Abstract
Objective
Obesity and shorter telomeres are commonly associated with elevated risk for age-related diseases and mortality. Whether telomere length (TL) may be associated with obesity or variations in adiposity is not well established. Therefore, we set out to test the hypothesis that TL may be a risk factor for increased adiposity using data from a large population-based cohort study.
Design
Levels of adiposity were assessed in 6 ways (obesity status, body mass index or BMI, the percentage of body fat or % body fat, leptin, visceral and subcutaneous fat mass) in 2,721 elderly subjects (42% black and 58% white). Associations between TL measured in leukocytes at baseline and adiposity traits measured at baseline and 3 of these traits after 7 years of follow-up were tested using regression models adjusting for important covariates. Additionally, we look at weight changes and relative changes in BMI and % body fat between baseline and follow-up.
Results
At baseline, TL was negatively associated with % body fat (β = −0.35 ± 0.09, p = 0.001) and subcutaneous fat (β = −2.66 ± 1.07, p = 0.01), and positively associated with leptin after adjusting for % body fat (β = 0.32 ± 0.14, p = 0.001), but not with obesity, BMI or visceral fat. Prospective analyses showed that longer TL was associated with positive percent change between baseline and 7-year follow-up for both BMI (β = 0.48 ± 0.20, p = 0.01) and % body fat (β = 0.42 ± 0.23, p = 0.05).
Conclusion
Our study suggests that shorter TL may be a risk factor for increased adiposity. Coupling with previous reports on their reversed roles, the relationship between adiposity and TL may be complicated and warrant more prospective studies.
Keywords: Obesity, telomere length, adiposity, telomeres
INTRODUCTION
Obesity is a common risk factor for increased morbidity and mortality, including many aging-related pathologies (1–3). Obesity has been consistently associated with increased systemic inflammation and oxidative stress (4–6), which are also known to lead to shorter telomere length (TL) in cells (7–9). Telomeres are DNA-protein complexes that cap the ends of eukaryotic chromosomes. In humans, TL varies with age but in general, is progressively shortened as we age (10–12). Like obesity, TL has been associated with oxidative stress, inflammation and many age-related diseases (13–15). Whether TL may be associated with obesity as well as the direction of their relationship is unclear. In addition, the biological mechanism underlying these associations may be complicated and even involves feed-back loops. A small number of studies have investigated the relationship between TL and obesity. Some cross-sectional studies have reported significant associations between TL and obesity (16–18) but some did not (11, 19, 20). The only prospective study reported an increased TL in rectal mucosa of people who lost weight (21). All of these studies considered obesity, as a proxy of oxidative stress levels, being a risk factor for telomere shortening. While this may be plausible, the reverse, i.e. telomere erosion contributes to an increased risk of disorders related to fat metabolism is also possible but has been rarely investigated.
We hypothesize that short telomeres are a risk factor for elevated levels of adiposity. The aim of this study was to evaluate the relation between TL, obesity and its related traits in a large bi-racial cohort of elderly individuals. We tested our hypothesis by using more extensive measures of regional and global adiposity in both cross-sectional and prospective analysis. Our findings provide new insight into the complex relationship between adiposity and TL.
RESEARCH DESIGN AND METHODS
Study Participants
The Health, Aging, and Body Composition (Health ABC) Study is a prospective cohort study aimed at studying the relation of age-related changes in health and body composition with incident functional limitations in initially well-functioning elderly black and white individuals. At baseline, the cohort included 3,075 persons aged 70 to 79 years; 41.6% were black and 51.6% were female. Participants were recruited from Medicare listings in Pittsburgh, Pennsylvania, and Memphis, Tennessee between April 1997 and June 1998. Eligibility criteria included: 1) reported ability to walk one quarter mile (0.4 km), climb 10 steps, and perform basic activities of daily living without difficulty; 2) absence of life-threatening illness; and 3) intention to remain in the current geographic area for at least 3 years. All participants gave informed written consent and the study protocol was approved by the institutional review boards of the clinical sites and the Data Coordinating Center (University of California, San Francisco).
Phenotypic measurements
Race, sex, age and other socio-demographic variables were self-reported during the initial clinic visit. Body weight was measured with a standard balance beam scale to the nearest 0.1 kg. Height was measured barefoot using a Harpenden stadiometer (Holtain, UK) to the nearest 0.1 cm. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). Obesity was defined as having a BMI of ≥30 kg/m2. A total body DXA scan was performed to measure the percentage of total body fat (% body fat) using fan-beam technology (Hologic QDR4500A, NY, USA). Abdominal computed tomography (CT) scans were performed to determine abdominal and subcutaneous fat masses at the L4-L5 level. Fat areas were calculated by multiplying the number of pixels of a given tissue type by the pixel area using Interactive Data Language software (ITT Visualization Solutions, Boulder, Colorado).
Visceral fat tissue was manually distinguished from subcutaneous fat tissue by tracing along the facial plane defining the internal abdominal wall and measured in cm2. Total circulating level of serum leptin was measured in duplicate by radioimmuno-assay (RIA, Linco Research, St. Charles, MO). BMI and % body fat were re-assessed after 7 years of follow-up and the obesity status was also determined. We calculated the percentage of change in these 2 traits between baseline and follow-up as follows: 100 × (baseline value − follow-up value)/baseline value. The weight change status was classified into 3 categories: weight gain, stable weight, and weight loss if the individuals gained ≥ 3kg, gained or lost within 3kg, or lost ≥ 3kg, respectively.
Average TL in leukocytes (peripheral blood mononucleocytes) was measured using a validated Q-PCR method (22). This method measures the relative average TLs in genomic DNA by determining the ratio of telomere repeat copy number to single copy gene copy number (T/S ratio) in experimental samples relative to a reference sample. All samples were measured in triplicate, and their mean was used. Results obtained using this method correlate well with those obtained with the traditional terminal restriction fragment (TRF) length by Southern blot technique (r = 0.7) (22). For this study the T/S ratio was converted to a TL in kilo basepairs (kbp) by multiplying the t/s value by the known TL of the reference DNA, 4,270 bp. To obtain the TL for the reference DNA, we used the T/S ratios of 64 DNA samples from Utah Caucasians with known mean TRF lengths. The slope of the linear regression line through a plot of T/S ratio (x axis) vs. mean TRF length (y axis) is the number of basepairs of telomeric DNA corresponding to a single T/S unit. Since the reference DNA has a T/S of 1.0, by definition, this slope is also the average telomere length of the reference DNA sample, 4,270 bp in our case.
Statistical analysis
The association between TL and quantitative traits was examined by fitting multiple linear regression models adjusting for age, sex, race, type 2 diabetes status, TL assay plates (modeled as a random factor) and subjects’ recruitment site. In addition, we adjusted for factors commonly known to influence adiposity, including physical activity and smoking habits. Furthermore, in the analysis of abdominal subcutaneous and visceral fat mass, we further adjusted for body height and weight. In the analysis of leptin, the effect of % body fat was also adjusted. Similar modeling approaches were used for analyses of discrete traits (obesity status and weight gain/loss) in logistic regression models.
Effect modification by sex and race was evaluated by adding interaction terms to the regression models. Co-linearity of the covariates was checked and determined not to affect inclusion in the models. As race and sex were not found to be effect modifiers, no stratified analysis by these factors was performed. A p value < 0.05 was considered significant. All analyses were performed using SPSS for Windows version 14 (SPSS, Chicago, IL, USA).
RESULTS
Measurements of TL at baseline were available in 2,721 individuals. Information on adiposity measures was available in all 2,721 subjects at baseline and in 1,958 subjects after 7 years of follow-up. Table 1a summarizes the baseline characteristics of the study population. The mean age at recruitment was similar for men and women. On average, TL was significantly longer in women compared to men, whereas no apparent difference in TL between blacks and whites within the age range of our study population (70 – 80 years old). Approximately one quarter (25.8%) and 24.2% of individuals were identified as obese at baseline and after 7 years of follow-up, respectively. The prevalence of obesity was significantly higher in blacks compared to whites. Among blacks, there were significantly more obese women than men whereas among whites, there were more obese men than women. Mean levels of % body fat, leptin, and subcutaneous fat were significantly higher in women compared to men in both races. As expected in an elderly population, more people lost rather than gained weight during the 7 years of follow-up. The relative change in BMI and % body fat did not follow any pattern and no significant difference was observed but in general women had less change in % fat.
Table 1a.
Characteristics of the study population by race and sex at baseline
| Traits (Mean ± SD or number, %) | Whites | Blacks | ||
|---|---|---|---|---|
|
| ||||
| Male (933) | Female (844) | Male (543) | Female (717) | |
| Age, years | 73.9 ± 2.9 | 73.6 ± 2.8 | 73.5 ± 2.8 | 73.4 ± 3.0 |
| Telomere length, kbp | 4.6 ± 1.2 | 5.0 ± 1.3* | 4.6 ± 1.1 | 5.0 ± 1.2* |
| Obese (n, %) | 180 (19.3%) | 143 (16.9%)* | 138 (25.4%) | 317 (44.2%)* |
| BMI, kg/m2 | 27.0 ± 3.7 | 26.0 ± 4.5 | 27.2 ± 4.4 | 29.7 ± 5.9* |
| Percentage total body fat, % | 28.7 ± 4.8 | 39.0 ± 5.6* | 26.8 ± 5.3 | 40.0 ± 6.1* |
| Leptin, ng/ml | 7.6 ± 6.3 | 16.1 ± 10.5* | 8.0 ± 6.4 | 21.3 ± 11.8* |
| Visceral fat, cm2 | 170 ± 71 | 132 ± 63* | 130 ± 67 | 130 ± 59 |
| Subcutaneous fat, cm2 | 229 ± 84 | 308 ± 109* | 237 ± 100 | 372 ± 138* |
Men vs. Women, p<0.001
As race and sex were not found to be effect modifiers, no stratified analysis by these factors was performed. Using adiposity measures obtained at baseline, TL was not associated with obesity status (OR = 1.0, 95% CI = 0.9 – 1.1) (Table 2). Longer TL was significantly associated with less % body fat (β = −0.35 ± 0.09, p = 0.001), less abdominal subcutaneous fat (β = −2.66 ± 1.07, p = 0.01), and higher levels of fasting leptin after adjusting for the effect of % body fat (β = 0.32 ± 0.14, p = 0.02). At follow-up, TL was also not significantly associated with obesity (OR = 1.0, 95% CI = 0.9 – 1.1), BMI (β = −0.06 ± 0.09, p = 0.5). The relationship between TL and % body fat became weaker, albeit remained in the same direction (β = −0.14 ± 0.11, p = 0.4) using % body fat measured at follow-up.
Table 2.
Association between TL (kbp) and adiposity at baseline and after 7 years of follow-up
| Trait | n | Baseline | n | Follow-up | n | % change^ |
|---|---|---|---|---|---|---|
| BMI | 2,715 | −0.12 ± 0.08 | 1,958 | −0.06 ± 0.09 | 1,958 | 0.48 ± 0.20* |
| % total body fat | 2,614 | −0.35 ± 0.09** | 1,755 | −0.14 ± 0.11 | 1,755 | 0.42 ± 0.23* |
| Leptin | 2,459 | 0.32 ± 0.14* | NA | NA | ||
| Visceral fat | 2,669 | −1.05 ± 0.82 | NA | NA | ||
| Subcutaneous fat | 2,648 | −2.66 ± 1.07* | NA | NA |
P<0.05,
p < 0.001
% change = (follow-up − baseline)/baseline
Adiposity related trait = age + sex + race + site + type 2 diabetes + smoking + physical activity + TL.
We further examined whether TL measured at baseline was associated with the change in adiposity traits between baseline and follow-up. TL was significantly associated with the percentage of change in BMI and % total body fat between baseline and follow-up. In other words, the longer the TL at baseline, the greater increase in BMI (β = 0.48 ± 0.20, p = 0.01) and % body fat (β = 0.42 ± 0.23, p = 0.05) during the 7-year follow-up (Table 2). Figure 1 shows the mean TL in people who gained weight compared to people who lost weight during the follow-up period. We observed that the mean TL was significantly longer in individuals who gained weight compared to individuals who lost weight (5.01 vs. 4.75 kbp; p = 0.01). In other words, for individuals who lost weight during the follow-up period, their TL was shorter by an average of 260 basepairs. This observation is in concordance with results from analyses of changes in BMI and % body fat.
Figure 1.
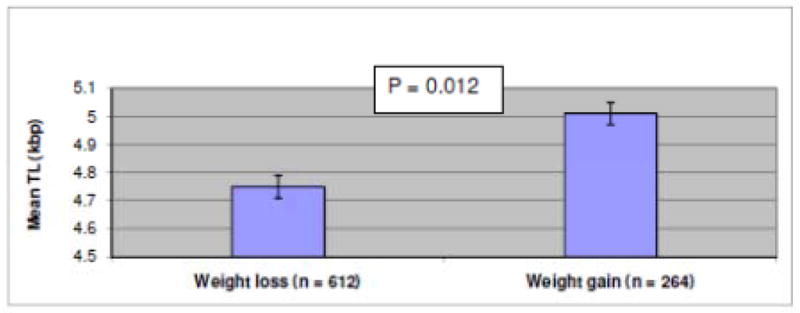
Adjusted mean TL by weight gain or loss at follow-up
¶ Error bars represent one standard error
DISCUSSION AND CONCLUSION
In this study, we investigated the relationship between TL and adiposity using both cross-sectional and follow-up data. Our findings suggest that shorter TL may be a risk factor for increased adiposity. TL measured at baseline was significantly associated with several adiposity measures, including, % body fat, subcutaneous fat and leptin, but not with obesity, BMI or visceral fat. In our prospective analyses of 3 available adiposity measures, the direction of their relationship with TL remained the same but the significance was weaker. Furthermore, we also found TL to be associated with positive change in BMI and % body fat after 7 years of follow-up.
It might appear somewhat puzzling as to why only certain adiposity measures were associated with TL while some others were not. In our cohort composed of elderly individuals, quantitative traits showing stronger association with TL were also in higher correlations with each other (r = 0.66 – 0.80), whereas all other pair-wise correlation estimates were weaker (≤ 0.56) except for that between BMI and subcutaneous fat (0.76). There were also varying degrees of statistical power in analyses of different traits. Although our sample size was large, we still did not have sufficient power to detect the observed effects for visceral fat and BMI (power ≈ 0.6) while the power was sufficient for the other three adiposity measures. In addition, one question that comes to mind is whether BMI is a good measure of adiposity in the elderly. Some previous studies have suggested that BMI may not be as informative as a proxy for adiposity or an appropriate measure for defining obesity in older individuals (23, 24). This may help explain the lack of association between BMI/obesity and TL.
There are a handful of previous investigations on the association between TL and obesity-related traits, all of which considered obesity being a risk factor for shorter TL. Some studies found significant associations between shorter TL and obesity or higher levels of its related quantitative measures (16, 17, 25, 26) whereas others did not (19, 20). Yet no study has been conducted in the elderly. Some studies only provided correlation coefficients (16, 21, 25), which tends to be less informative compared to association testing as it does not evaluate the magnitude of the effects. Weight, BMI, leptin and waist-to-hip ratio were most commonly investigated in earlier studies. BMI was inversely associated with TL in middle aged individuals (16, 18, 25, 27). We also observed a weak but negative relationship between TL and BMI, and a significantly negative association with % body fat in our cohort of elderly. Using BMI ≥ 30 kg/m2 to define obesity, MacEneaney et al. (19) reported no difference in TL between normal and overweight/obese individuals. One study by Valdes et al. (16) reported a negative correlation (r = −0.124, p < 0.0001) between TL and leptin, whereas another smaller study did not (27). In our analysis of leptin, when unadjusted for % body fat, we observed a similar result as those reported by Valdes et al. (16). However, we considered it informative to evaluate such as relationship independent of body fat, since leptin is produced by fat cells. As expected, we observed that shorter TL was associated with lower leptin levels after adjusting for the effect of % body fat. Leptin plays a role in appetite suppression and one would expect that higher leptin level be associated with better health, thus with longer TL. Two studies examined the effect of waist-to-hip ratio in TL and reported significantly negative association with TL (27, 28). We used measurements from CT scans to obtain more precise measures of regional adiposity and observed a significantly negative association of TL with subcutaneous fat, but not with visceral fat. Our study is novel in that we treated TL as a risk factor. It is interesting to see that while our study treated TL as a risk factor contrary to previous studies, we could still observe comparable findings mainly on the direction of the associations.
There are several advantages of our study. One of the main strengths is the prospective nature of our study with several repeated adiposity measures ascertained 7 years apart. This enabled us to test our hypothesis that TL may be a risk factor for increased adiposity in a prospective fashion. In addition, our study is the first large study to examine the association between TL and adiposity in a non-Caucasian sample, and our findings suggest that race did not appear to affect the association between adiposity and TL. The ages of our study subjects were notably older than previous studies, which provided evidence that the association between TL and adiposity observed in younger populaitons was also present in the elderly. However, as we did not have TL measured at follow-up, our study could not examine whether adiposity could be a risk factor for telomere shortening in a prospective fashion. Nevertheless, our observations suggest that TL may be considered a risk factor for adiposity. One possible limitation to the current study is that we measured TL in leukocytes as a proxy for TL in other tissues. However is well documented that TL measurement in easily accessible tissues such as blood could serve as a surrogate parameter for the relative telomere length in other tissues (29,30).
In summary, we found that shortening of TL was associated with adiposity. However, whether telomere shortening is a cause or consequence of increased adiposity could not be determined based on our data alone. Although it may be possible that TL shortening is a consequence of increased adiposity due to elevated levels of oxidative stress (9, 29) and other factors, our study suggests their relationship may be more complicated. The possible biological mechanism(s) for these associations deserves further investigations. A better understanding of their relationship may have implication on our effort to reduce obesity burden and to promote healthy aging.
Table 1b.
Characteristics of the study population by race and sex at follow-up
| Traits (Mean ± SD or number, %) | Whites | Blacks | ||
|---|---|---|---|---|
|
| ||||
| Male (933) | Female (844) | Male (543) | Female (717) | |
| Obese (n, %) | 137 (19.4%) | 128 (18.8%)* | 81 (24.7%) | 216 (43.4%)* |
| BMI, kg/m2 | 27.0 ± 3.7 | 26.1 ± 4.7 | 27.1 ± 4.7 | 29.4 ± 6.2* |
| Percentage total body fat, % | 29.6 ± 5.0 | 39.2 ± 5.4* | 28.0 ± 5.7 | 39.8 ± 6.2* |
| Weight gain/weight loss (n, %) | 95(13.8%)/226(32.9%) | 91(14%)/189(29.1%) | 50 (16.2%)/108 (35.1%) | 72(15.7%)/167(36.3%) |
| % change in BMI | −0.2 ± 6.5 | 0.8 ± 9.1 | −0.5 ± 7.7 | −1.3 ± 8.7 |
| % change in the percentage of body fat | 3.1 ± 8.9 | 0.5 ± 7.2* | 4.2 ± 12 | −0.6 ± 8.9* |
Men vs. Women, p<0.001
Acknowledgments
This study was supported in part by the Intramural Research Program of the National Institute on Aging, NIH contracts (N01-AG-6-2101, N01-AG-6-2103 and N01-AG-6-2106) and grants (K01 AG022782 and R01 AG023692). Dr Omer T. Njajou is supported by the Training in Molecular & Genetic Epidemiology of Cancer, National Institutes of Health, National Cancer Institute grant (R25 CA112355).
Footnotes
Conflict of interest statement: There is no conflict of interest
References
- 1.Lahmann PH, Lissner L, Gullberg B, Berglund G. A prospective study of adiposity and all-cause mortality: the Malmo Diet and Cancer Study. Obes Res. 2002;10:361–369. doi: 10.1038/oby.2002.50. [DOI] [PubMed] [Google Scholar]
- 2.Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes (Lond) doi: 10.1038/ijo.2010.222. [DOI] [PubMed] [Google Scholar]
- 3.Reis JP, Araneta MR, Wingard DL, Macera CA, Lindsay SP, Marshall SJ. Overall obesity and abdominal adiposity as predictors of mortality in u.s. White and black adults. Ann Epidemiol. 2009;19:134–142. doi: 10.1016/j.annepidem.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Rankin JW, Andreae MC, Oliver Chen CY, O’Keefe SF. Effect of raisin consumption on oxidative stress and inflammation in obesity. Diabetes Obes Metab. 2008;10:1086–1096. doi: 10.1111/j.1463-1326.2008.00867.x. [DOI] [PubMed] [Google Scholar]
- 5.Sohet FM, Neyrinck AM, Pachikian BD, de Backer FC, Bindels LB, Niklowitz P, et al. Coenzyme Q10 supplementation lowers hepatic oxidative stress and inflammation associated with diet-induced obesity in mice. Biochem Pharmacol. 2009;78:1391–1400. doi: 10.1016/j.bcp.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki K, Ito Y, Ochiai J, Kusuhara Y, Hashimoto S, Tokudome S, et al. Relationship between obesity and serum markers of oxidative stress and inflammation in Japanese. Asian Pac J Cancer Prev. 2003;4:259–266. [PubMed] [Google Scholar]
- 7.Halvorsen TL, Beattie GM, Lopez AD, Hayek A, Levine F. Accelerated telomere shortening and senescence in human pancreatic islet cells stimulated to divide in vitro. J Endocrinol. 2000;166:103–109. doi: 10.1677/joe.0.1660103. [DOI] [PubMed] [Google Scholar]
- 8.Kurz DJ, Decary S, Hong Y, Trivier E, Akhmedov A, Erusalimsky JD. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J Cell Sci. 2004;117:2417–2426. doi: 10.1242/jcs.01097. [DOI] [PubMed] [Google Scholar]
- 9.von Zglinicki T, Saretzki G, Docke W, Lotze C. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp Cell Res. 1995;220:186–193. doi: 10.1006/excr.1995.1305. [DOI] [PubMed] [Google Scholar]
- 10.Njajou OT, Cawthon RM, Damcott CM, Wu SH, Ott S, Garant MJ, et al. Telomere length is paternally inherited and is associated with parental lifespan. Proc Natl Acad Sci U S A. 2007;104:12135–12139. doi: 10.1073/pnas.0702703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, et al. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol. 2007;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 12.Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 13.Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009;361:2353–2365. doi: 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carroll KA, Ly H. Telomere dysfunction in human diseases: the long and short of it! Int J Clin Exp Pathol. 2009;2:528–543. [PMC free article] [PubMed] [Google Scholar]
- 15.Terasaki Y, Okumura H, Ohtake S, Nakao S. Accelerated telomere length shortening in granulocytes: a diagnostic marker for myeloproliferative diseases. Exp Hematol. 2002;30:1399–1404. doi: 10.1016/s0301-472x(02)00969-4. [DOI] [PubMed] [Google Scholar]
- 16.Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 17.Zannolli R, Mohn A, Buoni S, Pietrobelli A, Messina M, Chiarelli F, et al. Telomere length and obesity. Acta Paediatr. 2008 doi: 10.1111/j.1651-2227.2008.00783.x. [DOI] [PubMed] [Google Scholar]
- 18.Al-Attas OS, Al-Daghri NM, Alokail MS, Alfadda A, Bamakhramah A, Sabico S, et al. Adiposity and insulin resistance correlate with telomere length in middle-aged Arabs: the influence of circulating adiponectin. Eur J Endocrinol. 163:601–607. doi: 10.1530/EJE-10-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacEneaney OJ, Kushner EJ, Westby CM, Cech JN, Greiner JJ, Stauffer BL, et al. Endothelial progenitor cell function, apoptosis, and telomere length in overweight/obese humans. Obesity (Silver Spring) 18:1677–1682. doi: 10.1038/oby.2009.494. [DOI] [PubMed] [Google Scholar]
- 20.Diaz VA, Mainous AG, Player MS, Everett CJ. Telomere length and adiposity in a racially diverse sample. Int J Obes (Lond) 34:261–265. doi: 10.1038/ijo.2009.198. [DOI] [PubMed] [Google Scholar]
- 21.O’Callaghan NJ, Clifton PM, Noakes M, Fenech M. Weight loss in obese men is associated with increased telomere length and decreased abasic sites in rectal mucosa. Rejuvenation Res. 2009;12:169–176. doi: 10.1089/rej.2008.0819. [DOI] [PubMed] [Google Scholar]
- 22.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rimm EB, Stampfer MJ, Giovannucci E, Ascherio A, Spiegelman D, Colditz GA, et al. Body size and fat distribution as predictors of coronary heart disease among middle-aged and older US men. Am J Epidemiol. 1995;141:1117–1127. doi: 10.1093/oxfordjournals.aje.a117385. [DOI] [PubMed] [Google Scholar]
- 24.Folsom AR, Kushi LH, Anderson KE, Mink PJ, Olson JE, Hong CP, et al. Associations of general and abdominal obesity with multiple health outcomes in older women: the Iowa Women’s Health Study. Arch Intern Med. 2000;160:2117–2128. doi: 10.1001/archinte.160.14.2117. [DOI] [PubMed] [Google Scholar]
- 25.Moreno-Navarrete JM, Ortega F, Sabater M, Ricart W, Fernandez-Real JM. Telomere length of subcutaneous adipose tissue cells is shorter in obese and formerly obese subjects. Int J Obes (Lond) 34:1345–1348. doi: 10.1038/ijo.2010.49. [DOI] [PubMed] [Google Scholar]
- 26.Nordfjall K, Eliasson M, Stegmayr B, Melander O, Nilsson P, Roos G. Telomere length is associated with obesity parameters but with a gender difference. Obesity (Silver Spring) 2008;16:2682–2689. doi: 10.1038/oby.2008.413. [DOI] [PubMed] [Google Scholar]
- 27.Farzaneh-Far R, Lin J, Epel E, Lapham K, Blackburn E, Whooley MA. Telomere length trajectory and its determinants in persons with coronary artery disease: longitudinal findings from the heart and soul study. PLoS One. 5:e8612. doi: 10.1371/journal.pone.0008612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Attas OS, Al-Daghri N, Bamakhramah A, Shaun Sabico S, McTernan P, Huang TT. Telomere length in relation to insulin resistance, inflammation and obesity among Arab youth. Acta Paediatr. 99:896–899. doi: 10.1111/j.1651-2227.2010.01720.x. [DOI] [PubMed] [Google Scholar]
- 29.Mohn A, Catino M, Capanna R, Giannini C, Marcovecchio M, Chiarelli F. Increased oxidative stress in prepubertal severely obese children: effect of a dietary restriction-weight loss program. J Clin Endocrinol Metab. 2005;90:2653–2658. doi: 10.1210/jc.2004-2178. [DOI] [PubMed] [Google Scholar]
- 30.Friedrich U, Griese E, Schwab M, Fritz P, Thon K, Klotz U. Telomere length in different tissues of elderly patients. Mech Ageing Dev. 2000;119(3):89–99. doi: 10.1016/s0047-6374(00)00173-1. [DOI] [PubMed] [Google Scholar]


