Abstract
Currently there is no treatment for juvenile Batten disease, a fatal childhood neurodegenerative disorder caused by mutations in the CLN3 gene. The Cln3-knockout (Cln3Δex1-6) mouse model recapitulates several features of the human disorder. Cln3Δex1-6 mice, similarly to juvenile Batten disease patients, have a motor coordination deficit detectable as early as postnatal day 14. Previous studies demonstrated that acute attenuation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA)-type glutamate receptor activity by the non-competitive AMPA antagonist, EGIS-8332, in both 1- and 6–7-month-old Cln3Δex1-6 mice results in improvement in motor coordination. Here we show that acute inhibition of N-methyl-D-aspartate (NMDA)-type glutamate receptors by memantine (1 and 5 mg/kg i.p.) had no effect on the impaired motor coordination of one-month-old Cln3Δex1-6 mice. At a later stage of the disease, in 6–7-month-old Cln3Δex1-6 mice, memantine induced a delayed but extended (8 days) improvement of motor skills similarly to that observed previously with EGIS-8332 treatment. An age-dependent therapeutic effect of memantine implies that the pathomechanism in juvenile Batten disease changes during disease progression. In contrast to acute treatment, repeated administration of memantine or EGIS-8332 (1 mg/kg, once a week for 4 weeks) to 6-month-old Cln3Δex1-6 mice had no beneficial effect on motor coordination. Moreover, repeated treatments did not impact microglial activation or the survival of vulnerable neuron populations. Memantine did not affect astrocytosis in the cortex. EGIS-8332, however, decreased astrocytic activation in the somatosensory barrelfield cortex.
Acute inhibition of NMDA receptors can induce a prolonged therapeutic effect, identifying NMDA receptors as a new therapeutic target for juvenile Batten disease.
Keywords: juvenile Batten disease, neuronal ceroid lipofuscinoses, Cln3, NMDA receptor, AMPA receptor, rotarod
1. Introduction
Neuronal Ceroid Lipofuscinoses (NCLs) are a group of rare, recessively inherited lysosomal storage disorders characterized by progressive neurodegeneration (Goebel, 1995). Mutations in one of ten genes, CLN1–10, cause the different forms of NCL that vary in age of onset and speed of disease progression (Jalanko and Braulke, 2009). Mutations in the CLN3 gene are responsible for the development of the most common, juvenile onset form of NCL, also known as juvenile Batten disease (Consortium, 1995). CLN3 encodes a lysosomal membrane protein with unknown function (Getty and Pearce, 2011) and accordingly, the mechanism of the selective neurodegeneration induced by CLN3 mutations remains elusive. Juvenile Batten disease begins between five and eight years of age with visual impairment and seizures. As the disease progresses, visual impairment leads to blindness and the seizures become more frequent and intense. The disease also causes loss of motor skills and progressive cognitive decline. Juvenile Batten disease patients die in their late teens or early 20s (Goebel and Wisniewski, 2004). No specific therapy is currently available that could stop or slow down the progression of the disease.
The Cln3-knockout (Cln3Δex1-6) mouse model of juvenile Batten disease has been studied extensively and provided important information about the neuropathological changes occurring during disease progression (Benedict et al., 2007; Chan et al., 2009; Kovacs et al., 2006; Lim et al., 2007; Mitchison et al., 1999; Pears et al., 2005; Pontikis et al., 2004; Weimer et al., 2007; Weimer et al., 2009; Weimer et al., 2006). Cln3Δex1-6 mice, similarly to juvenile Batten disease patients, have a deficit in motor coordination that can be detected as early as 14 days of age (Kovacs et al., 2006; Mitchison et al., 1999; Weimer et al., 2009).
Recent studies indicate that glutamate neurotransmission is dysregulated in several fatal, neurodegenerative lysosomal storage disorders such as infantile, late infantile and juvenile Batten diseases (Ahtiainen et al., 2007; Finn et al., 2012; Kovacs et al., 2006; Macauley et al., 2009; Pears et al., 2005; Pears et al., 2007; Seitz et al., 1998; Sitter et al., 2004), Niemann-Pick disease (Byun et al., 2006; Chiulli et al., 2007; D’Arcangelo et al., 2011; Yadid et al., 1998) and Gaucher disease (Korkotian et al., 1999). The aberrant glutamate neurotransmission may cause the neurological deficits and progressive neurodegeneration observed in these lysosomal storage disorders. In fact, our recent results demonstrated that an abnormally increased AMPA-type glutamate receptor activity largely contributes to the motor coordination deficit in the Cln3Δex1-6 mouse model of juvenile Batten disease: acute attenuation of AMPA receptor activity by the non-competitive AMPA antagonist, EGIS-8332, in both 1- and 6–7-month-old Cln3Δex1-6 mice resulted in a significant improvement in motor coordination (Kovacs and Pearce, 2008; Kovacs et al., 2011). In the present study we tested if attenuation of NMDA-type glutamate receptors can improve the motor coordination of Cln3Δex1-6 mice. Our results show that in 6–7-month-old Cln3Δex1-6 mice, acute inhibition of NMDA receptors can induce a prolonged (8 days) therapeutic effect, identifying NMDA receptors as a new therapeutic target for juvenile Batten disease.
2. Materials and methods
2.1. Animals
In this study 129S6/SvEv wild type (WT) and homozygous Cln3-knockout (Cln3Δex1-6) mice (Mitchison et al., 1999) inbred on a 129S6/SvEv background were used. One-month-old male and female mice and 6–7-month-old female mice were used in the experiments. Female patients with Batten disease have a more severe disease course (Cialone et al., 2012). Although, this gender-specific difference has not been shown in the Cln3Δex1-6 mouse model of Batten disease, we used female 6–7-month-old Cln3Δex1-6 and WT mice in our study.
Mice were genotyped as described by Mitchison et al. (1999). All procedures were carried out according to the guidelines of the Animal Welfare Act, NIH policies and the University of Rochester Animal Care and Use Committee.
2.2. Drugs
Memantine was purchased from Tocris Bioscience (Bristol, UK), EGIS-8332 was a generous gift of EGIS Pharmaceuticals Plc (Budapest, Hungary). The stock solution of memantine was prepared in ultrapure water. To achieve the appropriate drug concentration for injection, the memantine stock solution was diluted in 0.9% NaCl. EGIS-8332 was dissolved in 20 mM HCl containing 10% DMSO for injection. Mice were injected with sterile solutions of the drugs in an injection volume of 10 ml/kg.
2.3. Rotarod test and drug administration
An accelerating rotarod (0–24 rpm in 240 s; AccuScan Instruments, Inc., Columbus, OH) was used to measure the motor skills of mice. The rotarod measures the ability of the mouse to maintain balance on a motor-driven, rotating rod. Thus, the fore- and hind limb motor coordination and balance can be analyzed (Karl et al., 2003). Due to the repeated, multiple test trials used in our rotarod protocol, motor learning also contributed to the rotarod performance of mice.
2.3.1. Acute treatment with memantine
Mice were trained on the rotarod in three consecutive runs. Following training, mice rested for 1 h and then were tested on the rotarod in three Pre-treatment test trials each consisting of three consecutive runs, with 15 min of rest between the trials. Two hours and thirty minutes afte.r the end of the Pre-treatment test, mice were intraperitoneally injected with memantine (1 or 5 mg/kg; injection volume: 10 ml/kg) or with the vehicle of memantine (sterile 0.9% NaCl). Thirty minutes after the injection (1-month-old mice) or 30 minutes, 1, 4, 6 and 8 days after the injection (6–7-month-old mice), animals were tested on the rotarod in three test trials each consisting of three consecutive runs, with 15 min of rest between the trials. The latencies to fall from the rotating rod during the testing periods were calculated for each mouse.
2.3.2. Repeated treatment
Six-month-old Cln3Δex1-6 mice were intraperitoneally injected with memantine (1 mg/kg), EGIS-8332 (1 mg/kg), or sterile 0.9% NaCl (injection volume: 10 ml/kg) once a week for 4 weeks. One day after the last injection, mice were trained on the rotarod in three consecutive runs. Following training, mice rested for 1 h and then were tested on the rotarod in three test trials each consisting of three consecutive runs, with 15 min of rest between the trials. Three and 7 days after the last injection mice were also tested on the rotarod. The latencies to fall from the rotating rod during the testing periods were calculated for each mouse.
2.4. Histological processing
Acute treatment groups
Six-month-old Cln3Δex1-6 mice acutely treated with memantine (1 or 5 mg/kg) or the vehicle of memantine (sterile 0.9% NaCl) were perfusion-fixed with 4% paraformaldehyde (in DPBS, pH 7.4) 8 days after the treatment (4 mice from each treatment group). Four 6-month-old, untreated WT mice were also perfusion-fixed.
Repeated treatment groups
Six-month-old Cln3Δex1-6 mice were treated with the NMDA receptor antagonist, memantine (1 mg/kg), the AMPA receptor antagonist, EGIS-8332, or sterile 0.9% NaCl (Vehicle) once a week for 4 weeks. Motor coordination of the mice was tested 1, 3 and 7 days after the last injection. Two days after the last rotarod test, four vehicle-injected, four memantine-treated and four EGIS-8332-treated Cln3Δex1-6 mice were perfusion-fixed with 4% paraformaldehyde. Four 7-month-old, untreated WT mice were also perfusion-fixed.
The brains were post-fixed for 2 h at room temperature and cryoprotected at 4°C in a solution of 30% sucrose in DPBS containing 0.05% sodium azide. Brains were bisected along the midline, and 40μm coronal sections were cut from the left hemisphere on a microtome (Leitz 1321 freezing microtome) and stored in 96 well plates that contained a cryoprotectant solution (30% ethylene glycol, 15% sucrose and 0.05% sodium azide in Tris buffered saline (TBS: 50 mM Tris, 150 mM NaCl, pH 7.6)).
2.5. Nissl staining and stereological estimation of neuron number
To visualize neuronal cytoarchitecture every sixth section of each brain (4 mice from each treatment group) was mounted on gelatine/chrome alum-coated Superfrost microscope slides (VWR, Poole, UK), air dried overnight and stained for 30 min at 60°C with cresyl fast violet solution (0.05% solution with 0.5 ml of 10% acetic acid per 100 ml of solution were mixed and preheated to 56°C directly before use). Slides were next rinsed in distilled water and differentiated through a graded series of IMS. Finally sections were cleared in xylene and coverslipped with DPX (VWR) (Pontikis et al., 2004).
To survey the survival of neuron populations that are vulnerable in Cln3Δex1-6 mice, counts of the large projection neurons in the thalamus (dorsal lateral geniculate nucleus) and in the medial deep cerebellar nucleus were made. These counts were obtained using StereoInvestigator software (Microbright Field, Williston, VT) as previously described (Bible et al., 2004). The boundaries of nuclei were defined by reference to landmarks in a mouse brain atlas (Paxinos and Franklin, 2001). The mean coefficient of error (CE) for all individual optical fractionator and nucleator estimates was calculated according to the method of Gundersen (Gundersen and Jensen, 1987) and was less than 0.09 in all these analyses. Images for neuronal cell counts were taken with a 100X oil objective on a Zeiss Axioskop2 MOT microscope (Carl Zeiss Ltd, Welwyn, Garden City, UK), as described previously (Weimer et al., 2009).
2.6. Quantification of GFAP and F4/80 immunoreactivity
To assess the degree of astrocytic and microglial activation every sixth section of each brain (4 mice from each treatment group) was immunohistochemically stained for the astrocytic marker glial fibrillary acidic protein (GFAP) and the microglia marker F4/80 as described previously (Weimer et al., 2009). Briefly, sections were first treated with 1% H2O2 in Tris-buffered saline (TBS: 50mM Tris, pH 7.6) for 30 min to block endogenous peroxidase activity and subsequently rinsed three times for 5 min in TBS. To minimize non-specific protein binding the sections were incubated with 15% normal serum (serum from the host species of the secondary antibody) in TBS-T (TBS containing 0.3% w/v Triton X-100) for 30 min. Sections were then labeled overnight at 4°C with either a rabbit anti-GFAP (Dako, 1:5000) or a rat anti-F4/80 (Serotec, 1:100) antibody diluted in TBS-T containing 10% normal serum (normal swine serum for GFAP, normal rabbit serum for F4/80). After washing in TBS (3 times, 5 min each), sections were incubated for 2 hours at room temperature with the appropriate biotinylated secondary antibody (for GFAP: swine anti-rabbit, 1:1000, Vector Laboratories; for F4/80: rabbit anti-rat, 1:200, Vector Laboratories) diluted in TBS-T containing 10% normal serum. After washing in TBS (3 times, 5 min each), sections were incubated for 2 h at room temperature in ABC reagent (avidin-biotinylated enzyme complex) diluted 1:1000 in TBS (Vectastatin Elite ABC kit, Vector Laboratories). After washing in TBS 3 times (5 min each), a standard diaminobenzidine reaction was used to visualize immunoreactivity. Afterwards sections were mounted on Superfrost microscope slides, air dried overnight, cleared in xylene and coverslipped with DPX (VWR).
All photomicrographs were taken with Zeiss Axiocam HR digital camera and Axiovision 4.6 software (Carl Zeiss UK Ltd, Welwyn Garden City, UK). All following analyses were performed with no previous knowledge of treatment. Assessment of GFAP and F4/80 staining was done with a semi-automated thresholding image analysis (Bible et al., 2004; Pontikis et al., 2004). Accordingly, forty non-overlapping pictures from the region of interest were captured from four sequential stained sections. Images were captured under constant conditions (lamp intensity, video camera set up and calibration). Thresholding analysis was then performed using Image Pro Plus image analysis software (Media Cybernatics, Chicago, IL). An appropriate threshold was determined to discriminate specific immunoreactivity from background staining, and was applied subsequently for all analysis.
2.7. Statistical analysis
Statistical analysis was performed using GraphPad Prism 5. The data from the rotarod test passed the normality test and therefore, repeated measures two-way ANOVA with Bonferroni’s post-test was applied to compare rotarod performances. Histological data were analyzed by one-way ANOVA with Bonferroni’s post-test.
3. Results
3.1. Acute inhibition of NMDA receptors has no effect on the impaired motor coordination of 1-month-old Cln3Δex1-6 mice
Cln3Δex1-6 mice, similarly to patients with juvenile Batten disease, have a deficit in motor coordination as measured by the rotarod test (Kovacs and Pearce, 2008). This motor coordination deficit can be detected as early as postnatal day 14 (Weimer et al., 2009). We have previously shown that a single intraperitoneal injection of the selective, non-competitive AMPA receptor antagonist, EGIS-8332, significantly improves the motor coordination of 1-month-old Cln3Δex1-6 mice (Kovacs and Pearce, 2008).
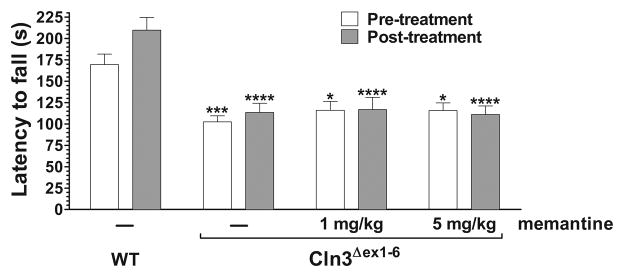
A recent study demonstrated increased glutamate levels in cerebellar and cerebral extracts of 1-, 2-, 3- and 6-month-old Cln3Δex1-6 mice by NMR spectroscopy (Pears et al., 2005) suggesting that Cln3Δex1-6 neurons may release more glutamate and extracellular glutamate level may abnormally be increased in the Cln3Δex1-6 brain. An increased extracellular glutamate level would abnormally enhance both AMPA and NMDA receptor activity, and therefore, administration of an NMDA receptor antagonist should also improve the motor coordination of Cln3Δex1-6 mice. We tested this possibility using the uncompetitive NMDA receptor antagonist, memantine, which is an FDA approved drug used to treat the symptoms of moderate to severe Alzheimer’s disease (Lipton, 2007). Memantine, injected intraperitoneally in doses of 1 and 5 mg/kg had no effect on the impaired motor coordination of one-month-old Cln3Δex1-6 mice (Fig. 1.). We chose the higher dose based on a recent study in which attenuation of NMDA receptor activity by acute intraperitoneal injection of 5 mg/kg memantine rescued the learning and memory deficit in a mouse model of Down syndrome (Costa et al., 2008).
Fig. 1. Acute treatment with the NMDA receptor antagonist, memantine, has no effect on the impaired motor coordination of one-month-old Cln3Δex1-6 mice.
An accelerating rotarod (from 0 to 24 rpm in 240 s) was used to measure the motor coordination of one-month-old wild type (WT) and Cln3Δex1-6 mice. Two hours and thirty minutes after the end of the Pre-treatment rotarod test, mice were intraperitoneally injected with either the uncompetitive NMDA receptor antagonist, memantine (1 and 5 mg/kg), or the vehicle of the drug (sterile 0.9% NaCl). The Post-treatment rotarod test began thirty minutes after the treatment. Columns and bars represent mean ± S.E.M. of the time (s) mice were able to stay on the rotating rod (n=9–14). Repeated measures two-way ANOVA with Bonferroni’s test for pairwise multiple comparison was applied to compare the different treatment groups (*p<0.05, ***p <0.001 and ****p <0.0001 as compared to vehicle-injected WT mice).
3.2. Acute inhibition of NMDA receptors by memantine induces a delayed but prolonged improvement of motor coordination in 6–7-month-old Cln3Δex1-6 mice
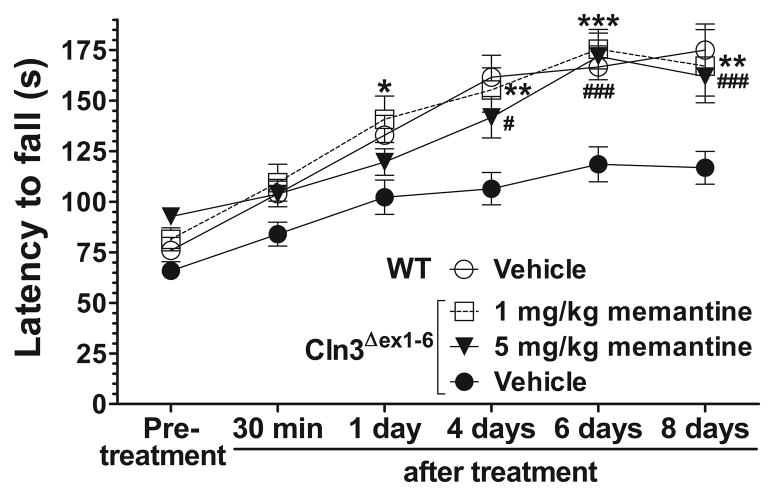
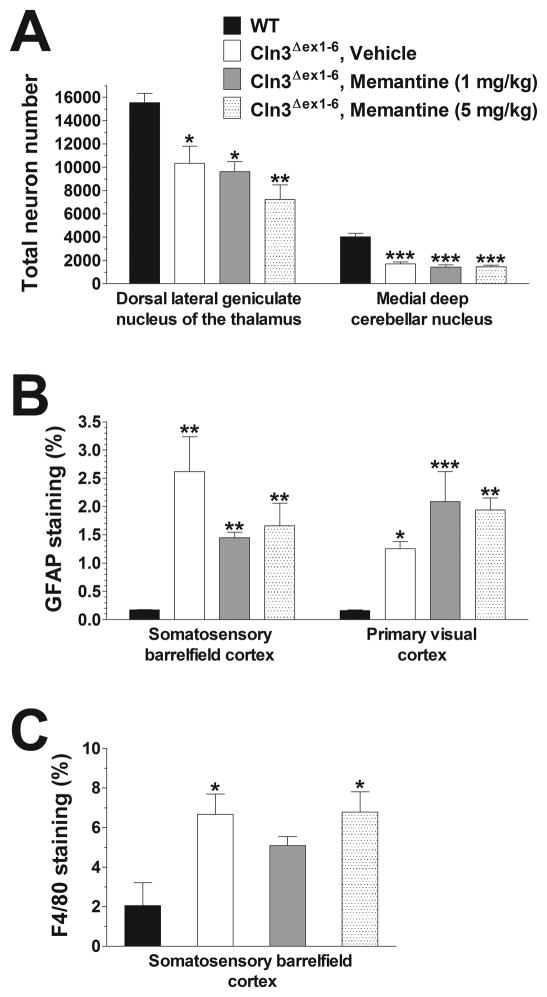
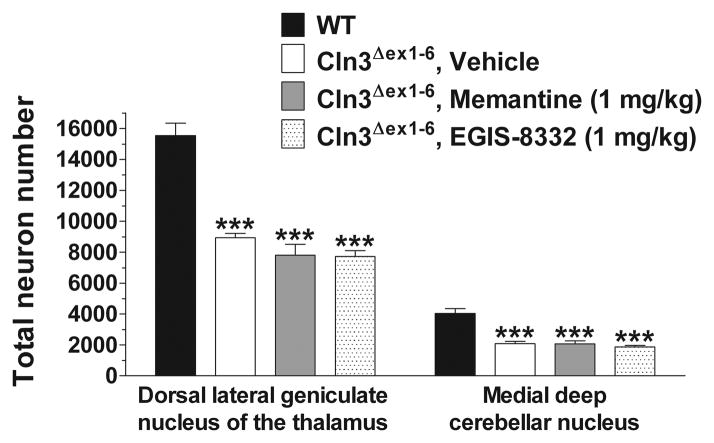
In neurodegenerative disorders the pathomechanism may change during disease progression (Graham et al., 2009). Therefore, we examined the effect of a single administration of memantine on the motor coordination of 6–7-month-old Cln3Δex1-6 mice to see if an acute treatment with the drug can be beneficial at a later stage of the disease. Memantine injected intraperitoneally in doses of 1 and 5 mg/kg did not improve the motor coordination 30 minutes after the treatment. The lower dose of memantine (1 mg/kg), however, induced a significant improvement 1 day after its injection, whereas the higher dose (5 mg/kg) resulted in an enhancement of motor skills days later (Fig. 2.). Memantine restored the motor coordination of Cln3Δex1-6 mice to the WT level, and its therapeutic effect could be observed even eight days after the treatment (Fig. 2). The memantine-induced delayed but prolonged improvement is very similar to that we observed previously when 6–7-month-old Cln3Δex1-6 mice were acutely treated with the AMPA receptor antagonist, EGIS-8332 (Kovacs et al., 2011). In that study, though EGIS-8332 induced an improvement in the motor coordination, it did not prevent the localized neuronal loss or the astrocytic and microglial activation (Kovacs et al., 2011). To examine if the acute treatment with memantine affects the previously described neuropathological changes in Cln3Δex1-6 mice (Pontikis et al., 2004; Weimer et al., 2009; Weimer et al., 2006), histological analysis was performed 8 days after the memantine injection. Acute memantine treatment (1 or 5 mg/kg) did not affect the loss of large projection neurons in the dorsal lateral geniculate nucleus of the thalamus or in the medial deep cerebellar nucleus (Fig. 3A). The acute treatment with memantine had no effect on astrocytic and microglial activation in the cortex (Fig. 3B–C) and cerebellum (data not shown).
Fig. 2. Acute treatment with memantine induces a delayed but extended improvement of motor coordination in 6–7-month-old Cln3Δex1-6 mice.
An accelerating rotarod (from 0 to 24 rpm in 240 s) was used to measure the motor coordination of 6–7-month-old Cln3Δex1-6 and WT mice. Mice were intraperitoneally injected with either the uncompetitive NMDA receptor antagonist, memantine (1 and 5 mg/kg) or the vehicle of the drug (sterile 0.9% NaCl). Data points represent mean ± S.E.M. of the time (s) mice were able to stay on the rotating rod (n=10–15). Repeated measures two-way ANOVA with Bonferroni’s test for pairwise multiple comparison was applied to compare the vehicle- and memantine-treated Cln3Δex1-6 mice (Vehicle vs. 1 mg/kg memantine: *p<0.05, **p<0.01 and ***p <0.001; Vehicle vs. 5 mg/kg memantine: #p<0.05 and ###p <0.001).
Fig. 3. Acute treatment with memantine has no effect on the neuropathological changes in 6-month-old Cln3Δex1-6 mice.
Six-month-old Cln3Δex1-6 mice acutely treated with memantine (1 or 5 mg/kg) or the vehicle of memantine (sterile 0.9% NaCl) were perfusion-fixed 8 days after the treatment (4 mice from each treatment group), and their brains were histologically analyzed. Four 6-month-old, untreated WT mice were also perfusion-fixed, and their brains were histologically analyzed, as well. (A) Localized neuronal loss in the thalamus and cerebellum. To survey the survival of neuron populations that are vulnerable in Cln3Δex1-6 mice, counts of the large projection neurons in the thalamus (dorsal lateral geniculate nucleus) and in the medial deep cerebellar nucleus were made. These counts were obtained using the StereoInvestigator software. (B) Astrocytosis in the cortex. Quantitative determination of astrocytosis in the somatosensory barrelfield cortex and the primary visual cortex was performed by thresholding image analysis following immunohistochemical staining for the astrocytic marker, GFAP. (C) Microgliosis in the cerebellum. Quantitative determination of microgliosis in the somatosensory barrelfield cortex and the primary visual cortex was performed by thresholding image analysis following immunohistochemical staining for the microglial marker F4/80.
Statistical significance was calculated by one-way ANOVA with Bonferroni’s post-test: *p<0.05, **p<0.01 and ***p<0.001 as compared to WT.
3.3. Effects of repeated administration of memantine and EGIS-8332 on the motor coordination and neuropathology of 7-month-old Cln3Δex1-6
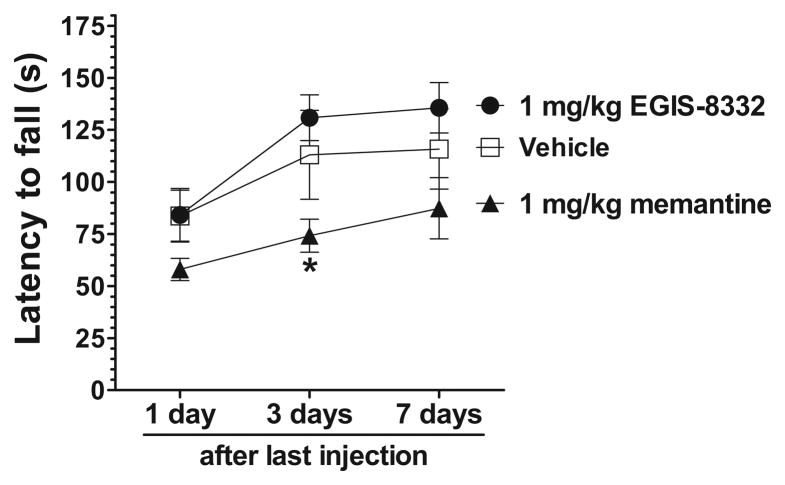
Next we tested the effect of repeated administration of memantine and the AMPA receptor antagonist, EGIS-8332, in 6-month-old Cln3Δex1-6 mice. Since the beneficial effect of an acute treatment with memantine or EGIS-8332 lasted at least for a week (Fig. 2.; Kovacs et al., 2011), 6-month-old Cln3Δex1-6 mice were intraperitoneally injected with the drugs once a week for 4 weeks. A group of mice were injected with sterile 0.9% NaCl solution (Vehicle). Motor coordination of the mice was tested 1, 3 and 7 days after the last injection. Repeated administration of EGIS-8332 did not result in a statistically significant improvement (Fig. 4.). Although, the repeated treatment with memantine seemingly impaired the motor coordination, the difference between vehicle-injected and memantine-treated mice was not statistically significant at any time points (Fig. 4). In comparison to EGIS-8332-treated mice, however, memantine-treated mice performed significantly worse on the rotarod three days after the last drug injection. The difference became statistically insignificant four days later (Fig. 4).
Fig. 4. Treatment of 6-month-old Cln3Δex1-6 mice once a week for four weeks with memantine or EGIS-8332 does not improve the motor coordination.
Six-month-old Cln3Δex1-6 mice were intraperitoneally injected with the NMDA receptor antagonist, memantine (1 mg/kg), the AMPA receptor antagonist, EGIS-8332 (1 mg/kg), or sterile 0.9% NaCl (Vehicle) once a week for 4 weeks. Motor coordination of the mice was tested 1, 3 and 7 days after the last injection using an accelerating rotarod (from 0 to 24 rpm in 240 s). Data points represent mean ± S.E.M. of the time (s) mice were able to stay on the rotating rod (n=6). Repeated measures two-way ANOVA with Bonferroni’s test for pairwise multiple comparison was applied to compare the rotarod performances of the three treatment groups (1 mg/kg memantine vs. 1 mg/kg EGIS-8332: *p<0.05).
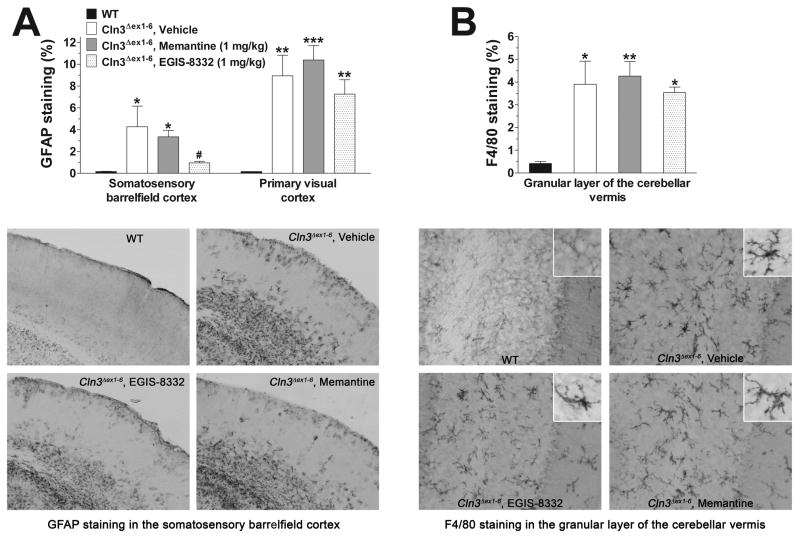
Two days after the last rotarod test, mice treated with 0.9% NaCl (Vehicle), memantine or EGIS-8332 were perfusion-fixed and their brain was histologically analyzed in comparison to 7-month-old WT mice. Optical fractionator counts of neuron number revealed that the repeated treatment with memantine or EGIS-8332 did not prevent the previously described loss of large projection neurons either in the dorsal lateral geniculate nucleus of the thalamus or in the medial deep cerebellar nucleus (Fig. 5). Treatment with memantine had no effect on astrocytosis in the cortex (Fig. 6A). EGIS-8332 significantly decreased astroglial activation in the somatosensory barrelfield cortex but it did not affect astrocytosis in the primary visual cortex (Fig. 6A). Neither memantine nor EGIS-8332 affected astroglial activation in the cerebellum (data not shown). The levels of microglial activation in the cerebellum and cortex of Cln3Δex1-6 mice were unaffected by memantine or EGIS-8332 (Fig. 6B; data are not shown for the cortex).
Fig. 5. Treatment of 6-month-old Cln3Δex1-6 mice once a week for four weeks with memantine or EGIS-8332 does not prevent the selective, localized neuronal loss in the thalamus and cerebellum.
Six-month-old Cln3Δex1-6 mice were intraperitoneally injected with the NMDA receptor antagonist, memantine (1 mg/kg), the AMPA receptor antagonist, EGIS-8332, or sterile 0.9% NaCl (Vehicle) once a week for 4 weeks. Motor coordination of the mice was tested 1, 3 and 7 days after the last injection. Two days after the last rotarod test, four vehicle-injected, four EGIS-8332-treated and four memantine-treated Cln3Δex1-6 mice were perfusion-fixed, and their brains were histologically analyzed. Four 7-month-old WT mice were also perfusion-fixed and their brains were histologically analyzed, as well. To survey the survival of neuron populations that are vulnerable in Cln3Δex1-6 mice, counts of the large projection neurons in the thalamus (dorsal lateral geniculate nucleus) and in the medial deep cerebellar nucleus were made. These counts were obtained using the StereoInvestigator software. Columns and bars represent mean ± S.E.M. Statistical significance was calculated by one-way ANOVA with Bonferroni’s post-test: ***p<0.001 as compared to WT.
Fig. 6. Astrocytic and microglial activation in Cln3Δex1-6 mice treated once a week for four weeks with memantine or EGIS-8332.
Six-month-old Cln3Δex1-6 mice were intraperitoneally injected with the NMDA receptor antagonist, memantine (1 mg/kg), the AMPA receptor antagonist, EGIS-8332, or sterile 0.9% NaCl (Vehicle) once a week for 4 weeks. Motor coordination of the mice was tested 1, 3 and 7 days after the last injection. Two days after the last rotarod test, four vehicle-injected, four EGIS-8332-treated and four memantine-treated Cln3Δex1-6 mice were perfusion-fixed, and the astrocytic and microglial activation in their brains were analyzed by immunohistological staining. Four 7-month-old WT mice were also perfusion-fixed and their brains were analyzed by immunohistological staining, as well. (A) Effect of repeated treatment with memantine orEGIS-8332 on astrocytosis in the cortex. Quantitative determination of astrocytosis in the somatosensory barrelfield cortex and the primary visual cortex was performed by thresholding image analysis following immunohistochemical staining for the astrocytic marker, GFAP. The representative photomicrographs show sections of the somatosensory barrelfield cortex immunohistochemically stained for the astrocytic marker, GFAP. Note that the repeated treatment with EGIS-8332 markedly reduced astrocytosis. (B) Effect of repeated treatment with memantine orEGIS-8332 on microgliosis in the cerebellum. Quantitative determination of microgliosis in the granular layer of the cerebellar vermis was performed by thresholding image analysis following immunohistochemical staining for the microglial marker, F4/80. The representative photomicrographs show the granular layer of the cerebellar vermis immunohistochemically stained for the microglial marker, F4/80. The inserts show single microglial cells at higher magnification.
Statistical significance was calculated by one-way ANOVA with Bonferroni’s post-test: *p<0.05, **p<0.01 and ***p<0.001 as compared to WT; #p<0.05 as compared to vehicle-injected Cln3Δex1-6 mice.
4. Discussion
In the present study we showed that NMDA receptors play an age-dependent role in the neurological deficit in the Cln3Δex1-6 mouse model of juvenile Batten disease. Acute inhibition of NMDA receptors by memantine (1 and 5 mg/kg i.p.) had no effect on the impaired motor coordination of one-month-old Cln3Δex1-6 mice, which is in striking contrast with the recently described therapeutic effect of AMPA receptor inhibition at this age (Kovacs and Pearce, 2008). These findings indicate that in one-month-old Cln3Δex1-6 mice only AMPA, but not NMDA, receptor function is abnormally enhanced. Our previous results, showing that Cln3Δex1-6 neurons in dissociated cultures and in organotypic slice cultures are significantly more sensitive to AMPA, but not NMDA, receptor-mediated toxicity than their wild type counterparts (Kovacs et al., 2006), also suggested that at early ages only AMPA receptor function is abnormally enhanced in Cln3Δex1-6 mice.
At a later stage of the disease, in 6–7-month-old Cln3Δex1-6 mice, memantine induced a delayed but prolonged (8 days) improvement of motor skills, restoring motor coordination to the WT level for several days (Fig. 2). Similar restoration of motor coordination was observed previously when Cln3Δex1-6 mice were acutely treated with the AMPA receptor antagonist, EGIS-8332 (Kovacs et al., 2011). Since memantine has a relatively short half-life in rodents (Parsons et al., 1999), it cannot provide effective NMDA receptor inhibition for several days. The most likely explanation for the prolonged (8 days) therapeutic effect of an acute memantine treatment is that temporal inhibition of NMDA receptors in the Cln3Δex1-6 brain initiates long-lasting beneficial changes in glutamatergic neurotransmission and synaptic plasticity. This concept is supported by recent reports that showed that a single administration of the NMDA receptor blocker, MK-801 (5 mg/kg), in rats induced changes in synaptic plasticity lasting for four weeks (Manahan-Vaughan et al., 2008; Wohrl et al., 2007).
While 6–7-month-old WT mice displayed effective motor learning clearly improving their motor performance with each rotarod testing, Cln3Δex1-6 mice showed only limited improvements during the repeated rotarod tests (Fig. 2), indicative of a motor learning deficit. Memantine primarily inhibits extrasynaptic NMDA receptors (Milnerwood et al., 2010; Xia et al., 2010) and enhances learning and memory(Beracochea et al., 2008; Minkeviciene et al., 2008; Rammes et al., 2008; Zoladz et al., 2006). Therefore it is likely that the acute treatment with memantine corrected the learning defect of Cln3Δex1-6 mice (Fig. 2).
The age-dependent therapeutic effect of memantine in the Cln3Δex1-6 mouse model of juvenile Batten disease implies that the pathomechanism in juvenile Batten disease changes during disease progression. It is now recognized that the disease causing mechanism in neurodegenerative disorders may remarkably change as the disease progresses. A recent study using the YAC128 mouse model of Huntington’s disease, e.g., revealed that while striatal neurons had high NMDA receptor activity and were very vulnerable to NMDA receptor-mediated toxicity in young, pre-symptomatic mice, striatal neurons in old, symptomatic mice displayed low NMDA receptor activity and became resistant to NMDA receptor-mediated cell death (Graham et al., 2009).
In contrast to the acute treatment with memantine (Fig. 2) or EGIS-8332 (Kovacs et al., 2011), repeated administration of the two drugs to 6-month-old Cln3Δex1-6 mice, once a week for 4 weeks, did not improve the motor coordination (Fig. 4) and did not prevent the well-characterized neuropathological changes (Fig. 5–6). These results indicate that the prolonged beneficial effect of the first drug injection was interfered and quenched by the further drug injections repeated weekly.
Activation of astrocytes and microglial cells is a fairly common event in neurodegenerative disorders including Batten disease (Danton and Dietrich, 2003; Farfara et al., 2008; Venneti et al., 2009). However, differently from many other neurodegenerative diseases, activation of glial cells in Batten disease occurs before the loss of neurons (Bible et al., 2004; Kielar et al., 2007; Pontikis et al., 2004). Recent studies revealed that chronic inhibition of AMPA or NMDA receptor can prevent neuroinflammation manifested as astrocytic and microglial activation (Greene et al., 2008). The repeated treatment with memantine and EGIS-8332 we applied once a week for 4 weeks had no effect on glial activation in the brain of Cln3Δex1-6 mice with the only exception of EGIS-8332 selectively decreasing astrocytic activation in the somatosensory barrelfield cortex (Fig. 6). Memantine and EGIS-8332 have relatively short half-lives in rodents (Gigler et al., 2007; Parsons et al., 1999), and as our results show the temporary, brief inhibition of NMDA or AMPA receptors achieved by the weekly, repeated treatments is not sufficient to inhibit the glial activation in Cln3Δex1-6 mice. Currently it is unclear why the repeated treatment with EGIS-8332 reduced astrocytic activation in the somatosensory barrelfield cortex but had no effect in the primary visual cortex or in the cerebellum. If the expression level and functional properties of AMPA receptors in the somatosensory barrelfield cortex markedly differ from that in the primary visual cortex and cerebellum that could explain the observed, selective effect of EGIS-8332. To our knowledge such a subregion-specific comparison of AMPA receptors has not been carried out, yet.
In summary, we demonstrated an age-dependent therapeutic effect of the NMDA receptor blocker, memantine, in the Cln3Δex1-6 mouse model of juvenile Batten disease. Our finding that acute inhibition of NMDA receptors can induce a prolonged (8 days) improvement of the neurological function in Cln3Δex1-6 mice identifies NMDA receptors as a new therapeutic target for juvenile Batten disease.
Research highlights.
We studied the therapeutic effect of memantine in a mouse model of Batten disease.
Acute treatment had no effect on the impaired motor skills of 1-month-old mice.
Acute treatment induced a prolonged improvement in 6–7-month-old Cln3Δex1-6 mice.
Acute treatment in 6–7-month-old Cln3Δex1-6 mice did not affect neuropathology.
Repeated treatment in 6-month-old Cln3Δex1-6 mice did not improve the motor skills.
Acknowledgments
This work was supported by the Luke and Rachel Batten Foundation and the National Institutes of Health (NIH) grants R01 NS044310 and R21 NS067147.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate
- NMDA
N-methyl-D-aspartate
- WT
wild type
- DPBS
Dulbecco’s phosphate-buffered saline
- TBS
Tris-buffered saline
- S1BF
somatosensory barrelfield cortex
- GFAP
glial fibrillary acidic protein
Footnotes
Conflict of interest
None of the authors of this paper has any conflict of interest relating to the publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahtiainen L, Kolikova J, Mutka AL, Luiro K, Gentile M, Ikonen E, Khiroug L, Jalanko A, Kopra O. Palmitoyl protein thioesterase 1 (Ppt1)-deficient mouse neurons show alterations in cholesterol metabolism and calcium homeostasis prior to synaptic dysfunction. Neurobiol Dis. 2007;28:52–64. doi: 10.1016/j.nbd.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Benedict JW, Sommers CA, Pearce DA. Progressive oxidative damage in the central nervous system of a murine model for juvenile Batten disease. J Neurosci Res. 2007;85:2882–2891. doi: 10.1002/jnr.21416. [DOI] [PubMed] [Google Scholar]
- Beracochea D, Boucard A, Trocme-Thibierge C, Morain P. Improvement of contextual memory by S 24795 in aged mice: comparison with memantine. Psychopharmacology (Berl) 2008;196:555–564. doi: 10.1007/s00213-007-0987-5. [DOI] [PubMed] [Google Scholar]
- Bible E, Gupta P, Hofmann SL, Cooper JD. Regional and cellular neuropathology in the palmitoyl protein thioesterase-1 null mutant mouse model of infantile neuronal ceroid lipofuscinosis. Neurobiol Dis. 2004;16:346–359. doi: 10.1016/j.nbd.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Byun K, Kim J, Cho SY, Hutchinson B, Yang SR, Kang KS, Cho M, Hwang K, Michikawa M, Jeon YW, Paik YK, Lee B. Alteration of the glutamate and GABA transporters in the hippocampus of the Niemann-Pick disease, type C mouse using proteomic analysis. Proteomics. 2006;6:1230–1236. doi: 10.1002/pmic.200500412. [DOI] [PubMed] [Google Scholar]
- Chan CH, Ramirez-Montealegre D, Pearce DA. Altered arginine metabolism in the central nervous system (CNS) of the Cln3−/− mouse model of juvenile Batten disease. Neuropathol Appl Neurobiol. 2009;35:189–207. doi: 10.1111/j.1365-2990.2008.00984.x. [DOI] [PubMed] [Google Scholar]
- Chiulli N, Codazzi F, Di Cesare A, Gravaghi C, Zacchetti D, Grohovaz F. Sphingosylphosphocholine effects on cultured astrocytes reveal mechanisms potentially involved in neurotoxicity in Niemann-Pick type A disease. Eur J Neurosci. 2007;26:875–881. doi: 10.1111/j.1460-9568.2007.05732.x. [DOI] [PubMed] [Google Scholar]
- Cialone J, Adams H, Augustine EF, Marshall FJ, Kwon JM, Newhouse N, Vierhile A, Levy E, Dure LS, Rose KR, Ramirez-Montealegre D, de Blieck EA, Mink JW. Females experience a more severe disease course in batten disease. J Inherit Metab Dis. 2012;35:549–555. doi: 10.1007/s10545-011-9421-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium TIBD. Isolation of a novel gene underlying Batten disease, CLN3. Cell. 1995;82:949–957. doi: 10.1016/0092-8674(95)90274-0. [DOI] [PubMed] [Google Scholar]
- Costa AC, Scott-McKean JJ, Stasko MR. Acute injections of the NMDA receptor antagonist memantine rescue performance deficits of the Ts65Dn mouse model of Down syndrome on a fear conditioning test. Neuropsychopharmacology. 2008;33:1624–1632. doi: 10.1038/sj.npp.1301535. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Grossi D, De Chiara G, de Stefano MC, Cortese G, Citro G, Rufini S, Tancredi V, Merlo D, Frank C. Glutamatergic neurotransmission in a mouse model of Niemann-Pick type C disease. Brain Res. 2011;1396:11–19. doi: 10.1016/j.brainres.2011.04.020. [DOI] [PubMed] [Google Scholar]
- Danton GH, Dietrich WD. Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol. 2003;62:127–136. doi: 10.1093/jnen/62.2.127. [DOI] [PubMed] [Google Scholar]
- Farfara D, Lifshitz V, Frenkel D. Neuroprotective and neurotoxic properties of glial cells in the pathogenesis of Alzheimer’s disease. J Cell Mol Med. 2008;12:762–780. doi: 10.1111/j.1582-4934.2008.00314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn R, Kovacs AD, Pearce DA. Altered glutamate receptor function in the cerebellum of the Ppt1(−/−) mouse, a murine model of infantile neuronal ceroid lipofuscinosis. J Neurosci Res. 2012;90:367–375. doi: 10.1002/jnr.22763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getty AL, Pearce DA. Interactions of the proteins of neuronal ceroid lipofuscinosis: clues to function. Cell Mol Life Sci. 2011;68:453–474. doi: 10.1007/s00018-010-0468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigler G, Moricz K, Agoston M, Simo A, Albert M, Benedek A, Kapus G, Kertesz S, Vegh M, Barkoczy J, Marko B, Szabo G, Matucz E, Gacsalyi I, Levay G, Harsing LG, Jr, Szenasi G. Neuroprotective and anticonvulsant effects of EGIS-8332, a non-competitive AMPA receptor antagonist, in a range of animal models. British Journal of Pharmacology. 2007;152:151–160. doi: 10.1038/sj.bjp.0707362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel HH. The neuronal ceroid-lipofuscinoses. J Child Neurol. 1995;10:424–437. doi: 10.1177/088307389501000602. [DOI] [PubMed] [Google Scholar]
- Goebel HH, Wisniewski KE. Current state of clinical and morphological features in human NCL. Brain Pathol. 2004;14:61–69. doi: 10.1111/j.1750-3639.2004.tb00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham RK, Pouladi MA, Joshi P, Lu G, Deng Y, Wu NP, Figueroa BE, Metzler M, Andre VM, Slow EJ, Raymond L, Friedlander R, Levine MS, Leavitt BR, Hayden MR. Differential susceptibility to excitotoxic stress in YAC128 mouse models of Huntington disease between initiation and progression of disease. J Neurosci. 2009;29:2193–2204. doi: 10.1523/JNEUROSCI.5473-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene IP, Lee EY, Prow N, Ngwang B, Griffin DE. Protection from fatal viral encephalomyelitis: AMPA receptor antagonists have a direct effect on the inflammatory response to infection. Proc Natl Acad Sci U S A. 2008;105:3575–3580. doi: 10.1073/pnas.0712390105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Jalanko A, Braulke T. Neuronal ceroid lipofuscinoses. Biochim Biophys Acta. 2009;1793:697–709. doi: 10.1016/j.bbamcr.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Karl T, Pabst R, von Horsten S. Behavioral phenotyping of mice in pharmacological and toxicological research. Exp Toxicol Pathol. 2003;55:69–83. doi: 10.1078/0940-2993-00301. [DOI] [PubMed] [Google Scholar]
- Kielar C, Maddox L, Bible E, Pontikis CC, Macauley SL, Griffey MA, Wong M, Sands MS, Cooper JD. Successive neuron loss in the thalamus and cortex in a mouse model of infantile neuronal ceroid lipofuscinosis. Neurobiol Dis. 2007;25:150–162. doi: 10.1016/j.nbd.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkotian E, Schwarz A, Pelled D, Schwarzmann G, Segal M, Futerman AH. Elevation of intracellular glucosylceramide levels results in an increase in endoplasmic reticulum density and in functional calcium stores in cultured neurons. J Biol Chem. 1999;274:21673–21678. doi: 10.1074/jbc.274.31.21673. [DOI] [PubMed] [Google Scholar]
- Kovacs AD, Pearce DA. Attenuation of AMPA receptor activity improves motor skills in a mouse model of juvenile Batten disease. Exp Neurol. 2008;209:288–291. doi: 10.1016/j.expneurol.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs AD, Saje A, Wong A, Szenasi G, Kiricsi P, Szabo E, Cooper JD, Pearce DA. Temporary inhibition of AMPA receptors induces a prolonged improvement of motor performance in a mouse model of juvenile Batten disease. Neuropharmacology. 2011;60:405–409. doi: 10.1016/j.neuropharm.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs AD, Weimer JM, Pearce DA. Selectively increased sensitivity of cerebellar granule cells to AMPA receptor-mediated excitotoxicity in a mouse model of Batten disease. Neurobiol Dis. 2006;22:575–585. doi: 10.1016/j.nbd.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Lim MJ, Alexander N, Benedict JW, Chattopadhyay S, Shemilt SJ, Guerin CJ, Cooper JD, Pearce DA. IgG entry and deposition are components of the neuroimmune response in Batten disease. Neurobiol Dis. 2007;25:239–251. doi: 10.1016/j.nbd.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Pathologically-activated therapeutics for neuroprotection: mechanism of NMDA receptor block by memantine and S-nitrosylation. Curr Drug Targets. 2007;8:621–632. doi: 10.2174/138945007780618472. [DOI] [PubMed] [Google Scholar]
- Macauley SL, Wozniak DF, Kielar C, Tan Y, Cooper JD, Sands MS. Cerebellar pathology and motor deficits in the palmitoyl protein thioesterase 1-deficient mouse. Exp Neurol. 2009;217:124–135. doi: 10.1016/j.expneurol.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan-Vaughan D, von Haebler D, Winter C, Juckel G, Heinemann U. A single application of MK801 causes symptoms of acute psychosis, deficits in spatial memory, and impairment of synaptic plasticity in rats. Hippocampus. 2008;18:125–134. doi: 10.1002/hipo.20367. [DOI] [PubMed] [Google Scholar]
- Milnerwood AJ, Gladding CM, Pouladi MA, Kaufman AM, Hines RM, Boyd JD, Ko RW, Vasuta OC, Graham RK, Hayden MR, Murphy TH, Raymond LA. Early increase in extrasynaptic NMDA receptor signaling and expression contributes to phenotype onset in Huntington’s disease mice. Neuron. 2010;65:178–190. doi: 10.1016/j.neuron.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Minkeviciene R, Banerjee P, Tanila H. Cognition-enhancing and anxiolytic effects of memantine. Neuropharmacology. 2008;54:1079–1085. doi: 10.1016/j.neuropharm.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Mitchison HM, Bernard DJ, Greene ND, Cooper JD, Junaid MA, Pullarkat RK, de Vos N, Breuning MH, Owens JW, Mobley WC, Gardiner RM, Lake BD, Taschner PE, Nussbaum RL. Targeted disruption of the Cln3 gene provides a mouse model for Batten disease. The Batten Mouse Model Consortium [corrected] Neurobiol Dis. 1999;6:321–334. doi: 10.1006/nbdi.1999.0267. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Quack G. Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist--a review of preclinical data. Neuropharmacology. 1999;38:735–767. doi: 10.1016/s0028-3908(99)00019-2. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Academic Press; San Diego: 2001. [Google Scholar]
- Pears MR, Cooper JD, Mitchison HM, Mortishire-Smith RJ, Pearce DA, Griffin JL. High resolution 1H NMR-based metabolomics indicates a neurotransmitter cycling deficit in cerebral tissue from a mouse model of Batten disease. Journal of Biological Chemistry. 2005;280:42508–42514. doi: 10.1074/jbc.M507380200. [DOI] [PubMed] [Google Scholar]
- Pears MR, Salek RM, Palmer DN, Kay GW, Mortishire-Smith RJ, Griffin JL. Metabolomic investigation of CLN6 neuronal ceroid lipofuscinosis in affected South Hampshire sheep. J Neurosci Res. 2007;85:3494–3504. doi: 10.1002/jnr.21343. [DOI] [PubMed] [Google Scholar]
- Pontikis CC, Cella CV, Parihar N, Lim MJ, Chakrabarti S, Mitchison HM, Mobley WC, Rezaie P, Pearce DA, Cooper JD. Late onset neurodegeneration in the Cln3−/− mouse model of juvenile neuronal ceroid lipofuscinosis is preceded by low level glial activation. Brain Res. 2004;1023:231–242. doi: 10.1016/j.brainres.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Rammes G, Danysz W, Parsons CG. Pharmacodynamics of memantine: an update. Curr Neuropharmacol. 2008;6:55–78. doi: 10.2174/157015908783769671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz D, Grodd W, Schwab A, Seeger U, Klose U, Nagele T. MR imaging and localized proton MR spectroscopy in late infantile neuronal ceroid lipofuscinosis. AJNR Am J Neuroradiol. 1998;19:1373–1377. [PMC free article] [PubMed] [Google Scholar]
- Sitter B, Autti T, Tyynela J, Sonnewald U, Bathen TF, Puranen J, Santavuori P, Haltia MJ, Paetau A, Polvikoski T, Gribbestad IS, Hakkinen AM. High-resolution magic angle spinning and 1H magnetic resonance spectroscopy reveal significantly altered neuronal metabolite profiles in CLN1 but not in CLN3. J Neurosci Res. 2004;77:762–769. doi: 10.1002/jnr.20123. [DOI] [PubMed] [Google Scholar]
- Venneti S, Wiley CA, Kofler J. Imaging microglial activation during neuroinflammation and Alzheimer’s disease. J Neuroimmune Pharmacol. 2009;4:227–243. doi: 10.1007/s11481-008-9142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer JM, Benedict JW, Elshatory YM, Short DW, Ramirez-Montealegre D, Ryan DA, Alexander NA, Federoff HJ, Cooper JD, Pearce DA. Alterations in striatal dopamine catabolism precede loss of substantia nigra neurons in a mouse model of juvenile neuronal ceroid lipofuscinosis. Brain Res. 2007;1162:98–112. doi: 10.1016/j.brainres.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer JM, Benedict JW, Getty AL, Pontikis CC, Lim MJ, Cooper JD, Pearce DA. Cerebellar defects in a mouse model of juvenile neuronal ceroid lipofuscinosis. Brain Res. 2009;1266:93–107. doi: 10.1016/j.brainres.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer JM, Custer AW, Benedict JW, Alexander NA, Kingsley E, Federoff HJ, Cooper JD, Pearce DA. Visual deficits in a mouse model of Batten disease are the result of optic nerve degeneration and loss of dorsal lateral geniculate thalamic neurons. Neurobiol Dis. 2006;22:284–293. doi: 10.1016/j.nbd.2005.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohrl R, Eisenach S, Manahan-Vaughan D, Heinemann U, von Haebler D. Acute and long-term effects of MK-801 on direct cortical input evoked homosynaptic and heterosynaptic plasticity in the CA1 region of the female rat. European Journal of Neuroscience. 2007;26:2873–2883. doi: 10.1111/j.1460-9568.2007.05899.x. [DOI] [PubMed] [Google Scholar]
- Xia P, Chen HS, Zhang D, Lipton SA. Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses. J Neurosci. 2010;30:11246–11250. doi: 10.1523/JNEUROSCI.2488-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadid G, Sotnik-Barkai I, Tornatore C, Baker-Cairns B, Harvey-White J, Pentchev PG, Goldin E. Neurochemical alterations in the cerebellum of a murine model of Niemann-Pick type C disease. Brain Res. 1998;799:250–256. doi: 10.1016/s0006-8993(98)00449-1. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Campbell AM, Park CR, Schaefer D, Danysz W, Diamond DM. Enhancement of long-term spatial memory in adult rats by the noncompetitive NMDA receptor antagonists, memantine and neramexane. Pharmacol Biochem Behav. 2006;85:298–306. doi: 10.1016/j.pbb.2006.08.011. [DOI] [PubMed] [Google Scholar]