Sakhaee et al in this issue have investigated whether the risk of the common calcium nephrolithiasis is associated with the metabolic syndrome (MS). This question is interesting since it deals with a more general problem on whether calcium nephrolithiasis is a ‘systemic disorder’ and entails a cardiovascular risk..
Keywords: kidney stone, metabolic syndrome, nephrolithiasis, obesity
Abstract
Background
The metabolic syndrome (MS) is associated with increased prevalence of kidney stones, yet the specific stone type remains largely unknown. This study was conducted to assess whether risk factors associated with calcium nephrolithiasis increase with individual characteristics of the MS.
Methods
A retrospective analysis was performed in 109 non-stone-forming subjects and 128 recurrent calcium stone formers from Dallas, Texas. A separate analysis was performed in 140 recurrent calcium stone formers from Bern, Switzerland. Demographic, anthropometric, serum and urinary profiles were measured.
Results
In non-stone formers from Dallas, urinary calcium (3.6 ± 1.8 to 6.0 ± 2.9 mmol/day, P = 0.0003 for trend, zero to four features) increased with increasing features of the MS. This change was attendant with a significant rise in supersaturation index (SI) of calcium oxalate (CaOx) (2.76 ± 1.21 to 4.45 ± 1.65, P < 0.0001; zero to four features). In calcium stone formers from Dallas, urinary calcium marginally increased (5.2 ± 2.3 to 7.0 ± 4.0 mmol/day, P = 0.09; zero to four features), while urinary oxalate (356 ± 141 to 504 ± 203 μmol/day, P = 0.001; zero to four features) and SI CaOx (4.46 ± 1.80 to 6.16 ± 3.71, P = 0.009; zero to four features) significantly increased with features of the MS. However, when adjusted for confounding variables such as total volume, age, gender, urine sodium and urine sulfate, urinary calcium and SI CaOx showed no significant changes in stone formers yet remained significant in non-stone formers. In a separate cohort from Bern, Switzerland urinary calcium (6.9 ± 3.6 versus 7.0 ± 3.2, P = 0.8) and SI CaOx (3.37 ± 1.98 versus 4.04 ± 2.78, P = 0.5) did not differ between subjects with and without the MS.
Conclusions
In non-stone formers, the risk of CaOx stone formation increases with the number of features of the MS. However, in stone-forming subjects, the propensity for CaOx precipitation is much higher but is not independently associated with increasing features of the MS.
Introduction
The metabolic syndrome (MS) and obesity are formidable health burdens in the USA, affecting 20–30 % of the general population [1, 2]. A large cross-sectional study utilizing data from the third National Health and Nutrition Examination Survey (NHANES III) found that an increasing number of features of the MS [3] was associated with an increase in prevalence of self-reported kidney stones [4]. Similarly, a prospective study in three large cohorts demonstrated that obesity and weight gain increase the risk of kidney stone formation [5]. However, the type of stone and underlying risk factors in individuals with the MS has yet to be characterized. Previous studies have shown that excessively acidic urine, the major abnormality in patients with uric acid nephrolithiasis, is also seen in non-stone-forming patients with Type II diabetes mellitus (T2DM), suggesting that unduly low urine pH may be an underlying renal manifestation of the MS [6–10]. Furthermore, it has been demonstrated that higher body mass index (BMI) and T2DM are independent risk factors for the development of uric acid kidney stones, and the proportion of uric acid stone formation in these individuals is three times higher than those without such features [11]. However, it is not known whether the reported increase in prevalence of kidney stones in obese subjects [3, 4] is due to uric acid nephrolithiasis alone. Since 80 % of kidney stones consist of calcium oxalate (CaOx) [12, 13], studies exploring the relationship between urinary risk factors for calcium stone formation and features of the MS are critical. Here, we present the biochemical and physicochemical characteristics of calcium stone risk in non-stone-forming subjects and in patients with calcium stones and their relationship to features of the MS.
Materials and methods
Study participants
We examined three separate cohorts: stone formers and non-stone formers from Dallas, Texas, USA and stone-formers from Inselspital, Bern, Switzerland. Data from calcium stone-forming subjects in Dallas were obtained retrospectively from the Stone Registries at The University of Texas Southwestern Medical Center. Local advertisements were used to prospectively recruit non-stone-forming subjects for this study. Stone analysis showed predominantly CaOx composition. A separate analysis was performed on a cohort of kidney stone formers from the Inselspital at the University of Bern, Switzerland. Included in the study were subjects > 21 years old. Excluded were individuals with chronic kidney disease (creatinine clearance < 40 mL/min), renal tubular acidosis, chronic diarrhea, primary hyperparathyroidism and urinary tract infection. All subjects were instructed to hold all medications known to influence urinary kidney stone profiles, such as alkali treatment and thiazide diuretics, for 1 week prior to inclusion in the study. In all cohorts, subjects with T2DM were permitted to participate in this study if they were not taking insulin or thiazolidinediones. This study was approved by the institutional review board at the University of Texas Southwestern Medical Center at Dallas and the University of Bern. Informed consent was obtained from all participants. Retrospective and prospective enrollment was conducted sequentially over a 5-year time period.
Data collection and measurements
All participants collected a 24-h urine sample, and the urine container was kept refrigerated or maintained in an ice chest during the collection. The urine specimen was obtained while subjects were maintained on an ad-lib random home diet. At completion of the collection, a fasting blood specimen was drawn and height, weight and blood pressure (BP) were measured. The updated definition of the MS from the American Heart Association/National Heart, Lung and Blood Institute in 2005 was utilized to classify features of the MS [3]. The MS was defined by the presence of three or more of the following: (i) elevated fasting blood glucose ≥ 6 mmol/L or drug treatment for elevated serum glucose, (ii) elevated BP as systolic BP > 135 mmHg, diastolic BP > 85 mmHg or drug treatment for hypertension, (iii) elevated fasting serum triglycerides > 1.70 mmol/L or drug treatment for hypertriglyceridemia, (iv) low fasting serum high-density lipoprotein (HDL) cholesterol < 1.30 mmol/L in women and < 1.04 mmol/L in men or drug treatment for low HDL, (v) abdominal obesity with a waist circumference of > 88 cm in women and > 102 cm in men. Because waist circumference was not available in all participants of this study, BMI of ≥ 30 kg/m2 was used as criterion for obesity. BMI is considered a reliable substitute for waist circumference since they are highly correlated [14]. Indeed, BMI has been utilized instead of waist circumference for the definition of MS in past studies [15, 16]. Insulin resistance was calculated from fasting serum glucose concentration and plasma insulin concentration using the homeostasis model assessment for insulin resistance (HOMA-IR) [17, 18]. This measurement was only performed in non-stone-forming subjects who had serum insulin measured prospectively but was not available in the retrospective evaluation of calcium kidney stone formers.
Analytical procedures
Serum electrolytes, glucose, triglycerides, total cholesterol, HDL cholesterol, creatinine and uric acid concentrations were measured using an automated system (CX9ALX; Beckman, Fullerton, CA). Plasma insulin was determined by enzyme-linked immunosorbent assay (Mercodia, Metuchen, NJ). Urine creatinine was determined using the picric acid method. Urinary calcium was determined by atomic absorption spectroscopy, oxalate by a chromatography system using a carbonate–bicarbonate eluent and an anion column and phosphorus by ammonium molybdate-based reaction. Urinary uric acid was analyzed by the urate oxidase method using an alkalinized aliquot to prevent precipitation. Urinary sodium and potassium were analyzed by flame emission photometry, chloride by Labconco buchler chloridometer and sulfate by ion chromatography. Urine pH and Pco2 were measured by an electrode. Urinary bicarbonate (HCO − 3was calculated from urine pH and Pco2. Urinary NH4+ was determined by glutamate dihydrogenase method. Titratable acidity (TA) was measured directly using automated burette end point titration system (Radiometer, Copenhagen, Denmark). Urinary TA in non-stone-forming subjects was also indirectly calculated from urinary constituents according to the method described by Kok et al. [19]. Citrate was calculated by citrate lyase assay, and milliequivalents of citrate were calculated from urine pH and a pKa of citrate2 −/citrate3− of 5.6. Net acid excretion (NAE) was calculated as urine (NH4+ + TA) − (citrate2− /3− + HCO3−), all expressed in milliequivalents. From these urinary measures, urinary saturation with respect to CaOx and brushite (CaHPO4·2H2O) was calculated as supersaturation index (SI) by using the JESS program [20]. SI value of 1 indicates saturation, > 1 supersaturation and < 1 undersaturation. In order to account for differences in urinary volume, SI values were calculated for a theoretical 2 L/day of urine volume.
Statistical analyses
Categorical comparisons were performed using the χ2 test. Serum triglycerides, HOMA-IR and skewed urinary variables were log transformed before performing parametric statistical analyses. For normally distributed continuous variables, comparisons of participants with and without MS were made with two-sample t-test. For urine NH4+/NAE, the Wilcoxon rank sum test was used. Tests of linear trend were conducted by one-way analysis of variance with polynomial contrasts. The urine NH4+/NAE trend was analyzed with the Jonckheere–Terpstra test. Linear models were used to examine the relationship between the number of MS features, the independent variable and the dependent variables urine calcium and SI CaOx. The linear trends were compared between non-stone formers and stone formers by evaluating the group-by-MS interaction and, in the absence of an interaction, by the group main effect from these models. Analysis of covariance was also used to further examine the relationship between SI CaOx and increasing MS features while adjusting for potential confounding variables. BMI, age, gender, urine sulfate and creatinine clearance were included as covariates. Results are expressed as mean and SD unless and otherwise specified. Statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, NC).
Results
Characteristics of non-stone formers from Dallas
A total of 109 non-stone-forming subjects participated in this study. Demographic characteristics are presented in Table 1. The mean ( ± SD) age was 43 ± 15 years, and the mean BMI was 27.6 ± 6.3 kg/m2. The median number of MS features was 1, and 29.4 % of subjects met criteria for the MS (i.e. three or more MS features).
Table 1.
Demographic characteristics of non-stone-forming subjects from Dallas
| Characteristic | Value |
|---|---|
| No. of participants | 109 |
| Gender [male/female; n ( % )] | 57/52 (52/48) |
| Ethnicity (White/Asian/Hispanic) | 85/12/12 |
| Age (years; mean ± SD) | 43 ± 15 |
| Height (cm; mean ± SD) | 168 ± 11 |
| Weight (kg; mean ± SD) | 79 ± 20 |
| BMI (kg/m2; mean ± SD) | 27.6 ± 6.3 |
| Elevated BMI [n ( % )] | 31 (28.4) |
| Elevated fasting blood glucose [n ( % )] | 38 (34.9) |
| Elevated triglycerides [n ( % )] | 31 (28.4) |
| Low HDL [n ( % )] | 56 (51.4) |
| Elevated BP [n ( % )] | 35 (32.1) |
| No. of MS features [median (IQR)] | 1 (0–3) |
| MS [n ( % )] | 32 (29.4) |
Relationship of biochemical profiles to the MS in non-stone-forming subjects from Dallas
A comparison of the anthropometric and biochemical characteristics of participants with and without the MS is shown in Table 2. There was no significant difference in gender and ethnic distributions between the two groups.
Table 2.
Demographic, anthropometric, serum and urinary characteristics of non-stone-forming subjects from Dallas with and without the MSa
| No of MS (N = 77) | MS (N = 32) | P | |
|---|---|---|---|
| Gender, male/female; n ( % ) | 38/39 (49/51) | 19/13 (59/41) | 0.3 |
| Ethnicity, White/Asian/Hispanic; n ( % ) | 61/10/6 (79/13/8) | 24/2/6 (75/6/19) | 0.2 |
| Age, years | 39 ± 14 | 52 ± 14 | < 0.0001 |
| BMI, kg/m2 | 24.9 ± 4.1 | 34.3 ± 5.6 | < 0.0001 |
| Systolic BP, mmHg | 116 ± 13 | 132 ± 16 | < 0.0001 |
| Diastolic BP, mmHg | 73 ± 10 | 81 ± 9 | 0.0005 |
| Serum | |||
| Glucose, mmol/L | 5.1 ± 0.8 | 6.6 ± 2.1 | 0.0007 |
| Triglycerides, mmol/L | 1.10 ± 0.81 | 2.35 ± 1.46 | < 0.0001 |
| HDL cholesterol, mmol/L | 1.26 ± 0.29 | 1.00 ± 0.20 | < 0.0001 |
| Insulin, pmol/L | 53 ± 30 | 114 ± 62 | < 0.0001 |
| HOMA-IR | 1.0 ± 0.6 | 2.2 ± 1.2 | < 0.0001 |
| Urine | |||
| Total volume, L/day | 1.87 ± 0.87 | 2.18 ± 1.34 | 0.2 |
| Calcium, mmol/day | 3.8 ± 1.9 | 5.4 ± 2.7 | 0.003 |
| Oxalate, mmol/day | 306 ± 98 | 340 ± 119 | 0.1 |
| Uric acid, mmol/day | 2.98 ± 1.12 | 3.25 ± 0.99 | 0.2 |
| pH | 6.13 ± 0.44 | 5.67 ± 0.50 | < 0.0001 |
| Citrate, mEq/day | 9.8 ± 4.1 | 8.8 ± 3.9 | 0.2 |
| Sulfate, mmol/day | 18.3 ± 7.7 | 22.5 ± 8.5 | 0.01 |
| NAE, mEq/day | 37.5 ± 26.5 | 60.0 ± 28.4 | 0.0004 |
| NAE, mEq/day (calculated) | 35.4 ± 23.7 | 58.4 ± 31.7 | 0.0006 |
| NH4+/NAE. | 0.73 (0.57–0.94) | 0.84 (0.38–1.43) | 0.0008 |
| Creatinine clearance, mL/s | 1.88 ± 0.53 | 2.17 ± 0.75 | 0.06 |
| Supersaturation indices | |||
| SI CaOx (volume adjusted) | 2.89 ± 1.29 | 4.26 ± 1.73 | 0.0002 |
| SI Br (volume adjusted) | 0.93 ± 0.56 | 0.95 ± 0.70 | 0.9 |
aValues are expressed as mean ± SD.
Subjects with the MS were significantly older and had significantly greater serum insulin and HOMA-IR. Compared to participants without the MS, subjects with the MS had significantly higher urinary calcium (5.4 ± 2.7 mmol/day versus 3.8 ± 1.9 mmol/day; P = 0.003). The 24-h urinary pH was significantly lower in subjects with the MS (5.67 ± 0.50 versus 6.13 ± 0.44; P < 0.0001). Urinary sulfate (22.5 ± 8.5 mmol/day versus 18.3 ± 7.7 mmol/day; P = 0.01) and NAE (60.0 ± 28.4 mEq/day versus 37.5 ± 26.5 mEq/day; P = 0.0004) were also significantly higher in subjects with the MS. Urinary oxalate, uric acid and citrate did not differ between the two groups. SI of CaOx (4.26 ± 1.73 versus 2.89 ± 1.29; P = 0.0002) was significantly higher in subjects with the MS compared to those without. However, SI of brushite did not differ between the two groups.
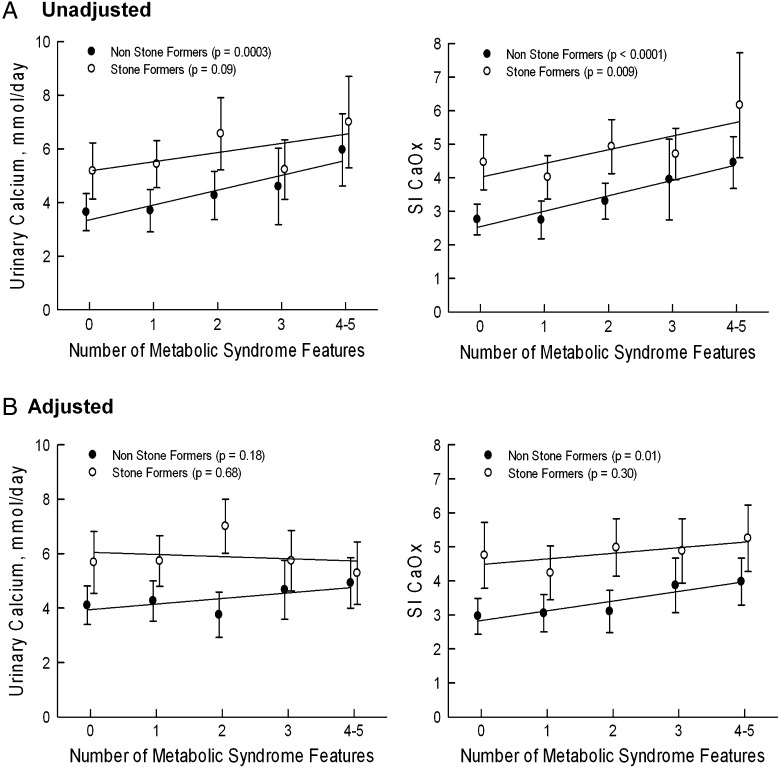
Demographics, biochemical and physicochemical characteristics were further stratified according to the number of features of the MS as illustrated in Table 3. Gender and ethnicity did not differ according to the number of MS features. Age, serum insulin and HOMA-IR increased with increasing number of features of the MS. Twenty-four-hour urinary calcium rose with increasing number of features of the MS (P = 0.0003 for trend) (Figure 1A) but was not significant after covariate adjustment for age, gender, urine sulfate and creatinine clearance (Figure 1B). Urinary pH decreased with higher number of MS characteristics (P < 0.0001 for trend). Urinary oxalate increased numerically but was not statistically significant (P = 0.06). Uric acid and citrate did not change significantly with increasing numbers of features of the MS (Table 3). SI of CaOx increased with increasing MS characteristics Figure (1A and B). SI of brushite did not change significantly with number of characteristics of the MS (Table 3).
Table 3.
Demographic, anthropometric, serum and urinary characteristics of non-Stone-forming subjects from Dallas and number of features of the MSa
| Number of MS features |
P for trend | |||||
|---|---|---|---|---|---|---|
| 0 (N = 29) | 1 (N = 28) | 2 (N = 20) | 3 (N = 12) | ≥4 (N = 20) | ||
| Gender, male/female; n ( % ) | 13/16 (45/55) | 14/14 (50/50) | 11/9 (55/45) | 7/5 (58/42) | 12/8 (60/40) | 0.8 |
| Ethnicity, White/Asian/Hispanic | 24/3/2 | 22/4/2 | 15/3/2 | 10/1/1 | 14/1/5 | 0.6 |
| Age, years | 38 ± 12 | 35 ± 13 | 47 ± 15 | 50 ± 18 | 54 ± 11 | < 0.0001 |
| BMI, kg/m2 | 22.9 ± 3.5 | 24.6 ± 3.7 | 28.1 ± 3.6 | 32.8 ± 4.5 | 35.2 ± 6.1 | < 0.0001 |
| Systolic BP, mmHg | 113 ± 9 | 111 ± 11 | 126 ± 16 | 131 ± 18 | 133 ± 15 | < 0.0001 |
| Diastolic BP, mmHg | 72 ± 9 | 70 ± 11 | 78 ± 10 | 77 ± 10 | 82 ± 8 | < 0.0001 |
| Serum | ||||||
| Glucose, mmol/L | 4.9 ± 0.4 | 4.9 ± 0.6 | 5.7 ± 1.1 | 5.8 ± 1.4 | 7.0 ± 2.4 | < 0.0001 |
| Triglycerides, mmol/L | 0.73 ± 0.35 | 0.96 ± 0.62 | 1.81 ± 1.07 | 1.23 ± 0.49 | 3.01 ± 1.43 | < 0.0001 |
| HDL cholesterol, mmol/L | 1.46 ± 0.27 | 1.14 ± 0.25 | 1.16 ± 0.22 | 1.05 ± 0.26 | 0.97 ± 0.16 | < 0.0001 |
| Insulin, pmol/L | 42 ± 24 | 50 ± 38 | 72 ± 33. | 91 ± 34. | 129 ± 72 | < 0.0001. |
| HOMA-IR | 0.8 ± 0.4 | 0.9 ± 0.5 | 1.3 ± 0.6 | 1.7 ± 0.6 | 2.5 ± 1.4 | < 0.0001. |
| Urine | ||||||
| Total volume, L/day | 1.95 ± 0.94 | 1.66 ± 0.79 | 2.05 ± 0.85 | 2.27 ± 1.29 | 2.13 ± 1.39 | 0.2 |
| Calcium, mmol/day | 3.6 ± 1.8 | 3.7 ± 2.0 | 4.3 ± 1.9 | 4.6 ± 2.2 | 6.0 ± 2.9 | 0.0003 |
| Oxalate, mmol/day | 292 ± 101 | 302 ± 86 | 329 ± 109 | 341 ± 113 | 340 ± 124 | 0.06 |
| Uric acid, mmol/day | 2.84 ± 0.91 | 3.06 ± 1.31 | 3.07 ± 1.17 | 3.30 ± 0.83 | 3.21 ± 1.09 | 0.2 |
| pH | 6.23 ± 0.44 | 6.19 ± 0.44 | 5.91 ± 0.38 | 5.87 ± 0.51 | 5.55 ± 0.47 | < 0.0001 |
| Citrate, mEq/day | 8.9 ± 4.2 | 10.9 ± 4.2 | 9.8 ± 3.8 | 7.5 ± 4.2 | 9.6 ± 3.5 | 0.5 |
| Sulfate, mmol/day | 17.0 ± 6.7 | 17.3 ± 8.4 | 21.6 ± 7.3 | 19.3 ± 4.8 | 24.5 ± 9.6 | 0.002 |
| NAE, mEq/day | 33.6 ± 25.3 | 32.2 ± 25.9 | 50.8 ± 25.5 | 52.4 ± 26.7 | 64.8 ± 29.1 | < 0.0001 |
| NAE, mEq/day (calculated) | 32.5 ± 22.8. | 31.1 ± 22.8. | 45.7 ± 24.1. | 47.7 ± 21.8. | 64.8 ± 35.4. | < 0.0001. |
| NH4+/NAE | 0.85 (0.66–0.97) | 0.74 (0.53–1.09) | 0.62 (0.52–0.79) | 0.58 (0.54–0.69) | 0.56 (0.46–0.65) | < 0.0001 |
| Creatinine clearance, mL/s | 1.77 ± 0.32 | 1.87 ± 0.55 | 2.08 ± 0.68 | 2.10 ± 0.90 | 2.20 ± 0.67 | 0.008 |
| Supersaturation indices | ||||||
| SI CaOx (vol adjusted) | 2.76 ± 1.21 | 2.74 ± 1.45 | 3.30 ± 1.15 | 3.95 ± 1.89 | 4.45 ± 1.65 | < 0.0001 |
| SI Br (volume adjusted) | 0.91 ± 0.50 | 0.97 ± 0.67 | 0.92 ± 0.50 | 1.07 ± 0.90 | 0.88 ± 0.57 | 0.9 |
aValues are expressed as mean ± SD except where otherwise noted.
Fig. 1.
Trends in urinary calcium and Supersaturation index of calcium oxalate (CaOx). (A) Unadjusted. (B) Adjusted for total volume, age, gender, urine sodium and urine sulfate. Data are presented as mean and 95 % confidence interval. P-values represent tests of linear trends for each group. Unadjusted and adjusted urinary calcium and SI CaOx values were higher in stone formers than in non-stone formers at across of features of the MS (P ≤ 0.001).
Characteristics of calcium stone-forming subjects from Dallas
A total of 128 recurrent calcium kidney stone formers were retrospectively evaluated. Comprehensive demographic characteristics of the stone-forming population are shown in Table 4. The mean ( ± SD) age was 43 ± 12 years, and the mean BMI was 29.3 ± 8.4 kg/m2. The median number of MS features was 2 and 35.9 % of subjects met the criteria for the MS. A number of features of the MS were retrieved at the time of initial evaluation. Participants were further subdivided according to the presence or absence of the MS. Demographic, anthropometric and biochemical characteristics of these groups are illustrated in Table 5. There was no significant difference in gender and ethnic distributions between the two groups.
Table 4.
Demographic characteristics of calcium stone-forming subjects from Dallas
| Characteristic | Value |
|---|---|
| No of participants | 128 |
| Gender [male/female; n ( % )] | 72/56 (56/44) |
| Ethnicity (White/Asian/Hispanic/American Indian) | 118/2/6/2 |
| Age (years; mean ± SD) | 43 ± 12 |
| Height (cm; mean ± SD) | 171 ± 10 |
| Weight (kg; mean ± SD) | 87 ± 27 |
| BMI (kg/m2; mean ± SD) | 29.3 ± 8.4 |
| Elevated BMI [n ( % )] | 42 (32.8) |
| Elevated fasting blood glucose [n ( % )] | 31 (24.2) |
| Elevated triglycerides [n ( % )] | 42 (32.8) |
| Low HDL [n ( % )] | 88 (68.8) |
| Elevated BP [n ( % )] | 56 (43.8) |
| No. of MS features [median (IQR)] | 2 (0–4) |
| MS [n ( % )] | 46 (35.90) |
Table 5.
Demographic, anthropometric, serum and urinary characteristics of calcium stone-forming subjects from Dallas with and without the MSa
| No MS (N = 82) | MS (N = 46) | P | |
|---|---|---|---|
| Gender, male/female; n ( % ) | 44/38 (52/48) | 28/18 (60/40) | 0.4 |
| Ethnicity, White/Asian/Hispanic/American Indian | 75/1/4/2 | 43/1/2/0 | 0.7 |
| Age, years | 42 ± 12 | 45 ± 11 | 0.2 |
| BMI, kg/m2 | 25.9 ± 5.1 | 35.4 ± 9.6 | < 0.0001 |
| Systolic BP, mmHg | 121 ± 14 | 128 ± 13 | 0.01 |
| Diastolic BP, mmHg | 75 ± 9 | 82 ± 9 | 0.001 |
| Serum | |||
| Glucose, mmol/L | 5.0 ± 0.4 | 5.8 ± 1.5 | 0.0004 |
| Triglycerides, mmol/L | 1.14 ± 0.58 | 2.70 ± 1.58 | < 0.0001 |
| HDL cholesterol, mmol/L | 1.35 ± 0.45 | 1.07 ± 0.20 | < 0.0001 |
| Urine | |||
| Total volume, L/day | 1.87 ± 1.00 | 2.19 ± 1.33 | 0.2 |
| Calcium, mmol/day | 5.8 ± 2.9 | 6.1 ± 3.5 | 0.50 |
| Oxalate, mmol/day | 353 ± 141 | 454 ± 206 | 0.004 |
| Uric acid, mmol/day | 3.28 ± 1.12 | 3.79 ± 1.52 | 0.05 |
| pH | 6.14 ± 0.42 | 5.91 ± 0.44 | 0.005 |
| Citrate, mEq/day | 7.5 ± 4.6 | 8.0 ± 5.7 | 0.6 |
| Sulfate, mmol/day | 17.9 ± 7.6 | 20.1 ± 10.2 | 0.2 |
| NAE, mEq/day (calculated) | 43.7 ± 22.0 | 58.0 ± 32.0 | 0.009 |
| NH4+/NAE | 0.79 (0.68–0.92) | 0.68 (0.60–0.82) | 0.02 |
| Creatinine clearance, mL/s | 1.77 ± 0.47 | 2.05 ± 0.73 | 0.02 |
| Supersaturation indices | |||
| SI CaOx (volume adjusted) | 4.45 ± 1.94 | 5.47 ± 3.00 | 0.04 |
| SI Br (volume adjusted) | 1.53 ± 0.87 | 1.45 ± 0.91 | 0.6 |
aValues are expressed as mean ± SD.
Relationship of biochemical profiles to the MS in calcium stone-forming subjects from Dallas
Urinary calcium, sulfate and citrate did not differ between the two groups. However, urinary pH (6.14 ± 0.42 versus 5.91 ± 0.44; P = 0.005) was lower, while urinary oxalate (353 ± 141 versus 454 ± 206 mmol/day; P = 0.004) and NAE (43.7 ± 22.0 mEq/day versus 58.0 ± 32.0 mEq/day; P = 0.009) were higher in kidney stone formers with the MS than in those without. NH4+/NAE was lower in kidney stone formers with the MS [0.79 (0.68–0.92) versus 0.68 (0.60–0.82); P = 0.02]. Creatinine clearance significantly differed between those with or without the MS (1.77 ± 0.47 versus 2.05 ± 0.73 mL/min; P = 0.02). However, creatinine clearance ranged between 40 and 60 mL/min in only five patients. SI of CaOx was higher in stone formers with the MS than those without (5.47 ± 3.0 versus 4.45 ± 1.94; P = 0.04). SI of brushite did not differ between the two groups.
Calcium stone-forming subjects were further stratified according to the number of features of the MS as represented in Table 6. Age and ethnicity did not differ according to the number of MS features. BMI, systolic and diastolic BP increased according to the number of features of the MS. Urinary pH decreased (P = 0.002 for trend), urinary NAE increased (P = 0.0004 for trend) but NH4+/NAE decreased (P = 0.001 for trend) with increasing number of features of the MS. Creatinine clearance also progressively increased (from 101 ± 27 to 143 ± 43 mL/min; P = 0.0004 for trend) with increasing features of the MS (Table 6). Mean 24-h urinary calcium did not change with increasing MS features (Figure 1A and 1B) but urinary oxalate increased significantly (P = 0.001 for trend). SI of CaOx also increased significantly with increasing characteristics of the MS (4.46 ± 1.80–6.16 ± 3.7; P = 0.009 for trend) (Figure 1A) but was not significant after covariate adjustment for age, gender, urine sodium, urine sulfate and creatinine clearance (Figure 1B).
Table 6.
Demographic, anthropometric, serum and urinary characteristics of calcium stone-forming subjects from Dallas and number of features of the MSa
| Number of MS features |
P for trend | |||||
|---|---|---|---|---|---|---|
| 0 (N = 21) | 1 (N = 32) | 2 (N = 29) | 3 (N = 22) | ≥4 (N = 24) | ||
| Gender, male/female; n ( % ) | 7/14 (36/67) | 14/18 (44/56) | 23/6 (79/21) | 13/9 (59/41) | 15/9 (63/37) | 0.01 |
| Ethnicity, White/Asian/Hispanic/American Indian | 20/0/1/0 | 30/1/1/0 | 25/2/0/2 | 19/1/2/0 | 24/0/0/0 | 0.4 |
| Age, years | 41 ± 11 | 39 ± 11 | 46 ± 13 | 47 ± 12 | 43 ± 10 | 0.1 |
| BMI, kg/m2 | 22.3 ± 2.8 | 25.0 ± 3.2 | 29.4 ± 5.9 | 30.5 ± 6.3 | 40.0 ± 9.9 | < 0.0001 |
| Systolic BP, mmHg | 114 ± 9 | 121 ± 17 | 125 ± 12. | 128 ± 13 | 128 ± 13 | 0.001 |
| Diastolic BP, mmHg | 70 ± 5 | 74 ± 10 | 79 ± 10 | 81 ± 8 | 83 ± 11 | < 0.0001 |
| Serum | ||||||
| Glucose, mmol/L | 4.9 ± 0.3 | 4.9 ± 0.4 | 5.1 ± 0.5 | 5.2 ± 0.4 | 6.4 ± 1.9 | < 0.0001 |
| Triglycerides, mmol/L | 0.81 ± 0.27 | 1.00 ± 0.42 | 1.54 ± 0.69 | 2.46 ± 1.29 | 2.91 ± 1.82 | < 0.0001 |
| HDL cholesterol, mmol/L | 1.76 ± 0.52 | 1.33 ± 0.37 | 1.07 ± 0.18 | 1.07 ± 0.21 | 1.06 ± 0.19 | < 0.0001 |
| Urine | ||||||
| Total volume, L/day | 1.7. ± 0.97 | 1.84 ± 1.16 | 2.00 ± 0.86 | 1.66 ± 0.70 | 2.68 ± 1.58 | 0.02 |
| Calcium, mmol/day | 5.2 ± 2.3 | 5.4 ± 2.4 | 6.6 ± 3.5 | 5.2 ± 2.5 | 7.0 ± 4.0 | 0.09 |
| Oxalate, mmol/day | 356 ± 141 | 336 ± 126 | 373 ± 159 | 399 ± 198 | 504 ± 203 | 0.001 |
| Uric acid, mmol/day | 2.96 ± 0.90 | 3.23 ± 1.14 | 3.57 ± 1.20 | 3.20 ± 1.31 | 4.34 ± 1.50 | 0.001 |
| pH | 6.23 ± 0.40 | 6.15 ± 0.44 | 6.05 ± 0.41 | 5.95 ± 0.37 | 5.88 ± 0.50 | 0.002 |
| Citrate, mEq/day | 7.0 ± 3.3 | 8.3 ± 4.8 | 7.0 ± 5.0 | 6.1 ± 3.3 | 9.6 ± 6.9 | 0.3 |
| Sulfate, mmol/day | 17.2 ± 7.4 | 17.2 ± 7.9 | 19.1 ± 7.6 | 16.3 ± 9.3 | 23.5 ± 9.8 | 0.03 |
| NAE, mEq/day (calculated) | 37.4 ± 18.8 | 41.3 ± 22.7 | 50.8 ± 22.0 | 52.7 ± 26.5 | 63.1 ± 36.3 | 0.0004 |
| NH4+/NAE | 0.87 (0.73–0.98) | 0.83 (0.69–0.96) | 0.72 (0.65–0.82) | 0.74 (0.61–0.81) | 0.65 (0.60–0.93) | 0.001 |
| Creatinine clearance, mL/s | 1.68 ± 0.45 | 1.80 ± 0.48 | 1.77 ± 0.45 | 1.67 ± 0.57 | 2.38 ± 0.72 | 0.0004 |
| Supersaturation indices | ||||||
| SI CaOx (volume adjusted) | 4.46 ± 1.80 | 4.01 ± 1.81 | 4.93 ± 2.12 | 4.71 ± 1.74 | 6.16 ± 3.71 | 0.009 |
| SI Br (volume adjusted) | 1.39 ± 0.81 | 1.47 ± 0.80 | 1.71 ± 0.98 | 1.36 ± 0.78 | 1.54 ± 1.02 | 0.7 |
aValues are expressed as mean ± SD except where otherwise noted.
Our tests of group-by-MS interaction do not allow us to conclude that non-stone formers and stone formers have differing trends, whether adjusted or unadjusted (interaction P-values for urine calcium: P = 0.5 unadjusted, P = 0.4 adjusted; SI CaOx: P = 0.9 unadjusted, P = 0.9 adjusted). However, the differences between non-stone formers and stone formers remained irrespective of the number of MS features (group main effect for urine calcium: P = 0.001 unadjusted, P = 0.0004 adjusted; SI CaOx: P = 0.0005 unadjusted, P = 0.0007 adjusted) (Figure 1A and 1B).
Anthropometric characteristics and biochemical profiles from Bern, Switzerland
A total of 140 additional recurrent calcium kidney stone formers from a separate cohort were retrospectively evaluated and divided into subjects without the MS and those with the MS (Table 7). BMI, systolic BP and diastolic BP were significantly higher in subjects with the MS. Serum glucose and triglyceride concentrations were significantly higher, and serum HDL was significantly lower in those with the MS than in those without (Table 7). Urinary calcium and oxalate did not differ between the two groups of calcium stone formers. Accordingly, SI of CaOx and brushite did not differ between the two groups.
Table 7.
Demographic, anthropometric, serum and urinary characteristics of calcium stone-forming subjects from Bern with and without the MSa
| No MS (N = 111) | MS (N = 29) | P | |
|---|---|---|---|
| Gender, male/female; n ( % ) | 90/21 (81/19) | 26/3 (90/10) | 0.4 |
| Age, years | 48 ± 14 | 49 ± 10 | 0.8 |
| BMI, kg/m2 | 25.0 ± 3.8 | 30.7 ± 6.0 | < 0.0001 |
| Systolic BP, mmHg | 137 ± 20 | 146 ± 17 | 0.03 |
| Diastolic BP, mmHg | 86 ± 11 | 95 ± 15 | 0.003 |
| Serum | |||
| Glucose, mmol/L | 5.1 ± 0.8 | 5.7 ± 1.1 | 0.01 |
| Triglycerides, mmol/L | 1.11 ± 0.45 | 2.24 ± 0.80 | < 0.0001 |
| HDL cholesterol, mmol/L | 1.39 ± 0.32 | 0.99 ± 0.18 | < 0.0001 |
| Urine | |||
| Total volume, L/day | 2.08 ± 0.77 | 2.19 ± 1.23 | 0.7 |
| Calcium, mmol/day | 6.9 ± 3.7 | 7.0 ± 3.2 | 0.8 |
| Oxalate, mmol/dayb | 298 ± 138 | 327 ± 116 | 0.6 |
| Uric acid, mmol/day | 3.27 ± 1.21 | 3.68 ± 1.12 | 0.09 |
| pH | 6.07 ± 0.81 | 6.14 ± 0.68 | 0.6 |
| Citrate, mEq/day | 9.4 ± 8.3 | 9.4 ± 5.9 | 0.9 |
| Sulfate, mmol/day | 24.5 ± 17.7 | 25.4 ± 11.5 | 0.7 |
| NAE, mEq/day (calculated) | 66.8 ± 40.8 | 69.5 ± 43.7 | 0.9 |
| Creatinine clearance, mL/s | 1.97 ± 0.58 | 2.27 ± 0.67 | 0.02 |
| Supersaturation indices | |||
| SI CaOx (volume adjusted) | 3.37 ± 1.98 | 4.04 ± 2.78 | 0.5 |
| SI Br (volume adjusted) | 1.35 ± 0.92 | 1.59 ± 0.73 | 0.6 |
aValues are expressed as mean ± SD.
bOxalate only available in 25 subjects with no MS and six subjects with the MS.
Discussion
One principal finding of this study is increased risk of CaOx crystallization (SI CaOx) with increasing features of the MS (Table 3 and 6). However, this risk cannot be independently attributed to the MS. The MS likely acts through other factors such as age [21], gender [22, 23], urine sodium [24] and urine sulfate [25–27], which is known to influence the risk of CaOx stone formation (Figure 1B). Furthermore, differences in SI CaOx between non-stone formers and stone formers are maintained irrespective of the number of features of the MS (Figure 1A and 1B). Urinary calcium and SI CaOx were higher in stone formers than in non-stone formers as the number of features of the MS increased. Although the slope remained similar, the difference between the two groups was significant (P ≤ 0.001). This strongly supports that a plethora of underlying heterogeneous pathophysiological abnormalities in hypercalciuric calcium stone formers, such as intestinal hyperabsorption of calcium, disturbed bone calcium balance and renal calcium leak prevail diminishing the contribution of MS features on hypercalciuria [28–31].
Therefore, while we can demonstrate that the MS is associated with increased urinary calcium excretion and the physicochemical risk of CaOx stone formations in both non-stone formers and stone formers, such an effect cannot be consistently detected on urinary calcium excretion and/or CaOx saturation in these subjects when adjusted for variables known to affect urinary calcium [21–27]. We propose that while the MS influences calcium excretion and CaOx supersaturation, it does not independently exert a large effect on calciuria. This is unlike unduly acidic urine, the principle determinant of urinary uric acid crystallization, which was found to be independently related to the MS in the general kidney stone-forming population and more specifically in patients with established uric acid nephrolithiasis [6, 32]. These results were supported by additional studies in normal subjects and diabetic non-stone formers who exhibited an association between the MS and low urinary pH [9, 10]. In animal and cell culture models, the potential pathophysiological link between the MS and low urinary pH was attributed to increased fatty acid provision to the renal proximal tubule leading to lipotoxicity and resulting in impaired ammonium production and secretion [33, 34].
A separate unadjusted analysis was performed in the stone-forming population from Bern, Switzerland. Similar to the results from Dallas, urinary calcium did not significantly differ between stone formers with and without the MS (Table 7). On the contrary, no difference in urinary oxalate, and consequently SI CaOx, was seen in Bern stone formers with and without the MS. This result was unlike the significantly higher urinary oxalate in Dallas stone formers with the MS. A limitation was that only 31 urinary oxalate measurements were able to be measured with an established assay.
Our result is significant since it was performed in a cohort of established kidney stone formers followed in kidney stone clinics. Although previous population-based studies [4, 5, 35] established the relationship between the MS and kidney stone disease, the diagnosis of kidney stones was based on self-reported history of kidney stones. In addition, validation of self-reported kidney stone history was only performed in a very small percentage of participants in the Health Professionals Follow-up Study and Nurse’s Health Study I [5, 35]. On the contrary, one report in a large number of stone-forming diabetic and non-diabetic patients showed that the relative percentage of CaOx stone formation was lower in diabetic stone formers in whom BMI was significantly higher than in non-diabetics [36].
One potential link between the MS and nephrolithiasis is hyperinsulinemia, an invariable feature of the MS. Hyperinsulinemia and obesity act in opposing directions on renal tubular calcium handling. While hyperinsulinemia could potentially cause hypercalciuria [37, 38], this effect can be opposed by body weight [39] and calciotropic hormone status [40]. Additional studies using the hyperinsulinemic euglycemic clamp in both non-stone-forming and calcium stone-forming subjects are needed to understand the potential role of insulin in urinary calcium excretion.
The association of MS features with urinary oxalate excretion has not been studied. One study suggested that larger body size is associated with higher urinary oxalate excretion in normal subjects, which may be attributed to increased endogenous oxalate production [41]. In the present study, urinary oxalate was much higher in calcium stone formers with the MS (Table 5). The increase in urinary oxalate with features of the MS was also higher in the kidney stone-forming population (Table 6), which may be responsible for significantly higher unadjusted SI CaOx (Figure 1A). However, the magnitude of this rise was not sufficient to change the urinary supersaturation of CaOx when adjusted for age, gender, urine sodium, urine sulfate and creatinine clearance in stone formers (Figure 1B). One advantage of epidemiological studies is the inclusion of a large number of subjects, but a limitation is the absence of urinary stone risk profiles. Moreover, in many epidemiological studies, kidney stone incidence was based solely on self-report and there was a lack of identification of kidney stone composition. Our study overcame some of these limitations, yet the sample size was smaller in comparison. However, a notable limitation of our study was that it is cross-sectional in nature. Nevertheless, in general the risk of CaOx nephrolithiasis coincides with urinary supersaturation profiles [42]. To substantiate this link, long-term studies with kidney stone incidence as a primary end point are needed.
Conclusions
In subjects without a history of kidney stones, the risk of CaOx stone formation increases with the number of features of the MS. In subjects with established history of calcium stones, the risk of CaOx precipitation is much higher overall but is not independently associated with the number of features of the MS.
Acknowledgements
The authors were supported by the National Institutes of Health grants R01-DK081423, R01-DK081523, M01-RR00633, P01-DK20543; Seldin-Pak Center of Metabolic Research, Beauticontrol Cosmetics, Inc. Professorship in Mineral Metabolism & Osteoporosis. The authors would like to acknowledge Hadley Palmer for her primary role in the preparation of this manuscript.
Conflict of interest statement. None declared.
(See related article by Gambaro. Calcium nephrolithiasis, metabolic syndrome and the cardiovascular risk. Nephrol Dial Transplant 2012; 27: 3008–3010.)
References
- 1.Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among U.S. adults. Diabetes Care. 2004;27:2444–2449. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 4.West B, Luke A, Durazo-Arvizu RA, et al. Metabolic syndrome and self-reported history of kidney stones: the National Health and Nutrition Examination Survey (NHANES III) 1988–1994. Am J Kidney Dis. 2008;51:741–747. doi: 10.1053/j.ajkd.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 5.Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293:455–462. doi: 10.1001/jama.293.4.455. [DOI] [PubMed] [Google Scholar]
- 6.Sakhaee K, Adams-Huet B, Moe OW, et al. Pathophysiologic basis for normouricosuric uric acid nephrolithiasis. Kidney Int. 2002;62:971–979. doi: 10.1046/j.1523-1755.2002.00508.x. [DOI] [PubMed] [Google Scholar]
- 7.Abate N, Chandalia M, Cabo-Chan AV, Jr, et al. The metabolic syndrome and uric acid nephrolithiasis: novel features of renal manifestation of insulin resistance. Kidney Int. 2004;65:386–392. doi: 10.1111/j.1523-1755.2004.00386.x. [DOI] [PubMed] [Google Scholar]
- 8.Cameron MA, Maalouf NM, Adams-Huet B, et al. Urine composition in type 2 diabetes: predisposition to uric acid nephrolithiasis. J Am Soc Nephrol. 2006;17:1422–1428. doi: 10.1681/ASN.2005121246. [DOI] [PubMed] [Google Scholar]
- 9.Maalouf NM, Cameron MA, Moe OW, et al. Low urine pH: a novel feature of the metabolic syndrome. Clin J Am Soc Nephrol. 2007;2:883–888. doi: 10.2215/CJN.00670207. [DOI] [PubMed] [Google Scholar]
- 10.Maalouf NM, Cameron MA, Moe OW, et al. Metabolic basis for low urine pH in type 2 diabetes. Clin J Am Soc Nephrol. 2010;5:1277–1281. doi: 10.2215/CJN.08331109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daudon M, Traxer O, Conort P, et al. Type 2 diabetes increases the risk for uric acid stones. J Am Soc Nephrol. 2006;17:2026–2033. doi: 10.1681/ASN.2006030262. [DOI] [PubMed] [Google Scholar]
- 12.Coe FL, Parks JH, Asplin JR. The pathogenesis and treatment of kidney stones. N Engl J Med. 1992;327:1141–1152. doi: 10.1056/NEJM199210153271607. [DOI] [PubMed] [Google Scholar]
- 13.Levy FL, Adams-Huet B, Pak CY. Ambulatory evaluation of nephrolithiasis: an update of a 1980 protocol. Am J Med. 1995;98:50–59. doi: 10.1016/S0002-9343(99)80080-1. [DOI] [PubMed] [Google Scholar]
- 14.Ford ES, Mokdad AH, Giles WH. Trends in waist circumference among U.S. adults. Obes Res. 2003;11:1223–1231. doi: 10.1038/oby.2003.168. [DOI] [PubMed] [Google Scholar]
- 15.Sundstrom J, Riserus U, Byberg L, et al. Clinical value of the metabolic syndrome for long term prediction of total and cardiovascular mortality: prospective, population based cohort study. BMJ. 2006;332:878–882. doi: 10.1136/bmj.38766.624097.1F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malik S, Wong ND, Franklin SS, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 17.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 18.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 19.Kok DJ, Poindexter J, Pak CY. Calculation of titratable acidity from urinary stone risk factors. Kidney Int. 1993;44:120–126. doi: 10.1038/ki.1993.221. [DOI] [PubMed] [Google Scholar]
- 20.Rodgers A, Allie-Hamdulay S, Jackson G. Therapeutic action of citrate in urolithiasis explained by chemical speciation: increase in pH is the determinant factor. Nephrol Dial Transplant. 2006;21:361–369. doi: 10.1093/ndt/gfi211. [DOI] [PubMed] [Google Scholar]
- 21.Taylor EN, Curhan GC. Demographic, dietary, and urinary factors and 24-h urinary calcium excretion. Clin J Am Soc Nephrol. 2009;4:1980–1987. doi: 10.2215/CJN.02620409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frumar AM, Meldrum DR, Geola F, et al. Relationship of fasting urinary calcium to circulating estrogen and body weight in postmenopausal women. J Clin Endocrinol Metab. 1980;50:70–75. doi: 10.1210/jcem-50-1-70. [DOI] [PubMed] [Google Scholar]
- 23.Heller HJ, Sakhaee K, Moe OW, et al. Etiological role of estrogen status in renal stone formation. J Urol. 2002;168:1923–1927. doi: 10.1016/S0022-5347(05)64264-4. [DOI] [PubMed] [Google Scholar]
- 24.Sakhaee K, Harvey JA, Padalino PK, et al. The potential role of salt abuse on the risk for kidney stone formation. J Urol. 1993;150(2 Pt 1):310–312. doi: 10.1016/s0022-5347(17)35468-x. [DOI] [PubMed] [Google Scholar]
- 25.Lemann J, Litzow JR, Lennon EJ. Studies of the mechanism by which chronic metabolic acidosis augments urinary calcium excretion in man. J Clin Invest. 1967;46:1318–1328. doi: 10.1172/JCI105624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breslau NA, Brinkley L, Hill KD, et al. Relationship of animal protein-rich diet to kidney stone formation and calcium metabolism. J Clin Endocrinol Metab. 1988;66:140–146. doi: 10.1210/jcem-66-1-140. [DOI] [PubMed] [Google Scholar]
- 27.Borghi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med. 2002;346:77–84. doi: 10.1056/NEJMoa010369. [DOI] [PubMed] [Google Scholar]
- 28.Brannan PG, Vergne-Marini P, Pak CY, et al. Magnesium absorption in the human small intestine. Results in normal subjects, patients with chronic renal disease, and patients with absorptive hypercalciuria. J Clin Invest. 1976;57:1412–1418. doi: 10.1172/JCI108410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broadus AE, Dominguez M, Bartter FC. Pathophysiological studies in idiopathic hypercalciuria: use of an oral calcium tolerance test to characterize distinctive hypercalciuric subgroups. J Clin Endocrinol Metab. 1978;47:751–760. doi: 10.1210/jcem-47-4-751. [DOI] [PubMed] [Google Scholar]
- 30.Brannan PG, Morawski S, Pak CY, et al. Selective jejunal hyperabsorption of calcium in absorptive hypercalciuria. Am J Med. 1979;66:425–428. doi: 10.1016/0002-9343(79)91063-5. [DOI] [PubMed] [Google Scholar]
- 31.Coe FL, Favus MJ, Crockett T, et al. Effects of low-calcium diet on urine calcium excretion, parathyroid function and serum 1,25(OH)2D3 levels in patients with idiopathic hypercalciuria and in normal subjects. Am J Med. 1982;72:25–32. doi: 10.1016/0002-9343(82)90567-8. [DOI] [PubMed] [Google Scholar]
- 32.Maalouf NM, Sakhaee K, Parks JH, et al. Association of urinary pH with body weight in nephrolithiasis. Kidney Int. 2004;65:1422–1425. doi: 10.1111/j.1523-1755.2004.00522.x. [DOI] [PubMed] [Google Scholar]
- 33.Bobulescu IA, Dubree M, Zhang J, et al. Effect of renal lipid accumulation on proximal tubule Na+/H+ exchange and ammonium secretion. Am J Physiol Renal Physiol. 2008;294:F1315–F1322. doi: 10.1152/ajprenal.00550.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bobulescu IA, Dubree M, Zhang J, et al. Reduction of renal triglyceride accumulation: effects on proximal tubule Na+/H+ exchange and urinary acidification. Am J Physiol Renal Physiol. 2009;297:F1419–F1426. doi: 10.1152/ajprenal.00177.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curhan GC, Willett WC, Rimm EB, et al. Body size and risk of kidney stones. J Am Soc Nephrol. 1998;9:1645–1652. doi: 10.1681/ASN.V991645. [DOI] [PubMed] [Google Scholar]
- 36.Daudon M, Lacour B, Jungers P. High prevalence of uric acid calculi in diabetic stone formers. Nephrol Dial Transplant. 2005;20:468–469. doi: 10.1093/ndt/gfh594. [DOI] [PubMed] [Google Scholar]
- 37.DeFronzo RA, Cooke CR, Andres R, et al. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest. 1975;55:845–855. doi: 10.1172/JCI107996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kerstetter J, Caballero B, O'Brien K, et al. Mineral homeostasis in obesity: effects of euglycemic hyperinsulinemia. Metabolism. 1991;40:707–713. doi: 10.1016/0026-0495(91)90088-e. [DOI] [PubMed] [Google Scholar]
- 39.Pitroda AP, Harris SS, Dawson-Hughes B. The association of adiposity with parathyroid hormone in healthy older adults. Endocrine. 2009;36:218–223. doi: 10.1007/s12020-009-9231-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bell NH, Epstein S, Greene A, et al. Evidence for alteration of the vitamin D-endocrine system in obese subjects. J Clin Invest. 1985;76:370–373. doi: 10.1172/JCI111971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lemann J, Jr, Pleuss JA, Worcester EM, et al. Urinary oxalate excretion increases with body size and decreases with increasing dietary calcium intake among healthy adults. Kidney Int. 1996;49:200–208. doi: 10.1038/ki.1996.27. [DOI] [PubMed] [Google Scholar]
- 42.Parks JH, Coward M, Coe FL. Correspondence between stone composition and urine supersaturation in nephrolithiasis. Kidney Int. 1997;51:894–900. doi: 10.1038/ki.1997.126. [DOI] [PubMed] [Google Scholar]