Abstract
Background
Although chronic rhinosinusitis with nasal polyps (CRSwNP) is characterized by Th2 inflammation, the role of mast cells is poorly understood.
Objective
The objective of this study was to investigate the presence, localization and phenotype of mast cells in patients with chronic rhinosinusitis (CRS).
Methods
We collected nasal tissue and nasal lavage fluid from patients with CRS and control subjects. We analyzed mRNA for the mast cell proteases tryptase, chymase and carboxypeptidase A3 (CPA3), using real-time PCR, and measured mast cell protease proteins using ELISA, immunohistochemistry, and immunofluorescence.
Results
Tryptase mRNA was significantly increased in nasal polyps (NPs) from patients with CRSwNP (P < .001) compared with uncinate tissue (UT) from patients with CRS or control subjects. Tryptase protein was also elevated in NPs and in nasal lavage fluids from patients with CRSwNP. Immnohistochemistry showed increased numbers of mast cells in epithelium and glands but not within the lamina propria in NPs. The mast cells detected in epithelium in NPs were characterized by expression of tryptase and CPA3 but not chymase. Mast cells expressing all three proteases were abundant within glandular epithelium of NPs but were not found in normal glandular structures. Conclusion: Herein we demonstrate a unique localization of mast cells within glandular epithelium of NPs, and show that NPs mast cells have distinct phenotypes that vary by tissue location. Glandular mast cells and the diverse subsets of mast cells detected may contribute to the pathogenesis of CRSwNP.
Keywords: Chronic rhinosinusitis, nasal polyps, mast cells, tryptase, chymase, carboxypeptidase A3 (CPA3)
Introduction
Chronic rhinosinusitis (CRS) is one of the most common chronic diseases in adults in the United States and affects up to 15% of the population.1, 2 The prevalence and medical costs of CRS are increasing and have become important social issues. This disease is typically classified into 2 types: CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP). The etiology and pathogenesis of CRS remain controversial, but bacteria, viruses, and fungi have all been implicated in the establishment of an inflammatory process.3–5 Particularly, CRS is often comorbid with asthma and is resistant to therapeutic interventions.6 Surgical intervention is frequently necessary to clear the nasal and sinus passages, and repeat endoscopic sinus surgery is often required. Recent studies suggest that CRSwNP is characterized by a Th2 skewed eosinophilic inflammation characterized by significantly elevated levels of IL-5, IL-13, eotaxin, and eosinophil cationic protein (ECP).7–9 Taken together, these results point to a critical role for eosinophils in the pathophysiology of CRSwNP and further suggest that factors triggering eosinophil degranulation may also be associated with formation of NPs.10
The potential importance of mast cells has been considered in CRS disease pathogenesis because they produce an abundance of cytokines that activate eosinophils, molecules that directly promote tissue remodeling and chemical mediators that can produce profound tissue edema.11 Mast cells play key roles in host defense, homeostasis, tissue repair, and mechanisms of allergic inflammation.12 In the case of human mast cells, two subtypes have been recognized by variable expression of granular neutral proteases: mast cell-tryptase (MC-T) and mast cell-tryptase/chymase (MC-TC). MC-T type cells predominantly express high levels of tryptase but not chymase or CPA3, whereas MC-TC cells express all three proteases.13 MC-TC cells are essentially the exclusive type of mast cell found in normal skin and account for a small minority of mast cells in the lung. MC-T cells predominate in the alveolar wall and epithelium of the lung, whereas MC-TC cells favor the bronchial smooth muscle of patients with asthma and correlate with their bronchial hyperreactivity.14 MC-T cell numbers in respiratory epithelium increase during the pollen season in sensitive subjects.15, 16 These distinct tissue distributions and disease associations suggest a purposeful presence for each type of mast cell. Recent studies have demonstrated increased levels of mast cells in the presence of highly eosinophilic allergic inflammatory diseases such as asthma and eosinophilic esophagitis (EoE).17, 18 Moreover, mast cells in these studies have been shown to have a unique protease phenotype (tryptase and CPA3 high and chymase low).19, 20 The expression, phenotype, regulation, and protease expression patterns of mast cells in CRS have not been explored in detail.
In this study, we examined the distribution and phenotype of mast cells in UT from control as well as patients with CRS, including both CRSsNP and CRSwNP; we also evaluated NPs in patients with CRSwNP. We used quantitative RT-PCR, ELISA and immunohistochemistry to determine the expression and distribution of mast cells as well as mast cell tryptase, chymase, and CPA3 in CRS subjects. We detected a profound elevation of mast cells located within glands in nasal polyps, an observation not previously described.
METHODS
Patients
Patients with CRS were recruited from the allergy and otolaryngology clinics at Northwestern University and the Northwestern Sinus Center. Sinonasal and polyp tissues were obtained from routine functional endoscopic sinus surgery in patients with CRS. All subjects met the criteria for CRS as defined by the Sinus and Allergy Health Partnership.1
Patients with an isolated antrochoanal polyp, cystic fibrosis, or unilateral NPs were excluded from the study. Details of subjects’ characteristics are included in table I and in the Methods section of this article’s Online Repository at www.jacionline.org. All subjects signed informed consent, and the protocol and consent forms governing procedures for study have been approved by the Institutional Review Board of Northwestern University Feinberg School of Medicine.
Real time PCR
Total RNA from sinus tissue was extracted using QIAzol (Qiagen, Valencia, Calif), and the quality of total RNA from sinus tissue was assessed with a 2100 Bioanalyzer (Agilent Technologies, Santa Clara, Calif). Semiquantitative real-time RT-PCR was performed using the TaqMan method. The mRNA expression levels were normalized to the median expression of the housekeeping gene β-glucuronidase (GUSB). Further details can be found in the Methods section of this article’s Online Repository.
ELISA
Tryptase was assayed using a commercially available assay kit (Uscn Life Science Inc, Wuhan, China). The color intensity was measured with a Bio-Rad Spectrophotometer Model 680 Microplate Reader (Bio-Rad Laboratories, Hercules, Calif). Concentrations of tryptase protein were normalized to the concentration of total protein. Details can be found in the Methods section of this article’s Online Repository.
Immunohistochemistry
Immunohistochemistry was performed as described previously.21 Tissue sections were incubated with mouse anti-human tryptase mAb (Thermo scientific, Fremont, Calif) or mouse anti-human chymase mAb (Thermo scientific) or rabbit anti-human CPA3 pAb HPA008689, SIGMA, St. Louis, Mo) overnight at 4°C. Sections were rinsed and then incubated in biotinylated secondary horse anti-mouse or goat anti-rabbit antibody (Jackson ImmunoResearch Laboratories, West Grove, Pa) at a 1:500 dilution for 1 hour at room temperature. After another rinse, sections were incubated in ABC reagent (Vector Laboratories, Burlingame, Calif) for 1 hour. Sections were rinsed again and incubated in DAB reagent (Invitrogen, Carlsbad, Calif) and then counterstained with hematoxylin. Slides were blinded, and 10 pictures were randomly taken from each slide. The number of positive cells in epithelium, glands, and submucosa was counted by 2 independent observers. Details of the methods for immunofluorescence and immunohistochemistry are described in the Methods section of this article’s Online Repository.
Statistical analysis
All data are reported as the median. Differences between groups were analyzed by using the 1-way ANOVA Kruskal-Wallis test. Correlations were assessed by using the Spearman’s rank correlation. A P value of less than .05 was considered statistically significant.
RESULTS
Mast cell expression and distribution in patients with CRS
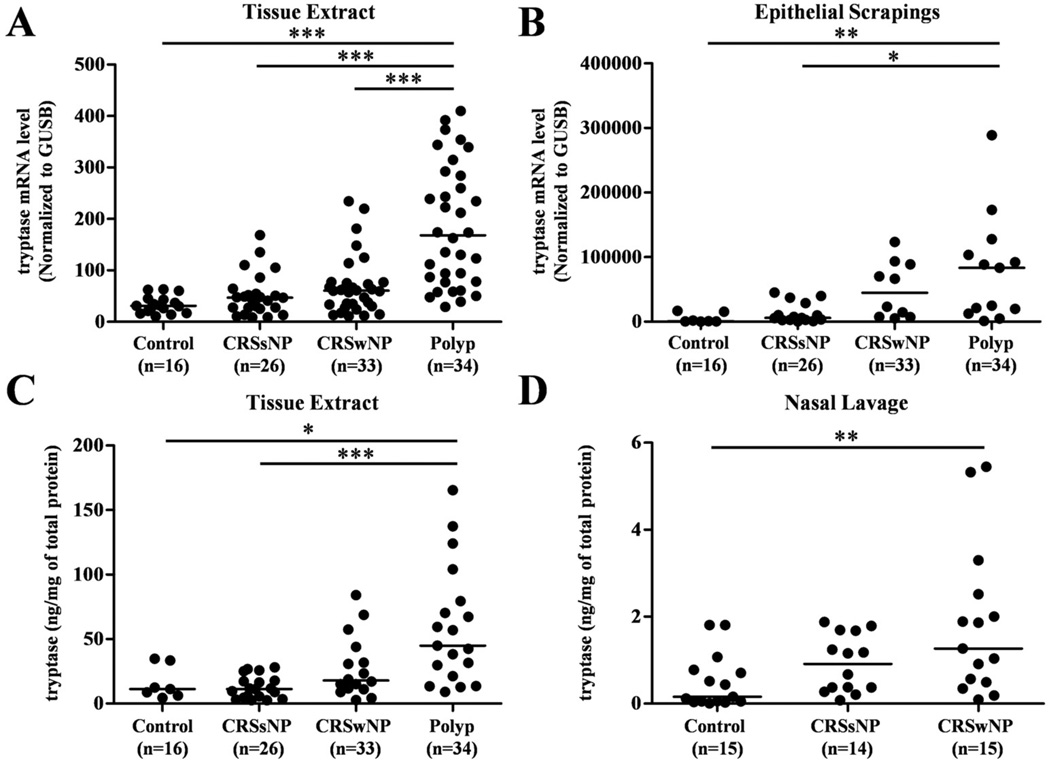
Sinonasal and polyp tissues were collected from 70 subjects with CRSsNP, 91 subjects with CRSwNP, and 42 control subjects, to determine the levels of expression of mast cells and their products in patients with CRS. Subject characteristics are shown in Table I. To estimate the levels of mast cells in nasal mucosa, we first assessed the expression of mRNA for tryptase in UT from patients with CRSsNP, CRSwNP and controls, as well as in NPs from patients with CRSwNP. Expression of the housekeeping gene GUSB was not significantly different among the 4 groups (data not shown). Tryptase mRNA was significantly increased in NPs from patients with CRSwNP (P < .0001) in comparison with UT from either patients with CRS or control subjects (Fig 1, A). Likewise, the levels of tryptase mRNA were significantly elevated in NPs in 17 patients from which we had matched sets of both polyp and UT for RT-PCR evaluation (P<.05 data not shown). To assess the gene expression level in epithelium, we used nasal scraping derived epithelial cells. Tryptase mRNA was significantly increased in NPs scrapings in comparison with scrapings of the UT from either patient with CRS or control subjects (Fig 1, B). To confirm this observation at the protein level, we made detergent extracts from homogenates of UT and NPs and then measured the concentration of tryptase using ELISA. Tryptase protein was significantly increased in NPs (P < .05) compared with UT from either patients with CRSsNP or control subjects (Fig 1, C). We also determined tryptase protein expression in nasal lavage fluids collected from a separate group of subjects. Tryptase protein was significantly increased in lavage fluids from CRSwNP (P=.0079) compared to lavage of control subjects (Fig 1, D).
TABLE 1.
Subjects’ characteristics
| Normal | CRSsNP | CRSwNP | CRSwNP polyp | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total no. of subjects | n=42 (17 M/ 25 F) | n=70 (26 M/ 44 F) | n=91 (56 M/ 35 F) | — | ||||||
| Age (y), median (range) | 37 (16–63) | 36 (18–69) | 44 (22–75) | — | ||||||
| Y | N | U | Y | N | U | Y | N | U | — | |
| Atopy | 2 | 32 | 8 | 35 | 27 | 8 | 45 | 24 | 22 | — |
| Asthma | 0 | 35 | 7 | 17 | 46 | 7 | 44 | 44 | 3 | — |
| Methodology used | ||||||||||
| Tissue RNA | n=16 (7M/ 9F) | n=26 (10M/ 16F) | n=33 (21M/ 12F) | n=34 (22M/ 12F) | ||||||
| Age (y), median (range) | 45 (16–62) | 35 (20–59) | 38 (23–67) | 39 (23–67) | ||||||
| Nasal scraping | ||||||||||
| RNA | n=7 (4M/ 3F) | n=15 (8M/ 7F) | n=10 (6M/ 4F) | n=13 (7M/ 6F) | ||||||
| Age (y), median (range) | 47 (29–62) | 37 (18–61) | 54 (27–73) | 48 (22–73) | ||||||
| Tissue extract | n=7 (5M/ 7F) | n=19 (7M/ 12F) | n=16 (9M/ 7F) | n=19 (13M/ 6F) | ||||||
| Age (y), median (range) | 49 (19–64) | 36 (26–69) | 46 (26–73) | 45 (29–71) | ||||||
| Nasal lavage | n=15 (5M/ 10F) | n=14 (5M/ 9F) | n=15 (9M/ 6F) | — | ||||||
| Age (y), median (range) | 31 (21–63) | 38 (24–64) | 46 27–73) | — | ||||||
| Immunohistochemistry | n=10 (3M/7F) | n=10 (4M/ 6F) | n=10 (6M/ 4F) | n=10 (7M/ 3F) | ||||||
| Age (y), median (range) | 28 (16–62) | 31 (21–58) | 45 (26–75) | 50 (30–75) | ||||||
F, Female; M, male; N, no; U, unknown; Y, yes.
Figure 1.
Increased expression of tryptase in NPs. The gene expression of tryptase in UT and NPs (A) and nasal scrapings (B) was measured using real-time PCR. The concentration of tryptase in tissue homogenates of UT and NPs (C) and nasal lavage (D) was measured by ELISA. Tryptase concentration was normalized to the concentration of total protein. *P< .05, **P< .001, ***P< .0001.
To further characterize the mast cells in nasal mucosa of patients with CRS, we performed immunohistochemistry using surgical samples from control subjects and patients with CRS to detect tryptase-expressing cells. Consistent with the real-time PCR and ELISA data, increased numbers of tryptase positive cells were found in epithelium and glands of NPs from patients with CRSwNP (Fig 2). We also counted the number of tryptase positive cells using a semiquantitative method. Tryptase positive cells were significantly elevated (P<.0001) in both epithelium and glands of NPs from patients with CRSwNP compared with either UT from control or patients with CRSsNP (Fig 3, A, left). We did not observe a significant difference in the number of tryptase positive cells in the submucosa (excluding those within the glands) among the 4 groups of subject tissues (Fig 3, A, center). These results suggest that mast cells were significantly increased in NPs but not in UT tissue from patients with either form of CRS. Importantly, the increase of the mast cells was only localized to within epithelium and epithelium of submucosal glands within NPs.
Figure 2.
Immunohistochemical detection of tryptase was performed by using anti-human tryptase mAb. Representative immunostaining for tryptase within the epithelium of UT from a control subject (A), a patient with CRSsNP (B), a patient with CRSwNP (C), and a NPs (D). Tryptase positive cells in the glands of UT from a control subject (E) and a NPs (F). Magnification ×400.
Figure 3.
Quantitation of mast cell protease positive cells in nasal mucosal tissue from control subject and patients with CRS and distribution in epithelium, submucosa, and glands. Immunostaining for tryptase (A), chymase (B), and CPA3 (C). **P< .001, ***P< .0001, NS, Not significant.
Chymase expression and distribution in patients with CRS
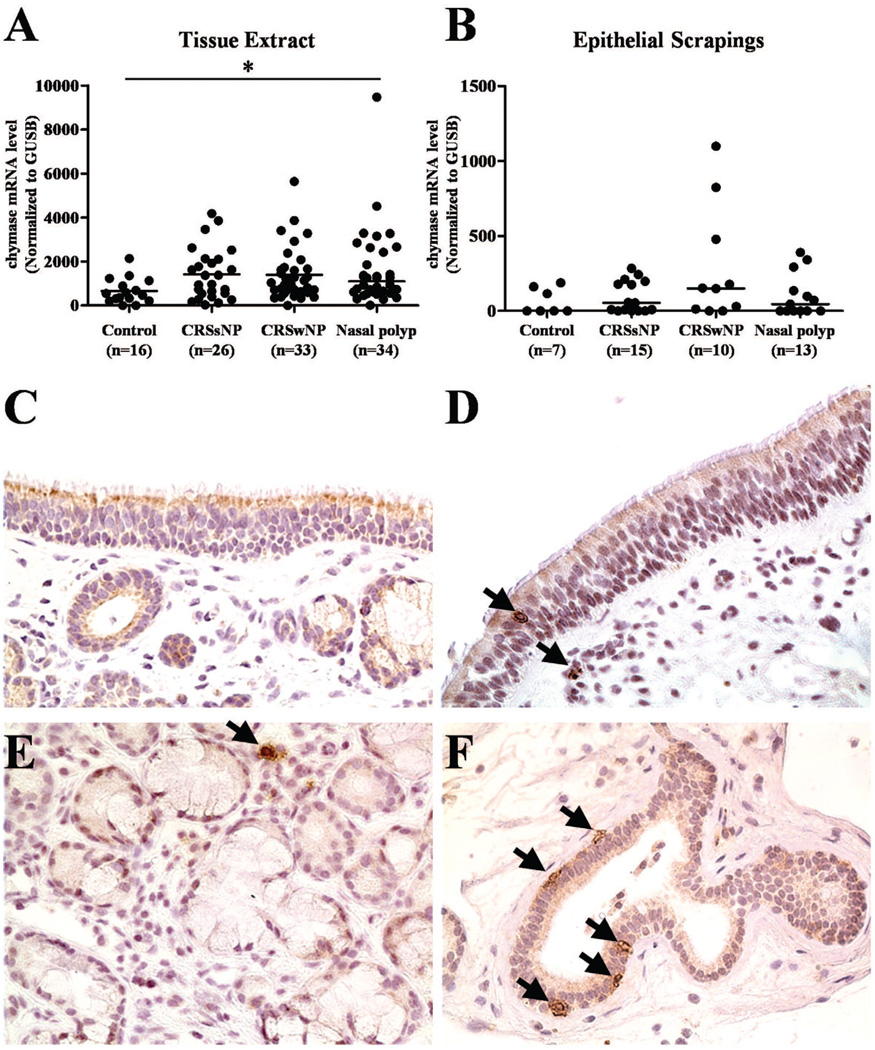
To determine the phenotype of mast cells in nasal mucosa, we assessed the expression of chymase. The expression of mRNA for chymase was elevated in CRS subjects compared to control UT, but there were no differences among CRS subjects (Fig 4, A). Likewise, the level of Chymase was not different between UT and NPs in 17 patients for whom we had matched sets of both polyp and UT for RT-PCR evaluation (data not shown). The expression of mRNA for chymase in nasal scraping samples was not different among these groups (Fig , 4B). To examine the chymase positive mast cells in nasal mucosa of patients with CRS, we performed semiquantitative immunohistochemical evaluation using UT from control subjects and patients with CRS (Fig 4, C-F and Fig 3, B). In epithelium, chymase positive cells were slightly increased in NPs compared to control UT, but there were no differences among CRS groups (Fig 3, B, left panel). On the other hand, chymase positive mast cells located in glands were profoundly elevated in NPs from patients with CRSwNP (Fig 3, B, right panel). When we counted cells outside of the glands but still within the submucosa, we did not observe a significant difference among these four groups of tissue samples (Fig 3, B, middle panel).
Figure 4.
The expression of chymase in NPs. The gene expression of chymase in tissue (A) and nasal scrapings (B) was measured using real-time PCR. Representative immunostaining for chymase within the epithelium of UT from a control subject (C) and a NPs (D) and within the glands of UT from a control subject (E) and a NPs (F). Magnification ×400. **P< .001, NS, Not significant.
CPA3 expression and distribution in patients with CRS
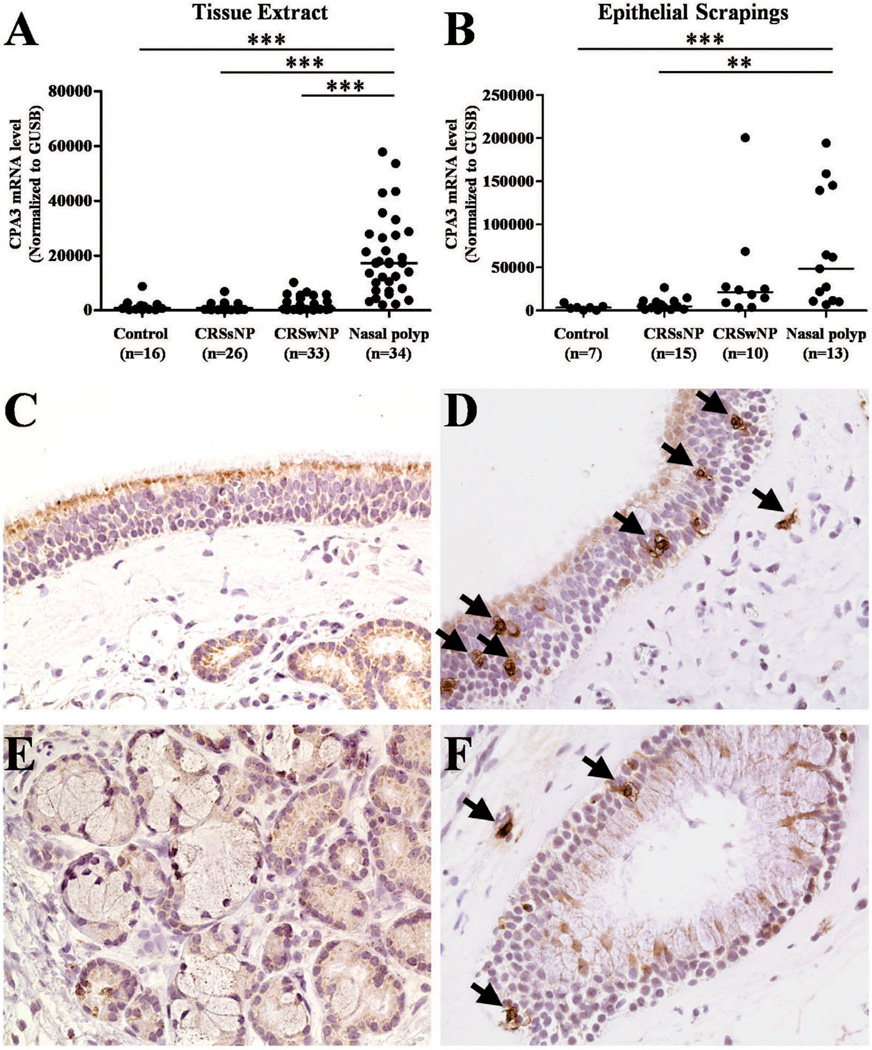
We next assessed the expression of CPA3. The expression of mRNA for CPA3 was significantly increased in NPs from patients with CRSwNP (P < .0001) in comparison with UT from either patients with CRS or control subjects (Fig 5, A). Levels of CPA3 mRNA were significantly elevated in NPs in 17 patients with matched polyp and UT (P<.05 data not shown). To measure the gene expression in epithelium, we evaluated nasal scraping samples and found that CPA3 mRNA was significantly increased in NPs epithelium in comparison with epithelium from UT of either patients with CRSsNP or control subjects (P <.001). To further examine the CPA3 positive mast cells in nasal mucosa of patients with CRS, we performed immunohistochemistry in samples from control subjects and patients with CRS. As shown in Fig 5 C-F, CPA3 positive cells were found in mucosal and glandular epithelium within NPs from patients with CRS. Lack of staining in NP with a negative control antibody is shown in Figure E2. We also counted the number of CPA3 positive cells using a semiquantitative method and found that CPA3 positive cells were significantly elevated in both epithelium (P<.0001) and glands (P<.001) of NPs compared with levels seen in those locations in UT from control subjects. We did not observe a significant difference in CPA3 cells among these four groups of samples in submucosal locations outside of the glands (Fig 3, C).
Figure 5.
The expression of CPA3 in NPs. The gene expression of CPA3 in tissue (A) and nasal scrapings (B) was measured using real-time PCR. Representative immunostaining for CPA3 within the epithelium of UT from a contol subject (C) and a NPs (D) and within the gland of UT from a control subject (E) and a NPs (F). Magnification ×400. **P< .001, ***P< .0001.
Colocalization of mast cell serine proteases
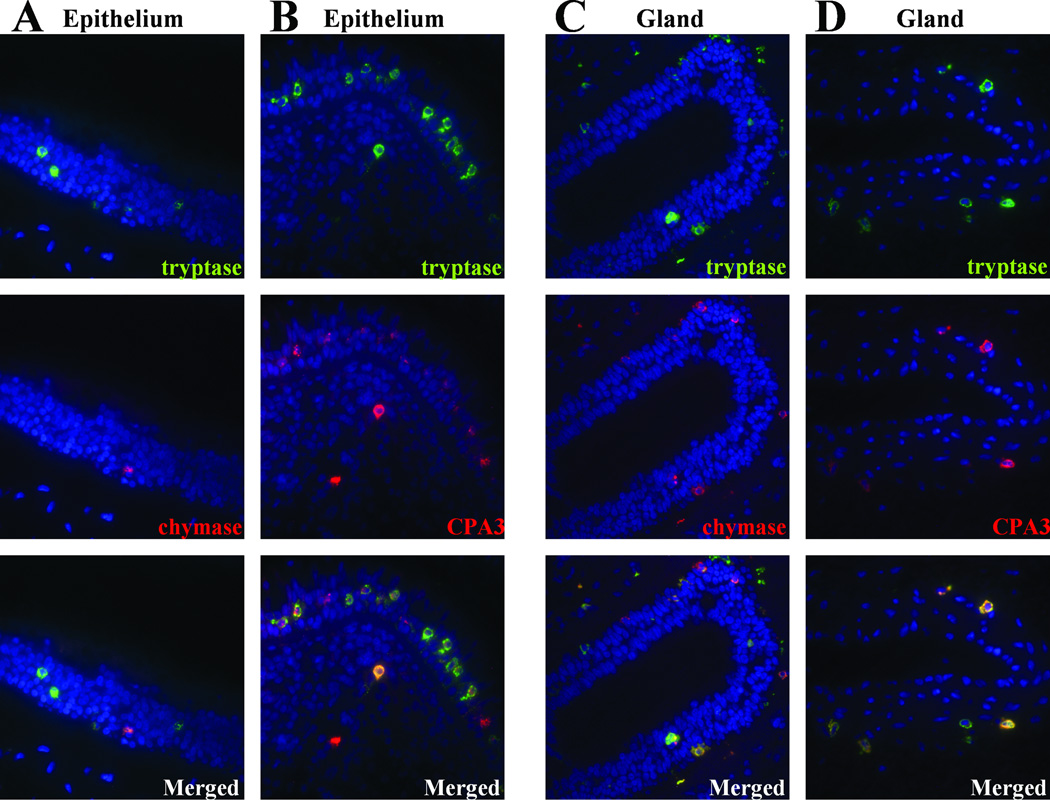
To determine whether the three mast cell proteases under investigation are present in the same or different mast cells, we utilized dual label immunofluorescence and characterized the mast cells localized within epithelium or glands of NPs from patients with CRSwNP. As illustrated in Fig 6, A, of the tryptase positive mast cells, most did not express chymase (74.8 % ± 3.7, mean and SEM tryptase single positive, n=10) in epithelium. On the other hand, most mast cells within epithelium that expressed tryptase were also CPA3 positive (84.2 % ± 2.9 SEM double positive, n=10) (Fig 6, B).
Figure 6.
Immunofluorescence of mast cell proteases in NPs. Immunofluorescence assay was performed with anti-tryptase(green fluorescence) and anti-chymase (red fluorescence) (A), and anti-CPA3 (red fluorescence) (B) in epithelium, anti-tryptase (green fluorescence), and anti-chymase (red fluorescence) (C), and CPA3 (red fluorescence) (D) in glands. Nuclei ware counterstained with 4’,6-diamidino-2-phenylindole (DAPI; blue fluorescence). The results are representative of 5 separate subjects.
We also determined the co-existence of these proteases in the mast cells found in glands of NPs. As shown Fig 6, most mast cells in glands within NPs expressed both tryptase and chymase (79.5% ± 4.0 SEM double positive, n=10, Fig 6, C) and most mast cells expressed both tryptase and CPA3 (87.7 % ± 2.7 SEM double positive, n=10, Fig 6, D). These results suggest that the mast cells found within submucosal glands of NPs were primarily double and triple positive for these proteases. In contrast, mast cells contained within mucosal epithelium predominantly expressed high levels of tryptase and CPA3, but not chymase. Thus, the main distinction between the mast cells found located within the glands and those found elsewhere was that the glandular mast cells were chymase positive. We note that the immunohistochemistry counts suggest that there are approximately 50% as many chymase of CPA3 positive cells as tryptase positive cells. However, using IF, the percentage of tryptase positive cells that were positive for chymase or CPA3 was approximately 80 or 90%. This apparent discrepancy may reflect the different sensitivity of immunofluorescence and immunohistochemical approaches.
Discussion
The current study provides the first in depth description of the expression, distribution and phenotype of mast cells in NPs from patients with CRSwNP. We found increased levels of mast cells in NPs compared to UT from control subjects and patients with CRS (Fig 1). We made the striking observation that mast cells were present within glandular tissues contained in NPs but were almost never detected within glands in UT from either patients or controls. Another important original finding was that these mast cells within glands of NPs, unlike mast cells in control tissue or outside of NPs, expressed the potent glandular secretagogue chymase, along with tryptase and/or CPA3.
Mast cells are classically important in acute IgE-mediated reactions as observed in anaphylaxis, asthma and rhinitis. However, recently, considerable evidence suggests that the roles of mast cells in health and disease are complex, and extend to include innate and adaptive immune responses as well as the control of physiological and homeostatic responses.11 Previous studies demonstrated that NPs mucosa exhibits a high degree of tissue eosinophilia as well as T cells demonstrating skewing toward Th2 cytokine expression3. Mast cells can produce an abundance of cytokines that activate eosinophils such as IL-5, Granulocyte Macrophage colony-stimulating Factor, eotaxin and RANTES.22 In addition, ECP can activate mast cell degranulation and cytokine production.23, 24 In this study we found a strong correlation between the levels of tryptase and ECP in nasal tissue extracts (Fig. E1). It is thus reasonable to speculate that either mast cells and eosinophils employ the same pathways for recruitment or that they may play a role in the accumulation and activation of one another.
Previous studies of mast cell abundance and distribution in NPs have reported contradictory findings; it is described that the number of epithelial mast cells in NPs is elevated 25–28 or that there is no difference in the number of mast cells in NPs compared to control tissue.8, 29, 30 One possible explanation may be that most studies used inferior turbinate (IT) tissue as a control tissue. We know that turbinate and UT differ with respect to expression of numerous host defense and inflammatory molecules (Tieu et al and unpublished observations). NPs usually arise from mucous membranes in the middle nasal meatus and thus we contend that UT or other ethmoid tissue represents a better control than IT. In this study, we used UT resected at skull base surgery as a control tissue and compared with nasal polyps. To our knowledge, this is the first report to use UT as a control for studying mast cells in CRS. We also utilized numerous complementary methods, including RT-PCR, ELISA and double immunofluorescence, which have not been previously employed to study mast cells in CRS.
We observed unequivocally increased numbers of mast cells in epithelium and glands but not submucosa in NPs by immunohistochemistry (Fig 2). These results were reinforced by findings utilizing real-time PCR and ELISA assays in nasal epithelial scraping samples (Fig 1). Although there is not a statistically significant difference between NPs and UT from CRSwNP patients, we observed the same trend. Previous studies in allergic rhinitis and asthma support a model in which deeper tissue mast cells migrate to the mucosal surface during the allergy season, while the total content of mast cells (as measured by total tissue histamine) does not increase greatly.31 In our studies, we show a very clear expansion of the total number of mast cells within NPs, as measured by several techniques. This suggests that local mechanisms are promoting the expansion of mast cells within NPs, either by recruitment or proliferation or both mechanisms.
The mechanism for this accumulation of mast cells remains unclear. Airway epithelial cell derived stem cell factor (SCF) has been shown to be both chemoattractant and essential survival factor for mast cells.20 Previous reports demonstrated a significant increase in expression of SCF in NPs epithelium compared with normal nasal mucosa.27, 32 Th2 cytokine induced epithelial eotaxins could also lead to mast cell recruitment and activation by CCR3.33 In this study, we found an upregulation of mRNA for SCF and eotaxins 1, 2 and 3 in nasal polyps from patients with CRSwNP in comparison with UT from either patients with CRS or control subjects (Fig. E3, A). We also found strong correlations between each of these cytokines and the level of tryptase in nasal tissue (Fig. E3, B). Although these correlations do not prove cause and effect, it is reasonable to speculate that mast cells may be recruited via CCR3 active chemokines and their survival maintained via SCF expressed locally in sinonasal tissue. Other possible mediators of the mast cell response could include neuropeptides such as substance P (SP) and vasoactive intestinal polypeptide (VIP) or leukotriene B4, all of which have been shown to influence mast cell responses.34, 35, 36
Clearly further study will be required to determine the mechanism of mast cell accumulation within NPs epithelium, both at the mucosal surface and within submucosal glands.
Although we did not directly establish mast cell activation (e.g. by using electron microscopy), we detected elevated tryptase in nasal lavage fluids, suggesting that mast cell activation had been taking place in patients with NPs. There are several possible mechanisms that might activate mucosal mast cells in CRS. Staphylococcal colonization is common in CRS and staph enterotoxin may act as an allergen, along with aeroallergens, to induce activation of mast cells.37, 38 Neuropeptides can also activate mast cells independent of crosslinking of FcεRI. A recent study from our laboratories demonstrated that SP and VIP induce degranulation and chemokine production in human mast cells.39 SP can also up-regulate Toll-like receptor (TLR) 2 on human mast cells and increase TLR2-dependent production of leukotriene C4 (LTC4) and IL-8.34 In the rat, 80% of neurons projecting to the nasal epithelium were positive for SP.40 It is thus worthy of consideration that neuropeptides may play an important role in activation of mast cells in NP epithelium and within glands.
Human mast cells can also be activated by thymic stromal lymphopoietin (TSLP), an epithelial cell-derived cytokine present in CRS, resulting in production by mast cells of high levels of Th2 cytokines in the presence of proinflammatory cytokines, IL-1 and tumor necrosis factor.41
Activated mast cells can release a variety of preformed mediators and de novo synthesized proinflammatory mediators that might contribute to NPs development. One such preformed mediator is histamine, which can promote vasodilatation and increased vascular permeability. Mast cell derived chemokines may play a role in the recruitment of eosinophils and other cells found within NPs.22
In the current study we found that mast cells within the NPs epithelium were tryptase and CPA3 double positive and chymase negative using double immunofluorescence (Fig 6). This pattern of mast cell protease expression is not consistent with the patterns observed for either MC-T or MC-TC cells. Recently, a similar unique phenotype of mast cells was reported in epithelium of Th2 high asthma and EoE.19, 20 In contrast, severe asthma is associated with a predominance of MC-TC in the airway epithelium and submucosa and increased prostaglandin D2 (PGD2).42 It has also been reported that arachidonic acid metabolism in MC-TC preferentially uses the cyclooxygenase pathway.43 The unique tryptase and CPA3 double positive, chymase negative mast cell type thus might utilize the lipoxygenase pathway as opposed to the mast cells reported in severe asthma. We did not find any difference in mast cell populations in NPs when comparing CRS patients with or without asthma (data not shown), suggesting that our findings do not simply reflect a large subpopulation of patients in our cohort that have Th2 high asthma. To our knowledge, this is the first report showing the up-regulation of a unique population of mast cells in NPs epithelium.
The underlying mechanism by which the tryptase +/CPA3+/chymase- cells develop is unknown. It has been previously reported that mast cells grown in coculture with epithelial cells down-regulate chymase and the conditioned medium from IL-13 stimulated epithelial cells down-regulates chymase while maintaining tryptase and CPA3 expression in cultured mast cells.20, 44 There are many potential sources of IL-13 in NPs, including Th2 cells, innate type 2 cells, and even mast cells themselves.41 We speculate that Th2 skewing, and IL-13 in particular, might be involved in the development of the unique tryptase+/CPA3+/chymase- phenotype of mast cells in the lungs in Th2 asthma, in the esophagus in EoE, and in the mucosal epithelium of NPs.
We are particularly intrigued by the increase of mast cells within submucosal glands in CRS, as this only occurred in CRSwNP patients and within the NPs. Interestingly, most of the mast cells were triple positive, i.e. traditional MC-TC cells. Hypersecretion of mucus is a hallmark feature of CRS and mucus glands may thus play an important role in the pathogenesis of NPs.45 The expression of mucin gene MUC5B was reported to be upregulated in submucosal glands of NPs compared with normal nasal mucosa and numerous inflammatory mediators can upregulate these genes.46 It has been reported that the expression of PGD2 was upregulated in NPs compared to normal nasal mucosa, which in turn induced MUC5B overproduction.47 Since MC-TC cells contribute to the increase of PGD2 levels,42 and since chymase, but not tryptase, is a potent inducer of mucus secretion 20, 48, 49, the specific accumulation of MC-TC cells within the submucosal glands of NPs should be suspected to play a role in the activation of the secretory response in these glands.
In summary, we report here a profound increase of mast cells in epithelium and glands of NPs as compared with UT from control subjects and patients with CRS. The mast cells that have accumulated in NPs epithelium have a recently recognized unique phenotype, displaying upregulation of tryptase and CPA3 but not chymase. On the other hand, those in glands have the typical MC-TC phenotype in which all three proteases are expressed. Our results raise the exciting hypothesis that expansion of distinct populations of mast cells within the mucosal epithelium and the submucosal glands may play an important role in pathophysiology and development of NPs.
Acknowledgments
Supported by NIH grants R37HL068546-27, R01HL078860, RO1AI072570 and the Ernest S. Bazley Trust
Abbreviations
- CRS
Chronic rhinosinusitis
- NPs
nasal polyps
- CRSsNP
CRS without nasal polyps
- CRSwNP
CRS with nasal polyps
- ECP
eosinophil cationic protein
- MC-T
Mast cell-tryptase
- MC-TC
Mast cell-tryptase/chymase
- CPA3
Carboxypeptidase A3
- GUSB
β-Glucuronidase
- UT
Uncinate tissue
- IT
Inferior turbinate
- HPF
High-powered field
- TSLP
Thymic stromal lymphopoietin
- SCF
Stem cell factor
- SP
Substance P
- VIP
Vasoactive intestinal polypeptide
- TLR
Toll-like receptor
- LTC4
Leukotriene C4
- PGE2
Prostaglandin E2
- EoE
Eosinophilic esophagitis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of potential conflict interest: None – the work has no commercial value.
Clinical implications: A unique phenotype of mast cells in nasal polyp glands may have a pathogenic role in Chronic Rhinosinusitis.
References
- 1.Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. The Journal of allergy and clinical immunology. 2004;114:155–212. doi: 10.1016/j.jaci.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamilos DL. Chronic rhinosinusitis patterns of illness. Clinical allergy and immunology. 2007;20:1–13. [PubMed] [Google Scholar]
- 3.Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61:1280–1289. doi: 10.1111/j.1398-9995.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 4.Bachert C, Gevaert P, van Cauwenberge P. Staphylococcus aureus superantigens and airway disease. Current allergy and asthma reports. 2002;2:252–258. doi: 10.1007/s11882-002-0027-9. [DOI] [PubMed] [Google Scholar]
- 5.Shin SH, Ponikau JU, Sherris DA, Congdon D, Frigas E, Homburger HA, et al. Chronic rhinosinusitis: an enhanced immune response to ubiquitous airborne fungi. The Journal of allergy and clinical immunology. 2004;114:1369–1375. doi: 10.1016/j.jaci.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Schleimer RP, Kato A, Peters A, Conley D, Kim J, Liu MC, et al. Epithelium, inflammation, and immunity in the upper airways of humans: studies in chronic rhinosinusitis. Proceedings of the American Thoracic Society. 2009;6:288–294. doi: 10.1513/pats.200808-088RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Bruaene N, Perez-Novo CA, Basinski TM, Van Zele T, Holtappels G, De Ruyck N, et al. T-cell regulation in chronic paranasal sinus disease. The Journal of allergy and clinical immunology. 2008;121:1435–1441. 41 e1–41 e3. doi: 10.1016/j.jaci.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 8.Polzehl D, Moeller P, Riechelmann H, Perner S. Distinct features of chronic rhinosinusitis with and without nasal polyps. Allergy. 2006;61:1275–1279. doi: 10.1111/j.1398-9995.2006.01132.x. [DOI] [PubMed] [Google Scholar]
- 9.Perez-Novo CA, Claeys C, Van Cauwenberge P, Bachert C. Expression of eicosanoid receptors subtypes and eosinophilic inflammation: implication on chronic rhinosinusitis. Respiratory Research. 2006;7 doi: 10.1186/1465-9921-7-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gevaert P, Lang-Loidolt D, Lackner A, Stammberger H, Staudinger H, Van Zele T, et al. Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. Journal of Allergy and Clinical Immunology. 2006;118:1133–1141. doi: 10.1016/j.jaci.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 11.Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nature reviews. Immunology. 2010;10:440–452. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beaven MA. Our perception of the mast cell from Paul Ehrlich to now. European Journal of Immunology. 2009;39:11–25. doi: 10.1002/eji.200838899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz LB. Analysis of MC(T) and MC(TC) mast cells in tissue. Methods in molecular biology. 2006;315:53–62. [PubMed] [Google Scholar]
- 14.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. New England Journal of Medicine. 2002;346:1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 15.Gibson PG, Allen CJ, Yang JP, Wong BJO, Dolovich J, Denburg J, et al. Intraepithelial Mast-Cells in Allergic and Nonallergic Asthma - Assessment Using Bronchial Brushings. American Review of Respiratory Disease. 1993;148:80–86. doi: 10.1164/ajrccm/148.1.80. [DOI] [PubMed] [Google Scholar]
- 16.Juliusson S, Pipkorn U, Karlsson G, Enerback L. Mast-Cells and Eosinophils in the Allergic Mucosal Response to Allergen Challenge - Changes in Distribution and Signs of Activation in Relation to Symptoms. Journal of Allergy and Clinical Immunology. 1992;90:898–909. doi: 10.1016/0091-6749(92)90462-b. [DOI] [PubMed] [Google Scholar]
- 17.Balzar S, Fajt ML, Comhair SA, Erzurum SC, Bleecker E, Busse WW, et al. Mast cell phenotype, location, and activation in severe asthma: data from the severe asthma research program. American Journal of Respiratory and Critical Care Medicine. 2011;183:299–309. doi: 10.1164/rccm.201002-0295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu Blatman M Karen S, Gonsalves Nirmala, MD, Hirano Ikuo, MD, Bryce Paul J., PhD Expression of mast cell–associated genes is upregulated in adult eosinophilic esophagitis and responds to steroid or dietary therapy. Journal of Allergy and Clinical Immunology. 2011;125:1307–1308. doi: 10.1016/j.jaci.2010.12.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abonia JP, Blanchard C, Buckmeier BK, Collins MH, Stringer KF, Putnam PE, et al. Involvement Of Mast Cells In Eosinophilic Esophagitis. Journal of Allergy and Clinical Immunology. 2010;125 doi: 10.1016/j.jaci.2010.04.009. Ab132-Ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dougherty RH, Sidhu SS, Raman K, Solon M, Solberg OD, Caughey GH, et al. Accumulation of intraepithelial mast cells with a unique protease phenotype in T(H)2-high asthma. Journal of Allergy and Clinical Immunology. 2010;125:1046–1053. doi: 10.1016/j.jaci.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato A, Peters A, Suh L, Carter R, Harris KE, Chandra R, et al. Evidence of a role for B cell-activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. Journal of Allergy and Clinical Immunology. 2008;121:1385–1392. doi: 10.1016/j.jaci.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nature immunology. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 23.Piliponsky AM, Gleich GJ, Bar I, Levi-Schaffer F. Effects of eosinophils on mast cells: a new pathway for the perpetuation of allergic inflammation. Molecular immunology. 2002;38:1369–1372. doi: 10.1016/s0161-5890(02)00090-1. [DOI] [PubMed] [Google Scholar]
- 24.Zheutlin LM, Ackerman SJ, Gleich GJ, Thomas LL. Stimulation of basophil and rat mast cell histamine release by eosinophil granule-derived cationic proteins. Journal of Immunology. 1984;133:2180–2185. [PubMed] [Google Scholar]
- 25.Pawankar R. Mast cells in allergic airway disease and chronic rhinosinusitis. Chemical immunology and allergy. 2005;87:111–129. doi: 10.1159/000087639. [DOI] [PubMed] [Google Scholar]
- 26.Kowalski ML, Grzegorczyk J, Pawliczak R, Kornatowski T, Wagrowska-Danilewicz M, Danilewicz M. Decreased apoptosis and distinct profile of infiltrating cells in the nasal polyps of patients with aspirin hypersensitivity. Allergy. 2002;57:493–500. doi: 10.1034/j.1398-9995.2002.13508.x. [DOI] [PubMed] [Google Scholar]
- 27.Kowalski ML, Lewandowska-Polak A, Wozniak J, Ptasinska A, Jankowski A, Wagrowska-Danilewicz M, et al. Association of stem cell factor expression in nasal polyp epithelial cells with aspirin sensitivity and asthma. Allergy. 2005;60:631–637. doi: 10.1111/j.1398-9995.2005.00753.x. [DOI] [PubMed] [Google Scholar]
- 28.Di Lorenzo G, Drago A, Pellitteri ME, Candore G, Colombo A, Gervasi F, et al. Measurement of inflammatory mediators of mast cells and eosinophils in native nasal lavage fluid in nasal polyposis. International Archives of Allergy and Immunology. 2001;125:164–175. doi: 10.1159/000053811. [DOI] [PubMed] [Google Scholar]
- 29.Patou J, Holtappels G, Affleck K, Gevaert P, Perez-Novo C, Van Cauwenberge P, et al. Enhanced release of IgE-dependent early phase mediators from nasal polyp tissue. Journal of Inflammation-London. 2009;6 doi: 10.1186/1476-9255-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ediger D, Sin BA, Heper A, Anadolu Y, Misirligil Z. Airway inflammation in nasal polyposis: immunopathological aspects of relation to asthma. Clinical and Experimental Allergy. 2005;35:319–326. doi: 10.1111/j.1365-2222.2005.02194.x. [DOI] [PubMed] [Google Scholar]
- 31.Enerback L, Karisson G, Pipkorn U. Nasal mast cell response to natural allergen exposure. International Archives of Allergy and applied Immunology. 1989;88:209–211. doi: 10.1159/000234788. [DOI] [PubMed] [Google Scholar]
- 32.Otsuka H, Kusumi T, Kanai S, Koyama M, Kuno Y, Takizawa R. Stem cell factor mRNA expression and production in human nasal epithelial cells: Contribution to the accumulation of mast cells in the nasal epithelium of allergy. Journal of Allergy and Clinical Immunology. 1998;102:757–764. doi: 10.1016/s0091-6749(98)70015-6. [DOI] [PubMed] [Google Scholar]
- 33.Price KS, Friend DS, Mellor EA, De Jesus N, Watts GF, Boyce JA. CC chemokine receptor 3 mobilizes to the surface of human mast cells and potentiates immunoglobulin E-dependent generation of interleukin 13. American Journal of Respiratory Cell and Molecular Biology. 2003;28:420–427. doi: 10.1165/rcmb.2002-0155OC. [DOI] [PubMed] [Google Scholar]
- 34.Tancowny BP, Karpov V, Schleimer RP, Kulka M. Substance P primes lipoteichoic acid- and Pam3CysSerLys4-mediated activation of human mast cells by up-regulating Toll-like receptor 2. Immunology. 2010;131:220–230. doi: 10.1111/j.1365-2567.2010.03296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maintz L, Wardelmann E, Walgenbach K, Fimmers R, Bieber T, Raap U, et al. Neuropeptide blood levels correlate with mast cell load in patients with mastocytosis. Allergy. 2011;66:862–869. doi: 10.1111/j.1398-9995.2011.02550.x. [DOI] [PubMed] [Google Scholar]
- 36.Weller CL, Collington SJ, Brown JK, Miller HRP, Al-Kashi A, Clark P, et al. Leukotriene B4, an activation product of mast cells, is a chemoattractant for their progenitors. The Journal of experimental medicine. 2005;201:1961–1971. doi: 10.1084/jem.20042407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Zele T, Gevaert P, Holtappels G, van Cauwenberge P, Bachert C. Local immunoglobulin production in nasal polyposis is modulated by superantigens. Clinical and Experimental Allergy. 2007;37:1840–1847. doi: 10.1111/j.1365-2222.2007.02838.x. [DOI] [PubMed] [Google Scholar]
- 38.Verbruggen K, Van Cauwenberge P, Bachert C. Anti-IgE for the Treatment of Allergic Rhinitis and Eventually Nasal Polyps? International Archives of Allergy and Immunology. 2009;148:87–98. doi: 10.1159/000155739. [DOI] [PubMed] [Google Scholar]
- 39.Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 2008;123:398–410. doi: 10.1111/j.1365-2567.2007.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hunter DD, Dey RD. Identification and neuropeptide content of trigeminal neurons innervating the rat nasal epithelium. Neuroscience. 1998;83:591–599. doi: 10.1016/s0306-4522(97)00324-2. [DOI] [PubMed] [Google Scholar]
- 41.Allakhverdi Z, Comeau M, Ziegler SF, Sarfati M, Delespesse G. Thymic stromal lymphopoietin (TSLP) is released by human epithelial cells in response to microbes and potently activates mast cells. Journal of Allergy and Clinical Immunology. 2007;119:523. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balzar S, Fajt ML, Comhair SAA, Erzurum SC, Bleecker E, Busse WW, et al. Mast Cell Phenotype, Location, and Activation in Severe Asthma Data from the Severe Asthma Research Program. American Journal of Respiratory and Critical Care Medicine. 2011;183:299–309. doi: 10.1164/rccm.201002-0295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boyce JA. Mast cells and eicosanoid mediators: a system of reciprocal paracrine and autocrine regulation. Immunological Reviews. 2007;217:168–185. doi: 10.1111/j.1600-065X.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- 44.Hsieh FH, Sharma P, Gibbons A, Goggans T, Erzurum SC, Haque SJ. Human airway epithelial cell determinants of survival and functional phenotype for primary human mast cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14380–14385. doi: 10.1073/pnas.0503948102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tos M, Mogensen C. Mucous Glands in Nasal Polyps. Archives of Otolaryngology-Head & Neck Surgery. 1977;103:407–413. doi: 10.1001/archotol.1977.00780240065009. [DOI] [PubMed] [Google Scholar]
- 46.Ali MS, Wilson JA, Bennett M, Pearson JP. Mucin gene expression in nasal polyps. Acta Oto-Laryngologica. 2005;125:618–624. doi: 10.1080/00016480510027538. [DOI] [PubMed] [Google Scholar]
- 47.Choi YH, Lee SN, Aoyagi H, Yamasaki Y, Yoo JY, Park B, et al. The Extracellular Signal-regulated Kinase Mitogen-activated Protein Kinase/Ribosomal S6 Protein Kinase 1 Cascade Phosphorylates cAMP Response Element-binding Protein to Induce MUC5B Gene Expression via D-Prostanoid Receptor Signaling. J Biol Chem. 2011;286:34199–34214. doi: 10.1074/jbc.M111.247684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sommerhoff CP, Caughey GH, Finkbeiner WE, Lazarus SC, Basbaum CB, Nadel JA. Mast cell chymase. A potent secretagogue for airway gland serous cells. Journal of Immunology. 1989;142:2450–2456. [PubMed] [Google Scholar]
- 49.Nadel JA. Role of Enzymes from Inflammatory Cells on Airway Submucosal Gland Secretion. Respiration. 1991;58:3–5. doi: 10.1159/000195961. [DOI] [PubMed] [Google Scholar]