Abstract
Cocaine dependence is a significant public health problem for which there are currently no FDA-approved medications. Hence, identifying candidate compounds and employing an efficient evaluation process is crucial. This paper describes key design decisions made for a National Institute on Drug Abuse (NIDA) Clinical Trials Network (CTN) study that uses a novel two-stage process to evaluate buspirone (60 mg/day) for cocaine-relapse prevention. The study includes pilot (N=60) and full-scale (estimated N=264) trials. Both trials will be randomized, double-blind, and placebo-controlled and both will enroll treatment-seeking cocaine-dependent participants engaged in inpatient/residential treatment and scheduled for outpatient treatment post-discharge. All participants will receive contingency management in which incentives are given for medication adherence as evaluated by the Medication Events Monitoring System (MEMS). The primary outcome measure is maximum days of continuous cocaine abstinence, as assessed by twice-weekly urine drug screens (UDS) and self-report, during the 15-week outpatient treatment phase. Drug-abuse outcomes include cocaine use as assessed by UDS and self-report of cocaine use, other substance use as assessed by UDS and self-report of substance use (i.e., alcohol and/or illicit drugs), cocaine bingeing, HIV risk behavior, quality of life, functioning, and substance-abuse treatment attendance. Unique aspects of the study include conducting an efficacy trial in community treatment programs, a two-stage process to efficiently evaluate buspirone, and an evaluation of mediators by which buspirone might exert a beneficial effect on relapse prevention.
Keywords: cocaine, buspirone, relapse-prevention, contingency management
1. Introduction
Cocaine addiction is a significant problem based on the number of lives affected and its associated medical and legal consequences [1]. In 2009, over one million people in the U.S. were abusing or dependent on cocaine [2] and in Europe cocaine use has increased significantly in recent years [3]. Though psychosocial interventions can help, treatment dropout followed by relapse to cocaine use is high. Despite extensive work, there still is no FDA-approved treatment for cocaine dependence [4]. Pre-clinical research has found that dopamine D3 receptor antagonists reduce the rewarding effects of cocaine and the reinstatement of cocaine seeking [5–7]. In addition, imaging research suggests that dopamine D3 receptors may be upregulated in stimulant abusers [8]. Heidbreder and Newman [7] note that while there is insufficient evidence to suggest that dopamine D3 antagonists would be effective in stopping on-going use, there is evidence that they may be effective in relapse-prevention. Identifying an effective relapse-prevention medication for cocaine dependence would be beneficial in that relapse following discharge from inpatient settings is substantial. For example, studies of shorter stays (2–10 days) report relapse rates of 72% within one month [9] and 86% within 12 weeks [10], and studies of longer inpatient/residential stays (2–4 weeks) have reported relapse rates of 65% [11] and 72% [12,13] within 90 days following discharge.
Buspirone has attracted interest for this indication. It is an FDA-approved treatment for generalized anxiety disorder with little abuse potential [14] and a well-established safety profile [15]. It is a 5HT1A agonist [14], but both published data [16] and unpublished data from the National Institute on Drug Abuse (NIDA) show that it is also a dopamine D3 antagonist. Of interest, a pre-clinical protocol utilized by NIDA to screen potential cocaine-dependence treatment compounds yielded positive results for buspirone. In fact, buspirone was one of only five compounds with a positive result among approximately 81 tested, and the only one deemed safe enough to test clinically. In addition, NIDA has unpublished studies in which buspirone was found to reduce footshock-induced cocaine reinstatement in an animal model of stress-induced relapse and was also effective in blocking cue-and priming-induced relapse to methamphetamine self-administration in rats. Buspirone has been studied in substance abusing populations including alcohol-dependent [17–21], opioid-dependent [22], and marijuana-dependent [23,24] individuals and was safe and well tolerated, though efficacy results were mixed. For cocaine abusers, Giannini et al. [25] reported that buspirone, compared to placebo, significantly reduced cocaine withdrawal symptoms. Moeller et al. [26] found no significant effect of buspirone in a small (n=35) 12-week double-blind, placebo-controlled trial evaluating the association between impulsivity, severity of cocaine use, and buspirone treatment. This finding is not surprising given the current hypothesis, based on D3 antagonist data and unpublished pre-clinical data, that buspirone will be effective for relapse-prevention but may not reduce use in active users such as those in the Moeller et al. [26] study.
To evaluate buspirone for relapse prevention, the NIDA Clinical Trials Network (CTN) has developed the study: “A Randomized Controlled Evaluation of Buspirone for Relapse-Prevention in Adults with Cocaine Dependence (BRAC).” BRAC (pronounced “brake”) is somewhat unique in that it is being conducted at community treatment programs as opposed to traditional research/academic settings and will evaluate potential mediators through which buspirone may exert beneficial effects. This paper describes the key design considerations associated with this study.
2. Research design and study organization
2.1 Research questions
The primary question addressed by BRAC is whether buspirone, relative to placebo, prevents relapse in cocaine-dependent adults who are planning to enter outpatient treatment upon discharge from inpatient/residential units. Secondary questions include 1) Does buspirone, relative to placebo, impact other drug-abuse outcomes; and 2) What is the impact of buspirone, relative to placebo, on factors that may mediate its efficacy for preventing relapse? This latter question is somewhat unique in that trials typically do not test the mediators by which a medication is hypothesized to work (e.g.,[27]).
2.2 Research design
Buspirone has not been previously evaluated as a relapse-prevention treatment for cocaine dependence, and, thus, there are minimal empirical data upon which to base the design of a full-scale trial. Traditionally, this would result in buspirone being evaluated in a pilot study at an academic site, with a positive signal leading to a larger study conducted at multiple academic or clinical sites. However, buspirone’s well-known safety profile raised the issue of whether earlier evaluation in clinical sites might be more beneficial. Specifically, the results from studies conducted at clinical sites, which include participants seeking treatment at the site, may differ from those conducted by typical academic sites, which typically rely heavily on advertising for participant recruitment [28]. The use of clinical sites should, thus, improve the generalizability of the results to treatment-seeking patients, which is the population of ultimate interest.
In addition, completing a small pilot trial, followed by one that is larger and more definitive was questioned in terms of efficiency. The conduct of two separate trials is associated with greater financial resources, including the need to hire and train staff and to find and initiate study sites for each trial. In addition, a significant amount of time typically elapses between the end of a pilot trial and the initiation of a larger follow-up study. For example, the time between completion of a pilot trial of reserpine for treating cocaine dependence [29] and the initiation of the larger follow-up study was approximately 2.5 years [30]. Similarly, the time between completion of a pilot trial of tiagabine for treating cocaine dependence [31] and the initiation of the larger follow-up trial of tiagabine [1] was over two years. Given that there is no FDA approved medication for cocaine dependence, reducing the time required to evaluate compounds would be beneficial. To this end, BRAC uses a novel design entailing a two-stage process in which a pilot trial will first be completed to obtain information needed to address important operational aspects critical to the design of the full-scale clinical trial (e.g., medication tolerability, adherence, missing data rates, eligibility criteria, etc.). However, the treatment effect estimate from the pilot trial is not intended to guide decisions about efficacy for the full-scale trial, consistent with the recommendations of Kraemer et al. [32].
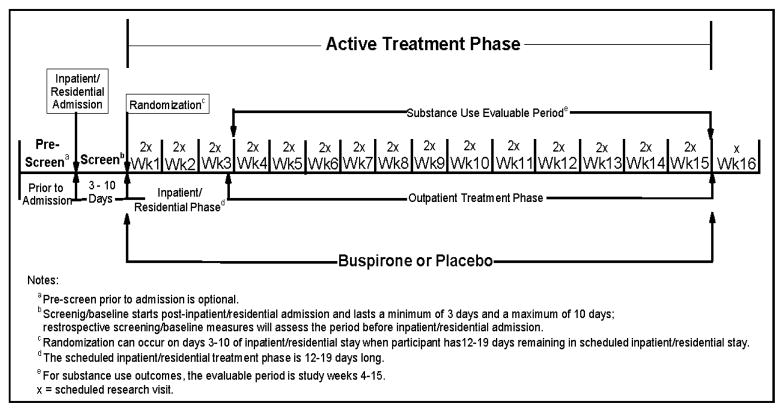
The pilot (N=60) and full-scale (estimated N=264) trials will utilize similar treatment phases and outcomes with adjustments made as needed (e.g., to medication dosing, sample size estimates, etc.) to the full-scale trial based on the pilot data. It is estimated that only 4.5 months will elapse between the end of data collection for the pilot study and the initiation of the full-scale trial and the same sites will be used for both trials, thus saving start-up costs for the full-scale trial. Both trials will be randomized, 16-week, intent-to-treat, double-blind, and placebo-controlled. Eligible participants will be randomized to buspirone or matching placebo and scheduled to receive study medication and to attend two research visits per week throughout the active treatment phase. This phase will begin with randomization and end on day 7 of study week 15. A single visit will be scheduled in week 16 to complete retrospective data for week 15. Participants will be screened after being admitted to inpatient/residential treatment and randomized when they have 12–19 days remaining of their scheduled inpatient/residential stay, allowing a 10-day dose escalation period to be completed in the inpatient/residential setting. The allowed range for the length of the inpatient/residential stay reflects the need for flexibility in conducting the trial in real world settings, which tend to vary in their standard lengths of stay. The study schema is provided in Figure 1. Participants will be reimbursed for transportation, inconvenience, and time; a participant attending all 31 post-randomization research visits will receive $955.
Figure 1.
Study Schema
2.3 Study setting
BRAC is being conducted by NIDA’s CTN. The CTN was established in 1999 in response to an Institute of Medicine report [33] delineating the relative lack of information exchange between substance abuse researchers and substance abuse community treatment providers. In the CTN, substance abuse researchers work with community treatment providers to design clinically meaningful trials and implement them in “real world” settings utilizing existing staff as interventionists and clinic patients as participants. The CTN links substance abuse researchers and treatment providers through nodes; each node consists of a Regional Research and Training Center (RRTC) affiliated with at least one university and multiple Community Treatment Programs (CTPs) that work with the RRTC.
2.4 Site selection process
BRAC will recruit cocaine-dependent patients with a planned inpatient/residential stay of 14–28 days. Recent years have seen shorter inpatient/residential stays and a decline in inpatient/ residential admissions for cocaine dependence. In addition, because the full-scale trial is an efficacy trial, relatively narrow eligibility criteria are being used (see section 2.6), further reducing the pool of potential participants. For example, the psychotropic medication exclusion significantly reduces the pool of potential participants for this study population. To be eligible for study participation, a CTP needed to treat a sufficient number of stimulant-dependent individuals that are likely to meet criteria to randomize at least 1.6 per month on average, and to have medical staffing with enough time available to safely and effectively conduct a medication trial. Site selection was completed using the following process: 1) A call for site nominations was sent to the Principal Investigators of 13 RRTCs, 2) 27 sites nominated in this process completed surveys describing their programs and the patients that present for treatment, 3) the BRAC Lead Team reviewed the surveys and obtained more detailed survey information from nine sites that appeared to best meet study criteria, and 4) final site selections were made by the study Executive Committee. The selection process resulted in six diverse sites being selected for participation: Addiction Medicine Services (Pittsburgh, PA), Gateway Community Services (Jacksonville, FL), Morris Village/Lexington Richland Alcohol and Drug Abuse Council (Columbia, SC), Maryhaven (Columbus, OH), Nexus Recovery Center (Dallas, TX), and Penn Presbyterian Medical Center (Philadelphia, PA). All six sites have successfully participated in past clinical trials and, thus, are particularly well-suited for BRAC.
2.5 Study population
The study population includes adults (≥18 years old) who meet DSM-IV-TR criteria for cocaine dependence, self-report having used crack cocaine a minimum of four times in the 28 days prior to inpatient/residential admission, are scheduled for 12–19 days of inpatient/residential SUD treatment at time of randomization, and planning to enroll in local outpatient treatment through the end of the active treatment phase (i.e., study week 15).
2.6 Inclusion and exclusion criteria
The eligibility criteria, listed in Table 1, were developed to ensure the safety of participants and lower variance to increase the likelihood of detecting a medication effect should one be present.
Table 1.
Eligibility criteria for the BRAC study
Inclusion CriteriaPotential participants must:
|
Exclusion CriteriaPotential participants must not:
|
2.7 Randomization
Randomization strata include study site and baseline cocaine use frequency (<10 days or ≥ 10 days in the 28 days prior to inpatient/residential admission).
3. Study treatments
3.1 Buspirone/Placebo
Study participants will be randomly assigned to receive either buspirone or matching placebo. The target dose for buspione is 60 mg per day, which is based on the maximum dose approved by the FDA for treatment of generalized anxiety disorder. The dose escalation schedule for this study, conducted under observation on inpatient/residential units, is given in Table 2. The schedule is somewhat aggressive with the target of reaching 60 mg per day by study day 10. The relative aggressiveness of the dose escalation schedule is based on buspirone’s well-known side-effects profile, one of the authors’ (KTB) expertise and experience with using buspirone in substance-abusing populations, and a desire to have participants reach full dose prior to discharge from the inpatient/residential setting. Participants who are unable to reach the 60 mg dose or need to be reduced from 60 mg due to tolerability will be maintained on 15 mg, 30 mg, or 45 mg, whichever is the maximum dose tolerated.
Table 2.
Buspirone/placebo dose escalation for the BRAC study
| Study Day | Dose |
|---|---|
| 1–3 | 10 mg (5 each am, 5 each pm) |
| 4–6 | 20 mg (10 each am, 10 each pm) |
| 7–9 | 40 mg (20 each am, 20 each pm) |
| 10 | 60 mg (30 each am, 30 each pm) |
BRAC medication adherence measures include the Medication Events Monitoring System (MEMS) which is a medication bottle with a microchip that records the times and dates of bottle opening, pill counts, and participant self-reported adherence. A participant’s medication compliance for each study week will be defined as the most conservative estimate yielded from the three measures of medication adherence. In addition, a biological measure of adherence will be obtained for participants in the busprione arm. Specifically, urine samples collected during the treatment period will be shipped to a central lab and the samples from the buspirone group will be assayed for buspirone and/or its metabolite (1-PP) using a liquid chromatography/ mass spectrometry/mass spectrometry method.
3.2 Contingency Management for medication adherence
Past research suggests that complete adherence to a prescribed medication regimen frequently fails to occur in clinical trials and that cocaine-dependence trials are no exception [34]. A lack of adherence in medication clinical trials negatively impacts the internal validity of the study. In the present study, assuring good medication adherence likely will be more challenging due to buspirone’s required twice daily dosing. Contingency management (CM) is an intervention in which patients receive rewards contingent on a target behavior, such as providing drug-free urines, attending treatment, or performing tasks associated with taking medication. A randomized controlled trial by Sorensen et al. [35] found that CM significantly increased medication adherence in HIV-positive, methadone-maintained individuals. More specifically, the MEMS was used to assess adherence, with adherence defined as a bottle opening occurring within four hours of a prescribed medication dose (i.e., two hours before or after the dose was to be taken; [35]). Patients report that a primary reason for failed medication adherence is that they forget to take it [36,37]. Reinforcing bottle openings should provide an incentive to take study medication as prescribed and thus facilitate adherence.
The BRAC study will utilize a procedure similar to that of Sorensen et al. [35] but with the addition of bonuses for consecutive instances of apparent adherence as measured by MEMS, a strategy that has been used in other CM protocols [38]. The target behavior for the present protocol is bottle opening within six hours of a prescribed dose (i.e., three hours before or after the dose was to be taken). The payment protocol begins with the first day of medication and continues for 15 weeks of dosing. Consistent with Sorensen et al. [35], the CM plan for the present study will involve a relatively quick escalation of the reinforcement earnings as a strategy to promote consistent opening of the medication bottle. To emphasize the importance of adherence to the twice-daily dosing regimen, payment bonuses are used to reinforce this aspect of the target behavior. Thus, throughout the trial, participants can earn 50 cents for each day on which they have a single bottle opening (indicating partial adherence). However, bonuses for full adherence (2 bottle openings within the prescribed time limits on a given day) escalate over the first two weeks from $0.50 to $5 per day. Thus, by the end of week 2 (while still on the inpatient/residential unit), participants will be earning $5 per day for bottle-openings that we hope will be associated with adherence. An additional $20 bonus will be awarded for each week in which the participant was fully adherent - 2 bottle openings per day for 7 consecutive days. Under this scheme, which continues throughout the 15-week active treatment phase, a fully adherent participant would be able to earn a total of $798.50.
It is also possible that participants will fail to open the bottle on a given day. Under this circumstance, the reinforcement amount will be reset to $0.50 the next time MEMS data show that the bottle was opened. Following such a reset, escalation of the reinforcement value will follow the original schedule (i.e., as if the participant was restarting at study day 1) and continue escalating until the end of week 15 or another reset occasion occurs. Thus, the actual amount that a participant earns will depend on their individual pattern and consistency of bottle-opening adherence. Past studies of CM for increasing medication adherence have provided reinforcement for medication-related behaviors in the form of gift cards or other goods [35,39–41], cash [42–43], or both [38]. Reinforcements in BRAC will be provided in the form of retail gift cards (minimum $5 value), with the provision of cash for reinforcements less than $5.
4. Assessments
4.1 Primary outcome measure
There is no agreed-upon operational definition of cocaine relapse [10] and, thus, no standard upon which to base the primary outcome measure for BRAC. As noted by Havassy et al. [44], defining relapse as any use is appealing in that it is relatively easy to measure and unambiguous to interpret (i.e., use did or did not occur) but this definition is problematic in that a single use is quite conservative as a definition and one that may not predict continued use. Indeed, this potential downside was found in a cocaine-relapse-prevention trial completed by Schmitz et al. [10] in which no medication effect was found for time to first use while a medication effect was found for percentage of cocaine-negative urines. A trial evaluating a pharmacological treatment (tryptophan) plus vouchers for relapse-prevention in cocaine-dependent patients revealed a significant effect for vouchers on continuous cocaine abstinence, suggesting that length of continuous cocaine abstinence is a measure that is sensitive to treatment effects [45].
The primary outcome measure for BRAC is the maximum days of continuous cocaine abstinence during outpatient treatment (e.g., study weeks 4–15), since this appears to be a better proxy for relapse than a single episode of use. Cocaine use will be determined by a combination of self-report assessed by the Timeline Follow-back (TLFB) and qualitative urine drug screens (UDS). The TLFB [46,47] is a widely employed and well-validated method to capture self-report drug use information for each day elapsed since the previous visit. During the active treatment phase, participants are scheduled to provide two urine samples per week on nonconsecutive days. To avoid falsification, urine samples will be collected using temperature monitoring, and the validity of urine samples will be checked with the use of a commercially available adulterant test. In cases where the temperature reading/adulterant test indicates a non-valid sample, an attempt will be made to obtain a second urine sample. A rapid UDS system that screens for drugs of abuse including cocaine, methamphetamine, amphetamine, opioids, benzodiazepines, marijuana, barbiturates, methadone, oxycodone, and methylenedioxymethamphetamine (MDMA, Ecstasy) will be used to analyze the urine samples (Branan Medical Corporation).
4.2 Secondary outcome measures
The impact of buspirone, relative to placebo, on drug-abuse outcomes include measures of treatment utilization and reduction in drug use (e.g., cocaine-use and substance-use days, cocaine bingeing), and adverse consequences related to drug use (e.g., Addiction Severity Index (ASI)-Lite, Quality of Life). Substance-use treatment utilization is defined as the percent of scheduled treatment hours attended as obtained from clinic records. The TLFB will be used at each study visit to capture self-report drug information for each day elapsed since the previous study visit. For each day on which cocaine use is reported, the amount of cocaine used and the length of time spent using will also be assessed. The ASI-Lite is derived from the Fifth Edition of the ASI [48], a structured clinical interview that yields scores for seven areas of functioning typically impacted by addiction, including medical status, employment status, drug use, alcohol use, family status, legal status, and psychiatric status. The CTN Treatment Effect and Assessment Measures (TEAM) Task Force noted that effective substance abuse treatment should improve patients’ quality of life in addition to decreasing substance use and recommended that the World Health Organization Quality of Life-BREF (WHOQOL-BREF) [49] be included in CTN trials. The WHOQOL-BREF provides a score for four domains: physical health, psychological, social relationship, and environment. The ASI-Lite and WHOQOL-BREF will be completed at baseline and study week 15.
4.3 Process Measures
Factors that could mediate buspirone’s efficacy for relapse-prevention are those related to relapse/use that could be affected by buspirone, which include drug attentional bias, compulsivity, and craving. The incentive salience hypothesis postulates that the pathological wanting of the drug, which is distinct from drug liking, is what drives the compulsive use that marks addiction [50–52]. This pathological wanting leads to the focus on substance use seen in addiction [53], is particularly triggered by exposure to drug-related cues [54], and is associated with activation in a broad range of brain regions [52]. A measure of the increased salience of drug cues is the Drug Stroop task that assesses attentional bias for drug cues [55,56]. Increased attentional bias for drug cues, as measured by the Drug Stroop, has been found to be significantly greater in stimulant-dependent, relative to normal control, participants [57] and to be associated with poorer treatment outcomes in cocaine-dependent individuals [55]. Past research has found that dopamine antagonists can improve performance on the Drug Stroop in heroin-dependent individuals [58]. As a dopamine antagonist, buspirone could be expected to decrease drug attentional bias, which, in turn, could reduce the likelihood of relapse. The computerized Drug Stroop [55,56] will be obtained at baseline and study week 2, prior to discharge from inpatient/residential treatment.
Compulsive drug use is a defining characteristic of addiction [59]. Ersche et al [57] recently reported that the impact of dopaminergic agents in stimulant-dependent participants differed as a function of compulsivity, measured by the Obsessive Compulsive Drug Use Scale (OCDUS) [60,61], and noted that research evaluating dopaminergic agents should include a measure of compulsivity. The OCDUS will be obtained at baseline and study week 2, prior to discharge from inpatient/residential treatment, and at study weeks 8 and 15. The Cocaine Craving Questionnaire-Brief (CCQ-Brief) [62], which captures the multidimensional nature of craving including perceived ability to resist using, has been found to be predictive of time to cocaine relapse following inpatient treatment [12]. Moeller et al. [26] reported no significant effect for buspirone on cocaine craving in their small trial of buspirone. However, based on the means and standard deviations reported for endpoint craving [26], buspirone’s effect size (d= .75; Cohen, 1988) was in the medium to large range, and, thus, we predict that buspirone, relative to placebo, will significantly decrease craving. The CCQ-Brief will be collected at baseline and weekly through week 15.
5. Data and Safety Monitoring
Although buspirone is expected to be well tolerated with minimal side-effects, safety measures to be assessed weekly including vital signs, adverse events, suicide assessment and the Hospital Anxiety and Depression Scale [64], a brief, validated instrument that screens for both depression and anxiety [65]. An independent CTN Data and Safety Monitoring Board (DSMB) will examine accumulating data to assure protection of participants’ safety while the study’s scientific goals are being met. The DSMB recommends to the sponsor (NIDA) whether there is support for continuation of the trial, or evidence that study procedures should be changed, or if the trial should be halted, for reasons relating to the safety of the study participants, the efficacy of the treatment under study, or inadequate trial performance (e.g., poor recruitment).
6. Sample size estimation
Days of continuous cocaine abstinence is the primary outcome measure for both the pilot and full-scale trials of BRAC. Sample size estimates for the full-scale trial were calculated via simulation, where the maximum days of continuous cocaine abstinence were assumed to follow a gamma distribution, and simulated data of 10,000 iterations were analyzed using generalized estimating equations (GEE) that included the following variables consistent with the method of analysis for the primary outcome: Treatment group, Site, baseline Cocaine use frequency (CUF), and Length of Residential Stay prior to randomization (see Section 7.1). Treatment effect (effect size) for maximum days of continuous cocaine abstinence in the buspirone, compared to placebo, group was defined in terms of Cohen’s d. From these analyses, the required full-scale trial sample size is estimated to be 250 (125 per arm), which is consistent with detecting a moderate difference between the two arms (effect size of a Cohen’s d = .4), and achieves at least 89% power over varying assumptions on the standard deviation of distribution of the primary outcome (see Table 3). This sample size estimate assumed the mean maximum days of continuous cocaine abstinence for the placebo arm to be 14, based on the findings from a cocaine- relapse-prevention trial completed by Jones et al. [45], and the same standard deviations in both the arms. Table 3 provides additional information on sample size and power for varying assumptions on treatment effect size (Cohen’s d of 0.3 and 0.4 and its corresponding treatment difference) and standard deviation. Accounting for a 5% loss-to-follow-up from study participants who do not provide any primary outcome data after release from the residential program, the sample size for the full-scale trial was increased to 264 participants. The estimated 5% loss-to-follow-up was based on a relapse-prevention study in which 92% of cocaine-dependent patients in the residential phase started the outpatient treatment phase [45], combined with the superior retention record of the sites participating in CTN-0052. Specifically, five of the six CTN-0052 sites participated in an outpatient trial of cocaine- and/or methamphetamine-dependent cigarette smokers [66] and had an average completion rate of 86% for the 10-week active treatment phase.
Table 3.
Power to detect a treatment effect size of .4 or .3 as a function of sample size, difference between treatment arms, and standard deviation
| Mean and SD of Buspirone Arm for Cohen’s d of .4
| |||
|---|---|---|---|
| Sample Size | Mean=20, SD =15 Diff* = 6 days |
Mean=18, SD=10 Diff = 4 days |
Mean=16, SD =5 Diff = 2 days |
| 150 | 77% (19%) | 71% | 69% |
| 200 | 86% (12%) | 81% | 81% |
| 250 | 92% (7%) | 89% | 89% |
|
| |||
| Mean and SD of Buspirone Arm for Cohen’s d of .3
| |||
| Sample Size | Mean=18.5, SD =15 Diff* = 4.5 days |
Mean=17, SD=10 Diff = 3 days |
Mean=15.5, SD =5 Diff = 1.5 days |
| 250 | 74% | 68% | 67% |
| 300 | 81% | 75% | 74% |
| 350 | 86% | 81% | 80% |
Note:
Difference in days is equal to the difference between the mean maximum days of continuous cocaine abstinence in the buspirone and placebo arms. Parenthesized figures indicate percent of cases that failed to converge, when convergence failure rates exceeded 5%.
The pilot trial will provide information about the primary outcome measure and estimates of the percentage of participants who provide no outcome data after release from the residential program. Findings from the pilot trial in these two areas that differ from our original assumptions may require modifications to the sample size estimate for the full-scale trial. We selected a sample size of 60 (30 per arm) for the pilot trial as this sample size provides reasonably-sized margins of error as a function of standard deviation and differences between the mean maximum days of continuous cocaine abstinence in the two arms (See Table 4). Another important aspect of the pilot trial is assessing medication tolerability, where we are interested in assessing the proportions of participants reaching maximum dose, reaching target dose in 10 days, having a permanent dose reduction or medication discontinuation, and achieving a sustained dose at maximum. A sample size of 60 will achieve a margin of error below 0.2 for these outcomes.
Table 4.
Margin of error for primary treatment effect as a function of treatment group difference and standard deviation
| Mean and SD of Buspirone Arm and Difference in Days* | |||
|---|---|---|---|
| Mean=20, SD=15 Diff*=6 days |
Mean=18, SD=10 Diff*=4 days |
Mean=16, SD=5 Diff*=2 days |
|
| 95% Confidence Interval Margin of Error | 4.13 days | 2.76 days | 1.37 days |
Note:
Difference in days is equal to the difference between the mean maximum days of continuous cocaine abstinence in the buspirone and placebo arms.
7. Analytic Plan
7.1 Primary Outcome Analysis
The primary hypothesis is that buspirone-treated participants, relative to placebo-treated participants, will have a significantly longer mean period of continuous cocaine abstinence as assessed by TLFB and twice-weekly UDS. For this outcome, each day during the 12-week outpatient treatment phase must be assigned as an abstinent or a cocaine use day based on information collected on both TLFB and UDS. An algorithm has been developed to combine UDS and TLFB for classifying each day, and follows the general principle that a UDS covers a four day look-back period spanning days -3 to 0, where day 0 is when the urine is donated. A positive UDS for which the associated TLFB reports are negative will result in “correcting” TLFB days to be cocaine use days. Assumptions are also required for assigning missing UDS dates in scenarios when a UDS is missing. Per the protocol, all participants are expected to contribute two UDS per week. If a participant contributes only one UDS in a week, we specify a rule for assigning the day for the missed second urine collection in order to assess whether the imputed UDS is consistent with self-report. For example, if a participant only provides a UDS on Day 2, we assume the second UDS is collected on Day 5. Table 5 describes this rule for assigning a day of collection for a missing UDS within a week when there is only one urine specimen collected. If no urine samples are collected in a week, we assume the collection for missing urine samples occur on Days 4 and 7. Table 6 provides the approach we plan to follow for combining TLFB and UDS, and accounts for the potential of missing UDS results.
Table 5.
UDS collection day assignment for a study week with only one urine sample provided
| Day in a Given Week | |||||||
|---|---|---|---|---|---|---|---|
| Observed Collection Day | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Imputed Collection Day | 5 | 5 | 6 | 7 | 7 | 3 | 4 |
Table 6.
Algorithm to combine TLFB and UDS for classification of days as cocaine-free or not cocaine-free
| Associated TLFB in days 0 to −3 | ||||
|---|---|---|---|---|
| At least one positive day | All days are negative | Missing | ||
| UDS on day 0 | + | Use TLFB | Change Day −3 in TLFB to positive | Assumed Impossible |
| − | Use TLFB | Use TLFB | Assumed Impossible | |
| Missing | Use TLFB | Missing | Missing | |
Once the classification of cocaine-use days is determined for each participant, continuous cocaine abstinence will be calculated from the beginning of week 4 (day 22) through day 7 of study week 15. Data will be analyzed using generalized estimating equations (GEE) assuming a gamma distribution, identity link function and fixed site effect. The following statistical model will be used for the analysis.
where, Yij is the maximum days of continuous cocaine abstinence for the kth participant (subscript k suppressed for clarity in the model statement above) in the jth site on the ith treatment, and CUF is the baseline cocaine use frequency (<10 days or ≥10 days of use in the 28 days prior to inpatient/residential admission) and LOS is the length of stay prior to randomization (continuous variable 2–9 days). The statistical significance of β2 will determine the primary outcome of the trial.
To handle missing cocaine-use days (Table 6) in the analysis, a multiple imputation strategy will be performed under a Missing at Random (MAR) assumption, with the analysis repeated 5 times. Statistical inferences about the parameters of interest will be made after combining the results from the 5 repeated analyses. Although the primary analysis for the parameter of interest (maximum days of continuous cocaine abstinence during the 12 week outpatient treatment phase weeks 4–15) is based on the MAR assumption, secondary analyses of maximum days of continuous cocaine abstinence during the 12 week outpatient treatment phase will also be performed to allow us to assess robustness of conclusions to the deviation from the MAR missing data assumption.
7.2 Secondary outcome and process measure analyses
Discrete (use or no-use) outcomes will be analyzed using GEE analyses. For continuous outcomes, a mixed effects model will be used to analyze the data incorporating appropriate covariates. Adverse consequences related to drug use will be measured via ASI-Lite and WHOQOL-BREF and the analyses of these (at week 15) will be performed using mixed models adjusting for respective baseline values as covariates in the model. Process measures (Drug Stroop, CCQ-Brief, etc.) will be analyzed using mixed models adjusting for the baseline values as covariates in the model. Before modeling is commenced, assumptions of linearity will be examined. Process measures will also be evaluated as potential mediators of treatment effect using methods proposed by Baron and Kenny [67] and Judd and Kenny [68] and the statistical test proposed by Sobel [69].
8. Current status of the trial
Study recruitment for the pilot phase is scheduled to start in August 2012.
9. Summary
BRAC will evaluate the efficacy of buspirone, relative to placebo, as a cocaine-dependence-relapse-prevention treatment using a novel two-stage process in which both a pilot and full-scale trial are completed in clinical sites to maximize the efficiency of testing and the generalizability of the results. Because of this unique design, BRAC might serve as a useful model for future medication development efforts. BRAC is also unique in its evaluation of potential mediators (e.g., drug cue saliency, compulsivity, and drug craving) by which buspirone may exert its beneficial effects. Having a greater understanding of these mediators could facilitate future medication development efforts for a condition with serious consequences and for which there is currently no widely used, safe and effective medication.
Acknowledgments
This work was supported by the National Institute on Drug Abuse, National Drug Abuse Treatment Clinical Trials Network, National Institutes of Health, by grants: U10-DA013732 to the University of Cincinnati (Theresa Winhusen), U10DA013727 (Kathleen Brady and John March), U10 DA013034 (Maxine L. Stitzer and Robert P. Schwartz), U10DA013043 (George Woody), U10DA020024-07 (Madhukar Trivedi) and through contract nos. HHSN271200522081C and HHSN271200900034C.
Dr. Adinoff has served as a consultant for Shook, Hardy & Bacon LLP (medical malpractice consultant, tobacco companies) and Teva Pharmaceutical Industries Ltd. and has received funding from the National Institute on Drug Abuse, National Institute on Alcohol Abuse and Alcoholism, and the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Winhusen T, Somoza E, Ciraulo D, Harrer JM, Goldsmith RJ, Grabowski J, et al. A double-blind, placebo-controlled trial of tiagabine for the treatment of cocaine dependence. Drug Alcohol Depend. 2007;91:141–8. doi: 10.1016/j.drugalcdep.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 2.Substance Abuse and Mental Health Services Administration. NSDUH Series H-38A, HHS Publication No SMA 10–4856 Findings. Office of Applied Studies; Rockville, MD: 2010. Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings. [Google Scholar]
- 3.UNODC. World Drug Report. United Nations Publication; 2010. Sales No. E.10.XI.13. [Google Scholar]
- 4.Kuehn BM. Scientists target cocaine addiction. JAMA. 2009;302(24):2641–2. doi: 10.1001/jama.2009.1837. [DOI] [PubMed] [Google Scholar]
- 5.Vorel SR, Ashby CR, Jr, Paul M, Liu X, Hayes R, Hagan JJ, Middlemiss DN, Stemp G, Gardner EL. Dopamine d3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci. 2002;22(21):9595–603. doi: 10.1523/JNEUROSCI.22-21-09595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xi Z, Newman AH, Gilbert JG, Pak AC, Peng X, Ashby CR, Jr, et al. The novel dopamine d3 receptor antagonist NGB 2904 inhibits cocaine’s rewarding effects and cocaine-induced reinstatement of drug-seeking behavior in rats. Neuropsychopharmacology. 2006;31:1393–1405. doi: 10.1038/sj.npp.1300912. [DOI] [PubMed] [Google Scholar]
- 7.Heidbreder CA, Newman AH. Current perspectives on selective dopamine d3 receptor antagonists as pharmacotherapeutics for addictions and related disorders. Ann N Y Acad Sci. 2010;1187:4–34. doi: 10.1111/j.1749-6632.2009.05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boileau I, Payer D, Houle S, Behzadi A, Rusjan PM, Tong J, et al. Higher binding of the dopamine d3 receptor-preferring ligand [11C]-(+)-propyl-hexahydro-naphtho-oxazin in methamphetamine polydrug users: a positron emission tomography study. J Neurosci. 2012;32(4):1353–9. doi: 10.1523/JNEUROSCI.4371-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Back SE, Hartwell K, DeSantis SM, Saladin M, McRae-Clark AL, Price KL, et al. Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug Alcohol Depend. 2010;106(1):21–7. doi: 10.1016/j.drugalcdep.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitz JM, Stotts AL, Rhoades HM, Grabowski J. Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict Behav. 2001;26:167–80. doi: 10.1016/s0306-4603(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 11.Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–31. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- 12.Paliwal P, Hyman S, Sinha R. Craving predicts time to cocaine relapse: further validation of the now and brief versions of the cocaine craving questionnaire. Drug Alcohol Depend. 2008;93:252–9. doi: 10.1016/j.drugalcdep.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hyman SM, Paliwal P, Chaplin TM, Mazure CM, Rounsaville BJ, Sinha R. Severity of childhood trauma is predictive of cocaine relapse outcomes in women but not men. Drug Alcohol Depend. 2008;92(1–3):208–16. doi: 10.1016/j.drugalcdep.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lader M. Can buspirone induce rebound, dependence, or abuse? Br J Psychiatry. 1991;159(Suppl):45–51. [PubMed] [Google Scholar]
- 15.Julien RM. A primer of drug action: A concise, nontechnical guide to the actions, uses, and side effects of psychoactive drugs. 10. New York: WH Freeman; 200. [Google Scholar]
- 16.Kula NS, Baldessarini RJ, Kebabian JW, Neumeyer JL. S(+)-Aporphines are not selective for human D3 dopamine receptors. Cell Mol Neurobiol. 1994;14:185–91. doi: 10.1007/BF02090784. [DOI] [PubMed] [Google Scholar]
- 17.Bruno F. Buspirone in the treatment of alcoholic patients. Psychopathology. 1989;22(Suppl 1):49–59. doi: 10.1159/000284626. [DOI] [PubMed] [Google Scholar]
- 18.Malcolm R, Anton RF, Randall CL, Johnston A, Brady K, Thevos A. A placebo-controlled trial of buspirone in anxious inpatient alcoholics. Alcohol Clin Exp Res. 1992;16:1007–13. doi: 10.1111/j.1530-0277.1992.tb00691.x. [DOI] [PubMed] [Google Scholar]
- 19.Tollefson GD, Montague-Clouse J, Tollefson SL. Treatment of comorbid generalized anxiety in a recently detoxified alcoholic population with a selective serotonergic drug (buspirone) J Clin Psychopharmacol. 1992;12(1):19–26. doi: 10.1097/00001573-199202000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Kranzler HR, Burleson JA, Del Boca FK, Babor TF, Korner P, Brown J, et al. Buspirone treatment of anxious alcoholics. A placebo-controlled trial. Arch Gen Psychiatry. 1994;51(9):720–31. doi: 10.1001/archpsyc.1994.03950090052008. [DOI] [PubMed] [Google Scholar]
- 21.Malec E, Malec T, Gagné MA, Dongier M. Buspirone in the treatment of alcohol dependence: a placebo-controlled trial. Alcohol Clin Exp Res. 1996;20:307–12. doi: 10.1111/j.1530-0277.1996.tb01644.x. [DOI] [PubMed] [Google Scholar]
- 22.McRae AL, Sonne SC, Brady KT, Durkalski V, Palesch Y. A randomized, placebo-controlled trial of buspirone for the treatment of anxiety in opioid-dependent individuals. Am J Addict. 2004;13(1):53–63. doi: 10.1080/10550490490265325. [DOI] [PubMed] [Google Scholar]
- 23.McRae AL, Brady KT, Carter RE. Buspirone for treatment of marijuana dependence: a pilot study. Am J Addict. 2006;15(5):404. doi: 10.1080/10550490600860635. [DOI] [PubMed] [Google Scholar]
- 24.McRae-Clark AL, Carter RE, Killeen TK, Carpenter MJ, Wahlquist AE, Simpson SA, et al. A placebo-controlled trial of buspirone for the treatment of marijuana dependence. Drug Alcohol Depend. 2009;105(1–2):132–8. doi: 10.1016/j.drugalcdep.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giannini AJ, Loiselle RH, Graham BH, Folts DJ. Behavioral response to buspirone in cocaine and phencyclidine withdrawal. J Sub Abuse Treat. 1993;10(6):523–7. doi: 10.1016/0740-5472(93)90055-7. [DOI] [PubMed] [Google Scholar]
- 26.Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. J Subst Abuse Treat. 2001;21(4):193–8. doi: 10.1016/s0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- 27.Brady KT, Gray KM, Tolliver BK. Cognitive enhancers in the treatment of substance use disorders: clinical evidence. Pharmacol Biochem Behav. 2011;99(2):285–94. doi: 10.1016/j.pbb.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winhusen T, Winstanley E, Somoza E, Brigham G. The impact of recruitment method on sample characteristics and treatment outcomes in a psychosocial trial for women with co-occurring substance use disorder and PTSD. Drug Alcohol Depend. 2012;120:225–8. doi: 10.1016/j.drugalcdep.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berger SP, Winhusen TM, Somoza EC, Harrer JM, Mezinskis JP, Leiderman DB, et al. A medication screening trial evaluation of reserpine, gabapentin, and lamotrigine pharmacotherapy of cocaine dependence. Addiction. 2005;100(Suppl 1):58–67. doi: 10.1111/j.1360-0443.2005.00983.x. [DOI] [PubMed] [Google Scholar]
- 30.Winhusen T, Somoza E, Sarid-Segal O, Goldsmith RJ, Harrer JM, Coleman FS, et al. A double-blind, placebo-controlled trial of reserpine for the treatment of cocaine dependence. Drug Alcohol Depend. 2007;91(2–3):205–12. doi: 10.1016/j.drugalcdep.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winhusen TM, Somoza EC, Harrer JM, Mezinskis JP, Montgomery MA, Goldsmith RJ, et al. A placebo-controlled screening trial of tiagabine, sertraline, and donepezil as cocaine dependence treatments. Addiction. 2005;100(Suppl 1):68–77. doi: 10.1111/j.1360-0443.2005.00992.x. [DOI] [PubMed] [Google Scholar]
- 32.Kraemer HC, Mintz J, Noda A, Tinklenberg J, Yesavage JA. Caution regarding the use of pilot studies to guide power calculations for study proposals. Arch Gen Psychiatry. 2006;63(5):484–9. doi: 10.1001/archpsyc.63.5.484. [DOI] [PubMed] [Google Scholar]
- 33.Lamb S, Greenlick MR, McCarty D, editors. Bridging the gap between practice and research, forging partnerships with community-based drug and alcohol treatment. Washington, DC: National Academy Press; 1998. [PubMed] [Google Scholar]
- 34.Mooney M, Sayre S, Green C, Rhoades H, Schmitz J. Comparing measures of medication taking in a pharmacotherapy trial for cocaine dependence. Addict Disord Their Treat. 2004;3:165–73. [Google Scholar]
- 35.Sorensen JL, Haug NA, Delucchi KL, Gruber V, Kletter E, Batki SL, et al. Voucher reinforcement improves medication adherence in HIV-positive methadone patients: a randomized trial. Drug Alcohol Depend. 2007;88(1):54–63. doi: 10.1016/j.drugalcdep.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stone VE. Strategies for optimizing adherence to highly active antiretroviral therapy: lessons from research and clinical practice. Clin Infect Dis. 2001;33(6):865–72. doi: 10.1086/322698. [DOI] [PubMed] [Google Scholar]
- 37.Osterberg L, Blaschke T. Drug therapy adherence to medication. N Engl J Med. 2005;353:487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 38.Schmitz JM, Lindsay JA, Stotts AL, Green CE, Moeller FG. Contingency management and levodopa-carbidopa for cocaine treatment: a comparison of three behavioral targets. Exp Clin Psychopharmacol. 2010;18(3):238–44. doi: 10.1037/a0019195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carroll KM, Sinha R, Nich C, Babuscio T, Rounsaville BJ. Contingency management to enhance naltrexone treatment of opioid dependence: a randomized clinical trial of reinforcement magnitude. Exp Clin Psychopharmacol. 2002;10:54–63. doi: 10.1037//1064-1297.10.1.54. [DOI] [PubMed] [Google Scholar]
- 40.Carroll KM, Ball SA, Nich C, O’Connor PG, Eagan DA, Frankforter TL, et al. Targeting behavioral therapies to enhance naltrexone treatment of opioid dependence. Arch Gen Psychiatry. 2001;58:755–61. doi: 10.1001/archpsyc.58.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Preston KL, Silverman K, Umbricht A, DeJusus A, Montoya ID, Schuster CR. Improvement in naltrexone treatment compliance with contingency management. Drug Alcohol Depend. 1999;54:127–35. doi: 10.1016/s0376-8716(98)00152-5. [DOI] [PubMed] [Google Scholar]
- 42.Seal KH, Kral AH, Lorvick J. A randomized controlled trial of monetary incentives vs. outreach to enhance adherence to the hepatitis B vaccine series among injection drug users. Drug Alcohol Depend. 2003;71:127–31. doi: 10.1016/s0376-8716(03)00074-7. [DOI] [PubMed] [Google Scholar]
- 43.Rigsby MO, Rosen MI, Beauvais JE, Cramer JA, Rainey PM, O’Malley SS, et al. Cue-dose training with monetary reinforcement: pilot study of an antiretroviral adherence intervention. J Gen Intern Med. 2000;15(12):841–7. doi: 10.1046/j.1525-1497.2000.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Havassy B, Wasserman D, Hall S. Relapse to cocaine use: conceptual issues. In: Tims FM, Leukefeld CG, editors. Cocaine treatment: research and clinical perspectives. NIDA Research Monograph No. 135. Rockville, MD: National Institute on Drug Abuse; 1993. pp. 203–17. [PubMed] [Google Scholar]
- 45.Jones HE, Johnson RE, Bigelow GE, Silverman K, Mudric T, Strain EC. Safety and efficacy of L-tryptophan and behavioral incentives for treatment of cocaine dependence: a randomized clinical trial. Am J Addict. 2004;13:421–37. doi: 10.1080/10550490490512753. [DOI] [PubMed] [Google Scholar]
- 46.Sobell LC, Sobell MB. Timeline follow back: a technique for assessing self-reported ethanol consumption. In: Allen J, Litten R, editors. Techniques to Assess Alcohol Consumption. New Jersey: Humana Press, Inc; 1992. pp. 19–28. [Google Scholar]
- 47.Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. J Consult Clin Psychol. 2000;68:134–44. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- 48.McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 49.WHOQOL Group. Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol Med. 1998;28:551–8. doi: 10.1017/s0033291798006667. [DOI] [PubMed] [Google Scholar]
- 50.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 51.Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3137–46. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr Opin Pharmacol. 2009;9(1):65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- 54.Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3125–35. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carpenter KM, Schreiber E, Church S, McDowell D. Drug Stroop performance: relationships with primary substance of use and treatment outcome in a drug-dependent outpatient sample. Addict Behav. 2006;31(1):174–81. doi: 10.1016/j.addbeh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 56.Vadhan NP, Carpenter KM, Copersino ML, Hart CL, Foltin RW, Nunes EV. Attentional bias towards cocaine-related stimuli: relationship to treatment-seeking for cocaine dependence. Am J Drug Alcohol Abuse. 2007;33(5):727–36. doi: 10.1080/00952990701523722. [DOI] [PubMed] [Google Scholar]
- 57.Ersche KD, Bullmore ET, Craig KJ, Shabbir SS, Abbott S, Müller U, et al. Influence of compulsivity of drug abuse on dopaminergic modulation of attentional bias in stimulant dependence. Arch Gen Psychiatry. 2010;67(6):632–44. doi: 10.1001/archgenpsychiatry.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Franken IH, Hendriks VM, Stam CJ, Van den Brink W. A role for dopamine in the processing of drug cues in heroin dependent patients. Eur Neuropsychopharmacol. 2004;14(6):503–8. doi: 10.1016/j.euroneuro.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 59.Baler RD, Volkow ND. Drug Addiction: the neurobiology of disrupted self-control. Trends Mol Med. 2006;12(12):559–66. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 60.Franken IH, Kroon LY, Hendriks VM. Influence of individual differences in craving and obsessive cocaine thoughts on attentional processes in cocaine abuse patients. Addict Behav. 2000;25(1):99–102. doi: 10.1016/s0306-4603(98)00112-9. [DOI] [PubMed] [Google Scholar]
- 61.Franken IH, Hendriksa VM, van den Brink W. Initial validation of two opiate craving questionnaires: The Obsessive Compulsive Drug Use Scale and the Desires for Drug Questionnaire. Addict Behav. 2002;27(5):675–85. doi: 10.1016/s0306-4603(01)00201-5. [DOI] [PubMed] [Google Scholar]
- 62.Sussner BD, Smelson DA, Rodrigues S, Kline A, Losonczy M, Ziedonis D. The validity and reliability of a brief measure of cocaine craving. Drug Alcohol Depend. 2006;83(3):233–7. doi: 10.1016/j.drugalcdep.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 63.Cohen J. Statistical power analysis of the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 64.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 65.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 66.Winhusen T, Stitzer M, Woody G, Brigham G, Kropp F, Ghitza U, Lindblad R, Adinoff B, Green C, Sharma G, Somoza E. Design considerations for a study to evaluate the impact of smoking cessation treatment on stimulant use outcomes in stimulant-dependent individuals. Contemp Clin Trials. 2012;33 (1):197–205. doi: 10.1016/j.cct.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 68.Judd CM, Kenny DA. Process analysis: estimating mediation in treatment evaluations. Eval Rev. 1981;5:602–19. [Google Scholar]
- 69.Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. Sociol Methodol. 1982;13:290–312. [Google Scholar]