficient macrophages, we examined the trafficking of the L. pneumophila dotA−/− which is known to co-localize with the lysosomes in restrictive WT and in permissive Casp1−/− macrophages (Supplementary Figure 4b). Caspase-11-deficient macrophages delivered most of the dotA−/− mutant to lysotracker-labeled vacuoles within 1 hr of infection (Supplementary Figure 4b). In agreement with these results, phagosomes harboring E. coli were effectively (>95%) and promptly (1 hr post infection) fused with the lysosomes in macrophages lacking caspase-11 similar to WT macrophages (Figure 5e and f). Hence, caspase-11 modulates the fusion of phagosomes harboring intracellular pathogens such as L. pneumophila but not those enclosing non-pathogenic bacteria.
L. pneumophila replicates in endoplasmic reticulum (ER)-labeled vacuoles (Vogel and Isberg, 1999), thus, we examined the recruitment of the ER marker calreticulin to the L. pneumophila vacuole (Supplementary Figure 4c and d). In caspase-11-deficient macrophages, 30% of the L. pneumophila localized with calreticulin within 6 hrs of infection, whereas in WT macrophages, less than 5% bacteria did so (Supplementary Figure 4c and d). Therefore, caspase-11-deficient macrophages allow L. pneumophila replication within ER-labeled vacuoles.
F-actin network formation around L. pneumophila-containing vacuole is essential for fusion with the lysosome and requires caspase-11
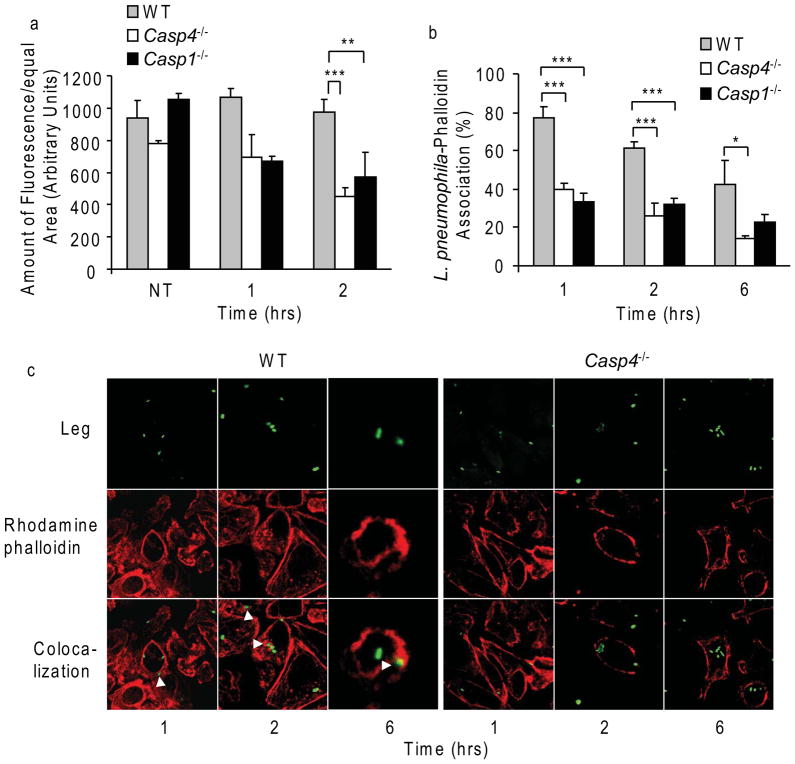
Phagosomes bind and move along microtubules and actin filaments to encounter and interact with other compartments within the cell. Thus, F-actin remodeling promotes the fusion of specific vesicular compartments including lysosomes (Desjardins et al., 1994; Jahraus et al., 2001; Kjeken et al., 2004; Marion et al., 2011; Stockinger et al., 2006; Tjelle et al., 2000). To determine whether caspase-11 mediates actin remodeling in macrophages infected with L. pneumophila, the amount of red fluorescent phalloidin, which reflects the quantity of polymerized F-actin, was determined by confocal microscopy (Figure 6a). Polymerized F-actin was higher in WT macrophages than in caspase-11-deficient macrophages throughout 2 and 6 hrs of infection (Figure 6a and c). These findings prompted us to determine whether L. pneumophila-containing phagosomes are surrounded by polymerized actin. Notably, we found that L. pneumophila-containing phagosomes are frequently surrounded by polymerized F-actin structures in WT macrophages (Figure 6b and c). This actin staining around the L. pneumophila-containing vacuoles correlated with L. pneumophila degradation (Figure 6c, white arrow heads, and Supplementary Figure 4a). In stark contrast, scarce amounts of F-actin were found around the L. pneumophila-containing phagosomes in caspase-11-deficient macrophages (Figure 6b and c). Notably, the failure to form the F-actin network was accompanied by a prominent defect in L. pneumophila clearance and the accumulation of replicative vacuoles. To investigate if actin remodeling is needed for the proper fusion of the L. pneumophila vacuoles with lysosomes, WT macrophages were treated with cytochalasin-D after 30 min of L. pneumophila infection to allow the uptake of the organism. Cytochalasin-D hindered the acquisition of lysotracker by L. pneumophila vacuoles, indicating that actin remodeling is required for the proper fusion of the L. pneumophila-containing vacuole with the lysosome (Supplementary Figure 5a and c).
Figure 6. Caspase-11 is required for the dynamic formation of polymerized actin around phagosomes.
(a–c) WT, caspase-11-deficient (Casp4−/−) (a and b), and Casp1−/− BMDMs (c) were infected with L. pneumophila (Leg) constitutively expressing GFP. Polymerized actin was stained with rhodamine-phalloidin. (a) The amount of rhodamine-phalloidin within equal areas was quantified by confocal microscopy and expressed as arbitrary units. (b) The percentage of phalloidin-labelled Leg-containing phagosomes was quantified by confocal microscopy. (c) Confocal microscopy showing rhodamine-phalloidin staining (red) around GFP-expressing (green) Leg. Phagosomes containing degraded bacteria are heavily labeled for polymerized actin (white arrow heads). Data in a and b are representative of three independent experiments and presented as the means ±S.D. Asterisks indicate significant differences (*P<0.05; **P<0.01; ***P<0.001).
The nucleation of actin on the phagosomal membrane requires flotillin-1 (Dermine et al., 2001; Desjardins et al., 1994). Supplementary Figure 6 demonstrated that in caspase-11-deficient macrophages, flotilin-1 expression is too scarce to promote actin nucleation which is required for the fusion of the L. pneumophila-containing vacuole with the lysosome.
The change of phosphorylation state of cofilin during L. pneumophila infection requires caspase-11
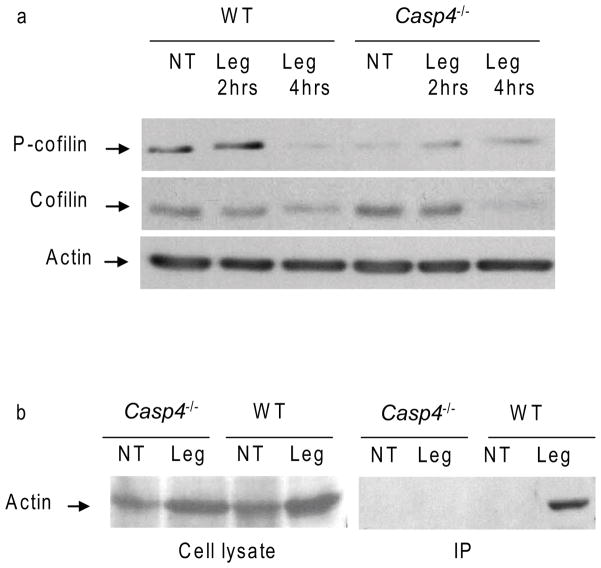
Dynamic phosphorylation and dephosphorylation of cofilin mediates cyclic actin polymerization and depolymerization that promotes phagosome-lysosome fusion (Bamburg and Bernstein, 2010; Ghosh et al., 2004). To determine the mechanism by which caspase-11 modulates actin remodeling, we first examined the phosphorylation of cofilin in WT and caspase-11-deficient macrophages. Uninfected WT macrophages allowed the phosphorylation of basal amounts of cofilin (Figure 7a). Then, L. pneumophila infection of WT macrophages led to gradual dephosphorylation of cofilin (Figure 7a). Nevertheless, cofilin was unphosphorylated in uninfected caspase-11-deficient macrophages and remained unphosphorylated throughout L. pneumophila infection (Figure 7a), thus maintaining actin in the depolymerized form (Figure 7a). Second, we determined if caspase-11 interacted with actin in the presence of L. pneumophila infection. Immunoprecipitation of caspase-11 was accompanied by the precipitation of actin only during L. pneumophila infection (Figure 7b). Thus, caspase-11 interacts with actin and is required for modulation of the phosphorylation state of cofilin during infection with pathogenic L. pneumophila.
Figure 7. Caspase-11 is required for the phosphorylation of cofilin and interacts with actin upon L. pneumophila infection.
(a) WT and caspase-11-deficient (Casp4−/−) BMDMs were infected with L. pneumophila (Leg) for 2 and 4 hrs or left untreated (NT). BMDMs lysates were immunoblotted with antibodies against phosphorylated cofilin (P-cofilin), cofilin and actin. (b) WT and Casp4−/− BMDMs were infected with Leg or left NT, lysed (cell lysates), and immunoprecipitated (IP) with beads coated with caspase-11 antibody and immunoblotted with actin antibody.
To determine if the human homologs of mouse caspase-11, caspase-4 and -5 alter cofilin phosphorylation in human macrophages, THP-1 cells were transfected with the empty plasmid (vector) or plasmids incorporating caspase-4 (CASP4) and -5 (CASP5), then infected with L. pneumophila. THP-1 cells transfected with vector alone maintained cofilin in the phosphorylated form before and during L. pneumophila infection. Cofilin was dephosphorylated only when caspases-4 and -5 were ectopically expressed in THP-1 cells before L. pneumophila infection (Supplementary Figure 2 d). Thus, alteration of the phosphorylation state of cofilin during L. pneumophila infection requires the expression of caspase-11 in the mouse and caspase-4 and -5 in human macrophages.
Discussion
Inflammasomes are protein complexes that include members of the NLR family of proteins and lead to caspase-1 activation when assembled (Lamkanfi and Dixit, 2009). Murine caspase-11 contributes to caspase-1 activation in response to bacterial toxins and LPS and seems to be induced through Toll-Like receptor 4 (TLR4) during LPS treatment (Choi et al., 2009; Kayagaki et al., 2011; Wang et al., 1998). However, little is known about its role in response to pathogenic bacteria. We have demonstrated that caspase-1 is activated in caspase-11-deficient macrophages by L. pneumophila, Salmonella, Francisella and Listeria suggesting the existence of another protease mediating caspase-1 activation at least during intracellular infection (Mueller et al., 2002). However, it is still possible that caspase-1 is autoactivated upon assembly and oligomerization (Mariathasan et al., 2004; Yu and Finlay, 2008).
Caspase-11 expression is undetectable and is inducible by stress or apoptotic signals (Kang et al., 2002). Here, we have shown that endogenous caspase-11 was induced upon L. pneumophila infection, then interacted with the members of the inflammasome such as Nlrc4, caspase-1, Asc, and also with actin. This interaction required bacterial flagellin. Therefore, restriction of L. pneumophila was not mediated by the mere induction of caspase-11 but by its interaction with the inflammasome complex. Yet, caspase-11 was not a prerequisite for inflammasome assembly since caspase-1 was activated in the absence of caspase-11. Flagellin, however, was required for caspase-1 activation by the inflammasome whether caspase-11 is included in the complex or not. Therefore, although caspase-11 seemed to be a member of the Nlrc4 inflammasome complex, it was not required for caspase-1 activation during infection with pathogenic bacteria.
As an initiator caspase, caspase-11 is predicted to undergo autocatalytic intrachain cleavage that may have only a modest effect on its catalytic activity (Srinivasula et al., 1999; Stennicke et al., 1999; Stennicke and Salvesen, 2000). Here we have shown that caspase-11 enzymatic activity was required for restriction of L. pneumophila infection. Furthermore, the ectopic expression of native caspase-11 in Casp1−/− macrophages partially restricted L. pneumophila infection. These data suggested that caspase-11 function did not require caspase-1. Similarly, depletion of caspase-11 from WT macrophages allowed moderate L. pneumophila growth. Together, these results indicated that whereas the activity of both caspase-1 and caspase-11 efficiently suppressed L. pneumophila replication, the absence of either caspases allowed for bacterial growth. These data also suggested that many phenotypes observed in Casp1−/− macrophages may actually be due to the lack of caspase-11.
Murine macrophages lacking caspase-11 are defective in migration and in phagocytosis (Li et al., 2007). This observation suggests that the uptake of L. pneumophila may be impaired in caspase-11-deficient macrophages. However, L. pneumophila uptake was not affected and the final bacterial burden in caspase-11-deficient macrophages and mice was higher than that in WT counterparts.
Intracellular growth of L. pneumophila requires halting of phagosome-lysosome fusion (Horwitz, 1983a; Vogel and Isberg, 1999). This trafficking defect is observed in permissive macrophages, whereas in restrictive WT macrophages, most L. pneumophila-containing vacuoles fuse with lysosomes and the bacteria are degraded (Coers et al., 2000; Horwitz, 1983a). However, the mechanism by which phagosome-lysosome fusion is modulated upon L. pneumophila infection is not fully understood. Therefore, one could propose that the reason for the permissiveness of caspase-11-deficient macrophages is due to a defect in phagosome maturation. It is unlikely that caspase-11 controls L. pneumophila-phagosome fusion with the lysosome through controlling the activation of caspase-7 (Akhter et al., 2009) since caspase-7 and caspase-3 were activated in caspase-11-deficient macrophages in response to L. pneumophila. Notably, phagosome-lysosome fusion required proper dynamic actin polymerization and depolymerization (Desjardins et al., 1994; Jahraus et al., 2001; Kjeken et al., 2004; Marion et al., 2011; Stockinger et al., 2006; Tjelle et al., 2000). Sustained accumulation of polymerized actin during Salmonella or Leishmania donovani infection prevents phagosome-lysosome fusion (Meresse et al., 2001). Disorganization of the F-actin network during Mycobacterium avium infection also prevents the fusion of its enclosing vacuole with the lysosome (Guerin and de Chastellier, 2000). Thus, it is possible that caspase-11 modulated phagosome-lysosome fusion by affecting actin polymerization since the lack of caspase-11 maintained cofilin in the unphosphorylated active form, sustaining actin depolymerization which hinders proper phagosome-lysosome fusion. Accordingly, we found that the low amount of polymerized actin in the vicinity of the L. pneumophila-containing phagosome in caspase-11-deficient macrophages was associated with defective fusion with the lysosome. This conclusion was further corroborated by the fact that caspase-11 interacted with actin upon infection with L. pneumophila that expressed flagellin. Interestingly, L. pneumophila-containing phagosomes in Casp1−/− macrophages (which also lack caspase-11) failed to acquire phalloidin staining and do not fuse with lysosomal compartments. However, phagosomes enclosing non-pathogenic bacteria such as E. coli acquired phalloidin staining regularly and fused with the lysosome in WT and caspase-11-deficient macrophages. Therefore, caspase-11 separates phagosomal fusion with lysosomes according to their cargo, uncoupling their trafficking pathways. It is also possible that endocytic pathways followed by pathogenic bacteria are distinct from general phagocytic pathways leading to lysosomal degradation.
Taken together, in WT murine macrophages, intracellular L. pneumophila replication was regulated by caspase-11 and caspase-1, and required a functional Dot system. This information in addition to the existence of an interaction between caspase-11 and members of the Nlrc4 inflammasome only in the presence of bacterial flagellin, led us to propose a working model where monomeric flagellin leaking through the Dot system engaging caspase-1 and caspase-11 within the inflammasome complex (Santic et al., 2007; Silveira and Zamboni, 2010). Both caspase-1 and caspase-11 converge on the fusion of the L. pneumophila vacuole with the lysosome yet function independently of each other. Nevertheless, in human macrophages, ectopic expression of caspase-4 and caspase-5 cooperated to activate caspase-1 and dephosphorylate cofilin during L. pneumophila infection thus, restricting bacterial growth. Therefore, the recapitulation of events taking place in restrictive murine macrophages such as caspase-1 activation (Abdelaziz et al., 2011a) and dynamic alteration of cofilin phosphorylation state renders permissive human macrophages restrictive to L. pneumophila.
Our study is not the first report describing the encounter between the inflammasome and cytoskeletal signaling (Waite et al., 2009a; Waite et al., 2009b) however, the contribution of caspase-11 to phagosome-lysosome fusion in the context of intracellular infection has not been previously reported. Our results also demonstrate the intriguing possibility of biological functions of caspase-11 during bacterial infections.
Material and Methods
Preparation of bone-marrow-derived macrophages (BMDM)
All animal experiments were performed according to protocols approved by the Animal Care Use Committee of The Ohio State University College of Medicine. Wild-type (WT) C57BL/6 and A/J mice were purchased from Jackson. Caspase-11-deficient (Casp4−/−) mice on C57BL/6 background were obtained from Dr. Yang at Harvard University. Caspase-1-deficient (Casp1−/−) mice on C57BL/6 background were obtained from Dr. Hise at Case Western University. BMDMs were prepared as previously described (Abdelaziz et al., 2011a; Abdelaziz et al., 2011b; Abdulrahman et al., 2011; Akhter et al., 2009; Amer et al., 2006; Kotrange et al., 2011).
Bacterial growth in vitro
L. pneumophila strain Lp02, the Dot type IV secretion mutant (dotA−/−) and SSK strain were previously described (Amer and Swanson, 2005; Brieland et al., 1994; Sturgill-Koszycki and Swanson, 2000). Infections and quantification of colony-forming-units (CFUs) was previously described. Escherichia coli strain DH5α, Listeria monocytogenes, Francisella novicida and Salmonella Typhimurium were grown as previously described (Abdelaziz et al., 2011a; Abdelaziz et al., 2011b; Abdulrahman et al., 2011; Akhter et al., 2009; Amer et al., 2006; Kotrange et al., 2011).
Immunoblotting
Proteins on Western blots were detected with specific antibodies against caspase-11 (Sigma Aldrich), caspase-1 (Cell Signaling), caspase-3 (Cell Signaling), caspase-7 (Cell Signaling), Asc (Alexis Biochemicals), Nlrc4 (Novus Biologicals), cofilin (Cell Signaling), phosphorylated-cofilin (Cell Signaling), flotillin-1 (Santa Cruz), Flag (Sigma) and actin (Abcam). Corresponding bands were visualized as previously described (Abdelaziz et al., 2011a; Abdelaziz et al., 2011b; Akhter et al., 2009).
Transmission Electron Microscopy
WT and caspase-11-deficient (Casp4−/−) primary murine macrophages were processed as previously described. (Abdelaziz et al., 2011a; Abdelaziz et al., 2011b; Abdulrahman et al., 2011; Akhter et al., 2009; Amer et al., 2006; Kotrange et al., 2011).
Fluorescence microscopy
Lysotracker Red (Invitrogen) was used to stain acidic vesicles. Calreticulin antibody (Stressgen) and Legionella antibody (Abcam) were used as previously described. Polymerized F-actin structures were visualized by staining with rhodamine-phalloidin (1:100 dilution, Molecular Probes) for 30 min (Li et al., 2007). Images were taken using laser scanning confocal fluorescence microscope using a 60x objective as previously described (Abdelaziz et al., 2011a; Abdelaziz et al., 2011b; Abdulrahman et al., 2011; Akhter et al., 2009; Amer et al., 2006; Kotrange et al., 2011)
Transfection of primary macrophages with small interfering RNA (siRNA)
siRNA treatment was performed using siRNA against mouse Casp4 (Dharmacon): GUGCAACAAUCAUUUGAAA, AAGCUAAUCUGGAAAUGGA, CGAAAGGCUCUUAUCAUAU, GAUGUGCUACAGUAUGAUA. siRNA was nucleofected into primary macrophages using Lonza Nucleofection kit and Amaxa equipment as described previously and according to the manufacturer’s protocol (Abdelaziz et al., 2011a; Abdelaziz et al., 2011b; Abdulrahman et al., 2011; Akhter et al., 2009; Gavrilin et al., 2006; Gavrilin et al., 2009; Kotrange et al., 2011).
Plasmids and transfection
Mouse caspase-11 plasmid (pCASGGS-Casp4), inactive caspase-11 plasmid (pCAGGS-Casp4m2) and pCAGGS vector (LMBP 3818) (PL- Casp11, PL-inactive Casp11, and PL respectively) were purchased from Gent University (Belgium). Plasmids encoding human caspase-4 (CASP4) and caspase-5 (CASP5) were purchased from Origene. THP-1 monocytes were treated with 200nM of phorbol-12-myristate-13-acetate (PMA) for 3 hrs to transform them to macrophage-like adherent cells. Plasmids were nucleofected (Lonza) into murine BMDMs and THP-1 human monocytes using Y-01 or V-01 program respectively and as described previously (Abdelaziz et al., 2011a; Abdelaziz et al., 2011b; Abdulrahman et al., 2011; Gavrilin et al., 2009; Hall et al., 2007; Kotrange et al., 2011). Bacteria were added after 16 hrs of recovery from nucleofection.
RT-PCR
Quantification of Casp4 expression was performed with SYBR Green I PCR Master Mix in the StepOne Plus Real Time PCR System (both, Applied Biosystems) and expressed in relative copy numbers (RCN) as we described earlier (Abdelaziz et al., 2011a; Abdelaziz et al., 2011b; Abdulrahman et al., 2011; Hughes et al., 2010; Kotrange et al., 2011). The following primers were used for murine Casp4: CATCACTAGACTCATTTCCTGCTT and CTGGAATTTCAGGAATAGAATGTG.
Immunoprecipitation of active caspases
Mouse macrophages were infected with L. pneumophila for 4 hrs. To label active caspase-11, 5 × 107 cells were lysed in KPM buffer in the presence of biotinylated-YVAD-CMK (AnaSpec, Frement, CA) as previously described (Fahy et al., 1999; Shoma et al., 2008). Next, lysates were incubated and immunoprecipitated using Streptavidin-beads (Thermo Fisher Scientific). Immunoprecipitates were analyzed by western blots. Membranes were immunoblotted using caspase-11 antibodies.
Statistical Analysis
All experiments were performed at least three independent times. Comparisons of groups for statistical difference were analyzed using Student’s two-tailed t-test. P value ≤ 0.05 is considered significant.
Supplementary Material
Acknowledgments
We thank Dr. Yuan, J. at Harvard Medical School for providing the breeding pairs for the caspase-11-deficient (Casp4−/−) mice used in these studies. We are grateful to Christie Newland for help with in vivo experiments. We thank Tim Eubank for supplying the graphical abstract. D.A. and B.A. are supported by a doctoral fellowship from the Egyptian Bureau of Education. Work in Amer’s Laboratory is supported by grants R01HL094586, RO1HL094586 (minority supplement), R21AI083871 from the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelaziz DH, Gavrilin MA, Akhter A, Caution K, Kotrange S, Khweek AA, Abdulrahman BA, Grandhi J, Hassan ZA, Marsh C, et al. Apoptosis-associated speck-like protein (ASC) controls Legionella pneumophila infection in human monocytes. J Biol Chem. 2011a;286:3203–3208. doi: 10.1074/jbc.M110.197681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelaziz DH, Gavrilin MA, Akhter A, Caution K, Kotrange S, Khweek AA, Abdulrahman BA, Hassan ZA, El-Sharkawi FZ, Bedi SS, et al. Asc-dependent and independent mechanisms contribute to restriction of legionella pneumophila infection in murine macrophages. Front Microbiol. 2011b;2:18. doi: 10.3389/fmicb.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulrahman BA, Abu Khweek A, Akhter A, Caution K, Kotrange S, Abdelaziz DHA, Newland C, Rosales-Reyes R, Kopp B, McCoy K, et al. Autophagy stimulation by Rapamycin suppresses lung inflammation and infection by Burkholderia cenocepacia in a model of cystic fibrosis Autophagy. 2011. p. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhter A, Gavrilin MA, Frantz L, Washington S, Ditty C, Limoli D, Day C, Sarkar A, Newland C, Butchar J, et al. Caspase-7 activation by the Nlrc4/Ipaf inflammasome restricts Legionella pneumophila infection. PLoS Pathog. 2009;5:e1000361. doi: 10.1371/journal.ppat.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer A, Franchi L, Kanneganti TD, Body-Malapel M, Ozoren N, Brady G, Meshinchi S, Jagirdar R, Gewirtz A, Akira S, et al. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem. 2006;281:35217–35223. doi: 10.1074/jbc.M604933200. [DOI] [PubMed] [Google Scholar]
- Bamburg JR, Bernstein BW. Roles of ADF/cofilin in actin polymerization and beyond. F1000 Biol Rep. 2010;2:62. doi: 10.3410/B2-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieland J, Freeman P, Kunkel R, Chrisp C, Hurley M, Fantone J, Engleberg C. Replicative Legionella pneumophila lung infection in intratracheally inoculated A/J mice. A murine model of human Legionnaires’ disease. Am J Pathol. 1994;145:1537–1546. [PMC free article] [PubMed] [Google Scholar]
- Case CL, Shin S, Roy CR. Asc and Ipaf Inflammasomes direct distinct pathways for caspase-1 activation in response to Legionella pneumophila. Infect Immun. 2009;77:1981–1991. doi: 10.1128/IAI.01382-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JR, Heo H, Lang Y, Shin KS, Kang SJ. Apoptosis signal-regulating kinase 1 regulates the expression of caspase-11. FEBS Lett. 2009;583:3016–3020. doi: 10.1016/j.febslet.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Coers J, Kagan JC, Matthews M, Nagai H, Zuckman DM, Roy CR. Identification of Icm protein complexes that play distinct roles in the biogenesis of an organelle permissive for Legionella pneumophila intracellular growth. Mol Microbiol. 2000;38:719–736. doi: 10.1046/j.1365-2958.2000.02176.x. [DOI] [PubMed] [Google Scholar]
- Dermine JF, Duclos S, Garin J, St-Louis F, Rea S, Parton RG, Desjardins M. Flotillin-1-enriched lipid raft domains accumulate on maturing phagosomes. J Biol Chem. 2001;276:18507–18512. doi: 10.1074/jbc.M101113200. [DOI] [PubMed] [Google Scholar]
- Derre I, Isberg RR. Macrophages from mice with the restrictive Lgn1 allele exhibit multifactorial resistance to Legionella pneumophila. Infect Immun. 2004;72:6221–6229. doi: 10.1128/IAI.72.11.6221-6229.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins M, Celis JE, van Meer G, Dieplinger H, Jahraus A, Griffiths G, Huber LA. Molecular characterization of phagosomes. J Biol Chem. 1994;269:32194–32200. [PubMed] [Google Scholar]
- Fahy RJ, Doseff AI, Wewers MD. Spontaneous human monocyte apoptosis utilizes a caspase-3-dependent pathway that is blocked by endotoxin and is independent of caspase-1. J Immunol. 1999;163:1755–1762. [PubMed] [Google Scholar]
- Gavrilin MA, Bouakl IJ, Knatz NL, Duncan MD, Hall MW, Gunn JS, Wewers MD. Internalization and phagosome escape required for Francisella to induce human monocyte IL-1beta processing and release. Proc Natl Acad Sci U S A. 2006;103:141–146. doi: 10.1073/pnas.0504271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilin MA, Mitra S, Seshadri S, Nateri J, Berhe F, Hall MW, Wewers MD. Pyrin critical to macrophage IL-1beta response to Francisella challenge. J Immunol. 2009;182:7982–7989. doi: 10.4049/jimmunol.0803073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M, Song X, Mouneimne G, Sidani M, Lawrence DS, Condeelis JS. Cofilin promotes actin polymerization and defines the direction of cell motility. Science. 2004;304:743–746. doi: 10.1126/science.1094561. [DOI] [PubMed] [Google Scholar]
- Guerin I, de Chastellier C. Pathogenic mycobacteria disrupt the macrophage actin filament network. Infect Immun. 2000;68:2655–2662. doi: 10.1128/iai.68.5.2655-2662.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MW, Gavrilin MA, Knatz NL, Duncan MD, Fernandez SA, Wewers MD. Monocyte mRNA phenotype and adverse outcomes from pediatric multiple organ dysfunction syndrome. Pediatr Res. 2007;62:597–603. doi: 10.1203/PDR.0b013e3181559774. [DOI] [PubMed] [Google Scholar]
- Horwitz MA. Formation of a novel phagosome by the Legionnaires’ disease bacterium (Legionella pneumophila) in human monocytes. J Exp Med. 1983a;158:1319–1331. doi: 10.1084/jem.158.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz MA. The Legionnaires’ disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983b;158:2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz MA, Silverstein SC. Legionnaires’ disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J Clin Invest. 1980;66:441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz MA, Silverstein SC. Interaction of the legionnaires’ disease bacterium (Legionella pneumophila) with human phagocytes. II. Antibody promotes binding of L. pneumophila to monocytes but does not inhibit intracellular multiplication. J Exp Med. 1981;153:398–406. doi: 10.1084/jem.153.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes T, Becknell B, Freud AG, McClory S, Briercheck E, Yu J, Mao C, Giovenzana C, Nuovo G, Wei L, et al. Interleukin-1beta selectively expands and sustains interleukin-22+ immature human natural killer cells in secondary lymphoid tissue. Immunity. 2010;32:803–814. doi: 10.1016/j.immuni.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahraus A, Egeberg M, Hinner B, Habermann A, Sackman E, Pralle A, Faulstich H, Rybin V, Defacque H, Griffiths G. ATP-dependent membrane assembly of F-actin facilitates membrane fusion. Mol Biol Cell. 2001;12:155–170. doi: 10.1091/mbc.12.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SJ, Wang S, Kuida K, Yuan J. Distinct downstream pathways of caspase-11 in regulating apoptosis and cytokine maturation during septic shock response. Cell Death Differ. 2002;9:1115–1125. doi: 10.1038/sj.cdd.4401087. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- Kjeken R, Egeberg M, Habermann A, Kuehnel M, Peyron P, Floetenmeyer M, Walther P, Jahraus A, Defacque H, Kuznetsov SA, et al. Fusion between phagosomes, early and late endosomes: a role for actin in fusion between late, but not early endocytic organelles. Mol Biol Cell. 2004;15:345–358. doi: 10.1091/mbc.E03-05-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotrange S, Kopp B, Akhter A, Abdelaziz D, Abu Khweek A, Caution K, Abdulrahman B, Wewers MD, McCoy K, Marsh C, et al. Burkholderia cenocepacia O polysaccharide chain contributes to caspase-1-dependent IL-1beta production in macrophages. J Leukoc Biol. 2011;89:481–488. doi: 10.1189/jlb.0910513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. Inflammasomes: guardians of cytosolic sanctity. Immunol Rev. 2009;227:95–105. doi: 10.1111/j.1600-065X.2008.00730.x. [DOI] [PubMed] [Google Scholar]
- Li J, Brieher WM, Scimone ML, Kang SJ, Zhu H, Yin H, von Andrian UH, Mitchison T, Yuan J. Caspase-11 regulates cell migration by promoting Aip1-Cofilin-mediated actin depolymerization. Nat Cell Biol. 2007;9:276–286. doi: 10.1038/ncb1541. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- Marion S, Hoffmann E, Holzer D, Le Clainche C, Martin M, Sachse M, Ganeva I, Mangeat P, Griffiths G. Ezrin promotes actin assembly at the phagosome membrane and regulates phago-lysosomal fusion. Traffic. 2011;12:421–437. doi: 10.1111/j.1600-0854.2011.01158.x. [DOI] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Meresse S, Unsworth KE, Habermann A, Griffiths G, Fang F, Martinez-Lorenzo MJ, Waterman SR, Gorvel JP, Holden DW. Remodelling of the actin cytoskeleton is essential for replication of intravacuolar Salmonella. Cell Microbiol. 2001;3:567–577. doi: 10.1046/j.1462-5822.2001.00141.x. [DOI] [PubMed] [Google Scholar]
- Mueller NJ, Wilkinson RA, Fishman JA. Listeria monocytogenes infection in caspase-11-deficient mice. Infect Immun. 2002;70:2657–2664. doi: 10.1128/IAI.70.5.2657-2664.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyet JL, Srinivasula SM, Tnani M, Razmara M, Fernandes-Alnemri T, Alnemri ES. Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J Biol Chem. 2001;276:28309–28313. doi: 10.1074/jbc.C100250200. [DOI] [PubMed] [Google Scholar]
- Ren T, Zamboni DS, Roy CR, Dietrich WF, Vance RE. Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog. 2006;2:e18. doi: 10.1371/journal.ppat.0020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvesen GS, Ashkenazi A. Snapshot: caspases. Cell. 2011;147:476–476. e471. doi: 10.1016/j.cell.2011.09.030. [DOI] [PubMed] [Google Scholar]
- Santic M, Asare R, Doric M, Abu Kwaik Y. Host-dependent trigger of caspases and apoptosis by Legionella pneumophila. Infect Immun. 2007;75:2903–2913. doi: 10.1128/IAI.00147-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoma S, Tsuchiya K, Kawamura I, Nomura T, Hara H, Uchiyama R, Daim S, Mitsuyama M. Critical involvement of pneumolysin in production of interleukin-1alpha and caspase-1-dependent cytokines in infection with Streptococcus pneumoniae in vitro: a novel function of pneumolysin in caspase-1 activation. Infect Immun. 2008;76:1547–1557. doi: 10.1128/IAI.01269-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RM. Caspases at the crossroads of immune-cell life and death. Nat Rev Immunol. 2006;6:308–317. doi: 10.1038/nri1809. [DOI] [PubMed] [Google Scholar]
- Silveira TN, Zamboni DS. Pore formation triggered by Legionella spp. is an Nlrc4 inflammasome-dependent host cell response that precedes pyroptosis. Infect Immun. 2010;78:1403–1413. doi: 10.1128/IAI.00905-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasula SM, Ahmad M, Guo Y, Zhan Y, Lazebnik Y, Fernandes-Alnemri T, Alnemri ES. Identification of an endogenous dominant-negative short isoform of caspase-9 that can regulate apoptosis. Cancer Res. 1999;59:999–1002. [PubMed] [Google Scholar]
- Stennicke HR, Deveraux QL, Humke EW, Reed JC, Dixit VM, Salvesen GS. Caspase-9 can be activated without proteolytic processing. J Biol Chem. 1999;274:8359–8362. doi: 10.1074/jbc.274.13.8359. [DOI] [PubMed] [Google Scholar]
- Stennicke HR, Salvesen GS. Properties of the caspases. Biochim Biophys Acta. 1998;1387:17–31. doi: 10.1016/s0167-4838(98)00133-2. [DOI] [PubMed] [Google Scholar]
- Stennicke HR, Salvesen GS. Caspase assays. Methods Enzymol. 2000;322:91–100. doi: 10.1016/s0076-6879(00)22010-7. [DOI] [PubMed] [Google Scholar]
- Stockinger W, Zhang SC, Trivedi V, Jarzylo LA, Shieh EC, Lane WS, Castoreno AB, Nohturfft A. Differential requirements for actin polymerization, calmodulin, and Ca2+ define distinct stages of lysosome/phagosome targeting. Mol Biol Cell. 2006;17:1697–1710. doi: 10.1091/mbc.E05-12-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgill-Koszycki S, Swanson MS. Legionella pneumophila replication vacuoles mature into acidic, endocytic organelles. J Exp Med. 2000;192:1261–1272. doi: 10.1084/jem.192.9.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterwala FS, Flavell RA. NLRC4/IPAF: a CARD carrying member of the NLR family. Clin Immunol. 2009;130:2–6. doi: 10.1016/j.clim.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjelle TE, Lovdal T, Berg T. Phagosome dynamics and function. Bioessays. 2000;22:255–263. doi: 10.1002/(SICI)1521-1878(200003)22:3<255::AID-BIES7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Vogel JP, Isberg RR. Cell biology of Legionella pneumophila. Curr Opin Microbiol. 1999;2:30–34. doi: 10.1016/s1369-5274(99)80005-8. [DOI] [PubMed] [Google Scholar]
- Waite AL, Schaner P, Hu C, Richards N, Balci-Peynircioglu B, Hong A, Fox M, Gumucio DL. Pyrin and ASC co-localize to cellular sites that are rich in polymerizing actin. Exp Biol Med (Maywood) 2009a;234:40–52. doi: 10.3181/0806-RM-184. [DOI] [PubMed] [Google Scholar]
- Waite AL, Schaner P, Richards N, Balci-Peynircioglu B, Masters SL, Brydges SD, Fox M, Hong A, Yilmaz E, Kastner DL, et al. Pyrin Modulates the Intracellular Distribution of PSTPIP1. PLoS One. 2009b;4:e6147. doi: 10.1371/journal.pone.0006147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Miura M, Jung YK, Zhu H, Li E, Yuan J. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell. 1998;92:501–509. doi: 10.1016/s0092-8674(00)80943-5. [DOI] [PubMed] [Google Scholar]
- Wright EK, Goodart SA, Growney JD, Hadinoto V, Endrizzi MG, Long EM, Sadigh K, Abney AL, Bernstein-Hanley I, Dietrich WF. Naip5 affects host susceptibility to the intracellular pathogen Legionella pneumophila. Curr Biol. 2003;13:27–36. doi: 10.1016/s0960-9822(02)01359-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Klein TW, Newton CA, Widen R, Friedman H. Growth of Legionella pneumophila in thioglycolate-elicited peritoneal macrophages from A/J mice. Infect Immun. 1988;56:370–375. doi: 10.1128/iai.56.2.370-375.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HB, Finlay BB. The caspase-1 inflammasome: a pilot of innate immune responses. Cell Host Microbe. 2008;4:198–208. doi: 10.1016/j.chom.2008.08.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.