Abstract
Objective
Previous functional magnetic resonance imaging (fMRI) studies in pediatric bipolar disorder (BD) have reported greater amygdala and less dorsolateral prefrontal cortex (DLPFC) activation to facial expressions compared to healthy controls. The current study investigates whether these differences are associated with the early or late phase of activation, suggesting different temporal characteristics of brain responses.
Method
Twenty euthymic adolescents with familial BD (14 male) and twenty-one healthy control subjects (13 male) underwent fMRI scanning during presentation of happy, sad, and neutral facial expressions. Whole brain voxel-wise analyses were conducted in SPM5, using a 3-way analysis of variance (ANOVA) with factors group (BD and healthy control [HC]), facial expression (happy, sad, and neutral versus scrambled), and phase (early and late, corresponding to the first and second half of each block of faces).
Results
There were no significant group differences in task performance, age, gender, or IQ. Significant activation from the Main Effect of Group included greater DLPFC activation in the HC group, and greater amygdala/hippocampal activation in the BD group. The interaction of Group X Phase identified clusters in the superior temporal sulcus/insula and visual cortex, where activation increased from the early to late phase of the block for the BD but not the HC group.
Conclusions
These findings are consistent with previous studies that suggest deficient prefrontal cortex regulation of heightened amygdala response to emotional stimuli in pediatric BD. Increasing activation over time in superior temporal and visual cortices suggests difficulty processing or disengaging attention from emotional faces in BD.
Keywords: bipolar, fMRI, pediatric, amygdala
INTRODUCTION
Retrospective studies of adults with bipolar disorder (BD) suggest that the first episode of this disorder begins in childhood or adolescence in about 50% to 67% of cases 1–2. Compared to adult onset illness, pediatric onset BD has a more adverse course 3. Early onset BD can negatively influence emotional, cognitive, and social development, thus, early identification and treatment is important for reducing its negative impact 4. Neuroimaging investigation of pediatric BD may expand our understanding of neural abnormalities that could be targeted by treatment.
Patients with BD exhibit emotion processing deficits, including difficulty labeling facial expressions5. Important components of the brain networks that process facial expressions include the amygdala and prefrontal cortex6–8. Several recent fMRI studies of adult patients with BD report prefrontal cortex abnormalities during face processing, particularly the dorsolateral prefrontal cortex (DLPFC)9, the ventrolateral prefrontal cortex (VLPFC)10–12, and the orbitofrontal cortex (OFC) 13–14, although the direction of the findings varies. Amygdala hyperactivation has been reported by several groups15–18 but not all9–10, 19–20. Meta-analyses of functional magnetic resonance imaging (fMRI) studies of adult patients with BD also highlight these regions, reporting less ventrolateral prefrontal and greater limbic (parahippocampal, hippocampal, amygdala, basal ganglia) activation during emotional tasks 21 and specifically during facial emotion processing tasks 22. In the meta-analysis comparison focused specifically on patients in a euthymic mood state performing facial affect tasks, hypoactivation in the DLPFC and hyperactivation in the amygdala, hippocampus, superior temporal gyrus, and insula was found compared to controls. 23 However, these patterns are not always unique to the euthymic mood state. The meta-analysis by Houenou et al.24 found increased parahippocampal/amygdala activation in both manic and euthymic patients compared to healthy control (HC), but decreased VLPFC activation in manic patients only. Different findings according to mood state were also reported by Hulvershorn et al.25, who found that amygdala hyperactivation in response to negative faces characterized euthymic and depressed BD patients, but not manic patients, while only manic patients showed increased DLPFC activation compared to all other groups. Taken together, these studies show somewhat consistent finding in terms of abnormalities in prefrontal and limbic regions, but are complicated by several patient factors, such as mood state, and also illness duration, medication exposure, and variations in task and task demands.
Studies of pediatric patients can provide important information about interactions between BD and brain development, and are less likely to be confounded by medication exposure, substance use, and the long-term effects of mood symptoms, compared to adult samples. In addition, if different brain abnormalities are found in child versus adult samples, this could suggest that the illness may manifest differently depending on developmental stage. So far, however, results from pediatric fMRI studies of pediatric patients with BD viewing facial expressions are mostly consistent with the adult BD literature, including a pattern of abnormal dorsolateral, ventrolateral, and/or orbital prefrontal activation in both euthymic patients 26–27 and a mixed group of mood states 28. Amygdala hyperactivation also has been reported in both euthymic patients 29–30 and patients from a mixed group of mood states 31. Interestingly, elevated amygdala activation in manic/hypomanic pediatric patients persists following treatment with lamotrigine, while VLPFC activation normalizes 32.
For both adult and pediatric samples, fMRI data have been interpreted as showing that amygdala responses to emotional stimuli are poorly inhibited by prefrontal regions. Functional connectivity analyses in children support this hypothesis 33. However, more neuroimaging studies of youth with BD are needed to understand the contributions of mood state and brain development to patterns of activation.
A potentially fruitful method for examining the relevance of amygdala/DLPFC abnormalities to pediatric BD is to examine the temporal characteristics of abnormal activation in these regions. Temporal characteristics of activation can be assessed by examining decreases (habituation) or increases (sensitization) in the magnitude of activation over time. Habituation and sensitization of amygdala activation have been reported in healthy adults 34–36, and may reflect the ability of prefrontal brain regions to modulate responses of the amygdala over time. In BD, poor emotion regulation may be seen as abnormal habituation of amygdala activation over a block of faces, accompanied by lack of sensitization in prefrontal activation. This idea is supported by the only previous neuroimaging study that examined early and late phase of activation in adults with BD while viewing fearful faces 37. In that study, activation in regulatory regions, including the OFC, anterior cingulate, and striatum, increased during the late versus early phase of the task in the healthy control group but not in the BD group, suggesting lack of regulatory control in BD. Temporal patterns of brain activation have not been studied in children and adolescents with BD. In the current study, we hypothesized that youth with BD will demonstrate abnormal amygdala activation during the late phase of the block of faces, and abnormal prefrontal activation during the early phase. Our study focuses on euthymic youth with BD, to build on previous studies of euthymic patients and to contrast with those in manic and depressed states.
METHOD
Subjects
The study was approved by the Stanford University Administrative Panel of Medical Research in Human Subjects. Written informed consent and assent were obtained from parents and children, and subjects were paid for participation. Participants included thirty-two children and adolescents with familial BD participating in an NIMH-sponsored study of offspring of adult patients with BD (MH64460-01). Participants were recruited from advertisements and clinician contacts in the Stanford University Adult Bipolar Disorders Clinic and Pediatric Mood Disorders Clinic, and from San Francisco Bay Area communities. Twenty-seven healthy control subjects were recruited from the same community by advertisement.
For both groups, subjects were required to be 9–17 years old. Exclusion criteria were pervasive developmental disorder, neurological conditions, substance use disorder, IQ less than 80, braces, and MRI contraindications (e.g. metal in the body). For all subjects, full scale IQ was measured using the Wechsler Abbreviated Scale of Intelligence, San Antonio (WASI).
Inclusion in the BD group required a diagnosis of bipolar I or bipolar II disorder and at least one parent meeting diagnostic criteria for bipolar I or II disorder by the Structured Clinical Interview for DSM-IV (SCID) 38. All children were assessed using the mood disorders module of the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS;39) and the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime (K-SADS-PL) 40, as reported in a previous publication 41. Subjects were deemed euthymic in that they were not experiencing a current manic, mixed, or depressive episode. However, there was variation in degree of subthreshold symptomatology as indicated by Young Mania Rating Scale (YMRS) and Childhood Depression Rating Scale (CDRS) scores. On the day of the scan, current symptoms of depression within the past 2 weeks were assessed using the CDRS42, and current symptoms of mania within the past week were assessed using the YMRS43–44.
Inclusion in the healthy control group required that the subject have no current or past DSM-IV psychiatric diagnosis, as confirmed by K-SADS-PL interview. A SCID interview confirmed that both parents had no psychiatric diagnosis. The Family History Research Diagnostic Criteria 45 confirmed that first and second degree relatives did not have BD.
Emotional Facial Expressions Stimuli and Task
Validation of the affective faces stimuli has been reported in a previous publication 46. Briefly, 96 pictures were taken from several sources 47–48. Pictures were edited to be monochromatic and of the same size. The faces displayed either a happy, sad, or neutral expression of moderate intensity. Half of the pictures showed male models, and half showed female models. Pictures were matched (across facial expression) for intensity of emotion and gender of model. Stimuli were rated by an independent group of subjects and verified to be perceived as intended by the investigators (happy, sad, or neutral). Scrambled pictures were created by randomly rearranging the voxels from these pictures into an unrecognizable pattern.
Emotional facial expressions were presented in a block design. Four (non-repeated) blocks of each facial expression were shown (happy, sad, neutral, scrambled) and each block contained 8 pictures, each of which was shown for 3 seconds with no interstimulus interval. Therefore each block lasted 24 seconds. To reduce effects related to the order of the blocks, 4 different orders of blocks were presented. The task utilized implicit emotion perception, as subjects were instructed to judge the gender of the model in each picture and press button 1 with the right index finger to indicate female and button 2 with the right second digit to indicate male pictures. During scrambled blocks, subjects alternated pushing buttons 1 and 2. Correct and incorrect responses and response times were recorded by the task presentation software, Psyscope (http://psyscope.psy.cmu.edu/). Group differences in accuracy and response time were assessed using t-tests in SPSS (spss.com).
Onset of scanner and task were synchronized using a transistor–transistor logic (TTL) pulse delivered to the scanner timing microprocessor. Stimuli were projected from the foot of the scanner onto a screen attached to the headcoil. The subject viewed the stimuli by looking directly up at a mirror reflecting the stimuli shown on the screen. Before the scan, all subjects underwent a training session in an MRI simulator in which they practiced performing the task, learned to lie motionless for up to 10–15 continuous minutes, and acclimated to the sounds and confines of the scanner. Subjects were allowed to continue current medications, except psychostimulants, which were temporarily discontinued 24–48 hours before the scan.
Functional data acquisition
Images were acquired on a 3T GE Signa scanner using a standard GE whole head coil (General Electric; Milwaukee). The following spiral pulse sequence parameters were used: field of view (FOV)=20 cm, repetition time (TR) = 2000 msec, time to echo (TE) = 30 msec, flip angle = 89° and 1 interleave 49. Twenty-eight axial slices were acquired (4 mm thick, 0.5 mm skip). An individually calculated automated high-order shimming method based on spiral acquisitions was used to reduce B0 inhomogeneities. To localize functional activation, a 3-D high resolution T1 weighted spoiled grass gradient recalled (SPGR) image was collected (124 coronal slices, 256 × 192 matrix; slices=1.5 mm thick, no skip, 240 mm FOV; TR = 35 msec; TE = 6 msec; and flip angle = 45°; acquired resolution=1.50 × 0.94 × 1.25 mm).
SPM functional image analysis
Functional Images were reconstructed by inverse Fourier transform for each of the 216 time points into 64 × 64 × 28 matrices (voxel size: 3.12 × 3.12 × 4.5 mm) and analyzed using SPM5 (http://www.fil.ion.ucl.ac.uk/spm). Images were spatially realigned and motion repaired using the ArtRepair toolbox (cibsr.stanford.edu/tools/ArtRepair/ArtRepair.htm), and normalized into an age-appropriate 2 × 2 × 2 mm stereotactic template (irc.cchmc.org/software/pedbrain.php), smoothed with a 7 mm Gaussian filter, and high-pass filtered.
The general linear model and the theory of Gaussian random fields implemented in SPM5 were used for voxel-wise whole brain statistical analyses 50. A fixed-effects model was used to identify activation associated with each facial expression compared to scrambled images. Affective facial expressions were compared to scrambled images in order to investigate responses to faces as emotional and social stimuli. Neutral faces were not used as the comparison condition because neutral faces can be interpreted as having (non-neutral) affective characteristics by children and adolescents, as evidenced by activation of emotion-related brain circuitry in children 51.
Each block of faces was divided into an early and late phase, so that the first 12 seconds of the faces block was modeled as the early phase, and the last 12 seconds was modeled as the late phase. The early phase of each facial expression block was contrasted with the early phase of the scrambled images block, and the late phase was contrasted with the late phase of the scrambled block. Voxel-wise t-statistics were normalized to Z scores.
Individual contrast images were combined into a random-effects analysis 52 in SPM5 using a multivariate analysis of variance (ANOVA). The ANOVA included the factors group (BD and HC), and face (happy, sad, neutral) and phase (early and late). Our primary hypothesis was tested by identifying activation associated with the interaction of group x phase. To provide consistency with previous studies in this population using this paradigm, we also investigated activation associated with the main effect of group. Finally, although we have no hypotheses about specific facial expressions, exploratory analyses tested for activation associated with group x phase x face, main effect of phase, and main effect of face. For all contrasts, significant clusters of activation were determined using the joint expected probability of height (p=.01) and extent (p=.01) of Z scores, yielding a cluster-wise significance level of p=.01, corrected for multiple comparisons 53. Cluster extent to reach this threshold was determined using the 3DclustSim/AlphaSim program (afni.nimh.nih.gov/pub/dist/doc/program_help/AlphaSim.html). MNI coordinates were converted to Talairach coordinates using procedures described by Brett (1999; www.mrc-cbu.cam.ac.uk/Imaging/mnispace.html). Activation foci were superimposed on high-resolution T1-weighted images and localized with reference to the stereotaxic atlas of Talairach and Tournoux 54. For significant group x phase interactions, post-hoc t-tests were conducted using SPSS software (spss.com) and using activation extracted by the MARSBAR program (http://marsbar.sourceforge.net/).
RESULTS
Subjects
All subjects tolerated the scanning session without difficulty. Twelve subjects from the BD group and 6 subjects from the HC group were removed from the analysis because of excessive head motion during the scan, leaving 20 subjects in the BD group and 21 subjects in the HC group for all analyses. For these remaining subjects, the number of head motion artifacts repaired during image processing did not differ between the BD (mean=14 of 216 timepoints) and HC groups (mean=9 of 216 timepoints) (p=.12).
Table 1 lists subject characteristics per group. The control and BD groups did not differ in full scale IQ (p=.28), age (p=.71), or gender (p=.59).
Table 1.
| Subject Characteristics | BD Group: n=20 | HC Group: n =21 | p |
|---|---|---|---|
| Sex | 6 female/14 male | 8 female/13 male | .59 |
| Number left-handed | 1 | 1 | - |
| Mean Age, years (SD) | 15.63(2.10) | 15.35(2.68) | .71 |
| Mean Full-scale IQ (SD) | 110.72(8.59) | 113.62(7.87) | .28 |
| Mean CDRS score (SD) | 36.6(13.1) | - | |
| Mean YMRS score (SD) | 16.3(8.7) | - | |
| Diagnoses | |||
| Bipolar Disorder I | 95%(n=19) | - | |
| Bipolar Disorder II | 5%(n=1) | - | |
| ADHD | 85%(n=17) | - | |
| ODD | 40%(n=8) | - | |
| Conduct Disorder | 5%(n=1) | - | |
| Anxiety Disordera | 75%(n=15) | - | |
| Medications | |||
| anticonvulsants | 60%(n=12) | ||
| valproate | 45%(n=9) | ||
| atypical antipsychotic | 30%(n=6) | ||
| lithium | 35%(n=7) | ||
| atypical antidepressant | 35%(n=7) | ||
| SSRI | 5%(n=1) | ||
| Task Performance | |||
| % correct happy (SD) | 82.97(10.6) | 88.69(9.45) | .08 |
| % correct sad (SD) | 82.81(11.1) | 87.80(6.16) | .09 |
| % correct neutral (SD) | 83.75(8.6) | 87.80(9.68) | .17 |
| mean RT happy (SD) | 822.31(172.70) | 906.45(187.55) | .14 |
| mean RT sad (SD) | 876.69(213.73) | 920.86(205.24) | .50 |
| mean RT neutral (SD) | 909.77(208.85) | 903.20(179.40) | .91 |
Note: ADHD=attention-deficit/hyperactivity disorder; BD=bipolar disorder; CDRS=Children’s Depression Rating Scale; HC=healthy control; ODD=oppositional defiant disorder; RT=response time; SSRI=selective serotonin reuptake inhibitor; YMRS=Young Mania Rating Scale.
Anxiety Disorders include: generalized anxiety disorder (n=7); separation anxiety disorder (n=2); obsessive compulsive disorder (n=3); panic disorder (n=1); specific phobia (n=1); posttraumatic stress disorder (n=1).
Task Performance
Descriptive statistics for task performance data per group are given in Table 1. There were no significant group differences in task performance. There was a trend for lower accuracy of the BD subjects when identifying the gender of the happy (p=.08) and sad (p=.09) faces.
Whole Brain Voxel-Wise Functional MRI Results
Group X Phase interaction
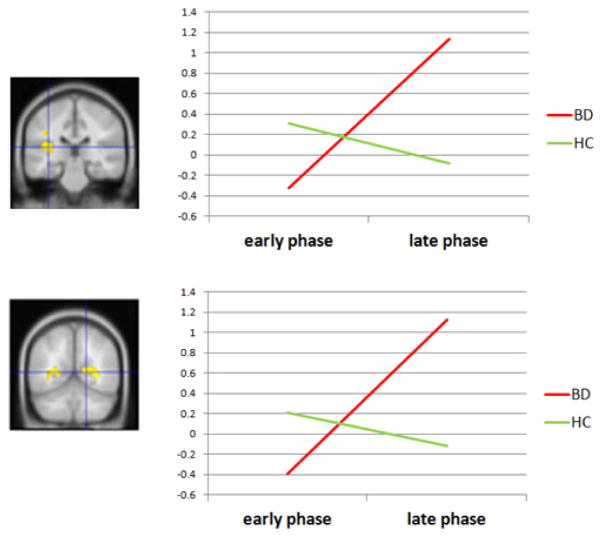
Significant activation associated with the interaction of Group X Phase is given in Table 2. Only 2 clusters were significant: one spanned the left superior temporal sulcus (STS) and part of the posterior insula. The other cluster included the right visual cortex and posterior cingulate cortex. These clusters are shown and graphed in Figure 1. For both regions, activation increases from the early to late phase in the BD group, but not in the HC group.
Table 2.
INTERACTION GROUP X PHASE.
| Brain Region | BA | k | Peak Z | x,y,z |
|---|---|---|---|---|
|
| ||||
| L STS/posterior insula | 13 | 1474 | 3.04 | −34,− 32,20 |
| L middle temporal gyrus | 19 | 2.99 | −53,−63,14 | |
| L visual cortex | 17 | |||
| R posterior cingulate | 23 | 416 | 2.88 | 16,−54,17 |
| R visual cortex | 17 | |||
Note: Significant clusters: p = .01 corrected for multiple comparisons. BA = Brodmann’s Area; L = left; R = right; STS=superior temporal sulcus.
Figure 1.
Significant clusters for Group X Phase: superior temporal sulcus (top) and visual cortex (bottom). Note: Graphs show that activation increases over time in the bipolar disorder (BD) group, but not in the healthy control (HC) group. DLPFC = dorsolateral prefrontal cortex; OFC = orbitofrontal cortex.
Main Effect of Group
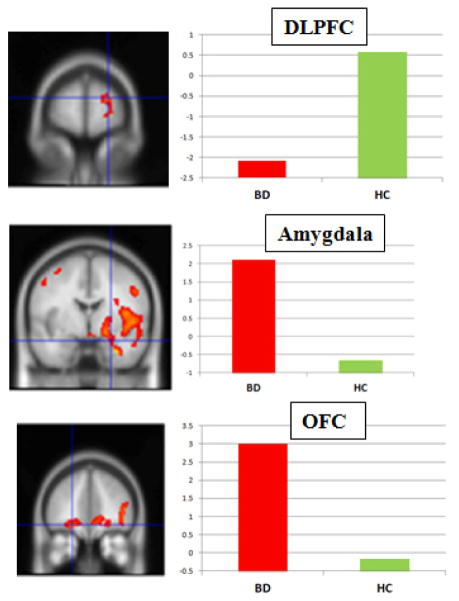
Significant activation associated with the Main Effect of Group is given in Table 3. When the one subject with bipolar II disorder was excluded, all ANOVA results remained unchanged. Clusters greater in BD than HC included the right amygdala and hippocampus, right and left OFC, bilateral parietal cortex, and anterior cingulate cortex. Clusters greater in HC versus BD included the DLPFC and bilateral visual cortex. These clusters are shown in Figure 2.
Table 3.
MAIN EFFECT OF GROUP: SIGNIFICANT CLUSTERS: p=.01 corrected for multiple comparisons
| Brain Region | BA | k | Peak Z | x,y,z | Direction |
|---|---|---|---|---|---|
|
| |||||
| R superior frontal (DLPFC) | 10 | 887 | 3.97 | 18, 65,17 | HC>BD |
| R middle frontal gyrus | 46 | 3.59 | 44,38,15 | ||
| R medial prefrontal | 10 | 4.32 | 12,66,4 | ||
| R middle occipital gyrus | 19/18 | 1595 | 3.86 | 46,−81,11 | HC>BD |
| R fusiform gyrus | 19 | 3.66 | 42, −72, −12 | ||
| R inferior occipital gyrus | 18 | 3.53 | 36, − 93,0 | ||
| L middle occipital gyrus | 19 | 789 | 5.68 | −40, −85,8 | HC>BD |
| L fusiform gyrus
|
19
|
|
4.59
|
−44, −83,6
|
|
| R amygdala | 441 | 4.68 | 30, −1, −18 | BD>HC | |
| R parahippocampal gyrus | 34 | 4.36 | 30,3, −17 | ||
| R superior temporal gyrus | 38 | 4.09 | 32,3, −17 | ||
| R uncus | 28 | 3.87 | 32,5, −19 | ||
| R globus pallidum | 3.60 | 20, −6, −3 | |||
| L superior parietal lobe | 7 | 536 | 4.96 | −28, −65,57 | BD>HC |
| L inferior parietal lobe | 40 | 4.27 | −65, −41,26 | ||
| L supramarginal gyrus | 40 | 3.76 | −63, −43,28 | ||
| L precentral gyrus | 4 | 3.53 | −51, −7,52 | ||
| L middle frontal gyrus | 6/8 | 3.40 | −50,8,46 | ||
| L Caudate Tail | 536 | 4.05 | −20, −32,15 | BD>HC | |
| L Thalamus (pulvinar nucleus) | 3.89 | −18, −32,13 | |||
| R superior parietal lobe | 7 | 2006 | 4.14 | 22, −61,62 | BD>HC |
| R inferior parietal lobe | 40 | 4.13 | 48, −33,35 | ||
| R postcentral gyrus | 5 | 4.04 | 36, −42,59 | ||
| R supramarginal gyrus | 40 | 3.82 | 65, −45,26 | ||
| R precentral gyrus | 44/6 | 1761 | 4.34 | 48,2,7 | BD>HC |
| R insula | 13 | 4.04 | 44,2,5 | ||
| R anterior cingulate cortex | 32 | 439 | 3.77 | 10,45,0 | BD>HC |
| R superior medial frontal gyrus | 10 | 3.67 | 14,45,3 | ||
| R orbital frontal | 11 | 3.67 | 24,37, −5 | ||
| L orbital frontal cortex | 11 | 3.48 | −20,42, −8 | ||
| L cerebellum | 652 | 3.55 | −22, −44, −21 | BD>HC | |
| L/R Brainstem and midbrain | 0, −27, −26 | ||||
Note: Significant clusters: p = .01 corrected for multiple comparisons. BA=Brodmann’s Area; BD=bipolar disorder; DLPFC=dorsolateral prefrontal cortex; HC=healthy control; L=left; R=right; STS=superior temporal sulcus.
Figure 2.
Selected clusters of significant activation from main effect of group. Note: y-axis denotes mean activation in cluster. BD = bipolar disorder; HC = healthy control.
Exploratory Interactions
There were no significant clusters for the main effect of phase, the main effect of valence, or the interaction of group x phase x valence.
Post-hoc follow-up t-tests for Group x Phase interaction
Significantly activated clusters from the interaction of group x phase (e.g. those listed in Table 2) were further evaluated by extracting mean activation in each cluster using the MARSBAR program (http://marsbar.sourceforge.net/). Extracted clusters were analyzed using SPSS software (www.spss.com) and a repeated-measures t-test, which tested for significant changes in activation (indicating habituation or sensitization) from the early to late phase of the block within each group, collapsed across facial expression. We found that changes within each group were not significant: Visual Cortex: for HC: t<1, for BD: t=−1.9, p=.07 (trend); for the STS/insula: for HC: t=1.01, p=.33; for BD: t= 1.75, p=.096 (trend). Between-group t-tests were used to compare BD and HC groups at each phase, collapsed across facial expression. There were no group differences at either phase: Visual cortex: Early phase: t=1.01, p=.32, Late phase: t=2.9, p=.097 (trend); STS/insula: Early phase: t=1.45, p=.24; Late phase: t=2.67, p=.11). In summary, although the post-hoc t-tests of extracted activation do not identify a particular phase responsible for this effect, the whole brain SPM analysis shows a significant interaction of group x phase in these regions.
Effects of Medication
Many of the subjects were taking psychotropic medications including lithium, anticonvulsants, and antipsychotics, which could affect fMRI data 55. We sought to determine whether our primary findings (main effect of group: DLPFC, amygdala, OFC; group X phase: visual cortex/posterior cingulate and STS/insula) were affected by these medications. For each region, we used extracted activation values and independent groups t-tests to compare (a) patients taking lithium (n=7) versus those not taking lithium (n=13); (b) patients taking atypical antipsychotics (n=9) versus those not taking atypical antipsychotics (n=11); and (c) patients taking anticonvulsants (n=12) versus those not taking anticonvulsants (n=8). Results showed that medication subgroups were not significantly different in activation of the amygdala, DLPFC, or orbital frontal cortex (p> .05). Also, medication subgroups were not significantly different in activation of the STS/insula or the visual cortex/posterior cingulate (p>.05).
Correlation with Symptom Severity and age
Within the BD group, Spearman’s correlation analyses were conducted using CDRS depression scores, YMRS mania scores, and extracted activation in 2 primary regions of interest: the amygdala and the DLPFC. None of the correlations passed the threshold of p=.05. We also correlated change in activation from phase 1 to 2 with YMRS and CDRS scores within the BD group. None of these correlations was significant (p>.05). Lastly, we correlated age with activation across both the BD and HC groups and found 1 significant correlation: Increasing age was associated with increasing activation in the DLPFC during the late phase of the sad faces (Spearman’s rho=.33, p=.037).
DISCUSSION
Our findings are consistent with previous fMRI studies of adult and pediatric BD that demonstrate less DLPFC and greater amygdala activation during perception of facial expressions. Our data does not support our hypothesis that abnormal amygdala activation would be seen during the late phase of the block, while abnormal prefrontal activation would be seen during the early phase of the block. However, our data shows a significant group x phase interaction in the STS/insula and visual cortex/posterior cingulate: For the BD group, activation increases from the early to late phase of the block, while for the HC group, activation decreases slightly. The STS is associated with perception of faces 8 and social aspects of face processing 56. Greater visual cortex activation may reflect greater attention to facial emotion 57. Thus, the interaction suggests that the HC group efficiently process the faces and then decreases allocation of neural resources, while the BD group has a gradually increasing response to faces, possibly reflecting difficulty processing emotional facial expressions, consistent with behavioral studies showing that patients with BD have difficulty identifying facial emotions 5.
Habituation or sensitization may indicate emotion regulation processes. In previous fMRI studies of changes in activation over time in healthy adults, amygdala activation decreases with exposure to repeated presentations of fearful and neutral facial expressions 35, 36. Presumably this is an adaptive response, such that after we perceive and process the meaning of a stimulus, we reallocate resources efficiently and appropriately based on the situation. On the other hand, a series of non-repeated angry faces have been shown to elicit increasing activation over time (sensitization) in healthy adults 34, suggesting that novel or threatening stimuli take longer to process, and/or grow in emotional significance over time. In summary, the ability to appropriately regulate responses to emotional stimuli may be reflected in gradual increases or decreases in activation over time, possibly mediated through prefrontal executive cortical regions.
In mood and anxiety disorders, including BD, abnormal increases in activation over time may reflect deficient control and/or dysregulation of emotional responses. An fMRI study of adults with BD viewing fearful faces found that activation in the OFC, anterior cingulate, and striatum increased from early to late in the task in healthy adults, but not in the BD group 37. This study was different from ours in defining early and late as the first versus last half of the experiment, while we used the early and late phases of each block. We did not observe habituation or sensitization per se (significant decreases or increases from early to late in the block) in either group, but rather, a significant interaction that was associated with increasing activation in the BD group, but decreasing activation in the HC group. This pattern was found in the STS and visual cortex, brain regions associated with processing faces, which may suggest that abnormalities in perception of emotional facial expressions in particular, rather than emotional responses in general, are reflected in this early/late phase analysis.
A related interpretation is that deficient control over emotion-related activation in BD may be related to an inability to disengage from negative stimuli, similar to that reported in unipolar depression 58. Behavioral data show that increasing symptoms of depression in patients with bipolar disorder are associated with inability to disengage attention in general, and that both euthymic and depressed patients showed a bias away from positive words 59. In another study, children who had BD and comorbid anxiety disorders showed attention bias toward threatening facial expressions 60. Therefore, in the current study, increasing STS and visual cortical activation in the BD group may reflect abnormal attention bias to faces, and difficulty disengaging from emotion-related stimuli.
Our study included pediatric patients with BD in a euthymic mood state, so these results may not hold for other mood states. Recent meta-analyses of adult BD studies indicate variations in profiles of abnormal activation depending on mood state 21, 23–24. Of these meta-analyses, the sub-analysis most similar to the current study: euthymic patients performing facial affect tasks, found results similar to ours: lower DLPFC and greater amygdala, hippocampus, insula, and superior temporal gyrus activation 23. Other mood states may show different findings, for example, functional connectivity between the amygdala and OFC is significantly different in adults with BD versus HC, but only for the depressed, not remitted, mood state 61. Two published studies have used a longitudinal design to examine the effects of mood state on brain activation in adults with BD. In the first, Kaladjian et al. showed that remission from mania is associated with decreased amygdala activation during a response inhibition task 62. The second study found greater amygdala/hippocampal activation during euthymia compared to mania, while OFC hyperactivation characterized BD versus HC across mood states 14.
Similarly to previous fMRI studies of BD patients, we did not discontinue medications because of the high risk of mood episode relapse, and the high morbidity and mortality of untreated BD. Thus, exposure to psychotropic medications may have contributed to group differences in activation, or may have obscured abnormalities. Previous studies suggest that mood stabilizer and antipsychotic medication tend to decrease amygdala activation 55, 63–64, so our findings of amygdala hyperactivation in BD were robust enough to survive this potential confound. Our analysis found that medication subgroups did not differ significantly in activation of our primary regions of interest.
The comorbid diagnoses of the subjects in this study may limit interpretations of our findings. Most of the BD subjects had a comorbid diagnosis of attention-deficit/hyperactivity disorder (ADHD), and the relatively small sample size prevented us from directly testing the effects of ADHD on brain activation in the BD group. Adler et al. showed that adolescents with BD and comorbid ADHD showed less prefrontal cortex activation than those with BD alone during performance of a simple attention task 65. Recently, children with ADHD were found to have greater amygdala activation than children with BD while viewing neutral faces 51. Passarotti et al. found that patients with pediatric BD, compared to those with ADHD, had greater activation in the subgenual anterior cingulate cortex (ACC) and OFC, and less activation in the DLPFC during a 2-back task presenting facial expressions 66. Together, these previous studies suggest that our results may represent abnormalities related to comorbid BD and ADHD. Future studies would benefit from including a larger number of subjects, including those with and without comorbid ADHD. This study focused on familial BD only, because this may provide a more homogeneous sample. However, our findings may not generalize beyond patients with familial BD. In summary, our findings support models of deficient prefrontal and excessive amygdala activation in patients with BD compared to controls, and add new information suggesting that youth with BD have aberrant timing of STS and visual cortical activation to facial expressions.
Acknowledgments
This work was supported in part by a Brain and Behavior Research Foundation Young Investigators Award, a Klingenstein Third Generation Foundation Fellowships (A.G., K.C.), National Institutes of Health (NIH) grants MH64460-01 (K.C.) and MH01142, MH19908, MH050047, and HD31715 (A.R.).
We thank Vinod Menon, Ph.D., of Stanford University for his assistance with task design.
Footnotes
Disclosure: Dr. Reiss has served as a consultant for Novartis. Dr. Chang has served on the advisory board for Eli Lilly and Co., has served as a consultant for Bristol-Myers Squibb and Merck and Co., and has received research support from GlaxoSmithKline. Drs. Garrett, Singh, and Adleman, Ms. Howe, Mr. Kelley, and Ms. Karchemskiy report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dr. Amy Garrett, Center for Interdisciplinary Brain Sciences Research and the Pediatric Bipolar Disorders Program at Stanford University.
Dr. Allan Reiss, Center for Interdisciplinary Brain Sciences Research at Stanford University.
Ms. Meghan Howe, Pediatric Bipolar Disorders Program at Stanford University.
Mr. Ryan Kelley, Center for Interdisciplinary Brain Sciences Research and the Pediatric Bipolar Disorders Program at Stanford University.
Dr. Manpreet Singh, Pediatric Bipolar Disorders Program at Stanford University.
Dr. Nancy Adleman, National Institute of Mental Health (NIMH).
Ms. Asya Karchemskiy, Center for Interdisciplinary Brain Sciences Research at Stanford University.
Dr. Kiki Chang, Pediatric Bipolar Disorders Program at Stanford University.
References
- 1.Perlis RH, Dennehy EB, Miklowitz DJ, et al. Retrospective age at onset of bipolar disorder and outcome during two-year follow-up: results from the STEP-BD study. Bipolar Disord. 2009 Jun;11(4):391–400. doi: 10.1111/j.1399-5618.2009.00686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005 Jun;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Post RM, Leverich GS, Kupka RW, et al. Early-onset bipolar disorder and treatment delay are risk factors for poor outcome in adulthood. J Clin Psychiatry. 2010 Jul;71(7):864–872. doi: 10.4088/JCP.08m04994yel. [DOI] [PubMed] [Google Scholar]
- 4.Sala R, Axelson D, Birmaher B. Phenomenology, longitudinal course, and outcome of children and adolescents with bipolar spectrum disorders. Child Adolesc Psychiatr Clin N Am. 2009 Apr;18(2):273–289. vii. doi: 10.1016/j.chc.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosen HR, Rich BA. Neurocognitive correlates of emotional stimulus processing in pediatric bipolar disorder: a review. Postgrad Med. 2010 Jul;122(4):94–104. doi: 10.3810/pgm.2010.07.2177. [DOI] [PubMed] [Google Scholar]
- 6.Dekowska M, Kuniecki M, Jaskowski P. Facing facts: neuronal mechanisms of face perception. Acta Neurobiol Exp (Wars) 2008;68(2):229–252. doi: 10.55782/ane-2008-1692. [DOI] [PubMed] [Google Scholar]
- 7.Pessoa L, Ungerleider LG. Neuroimaging studies of attention and the processing of emotion-laden stimuli. Prog Brain Res. 2004;144:171–182. doi: 10.1016/S0079-6123(03)14412-3. [DOI] [PubMed] [Google Scholar]
- 8.Fusar-Poli P, Placentino A, Carletti F, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009 Nov;34(6):418–432. [PMC free article] [PubMed] [Google Scholar]
- 9.Hassel S, Almeida JR, Kerr N, et al. Elevated striatal and decreased dorsolateral prefrontal cortical activity in response to emotional stimuli in euthymic bipolar disorder: no associations with psychotropic medication load. Bipolar Disord. 2008 Dec;10(8):916–927. doi: 10.1111/j.1399-5618.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foland-Ross LC, Bookheimer SY, Lieberman MD, et al. Normal amygdala activation but deficient ventrolateral prefrontal activation in adults with bipolar disorder during euthymia. Neuroimage. 2012 Jan 2;59(1):738–744. doi: 10.1016/j.neuroimage.2011.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawrence NS, Williams AM, Surguladze S, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004 Mar 15;55(6):578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Foland LC, Altshuler LL, Bookheimer SY, Eisenberger N, Townsend J, Thompson PM. Evidence for deficient modulation of amygdala response by prefrontal cortex in bipolar mania. Psychiatry Res. 2008 Jan 15;162(1):27–37. doi: 10.1016/j.pscychresns.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van der Schot A, Kahn R, Ramsey N, Nolen W, Vink M. Trait and state dependent functional impairments in bipolar disorder. Psychiatry Res. 2010 Dec 30;184(3):135–142. doi: 10.1016/j.pscychresns.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Chen CH, Suckling J, Ooi C, et al. A longitudinal fMRI study of the manic and euthymic states of bipolar disorder. Bipolar Disord. 2010 May;12(3):344–347. doi: 10.1111/j.1399-5618.2010.00801.x. [DOI] [PubMed] [Google Scholar]
- 15.Malhi GS, Lagopoulos J, Sachdev PS, Ivanovski B, Shnier R, Ketter T. Is a lack of disgust something to fear? A functional magnetic resonance imaging facial emotion recognition study in euthymic bipolar disorder patients. Bipolar Disord. 2007 Jun;9(4):345–357. doi: 10.1111/j.1399-5618.2007.00485.x. [DOI] [PubMed] [Google Scholar]
- 16.Altshuler LL, Bookheimer SY, Townsend J, et al. Blunted activation in orbitofrontal cortex during mania: a functional magnetic resonance imaging study. Biol Psychiatry. 2005 Nov 15;58(10):763–769. doi: 10.1016/j.biopsych.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Altshuler L, Bookheimer S, Proenza MA, et al. Increased amygdala activation during mania: a functional magnetic resonance imaging study. Am J Psychiatry. 2005 Jun;162(6):1211–1213. doi: 10.1176/appi.ajp.162.6.1211. [DOI] [PubMed] [Google Scholar]
- 18.Blumberg HP, Donegan NH, Sanislow CA, et al. Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology (Berl) 2005 Dec;183(3):308–313. doi: 10.1007/s00213-005-0156-7. [DOI] [PubMed] [Google Scholar]
- 19.Robinson JL, Monkul ES, Tordesillas-Gutierrez D, et al. Fronto-limbic circuitry in euthymic bipolar disorder: evidence for prefrontal hyperactivation. Psychiatry Res. 2008 Nov 30;164(2):106–113. doi: 10.1016/j.pscychresns.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Hassel S, Almeida JR, Frank E, et al. Prefrontal cortical and striatal activity to happy and fear faces in bipolar disorder is associated with comorbid substance abuse and eating disorder. J Affect Disord. 2009 Nov;118(1–3):19–27. doi: 10.1016/j.jad.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen CH, Suckling J, Lennox BR, Ooi C, Bullmore ET. A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disord. 2011 Feb;13(1):1–15. doi: 10.1111/j.1399-5618.2011.00893.x. [DOI] [PubMed] [Google Scholar]
- 22.Delvecchio G, Fossati P, Boyer P, et al. Common and distinct neural correlates of emotional processing in Bipolar Disorder and Major Depressive Disorder: a voxel-based meta-analysis of functional magnetic resonance imaging studies. Eur Neuropsychopharmacol. 2012 Feb;22(2):100–113. doi: 10.1016/j.euroneuro.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Kupferschmidt DA, Zakzanis KK. Toward a functional neuroanatomical signature of bipolar disorder: Quantitative evidence from the neuroimaging literature. Psychiatry Res. 2011 Aug 30;193(2):71–79. doi: 10.1016/j.pscychresns.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Houenou J, Frommberger J, Carde S, et al. Neuroimaging-based markers of bipolar disorder: evidence from two meta-analyses. J Affect Disord. 2011 Aug;132(3):344–355. doi: 10.1016/j.jad.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 25.Hulvershorn LA, Karne H, Gunn AD, et al. Neural Activation During Facial Emotion Processing in Unmedicated Bipolar Depression, Euthymia, and Mania [published online ahead of print December 28, 2011] Biol Psychiatry. 2012;71(7):603–10. doi: 10.1016/j.biopsych.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. An fMRI study of the neural correlates of incidental versus directed emotion processing in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2009 Mar;48(3):308–319. doi: 10.1097/CHI.0b013e3181948fc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dickstein DP, Rich BA, Roberson-Nay R, et al. Neural activation during encoding of emotional faces in pediatric bipolar disorder. Bipolar Disord. 2007 Nov;9(7):679–692. doi: 10.1111/j.1399-5618.2007.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ladouceur CD, Farchione T, Diwadkar V, et al. Differential patterns of abnormal activity and connectivity in the amygdala-prefrontal circuitry in bipolar-I and bipolar-NOS youth. J Am Acad Child Adolesc Psychiatry. 2011 Dec;50(12):1275–1289. e1272. doi: 10.1016/j.jaac.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pavuluri MN, O’Connor MM, Harral E, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry. 2007 Jul 15;62(2):158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 30.Rich BA, Vinton DT, Roberson-Nay R, et al. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci U S A. 2006 Jun 6;103(23):8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsavsky AK, Brotman MA, Rutenberg JG, et al. Amygdala hyperactivation during face emotion processing in unaffected youth at risk for bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2012 Mar;51(3):294–303. doi: 10.1016/j.jaac.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Passarotti AM, Sweeney JA, Pavuluri MN. Fronto-limbic dysfunction in mania pre-treatment and persistent amygdala over-activity post-treatment in pediatric bipolar disorder. Psychopharmacology (Berl) 2011 Aug;216(4):485–499. doi: 10.1007/s00213-011-2243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rich BA, Fromm SJ, Berghorst LH, et al. Neural connectivity in children with bipolar disorder: impairment in the face emotion processing circuit. J Child Psychol Psychiatry. 2008 Jan;49(1):88–96. doi: 10.1111/j.1469-7610.2007.01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strauss MM, Makris N, Aharon I, et al. fMRI of sensitization to angry faces. Neuroimage. 2005 Jun;26(2):389–413. doi: 10.1016/j.neuroimage.2005.01.053. [DOI] [PubMed] [Google Scholar]
- 35.Wright CI, Fischer H, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport. 2001 Feb 12;12(2):379–383. doi: 10.1097/00001756-200102120-00039. [DOI] [PubMed] [Google Scholar]
- 36.Breiter HC, Etcoff NL, Whalen PJ, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996 Nov;17(5):875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- 37.Killgore WD, Gruber SA, Yurgelun-Todd DA. Abnormal corticostriatal activity during fear perception in bipolar disorder. Neuroreport. 2008 Oct 8;19(15):1523–1527. doi: 10.1097/WNR.0b013e328310af58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.First MBSR, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P, version 2.0) New York, NY: Biometric Research, New York State Psychiatric Institute; 1995. [Google Scholar]
- 39.Geller BGWM, Zimerman B, Frazier J. WASH-U-KSADS (Washington University in St Louis Kiddie Schedule for Affective Disorders and Schizophrenia) St. Louis (Missouri): Washington University; 1996. [DOI] [PubMed] [Google Scholar]
- 40.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997 Jul;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 41.Chang K, Adleman N, Dienes K, Simeonova D, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder. Arch Gen Psychiatry. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- 42.Poznanski EO, Cook SC, Carroll BJ. A depression rating scale for children. Pediatrics. 1979 Oct;64(4):442–450. [PubMed] [Google Scholar]
- 43.Fristad MA, Weller RA, Weller EB. The Mania Rating Scale (MRS): further reliability and validity studies with children. Ann Clin Psychiatry. 1995 Sep;7(3):127–132. doi: 10.3109/10401239509149039. [DOI] [PubMed] [Google Scholar]
- 44.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978 Nov;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 45.Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria. Reliability and validity. Arch Gen Psychiatry. 1977 Oct;34(10):1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- 46.Yang TT, Menon V, Eliez S, et al. Amygdalar activation associated with positive and negative facial expressions. Neuroreport. 2002 Oct 7;13(14):1737–1741. doi: 10.1097/00001756-200210070-00009. [DOI] [PubMed] [Google Scholar]
- 47.Pictures of Facial Affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- 48.Japanese and Caucasian Facial Expressions of Emotion (JACFEE) and Japanese and Caucasian Neutral Faces (JACNeuF) (slides) San Francisco, CA: Intercultural and Emotion Research Laboratory, Department of Psychology, San Francisco State University; 1988. [Google Scholar]
- 49.Glover GH, Lai S. Self-navigated spiral fMRI: interleaved versus single-shot. Magn Reson Med. 1998 Mar;39(3):361–368. doi: 10.1002/mrm.1910390305. [DOI] [PubMed] [Google Scholar]
- 50.Friston KJ, Zarahn E, Josephs O, Henson RN, Dale AM. Stochastic designs in event-related fMRI. Neuroimage. 1999;10(5):607–619. doi: 10.1006/nimg.1999.0498. [DOI] [PubMed] [Google Scholar]
- 51.Brotman MA, Rich BA, Guyer AE, et al. Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. Am J Psychiatry. 2010 Jan;167(1):61–69. doi: 10.1176/appi.ajp.2009.09010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holmes AP, Friston KJ. Generalizability, random effects, and population inference. 1998;(7):754. Located at: NeuroImage. [Google Scholar]
- 53.Poline JB, Worsley KJ, Evans AC, Friston KJ. Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage. 1997 Feb;5(2):83–96. doi: 10.1006/nimg.1996.0248. [DOI] [PubMed] [Google Scholar]
- 54.Talairach J, Tournoux . Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- 55.Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry. 2008 Mar;165(3):313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ethofer T, Gschwind M, Vuilleumier P. Processing social aspects of human gaze: a combined fMRI-DTI study. Neuroimage. 2011 Mar 1;55(1):411–419. doi: 10.1016/j.neuroimage.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 57.Keil A, Costa V, Smith JC, et al. Tagging cortical networks in emotion: A topographical analysis. Hum Brain Mapp. doi: 10.1002/hbm.21413. [published online ahead of print Sep 2011] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annu Rev Clin Psychol. 2010 Apr 27;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jongen EM, Smulders FT, Ranson SM, Arts BM, Krabbendam L. Attentional bias and general orienting processes in bipolar disorder. J Behav Ther Exp Psychiatry. 2007 Jun;38(2):168–183. doi: 10.1016/j.jbtep.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 60.Brotman MA, Rich BA, Schmajuk M, et al. Attention bias to threat faces in children with bipolar disorder and comorbid lifetime anxiety disorders. Biol Psychiatry. 2007 Mar 15;61(6):819–821. doi: 10.1016/j.biopsych.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 61.Versace A, Thompson WK, Zhou D, et al. Abnormal left and right amygdala-orbitofrontal cortical functional connectivity to emotional faces: state versus trait vulnerability markers of depression in bipolar disorder. Biol Psychiatry. 2010 Mar 1;67(5):422–431. doi: 10.1016/j.biopsych.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaladjian A, Jeanningros R, Azorin JM, et al. Remission from mania is associated with a decrease in amygdala activation during motor response inhibition. Bipolar Disord. 2009 Aug;11(5):530–538. doi: 10.1111/j.1399-5618.2009.00722.x. [DOI] [PubMed] [Google Scholar]
- 63.Strakowski SM, Eliassen JC, Lamy M, et al. Functional magnetic resonance imaging brain activation in bipolar mania: evidence for disruption of the ventrolateral prefrontal-amygdala emotional pathway. Biol Psychiatry. 2011 Feb 15;69(4):381–388. doi: 10.1016/j.biopsych.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bell EC, Willson MC, Wilman AH, Dave S, Silverstone PH. Differential effects of chronic lithium and valproate on brain activation in healthy volunteers. Hum Psychopharmacol. 2005 Aug;20(6):415–424. doi: 10.1002/hup.710. [DOI] [PubMed] [Google Scholar]
- 65.Adler CM, Delbello MP, Mills NP, Schmithorst V, Holland S, Strakowski SM. Comorbid ADHD is associated with altered patterns of neuronal activation in adolescents with bipolar disorder performing a simple attention task. Bipolar Disord. 2005 Dec;7(6):577–588. doi: 10.1111/j.1399-5618.2005.00257.x. [DOI] [PubMed] [Google Scholar]
- 66.Passarotti AM, Sweeney JA, Pavuluri MN. Emotion processing influences working memory circuits in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010 Oct;49(10):1064–1080. doi: 10.1016/j.jaac.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]