Abstract
Background
In most patients, acute kidney injury (AKI) represents the combined effects of ischemic, toxic and inflammatory insults. No effective pharmacologic interventions have been developed to prevent AKI or to improve outcomes to date. Cyclosporine A (CsA) is a calcineurin inhibitor that mediates T-cell receptor signaling, suppresses inflammatory cytokine expression and inhibits leukocyte migration. It is also a potent inhibitor of mitochondrial permeability, protecting cells from death. These properties make it a potentially valuable drug to prevent or treat AKI. It does, however, carry a significant risk of nephrotoxicity, especially with chronic use. By contrast, a single dose of CsA may be protective while limiting the risk of nephrotoxicity.
Methods
We conducted a controlled animal experiment in male CD-1 mice. Specifically, mice were subjected to folic acid (FA)-induced AKI and then randomly assigned to sham operation or one of three dosage of CsA treatment groups.
Results
Intraperitoneal injection of FA consistently induced AKI. Serum interleukin (IL)-6 and urinary neutrophil gelatinase-associated lipocalin (NGAL) rose 1 day after FA injection. Compared to sham treatment, one dose (1 and 5 mg/kg body weight) of CsA significantly reduced kidney tubular cell apoptosis, serum creatinine, blood urea, serum IL-6 and urinary NGAL 2 days after FA injection. It was also shown to block the inflammatory mediator tumor necrosis factor (TNF)-like weak inducer of apoptosis (TWEAK) expression, nuclear factor kappa-B (NFκB) activation, inflammatory cell infiltration and interstitial fibrosis 14 days after treatment in a dose-dependent fashion. By contrast, a dose of 10 mg/kg body weight CsA resulted in nephrotoxicity in the setting of FA-induced AKI.
Conclusions
A single dose of CsA, currently used for organ transplant, significantly protects mice from FA-induced AKI, presumably through inhibition of cell death, inflammatory reaction, interstitial cell infiltration and fibrosis. The protective effects have the potential to open a completely new line of investigation in the prevention and treatment of AKI.
Keywords: acute kidney injury, apoptosis, cyclosporine A, folic acid, inflammation
Introduction
Acute kidney injury (AKI) remains a major clinical challenge, particularly in the critically ill and injured patients where it affects as many as 67% [1]. Severe AKI is associated with a 72% rate of renal replacement therapy and 60% of hospital mortality [2]. Furthermore, more than half of surviving patients may not recover their renal function [3]. Despite extensive efforts over the last two decades, treatment of AKI is still limited to supportive measures.
The pathogenesis of AKI may vary by type of injury. Drug-induced nephrotoxicity is a relatively common complication and attributes to up to 20% of hospital admissions due to AKI occurring in the community [4]. In the Acute Care Hospital setting, drug-induced nephrotoxicity has been implicated in 8–60% of all cases of in-hospital AKI and, as such, is a recognized source of significant morbidity and mortality [5]. As mechanisms of nephrotoxic AKI, injured renal tubular epithelial cells undergo necrosis and apoptosis [6], while other cells become sublethally injured and release immunological danger signals activating the innate immune system [7]. It is believed that activation of the immune response is an ultimate pathway that leads to leukocyte infiltration, ‘second hit’ injury to renal cells and worse outcome [8]. Nuclear factor kappa-B (NFκB) is a pleiotropic transcription factor that plays a central role in the immune response. Its activation results in upregulated expression of a variety of cytokines and chemokines which recruit inflammatory cells around the injured cells [9]. It is a well-known promoter in the pathogenesis of renal interstitial fibrosis and thus contributes to renal fibrosis and end-stage kidney disease [8].
Cyclosporine A (CsA), a calcineurin inhibitor with potent immunosuppressive effects, is a cornerstone in the management of solid organ transplantation. Long-term usage of CsA is associated with nephrotoxicity [10] and chronic kidney fibrosis. CsA toxicity is believed to be due to reduced DNA synthesis [11] and cell cycle blockade [12], but not tubular epithelial cell apoptosis or necrosis [12, 13]. CsA has immunosuppressive properties that are potentially useful for renal protection [14]. CsA is also an anti-apoptotic agent owing to prevention of the opening of the mitochondrial permeability transition (PT) pore [15] and thus can prevent mitochondrial cell death. Because of these characteristics, CsA was recently successfully used in patients with myocardial infarction to attenuate myocardial injury after ischemia–reperfusion [16].
We hypothesized that one dose of CsA (in the range currently used for organ transplantation) could attenuate AKI without inducing nephrotoxicity. We used a murine model of folic acid (FA)-induced AKI to assess the potential protective role of CsA.
Materials and methods
Animals
Male CD-1 mice weighing 25–35 g were purchased from Charles River Laboratories (Wilmington, MA). All protocols were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh. Mice were housed in the animal facilities of the University of Pittsburgh with free access to food and water.
Study protocol
Mice were randomly assigned to one of the following groups: (i) FA-induced AKI group: intraperitoneal injection of 250 mg/kg FA dissolved in 0.3 mM sodium bicarbonate vehicle, n = 12; (ii) Control: intraperitoneal injection of the same amount of 0.3 mM sodium bicarbonate as FA group, n = 12; (iii) FA-CsA 1 mg/kg of body weight (mg/kg.BW) group: tail vein injection of 1 mg/kg CsA 30 min after FA, n = 12; (iv) FA-CsA 5 mg/kg.BW group: tail vein injection of 5 mg/kg CsA 30 min after FA, n = 12; (v) FA-CsA 10 mg/kg.BW group: tail vein injection of 10 mg/kg CsA 30 min after FA, n = 12; (iv) FA-vehicle group: tail vein injection of the same amount of vehicle as CsA group after FA, n = 6. In addition, we randomly assigned healthy animals to receive CsA (n = 6 per group): (vii) CsA 1 mg/kg; (viii) CsA 5 mg/kg; (ix) CsA 10 mg/kg. This dosage range of CsA has been used in both rodents [17–19] and humans [20, 21] for immune suppression following organ transplant. FA-CsA 10 mg/kg after FA injection showed nephrotoxicity (Figure 2) and thus was not studied further in the mechanical investigations. FA was obtained from Sigma–Aldrich (St. Louis, MO). The dose of FA used reliably induces kidney injury, as assessed by significantly increased serum creatinine and blood urea nitrogen (BUN) and histological findings. Thirty minutes after FA administration, Groups 3–5 mice received an intravenous injection of CsA (Sigma–Aldrich). Serial blood was drawn from the saphenous vein at four time points: Days 2, 3, 7 and 14 after treatment. Urine was collected for 2 h in metabolic cages at 1 day before and Days 1–3, 7 and 14 after FA. Mice were sacrificed by both inhalation of high concentrations of CO2 and decapitation. Kidneys were harvested from sacrificed mice at Days 2 or 14. Left kidneys were used for routine histology and immunohistochemistry; right kidneys were snapped frozen in liquid nitrogen for additional analysis.
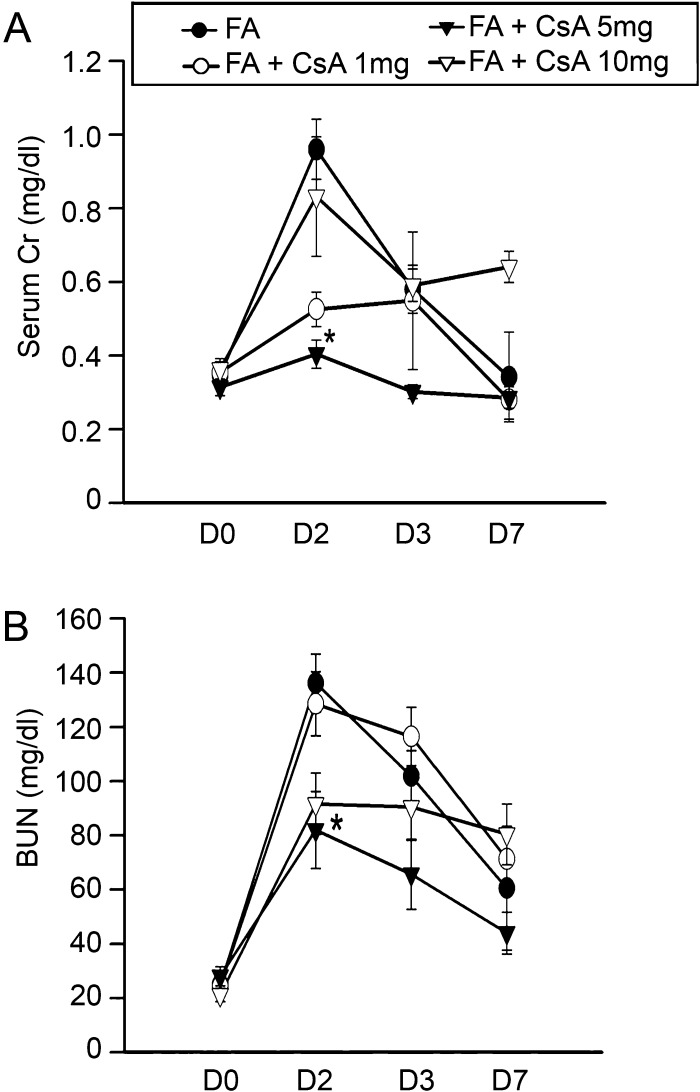
Fig. 2.
Kidney function was preserved following FA plus cyclosporine A (CsA) administration. CD1 mice were subjected to FA or FA + CsA. Serum creatinine (A) and BUN (B) are presented as mean ± SEM for each group of 12 animals. CsA at a dose of 5 mg/kg significantly decreased serum creatinine and BUN on Day 2. *P < 0.05 compared with FA only group on Day 2.
Blood and urine analyses
BUN, serum creatinine, interleukin (IL)-6 and urinary neutrophil gelatinase-associated lipocalin (NGAL) were measured by commercially available kits: Urea assay kit from BioAssay Systems (Hayward, CA), Creatinine Assay kit from BioVision (Mountain View, CA), IL-6 and urinary NGAL assay kit were purchased from R&D systems (Minneapolis, MN).
Immunohistochemistry and TUNEL staining
Kidney tissues were fixed in 10% neutral-buffered formalin and embedded in paraffin. Coronal sections were stained with hematoxylin and eosin (H&E) and Masson's trichrome reagent [22]. For immunohistochemistry staining, sections were deparaffinized, rehydrated and treated with proteinase K. After endogenous peroxidase was inactivated, slides were incubated with the primary antibody, rat anti-mouse F4/80 or rat anti-mouse neutrophil antibodies (Serotec, Raleigh, NC) at 4°C overnight. Signals of all primary antibodies were detected with biotinylated second antibody (anti-rat IgG Ab; Vector Laboratories) and were reacted with streptavidin-conjugated horseradish peroxidase, followed by a diaminobenzidine enhancement. Negative controls comprised the omission of primary antibody. Nuclei were counterstained with hematoxylin. For Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining, sections were stained as per the manufacturer's instructions (TdT In Situ Apoptosis Detection Kit #4810-30-K; R&D systems). Quantification of neutrophil, macrophage infiltration and TUNEL-positive cells was counted by average number of positive staining cells per field. The number of positive stains was determined from the mean of five randomly selected non-overlapping ×400 fields in each section blinded to the treatment. We used ImageJ to quantify Masson's trichrome stained fibrosis. Specifically, we converted the image to grayscale blue areas (which represent fibrotic areas in Masson's trichrome staining) were converted to dark, the fibrosis index was obtained by counting the dark pixels.
Western blotting
Frozen (−70°C) kidney tissues were processed for protein extraction. Protein extracts were prepared by homogenizing the whole cortex in 1 mL cold RIPA (Radio-Immunoprecipitation Assay) buffer containing protease inhibitors (Sigma–Aldrich) and a phosphatase inhibitor cocktail (Sigma–Aldrich) with Dounce homogenizer. After centrifugation at 4°C for 15 min at 15 000 g, the supernatants were collected and then centrifuged again to remove cellular debris. Protein concentration in the supernatant was determined with Micro Bicinchoninic Acid (BCA) assay kit (Sigma–Aldrich). Equal amounts of protein were resolved on 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (Bio-Rad, Richmond, CA) and then electroblotted to nitrocellulose membranes (Bio-Rad). The membranes were blocked for 1 h in TBS-T (10 mM Tris–HCl, pH 7.5, 150 mM NaCl, 0.05% Tween-20) containing 5% non-fat milk. Membranes were then incubated with rabbit polyclonal anti-mouse TWEAK antibody (Novus Biologicals, Littleton, CO) and diluted in the blocking buffer. The membranes were then washed thoroughly in TBS-T and incubated with anti-rabbit horseradish peroxidase-conjugated second antibody for 2 h. Detection of signal was visualized using the SuperSignal West Pico chemiluminescent substrate kit (Pierce Biotechnology, Rockford, IL).
Nuclear extract preparation and NFκB transcriptional activity assay
Nuclear extracts and NFκB activity measurement of kidney tissue were prepared with Nuclear Extract and TransAM NFκB p65 Transcription Factor Assay kit (Active Motif, Carlsbad, CA). Following weighing, tissue was diced into small pieces and homogenized in ice-cold complete lysis buffer with a Dounce homogenizer. The nuclear suspension was then centrifuged for 10 min at 10 000 g at 4°C, and the supernatant was used as the nuclear extract. The protein concentration of nuclear extracts was determined as described above, and 20 μg of protein per sample in triplicate was loaded in a 96-well plate. Following manufacturer recommendations, active NFκB activity was measured as optical density units and read at 450 nm with a reference wavelength of 650 nm.
Statistic analysis
All blood, urinary, cell number and fibrosis measurement values are expressed as mean ± SE. Statistical analysis was performed using SPSS 11.0 (SPSS Inc., Chicago, IL). Single comparisons were determined by the appropriate two-sided t-test or Mann–Whitney test, analysis of variance or Wilcoxon rank-sum tests was used for multiple comparisons. P-values < 0.05 were considered statistically significant.
Results
A single dose of CsA is not nephrotoxic
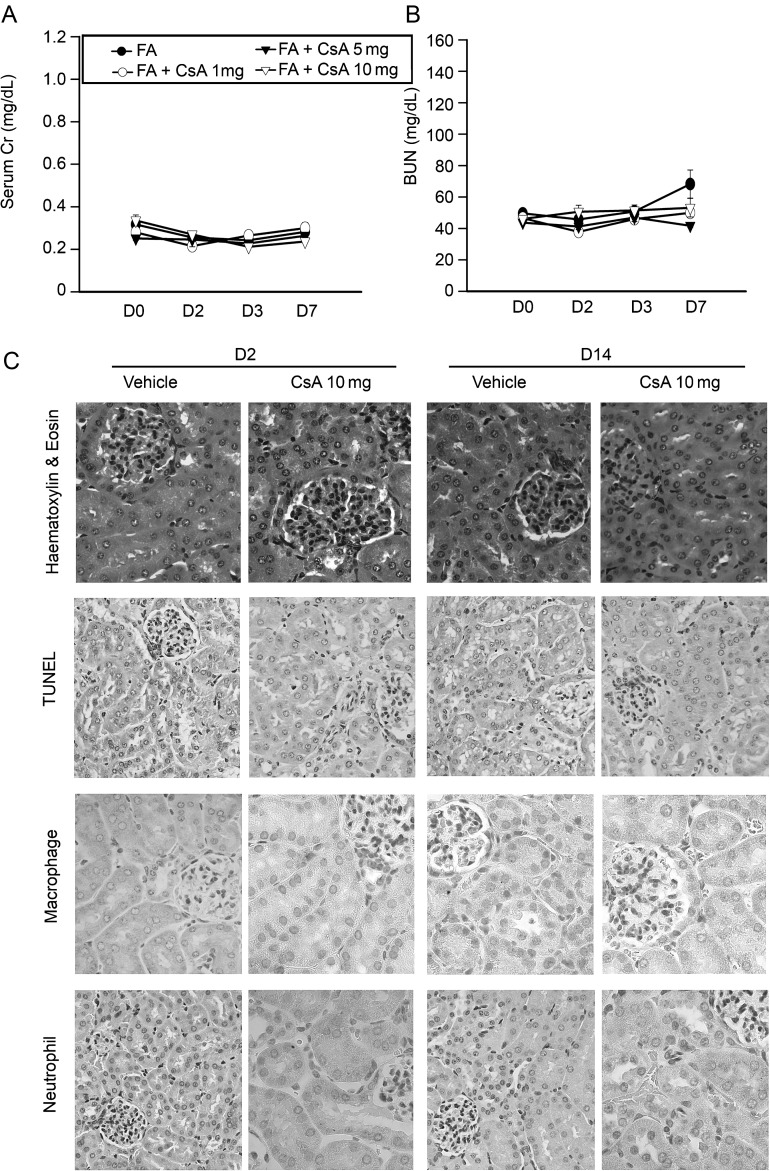
A single dose of CsA (1, 5 or 10 mg/kg) in study protocol for Groups 7, 8 and 9 had no significant effect on kidney function recorded by serum creatinine or BUN. Moreover, immunohistological examination of these kidneys did not show any signs of tubular epithelial cell apoptosis or significant inflammatory cell infiltration at 2 or 14 days after CsA administration (Figure 1).
Fig. 1.
A single dose of cyclosporine A (CsA) is not toxic to the kidney. Serum creatinine (A) and BUN (B) did not change and there were no differences between groups. H&E staining as well as TUNEL immunohistology staining (C) on Day 2 and Day 14 in kidney samples showed no structural defects, cell apoptosis or inflammatory cell infiltration even at a dose of CsA 10 mg/kg per body weight. Original magnification ×400.
FA resulted in a consistent pattern of AKI
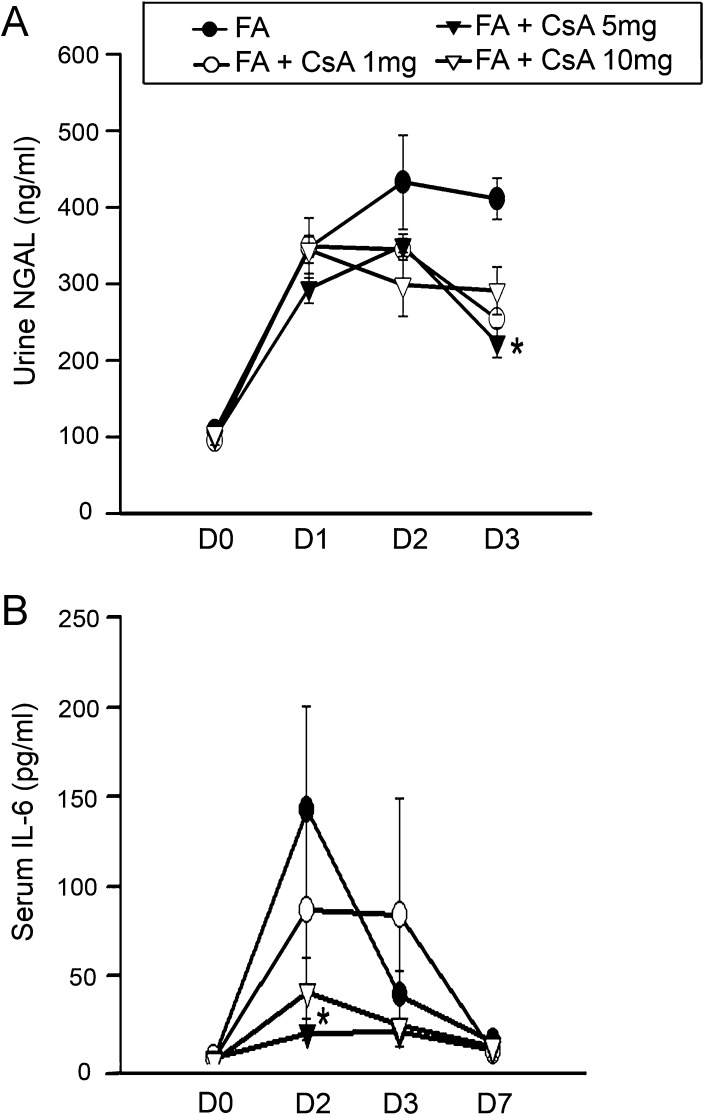
Intraperitoneal injection of a single dose of FA (250 mg/kg) consistently induced AKI at 48 h documented by a significant increase in serum creatinine and BUN 2- to 3-fold compared with administration of the vehicle alone (Figure 2). Urinary NGAL increase is a real-time indicator of active epithelial injury and therefore helps to quantify the extent of kidney injury [23]. Urinary NGAL was increased as early as 1 day after FA injection and remained at high levels consistently through Day 3 suggesting ongoing damage during this period of time.
CsA preserved renal function after FA administration
CsA at doses of 1 and 5 mg/kg in Groups 3 and 4 prevented FA-induced increases in serum creatinine and BUN on Day 2, whereas 10 mg/kg CsA plus FA (Group 5) showed nephrotoxicity and failed to improve kidney function (Figure 2) and thus was not studied further. Both serum creatinine and BUN in FA and CsA treatment groups were normalized by Day 7. Urinary NGAL was significantly reduced by Day 3 in the CsA treatment groups (Figure 3). After the acute phase, renal damage by FA was followed by partial regeneration and patchy tubulointerstitial fibrosis [24]. CsA (1 and 5 mg/kg) greatly reduced tubular epithelial cell apoptosis, a histological hallmark of FA-induced AKI. Comparing CsA-treated kidneys with FA-AKI histology, mice treated with 1 and 5 mg/kg of CsA 30 min after FA showed reductions in tubular epithelial cell flattening or loss at Day 2 (Figure 4).
Fig. 3.
Urinary NGAL and plasma IL-6 increases were inhibited by cyclosporine A (CsA) administration. Urine samples were collected before treatment and 1–3 days after FA. Urinary NGAL (A) was markedly increased starting from Day 1 following FA exposure. CsA decreased NGAL significantly on Day 3, n = 12. Serum IL-6 (B) was significantly inhibited by CsA 5 mg/kg on Day 2, n = 12 for each group. *P < 0.05 when compared with same time points as FA only.
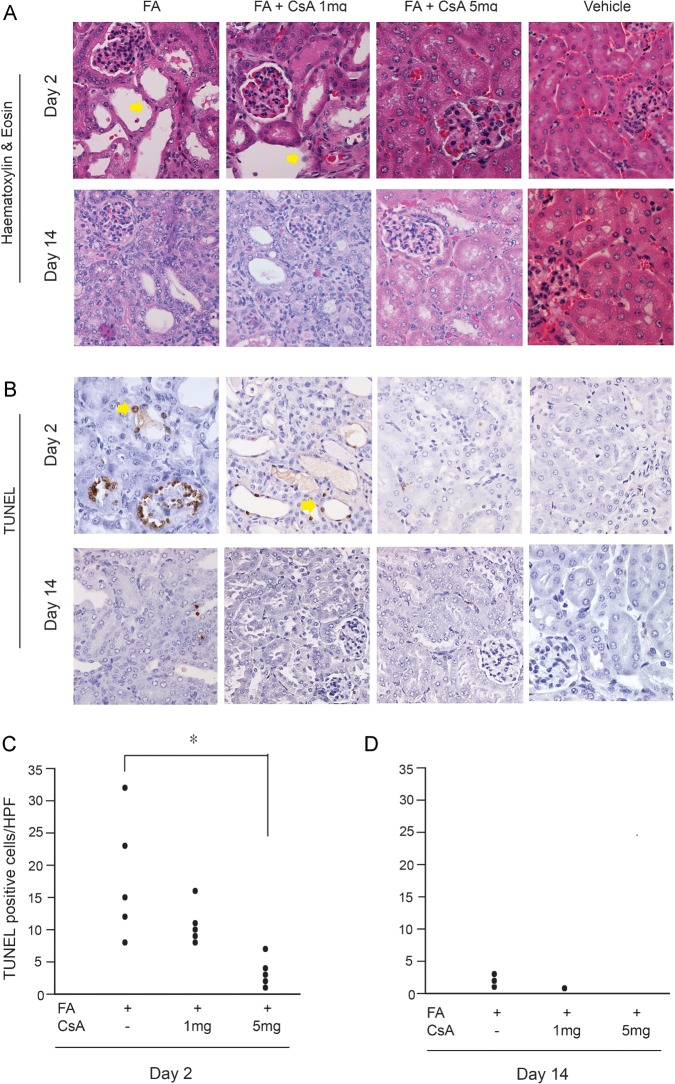
Fig. 4.
Cyclosporine A (CsA) protected tubular cells from apoptosis 2 days after FA administration. (A) H&E staining on representative FA + vehicle and FA + CsA at doses of 1 and 5 mg/kg in the kidney. CsA ameliorated proximal and distal tubular epithelial cell flattening in the kidney on Day 2 (see arrows). (B) TUNEL staining of representative kidneys. Kidneys of FA-exposed mice contained TUNEL-positive cells in the tubular epithelium area (see arrows). A marked decrease was noted in CsA 5 mg/kg group in the kidneys on day 2. Original magnification ×400. (C, D) TUNEL-positive cells per high-power field in FA and FA + CsA 1 and 5 mg/kg. *P < 0.05 compared with FA only mice.
Inflammatory cell infiltration and serum IL-6 expression were suppressed by CsA
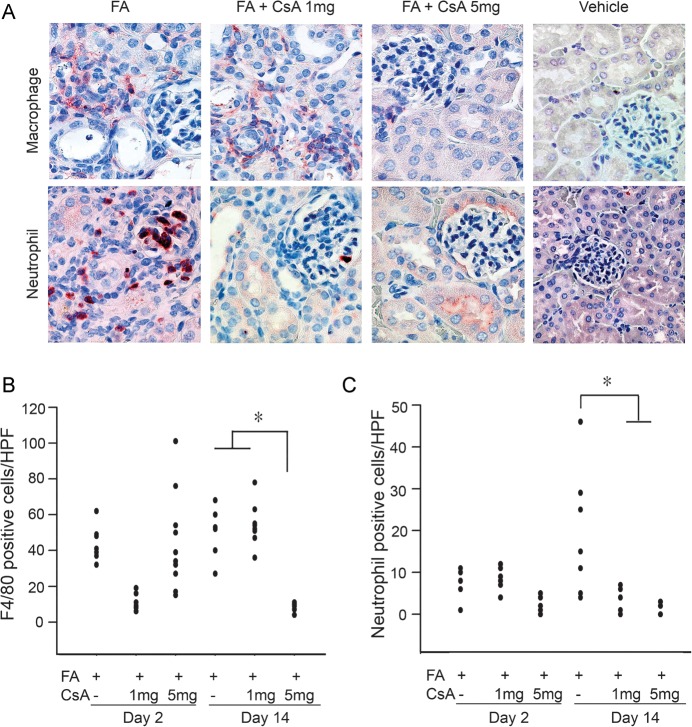
Given the negative association between IL-6 and survival [25] as well as higher levels of IL-6 predicting development of AKI [26] in human sepsis, we measured this cytokine in the plasma over time. We detected marked increases in plasma IL-6 in FA-induced AKI which was significantly attenuated by CsA in a dose-dependent manner prominently on Day 2 (Figure 3). FA injection led to significant macrophage infiltration, indicated by F4/80-positive staining and neutrophil infiltration, identified by neutrophil staining and was significantly increased after 14 days. Macrophage infiltration was significantly inhibited by CsA 5 mg/kg on Day 14. Although no significant differences were observed among control, AKI or CsA treatment groups on Day 2, kidney neutrophil infiltration was found to be significantly reduced by 1 and 5 mg/kg CsA on Day 14 (Figure 5); data for all time points are shown in Table 1.
Fig. 5.
Leukocyte infiltration was decreased by cyclosporine A (CsA) administration. Representative renal macrophage and neutrophil staining of kidneys obtained from treated mice on Day 14 (A). CsA at 5 mg/kg markedly decreased cell infiltration. Original magnification ×400. Qualification of macrophage (B) and neutrophil (C) positive stained cell numbers per high-power field from kidneys of treated mice on Days 2 and 14. CsA 5 mg/kg significantly decreased macrophage infiltration on Day 14. CsA at 1 and 5 mg/kg significantly decreased neutrophil infiltration on Day 14. *P < 0.05 was considered significant in each analysis.
Table 1.
Time point measurements of blood and urine samplesa
| Day | FA | FA-CsA 1 | FA-CsA 5 | FA-CsA 10 |
|---|---|---|---|---|
| S. Cr | ||||
| 0 | 0.40 ± 0.02 | 0.41 ± 0.02 | 0.37 ± 0.02 | 0.36 ± 0.03 |
| 2 | 0.87 ± 0.02 | 0.58 ± 0.27 | 0.49 ± 0.01* | 0.83 ± 0.16 |
| 3 | 0.58 ± 0.03 | 0.74 ± 0.05 | 0.35 ± 0.03 | 0.59 ± 0.04 |
| 7 | 0.38 ± 0.02 | 0.38 ± 0.01 | 0.35 ± 0.01 | 0.64 ± 0.04 |
| 14 | 0.34 ± 0.03 | 0.44 ± 0.02 | 0.34 ± 0.02 | 0.61 ± 0.04 |
| S. BUN | ||||
| 0 | 24.91 ± 1.39 | 24.19 ± 1.44 | 28.04 ± 3.54 | 21.16 ± 2.47 |
| 2 | 136.15 ± 10.68 | 128.50 ± 11.78 | 81.98 ± 14.12* | 91.65 ± 11.36 |
| 3 | 101.84 ± 9.47 | 116.45 ± 10.71 | 65.69 ± 12.94 | 90.48 ± 12.39 |
| 7 | 60.52 ± 22.82 | 71.29 ± 11.96 | 43.94 ± 7.74 | 80.42 ± 11.23 |
| 14 | 48.98 ± 16.86 | 57.42 ± 6.60 | 32.77 ± 2.44 | 65.08 ± 6.19 |
| S. IL-6 | ||||
| 0 | 4.21 ± 0.085 | 3.76 ± 0.84 | 3.66 ± 0.78 | 2.66 ± 0.82 |
| 2 | 142.61 ± 58.21 | 87.04 ± 60.17 | 18.63 ± 4.18* | 41.36 ± 18.69 |
| 3 | 39.65 ± 13.43 | 84.17 ± 64.66 | 18.82 ± 2.99 | 22.62 ± 11.87 |
| 7 | 13.73 ± 5.97 | 8.50 ± 1.21 | 9.37 ± 3.67 | 11.35 ± 3.82 |
| 14 | 31.45 ± 12.26 | 48.71 ± 33.11 | 16.95 ± 4.78 | 21.33 ± 9.35 |
| U. NGAL | ||||
| 0 | 108.41 ± 10.32 | 95.46 ± 5.53 | 99.92 ± 10.55 | 105.36 ± 8.90 |
| 2 | 432.97 ± 61.49 | 345.40 ± 23.86 | 350.41 ± 34.73 | 299.09 ± 41.73 |
| 3 | 411.43 ± 26.83 | 254.39 ± 33.72 | 223.55 ± 23.46* | 291.19 ± 31.15 |
| 7 | 420.62 ± 49.39 | 290.68 ± 45.89 | 168.25 ± 43.83 | 417.71 ± 49.50 |
| 14 | 472.13 ± 57.65 | 420.14 ± 59.99 | 210.07 ± 38.28 | 510.62 ± 60.62 |
aS. Cr, serum creatinine (mg/dL); S. BUN, serum BUN (mg/dL); S. IL-6, serum IL-6 (pg/mL); U. NGAL, urinary NGAL (ng/mL); FA (250 mg/kg intraperitoneally); CsA 1 (or 5, 10), cyclosporine A 1 (or 5, 10 mg/kg intravenously). Numbers are presented by mean ± SE.
*P < 0.05 when compared to FA only group.
CsA depressed TWEAK protein expression and NFκB activation
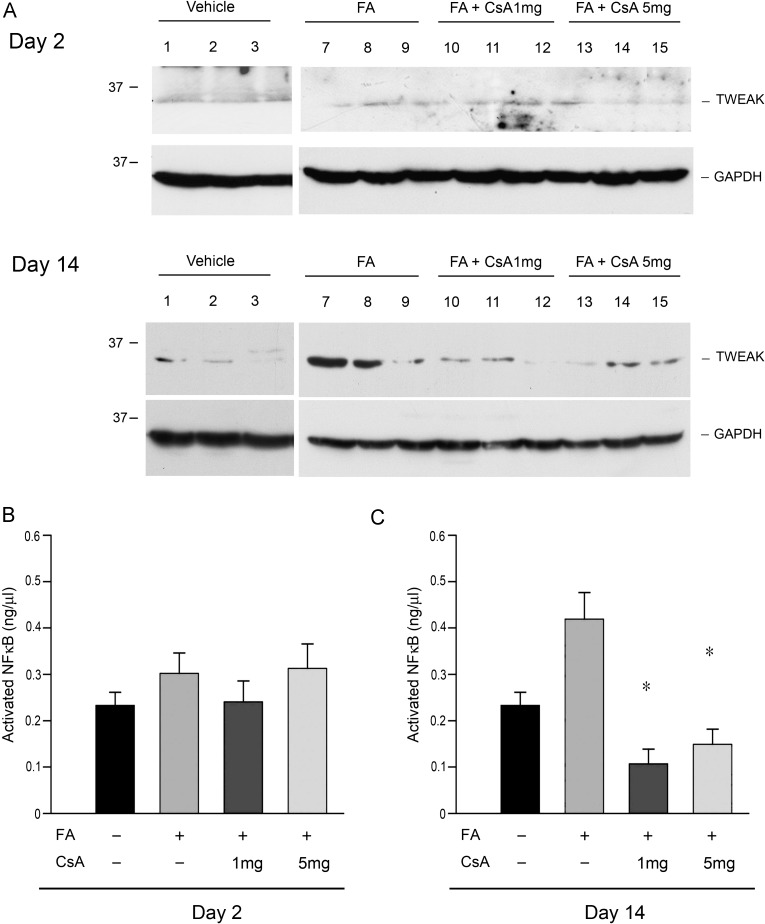
Tumor necrosis factor-like weak inducer of apoptosis (TWEAK) is a multifunctional cytokine affecting a variety of immune responses [27]. TWEAK and its cognate receptor human fibroblast growth factor-inducible molecule 14 (Fn14) are upregulated in FA-induced AKI [28] and mediate renal inflammation through activation of NFκB expression of pro-inflammatory cytokines and recruitment of leukocytes [29]. In our study, TWEAK protein expression in the kidney increased after FA injection. CsA inhibited activation of TWEAK as well as NFκB activation significantly on Day 14 (Figure 6).
Fig. 6.
Cyclosporine A (CsA) decreased TWEAK expression and NFκB activation after FA administration. (A) Western blotting for TWEAK protein expression of treated mice kidney. Bands of expected size were obtained below marker of 37 KDa. TWEAK expression decreased on Day 14. (B) Measurement of activated NFκB on Days 2 and 14. CsA significantly inhibited activation of NFκB on Day 14. n = 6 per each group. *P < 0.05 compared with FA only.
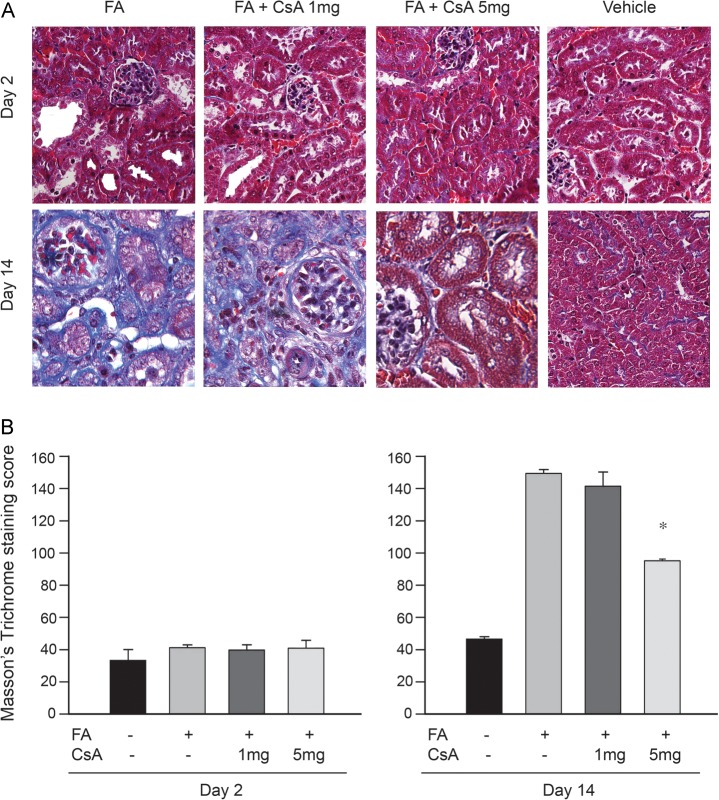
Interstitial fibrosis was attenuated by CsA
Masson's trichrome staining was used to identify interstitial fibrosis in kidney samples taken 14 days after FA. CsA ameliorated interstitial fibrosis (Figure 7). H&E staining also showed well-preserved kidney structure in CsA-treated mice (Figure 4).
Fig. 7.
Less interstitial fibrosis was observed with cyclosporine A (CsA) treatment in kidneys 14 days after FA administration. Extensive blue areas on Masson's trichrome staining (A) is notably improved by CsA administration. Original magnification ×400. Qualification of Masson's trichrome positive staining areas per high-power field (B). CsA 5 mg/kg significantly decreased the degree of kidney fibrosis on Day 14. *P ≤ 0.05 was considered statistically significant.
Discussion
Our study demonstrates that a single injection of CsA ameliorates FA-induced AKI, as evidenced by reduced tubular cell injury and inflammatory cell infiltration, decreased renal fibrosis and preserved renal function. Administration of one dose of 1 or 5 mg/kg CsA dramatically reduced FA-induced AKI. Kidney function, as measured by serum creatinine and BUN, was profoundly preserved in mice receiving 1 or 5 mg/kg CsA after FA injection. Mice that had received 5 mg/kg of CsA also showed decreased urinary NGAL secretion after FA injection. By contrast, 10 mg/kg worsened kidney function and was not studied further.
Drug-induced nephrotoxicity is a relatively common complication and accounts for up to 20% of hospital admissions due to AKI [4]. FA-induced AKI is a classic representation of nephrotoxic AKI and a generally accepted model for AKI mechanisms research. The FA-induced injury is limited to the kidney, thereby eliminating confounding extrarenal factors. The local inflammatory response in the kidney produced by FA administration recapitulates the upstream signaling of a wide range of cellular responses associated with inflammatory disorders crucial to tissue remodeling seen in many other forms of organ injury [30]. FA-AKI mimics important aspects of nephrotoxic AKI, such as tubular epithelial cell injury, apoptosis, inflammatory cell infiltration and inflammatory system activation.
We believe that the one important mechanism whereby CsA protected renal tubular cells was the preservation of mitochondrial function. Nephrotoxic AKI is recognized as a complex multifactorial process [31] in which mitochondrial impairments play important roles. Opening of the mitochondrial membrane PT pore is the final common pathway for the induction of cell apoptosis or necrosis [32]. PT inhibition represents an important mechanism known to be influenced by CsA [15, 33].
CsA inhibited systemic IL-6 release. Both experimental and clinical studies provide evidence for a crucial role of IL-6 in AKI [25, 26]. IL-6 is produced mainly by activated macrophages, which infiltrate the area of the vascular bundles of the outer medulla [34], and exerts its effects by recruiting neutrophils to the peritubular region [35]. Depending on mechanisms and intensity of kidney cell injury, the timing of IL-6 induction may vary. We documented an IL-6 increase on Day 2 of AKI relatively late compared to other reports [25]. Delayed chemokine responses with neutrophil retention are evidence of unresolved kidney inflammation and have been shown to be predictors of fibrosis [36]. CsA inhibited IL-6 secretion presumably because of its effect on both macrophage infiltration and possibly on IL-6 messenger RNA/protein expression in the macrophages [37].
Despite a significant protection from cell death, 5 mg/kg CsA barely affected cell infiltration on Day 2. This may be advantageous, as local macrophages that predominant on the first 1–4 days of injury phagocytose damaged tissue [38]. Engulfment of tissue debris by inflammatory cells can then trigger the release of inflammation-resolving cytokines [39, 40], facilitating resolution of inflammation.
FA is a known AKI model that leads to fibrosis. One of the driving factors in progressive interstitial fibrosis after FA injury seems to be chronic interstitial inflammation. TWEAK is an important upstream mediator that triggers inflammatory responses by linking cellular toxicity with NFκB activation, thus becoming an important therapeutic target [41–43]. Combination of TWEAK with its receptor Fn14 prolongs NFκB activation [44, 45], promotes the release of inflammatory mediators and thus emerges as a crucial factor in development of AKI [46, 47]. We documented a significant increase in NFκB activation after FA injection and the inhibition of TWEAK–NFκB activation after injection of CsA are in excellent agreement with these prior observations.
Both neutrophils and macrophages infiltrated into the kidney, persisting to Day 14. While this was unexpected, it can be understood from the available literature. Although neutrophils may apoptosis in a few days under normal conditions, their life span can be prolonged and function can be extended at sites of inflammation under pathophysiological conditions [48]. This dysregulation of cell apoptosis contributes to immune and organ dysfunction [49], known to be a predictor for fibrosis [36]. By 14 days, macrophages can promote extracellular collagen deposition in the kidney [38, 50]. Injections of 5 mg/kg of CsA significantly inhibited both neutrophil and macrophage infiltration on Day 14.
Our study has limitations. Exposure of tubular cells to nephrotoxic reagents, such as FA, activates complex signaling pathways that lead to tubular cell injury and death. Meanwhile, a robust inflammatory response is stimulated, further exacerbating renal tissue damage [31]. The protective function we find for CsA in AKI is to protect cells from initial injury (pathological changes seen at Days 2–7 in our study), ameliorates inflammatory reactions and thus improves AKI prognosis (damage seen at Day 14 in our study). A limitation of this study is that we were not able to identify the precise mechanism targeted by CsA in nephrotoxic AKI. Indeed, it seems likely that CsA affects multiple pathways and could be effective in inflammatory injury in multiple organs. Another limitation is that our results may not be generalizable to other forms of AKI. However, it is probable that a number of other compounds will have similar effects to CsA and our results likely represent a class effect. This could potentially open up a new approach to AKI management.
Many forms of AKI are associated with exposures that are known or predictable (e.g. drugs, contrast, cardiac surgery) and thus countermeasures given in the peri-exposure period, as was studied here, could be directly applicable to humans. Furthermore, even conditions traditionally thought of as uncontrolled exposures, such as sepsis, may still be amenable to such therapy. For example, inflammation is transiently unregulated by antibiotic administration [51] and CsA could reduce injury in such conditions. Of course, immune suppression in the setting of sepsis could be a liability as well and careful study will be needed before CsA can be translated into clinical use for sepsis-induced AKI.
In conclusion, we found that a single dose of CsA was effective for alleviating AKI in a mouse model of FA-induced nephrotoxicity, in which tubular cell apoptosis and intensive inflammation induced kidney damage. CsA protected renal tubular cells from apoptosis, suppressed inflammatory cytokines, abolished upregulation of TWEAK as well as NFκB activation and inhibited macrophage and neutrophil infiltration. As a result of these actions, CsA decreased interstitial fibrosis and preserved kidney structure and function. These results are important because they represent a potential ‘new’ therapy for AKI that is already clinically available and ready for clinical evaluation. Both nephrotoxic and primarily inflammatory (e.g. sepsis) types of AKI could be positively affected by CsA treatment. Should CsA or similar agents developed as anti-rejection drugs prove effective for various forms of human AKI, they could be rapidly transferred into clinical practice.
Acknowledgements
This study was supported in part by awards R01HL080926, R01DK070910 and K08GM081459 from the National Heart, Lung and Blood Institute (NHLBI), the National Institute of Diabetes, Digestive and Kidney Disease (NIDDK) and the National Institute of General Medical Sciences (NIGMS). The content is solely the responsibility of the authors and does not necessarily represent the office views of NHLBI, NIDDK, NIGMS or the National Institutes of Health. We wish to thank Jason Devlin in the Center for Biological Imaging (CBI), University of Pittsburgh for his help with the imaging studies.
Conflict of interest statement. None declared.
References
- 1.Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73. doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 3.Palevsky PM, Zhang JH, et al. VA/NIH Acute Renal Failure Trial Network. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359:7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elasy TA, Anderson RJ. Changing demography of acute renal failure. Semin Dial. 1996;9:438–443. [Google Scholar]
- 5.Schetz M, Dasta J, Goldstein S, et al. Drug-induced acute kidney injury. Curr Opin Crit Care. 2005;11:555–565. doi: 10.1097/01.ccx.0000184300.68383.95. [DOI] [PubMed] [Google Scholar]
- 6.Liu KD, Brakeman PR. Renal repair and recovery. Crit Care Med. 2008;36:S187–S192. doi: 10.1097/CCM.0b013e318168ca4a. [DOI] [PubMed] [Google Scholar]
- 7.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 8.Furuichi K, Kaneko S, Wada T. Chemokine/chemokine receptor-mediated inflammation regulates pathologic changes from acute kidney injury to chronic kidney disease. Clin Exp Nephrol. 2009;13:9–14. doi: 10.1007/s10157-008-0119-5. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Iturbe B, Garcia Garcia G. The role of tubulointerstitial inflammation in the progression of chronic renal failure. Nephron Clin Pract. 2010;116:c81–c88. doi: 10.1159/000314656. [DOI] [PubMed] [Google Scholar]
- 10.Puigmulé M, López-Hellin J, Suñé G, et al. Differential proteomic analysis of cyclosporine A-induced toxicity in renal proximal tubule cells. Nephrol Dial Transplant. 2009;24:2672–2686. doi: 10.1093/ndt/gfp149. [DOI] [PubMed] [Google Scholar]
- 11.Jennings P, Koppelstaetter C, Aydin S, et al. Cyclosporine A induces senescence in renal tubular epithelial cells. Am J Physiol Renal Physiol. 2007;293:F831–F838. doi: 10.1152/ajprenal.00005.2007. [DOI] [PubMed] [Google Scholar]
- 12.Healy E, Dempsey M, Lally C, et al. Apoptosis and necrosis: mechanisms of cell death induced by cyclosporine A in a renal proximal tubular cell line. Kidney Int. 1998;54:1955–1966. doi: 10.1046/j.1523-1755.1998.00202.x. [DOI] [PubMed] [Google Scholar]
- 13.Bakker RC, van kooten C, van de lagemaat-Paape ME, et al. Renal tubular epithelial cell death and cyclosporine A. Nephrol Dial Transplant. 2002;17:1181–1188. doi: 10.1093/ndt/17.7.1181. [DOI] [PubMed] [Google Scholar]
- 14.Dalmarco EM, Medeiros YS, Frode TS. Cyclosporin A inhibits CD11a/CD18 adhesion molecules due to inhibition of TNFalpha and IL-1 beta levels in the mouse model of pleurisy induced by carrageenan. Cell Adh Migr. 2008;2:231–235. doi: 10.4161/cam.2.4.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oka N, Wang L, Mi W, et al. Cyclosporine A prevents apoptosis-related mitochondrial dysfunction after neonatal cardioplegic arrest. J Thorac Cardiovasc Surg. 2008;135:123–130. doi: 10.1016/j.jtcvs.2007.05.009. 130e1–2. [DOI] [PubMed] [Google Scholar]
- 16.Piot C, Croisille P, Staat P, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 17.Mason DW, Morris PJ. Inhibition of the accumulation, in rat kidney allografts, of specific—but not nonspecific—cytotoxic cells by cyclosporine. Transplantation. 1984;37:46–51. doi: 10.1097/00007890-198401000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Ishida O, Ochi M, Miyamoto Y, et al. Suppression by cyclosporine of cellular and humoral reactivity after peripheral nerve allografts in mice. Transplantation. 1989;48:824–829. doi: 10.1097/00007890-198911000-00020. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz MZ, Flye MW, Storozuk RB. Growth and function of transplanted fetal rat intestine: effect of cyclosporine. Surgery. 1985;97:481–486. [PubMed] [Google Scholar]
- 20.Von Fliedner VE, Jeannet M, Gratwohl A, et al. Immunologic reactivity in marrow graft recipients receiving cyclosporine to prevent graft-versus-host disease. Transplantation. 1983;36:125–130. doi: 10.1097/00007890-198308000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Gratwohl A, Speck B, Wenk M, et al. Cyclosporine in human bone marrow transplantation. Serum concentration, graft-versus-host disease, and nephrotoxicity. Transplantation. 1983;36:40–44. doi: 10.1097/00007890-198307000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Sheehan DC, Hrapchak BB. Theory and Practice of Histotechnology. 2nd edn. St. Louis, MO: Battelle; 1987. [Google Scholar]
- 23.Mori K, Nakao K. Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney Int. 2007;71:967–970. doi: 10.1038/sj.ki.5002165. [DOI] [PubMed] [Google Scholar]
- 24.Ortega A, Ramila D, Ardura JA, et al. Role of parathyroid hormone-related protein in tubulointerstitial apoptosis and fibrosis after folic acid-induced nephrotoxicity. J Am Soc Nephrol. 2006;17:1594–1603. doi: 10.1681/ASN.2005070690. [DOI] [PubMed] [Google Scholar]
- 25.Kellum JA, Kong L, Fink MP, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167:1655–1663. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murugan R, Karajala-Subramanyam V, Lee M, et al. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int. 2010;77:527–535. doi: 10.1038/ki.2009.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maecker H, Varfolomeev E, Kischkel F, et al. TWEAK attenuates the transition from innate to adaptive immunity. Cell. 2005;123:931–944. doi: 10.1016/j.cell.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 28.Sanz AB, Sanchez-Niño MD, Izquierdo MC, et al. Tweak induces proliferation in renal tubular epithelium: a role in uninephrectomy induced renal hyperplasia. J Cell Mol Med. 2009;13:3329–3342. doi: 10.1111/j.1582-4934.2009.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanz AB, Justo P, Sanchez-Niño MD, et al. The cytokine TWEAK modulates renal tubulointerstitial inflammation. J Am Soc Nephrol. 2008;19:695–703. doi: 10.1681/ASN.2007050577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng TS, Burkly LC. No end in site: TWEAK/Fn14 activation and autoimmunity associated- end-organ pathologies. J Leukoc Biol. 2008;84:338–347. doi: 10.1189/jlb.0308165. [DOI] [PubMed] [Google Scholar]
- 31.Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 32.Seppet E, Gruno M, Peetsalu A, et al. Mitochondria and energetic depression in cell pathophysiology. Int J Mol Sci. 2009;10:2252–2303. doi: 10.3390/ijms10052252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irwin WA, Bergamin N, Sabatelli P, et al. Mitochondrial dysfunction and apoptosis in myopathic mice with collagen VI deficiency. Nat Genet. 2003;35:367–371. doi: 10.1038/ng1270. [DOI] [PubMed] [Google Scholar]
- 34.Kielar ML, John R, Bennett M, et al. Maladaptive role of IL-6 in ischemic acute renal failure. J Am Soc Nephrol. 2005;16:3315–3325. doi: 10.1681/ASN.2003090757. [DOI] [PubMed] [Google Scholar]
- 35.Nechemia-Arbely Y, Barkan D, Pizov G, et al. IL-6/IL-6R axis plays a critical role in acute kidney injury. J Am Soc Nephrol. 2008;19:1106–1115. doi: 10.1681/ASN.2007070744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Svensson M, Yadav M, Holmquist B, et al. Acute pyelonephritis and renal scarring are caused by dysfunctional innate immunity inmCxcr2 heterozygous mice. Kidney Int. 2011;80:1064–1072. doi: 10.1038/ki.2011.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams CM, Coleman JW. Induced expression of mRNA for IL-5, IL-6, TNF-alpha, MIP-2 and IFN-gamma in immunologically activated rat peritoneal mast cells: inhibition by dexamethasone and cyclosporin A. Immunology. 1995;86:244–249. [PMC free article] [PubMed] [Google Scholar]
- 38.Nahrendorf M, Swirski FK, Aikawa E, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kennedy AD, DeLeo FR. Neutrophil apoptosis and the resolution of infection. Immunol Res. 2009;43:25–61. doi: 10.1007/s12026-008-8049-6. [DOI] [PubMed] [Google Scholar]
- 41.Sanz AB, Sanchez-Nino MD, Ortiz A. TWEAK, a multifunctional cytokine in kidney injury. Kidney Int. 2011;80:708–718. doi: 10.1038/ki.2011.180. [DOI] [PubMed] [Google Scholar]
- 42.Sanz AB, Moreno JA, Sanchez-Nino MD, et al. TWEAKing renal injury. Front Biosci. 2008;13:580–589. doi: 10.2741/2703. [DOI] [PubMed] [Google Scholar]
- 43.Tamada S, Asai T, Kuwabara N, et al. Molecular mechanisms and therapeutic strategies of chronic renal injury: the role of nuclear factor kappaB activation in the development of renal fibrosis. J Pharmacol Sci. 2006;100:17–21. doi: 10.1254/jphs.fmj05003x4. [DOI] [PubMed] [Google Scholar]
- 44.Sanz AB, Sanchez-Niño MD, Izquierdo MC, et al. TWEAK activates the non-canonical NFkappaB pathway in murine renal tubular cells: modulation of CCL21. PLoS One. 2010;5:e8955. doi: 10.1371/journal.pone.0008955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ortiz A, Sanz AB, Muñoz García B, et al. Considering TWEAK as a target for therapy in renal and vascular injury. Cytokine Growth Factor Rev. 2009;20:251–258. doi: 10.1016/j.cytogfr.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 46.Dohi T, Borodovsky A, Wu P, et al. TWEAK/Fn14 pathway: a nonredundant role in intestinal damage in mice through a TWEAK/intestinal epithelial cell axis. Gastroenterology. 2009;136:912–923. doi: 10.1053/j.gastro.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 47.Haile WB, Echeverry R, Wu J, et al. The interaction between tumor necrosis factor-like weak inducer of apoptosis and its receptor fibroblast growth factor-inducible 14 promotes the recruitment of neutrophils into the ischemic brain. J Cereb Blood Flow Metab. 2010;30:1147–1156. doi: 10.1038/jcbfm.2009.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Francis N, Wong SH, Hampson P, et al. Lactoferrin inhibits neutrophil apoptosis via blockade of proximal apoptotic signaling events. Biochim Biophys Acta. 2011;1813:1822–1826. doi: 10.1016/j.bbamcr.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 49.Paunel-Gorgulu A, Flohe S, Scholz M, et al. Increased serum soluble Fas after major trauma is associated with delayed neutrophil apoptosis and development of sepsis. Crit Care. 2011;15:R20. doi: 10.1186/cc9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arnold L, Henry A, Poron F, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into anti-inflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peng ZY, Wang HZ, Srisawat N, et al. Bactericidal antibiotics temporarily increase inflammation and worsen acute kidney injury in experimental sepsis. Crit Care Med. 2012;40 doi: 10.1097/CCM.0b013e31822f0d2e. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]