Abstract
The reaction and coating kinetics for the glucose oxidase initiated interfacial polymerization are elaborated. The interfacial film grows rapidly and linearly with time, producing time-dependent controllable conformal coating thicknesses of up to a millimeter in less than 4 minutes. Bulk polymerization was only observed when the immersing media was stirred to induce higher mass transport rates. The dramatically different film thicknesses observed between different concentrations of glucose in the hydrogel and iron in the bulk media are demonstrated to be a result of an initial rapid growth phase following which the film grows at the same rate nearly independent of either the glucose or iron concentration. The polymerization rate and hence the thickness growth rate in this initial phase saturate at glucose and iron concentrations above 0.8 M and 0.63 mM, respectively. At iron concentrations above 0.05 mM, the film thickness at the end of 3 hours of reaction monotonically decreased with increasing iron concentration from 5.7 mm to 4.2 mm. The glucose oxidase is trapped by the growing polymerization front and can be used as the sole enzymatic precursor to coat a second polymeric layer. However, the rate of film growth of the second layer is 14-fold lower than the rate of film growth when bulk enzyme is present during the second stage coating process.
1. Introduction
Glucose oxidase-mediated initiation of radical polymerization reactions, first reported by Iwata et al. [1], has the advantage of being resistant to oxygen inhibition, the classical issue facing radical polymerizations [2], while proceeding rapidly at ambient temperature and pressure. Further, this technique represents an ideal biological reaction to use to facilitate interfacial polymerizations as it necessitates multiple components to achieve rapid, successful polymerization. In particular, glucose oxidase catalyzes the reaction between β-D-glucose and oxygen, producing hydrogen peroxide, which in the presence of ferrous ions (Fe2+), generates hydroxyl radicals that are highly efficient for initiating (meth)acrylate polymerization [3]. Since its original demonstration, this technique has been applied in a variety of systems that exploit the unique aspects of the initiation system. The light independent mechanism and absence of oxygen inhibition was utilized in fabricating hydrogels for cellular encapsulation that were several millimeters thick [3]. The ability of glucose oxidase to participate in radical generation while continually regenerating was used to fabricate highly sensitive polymerization-based amplification devices [4]. Recently, this initiation system was used to fabricate conformal coatings on 3-dimensional hydrogel structures by interfacial polymerization [5]. Unlike interfacial condensation polymerizations that result in polymer growth in an uncontrollable fashion, radical chain polymerizations allow for good control of polymerization kinetics to be achieved by simply changing the initiator concentration, whose effect on polymerization rate is very well understood. In addition, initiation reactions capable of generating radicals are compatible with a wide variety of monomers, and molecular design of chemical or biological properties of the resulting polymer is enabled by copolymerization of the desired functional monomers. This behavior was demonstrated recently in the fabrication of immunoactive barriers for implantable devices by covalent incorporation of thiolated-biomolecules as achieved by this interfacial polymerization method [6].
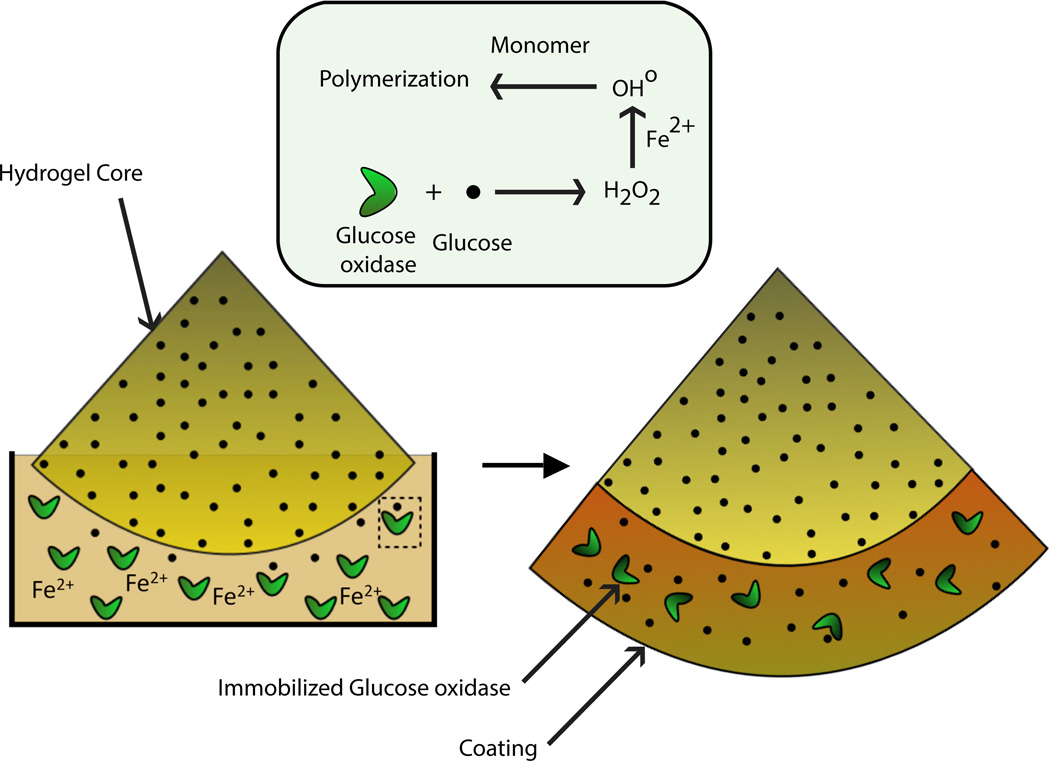
One method for forming interfacial coatings on hydrogel structures using this technique is realized by immersing a glucose-swollen hydrogel into an aqueous solution consisting of monomer, ferrous ion (Fe2+) and glucose-oxidase as shown in Figure 1. The hydrogel serves as a template that spatially confines glucose prior to polymerization. Thus, the locus of the enzymatic reaction involving glucose is initially at the hydrogel boundary but is subsequently delocalized due to the rapid diffusion of glucose into the bulk media. Depending on the conditions and species concentrations, hydrogen peroxide generated by this reaction can also diffuse into the surrounding media before reacting with ferrous ion to produce the hydroxyl radicals responsible for initiating the polymerization. The rapid mass transport of the initiating species results in the reaction zone extending well into the bulk, thereby enabling conformal coatings that are several hundreds of micrometers thick [5]. This outcome is difficult to achieve using conventional or other interfacial radical polymerizations that confine the initiator to the surface or interface [7–13] primarily because radicals have a very short lifetime and hence have significantly smaller diffusion length scales. However, if the interfacial polymerization process is reaction-controlled, high mass transport rates can cause undesired bulk polymerization. This behavior arises because the initiating species can avoid reaction at the interface and diffuse into the bulk, resulting in polymerization throughout the immersing solution. The choice of monomer that is used to fabricate the hydrogel and the polymeric coating also affects the resulting crosslinking density and hence the diffusion coefficient of the initiating species and the film growth rate. The efficiency of glucose oxidase encapsulation or capture by the propagating polymerization front influences the diffusion length of glucose and hence the degree of delocalization of the initiating reactions. The species concentrations affect the polymerization rate and consequently the likelihood of bulk polymerization induced by rapid diffusion of glucose and hydrogen peroxide. The current investigation attempts to understand the interplay of reaction and mass transport rates of this interfacial polymerization process as a means for controlling the physical, biological and chemical attributes of the coating process and achieving heightened capabilities from this technique.
Figure 1.
Schematic illustrating the formation of coating on hydrogel substrates by interfacial polymerization initiated by a glucose oxidase-mediated system. Reaction between GOx and glucose that subsequently triggers polymerization initially occurs near the hydrogel boundary but becomes delocalized due to rapid diffusion of glucose.
2. Experimental
2.1 Materials
Poly(ethylene glycol) diacrylate, MW 575 (PEGDA575), Iron (II) sulfate heptahydrate, glucose oxidase (GOx) from Aspergillus Niger, and D-(+)-glucose solutions were obtained from Sigma-Aldrich. 4-(2-Hydroxyethoxy) phenyl-(2-hydroxy-2-propyl) ketone (Irgacure 2959) was obtained from BASF. 2-(N-Morpholino) ethanesulfonic acid (MES) buffer pH 4.5 was obtained from Teknova. Methacryloxyethyl thiocarbamoyl rhodamine B (Rhodamine B acrylate) was obtained from Polysciences and suspended in DMSO before using volumes necessary to achieve the desired target concentration. The final volumes of DMSO in the precursor solution were 0.5 to 1 vol%. The hydrogen peroxide detection kit was obtained from Enzo Life Sciences.
2.2 Polymerization and formation of the core hydrogel substrate
The monomer formulation used was an aqueous solution of 15wt% PEGDA575, 0.1wt% Irgacure 2959 and glucose in appropriate quantities to achieve the final desired glucose concentration. This precursor solution was placed in a cylindrical mold ( 4mm diameter and 2 mm height) and polymerized by exposing to 320–390 nm light from an Acticure 4000 (Exfo) at an intensity of 15 mW/cm2 for 10 minutes.
2.3 Interfacial polymerization of hydrogel coating and characterization
The hydrogel substrates were immersed in a coating solution comprised of an aqueous solution of 15 wt% PEGDA575, 3.1µM GOx, 0.005wt% rhodamine-B acrylate and 10 mM MES pH 4.5, glucose and iron at the desired concentrations. The immersion process was carried out in an automated fashion using a VersArray ChipWriter Pro system (Bio-Rad laboratories). The pin that was used to hold the hydrogel substrate was punctured through the center of the substrate before being immersed into the bulk media. After the coating process, the hydrogels were thinly sliced to obtain a cross section followed by storage in water until further characterization. The coatings consisting of covalently immobilized fluorescent molecules, particularly rhodamine-B, were imaged using a Zeiss Pascal LSM 5 confocal microscope. The gels were excited with a 543 nm helium neon laser and fluorescence was monitored from 547–680nm.
3 Results and Discussion
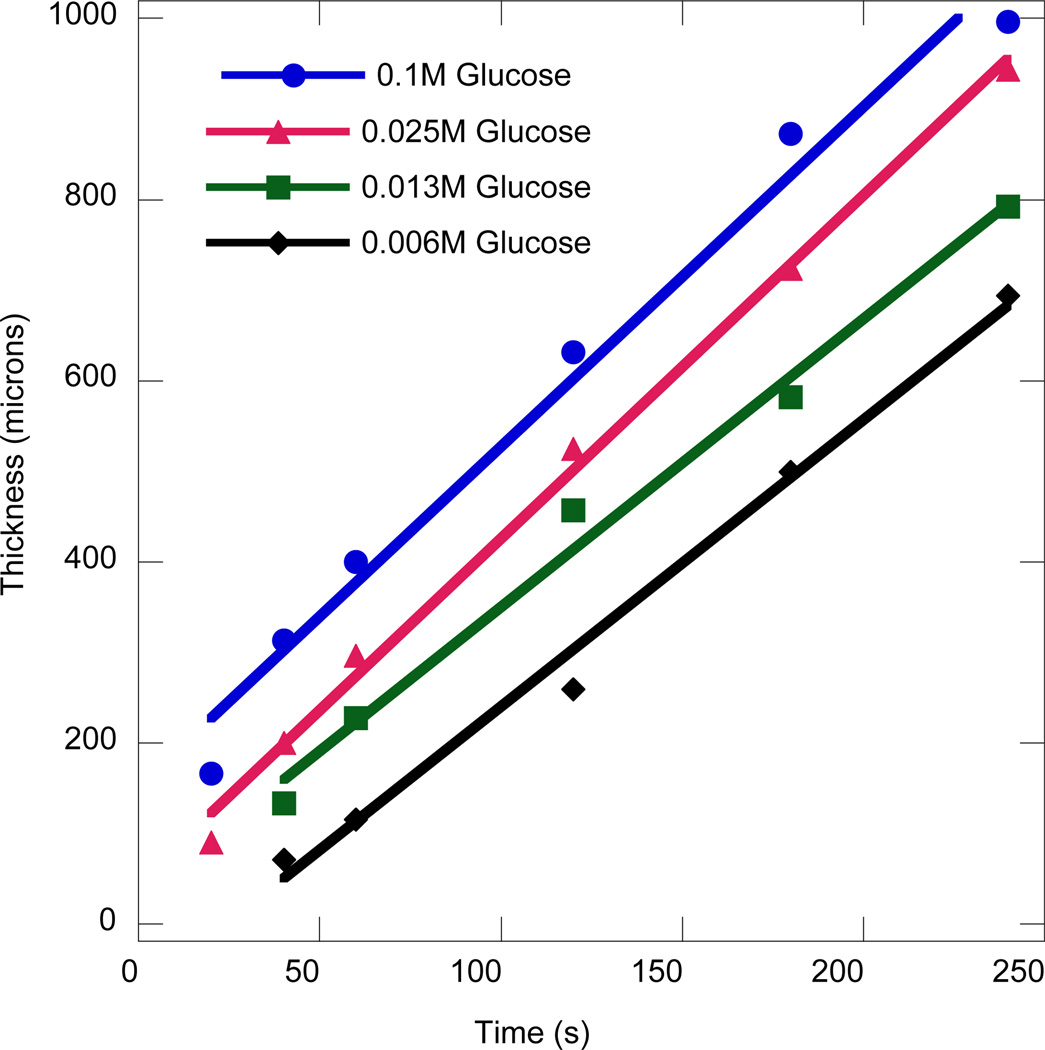
Interfacial polymerizations were conducted by immersing the hydrogel core in the bulk media for various times followed by evaluation of the coating extent and process. The coating thickness as a function of the immersion time is shown in Figure 2 for different concentrations of glucose present in the hydrogel precursor solution. The film growth rate, as measured by the slope of the thickness profile as a function of time, is a measure of the average polymerization rate within the coating. It is instructive to note that the polymerization reaction at the moving interface, which is defined as the transition from the gelled to the viscous region, contributes predominantly to film growth. The polymerization reaction happening within the coating increases the crosslinking density and hence alters the swelling and transport properties but is assumed to contribute minimally to the thickness, for the sake of interpretation of the experiments. After an initial period, the pseudo-linear dependence of film thickness on time indicates sufficiently rapid diffusion of glucose to the interface to maintain the average polymerization rate at a nearly constant level. The near-constant film growth rate could also be due to the rapid diffusion to the interface of hydrogen peroxide generated by the reaction between glucose and the enzyme trapped in the coating. In reality, the diffusion of both glucose and hydrogen peroxide influence the film growth rate. However the relative contributions depend on the thickness of the film, the fraction of glucose oxidase encapsulated within the already formed coating, and the concentration of iron in the bulk media. This complex interplay will be discussed later in this manuscript. In either case, the film growth rate is not limited by species diffusion. It is clear from Figure 2 that the film growth rate is nearly the same for different glucose concentrations and that the dramatically different thicknesses achieved at different glucose concentrations are a result of different initial film formation rates. For many of the conditions evaluated, at early times the absence of a significant coating results in lower resistance to mass transfer and hence a more rapid rate of film growth. Following this initial phase, the interfacial radical generation rate reaches a steady state as indicated by the constant rate of film growth, where this rate is almost independent of the initial glucose concentration. At lower glucose concentrations, the slower initiation rate delays gelation implying an effective inhibition time for the initial coating formation.
Figure 2.
Film thickness vs. time for various concentrations of glucose in the core hydrogel. The conditions used were: 3.1µM GOx, 0.25mM Fe2+, 0.005wt% rhodamine-B acrylate, 10 mM MES pH 4.5 and 15wt% PEGDA575
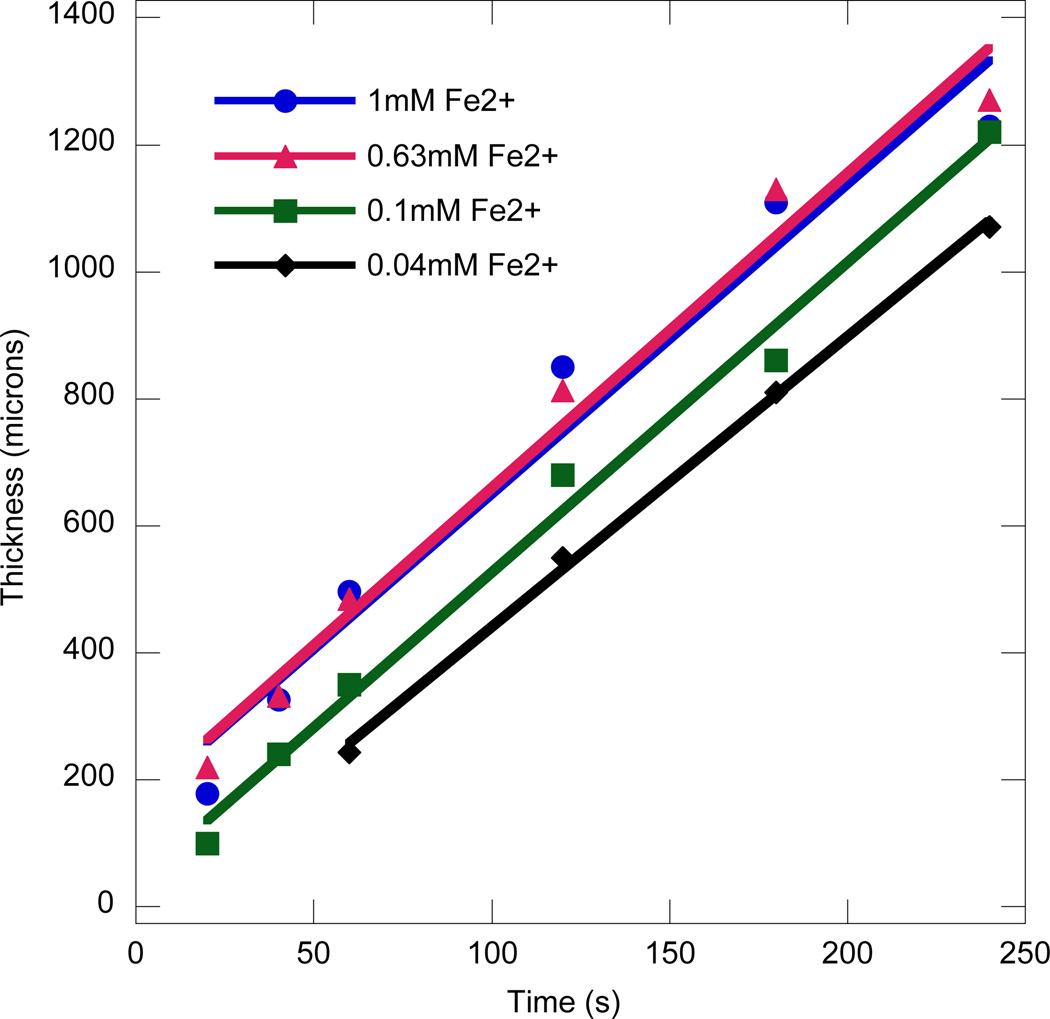
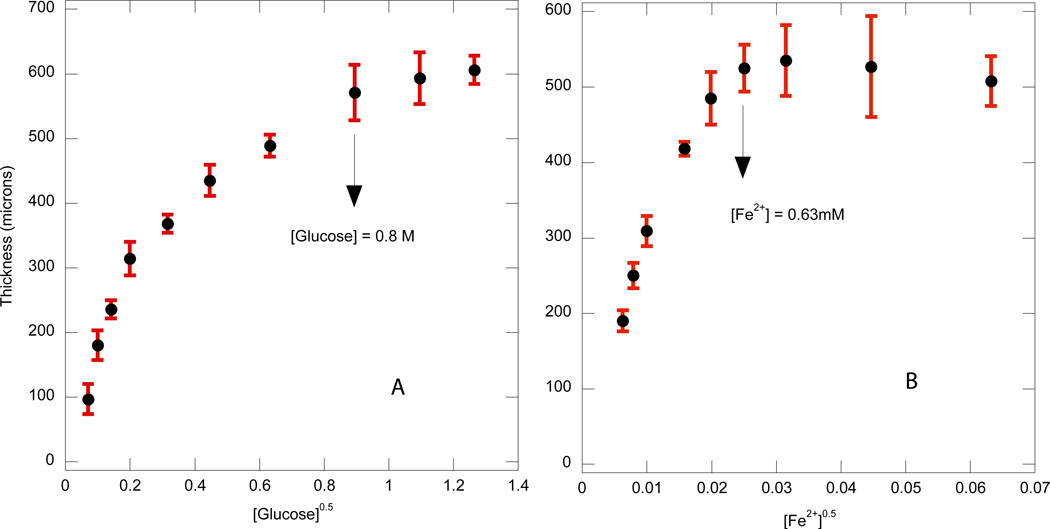
The thickness as a function of time for different iron solution concentrations is shown in Figure 3 with similar overall kinetic behavior observed. Furthermore, at the two highest iron concentrations, the thickness profiles overlap indicating that even the initial rapid initial growth phase is similar. This behavior is likely due to the presence of a rate-limiting reaction that does not involve iron and arises prior to the reaction of iron with the hydrogen peroxide to form the hydroxyl radicals. To explore further the reaction kinetics in this initial film growth region, the film thickness is plotted after 1 minute of immersion for different concentrations of glucose and iron, as shown in Figures 4a and 4b, respectively. Here, the abscissa is plotted as the square root of the species concentration. The exponent of 0.5 was chosen because previous studies on the bulk polymerization kinetics of a similar system indicated that the polymerization rate scaled with the half power of glucose and iron concentrations over a wide range of concentrations, likely due to bimolecular termination [3]. Therefore, the concentrations of these components of the initiation system are scaled to account for their influence on the ideal, bulk polymerization rate. It is seen that at glucose concentrations higher than 0.8 M and iron concentrations higher than 0.63 mM, the average polymerization rate in the rapid growth phase saturates. When the concentrations of iron, glucose and oxygen were increased further (not shown), there was no significant change in the film thickness, indicating that none of the abovementioned species limit the reaction rate. Thus, it appears that the turnover rate of the enzyme, a measure of the maximum substrate conversion rate achievable has been attained.
Figure 3.
Film thickness vs. time for various concentrations of iron in the bulk media. The conditions used were: 3.1µM GOx, 0.1M Glucose, 0.005wt% rhodamine-B acrylate, 10 mM MES pH 4.5 and 15wt% PEGDA575
Figure 4.
Variation of film thickness with square root of glucose concentration (a) and iron concentration (b) after 1 min immersion to form the coating. The conditions used were 3.1µM GOx, 0.005wt% rhodamine-B acrylate, 10 mM MES pH 4.5 and 15 wt% PEGDA575, and either 0.25 mM Fe2+ (Figure 4a) or 0.1 M glucose (Figure 4b)
In all these experiments, bulk polymerization was a minimal concern in spite of rapid diffusion rates of glucose and hydrogen peroxide. Bulk polymerization occurs if the initiating species escapes reaction at the interface and diffuses to the bulk causing polymerization in isolation from the adhered coating. Interestingly, when the bulk media is stirred at various rates (60, 150 and 400 rpm), an interfacial coating was not formed. Instead polymerization of the entire immersing solution occurred. This outcome represents a case where the interfacial mass transport rates are much higher than reaction rates at the interface. Without stirring, the interfacial reaction rate is fast enough to form an attached polymer at the interface prior to bulk diffusion of the initiating species. Therefore, if one were to use monomers with a much higher molecular weight to form the hydrogel, the resulting crosslinking density would be much lower, consequently increasing the likelihood of bulk polymerization due to lower mass transfer resistance. This pitfall can readily be countered simply by using a more viscous bulk solution or otherwise by enabling higher interfacial reaction rates.
3.1 Encapsulation of glucose oxidase (GOx) by the polymerization front
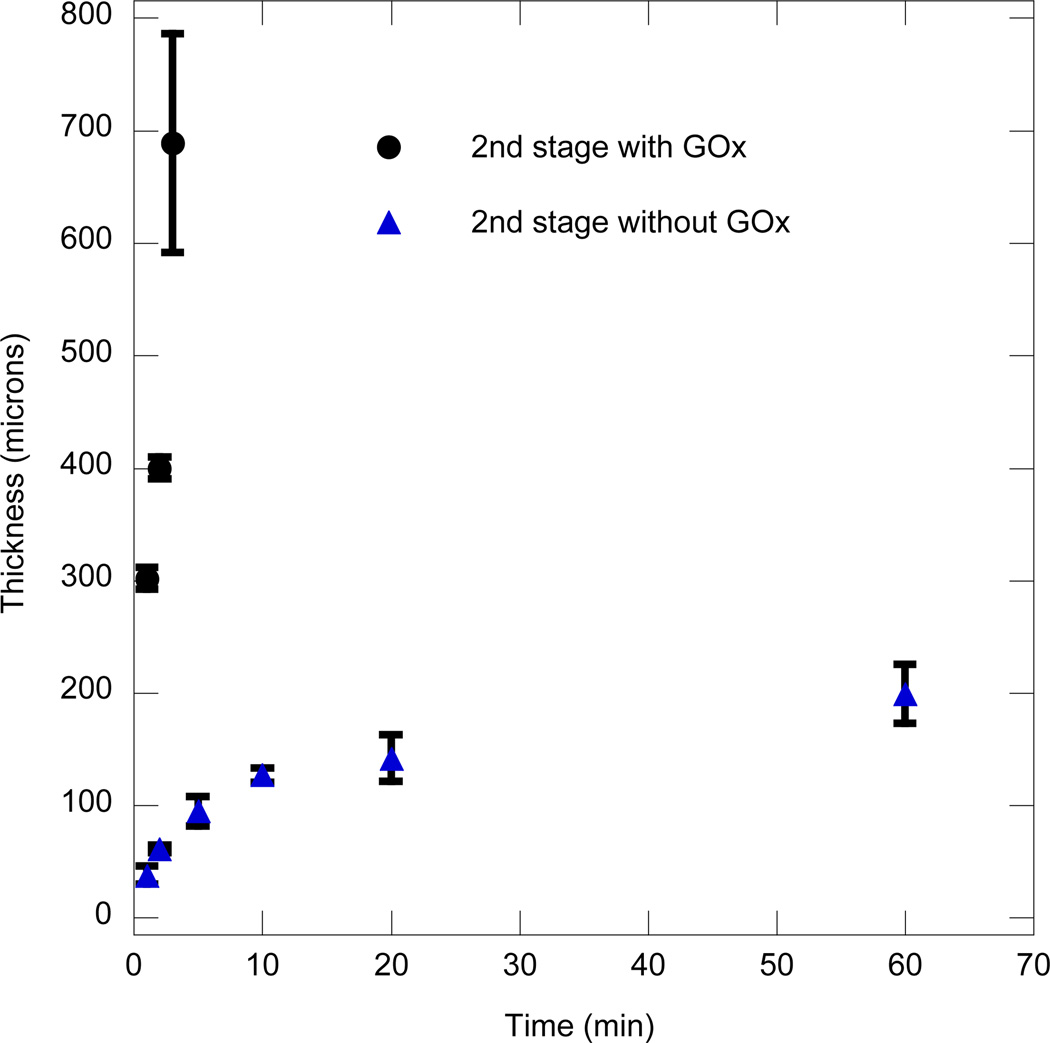
Glucose oxidase is a bulky enzyme (Hydrodynamic radius ~ 43A°) compared to the mesh size of the polymer network formed using PEGDA575 (<20A°). Once encapsulated by the growing polymerization front, the enzyme is effectively trapped. Immobilized glucose oxidase is capable of enzymatic activity associated with its reaction with glucose [14]. However, hydrogen peroxide generated by the initiation reaction is also capable of reacting with glucose-oxidase and rendering it inactive [15–17]. Therefore, the efficiency of glucose oxidase encapsulation in addition to enzyme inactivation by hydrogen peroxide dictates the effective active enzyme concentration trapped within the dynamically forming coating. To understand the trapped enzymatic activity, a two-stage interfacial coating was performed. In the first stage, a coating 600 microns thick is formed in the same manner as earlier. In the second stage, the coated hydrogel is immersed in a monomer solution without bulk enzyme. Therefore, for this particular experimental approach, any additional coating formation or polymerization that occurs during the 2nd stage would be entirely a result of the enzyme encapsulated within the coating formed during the 1st stage polymerization. The 2nd stage thickness profile as a function of time was plotted and compared to the control experiment, where the bulk enzyme was present in both stages (Figure 5). It is clearly seen that the interfacial polymerization rate without bulk GOx in the second stage is dramatically lower compared to when bulk GOx is present. This behavior indicates that the film growth is predominantly due to the diffusion of glucose to the interface and subsequent reaction with GOx in the bulk. It is also observed that the film growth rate, when bulk GOx is absent, decreases with increasing time. The locus of the initiation reaction in the 2nd stage that generates hydrogen peroxide is always within the 1st stage coating. This behavior means that, as the thickness in the 2nd stage process increases, the distance that H2O2 has to diffuse to reach the interface increases. The reaction of H2O2 with iron to produce hydroxyl radicals (OH°) is most productive at the interface and contributes least to the thickness when taking place far from the interface. This limitation arises because OH° generated within the coating take part in radical termination or chain transfer reactions with abstractable hydrogens on the polymer backbone in the absence of significant monomer. Furthermore, it is known that OH° are an extremely reactive species and cannot diffuse farther than 1–5 times their molecular diameter without reaction [18] and hence have minimal potential to diffuse to the interface. The result is a steadily decreasing film growth rate with increasing thickness. Additional evidence of the decreased contribution of OH° generated within the coating comes from Table 1. When the interfacial polymerization is allowed to proceed for 3 hours at three different iron concentrations, the thickness monotonically decreased with increasing iron concentration. At higher iron concentrations, H2O2 generated within the coating would not be able to diffuse far before reacting with iron to form OH°, and this reaction thus increases the likelihood of radicals being generated within the coating rather than at the interface.
Figure 5.
2nd stage film thickness vs. time with GOx (○) and without GOx (Δ) in the bulk media. The conditions used were: 3.1µM GOx, 0.005wt% rhodamine-B acrylate, 10 mM MES pH 4.5 and 15wt% PEGDA575, 0.05mM Fe2+, 0.1M glucose. The first stage thickness was 600 microns
Table 1.
Thickness of the interfacial film after 3 hours of reaction for three different bulk iron concentrations. The conditions used were: 3.1µM GOx, 0.005wt% rhodamine-B acrylate, 10 mM MES pH 4.5 and 15wt% PEGDA575, 0.1M glucose.
| [Fe2+] in bulk | Thickness |
|---|---|
| 0.05 mM | 5.7 mm |
| 0.25 mM | 4.6 mm |
| 2 mM | 4.2 mm |
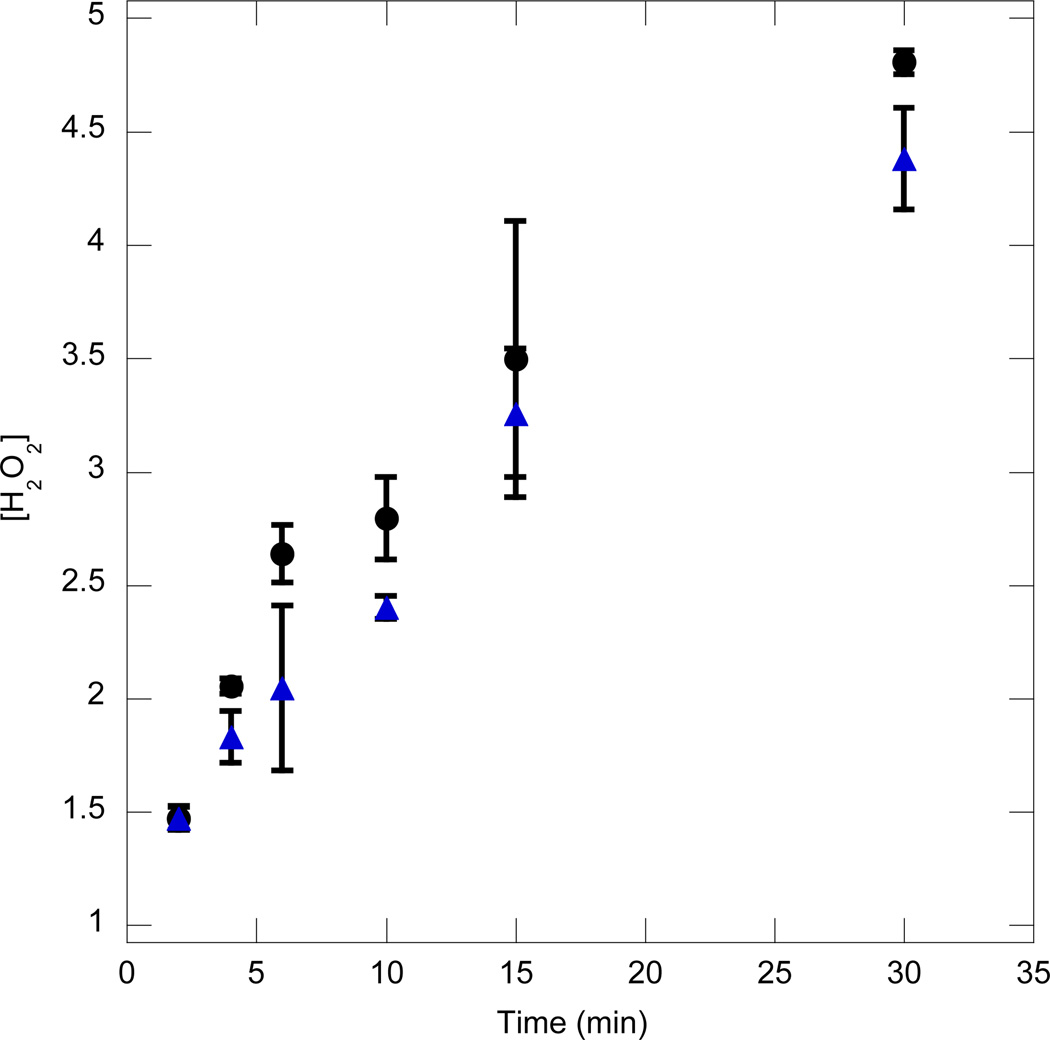
A persistent issue remains as to whether the low concentration of active GOx in the coating is due to an inefficient encapsulation process or due to deactivation of the enzyme. Alternatively, the enzyme activity might be negligible and the film growth in the 2nd stage without GOx in the bulk media is entirely as a result of the remnant hydrogen peroxide (H2O2) from the 1st stage coating process. To explore whether residual hydrogen peroxide was present and initiating the reaction, the coated hydrogel from the first stage was investigated for enzymatic activity. This evaluation was performed simply by immersing the hydrogel in water and evaluating the H2O2 released into the bulk by the coating. Figure 6 shows a plot of H2O2 concentration in the bulk as a function of time for just such a system. The circles represent the release from a coated construct just after the coating process. The triangles represent the release from the coated construct after it has been allowed to stand in an aqueous solution containing 0.5mM Fe2+ for 15 minutes before being investigated for H2O2 release. The purpose was to decompose any remnant hydrogen peroxide within the coating so that further H2O2 released would be due to the continuing reaction of glucose with the encapsulated enzyme. The similar slopes of the two curves indicate that the enzymatic reaction is predominantly responsible for the H2O2 release. The slightly lower concentration detected when the hydrogel is immersed in an iron solution before H2O2 release, is most likely due to the consumption of the remnant H2O2 by iron. The work of Hume et al. [6] provides additional evidence of encapsulated enzyme activity. In their work, when the coated hydrogel containing cells were placed in a cell culture media (containing glucose) overnight, low cell viability was observed, likely due to the reaction of encapsulated GOx with bulk glucose which generates H2O2 that is cytotoxic. This behavior implies that the 2nd stage coating thickness profile without bulk enzyme (Figure 5, Δ) is predominantly due to the encapsulated enzyme itself. The choice of monomers used for coating the hydrogel influences the glucose oxidase kinetics as well as its relative propensity to become trapped within the growing polymer film.
Figure 6.
Hydrogen peroxide concentration released into the bulk vs. time when the coated hydrogel construct is immersed in water just after the coating process (○) or after allowing the hydrogel to stand in a 0.05 mM Fe2+ solution for 15 min (Δ). The coating was 600 µm thick
Conclusions
The film formed by the interfacial glucose oxidase-mediated polymerization grows linearly with time for different concentrations of glucose and iron indicating that the interfacial polymerization process is not limited by the diffusion of glucose to the interface. The initial rapid film growth is due to the absence of significant polymer film at short times, thereby resulting in lower resistance to mass transfer. The diffusive flux of the initiating species to the interface reaches a steady state following this initial rapid growth phase and this behavior leads to a constant film growth rate that is independent of the initial concentrations of glucose and iron. The film thickness at the end of this initial rapid growth phase saturates at higher concentrations of iron and glucose, likely due to the enzymatic reaction achieving its maximum substrate conversion rate. When the interfacial polymerization is allowed to proceed for long times, higher bulk iron concentration leads to lower film thickness because the diffusion distance of hydrogen peroxide within the coating is significantly limited by reaction with iron to form hydroxyl radicals within the film rather than at the interface. Glucose oxidase that is trapped by the propagating polymerization front is active but significantly lower in overall activity as compared to the enzyme in the bulk media. This reduction results in a 14-fold lower initial film growth rate when the bulk enzyme is absent and the trapped enzyme is used as the sole precursor for further coating formation.
Acknowledgements
This work has been supported by the National Institutes of Health (Grant R21 EB 012188)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Iwata H, Hata Y, Matsuda T, Ikada Y. Initiation of radical polymerization by glucose-oxidase utilizing dissolved-oxygen. J Polym Sci A Polym Chem. 1991;29(8):1217–1218. [Google Scholar]
- 2.Decker C, Jenkins AD. Kinetic approach of oxygen inhibition in ultraviolet- and laser-induced polymerizations. Macromolecules. 1985;18(6):1241–1244. [Google Scholar]
- 3.Johnson LM, Fairbanks BD, Anseth KS, Bowman CN. Enzyme-mediated redox initiation for hydrogel generation and cellular encapsulation. Biomacromolecules. 2009;10(11):3114–3121. doi: 10.1021/bm900846m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berron BJ, Johnson LM, Ba X, McCall JD, Alvey NJ, Anseth KS, et al. Glucose oxidase-mediated polymerization as a platform for dual-mode signal amplification and biodetection. Biotechnol Bioeng. 2011;108(7):1521–1528. doi: 10.1002/bit.23101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson LM, DeForest CA, Pendurti A, Anseth KS, Bowman CN. Formation of three-dimensional hydrogel multilayers using enzyme-mediated redox chain initiation. ACS Appl Mater Interfaces. 2010;2(7):1963–1972. doi: 10.1021/am100275n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hume PS, Bowman CN, Anseth KS. Functionalized peg hydrogels through reactive dip-coating for the formation of immunoactive barriers. Biomaterials. 2011;32(26):6204–6212. doi: 10.1016/j.biomaterials.2011.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott C, Wu D, Ho CC, Co CC. Liquid-core capsules via interfacial polymerization: a free-radical analogy of the nylon rope trick. J Am Chem Soc. 2005;127(12):4160–4161. doi: 10.1021/ja044532h. [DOI] [PubMed] [Google Scholar]
- 8.Luo YW, Gu HY. A general strategy for nano-encapsulation via interfacially confined living/controlled radical miniemulsion polymerization. Macromol Rapid Commun. 2006;27(1):21–25. [Google Scholar]
- 9.Jiang D, Huang X, Qiu F, Luo C, Huang LL. Synthesis of polymer thin film gradient with nanometer thickness through water diffusion controlled surface polymerization. Macromolecules. 2009;43(1):71–76. [Google Scholar]
- 10.von Werne TA, Germack DS, Hagberg EC, Sheares VV, Hawker CJ, Carter KR. A versatile method for tuning the chemistry and size of nanoscopic features by living free radical polymerization. J Am Chem Soc. 2003;125(13):3831–3838. doi: 10.1021/ja028866n. [DOI] [PubMed] [Google Scholar]
- 11.Sikes HD, Hansen RR, Johnson LM, Jenison R, Birks JW, Rowlen KL, et al. Using polymeric materials to generate an amplified response to molecular recognition events. Nat Mater. 2008;7(1):52–56. doi: 10.1038/nmat2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshikawa C, Goto A, Tsujii Y, Fukuda T, Yamamoto K, Kishida A. Fabrication of high-density polymer brush on polymer substrate by surface-initiated living radical polymerization. Macromolecules. 2005;38(11):4604–4610. [Google Scholar]
- 13.Kizilel S, Perez-Luna VH, Teymour F. Photopolymerization of poly(ethylene glycol) diacrylate on eosin-functionalized surfaces. Langmuir. 2004;20(20):8652–8658. doi: 10.1021/la0496744. [DOI] [PubMed] [Google Scholar]
- 14.Fortier G, Belanger D. Characterization of the biochemical behavior of glucose-oxidase entrapped in a polypyrrole film. Biotechnol Bioeng. 1991;37(9):854–858. doi: 10.1002/bit.260370909. [DOI] [PubMed] [Google Scholar]
- 15.Krishnaswamy S, Kittrell JR. Deactivation studies of immobilized glucose oxidase. Biotechnol Bioeng. 1978;20(6):821–835. [Google Scholar]
- 16.Tse PHS, Gough DA. Time-dependent inactivation of immobilized glucose-oxidase and catalase. Biotechnol Bioeng. 1987;29(6):705–713. doi: 10.1002/bit.260290607. [DOI] [PubMed] [Google Scholar]
- 17.Greenfield PF, Kittrell JR, Lawrence RL. Inactivation of immobilized glucose oxidase by hydrogen-peroxide. Anal Biochem. 1975;65(1–2):109–124. doi: 10.1016/0003-2697(75)90497-2. [DOI] [PubMed] [Google Scholar]
- 18.Pryor WA. Oxyradicals and related species - their formation, lifetimes, and reactions. Annu Rev Physiol. 1986;48:657–667. doi: 10.1146/annurev.ph.48.030186.003301. [DOI] [PubMed] [Google Scholar]