Abstract
Background
The highest risk for stroke is among survivors of strokes or transient ischemic attacks (TIA). However, use of proven-effective cardiovascular medications to control stroke risk is suboptimal, particularly among the Black and Latino populations disproportionately impacted by stroke.
Methods
A partnership of Harlem and Bronx community representatives, stroke survivors, researchers, clinicians, outreach workers and patient educators used community-based participatory research to conceive and develop the Prevent Recurrence of All Inner-city Strokes through Education (PRAISE) trial. Using data from focus groups with stroke survivors, they tailored a peer-led, community-based chronic disease self-management program to address stroke risk factors. PRAISE will test, in a randomized controlled trial, whether this stroke education intervention improves blood pressure control and a composite outcome of blood pressure control, lipid control, and use of antithrombotic medications.
Results
Of the 582 survivors of stroke and TIA enrolled thus far, 81% are Black or Latino and 56% have an annual income less than $15,000. Many (33%) do not have blood pressures in the target range, and most (66%) do not have control of all three major stroke risk factors.
Conclusions
Rates of stroke recurrence risk factors remain suboptimal in the high risk, urban, predominantly minority communities studied. With a community-partnered approach, PRAISE has recruited a large number of stroke and TIA survivors to date, and may prove successful in engaging those at highest risk for stroke and reducing disparities in stroke outcomes in inner-city communities.
Keywords: Stroke, transient ischemic attack, community-based participatory research, peer education, disparities, clinical trial
1. Introduction
The greatest risk factor for recurrent stroke is a previous stroke or transient ischemic attack (TIA) [1–3]. Twenty-five to thirty percent of stroke survivors will have a recurrent stroke within five years, which is more than 9 times the stroke risk of the general population [4–6]. Roughly one fifth of TIA survivors will go on to have a stroke [1, 3, 7].
To lower stroke risk, stroke and TIA survivors can control hypertension and hyperlipidemia and use anti-thrombotic medication. The single most effective preventive therapy is blood pressure control. Reducing blood pressure to below 140/90 mmHg cuts recurrent stroke risk by 24–40% [2, 8, 9]. Other therapies have also proven important. Lowering LDL cholesterol below 100 mg/dl reduces strokes by 18% [10, 11]. The third pillar of secondary stroke prevention is anti-thrombotic medication. Anti-platelet agents lower stroke risk by 15–21% [12, 13], and anticoagulation for atrial fibrillation can lower risk by 68% [2, 14, 15]. Diabetes mellitus, smoking, sedentary behavior, and obesity are associated with increased risk for stroke. Control of these risk factors has been shown to reduce risk for first stroke, but has not been shown to similarly reduce risk for recurrent stroke [1, 2].
Medications are necessary to control the major recurrent stroke risk factors of hypertension, hyperlipidemia, and risk for thrombosis; lifestyle adaptations will not suffice. Unfortunately, there is profound underuse of proven secondary stroke prevention medications in all populations, with greatest underuse in Blacks and Latinos [16–20]. In fact, Blacks and Latinos are both more likely to have initial and recurrent strokes than Whites [1, 21, 22], and less likely to receive key therapies for secondary prevention [19, 23–25]. Interventions aimed at improving this disparity have, to date, met with only limited or short-term success [17, 26–31].
The neighborhoods of Northern Manhattan (East and Central Harlem and Washington Heights) and the South Bronx in New York City are representative of high risk stroke communities. People in these communities are predominantly Black or Latino, and their stroke risks have been studied [5, 18, 21]. Stroke recurrence rates are high and rates of taking preventive medications are low, and thus these communities are appropriate targets for stroke prevention interventions.
Peer education is a promising tool to promote self-efficacy for people with limited resources and education. Peer educators are widely available, cost effective, culturally appropriate, and may help overcome mistrust of the medical community [32–35]. Peer-led self-management courses, like the Stanford University Chronic Disease Self-Management Program, can improve pain and fatigue, self-efficacy, and health-related quality of life for people with chronic diseases [35–37]. To date, few studies have examined the role of a stroke-specific peer education course to modify recurrent stroke risk [38–40]. One pilot study demonstrated the feasibility of implementing a self-management program for stroke survivors, but was too small to affect outcomes [39]. A community-based exercise and education program improved social integration among stroke survivors, but other outcomes were not studied [40].
Given the heightened risk for recurrent strokes and poor risk factor control in minority communities, our community-academic collaboration, the East and Central Harlem Health Outcomes Community Action Board, was motivated to test an approach to decreasing the risk of stroke in their community. With the Prevent Recurrence of All Inner-city Strokes through Education (PRAISE) trial, our group aims to test the effectiveness of a community-based peer education program in reducing risk factors for recurrent stroke.
2. Methods
2.1 Study design and development
PRAISE is a community-based trial developed using a community-based participatory research approach that seeks to evaluate the efficacy of peer educators in teaching stroke survivors to lower risk for recurrent stroke. With this approach, researchers and community leaders are equitable partners in all phases of research, from project development through implementation, evaluation, and dissemination of results [41, 42]. The East and Central Harlem Health Outcomes Community Action Board includes community residents, stroke survivors, and community educators, who came together to create and test a program to help educate stroke and TIA survivors to lower risk for recurrent stroke.
2.2 Intervention development: qualitative methods
To develop the intervention, we held four focus groups with stroke survivors, two in English and two in Spanish, with 39 total participants recruited from community sites. We developed, piloted and revised moderator guides in English and Spanish. Trained moderators led the sessions, which were audio-taped and transcribed [43]. We developed coding schemes to inform intervention tailoring and assigned codes to the transcripts using ATLASti qualitative software. Emergent themes included participants’ desire to share their personal stroke story, social isolation following stroke, perceived vulnerability for future strokes but uncertainty about how to lower risk, low knowledge about the role of medications in reducing strokes, desire to communicate more effectively with clinicians, and lack of understanding about treating conditions like hypertension or hyperlipidemia that do not cause symptoms.
2.3 Curriculum development: partnership with Stanford University’s Patient Education Research Center
Our Board had experience using peer educators to improve self-efficacy and health behaviors among Harlem residents with chronic medical conditions [44, 45]. In addition, peer education is a promising approach to address the barriers to stroke prevention identified by focus groups. As a result, we partnered with Stanford University’s Patient Education Research Center, developers of the Chronic Disease Self-Management Program, to modify that course to be stroke-specific. Stanford’s Program is a widely used and extensively studied peer-education program [35, 37, 46]. This program enrolls people with any chronic condition into weekly workshop sessions, where participants learn and practice self-management skills in a supportive group environment. The course incorporates formation of weekly action plans for specific behaviors, feedback on progress, modeling of self-management and problem-solving techniques, social persuasion through group support, and guidance for self-management. Each workshop includes 10–15 participants, is taught by two peer leaders in English or Spanish, and is held at community sites whenever possible.
The program was built around the principles that patients can learn to take responsibility for daily management of their conditions, and that confident, knowledgeable patients have better health and use fewer health services. Stanford’s Program has been shown in randomized controlled trials to improve health behaviors and outcomes [35, 37, 46, 47], and disease-specific versions have also been effective [46–48]. One previous study using a stroke specific self- management program found high completion rates, but did not evaluate for efficacy [49, 50].
2.4 Curriculum development: stroke specific content
An intervention development team consisting of stroke survivors, outreach workers, patient educators, neurologists, primary care physicians, and researchers was charged with developing actionable, achievable education for stroke survivors. The team recognized the need for two major modifications to the Stanford program, while maintaining Stanford’s framework of weekly action plans and group problem-solving.
First, we needed to add a strong focus on demystifying stroke, as well as on medication adherence, as medications for blood pressure, lipids and anti-thrombosis are the cornerstones of stroke prevention in the target population. Thus, in contrast to Stanford’s focus on symptom management, PRAISE focuses primarily on improving adherence to medicines to control cardiovascular risk factors. We provide original didactic modules that explain what strokes and mini strokes are, and we teach participants about how to control risk factors for stroke, including blood pressure and LDL cholesterol control and the need for anti-thrombotic medication (See Table 1). Simple ways to increase medication adherence are included, like using pillboxes or keeping medications in an easy to remember place. In addition, suggestions for finding affordable medicine and tips for communicating with clinicians about medication problems are discussed.
Table 1.
Stroke specific didactics from the PRAISE curriculum
| Session number | PRAISE Didactic modules | Samples of text from peer leader script |
|---|---|---|
| Week 1 | What are strokes and mini strokes? | “A stroke is also called a brain attack. Arteries in our body carry blood to the brain like pipes carry water to our faucets… When blood is not able to reach our brain, there is no nourishment to the brain and a stroke happens.” |
| Week 2 | Preventing future strokes: blood pressure | “Blood pressure is the force that pushes blood through our arteries… When our blood pressure is too high, the walls of our bigger arteries get really thick. That’s because when your pressure is high, the force of the blood through your arteries makes the muscles in big arteries grow--just like your arms get bigger when you lift weights. However, it’s not good to build big muscles in the arteries, because they get narrower and can clog more easily…” |
| Week 3 | Preventing future strokes: LDL cholesterol | “Cholesterol is a fat like substance that is in the blood. Bad cholesterol, also called LDL, builds up on the inside of our arteries, which are similar to pipes. In the same way that grease builds up inside the pipe of your sink and clogs it—high LDL cholesterol can narrow your arteries and make them sticky so they can get blocked easily.” |
| Week 4 | Preventing future strokes: blood clumping | “When our arteries are narrowed by things like high blood pressure and high LDL cholesterol, why do they clog up? What happens is, blood cells clump together and get stuck in the arteries and block blood from flowing. This clump is called a clot. This is like when the pipes in your sink get clogged. One of the best ways to keep blood from clumping is to take medicines.” |
| Week 5 | Medicine responsibilities | “By now we all know that taking medicines for blood pressure, cholesterol, and to prevent blood clots, is the most important part of preventing another stroke. But taking medicines every day for the rest of our lives can be challenging… Do not stop taking a medicine because you feel good. Remember, the medicine could be what is making you feel good, and if you stop taking it you may get worse.” |
| Week 6 | Working with your health care team | “We learned how important it is to work closely with our doctors. To work well as a team with your doctor and other healthcare professionals, it is important to have good communication…” |
Second, we added two components to create a community of stroke survivors, and to help break through this population’s known sense of isolation, nihilism and tendency to withdraw [51–54]. We adapted the concept of Appreciative Inquiry [55], addressing root causes of success, by asking participants to share something they have accomplished, so that they begin on a note of optimism and an acknowledgement of their self-efficacy. In addition, participants begin by sharing the story of their strokes, so the group can begin to bond, socialize and engage.
2.5 Peer leader training
Peer leaders have backgrounds and health problems similar to the participants, and undergo training in the Stanford Program’s philosophy and methods. Many are stroke survivors or their family members. Leaders must have a high school education so they can effectively deliver the written content of the program. They receive five days of training, teach in pairs, and are compensated for training and for leading sessions. In addition, the trainers in peer education observe the first course and 20% of subsequent courses taught by new leaders to ensure quality control and to provide re-training if necessary. We plan to train 20 peer leaders.
2.6 Eligibility criteria
Community-dwelling adults, age 40 or older, who speak English or Spanish, are able to provide informed consent, and who experienced a stroke or TIA within five years prior to enrollment, are eligible for participation in PRAISE. The minimum age requirement excludes younger people, who are more likely to have strokes because of congenital conditions, sickle cell disease, arterial dissections, or drug use, rather than vascular disease [2, 56, 57]. After age 40, the presence of vascular risk factors increases and the incidence of strokes attributed to vascular risk factors increases significantly [57]. Because stroke survivors have a 25 to 30% risk for recurrence in the five years after an initial stroke [4–6], we chose a five year window. We exclude people with a terminal illness, pregnant women because of their unique needs for secondary stroke prevention, people who plan to move out of the area within one year, or people already enrolled in stroke-related research studies. We also exclude people with severe aphasia or significant cognitive deficits when these conditions would impede participation in a self-management program.
2.7 Recruitment strategies
2.7.1 Community based recruitment: partnership with the Community Action Board
Past efforts to recruit patients from inpatient stroke or rehabilitation units to attend peer education courses proved unsuccessful; patients were too overwhelmed by their acute condition to contemplate being part of classes after discharge. However, community partners, including stroke survivors, felt strongly that people who had strokes and TIAs would be very interested in these classes once they had re-settled into their communities. Accordingly, members of the Board welcome project staff into Harlem and South Bronx senior centers, community centers, churches, and community events where they have ties, so staff can advertise for the study and recruit stroke survivors. Staff build a local presence by repeatedly visiting and speaking at multiple sites and posting flyers and brochures in these sites and other public gathering places. In addition, staff focus on sites that provide services for seniors, by participating in health fairs, and building relationships with local independent pharmacies.
2.7.2 Clinic based recruitment: partnership with providers
Similar to Board members providing the requisite introductions to stroke and TIA survivors in the Harlem community, we rely on health care providers to champion the study in clinics. Project staff give presentations to clinicians, outlining the burden of stroke in the community and the study. At one academic hospital and one federally qualified neighborhood health center, the team uses the electronic medical record to create a database of patients with a diagnosis codes for stroke or TIA. A weekly list is generated that includes contact information, provider names, and which patients had had an upcoming appointment in the next week. Staff ask the patients’ providers to sign a letter to send to their patients with information about the study. These initial outreach letters, and all study communication, are sent in distinctive bright green envelopes to facilitate patients’ recognition. The letters include a page that patients could send back to decline participation in the study. Two weeks after the letters are mailed, project staff call patients to determine if they were eligible and interested in participating. We also engage in face-to-face recruitment at Mount Sinai clinics with clinicians’ permission.
To expand to a wider patient base, we partnered with the Visiting Nurse Service of New York to recruit stroke and TIA survivors. Again, potential participants are identified via electronic databases and receive an introductory letter in a distinctive green envelope, followed two weeks later by phone calls. Staff from each institution then contact the stroke and TIA survivors by telephone; if they agree to be contacted by the study, staff from these organizations notify project staff to schedule a recruitment visit.
The study was approved by the Institutional Review Board of each participating institution.
2.8 Recruitment visit: consent and baseline data collection
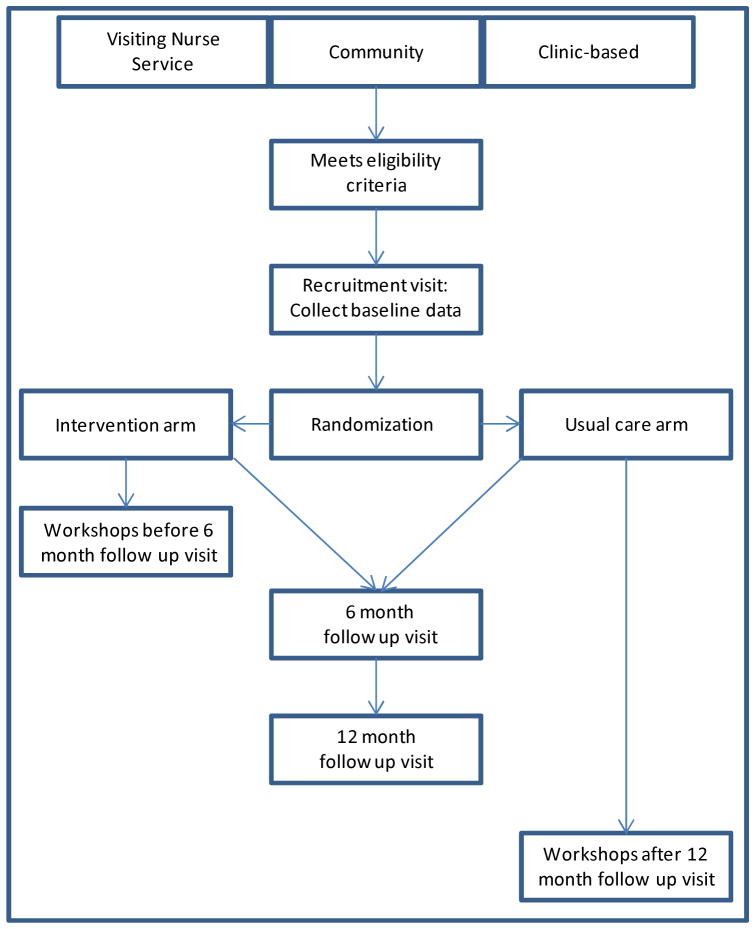
English- and Spanish-speaking project staff use laptops programmed with a pre-screening section to determine eligibility (see Figure 1). If eligible, research staff obtain written informed consent. Research staff then survey participants by reading questions from a laptop, to avoid challenges from low health literacy, and then enter responses into the laptop. All materials are read to participants in English or Spanish and are at a fourth grade educational level. Survey items include socio-demographic information adapted from the National Health and Nutrition Examination Survey or Behavioral Risk Factor Surveillance System, and validated scales to assess stroke severity [58], stroke knowledge [59, 60], beliefs about medicines [61], depression [62, 63], health-related quality of life [64], alcohol consumption [65], medication adherence [66], perceived racism [67], and perceived discrimination [68]. We record the medications that each participant is taking, and we explicitly ask if they were told by a health care provider not to take an antithrombotic medication. We also ask about their stroke history, including time since the most recent stroke or TIA and number of strokes or TIAs.
Figure 1.
Study enrollment, randomization, and follow up
Participants then have blood pressure measured by trained staff according to the American Heart Association guidelines with proper positioning of the participant including back support, arms supported at heart level, adequate pre-measurement rest of five minutes, appropriate selection of cuff size, and cuff placement. Obese adults with conical arms require blood pressure measured at the wrist. We measure blood pressure three times two minutes apart with a validated, automated, electronic sphygmomanometer (BPtru) [69, 70], and we use the average of the last two readings. Participants also have their height and weight measured. Finally, one tube of blood is drawn to measure direct LDL cholesterol, which is accurate and more practical than calculated LDL, and does not require a fasting state [71]. The laptop program for data entry has encoded logic to prevent out-of-range or inconsistent entries, and requires double entry of blood pressure, height, and weight.
2.9 Randomization
After completion of baseline assessments, participants are randomized via a computer-generated, random-number sequence, with block randomization of two, four, or six people per block, to the peer education study arm or to usual care (see Figure 1). Study arm assignments are in sealed, opaque envelopes. Randomization is by recruitment site; participants recruited from the community, academic medical center, neighborhood health center, and from the Visiting Nurse Service are randomized separately.
2.10 Post-randomization wrap up
Participants randomized to both groups receive a packet of culturally sensitive stroke education materials and a list of local health providers, including those who accept patients without health insurance. Each participant, regardless of study arm, receives a $20 gift card at enrollment as a thank you for their participation. All participants receive their height, weight, body mass index, and blood pressure results in writing, and each result is also reviewed verbally with staff. If body mass index is greater than 29 kg/m2, a staff member will inform the participant that his or her weight is too high, and will recommend that the participant discuss healthy weight goals with his or her health care providers. If systolic blood pressure is greater than 140 mmHg or diastolic blood pressure is greater than 90 mmHg, participants are told that their blood pressure is higher than recommended for a stroke or TIA survivor, and that they should discuss this result with their health care provider. For blood pressure results greater than 220/120, staff members recommend immediate care. If the participant does not have a primary care doctor, he or she is referred to emergency care. A staff member calls the participant within three days to follow up.
When LDL cholesterol results are available, letters are mailed to each participant. For LDL cholesterol, values above 100 mg/ dl are considered high. If LDL cholesterol is greater than 100 mg/ dl, each participant receives a phone call to notify him that his LDL cholesterol is too high and to recommend that he discuss this result with his health care provider. Participants are encouraged to bring the letter we send to their provider.
If participants screen positive for depression, they are told that the survey shows they have symptoms of depression. They are asked if they are being treated for depression, and are given a phone number to call to help locate a local mental health provider. A staff member will review with them the list of local health centers. These participants also receive a follow-up call within one week.
2.11 The intervention
Participants randomized to the PRAISE intervention are scheduled to attend the weekly peer-led workshops for six consecutive weeks, in ninety minute sessions. Workshops are led by a pair of peer leaders. At the end of each session, participants make an action plan and then report back to the group the next week. They are encouraged to build an action plan around something learned that week, like increasing physical activity or taking one’s medication as prescribed. Each session contains didactics specific to stroke and TIA survivors (see Table 1).
Participants randomized to usual care continue to receive the visits, medications, and tests routinely provided by their clinicians. Because Community Action Board members want all study enrollees to have the opportunity to benefit from the workshop, those in the usual care arm are offered the opportunity to attend the workshop after completing the one year of follow-up but are no longer surveyed as part of the study.
2.12 Follow up visits at 6 and 12 months
At 6 and 12 months from the date of randomization, participants are invited back for follow up data collection. Survey questions are read to participants from a laptop, and responses are entered into the laptop. Follow up surveys are shorter than the baseline assessment (see Table 2). Current medications are recorded. Blood pressure and weight are measured, and LDL cholesterol is checked. Similar to the enrollment visit, all results are reviewed with participants, and LDL cholesterol results are mailed to participants when they become available.
Table 2.
Data collection at 3 study time-points
| Domain | Baseline | 6 months | 12 months |
|---|---|---|---|
| Primary outcomes | |||
| Blood pressure | X | X | X |
| Direct LDL Cholesterol | X | X | X |
| Anti-thrombotic use (self-report) | X | X | X |
|
| |||
| Secondary outcomes | |||
| Body mass index (height, weight) | X | X | X |
| Smoking | X | X | X |
| Alcohol | X | X | X |
| Medication adherence | X | X | X |
| Health care utilization, cost-effectiveness | X | X | X |
|
| |||
| Other measures | |||
| Socio-demographics | X | ||
|
| |||
| Knowledge and beliefs | |||
| Stroke knowledge | X | X | X |
| Beliefs about Medicines | X | X | |
|
| |||
| Medical burden | |||
| Stroke impact | X | X | |
| Medical co-morbidities | X | X | X |
| Family history - stroke | X | ||
|
| |||
| Psychosocial burden | |||
| Depression | X | X | X |
| Post-traumatic stress disorder | X | X | |
| Health-related quality of life | X | X | X |
| Social support | X | X | X |
| Stress | X | X | X |
|
| |||
| Health behaviors | |||
| Use of medication reminders | X | X | X |
| Home blood pressure monitor | X | X | X |
|
| |||
| Environment | |||
| Access to care | X | X | |
| Neighborhood safety | X | ||
| Trust doctors | X | X | |
| Perceived racism/ discrimination | X | X | |
| Language barrier at doctor | X | X | |
2.13 Study retention
At enrollment, we distribute wallets and card magnifiers imprinted with the PRAISE logo and study phone number. We mail birthday cards and holiday cards to all participants. To facilitate convenient scheduling for follow up visits, we call participants 1–2 weeks before follow up visits are due. We offer evening and weekend time slots for workshops and follow up visits to accommodate participants’ schedules. Most important, in addition to training in recruitment, retention, surveying, and obtaining biological measures, project staff are trained in the importance of friendly customer service when interacting with study participants. Each participant is assigned one coordinator who shares his or her race, ethnicity, and/or primary language, to facilitate relationship-building
2.14 Outcome measures
Outcomes are measured at 6 and 12 months (see Table 2). There are two primary outcomes. The first is the control of hypertension (blood pressure <140/90 mmHg). The second is the composite of blood pressure control, control of lipids (LDL cholesterol <100 mg/dl) and regular use of anti-thrombotic medication (aspirin, clopidogrel, warfarin, dabigatran, enoxaparin, and others). Secondary outcomes include blood pressure and LDL cholesterol assessed as continuous variables, body mass index, smoking, alcohol use, medication adherence, stroke knowledge, health-related quality of life, health care utilization and cost effectiveness analysis. To assess medication adherence, in addition to measuring self-reported Morisky score at 6 and 12 months, we collect data from participants’ pharmacies regarding filling and re-filling of prescription medications to track medication adherence and use this information to calculate medication possession ratios [72, 73].
2.15 Sample size calculations
We calculated sample size using the more conservative of our two primary outcome measures: composite outcome of blood pressure control, LDL cholesterol control, and anti-thrombotic use. Six months after stroke, nearly three quarters of survivors do not have adequate control of these three pillar risk factors [18], with between one quarter and one third lacking control of each individual risk [16–20]. Assuming 70% underuse, to detect a 20% improvement in control of the composite outcome between the control group and the intervention group at six and twelve months (i.e., 70% in usual care vs. 56% in intervention), with 2 tailed test, 80% power and a 5% significance level, would require 186 participants in each trial arm, or 372 people. With a more conservative estimate of 60% underuse at baseline, we would need 270 participants per arm, or 540 total enrollees. Sample size was calculated with PASS 2008, Version: 08.0.13 [74]. Enrolling 600 participants would account for a 10% attrition rate even with the more conservative estimate of underuse, and so our recruitment target is 600.
2.16 Statistical analyses
We will evaluate the effect of the intervention on our primary outcomes using intention to treat analyses, adjusting for recruitment site. We will also conduct on-treatment analyses to evaluate whether participants who completed the intervention have different outcomes than those who did not. Intervention effects will be examined with chi-square tests at each follow up point to compare controlled risk factor proportions achieved by intervention and control groups, as well as using Generalized Estimation Equation models by controlling other possible covariates to assess the intervention effect over time. Because the outcome measure is the control or lack of control of risk factors, we chose GEE to model this longitudinal binary data to assess the effect of the intervention. By adding an interaction term of intervention with time and controlling for the baseline values, we can see if the change in risk factor control is due to the intervention [75]. We will also use a mixed model and compare the results obtained with the different models [76].
Another subgroup analysis will look at the relative effectiveness of the intervention on underuse in the three major racial and ethnic groups, namely Blacks, Latinos, and Whites. To account for type I error in all subgroup analyses, we will use the Bonferroni method, which reduces the probability value for defining statistical significance, by dividing the customary value of p=0.05 by the number of subgroups [77]. We will also report the power of the subgroup analysis given the obtained sample size.
We will use logistic regression or linear regression to test the relationship between intervention group as one of the independent variables along with covariates such as age, race, ethnicity, language spoken at home, insurance, time since stroke, and dependent variables such as knowledge, attitudes, beliefs and behaviors at six and twelve months. We will also use Generalized Estimation Equation models to evaluate possible changes over time in these dependent variables. Using three observations for each individual (baseline, 6 and 12 months), we will look for trends over the 12 months observation period.
3 Preliminary results
3.1 Baseline characteristics
From June 2009 until January 10, 2012, we enrolled 582 stroke and TIA survivors in the PRAISE trial (see Table 3), 57% from clinical sites, 28% from the Visiting Nurse Service of New York, and 15% from community sites. Enrollees have a mean age of 63 years, 60% are female, and 81% self-identify as Black or Latino (see Table 4). Most are low income with insurance coverage through Medicare and/or Medicaid. Rates of risk factor control are low, and two-thirds of enrollees do not have control of all the three major risk factors for recurrent stroke. Only 67% have blood pressure controlled, 58% have LDL cholesterol controlled, and 82% report regular use of anti-thrombotic medication.
Table 3.
Socio-demographics of stroke and TIA survivors in PRAISE (n=582)
| Total | |
|---|---|
| Age in years, mean ± SD | 63 ± 11 |
| Female gender | 60% |
| Race or ethnicity | |
| Black | 42% |
| Latino | 39% |
| White | 14% |
| Yearly income less than $15,000 | 56% |
| Less than high school education | 31% |
| Insurance | |
| Medicaid | 29% |
| Uninsured | 2% |
| Medicare | 52% |
| Commercial | 13% |
| Years since stroke, mean ± SD | 1.8 ± 1.5 |
Table 4.
Selected primary and secondary outcomes at baseline (n=582)
| Total | |
|---|---|
| Blood pressure controlled (< 140/90 mmHg) | 67% |
| LDL cholesterol controlled (<100 mg/ dl) | 58% |
| Regular use of anti-thrombotic medication | 82% |
| Control of all 3 risk factors | 34% |
| Smoking | 17% |
| Depression (PHQ-8 score ≥ 10) | 30% |
4 Discussion
In fewer than three years, we created a peer education course, developed a randomized trial to test its efficacy, and recruited nearly 600 stroke and TIA survivors to enroll in the trial. Our recruitment success may be credited to the enthusiasm of the stroke survivors in our Community Action Board and the enthusiasm of the community of clinicians who care for stroke survivors. The PRAISE participants are a racially and ethnically diverse group, of whom only one third have control of the three major risk factors for recurrent stroke and only two thirds have blood pressure at goal. The data collected for the study may reveal many of the barriers to recurrent stroke prevention in this vulnerable population. This study, with its novel use of peer education, may provide tools to help increase use of effective medical therapies in the post stroke population.
Acknowledgments
This work was supported by the National Institute on Minority Health and Health Disparities (5P60MD000270) and the National Center for Research Resources (UL1RR029887) of the National Institutes of Health. The funders were not involved in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2012 update: A report from the American Heart Association. Circulation. 2011 Dec 15; doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011 Jan;42(1):227–76. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- 3.Feinberg WM, Albers GW, Barnett HJ, Biller J, Caplan LR, Carter LP, et al. Guidelines for the management of transient ischemic attacks. From the ad hoc committee on guidelines for the management of transient ischemic attacks of the Stroke Council of the American Heart Association. Circulation. 1994 Jun;89(6):2950–65. doi: 10.1161/01.cir.89.6.2950. [DOI] [PubMed] [Google Scholar]
- 4.Burn J, Dennis M, Bamford J, Sandercock P, Wade D, Warlow C. Long-term risk of recurrent stroke after a first-ever stroke. The Oxfordshire community stroke project. Stroke. 1994 Feb;25(2):333–7. doi: 10.1161/01.str.25.2.333. [DOI] [PubMed] [Google Scholar]
- 5.Sacco RL, Shi T, Zamanillo MC, Kargman DE. Predictors of mortality and recurrence after hospitalized cerebral infarction in an urban community: The Northern Manhattan stroke study. Neurology. 1994 Apr;44(4):626–34. doi: 10.1212/wnl.44.4.626. [DOI] [PubMed] [Google Scholar]
- 6.Petty GW, Brown RD, Jr, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Survival and recurrence after first cerebral infarction: A population-based study in Rochester, Minnesota, 1975 through 1989. Neurology. 1998 Jan;50(1):208–16. doi: 10.1212/wnl.50.1.208. [DOI] [PubMed] [Google Scholar]
- 7.Thacker EL, Wiggins KL, Rice KM, Longstreth WT, Jr, Bis JC, Dublin S, et al. Short-term and long-term risk of incident ischemic stroke after transient ischemic attack. Stroke. 2010 Feb;41(2):239–43. doi: 10.1161/STROKEAHA.109.569707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: A systematic review. Stroke. 2003 Nov;34(11):2741–8. doi: 10.1161/01.STR.0000092488.40085.15. [DOI] [PubMed] [Google Scholar]
- 9.Lawes CM, Bennett DA, Feigin VL, Rodgers A. Blood pressure and stroke: An overview of published reviews. Stroke. 2004 Apr;35(4):1024. [PubMed] [Google Scholar]
- 10.Amarenco P, Bogousslavsky J, Callahan A, 3rd, Goldstein LB, Hennerici M, Rudolph AE, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006 Aug 10;355(6):549–59. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 11.Amarenco P, Goldstein LB, Szarek M, Sillesen H, Rudolph AE, Callahan A, 3rd, et al. Effects of intense low-density lipoprotein cholesterol reduction in patients with stroke or transient ischemic attack: The stroke prevention by aggressive reduction in cholesterol levels (SPARCL) trial. Stroke. 2007 Dec;38(12):3198–204. doi: 10.1161/STROKEAHA.107.493106. [DOI] [PubMed] [Google Scholar]
- 12.Johnson ES, Lanes SF, Wentworth CE, 3rd, Satterfield MH, Abebe BL, Dicker LW. A metaregression analysis of the dose-response effect of aspirin on stroke. Arch Intern Med. 1999 Jun 14;159(11):1248–53. doi: 10.1001/archinte.159.11.1248. [DOI] [PubMed] [Google Scholar]
- 13.Singer DE, Albers GW, Dalen JE, Fang MC, Go AS, Halperin JL, et al. Antithrombotic therapy in atrial fibrillation: American college of chest physicians evidence-based clinical practice guidelines (8th edition) Chest. 2008 Jun;133(6 Suppl):546S–92S. doi: 10.1378/chest.08-0678. [DOI] [PubMed] [Google Scholar]
- 14.Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. EAFT (European Atrial Fibrillation Trial) study group. Lancet. 1993 Nov 20;342(8882):1255–62. [PubMed] [Google Scholar]
- 15.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994 Jul 11;154(13):1449–57. [PubMed] [Google Scholar]
- 16.Hillen T, Dundas R, Lawrence E, Stewart JA, Rudd AG, Wolfe CD. Antithrombotic and antihypertensive management 3 months after ischemic stroke : A prospective study in an inner city population. Stroke. 2000 Feb;31(2):469–75. doi: 10.1161/01.str.31.2.469. [DOI] [PubMed] [Google Scholar]
- 17.Rahiman A, Saver JL, Porter V, Buxton W, McNair N, Razinia T, et al. In-hospital initiation of secondary prevention is associated with improved vascular outcomes at 3 months. J Stroke Cerebrovasc Dis. 2008 Jan-Feb;17(1):5–8. doi: 10.1016/j.jstrokecerebrovasdis.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Tuhrim S, Cooperman A, Rojas M, Brust JC, Koppel B, Martin K, et al. The association of race and sex with the underuse of stroke prevention measures. J Stroke Cerebrovasc Dis. 2008 Jul-Aug;17(4):226–34. doi: 10.1016/j.jstrokecerebrovasdis.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Bushnell CD, Zimmer LO, Pan W, Olson DM, Zhao X, Meteleva T, et al. Persistence with stroke prevention medications 3 months after hospitalization. Arch Neurol. 2010 Dec;67(12):1456–63. doi: 10.1001/archneurol.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bushnell CD, Olson DM, Zhao X, Pan W, Zimmer LO, Goldstein LB, et al. Secondary preventive medication persistence and adherence 1 year after stroke. Neurology. 2011 Sep 20;77(12):1182–90. doi: 10.1212/WNL.0b013e31822f0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheinart KF, Tuhrim S, Horowitz DR, Weinberger J, Goldman M, Godbold JH. Stroke recurrence is more frequent in blacks and Hispanics. Neuroepidemiology. 1998;17(4):188–98. doi: 10.1159/000026172. [DOI] [PubMed] [Google Scholar]
- 22.Morgenstern LB, Smith MA, Lisabeth LD, Risser JM, Uchino K, Garcia N, et al. Excess stroke in Mexican Americans compared with non-Hispanic whites: The brain attack surveillance in Corpus Christi project. Am J Epidemiol. 2004 Aug 15;160(4):376–83. doi: 10.1093/aje/kwh225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christian JB, Lapane KL, Toppa RS. Racial disparities in receipt of secondary stroke prevention agents among US nursing home residents. Stroke. 2003 Nov;34(11):2693–7. doi: 10.1161/01.STR.0000096993.90248.27. [DOI] [PubMed] [Google Scholar]
- 24.Birman-Deych E, Radford MJ, Nilasena DS, Gage BF. Use and effectiveness of warfarin in Medicare beneficiaries with atrial fibrillation. Stroke. 2006 Apr;37(4):1070–4. doi: 10.1161/01.STR.0000208294.46968.a4. [DOI] [PubMed] [Google Scholar]
- 25.Brenner DA, Zweifler RM, Gomez CR, Kissela BM, Levine D, Howard G, et al. Awareness, treatment, and control of vascular risk factors among stroke survivors. J Stroke Cerebrovasc Dis. 2010 Jul-Aug;19(4):311–20. doi: 10.1016/j.jstrokecerebrovasdis.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ovbiagele B, Saver JL, Fredieu A, Suzuki S, Selco S, Rajajee V, et al. In-hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow-up. Stroke. 2004 Dec;35(12):2879–83. doi: 10.1161/01.STR.0000147967.49567.d6. [DOI] [PubMed] [Google Scholar]
- 27.Touze E, Coste J, Voicu M, Kansao J, Masmoudi R, Doumenc B, et al. Importance of inhospital initiation of therapies and therapeutic inertia in secondary stroke prevention: IMplementation of prevention after a cerebrovascular evenT (IMPACT) study. Stroke. 2008 Jun;39(6):1834–43. doi: 10.1161/STROKEAHA.107.503094. [DOI] [PubMed] [Google Scholar]
- 28.Boysen G, Krarup LH, Zeng X, Oskedra A, Korv J, Andersen G, et al. ExStroke pilot trial of the effect of repeated instructions to improve physical activity after ischaemic stroke: A multinational randomised controlled clinical trial. BMJ. 2009 Jul 22;339:b2810. doi: 10.1136/bmj.b2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McManus JA, Craig A, McAlpine C, Langhorne P, Ellis G. Does behaviour modification affect post-stroke risk factor control? Three-year follow-up of a randomized controlled trial. Clin Rehabil. 2009 Feb;23(2):99–105. doi: 10.1177/0269215508095874. [DOI] [PubMed] [Google Scholar]
- 30.Johnston SC, Sidney S, Hills NK, Grosvenor D, Klingman JG, Bernstein A, et al. Standardized discharge orders after stroke: Results of the quality improvement in stroke prevention (QUISP) cluster randomized trial. Ann Neurol. 2010 May;67(5):579–89. doi: 10.1002/ana.22019. [DOI] [PubMed] [Google Scholar]
- 31.Wolfe CD, Redfern J, Rudd AG, Grieve AP, Heuschmann PU, McKevitt C. Cluster randomized controlled trial of a patient and general practitioner intervention to improve the management of multiple risk factors after stroke: Stop stroke. Stroke. 2010 Nov;41(11):2470–6. doi: 10.1161/STROKEAHA.110.588046. [DOI] [PubMed] [Google Scholar]
- 32.Persky V, Coover L, Hernandez E, Contreras A, Slezak J, Piorkowski J, et al. Chicago community-based asthma intervention trial: Feasibility of delivering peer education in an inner-city population. Chest. 1999 Oct;116(4 Suppl 1):216S–23S. doi: 10.1378/chest.116.suppl_2.216s. [DOI] [PubMed] [Google Scholar]
- 33.Rose MA. Evaluation of a peer-education program on heart disease prevention with older adults. Public Health Nurs. 1992 Dec;9(4):242–7. doi: 10.1111/j.1525-1446.1992.tb00108.x. [DOI] [PubMed] [Google Scholar]
- 34.Taylor T, Serrano E, Anderson J, Kendall P. Knowledge, skills, and behavior improvements on peer educators and low-income Hispanic participants after a stage of change-based bilingual nutrition education program. J Community Health. 2000 Jun;25(3):241–62. doi: 10.1023/a:1005160216289. [DOI] [PubMed] [Google Scholar]
- 35.Lorig KR, Ritter P, Stewart AL, Sobel DS, Brown BW, Jr, Bandura A, et al. Chronic disease self-management program: 2-year health status and health care utilization outcomes. Med Care. 2001 Nov;39(11):1217–23. doi: 10.1097/00005650-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Lorig KR, Sobel DS, Stewart AL, Brown BW, Jr, Bandura A, Ritter P, et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: A randomized trial. Med Care. 1999 Jan;37(1):5–14. doi: 10.1097/00005650-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Foster G, Taylor SJ, Eldridge SE, Ramsay J, Griffiths CJ. Self-management education programmes by lay leaders for people with chronic conditions. Cochrane Database Syst Rev. 2007 Oct 17;(4):CD005108. doi: 10.1002/14651858.CD005108.pub2. [DOI] [PubMed] [Google Scholar]
- 38.Jones F. Strategies to enhance chronic disease self-management: How can we apply this to stroke? Disabil Rehabil. 2006 Jul 15–30;28(13–14):841–7. doi: 10.1080/09638280500534952. [DOI] [PubMed] [Google Scholar]
- 39.Marsden D, Quinn R, Pond N, Golledge R, Neilson C, White J, et al. A multidisciplinary group programme in rural settings for community-dwelling chronic stroke survivors and their carers: A pilot randomized controlled trial. Clin Rehabil. 2010 Apr;24(4):328–41. doi: 10.1177/0269215509344268. [DOI] [PubMed] [Google Scholar]
- 40.Harrington R, Taylor G, Hollinghurst S, Reed M, Kay H, Wood VA. A community-based exercise and education scheme for stroke survivors: A randomized controlled trial and economic evaluation. Clin Rehabil. 2010 Jan;24(1):3–15. doi: 10.1177/0269215509347437. [DOI] [PubMed] [Google Scholar]
- 41.W.K. Kellogg Foundation Evaluation Handbook. Battle Creek. Michigan: W.K. Kellogg Foundation; 1998. [Google Scholar]
- 42.Horowitz CR, Robinson M, Seifer S. Community-based participatory research from the margin to the mainstream: Are researchers prepared? Circulation. 2009 May 19;119(19):2633–42. doi: 10.1161/CIRCULATIONAHA.107.729863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Plumb EJ, Chan PS, Villa FJ, Robinson V, Horowitz CR. Identifying patient barriers to recurrent stroke prevention in Harlem. Society of general internal medicine; 2010. [Google Scholar]
- 44.Horowitz CR, Arniella A, James S, Bickell NA. Using community-based participatory research to reduce health disparities in east and central Harlem. Mt Sinai J Med. 2004 Nov;71(6):368–74. [PMC free article] [PubMed] [Google Scholar]
- 45.Goldfinger JZ, Arniella G, Wylie-Rosett J, Horowitz CR. Project HEAL: Peer education leads to weight loss in Harlem. J Health Care Poor Underserved. 2008 Feb;19(1):180–92. doi: 10.1353/hpu.2008.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lorig K, Ritter PL, Villa FJ, Armas J. Community-based peer-led diabetes self-management: A randomized trial. Diabetes Educ. 2009 Jul-Aug;35(4):641–51. doi: 10.1177/0145721709335006. [DOI] [PubMed] [Google Scholar]
- 47.Gifford AL, Laurent DD, Gonzales VM, Chesney MA, Lorig KR. Pilot randomized trial of education to improve self-management skills of men with symptomatic HIV/AIDS. J Acquir Immune Defic Syndr Hum Retrovirol. 1998 Jun 1;18(2):136–44. doi: 10.1097/00042560-199806010-00005. [DOI] [PubMed] [Google Scholar]
- 48.Lorig K, Ritter PL, Plant K. A disease-specific self-help program compared with a generalized chronic disease self-help program for arthritis patients. Arthritis Rheum. 2005 Dec 15;53(6):950–7. doi: 10.1002/art.21604. [DOI] [PubMed] [Google Scholar]
- 49.Battersby M, Hoffmann S, Cadilhac D, Osborne R, Lalor E, Lindley R. ‘Getting your life back on track after stroke’: A phase II multi-centered, single-blind, randomized, controlled trial of the stroke self-management program vs the Stanford chronic condition self-management program or standard care in stroke survivors. Int J Stroke. 2009 Apr;4(2):137–44. doi: 10.1111/j.1747-4949.2009.00261.x. [DOI] [PubMed] [Google Scholar]
- 50.Cadilhac DA, Hoffmann S, Kilkenny M, Lindley R, Lalor E, Osborne RH, et al. A phase II multicentered, single-blind, randomized, controlled trial of the stroke self-management program. Stroke. 2011 Jun;42(6):1673–9. doi: 10.1161/STROKEAHA.110.601997. [DOI] [PubMed] [Google Scholar]
- 51.Boden-Albala B, Litwak E, Elkind MS, Rundek T, Sacco RL. Social isolation and outcomes post stroke. Neurology. 2005 Jun 14;64(11):1888–92. doi: 10.1212/01.WNL.0000163510.79351.AF. [DOI] [PubMed] [Google Scholar]
- 52.Brady MC, Clark AM, Dickson S, Paton G, Barbour RS. The impact of stroke-related dysarthria on social participation and implications for rehabilitation. Disabil Rehabil. 2011;33(3):178–86. doi: 10.3109/09638288.2010.517897. [DOI] [PubMed] [Google Scholar]
- 53.Haley WE, Roth DL, Kissela B, Perkins M, Howard G. Quality of life after stroke: A prospective longitudinal study. Qual Life Res. 2011 Aug;20(6):799–806. doi: 10.1007/s11136-010-9810-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taylor GH, Todman J, Broomfield NM. Post-stroke emotional adjustment: A modified social cognitive transition model. Neuropsychol Rehabil. 2011 Dec;21(6):808–24. doi: 10.1080/09602011.2011.598403. [DOI] [PubMed] [Google Scholar]
- 55.Suchman AL, Williamson PR, Litzelman DK, Frankel RM, Mossbarger DL, Inui TS, et al. Toward an informal curriculum that teaches professionalism. transforming the social environment of a medical school. J Gen Intern Med. 2004 May;19(5 Pt 2):501–4. doi: 10.1111/j.1525-1497.2004.30157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jordan LC, Hillis AE. Challenges in the diagnosis and treatment of pediatric stroke. Nat Rev Neurol. 2011 Apr;7(4):199–208. doi: 10.1038/nrneurol.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Putaala J, Metso AJ, Metso TM, Konkola N, Kraemer Y, Haapaniemi E, et al. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke: The Helsinki young stroke registry. Stroke. 2009 Apr;40(4):1195–203. doi: 10.1161/STROKEAHA.108.529883. [DOI] [PubMed] [Google Scholar]
- 58.Wilson JT, Hareendran A, Grant M, Baird T, Schulz UG, Muir KW, et al. Improving the assessment of outcomes in stroke: Use of a structured interview to assign grades on the modified Rankin scale. Stroke. 2002 Sep;33(9):2243–6. doi: 10.1161/01.str.0000027437.22450.bd. [DOI] [PubMed] [Google Scholar]
- 59.Reeves MJ, Hogan JG, Rafferty AP. Knowledge of stroke risk factors and warning signs among Michigan adults. Neurology. 2002 Nov 26;59(10):1547–52. doi: 10.1212/01.wnl.0000031796.52748.a5. [DOI] [PubMed] [Google Scholar]
- 60.Schneider AT, Pancioli AM, Khoury JC, Rademacher E, Tuchfarber A, Miller R, et al. Trends in community knowledge of the warning signs and risk factors for stroke. JAMA. 2003 Jan 15;289(3):343–6. doi: 10.1001/jama.289.3.343. [DOI] [PubMed] [Google Scholar]
- 61.Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999 Dec;47(6):555–67. doi: 10.1016/s0022-3999(99)00057-4. [DOI] [PubMed] [Google Scholar]
- 62.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001 Sep;16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009 Apr;114(1–3):163–73. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 64.Ware J, Jr, Kosinski M, Keller SD. A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996 Mar;34(3):220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 65.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): An effective brief screening test for problem drinking. ambulatory care quality improvement project (ACQUIP). alcohol use disorders identification test. Arch Intern Med. 1998 Sep 14;158(16):1789–95. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 66.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich) 2008 May;10(5):348–54. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.LaVeist TA, Nickerson KJ, Bowie JV. Attitudes about racism, medical mistrust, and satisfaction with care among African American and white cardiac patients. Med Care Res Rev. 2000;57( Suppl 1):146–61. doi: 10.1177/1077558700057001S07. [DOI] [PubMed] [Google Scholar]
- 68.Stewart AL, Napoles-Springer A, Perez-Stable EJ. Interpersonal processes of care in diverse populations. Milbank Q. 1999;77(3):305, 39, 274. doi: 10.1111/1468-0009.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves JW, Hill MN, et al. Recommendations for blood pressure measurement in humans: An AHA scientific statement from the council on high blood pressure research professional and public education subcommittee. J Clin Hypertens (Greenwich) 2005 Feb;7(2):102–9. doi: 10.1111/j.1524-6175.2005.04377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Myers MG, Godwin M, Dawes M, Kiss A, Tobe SW, Grant FC, et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: Randomised parallel design controlled trial. BMJ. 2011 Feb 7;342:d286. doi: 10.1136/bmj.d286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dungan KM, Guster T, DeWalt DA, Buse JB. A comparison of lipid and lipoprotein measurements in the fasting and nonfasting states in patients with type 2 diabetes. Curr Med Res Opin. 2007 Nov;23(11):2689–95. doi: 10.1185/030079907x233304. [DOI] [PubMed] [Google Scholar]
- 72.Monane M, Bohn RL, Gurwitz JH, Glynn RJ, Avorn J. Noncompliance with congestive heart failure therapy in the elderly. Arch Intern Med. 1994 Feb 28;154(4):433–7. [PubMed] [Google Scholar]
- 73.Avorn J, Monette J, Lacour A, Bohn RL, Monane M, Mogun H, et al. Persistence of use of lipid-lowering medications: A cross-national study. JAMA. 1998 May 13;279(18):1458–62. doi: 10.1001/jama.279.18.1458. [DOI] [PubMed] [Google Scholar]
- 74.Hintze J. PASS. NCSS, LLC; Kaysville, Utah: 2008. www.ncss.com. [Google Scholar]
- 75.Hubbard AE, Ahern J, Fleischer NL, Van der Laan M, Lippman SA, Jewell N, et al. To GEE or not to GEE: Comparing population average and mixed models for estimating the associations between neighborhood risk factors and health. Epidemiology. 2010 Jul;21(4):467–74. doi: 10.1097/EDE.0b013e3181caeb90. [DOI] [PubMed] [Google Scholar]
- 76.Koper N, Manseau M. Generalized estimating equations and generalized linear mixed-effects models for modelling resource selection. J Appl Ecol. 2009;46(3):590–9. [Google Scholar]
- 77.Yusuf S, Wittes J, Probstfield J, Tyroler HA. Analysis and interpretation of treatment effects in subgroups of patients in randomized clinical trials. JAMA. 1991 Jul 3;266(1):93–8. [PubMed] [Google Scholar]