Abstract
Objective
Sleep misperception is considered by some investigators a common characteristic of chronic insomnia, whereas others propose it as a separate diagnosis. The frequency and the determinants of sleep misperception in general population samples are unknown. In this study we examined the role of objective sleep duration, a novel marker in phenotyping insomnia, and psychological profiles on sleep misperception in a large, general population sample.
Methods
142 insomniacs and 724 controls selected from a general random sample of 1,741 individuals (age ≥ 20 years) underwent a polysomnographic evaluation, completed the Minnesota Multiphasic Personality Inventory-2, and were split into two groups based on their objective sleep duration: “normal sleep duration” (≥ 6 hours) and “short sleep duration” (< 6 hours).
Results
The discrepancy between subjective and objective sleep duration was determined by two independent factors. Short sleepers reported more sleep than they objectively had and insomniacs reported less sleep than controls with similar objective sleep duration. The additive effect of these two factors resulted in underestimation only in insomniacs with normal sleep duration. Insomniacs with normal sleep duration showed a MMPI-2 profile of high depression and anxiety, and low ego strength, whereas insomniacs with short sleep duration showed a profile of a medical disorder.
Conclusions
Underestimation of sleep duration is prevalent among insomniacs with objective normal sleep duration. Anxious-ruminative traits and poor resources for coping with stress appear to mediate the underestimation of sleep duration. These data further support the validity and clinical utility of objective sleep measures in phenotyping insomnia.
Keywords: Insomnia, objective sleep duration, personality, phenotypes, sleep misperception, subjective sleep duration
Introduction
Insomnia is the most common sleep disorder, yet little is known about the etiology, pathophysiology, clinical course, and consequences of this highly prevalent chronic condition (1,2). According to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), individuals with chronic insomnia complain of sleep difficulties and frequently underestimate their sleep duration (3). Some investigators in the field of insomnia consider the underestimation of sleep duration a trait feature of all insomniacs, in which extreme cases might exist (4), while others suggest that a more severe small subgroup of chronic insomnia patients that consistently underestimate their sleep duration deserves a separate diagnostic category (5). Indeed, this latter view is somewhat represented by the International Classification of Sleep Disorders (ICSD-2) (6), which allows the diagnosis of “paradoxical insomnia” (so-called “sleep state misperception”), whereas the DSM-IV does not include this diagnosis under “primary insomnia” (3) because of insufficient evidence to support its separate existence (4).
We have previously reported that objective sleep duration in insomnia in the general population may be a useful marker in phenotyping chronic insomnia. In the first phenotype, insomnia is associated with objective short sleep duration, activation of the hypothalamic-pituitary-adrenal (HPA) axis (7-11), hypertension (12), type 2 diabetes (13), and neurocognitive deficits (14). In the other phenotype, insomnia is associated with objective normal sleep duration, normal HPA axis activity, and lack of medical morbidity. Whether these two phenotypes differ in terms of estimation of sleep duration, i.e. sleep misperception, has not been examined. Furthermore, it is not known whether these two phenotypes are different in terms of their psychological profiles. The answer to these questions may have significant clinical implications, as these two phenotypes may benefit from different treatment approaches.
In this study we examined the role of objective sleep duration and psychological profiles in the association between chronic insomnia and sleep misperception in a large cross-sectional general random sample (The Penn State Cohort) using polysomnographic measures and psychological testing (i.e., Minnesota Multiphasic Personality Inventory-2).
Methods
Population
The data presented here were collected as part of a population-based cross-sectional study of sleep disorders, which used a two-phase protocol in order to recruit participants from various age groups (15-17). In the first phase of the study, a sample of adult men and women (age ≥ 20 years) was randomly selected from local telephone households in two counties of Central Pennsylvania (Dauphin and Lebanon) using the Mitofsky–Waksberg two-stage random digit dialing procedure (18). A within-household selection procedure described by Kish was used to select the specific man or woman to be interviewed (19). Telephone interviews were conducted with 4,364 age-eligible men and 12,219 age-eligible women residing in the sample households for a total sample of 16,583 with a response rate of 73.5% and 74.1%, respectively. The questionnaire employed in this interview included basic demographic and sleep information. In the second phase of this study, a subsample of 741 men and 1,000 women selected from those subjects previously interviewed by telephone were studied in our sleep laboratory. The response rate for this phase was 67.8% and 65.8% for men and women, respectively. Data were collected between January 1990 and March 1999. We contrasted those subjects who were recorded in the laboratory with those who were selected but not recorded in terms of age, BMI, and prevalence of sleep disorders. There were no significant differences between these two groups on any of these variables. After complete description of the study to the subjects, written informed consent was obtained. The study procedure was approved by the University’s Institutional Review Board.
Each subject selected for laboratory evaluation completed a comprehensive sleep history and physical examination. All subjects were evaluated for one night in the sleep laboratory in sound-attenuated, light- and temperature-controlled rooms. During this evaluation, each subject was continuously monitored for 8 hours (fixed-time period) using 16-channel polysomnography including electroencephalogram, electrooculogram, and electromyogram. Bedtimes were adjusted to conform to subjects’ usual bedtimes, and subjects were recorded between 10:00-11:00 p.m. and 6:00-7:00 a.m. In this random general sample of Central Pennsylvania the vast majority of individuals went to bed between 10:00 and 11:00 p.m., whereas only a small minority went to sleep outside of this time window and none for more than an hour. Thus, the maximum adjustment we had to do was of 1 hour. The sleep recordings were subsequently scored independently according to Rechtschaffen and Kales criteria (20). Percent of sleep time is total sleep time divided by recorded time in bed and multiplied by 100. Respiration was monitored throughout the night by use of thermocouples at the nose and mouth and thoracic strain gauges. All-night recordings of hemoglobin oxygen saturation (SaO2) were obtained with an oximeter attached to the finger.
Key measurements
As part of this protocol we also assessed for the presence of all sleep disorders, which was based on a standardized questionnaire completed by the subjects on the evening of their sleep laboratory visit. The characteristics and content of this questionnaire have been extensively presented elsewhere (12-17). The presence of “insomnia” was established by a complaint of insomnia with a duration of ≥ 1 year. “Poor sleep” was defined as a moderate to severe complaint of difficulty falling asleep, difficulty staying asleep, early final awakening, or unrefreshing sleep. “Normal sleeping” was defined as the absence of sleep complaints (i.e., insomnia, poor sleep, or excessive daytime sleepiness). The presence of excessive daytime sleepiness (EDS) was established based on a moderate or severe rating on either of the following two questions: “do you feel drowsy or sleepy most of the day but manage to stay awake?” and “do you have any irresistible sleep attacks during the day?”. “Subjective sleep duration” was ascertained by responses, recorded in hours, to the following questions: “how many hours do you usually sleep at night?” (i.e., habitual subjective sleep duration) and “How much sleep do you think you got last night?” (i.e., next-morning subjective sleep duration), with the latter being an item of a next-morning standardized questionnaire. The “discrepancy in sleep duration” was calculated by subtracting objective sleep duration (in hours) from subjective sleep duration (in hours), with positive scores indicating a tendency to overestimate and negative scores a tendency to underestimate sleep duration. A discrepancy index was obtained for each measure of habitual and next-morning subjective sleep duration. We defined an accurate estimation of sleep duration as a discrepancy score lying between -1.0h and +1.0h, overestimation as a discrepancy score ≥ +1.0h, and underestimation as a discrepancy score ≤ -1.0h. In the present study “sleep misperception” refers to the subjective underestimation of objective sleep, as it is commonly used in the existing literature (5).
The MMPI-2 was administered following the standardized rules and scored accordingly (21,22). The scores in 9 clinical scales [Hypochondriasis (1-HS), Depression (2-D), Hysteria (3-HY), Psychopathic Deviate (4-PD), Paranoia (6-PA), Psychasthenia (7-PT), Schizophrenia (8-SC), Hypomania (9-MA), Social Introversion (0-SI)] and 3 research scales [Depression (D), Anxiety (A), and Ego Strength (ES)] were studied. The Masculinity-Femininity (5-MF) scale was not studied because the sample was comprised of both men and women. The MMPI-2 composite total score was obtained from the average of the T scores of all MMPI-2 clinical scales. T scores with a mean of 50 and a standard deviation of 10 are generated for all scales with reference to standardized tables of the general population (21,22); scores ≥ 65 (1.5 SD above the mean) indicate a significant deviation from the original normal standardization pattern of responding and suggest an elevation at a clinically significant level. Furthermore, MMPI-2 profiles are usually examined in terms of the three most deviant scales, which together constitute the profile pattern or code-type (21,22). For each MMPI-2 profile, all clinical scales (except 5-MF and 0-SI) were ranked in order of T scores and the profile patterns were obtained. Thus, the scales that make up the profile pattern for a given individual were not necessarily in the pathological range. The analysis of code-types was performed following previous reports on clinical samples of chronic insomniacs (23,24).
To control for possible confounding variables influencing the relationships tested, we ascertained whether the respondent was currently being treated for physical (e.g., hypertension, diabetes, thyroidism, etc.) and/or mental (e.g., depression, alcohol use, drug use, etc.) health problems at the time of the interview. Depression, as a stand-alone variable, was considered to be present if the individual was currently treated for depression or had a history of suicidal thoughts or attempts. A composite variable for each general category of physical or mental health problems was calculated by indicating a positive response when at least one health problem within that category was present, as we have described elsewhere (14,17). Additional information obtained during the PSG included sleep apnea and periodic limb movement assessment. For the purpose of this study, sleep disordered breathing (SDB) was defined as an apnea or hypopnea index of 5 or greater (AHI ≥ 5). The condition of periodic limb movement (PLMS) was considered present when there were 5 or more movements per hour of sleep. A leg movement was scored when it lasted more than 0.5 seconds, less than 5.0 seconds, and in intervals of less than 90 seconds between movements (6). Body mass index was based on measured height (cm) and weight (kg) during the subjects’ sleep laboratory visit, and data are presented in terms of mean and percentage within each category.
Sample and subgroups
From the 1,741 individuals, a total of 1,300 validly completed the MMPI-2 and were classified as chronic insomniacs or controls. The total number of individuals in the “insomnia” group was 142. The total number of “normal sleepers” in the control group was 724. The remaining 434 subjects did not fulfill the criteria stated for each group, the vast majority of them (n = 383) because they reported “poor sleep” as defined above. From the objectively recorded percent of sleep time, we classified each participant into one of two groups of “normal sleep duration” (percent of sleep time ≥75%) and “short sleep duration” (percent of sleep time <75%). This cut-off point has been shown to be clinically meaningful (12-14,25) and corresponds to approximately 6 hours of objective sleep duration. We further classified the entire sample into 4 mutually exclusive subgroups according to insomnia status and objective sleep duration status: “insomniacs with normal sleep duration”, “insomniacs with short sleep duration”, “controls with normal sleep duration”, and “controls with short sleep duration”, respectively.
Statistical Analyses
The design of this study included oversampling of those at higher risk for SDB and women with markedly higher levels of BMI to increase the precision of the risk estimates. Because of this sampling strategy, numeric sampling weights were developed for the analysis so that the estimates could be inferred to the general population. Specifically, three weights were created for the men. First, in the telephone sample, 32 of the 963 clusters of phone numbers in the first stage were “exhausted” before the target sample size was obtained. A compensatory weight was computed which corrected for this problem. A second weight was computed because the within-household screening deliberately introduced unequal probabilities of selection across the three age groups in order to oversample the middle age group. The final weight for the men was computed to account for the oversampling of subjects for the sleep laboratory study (Phase II); those with larger counts of the four possible risk factors, i.e., snoring, daytime sleepiness, obesity, and hypertension, had substantially higher probability of being selected. For the women, the only weight required was to account for the oversampling of subjects for the sleep laboratory study. To eliminate any suggestion of possible sample bias, we calculated 32 unique weights for the women and 16 unique weights for the men corresponding to all possible combinations of the five risk factors for the women and four for the men. Any individual weight that had too small of a cell size was combined with adjacent cells so that less than 10% of the cells had a sample < 25 and no cell had a size less than 10. A comprehensive presentation of this sampling strategy has been presented elsewhere (12-17), including the use of the NHANES III laboratory data as the standard (26) to adjust both the men and women in terms of sociodemographics to be representative of the national population. Therefore, weighted analyses were performed to take into account the oversampling of those more-at-risk for SDB individuals in the second phase of the Penn State Cohort Study.
Two sets of multivariate analysis of covariance (MANCOVA) models were used. First, a full, 2 by 2 interaction MANCOVA model assessed the effects of insomnia (normal sleep vs. insomnia), objective sleep duration (< 6h vs. ≥ 6h), and their interaction on habitual subjective sleep duration, objective sleep duration, and discrepancy in habitual sleep duration. The second model assessed the effects of the 4 study subgroups (according to insomnia status and objective sleep duration status) on the same set of dependent variables. The same statistical approach was used to examine differences between subjective and objective sleep duration when we used the variable next-morning subjective sleep duration instead of habitual sleep duration. In a similar way, we analyzed all composite, clinical and research scales of the MMPI-2. In all models, we adjusted for major confounding factors expected to affect this relationship (i.e., age, race, gender, education, BMI, SDB, physical health, and mental health problems). Bonferroni corrections were applied to control for Type I errors when performing post-hoc planned comparisons in the second set of models. Multivariate logistic regression models were used to test significant differences between groups on nominal variables of subjective sleep duration, discrepancy in sleep duration, and MMPI-2 code-types, while controlling for all confounding factors.
Results
The demographic, clinical, and sleep characteristics of the entire sample and its groups, based on the presence of insomnia, and the two levels of objective sleep duration are presented in Table 1. As expected, insomniacs compared to controls reported subjectively less sleep duration (5.8 ± 1.40 vs. 6.9 ± 1.11; p < .001), more depression (38.1% vs. 7.7%; p < .001), and showed higher MMPI-2 total scores (55.6 ± 8.07 vs. 50.1 ± 6.33; p < .001).
Table 1.
Characteristics of study sample
| All | Insomnia | Objective sleep duration | Interactiona | Insomniaa | Objective sleep durationa | |||
|---|---|---|---|---|---|---|---|---|
| (N = 866) | No (n = 724) | Yes (n = 142) | < 6 h (n = 447) | ≥ 6 h (n = 419) | ||||
| Age, years | 50.0 (0.46) | 50.0 (0.51) | 50.5 (1.04) | 56.1 (0.63) | 44.9 (0.56) | .54 | .65 | <.001 |
| White, % | 90 | 93.1 | 91.8 | 93.8 | 92.0 | .47 | .48 | .10 |
| Male, % | 52.4 | 54.8 | 27.8 | 57.0 | 43.9 | .90 | <.001 | .06 |
| Education, years | 13.6 (0.09) | 13.6 (0.10) | 13.4 (0.19) | 13.2 (0.13) | 13.9 (0.12) | .07 | .65 | .02 |
| BMI, kg/m2 | 26.8 (0.17) | 26.6 (0.17) | 29.1 (0.63) | 27.0 (0.25) | 28.4 (0.25) | .004 | <.001 | .07 |
| BMI ≥ 30, % | 20.3 | 18.5 | 38.1 | 22.0 | 19.0 | .57 | <.001 | .97 |
| Physical health problems, % | 62.9 | 60.5 | 86.6 | 68.5 | 63.1 | .37 | <.001 | .75 |
| Mental health problems, % | 15.1 | 12.3 | 42.3 | 18.8 | 20.0 | .90 | <.001 | .24 |
| Depression symptoms, % | 10.6 | 7.7 | 38.1 | 14.8 | 15.4 | .69 | <.001 | .65 |
| SDB, % | 11.6 | 11.7 | 11.2 | 16.4 | 8.0 | .85 | .72 | .05 |
| Insomnia (%) | ||||||||
| No | 90.8 | --- | --- | 88.6 | 92.5 | --- | --- | --- |
| Yes | 9.2 | --- | --- | 11.4 | 7.5 | --- | --- | --- |
| Objective sleep duration (%) | ||||||||
| ≥ 6 hrs | 45.6 | 55.4 | 43.9 | --- | --- | --- | --- | --- |
| < 6 hrs | 54.4 | 44.6 | 56.1 | --- | --- | --- | --- | --- |
Where appropriate the standard error of the mean (SEM) is presented in parenthesis; SDB = apnea / hypopnea index ≥ 5; Discrepancy in total sleep time = subjective total sleep time − objective total sleep time;
P values from 2 by 2 ANOVA F test; All data are adjusted for sampling weight.
We first tested the effects of insomnia, objective sleep duration, and their interaction on measures of sleep estimation and MMPI-2 total score with a full, 2 by 2 MANCOVA while controlling for confounding factors. The interaction between chronic insomnia and objective sleep duration was not significant on habitual subjective sleep duration (p = .493), discrepancy in habitual sleep duration (p = .41), or MMPI-2 total score (p = .28). Consistently, insomnia showed significant effects on habitual subjective sleep duration (p < .001), discrepancy in habitual sleep duration (p < .001), and MMPI-2 total score (p < .001), but not on objective sleep duration (p = .38), whereas objective sleep duration showed significant effects on discrepancy in habitual sleep duration (p < .001), but not on habitual subjective sleep duration (p = .35) or MMPI-2 total score (p = .63).
Because of the observed additive, main effects of insomnia and objective sleep duration on subjective sleep duration and discrepancy in habitual sleep duration, we further examined differences between the 4 study subgroups in these variables, while controlling for potential confounders. As shown in Figure 1, insomniacs systematically rated their subjective relative to objective sleep duration shorter than controls, irrespective of their objective sleep duration. Also, individuals with normal sleep duration systematically rated their subjective relative to objective sleep duration as shorter than those with short sleep duration, irrespective of the presence of insomnia. The additive effects of insomnia and objective sleep duration resulted in a significant underestimation only in insomniacs with normal sleep duration. The mean scores, percentages, and post-hoc comparisons between subgroups in objective and subjective sleep duration and discrepancy in sleep duration are shown in Table 2. In general, both insomnia subgroups reported significantly less sleep duration compared to their respective controls. However and importantly, the group of insomniacs with normal sleep duration was the most likely group that significantly underestimated their sleep duration. Consistently, a significant interaction between insomnia and objective sleep duration was found on categorically defined sleep misperception (see Table 2).
Figure 1. Effects of insomnia and objective sleep duration on discrepancy in next-morning sleep duration.
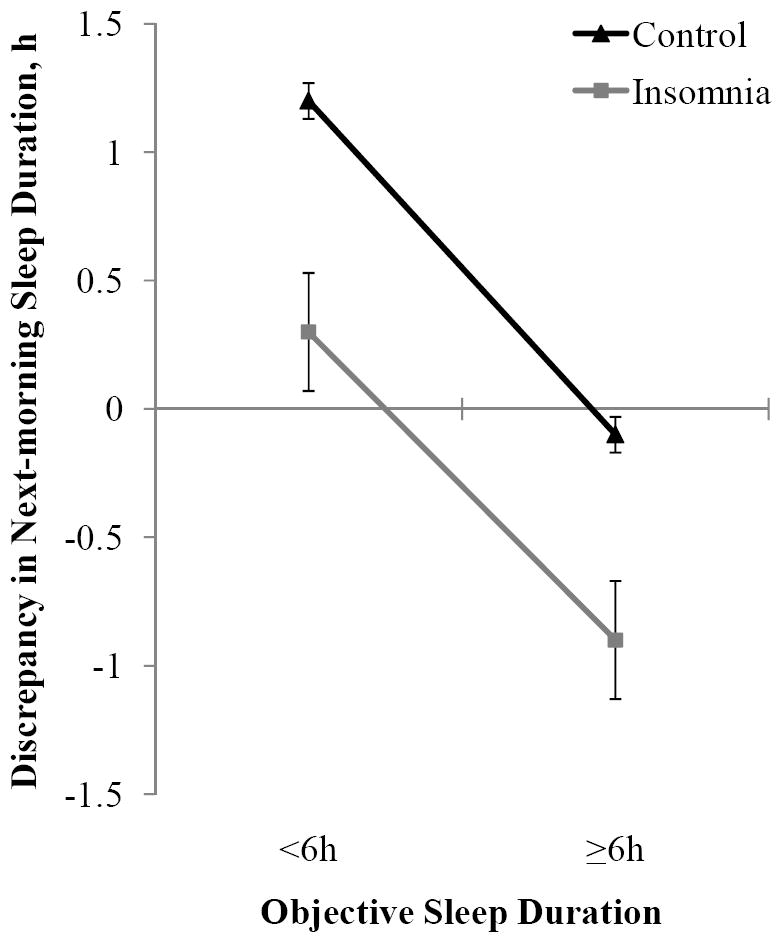
Line plot illustrating the absence of a significant interaction effect between insomnia and objective sleep duration on the discrepancy between objective sleep duration and next-morning subjective sleep duration. The additive effect of these two factors resulted in underestimation only in insomniacs with normal sleep duration. All data are adjusted for age, race, sex, education, BMI, SDB, physical health, and mental health. Error bars represent standard error of the mean (SEM).
Table 2.
Subjective and objective sleep estimation among study subgroups based on objective sleep duration
| 1. Control ≥ 6h (n = 359) | 2. Insomnia ≥ 6h (n = 60) | 3. Control < 6h (n = 365) | 4. Insomnia < 6h (n = 82) | Interactiona | Insomniaa | Objective sleep durationa | post-hoc b | |
|---|---|---|---|---|---|---|---|---|
| Percent of Sleep Time, % | 83.2 (0.52) | 82.5 (1.32) | 60.3 (0.52) | 58.6 (1.07) | .67 | .29 | <.001 | 1 > 3; 2 > 4 |
| Objective Sleep Duration, h | 6.6 (0.04) | 6.6 (0.10) | 4.8 (0.04) | 4.7 (0.09) | .93 | .38 | <.001 | 1 > 3; 2 > 4 |
| Habitual Sleep Duration, h | 6.8 (0.05) | 5.6 (0.18) | 6.9 (0.06) | 5.9 (0.15) | .49 | <.001 | .35 | 1 > 2; 3 > 4 |
| Habitual Sleep Duration, %c | ||||||||
| < 6:00 h | 12.8 | 48.3 | 13.0 | 37.8 | .77 | <.001 | .15 | 1 > 2; 3 > 4 |
| 6:00-6:59 h | 27.7 | 23.3 | 23.8 | 30.5 | ||||
| 7:00-8:00 h | 51.4 | 25.0 | 53.9 | 26.8 | ||||
| > 8:00 h | 8.1 | 3.3 | 9.2 | 4.9 | ||||
| Discrepancy in Habitual Sleep Duration, h | 0.2 (0.08) | -1.0 (0.20) | 2.1 (0.08) | 1.2 (0.17) | .41 | <.001 | <.001 | 1 > 2; 1 < 3; 2 < 4; 3 > 4 |
| Discrepancy in Habitual Sleep Duration, %a | ||||||||
| Overestimation | 22.6 | 10.7 | 81.8 | 57.3 | .04 | <.001 | <.001 | 1 < 2; 1 > 3; 2 > 4; 3 > 4 |
| Accuracy | 59.5 | 33.9 | 16.0 | 32.9 | ||||
| Underestimation | 17.9 | 55.4 | 2.2 | 9.8 | ||||
| Next-morning Sleep Duration, h | 6.5 (0.07) | 5.7 (0.27) | 6.0 (0.07) | 5.0 (0.23) | .19 | <.001 | <.001 | 1 > 2; 1 > 3; 2 > 4; 3 > 4 |
| Next-morning Sleep Duration, %c | ||||||||
| < 6:00 h | 14.6 | 34.8 | 37.7 | 76.3 | .57 | <.001 | <.001 | 1 > 2; 1 > 3; 3 > 4 |
| 6:00-6:59 h | 31.6 | 43.5 | 32.5 | 18.4 | ||||
| 7:00-8:00 h | 53.5 | 21.7 | 26.4 | 2.6 | ||||
| > 8:00 h | 0.3 | 0.0 | 3.4 | 2.6 | ||||
| Discrepancy in Next-morning Sleep Duration, h | -0.1 (0.07) | -0.9 (0.25) | 1.2 (0.07) | 0.3 (0.21) | .51 | <.001 | <.001 | 1 > 2; 1 < 3; 2 < 4; 3 > 4 |
| Discrepancy in Next-morning Sleep Duration, %a | ||||||||
| Overestimation | 7.1 | 8.3 | 47.9 | 35.6 | .045 | <.001 | <.001 | 1 < 2; 1 > 3; 2 > 4; 3 > 4 |
| Accuracy | 75.3 | 50.0 | 43.3 | 44.1 | ||||
| Underestimation | 17.6 | 41.7 | 8.8 | 15.3 | ||||
Where appropriate the standard error of the mean (SEM) is presented in parenthesis;
P values from 2 by 2 MANCOVA F test;
p < .05, post-hoc Bonferroni test for continuous variables and multivariate logistic regression for nominal variables, while adjusting for age, race, sex, education, BMI, SDB, physical health, and mental health.
% refers to the proportion of participants in the respective subgroups; Discrepancy in habitual /next-morning sleep duration = habitual/next-morning sleep duration − objective sleep duration; Overestimation = discrepancy in habitual /next-morning sleep duration ≥ +1.0h; Accuracy = discrepancy in habitual /next-morning sleep duration between -1.0h and +1.0h; Underestimation = discrepancy in habitual /next-morning sleep duration ≤ -1.0h.
Because habitual sleep duration might not fully reflect the subjectively experienced sleep duration of the recorded night, we performed the above mentioned analysis using next-morning subjective sleep duration. When we examined the effects of insomnia, objective sleep duration and their interaction on discrepancy in next-morning sleep duration while controlling for potential confounders, the results showed that there were significant additive, main effects in the absence of a significant interaction (see Table 2). The group that consistently and significantly underestimated their sleep duration was insomniacs with normal sleep duration, independent of whether habitual or next-morning sleep duration was used. Indeed, while 41.7% of insomniacs with normal sleep duration underestimated their sleep duration by 1 hour or more, only 17.6% of controls with normal sleep duration, 15.3% of insomniacs with short sleep duration, and 8.8% of controls with short sleep duration did.
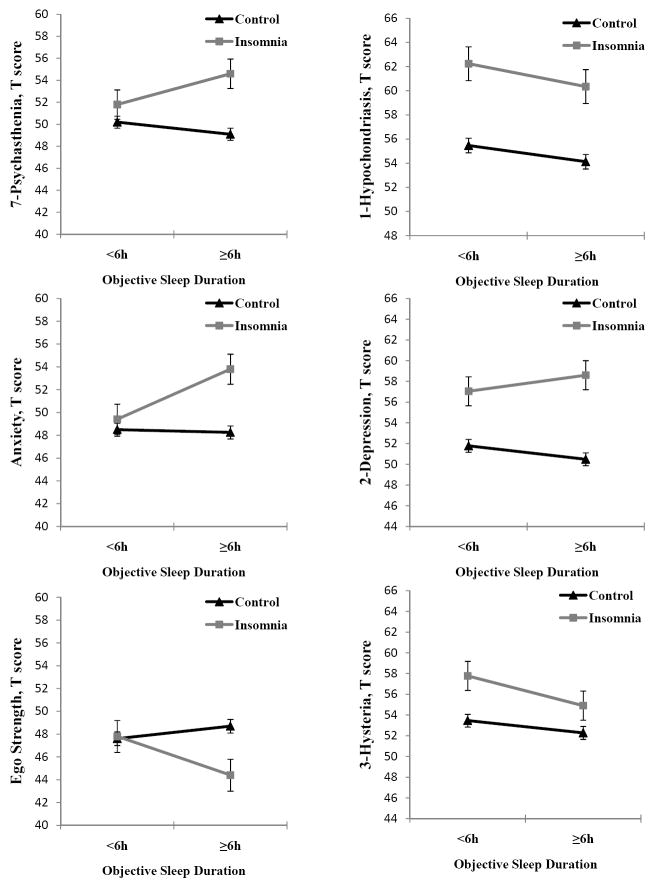
We further examined the effects of insomnia, objective sleep duration, and their interaction on MMPI-2 clinical and research scales, while controlling for potential confounders. Figure 2 shows that the interaction between chronic insomnia and objective sleep duration was significant on 7-psychasthenia (p = .03), anxiety (p = .046), and ego strength (p = .01). Insomnia showed significant main effects on all MMPI-2 clinical and research scales, except 9-hypomania, whereas objective sleep duration did not show significant main effects on any MMPI-2 scale. Table 3 shows the mean scores and post-hoc comparisons between the 4 subgroups. In general, insomniacs with normal sleep duration had significantly higher mean scores on nine clinical and research scales, whereas insomniacs with short sleep duration showed significantly elevated scores on only four scales compared to their respective controls. While both insomnia subgroups showed high depressive (2-D, D) personality traits, insomniacs with normal sleep duration also showed high anxious-ruminative (7-PT, A) personality traits, and poor resources for coping with stress (ES). Insomniacs with short sleep duration showed significantly higher scores in those MMPI-2 scales indicating somatic complaints (1-HS, 3-HY), as compared to their respective controls. The comparison within the two insomnia subgroups showed significant differences in scales measuring anxiety (A) and coping resources (ES). The two control subgroups did not differ in terms of MMPI-2 mean scores. Finally, these results were further replicated when differences on clinically significant elevations (T score ≥ 65) were examined with multivariate regression models between insomniacs and controls and within insomniacs’ and controls’ subgroups (data not shown).
Figure 2. Effects of insomnia and objective sleep duration on selected MMPI-2 clinical and research scales.
Line plot illustrating significant interaction effects between insomnia and objective sleep duration on 7-psychasthenia, anxiety and ego strength scales (left panel), and the absence of interaction effects on the other 3 most elevated clinical scales, i.e., 1-hypochondriasis, 2-depression, and 3-hysteria, (right panel). All data are adjusted for age, race, sex, education, BMI, SDB, physical health, and mental health. Error bars represent standard error of the mean (SEM).
Table 3.
MMPI-2 scores of study subgroups based on objective sleep duration
| 1. Control ≥ 6 h (n = 359) | 2. Insomnia ≥ 6 h (n = 60) | 3. Control < 6 h (n = 365) | 4. Insomnia < 6 h (n = 82) | Interactiona | Insomniaa | Objective sleep durationa | post-hocb | |
|---|---|---|---|---|---|---|---|---|
| Composite scores | ||||||||
| Total score | 50.00 (0.35) | 54.34 (0.86) | 50.74 (0.35) | 53.92 (0.71) | .86 | <.001 | .48 | 1 < 2; 3 < 4 |
| Number of elevations | 0.78 (0.08) | 1.64 (0.19) | 0.85 (0.08) | 1.38 (0.16) | .68 | <.001 | .98 | 1 < 2; 3 < 4 |
| Clinical scales | ||||||||
| 1-Hypochondriasis | 54.13 (0.60) | 60.35 (1.48) | 55.47 (0.60) | 62.24 (1.23) | .78 | <.001 | .11 | 1 < 2; 3 < 4 |
| 2-Depression | 50.49 (0.62) | 58.60 (1.53) | 51.79 (0.62) | 57.05 (1.27) | .59 | <.001 | .97 | 1 < 2; 3 < 4 |
| 3-Hysteria | 52.28 (0.62) | 54.91 (1.53) | 53.46 (0.62) | 57.78 (1.27) | .81 | <.001 | .13 | 1 < 2; 3 < 4 |
| 4-Psychopathic Deviate | 49.15 (0.54) | 54.05 (1.35) | 51.03 (0.54) | 52.62 (1.12) | .55 | .001 | .75 | 1 < 2 |
| 6-Paranoia | 49.04 (0.57) | 52.96 (1.41) | 49.62 (0.57) | 51.63 (1.17) | .65 | .003 | .90 | 1 < 2 |
| 7-Psychasthenia | 49.12 (0.55) | 54.55 (1.37) | 50.19 (0.55) | 51.78 (1.14) | .03 | <.001 | .83 | 1 < 2 |
| 8-Schizophrenia | 47.94 (0.55) | 51.53 (1.37) | 48.66 (0.55) | 50.76 (1.14) | .79 | .01 | .66 | --- |
| 9-Hypomania | 48.14 (0.54) | 48.51 (1.33) | 47.24 (0.54) | 50.03 (1.11) | .07 | .114 | .46 | --- |
| 0-Social Introversion | 49.68 (0.54) | 53.58 (1.35) | 49.24 (0.54) | 51.35 (1.12) | .30 | .003 | .40 | 1 < 2 |
| Research scales | ||||||||
| Depression | 48.16 (0.57) | 53.84 (1.40) | 47.99 (0.61) | 52.64 (1.32) | .17 | <.001 | .87 | 1 < 2; 3 < 4 |
| Anxiety | 48.25 (0.55) | 53.80 (1.35) | 48.50 (0.59) | 49.41 (1.28) | . 046 | .01 | .78 | 1 < 2; 2 > 4 |
| Ego Strength | 48.68 (0.58) | 44.38 (1.42) | 47.61 (0.62) | 47.80 (1.34) | .01 | .001 | .92 | 1 < 2; 2< 4 |
Data are in mean (SEM).
P values from 2 by 2 MANCOVA F test;
p < .05, post-hoc Bonferroni test while adjusting for age, race, sex, education, BMI, SDB, physical health, and mental health.
Furthermore, a MMPI-2 code-type analysis showed that 73.2% of our population-based insomniacs produced 1 of the 7 code-types reported to be most common in clinical insomniacs (23,24) (Table 4). Significant differences were found between insomniacs with normal and short sleep duration in the frequency of 278 (ruminative-depression), 237/273 (anxious-depression), and 127/271 (apprehensive/somatically focused-depression) code-types (24). Thus, the code-types of insomniacs with short sleep duration showed somatic concerns, depressive mood, and poor health status, whereas the code-types of insomniacs with normal sleep duration reflected depressive mood, introversion, anxiety, and rumination.
Table 4.
Percentage of MMPI-2 code-types among insomnia subgroups based on objective sleep duration
| Insomnia ≥ 6h (n = 60) | Insomnia < 6h (n = 82) | Pa | |
|---|---|---|---|
| 278 | 16.7 | 3.7 | .02 |
| 231/312 | 18.3 | 22.0 | --- |
| 237/273 | 15.0 | 4.9 | .04 |
| 127/271 | 6.7 | 24.4 | .02 |
| 234/432 | 6.7 | 7.3 | .71 |
| 247 | 5.0 | 4.9 | .98 |
| 248 | 6.7 | 4.9 | .83 |
Multivariate logistic regression for nominal variables while adjusting for age, race, sex, education, BMI, SDB, physical health, and mental health. The 231/312 code-type was used as the reference category.
Given the significant associations between objective sleep duration, sleep misperception, and psychological profiles we examined using discriminant analysis whether the differences between the two insomnia subgroups in sleep misperception and MMPI-2 scales could be explained by a subset of these variables. The analysis provided a canonical discriminant function that significantly distinguished insomniacs with short sleep duration from insomniacs with normal sleep duration (Wilks’ λ = .60; χ2 = 64.65; p < .001), correctly classifying 81.1% of the cases. The variables that significantly distinguished the two subgroups were: discrepancy in habitual sleep duration, 7-PT, anxiety, ego strength, and 4-PD. Table 5 shows the pooled correlations of the final discriminant function.
Table 5.
Discriminant analysis of insomnia subgroups based on objective sleep duration
| Discriminant Function a | |||
|---|---|---|---|
| F | Coefficients b | Structure Matrix c | |
| Discrepancy in Habitual Sleep Duration | 71.122 | -.946 | -.892* |
| 1-Hypochondriasis | .059 | -.078 | -.026 |
| 2-Depression | 1.151 | .011 | .113 |
| 3-Hysteria | .119 | -.363 | -.036 |
| 4-Psychopathic Deviate | 2.691 | .122 | .173* |
| 6-Paranoia | .613 | .016 | .083 |
| 7-Psychasthenia | 6.412 | .273 | .268* |
| 8-Schizophrenia | 1.571 | .018 | .133 |
| 9-Hypomania | .108 | .024 | .035 |
| 0-Social Introversion | 2.187 | .028 | .156 |
| Depression | 1.053 | .522 | .108 |
| Anxiety | 4.243 | .286 | .218* |
| Ego Strength | 3.627 | -.348 | -.201* |
Canonical correlation = .64; 81.1% of original grouped cases correctly classified.
Standardized canonical discriminant function coefficients.
Pooled within-groups correlations between discriminating variables and standardized canonical discriminant function.
P < .05
As objective sleep data is not always available for the clinician, we further performed a second discriminant analysis in order to examine whether sleep misperception could be predicted from non-PSG information. This analysis provided a canonical discriminant function that significantly distinguished insomniacs with sleep misperception (Wilks’ λ = .58; χ2 = 67.47; p < .001), correctly classifying 84.1% of the cases. The variables that significantly discriminated insomniacs with sleep misperception from those without were: habitual sleep duration (r = -.851), 7-PT (r = .314), 8-SC (r =.205), anxiety (r = .180), and ego strength (r = -.177).
These results remained significant and the mean scores and percentages very similar to those reported herein even after adjusting for the number of wakes, number of sleep stage changes, and percent stage 1 sleep, or when those subjects with PLMS or SDB were excluded from the analyses.
Discussion
This large, population-based study demonstrates that sleep misperception is prevalent in chronic insomniacs with objectively measured normal sleep duration but not in those with short sleep duration. Furthermore, sleep misperception is associated with depressive, anxious-ruminative personality traits and poor coping resources. These findings are independent of other factors frequently associated with insomnia or objective sleep duration, such as gender, age, race, education, obesity, SDB, hypertension, or depression.
Early studies of sleep misperception suggested that underestimation of sleep duration was a generic trait in insomnia (27-29). This view is reflected in the DSM-IV text of the diagnosis of “primary insomnia” (3). In contrast, the ICSD-2 states that “paradoxical insomnia” (i.e., “sleep state misperception” or “subjective insomnia”) is a rare condition accounting for fewer than 5% of all insomnia patients (6). In the present study, only insomniacs with normal sleep duration showed a significant underestimation of sleep duration. A recent review by Edinger and Krystal (5) showed that the relative prevalence of “subjective insomnia/sleep state misperception” in clinical and research samples varies between 9.2% and 50%. The present study suggests that insomnia with normal sleep duration, which accounts for about fifty percent of all chronic insomniacs in the general population, is strongly associated with sleep misperception.
The factors implicated in sleep misperception among insomniacs remain unknown. For example, several studies have failed to show an altered perception of time in insomniacs (30-34), suggesting that factors other than deficits in perceptual processing of time might be involved in sleep misperception. Personality traits, anxiety, rumination, pre-sleep worry (23,24,31,34-43), and their physiological correlates (40,44-50), have also been suggested to play a role in the underestimation of sleep duration in insomnia. The present study is the first to show that in a general population sample of chronic insomniacs sleep misperception is associated with MMPI-2 personality profiles characterized by “depressive mood, rumination, anxiety, intrusive thoughts, and poor resources for coping with stress” (22). These personality characteristics in a discriminant analysis differentiated with a sensitivity of approximately 84% the insomniacs with sleep misperception vs. insomniacs without.
Insomniacs with short sleep duration, similarly to their respective controls, significantly overestimated their sleep duration, a finding that is consistent with previous reports where insomniacs with objectively measured short sleep displayed overestimates of sleep duration (35,45,51-53). This group of insomniacs was associated with MMPI-2 profiles that reflect “depressive mood, fatigue, concerns about health and physical functioning, somatically focused anxiety, and poor health status” (22), which is a psychological profile typical of outpatients with a medical disorder (23). Previous reports have shown that insomnia with short sleep duration is associated with hypercortisolemia (7-11), increased catecholaminergic activity (54), sympathetic activity (55-57), and medical morbidity (12-14). It is very likely that the “somatic preoccupation” of these insomniacs is not “hypochondriac” in its nature, but reflects true physiological and physical changes as a result of chronic activation of the stress system. Alternatively, the activation of the stress system can be the result of physical and/or physiological sleep changes in insomnia subjects.
The distinct psychological profiles between insomnia subtypes based on objective sleep duration are consistent with previous studies in clinical samples which showed that “subjective insomniacs” have higher neuroticism (58), higher scores on psychasthenia (7-PT) and schizophrenia (8-SC) scales (42,44), higher anxiety, lower mood, more dysfunctional sleep-related cognitions (59), and fewer somatic complaints (11) when compared to “objective insomniacs”. Moreover, a previous cluster analytic study (38) found 2 insomnia subtypes based only on MMPI scores: an “anxious, ruminative, cognitively disorganized” group with a predominant 273/237 code-type, fewer somatic concerns (lower hypochondriasis -1-HS- scores), more worry and intrusive thoughts, and greater concern about not sleeping, and a 231/312 code-type group with “less anxiety and cognitive turmoil”. In the present study, the differences in anxiety and ego strength between the two insomnia subgroups were modest in terms of absolute values. Nevertheless, the present study suggests that objective sleep duration is a useful marker in separating insomniacs with and without sleep misperception and their associated psychological characteristics.
In the present population-based study, controls overestimated their sleep duration. This finding is consistent with epidemiological studies showing that individuals in the general population typically overestimate sleep time (60-62). The factors underlying the marked overestimation of sleep duration in controls with short sleep duration are not known or apparent and further investigation is needed.
The objective sleep duration in this study was based on one night of polysomnography, which may not be representative of the subjects’ typical objective sleep duration. However, in our previous studies, the association between objective short sleep duration and hyperactivity of the stress system in insomniacs was based on a 4 consecutive night sleep laboratory protocol, which should better represent the typical sleep profile of the subjects (7,8). The consistency of the findings on the role of objective short sleep duration in predicting insomnia severity between the physiological studies with multiple night recordings (7,8) and our previous epidemiological studies based on a single night recording (12-14,25) increases our confidence about the replicability and generalizability of the present findings. In large epidemiologic studies the average objective sleep duration is about 6 hours, which is independent of whether sleep is recorded at home with polysomnography, i.e., Sleep Heart Health Study (61), or for 3 consecutive nights with actigraphy, i.e., CARDIA study (60), or in the sleep laboratory, i.e., Penn State Cohort (12-14,25). Furthermore, the SHHS and CARDIA studies reported that in general population samples objective sleep duration is usually shorter by 1 hour (60,61) and by 18 minutes (61) than habitual and next-morning subjective sleep duration, respectively, which is very consistent with our findings, i.e., in the total sample mean discrepancy in habitual sleep duration was of 1.0 hours and next-morning sleep duration of 20 minutes. Thus, the consistency among these three large epidemiological studies in terms of objective sleep duration, subjective sleep duration and their discrepancy reinforces our belief that the inherent limitations of 1-night recording in large samples do not compromise the validity of the findings. In support of this view, in a recent study of clinical insomniacs based on 2-nights recording the frequency of insomniacs with sleep misperception was very similar to ours (45). From our study, we cannot exclude the possibility that the subjective-objective discrepancy may in part reflect the response of these individuals to sleeping in an unfamiliar environment (i.e., the sleep lab vs. their home environment). Future studies should explore the association between insomnia, objective sleep duration and sleep misperception using multiple night recordings obtained in the sleep laboratory or with easier-to-use methods, i.e., actigraphy.
The field of sleep disorders medicine has attempted to define subgroups within insomnia based on etiology (i.e., primary vs. secondary), age of onset (i.e., childhood vs. adult), and objective sleep findings (6). Although for years sleep specialists suggested that the sleep lab was of no use in the evaluation of insomnia (1,6), the previously published data on the association of insomnia combined with objective short sleep duration with the stress system (7-11,54), the autonomic system (44,55,56), and with medical morbidity (12-14), have led us to suggest two phenotypes of chronic insomnia. The first phenotype is associated with physiological hyperarousal, i.e., short sleep duration, activation of the stress system, and significant medical sequelae, e.g., hypertension, diabetes, neurocognitive deficits and increased mortality. The second phenotype is not associated with physiological hyperarousal, i.e., normal sleep duration, normal activity of the stress system, and lack of significant medical sequelae. The present study expands on the differential characteristics of these two phenotypes. The first one is associated with a psychological profile typical of medical outpatients, whereas the second one is associated with sleep misperception and an anxious-ruminative, poor coping skills profile.
Our findings on these proposed phenotypes may have a significant impact on how we diagnose and treat insomnia. Currently, the diagnosis of insomnia is based on subjective complaints. The introduction of objective measures of sleep in the evaluation of insomnia may be of relevance for the practicing physician in terms of prioritizing intervention based on severity. Furthermore, these 2 phenotypes may respond differentially to treatment approaches. The first phenotype may respond better to treatments that primarily aim at decreasing physiological hyperarousal (e.g., cortisol) and increasing sleep duration, such as medication or other biological treatments (9), whereas the second phenotype may respond better to treatments that primarily aim at decreasing cognitive-emotional hyperarousal (e.g., rumination) and altering sleep misperception (63), such as sleep scheduling, behavioral experiments, cognitive restructuring, or emotion regulation techniques.
In conclusion, the present study delineates even further these two chronic insomnia phenotypes based on objective sleep duration and provides further support for their potential clinical validity and usefulness. The diagnostic validity and clinical utility of this phenotyping should be tested in prospective studies and/or with treatment interventions in chronic insomniacs.
Acknowledgments
This research was in part funded by the National Institutes of Health grants RO1 51931 (E.O.B.), RO1 40916 (E.O.B.) and RO1 64415 (A.N.V.). The work was performed at the Sleep Research and Treatment Center at the Penn State University Milton Hershey Hospital, and the staff is especially commended for their efforts.
Acronyms
- AHI
apnea or hypopnea index
- BMI
body mass index
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders
- EDS
excessive daytime sleepiness
- HPA axis
hypothalamic-pituitary-adrenal axis
- ICSD-2
International Classification of Sleep Disorders
- MANCOVA
multivariate analysis of covariance
- MMPI-2
Minnesota Multiphasic Personality Inventory-2
- PLMS
periodic limb movements
- SDB
sleep disordered breathing
Footnotes
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.National Institutes of Health. National Institutes of Health State of the Science Conference statement on Manifestations and Management of Chronic Insomnia in Adults. Sleep. 2005;28:1049–57. doi: 10.1093/sleep/28.9.1049. [DOI] [PubMed] [Google Scholar]
- 2.Basta M, Chrousos GP, Vela-Bueno A, Vgontzas AN. Chronic insomnia and the stress system. Sleep Med Clin. 2007;2:279–91. doi: 10.1016/j.jsmc.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington: American Psychiatric Press; 2000. Text revision. [Google Scholar]
- 4.Reynolds CF, 3rd, Kupfer DJ, Buysse DJ, Coble PA, Yeager A. Subtyping DSM-III-R primary insomnia: a literature review by the DSM-IV Work Group on Sleep Disorders. Am J Psychiatry. 1991;148:432–38. doi: 10.1176/ajp.148.4.432. [DOI] [PubMed] [Google Scholar]
- 5.Edinger JD, Krystal AD. Subtyping primary insomnia: is sleep state misperception a distinct clinical entity? Sleep Med Rev. 2003;7:203–14. doi: 10.1053/smrv.2002.0253. [DOI] [PubMed] [Google Scholar]
- 6.American Academy of Sleep Medicine. The International Classification of Sleep Disorders (ICSD-2): diagnostic and coding manual. 2. Westchester: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 7.Vgontzas AN, Tsigos C, Bixler EO, Stratakis CA, Zachman K, Kales A, Vela-Bueno A, Chrousos GP. Chronic insomnia and activity of the stress system: a preliminary study. J Psychosom Res. 1998;45:21–31. doi: 10.1016/s0022-3999(97)00302-4. [DOI] [PubMed] [Google Scholar]
- 8.Vgontzas AN, Bixler EO, Lin HM, Prolo P, Mastorakos G, Vela-Bueno A, Kales A, Chrousos GP. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab. 2001;86:3787–94. doi: 10.1210/jcem.86.8.7778. [DOI] [PubMed] [Google Scholar]
- 9.Rodenbeck A, Cohrs S, Jordan W, Huether G, Rüther E, Hajak G. The sleep-improving effects of doxepin are paralleled by a normalized plasma cortisol secretion in primary insomnia. Psychopharmacology. 2003;170:423–28. doi: 10.1007/s00213-003-1565-0. [DOI] [PubMed] [Google Scholar]
- 10.Backhaus J, Junghanns K, Born J, Hohaus K, Faasch F, Hohagen F. Impaired declarative memory consolidation during sleep in patients with primary insomnia: influence of sleep architecture and nocturnal cortisol release. Biol Psychiatry. 2006;60:1324–30. doi: 10.1016/j.biopsych.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 11.Shaver JL, Johnston SK, Lentz MJ, Landis CA. Stress exposure, psychological distress, and physiological stress activation in midlife women with insomnia. Psychosom Med. 2002;64:793–802. doi: 10.1097/01.psy.0000024235.11538.9a. [DOI] [PubMed] [Google Scholar]
- 12.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32:491–97. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: a population-based study. Diabetes Care. 2009;32:1–6. doi: 10.2337/dc09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez-Mendoza J, Calhoun S, Bixler EO, Pejovic S, Karataraki M, Liao D, Vela-Bueno A, Ramos-Platon MJ, Sauder KA, Vgontzas AN. Insomnia with objective short sleep duration is associated with deficits in neuropsychological performance: a general population study. Sleep. 2010;33:459–65. doi: 10.1093/sleep/33.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–48. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 16.Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, Kales A. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163:608–13. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 17.Bixler EO, Vgontzas AN, Lin HM, Vela-Bueno A, Kales A. Insomnia in central Pennsylvania. J Psychosom Res. 2002;53:589–92. doi: 10.1016/s0022-3999(02)00450-6. [DOI] [PubMed] [Google Scholar]
- 18.Waksberg J. Sampling methods for random digit dialing. J Am Stat Assoc. 1978;73:40–6. [Google Scholar]
- 19.Kish L. Survey sampling. New York: John Wiley & Sons, Inc; 1965. [Google Scholar]
- 20.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Bethesda: National Institutes of Health; 1968. [Google Scholar]
- 21.Butcher JN, Graham JR, Ben-Porath YS, Tellegen A, Dahkstrom WG. MMPI-2: Manual for administration, scoring and interpretation. Minneapolis: University of Minnesota Press; 2001. Revised ed. [Google Scholar]
- 22.Nichols DS. Essentials of MMPI-2 assessment. New York: John Wiley & Sons, Inc; 2001. [Google Scholar]
- 23.Kales A, Caldwell AB, Preston TA, Healey S, Kales JD. Personality patterns in insomnia: theoretical implications. Arch Gen Psychiatry. 1976;33:1128–34. doi: 10.1001/archpsyc.1976.01770090118013. [DOI] [PubMed] [Google Scholar]
- 24.Kales A, Caldwell AB, Soldatos CR, Bixler EO, Kales JD. Biopsychobehavioral correlates of insomnia. II. Pattern specificity and consistency with the Minnesota Multiphasic Personality Inventory. Psychosom Med. 1983;45:341–56. doi: 10.1097/00006842-198308000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Basta M, Fernández-Mendoza J, Bixler EO. Insomnia with short sleep duration and mortality: the Penn State Cohort. Sleep. 2010 doi: 10.1093/sleep/33.9.1159. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.U.S. Department of Health and Human Services (DHHS), National Center for Health Statistics. NHANES III laboratory data file. Hyattsville: Centers for Disease Control and Prevention; 1996. Third National Health and Nutrition Examination Survey 1988-1994. [Google Scholar]
- 27.Carskadon MA, Dement WC, Mitler MM, Guilleminault C, Zarcone VP, Spiegel R. Self-reports versus sleep laboratory findings in 122 drug-free subjects with complaints of chronic insomnia. Am J Psychiatry. 1976;133:1382–88. doi: 10.1176/ajp.133.12.1382. [DOI] [PubMed] [Google Scholar]
- 28.Frankel BL, Coursey RD, Buchbinder R, Snyder F. Recorded and reported sleep in chronic primary insomnia. Arch Gen Psychiatry. 1976;33:615–23. doi: 10.1001/archpsyc.1976.01770050067011. [DOI] [PubMed] [Google Scholar]
- 29.Salin-Pascual RJ, Roehrs TA, Merlotti LA, Zorick F, Roth T. Long-term study of the sleep of insomnia patients with sleep state misperception and other insomnia patients. Am J Psychiatry. 1992;149:904–08. doi: 10.1176/ajp.149.7.904. [DOI] [PubMed] [Google Scholar]
- 30.Mercer JD, Bootzin RR, Lack LC. Insomniacs’ perception of wake instead of sleep. Sleep. 2002;25:564–71. [PubMed] [Google Scholar]
- 31.Tang NK, Harvey AG. Time estimation ability and distorted perception of sleep in insomnia. Behav Sleep Med. 2005;3:134–50. doi: 10.1207/s15402010bsm0303_2. [DOI] [PubMed] [Google Scholar]
- 32.Fichten CS, Creti L, Amsel R, Bailes S, Libman E. Time estimation in good and poor sleepers. J Behav Med. 2005;28:537–53. doi: 10.1007/s10865-005-9021-8. [DOI] [PubMed] [Google Scholar]
- 33.Rioux I, Tremblay S, Bastien CH. Time estimation in chronic insomnia sufferers. Sleep. 2006;29:486–93. doi: 10.1093/sleep/29.4.486. [DOI] [PubMed] [Google Scholar]
- 34.Tang NK, Anne Schmidt D, Harvey AG. Sleeping with the enemy: clock monitoring in the maintenance of insomnia. J Behav Ther Exp Psychiatry. 2007;38:40–55. doi: 10.1016/j.jbtep.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Means MK, Edinger JD, Glenn DM, Fins AI. Accuracy of sleep perceptions among insomnia sufferers and normal sleepers. Sleep Med. 2003;4:285–96. doi: 10.1016/s1389-9457(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 36.Borkovec TD, Grayson JB, O’Brien GT, Weerts TC. Relaxation treatment of pseudoinsomnia and idiopathic insomnia: an electroencephalographic evaluation. J Appl Behav Anal. 1979;12:37–54. doi: 10.1901/jaba.1979.12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levin D, Bertelson AD, Lacks P. MMPI differences among mild and severe insomniacs and good sleepers. J Pers Assess. 1984;48:126–29. doi: 10.1207/s15327752jpa4802_3. [DOI] [PubMed] [Google Scholar]
- 38.Edinger JD, Stout AL, Hoelscher TJ. Cluster analysis of insomniacs’ MMPI profiles: relation of subtypes to sleep history and treatment outcome. Psychosom Med. 1988;50:77–87. doi: 10.1097/00006842-198801000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Kalogjera-Sackellares D, Cartwright RD. Comparison of MMPI profiles in medically and psychologically based insomnias. Psychiatry Res. 1997;70:49–56. doi: 10.1016/s0165-1781(97)03078-3. [DOI] [PubMed] [Google Scholar]
- 40.Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res. 1997;6:179–88. doi: 10.1046/j.1365-2869.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- 41.Aikens JE, Vanable PA, Tadimeti L, Caruana-Montaldo B, Mendelson WB. Differential rates of psychopathology symptoms in periodic limb movement disorder, obstructive sleep apnea, psychophysiological insomnia, and insomnia with psychiatric disorder. Sleep. 1999;22:775–80. doi: 10.1093/sleep/22.6.775. [DOI] [PubMed] [Google Scholar]
- 42.Vanable PA, Aikens JE, Tadimeti L, Caruana-Montaldo B, Mendelson WB. Sleep latency and duration estimates among sleep disorder patients: variability as a function of sleep disorder diagnosis, sleep history, and psychological characteristics. Sleep. 2000;23:71–9. [PubMed] [Google Scholar]
- 43.Tsushima WT, Ingolfsdottir E. MMPI-2 scores of patients with insomnia. Psychol Rep. 2004;94:267–72. doi: 10.2466/pr0.94.1.267-272. [DOI] [PubMed] [Google Scholar]
- 44.Bonnet MH, Arand DL. Physiological activation in patients with sleep state misperception. Psychosom Med. 1997;59:533–40. doi: 10.1097/00006842-199709000-00011. [DOI] [PubMed] [Google Scholar]
- 45.Feige B, Al-Shajlawi A, Nissen C, Voderholzer U, Hornyak M, Spiegelhalder K, Kloepfer C, Perlis M, Riemann D. Does REM sleep contribute to subjective wake time in primary insomnia? A comparison of polysomnographic and subjective sleep in 100 patients. J Sleep Res. 2008;17:180–90. doi: 10.1111/j.1365-2869.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- 46.Schneider-Helmert D, Kumar A. Sleep, its subjective perception, and daytime performance in insomniacs with a pattern of alpha sleep. Biol Psychiatry. 1995;37:99–105. doi: 10.1016/0006-3223(94)00162-V. [DOI] [PubMed] [Google Scholar]
- 47.Smith S, Trinder J. The effect of arousals during sleep onset on estimates of sleep onset latency. J Sleep Res. 2000;9:129–35. doi: 10.1046/j.1365-2869.2000.00194.x. [DOI] [PubMed] [Google Scholar]
- 48.Krystal AD, Edinger JD, Wohlgemuth WK, Marsh GR. NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep. 2002;25:630–40. [PubMed] [Google Scholar]
- 49.Marchetti LM, Biello SM, Broomfield NM, Macmahon KM, Espie CA. Who is preoccupied with sleep? A comparison of attention bias in people with psychophysiological insomnia, delayed sleep phase syndrome and good sleepers using the induced change blindness paradigm. J Sleep Res. 2006;15:212–21. doi: 10.1111/j.1365-2869.2006.00510.x. [DOI] [PubMed] [Google Scholar]
- 50.Parrino L, Milioli G, De Paolis F, Grassi A, Terzano MG. Paradoxical insomnia: The role of CAP and arousals in sleep misperception. Sleep Med. 2009;10:1139–45. doi: 10.1016/j.sleep.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 51.Bonnet MH, Arand DL. The consequences of a week of insomnia II: patients with insomnia. Sleep. 1998;21:359–68. [PubMed] [Google Scholar]
- 52.Mendelson WB. Long-term follow-up of chronic insomnia. Sleep. 1995;18:698–701. doi: 10.1093/sleep/18.8.698. [DOI] [PubMed] [Google Scholar]
- 53.Edinger JD, Fins AI. The distribution and clinical significance of sleep time misperceptions among insomniacs. Sleep. 1995;18:232–39. doi: 10.1093/sleep/18.4.232. [DOI] [PubMed] [Google Scholar]
- 54.Irwin M, Clark C, Kennedy B, Christian Gillin J, Ziegler M. Nocturnal catecholamines and immune function in insomniacs, depressed patients, and control subjects. Brain Behav Immun. 2003;17:365–72. doi: 10.1016/s0889-1591(03)00031-x. [DOI] [PubMed] [Google Scholar]
- 55.Stepanski E, Glinn M, Zorick F, Roehrs T, Roth T. Heart rate changes in chronic insomnia. Stress Med. 1994;10:261–66. [Google Scholar]
- 56.Bonnet MH, Arand DL. 24-hour metabolic rate in insomniacs and matched normal sleepers. Sleep. 1995;18:581–88. doi: 10.1093/sleep/18.7.581. [DOI] [PubMed] [Google Scholar]
- 57.Bonnet MH, Arand DL. Heart rate variability in insomniacs and matched normal sleepers. Psychosom Med. 1998;60:610–15. doi: 10.1097/00006842-199809000-00017. [DOI] [PubMed] [Google Scholar]
- 58.Dorsey CM, Bootzin RR. Subjective and psychophysiologic insomnia: an examination of sleep tendency and personality. Biol Psychiatry. 1997;41:209–16. doi: 10.1016/0006-3223(95)00659-1. [DOI] [PubMed] [Google Scholar]
- 59.Edinger JD, Fins AI, Glenn DM, Sullivan RJ, Jr, Bastian LA, Marsh GR, Dailey D, Hope TV, Young M, Shaw E, Vasilas D. Insomnia and the eye of the beholder: are there clinical markers of objective sleep disturbances among adults with and without insomnia complaints? J Consult Clin Psychol. 2000;68:586–93. [PubMed] [Google Scholar]
- 60.Lauderdale DS, Knutson KL, Yan LL, Rathouz PJ, Hulley SB, Sidney S, Liu K. Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. Am J Epidemiol. 2006;164:5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- 61.Silva GE, Goodwin JL, Sherrill DL, Arnold JL, Bootzin RR, Smith T, Walsleben JA, Baldwin CM, Quan SF. Relationship between reported and measured sleep times: the sleep heart health study (SHHS) J Clin Sleep Med. 2007;3:622–30. [PMC free article] [PubMed] [Google Scholar]
- 62.Van Den Berg JF, Van Rooij FJ, Vos H, Tulen JH, Hofman A, Miedema HM, Neven AK, Tiemeier H. Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. J Sleep Res. 2008;17:295–302. doi: 10.1111/j.1365-2869.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- 63.Harvey AG, Sharpley AL, Ree MJ, Stinson K, Clark DM. An open trial of cognitive therapy for chronic insomnia. Behav Res Ther. 2007;45:2491–2501. doi: 10.1016/j.brat.2007.04.007. [DOI] [PubMed] [Google Scholar]