Abstract
Slow or insufficient enrollment in clinical research and a high demand for research participants raises questions about the need for and use of incentives to participate, including payment. Much of the available literature on payment to research participants focuses on ethical concerns, and rarely addresses guidelines, benchmarks, or formulas to assist investigators to assign or evaluate appropriate payment for individuals who take part in clinical research trials and procedures.
Using four years of data collected about the inconvenience units assigned by intramural investigators to selected clinical research procedures conducted at the National Institutes of Health (NIH) Clinical Center, this study provides payment benchmarks for commonly performed procedures.
Results were obtained from data collected on 36,273 incidents of payment made for procedures to research participants from August 2004 to August 2008. Analysis of the inconvenience units value assigned to specific procedures suggests that despite a wide distribution and frequent outliers, a convergence in practice around the center of distribution for most procedures does exist. As one of the first published studies reporting data reflecting payment amount for specific clinical research procedures, these data can guide investigators and institutional review boards as they establish and review an appropriate amount of payment to offer research participants. Our data may be useful in promoting payment standards for procedures, thereby complementing proposals or guidelines that advise payment calculations according to time and procedures.
Keywords: Paying Research Subjects, Remuneration, Compensating Participants
INTRODUCTION
Recruitment and subsequent participation of healthy and patient clinical research volunteers is essential for the success of a clinical trial, although the demand for research participants continues to exceed the number of individuals willing to take part. Often studies or investigational teams report the need to extend the recruitment period, or they find that they must reevaluate the cost and effort needed to enroll the required number of participants [1]. It has been estimated that recruitment difficulties cause clinical trials to take over 30% longer than anticipated, in addition to being delayed, over budget, or abandoned because of lack of participation [2]. Slow or insufficient enrollment in clinical research and a high demand for research participants raises questions about the need for and use of incentives to participate, including payment. Limited empirical data suggests that investigators pay to reimburse participants or compensate them for their inconvenience [3] and although some participants credit altruistic reasons as a motivational factor, financial compensation can ultimately improve participation [4]. Nonetheless, we know little about how payment is calculated and how much research participants are offered.
Investigators and institutional review boards (IRBs) grapple with decisions about the appropriate amount of payment to offer research participants without unduly influencing them [5]. Yet, IRBs and others disagree about the meaning and prevalence of undue influence as well as to what extent payment poses a risk of undue inducement [6]. Standards for determining an appropriate amount of payment in a given study are lacking, leaving many research institutions or IRBs to make decisions without clear guidance or benchmarks [7]. In addition, published journal articles rarely mention the amount of payment to research participants or even if there is any sort of remuneration at all [8]. Limited data or advice exists for research teams deciding whether and how much payment they should offer to study participants, as well as limited guidance for IRBs deciding whether or not to approve proposed amounts, despite previous calls for more specific guidance, “To ensure local standardization of payment, research institutions and institutional review boards should develop specific policies or guidelines outlining how investigators should determine which cases and in what manner to pay subjects who enroll in their studies,” [5, p. 202].
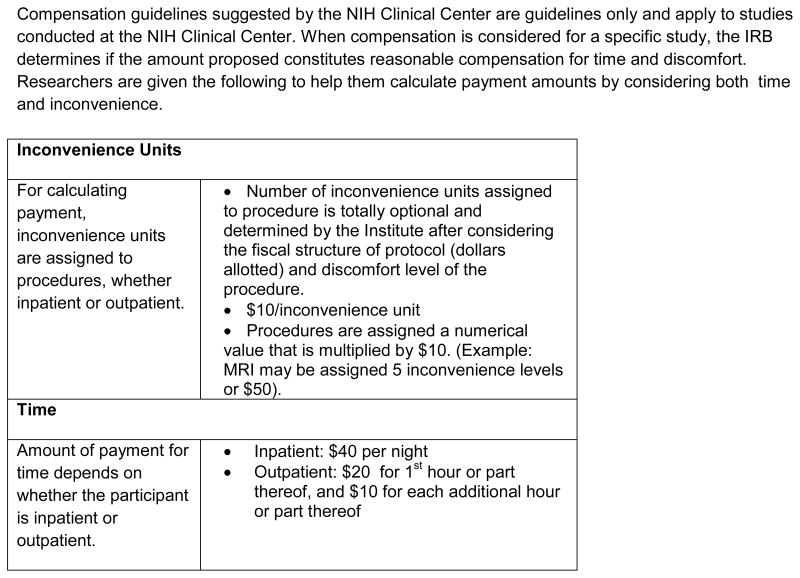
Guidelines for clinical trial remuneration do exist at the Clinical Center at the National Institutes of Health (NIH) in Bethesda, Maryland where there are approximately 1,500 intramural clinical research studies conducted annually [9]. Of the 1,500 intramural studies about 44% are clinical trials, 40% are natural history and the remainder are pharmacodynamics/kinetics, screening, training and data analysis protocols. The NIH intramural research program provides guidance for investigators regarding payment to research participants and recommends calculating payment by considering both time and inconvenience [19, 20]. The NIH guidance, originally established in 1981 with revisions last made in 1994 (Figure 1), first instructs investigators to calculate payment for time and provides a recommended dollar amount based on the amount of time for an outpatient visit and or an inpatient stay. In addition to time, the guidelines instruct investigators to assign a number of “inconvenience units” to the study-related procedures and interventions. The investigator determines a number of inconvenience units based on the research budget and the anticipated discomfort or inconvenience of the given procedure. Each inconvenience unit is $10.00. Based on this guidance, if an investigator assigns five inconvenience units to a magnetic resonance imaging scan (MRI) and a subject has a two hour clinic visit and an MRI, the total paid to this subject would be $80.00 ($30 for the time and $50 for the MRI) (Figure 1).
FIGURE 1.
NIH Guidance for compensation of research participants
Complete document can be found: http://www.medtran.ru/eng/trials/protomechanics/ch1.htm [20].
At this point, there are no rules, limits, benchmarks, or other tools to help NIH investigators at the Clinical Center assign inconvenience units, these are described as optional and the number is assigned at the discretion of each investigational team. Sparse data in the literature and from other institutions provides a limited opportunity for benchmark or comparison, but this fact remains: There is little available to investigators seeking assistance in how to determine the appropriate value of inconvenience units associated with common clinical research procedures.
Using four years of data collected about the inconvenience units assigned to procedures by investigators conducting intramural research at the NIH Clinical Center, this study provides useful benchmarks for calculating payment for commonly performed procedures in clinical research. These data will assist investigators by providing benchmarks for one part of the complex task of calculating payment to research participants.
METHODS
This report includes analysis of common and specific clinical research procedures for which inconvenience units were assigned by an investigator or study team. Results reported were extracted from data collected by the NIH Clinical Research Volunteer Program (CRVP) on inconvenience units assigned to procedures already completed in IRB approved studies. These inconvenience unit data describe payments made to research participants for specific procedures in the intramural research program of the NIH Clinical Center from August 2004 to August 2008.
The CRVP database created in 1996 with the cooperation of all NIH institutes, is a central repository through which research volunteer payments for subjects enrolled in IRB approved studies are tracked. The database has provided benefits to both investigators and research volunteers alike through, for example, reduced payment processing times and readily available volunteer payment history. Requests for payment, which is typically mailed to participants in the form of a check, are reported to the CRVP by individual research teams who have the option to itemize payment both by time and the inconvenience associated with study procedures consistent with the guidelines outlined in Figure 1. Payment for time alone, and payment for which the investigator proposed a total amount without itemizing time or inconvenience units to a specific procedure, were excluded from this analysis. This report analyzes the frequency with which specific procedures were reported, as well as the number of inconvenience units assigned to the procedure by the investigational teams.
We generated a list of all procedures to which inconvenience units were assigned, and reviewed and consolidated them such that procedures using similar but not identical terms were collapsed. For example, procedures entered as electrocardiogram, EKG, or ECG were collapsed into one category, and blood draw, venipuncture, or phlebotomy were collapsed into another. Listed procedures that incorporated a wide variation of types or were non-specific (e.g., clinic visit, evaluation, medication/treatment, or testing), listed multiple procedures that could not be collapsed (e.g., screening, blood ratings, or transmagnetic stimulation) or lacked identifiable categorization (e.g., movement restriction, task, or training) were excluded (total n=14,247 or 28.2%); leaving 36,273 (71.8%) entries for analysis of 21 procedures (Table 1). The final list of procedures summarized in Table 1 met the following criteria: procedures that were easily identifiable, used standard nomenclature, were frequently used in clinical research studies, and had inconvenience units assigned for payment purposes. We report the frequency of the procedures and the distribution [mean ± standard deviation, and median (inter-quartile range)] of the number of inconvenience units assigned for them. The data were analyzed using SAS v9.2 (SAS Institute, Inc, Cary, NC).
TABLE 1.
Frequency of procedures and details of their inconvenience units on payments made to research participants in the intramural program of the NIH Clinical Center from August 2004 to August 2008. Data collected by the NIH CRVP.
| PROCEDURE1 | OBSERVATIONS N (%) |
INCONVENIENCE UNITS Mean (± SD) Median (25th percentile, 75th percentile) Range (minimum-maximum) |
|---|---|---|
| Aphaeresis | 90 (0.25) | 8.06 ± 4.52 8.00 (4.00, 11.00) 2–20 |
| Biopsy2 | 65 (0.18) | 5.63 ± 1.61 5.00 (5.00, 8.00) 3–8 |
| Blood draw | 5,212 (14.37) | 2.25 ± 1.80 2.00 (1.00, 2.00) 0–21 |
| Dual energy x-ray absorptiometry scan (DEXA) | 291 (0.80) | 3.30 ± 2.19 2.00 (2.00, 6.00) 1–9 |
| Electrocardiography (EKG) | 1,156 (3.19) | 2.16 ± 2.01 1.00 (1.00, 4.00) 0–10 |
| Electroencephalogram (EEG) | 452 (1.25) | 5.35 ± 16.70 3.00 (2.00, 4.00) 0–128 |
| Electromyography (EMG) | 242 (0.67) | 3.37 ± 5.63 1.00 (1.00, 4.00) 0–39 |
| Functional magnetic resonance imaging scan (fMRI) | 204 (0.56) | 7.07 ± 3.47 10.00 (5.00, 10.00) 0–10 |
| History and physical | 3,098 (8.54) | 2.02 ± 2.10 1.00 (1.00, 2.00) 0–30 |
| Intravenous line (IV) | 1,976 (5.45) | 4.44 ± 2.59 4.00 (3.00, 5.00) 0–39 |
| Lumbar puncture (LP) | 48 (0.13) | 16.92 ± 19.49 5.00 (1.00, 29.00) 1–57 |
| Magnetic resonance imaging scan (MRI) | 9,434 (26.01) | 5.32 ± 3.69 5.00 (4.00, 5.00) 0–40 |
| Magnetoencephalogram (MEG) | 257 (0.71) | 3.72 ± 1.86 3.00 (3.00, 4.00) 0–8 |
| Positron emission tomography scan (PET) | 1,641 (4.52) | 10.41 ± 6.83 10.00 (5.00, 13.00) 1–65 |
| Psychological/cognitive testing | 3,883 (10.70) | 2.99 ± 2.11 3.00 (2.00, 3.00) 0–21 |
| Serial blood draw | 194 (0.53) | 4.85 ± 4.56 3.00 (2.00, 7.00) 1–40 |
| Structured clinical interview for DSM (SCID) | 385 (1.06) | 1.47 ± 0.59 1.00 (1.00, 2.00) 0–5 |
| 24 Hour urine collection | 224 (0.62) | 1.99 ± 1.35 2.00 (1.00, 2.00) 0–10 |
| Ultrasound | 116 (0.32) | 1.36 ± 0.75 1.00 (1.00, 2.00) 0–5 |
| Urinalysis | 873 (2.41) | 1.42 ± 0.92 1.00 (1.00, 2.00) 1–14 |
| Videofluoroscopy | 35 (0.10) | 7.17 ± 5.81 4.00 (4.00, 12.00) 2–21 |
Procedures were selected based on the following criteria: easily identifiable, used standard nomenclature, frequently used in clinical research studies, and had inconvenience units assigned for payment purposes. Medline Plus used for names of abbreviated procedures.
Does not include biopsies of major organs such as liver, kidney, etc.
RESULTS
We analyzed 36,273 procedures for which inconvenience units were assigned between 2004 and 2008 at the NIH. Table 1 details frequencies and summary statistics for procedures and inconvenience units. The five most frequently utilized procedures to which inconvenience units were assigned include, in descending order, MRI, blood draw, psychological or cognitive testing, history and physical, and intravenous line. The most commonly reported procedure during the study period was MRI entered in the database 9,434 times (26.0% of procedures) and the least frequently was videofluoroscopy (35 instances).
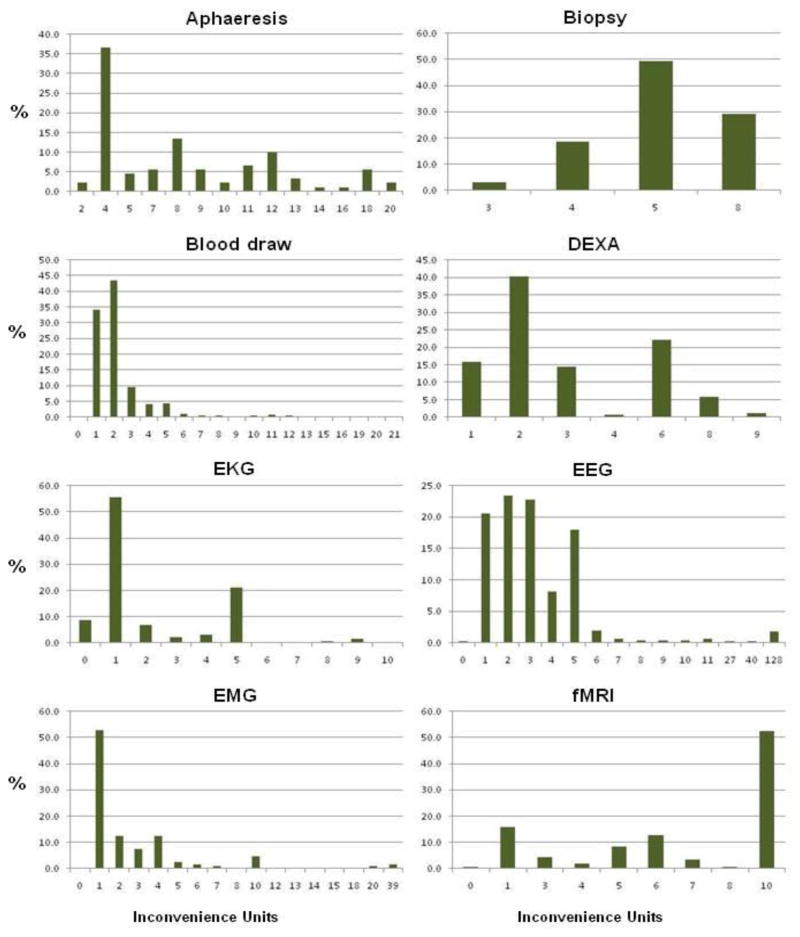
The number of assigned inconvenience units across procedures varied (Figure 2). Several procedures were assigned 1 as the median number of inconvenience, including history and physical, EKG, Electromyography (EMG), urinalysis, structured clinical interview with DSM (SCID), and ultrasound. For other procedures, the median number of inconvenience units assigned was 5 or above, including for example aphaeresis, biopsy, MRI and functional MRI (fMRI), positron emission tomography (PET), and lumbar puncture (LP). The distribution of inconvenience units assigned per procedure was also often wide (see Table 1), ranging from a minimum unit of 0 to a maximum of 128 for all procedures. The range, for example, for an LP was 1 to 57 inconvenience units, for PET scanning 1 to 65, and for electroencephalogram (EEG) 0 to 128.
FIGURE 2.
Histograms of the distributions of the inconveniences units (and their percentages) on payments made to research participants in the intramural program of the NIH Clinical Center from August 2004 to August 2008. These supplement detailed data in Table 1 and provide a visual description of the range and occurrence of inconvenience units assigned for each procedure that can also be compared to each other at a glance. Data collected by the NIH CRVP.
The range of inconvenience units for each procedure is unique to its distribution. Due to a wide range of units for some, the units presented on the x-axis are not equidistant and may jump numbers. In addition, depending on the distribution, some low frequencies may not be visible in the figure.
Despite the wide distribution, there was usually convergence in practice around the center of the distributions. For example, as shown by the summary statistics and histograms in Figure 2, the mean (5.35 ± 16.7) and median [3.00 (2.00–4.00)] for electroencephalogram (EEG), reflect the fact that, despite the wide range and skewed distribution, the majority of EEGs were assigned 5 or fewer inconvenience units. Although the range of assigned inconvenience units was broad for the two procedures for which inconvenience units were most frequently assigned, MRI (1–40) and blood draw (0–21), the mean and median were similar in each case (MRI: mean 5.3, median 5; blood draw: mean 2.3, median 2) reflecting convergence around the mean despite the outliers.
DISCUSSION
This is the first published study reporting data that reflect payment amounts for procedures commonly used in clinical research. By analyzing inconvenience units as the basis for calculating payment amounts for common study procedures, our data offer possible benchmarks for how much to pay research participants for commonly used procedures. These data should provide welcome guidance to investigators and IRBs when determining and reviewing an appropriate amount of payment to offer to research participants. These data may also be useful to promote standardization of payment for procedures, thereby complementing proposals or guidelines that advise payment calculations according to time and procedures.
Previous research provided limited information about practices related to payment of research participants, especially regarding dollar amounts or calculating payment. One study of 467 clinical research protocols in the U.S. found unexplained variation in dollar amounts for similar procedures across studies even when conducted at one site and also different dollar amounts between sites for the same multi-site study [10]. Another study that asked IRB chairs and investigators how much research participants should be offered in six hypothetical studies found that although most respondents consider inconvenience an important factor in deciding about payment for participants, there were inconsistencies in other factors considered for determining payment [11]. An earlier study showed that among a small number of research organizations that had written guidance about payment, the written guidance described payment almost exclusively as compensation for time and inconvenience [7]. Only a quarter of these organizations had formulas for calculating payment amounts, and the formulas varied both in their level of specificity and the basis for calculating payment. All such formulas included a payment amount per visit or per hour. The majority described supplemental payment for procedures or the inconvenience of procedures, although none specified payment amounts per procedure. Another study found that even when organizations had written policies regarding compensation, 38% of research ethics committee members were not aware and 38% were not sure if they existed [3].
Several models for payment have been proposed in the literature, including a market model, wage payment model, reimbursement model, [5] and a fair share model [12]. Data on common payment for procedures, such as those included in this paper, could facilitate implementation of a wage payment type model. The wage payment model recommends calculating payment according to the amount of time research participants contribute to research with additional amounts for the inconvenience of procedures and interventions [5]. One advantage expected with a wage payment type model is greater standardization of payment amounts across studies. Standardization of payment is valuable because it could reduce competition for research participants which in turn not only helps to contain research costs, but also does not privilege studies with larger research budgets over worthy studies with smaller budgets. Standardization also recognizes the importance of paying similar amounts for similar contributions, a key application of the principle of justice, and would help investigators and IRBs as they determine appropriate levels of payment in a given study. The data presented here could be used to develop a standard range of payment amounts for commonly used research procedures that would promote standardization across studies.
There continues to be a general admonition against considering payment as compensation for risk and some disagreement about how much risk should be a factor in determining payment amounts. Institutional guidance often warns against payment for risk, and IRB members and staff downplay the influence of risk on determining payment amounts in favor of payment as reimbursement and compensation for time and inconvenience [6]. Despite this, research participants appear to assume that more money in a research study implies that the study has more risk [13]. In our analysis, the highest number of inconvenience units (mean and median) was assigned to procedures such as aphaeresis, LP, and PET scan, procedures that are more invasive and have more potential risks than many of the other procedures listed. In the NIH guidelines, the term inconvenience is not explicitly defined; however, common meanings of inconvenience include aggravation, difficulty, troublesomeness, all of which could be compatible with risk or discomfort.
Of note the measures of central tendency, where conflicting, were likely influenced by a skewed distribution of assigned inconvenience units for some of the common procedures—see for example, LP. In addition, there were clearly outliers, as for example, EEG where 128 inconvenience units were assigned. There could be several reasons for outliers, but further investigation would be necessary in these cases to understand the reasons. It is possible, for example, that some investigators wanted to offer more money for a study than what could be calculated by strictly following the formula, in which case the investigators might have started with a preconceived dollar amount and used that as the basis for determining inconvenience units. Another possibility is that investigators think that inconvenience is influenced by characteristics of the participant pool, or by other features of the particular study such as whether the procedure is part of a complex study with multiple procedures. Future research to investigate how features of the participant pool or study complexity are accounted for when assigning inconvenience units would be helpful.
Despite the wide range of assigned inconvenience units and some clear outliers, convergence around the means and medians of assigned inconvenience units provide a possible benchmark for calculating and standardizing payment for similar procedures. Further support for using these numbers as possible benchmarks comes from their concordance with a few other available lists of per procedure payment amounts [14, 15] as well as one research study that asked investigators to propose a payment amount for common procedures [16, 17]. Although not all of the same procedures are included, each of these list similar payment amounts for some commonly performed procedures (Table 2). One important question for future research is to determine whether research participants find these amounts acceptable, how effective the amounts are at recruiting and compensating participants and if participants believe compensation should be calculated differently [18]. Future research investigating the extent to which IRBs agree with the assignment of inconvenience units and payment amounts, how disagreements between the IRB and investigators are resolved, and how IRBs might utilize benchmark data on inconvenience units would also be useful.
TABLE 2.
Payment amounts estimated by median inconvenience units for selected common procedures relative to other published data.
| PROCEDURE | NIH DATA | JOHNS HOPKINS DRUG DEVELOPMENT UNIT1 | PARTNERS2 | DATA FROM RIPLEY SURVEY3 |
|---|---|---|---|---|
| Clinic visit (return visit blood and physical) | $304 | $40.00/visit | $30–75 | $25–26 |
| Blood sample | $20 | $3.00/sample | $5–25 | $16–20 |
| Urine sample | $10 | Not available | Not available | $12 |
| Questionnaire (1 hour) | $305 | Not available | $5–30 | $21 |
| MRI scan | $50 | Not available | $50–200 | Not available |
| Lumbar Puncture | $50 | $75 | $100 | Not available |
Drug Development Unit Remuneration Rate Schedule Johns Hopkins
Average recommendations from 395 IRB chairs and 455 investigators as reported in reference number 17.
Clinic visit was not included in Table 1 because of the wide variety of types of clinic visits.
The median for questionnaires was calculated at $30.00 using the median inconvenience units for psychological/cognitive testing because the category “questionnaire” in the NIH database was too vague.
LIMITATIONS
As noted, we report data on inconvenience units and dollar amounts assigned to specific commonly used procedures. This does not reflect the total dollar amount that research participants received when participating in NIH intramural studies during the study period. NIH intramural investigators use the guidelines for many protocols, yet not all investigators who offer payment to research participants follow these guidelines. Some choose to describe the total amount of payment in a study without explicitly calculating time and inconvenience. Others partially use the guidance but make changes to accommodate for a different dollar amount, or sometimes add a completion bonus. Investigators that do follow the guidelines usually offer money based on both time and inconvenience.
We also recognize that research protocols vary in their complexity, some involving multiple procedures of different types and some involving the same procedure at various time points. We do not have data on how the complexity, type, number of procedures, or length of the study or the investigators’ specialty or years of experience might influence the assignment of inconvenience units.
As a federally funded national research facility, the NIH Clinical Center is somewhat unique. It is possible that private research funders or even academic medical centers offer payment for clinical research procedures that differs from what we documented here. However, concordance with other available lists of per procedure payment suggests that the NIH experience may not be much different from other facilities, and the NIH experience may be useful for other institutions that currently do not have guidelines.
CONCLUSIONS
The data presented here provide valuable benchmarks for investigators and IRB members in determining an appropriate amount of money to offer research participants for common procedures used in clinical research. Although our analysis shows that there is considerable variation even when guidelines exist, converging patterns for certain procedures suggest standard amounts for payment for common procedures. We recommend that institutions, including the NIH Clinical Center, use data such as these to develop more specific guidelines to help standardize payment amounts across similar procedures and to provide sought after guidance to investigators and IRBs. For example, institutions could develop a list of common procedures with an acceptable range of dollar amounts for the investigative team to select from, similar to billing codes used by insurance companies. Further, we recommend continued investigation into the practice of paying research participants, and especially how effective payment is in recruiting participants for clinical research and the extent to which offers of payment affect participants’ understanding, appreciation, or acceptance of study information including risks.
Acknowledgments
The authors would like to acknowledge the NIH Clinical Center’s partners in discovery—the thousands of clinical research volunteers that have contributed to a long list of medical milestones. They would also like to thank their colleagues for their support and assistance, in particular: Dee Koziol, Wendy Schubert and Alan Wertheimer for their review of this manuscript.
This research was supported in part by the Intramural Research Program of the NIH, Clinical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dinora Dominguez, Chief Patient Recruitment and Public Liaison Section, NIH Clinical Center.
Mandy Jawara, Program Coordinator, Clinical Research Volunteer Program, Office of Communications, Patient Recruitment, and Public Liaison, NIH Clinical Center.
Nicole Martino, Office of Communications, Patient Recruitment, and Public Liaison, NIH Clinical Center.
Ninet Sinaii, Biostatistics & Clinical Epidemiology Service, NIH Clinical Center.
Christine Grady, Chief, Department of Bioethics, NIH Clinical Center.
References
- 1.Sung NS, Crowley WF, Genel M, Salber P, Sandy L, Sherwood LM, et al. Central challenges facing the national clinical research enterprise. Jama-Journal of the American Medical Association. 2003;289:1278–87. doi: 10.1001/jama.289.10.1278. [DOI] [PubMed] [Google Scholar]
- 2.Avins AL, Goldberg H. Creating a culture of research. Contemporary Clinical Trials. 2007;28:557–62. doi: 10.1016/j.cct.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Ripley EBD. A review of paying research participants: It’s time to move beyond the ethical debate. Journal of Empirical Research on Human Research Ethics. 2006;1:9–19. doi: 10.1525/jer.2006.1.4.9. [DOI] [PubMed] [Google Scholar]
- 4.Stunkel L, Grady C. More than the money: A review of the literature examining healthy volunteer motivations. Contemporary Clinical Trials. 2011;32:342–52. doi: 10.1016/j.cct.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dickert N, Grady C. What’s the Price of a Research Subject? Approaches to Payment for Research Participation. New England Journal of Medicine. 1999;341:198–203. doi: 10.1056/NEJM199907153410312. [DOI] [PubMed] [Google Scholar]
- 6.Largent E, Grady C, Miller F, Wertheimer A. Money, Coercion, And Undue Inducement: A Survey of Attitudes about Payments to Research Participants. IRB: Ethics and Human Research. 2011 In Press. [PMC free article] [PubMed] [Google Scholar]
- 7.Dickert N, Emanuel E, Grady C. Paying research subjects: An analysis of current policies. Annals of Internal Medicine. 2002;136:368–73. doi: 10.7326/0003-4819-136-5-200203050-00009. [DOI] [PubMed] [Google Scholar]
- 8.Klitzman R, Albala I, Siragusa J, Nelson KN, Appelbaum PS. The reporting of monetary compensation in research articles. Journal of Empirical Research on Human Research Ethics. 2007;2:61–7. doi: 10.1525/jer.2007.2.4.61. [DOI] [PubMed] [Google Scholar]
- 9.National Institutes of Health Clinical Center. Director’s Annual Report, Profile. 2011. p. 16. [Google Scholar]
- 10.Grady C, Dickert N, Jawetz T, Gensler G, Emanuel E. An analysis of US practices of paying research participants. Contemporary Clinical Trials. 2005;26:365–75. doi: 10.1016/j.cct.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Ripley E, Macrina F, Markowitz M, Gennings C. Who’s doing the math? Are we really compensating research participants? Journal of Empirical Research on Human Research Ethics. 2010;5:57–65. doi: 10.1525/jer.2010.5.3.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saunders CA, Sugar AM. What’s the price of a research subject? New England Journal of Medicine. 1999;341:1550–1. doi: 10.1056/NEJM199911113412016. [DOI] [PubMed] [Google Scholar]
- 13.Cryder CE, London AJ, Volpp KG, Loewenstein G. Informative inducement: Study payment as a signal of risk. Social Science & Medicine. 2010;70:455–64. doi: 10.1016/j.socscimed.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 14.Partners Human Research Committee. Remuneration for Research Subjects. Policy and Guidence, Partners Human Research Office; [Google Scholar]
- 15.Johns Hopkins Drug Development Unit. Remuneration Rate Schedule. 1999. [Google Scholar]
- 16.Ripley EBD, Macrina FL, Markowitz M. Paying clinical research participants: One institution’s research ethics committees’ perspective. Journal of Empirical Research on Human Research Ethics. 2006;1:37–44. doi: 10.1525/jer.2006.1.4.37. [DOI] [PubMed] [Google Scholar]
- 17.Ripley E, Macrina F, Markowitz M, Gennings C. Why do we pay? A national survey of investigators and IRB chairpersons. Journal of Empirical Research on Human Research Ethics. 2010;5:43–56. doi: 10.1525/jer.2010.5.3.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breitkopf CR, Loza M, Vincent K, Moench T, Stanberry LR, Rosenthal SL. Perceptions of Reimbursement for Clinical Trial Participation. Journal of Empirical Research on Human Research Ethics. 2011;6:31–8. doi: 10.1525/jer.2011.6.3.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Institutes of Health. Office of Human Subjects Research. OHSR Information Sheets/Forms “Sheet 20—Guidelines for remuneration of research subjects in the intramural research program and registration in the clinical research volunteer program database”. [Google Scholar]
- 20.Decker John L. Protomechanics: Preparing and Conducting a Clinical Research Study. Warren Grant Magnuson Clinical Center of the National Institutes of Health; Bethesda, Md: [Google Scholar]