Abstract
Background
An elevated triglyceride level is associated with cardiovascular and all-cause mortality in the general population. The associations between serum triglyceride and all-cause mortality among patients with chronic kidney disease (CKD) are unclear.
Methods
Patients with Stage 3 and Stage 4 CKD (estimated glomerular filtration rate 15–59 mL/min/1.73 m2) who had serum triglycerides measured prior to being classified as CKD were included. We examined the associations of serum triglyceride levels with all-cause mortality among 25 641 Stage 3 and Stage 4 CKD patients using Cox proportional hazard models and Kaplan–Meier survival curves.
Results
In the Cox model, after adjusting for relevant covariates including other lipid parameters, serum triglyceride level 150–199 mg/dL was not associated with death [hazard ratio (HR) 1.00, 95% confidence interval (95% CI) 0.92–1.10] relative to serum triglyceride <150 mg/dL while serum triglyceride ≥200 mg/dL was associated with a 11% increased hazard for death (95% CI 1.01–1.22). Age modified the association between serum triglyceride levels ≥200 mg/dL and mortality with patients <65 years having a 38% higher hazard for death (95% CI 1.15–1.65) and ≥65 years with no increased risk for death (HR 0.97, 95% CI 0.88–1.08, P for interaction <0.001). When serum triglycerides were examined as a continuous log-transformed variable, similar associations with mortality were noted.
Conclusions
Serum triglyceride ≥200 mg/dL was independently associated with all-cause mortality in Stage 3 and Stage 4 CKD patients aged <65 years but not among patients of age ≥65 years. Future studies should confirm these findings and examine the mechanisms that may explain these associations.
Keywords: chronic kidney disease, mortality, serum triglycerides
Introduction
Chronic kidney disease (CKD) is common and a significant proportion of non-dialysis-dependent CKD patients die of cardiovascular disease even before they reach end-stage renal disease [1]. Dyslipidemia is a well-established risk factor for cardiovascular disease in the general population and is widely prevalent among non-dialysis-dependent CKD patients. The pattern of lipid abnormalities differs between CKD and non-CKD populations [2, 3]. CKD patients have a higher prevalence of hypertriglyceridemia and small dense low-density lipoprotein (LDL) particles. Increased serum triglyceride levels indicate the presence of increased chylomicron remnants, which can penetrate vascular endothelium and lead to the development of atherosclerosis [3, 4]. Recently, a large Mendelian study showed that triglyceride-mediated pathways are causally related to cardiovascular disease [5]. The American Heart Association has also issued new guidelines for the management of high serum triglycerides in the general population, acknowledging the fact that serum triglyceride is an important risk factor for cardiovascular disease and death [6–9].
Previous studies that examined the associations between serum triglyceride and cardiovascular disease and mortality in CKD patients provided conflicting results. A serum triglyceride level of ≥182 mg/dL was significantly associated with an increased risk for cardiovascular disease among CKD participants in the Atherosclerosis Risk in Communities (ARIC) cohort study [10]. A secondary analysis of the Modified Diet in Renal Disease (MDRD) study showed no association between serum triglycerides and cardiovascular disease, the progression of CKD or death among non-diabetic CKD patients [11]. In addition, it is unclear whether the effects of serum triglycerides are consistent within the CKD population. In particular, with the CKD population generally being of advanced age and have higher comorbid illnesses, an important unanswered question is whether the association between serum triglyceride level and outcomes differs based on patient age and presence or absence of different comorbid conditions. Thus, studying the association between serum triglyceride and mortality might provide an opportunity to develop and test interventions to lower serum triglycerides in CKD patients similar to general population [12].
Therefore, we examined the associations of serum triglyceride levels with all-cause mortality and whether these associations differ based on age and presence or absence of comorbid conditions in a large cohort of Stage 3 and Stage 4 CKD patients followed in our health care system.
Materials and methods
Study population
Patients who met the following criteria between 1 January 2005 and 4 April 2011 were included: (i) patients who had at least one face-to-face outpatient encounter with a Cleveland Clinic health care provider, (ii) had two estimated glomerular filtration rate (eGFR) values of <60 mL/min/1.73 m2 [the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation] > 90 days apart and (iii) patients who had outpatient serum triglyceride levels measured prior to being classified as CKD ( < 12 months) in our health care system. Patients aged <18 years old and those who were diagnosed with end-stage renal disease needing dialysis or renal transplant before their second eGFR < 60 mL/min/1.73 m2 were excluded. The study participants were identified from a previously validated Electronic Health Record (EHR)-based CKD registry. The EHR validation process of the kidney disease-related conditions and comorbid conditions included in this registry was performed by two reviewers using various sections of the EHR. Definitions and criteria for these conditions were based on prior definitions and criteria used in the literature from a combination of billing codes, use of relevant medications, laboratory values and imaging studies. The development and validation of our EHR-based CKD registry at Cleveland Clinic have been described in detail elsewhere [13].
Definitions and outcome measures
Demographic details of the study population were extracted from the EHR. Diabetes mellitus, hypertension, coronary artery disease and other comorbid conditions were defined using pre-specified criteria validated previously. These conditions existed prior to being classified as CKD. Serum triglyceride levels and other relevant outpatient laboratory details were obtained electronically from our laboratory records. We applied the CKD-EPI equation to patients who had two outpatient serum creatinine levels between 1 January 2005 and 4 April 2011 in our health system to calculate eGFR [14]. All creatinine measurements were performed by the modified kinetic Jaffe reaction, using a Hitachi 747-200 Chemistry Analyzer (1996–2001) or a Hitachi D 2400 Modular Chemistry Analyzer thereafter (Roche Diagnostics, Indianapolis, IN) in our laboratory. CKD was defined according to current guidelines as follows: Stage 3 CKD (eGFR 30–59 mL/min/1.73 m2) and Stage 4 CKD (eGFR 15–29 mL/min/1.73 m2). We further categorized Stage 3 into Stage 3a (eGFR 45–59 mL/min/1.73 m2) and Stage 3b (eGFR 30–44 mL/min/1.73 m2). Serum triglyceride was measured using an enzymatic colorimetric test run on the Roche Modular platform. Within- and between-run precision of human serum is stated at 1.5 and 1.8% , respectively. In our health care system, lipid profiles are customarily obtained in fasting state by standard protocol. However, there may have been instances where the lipid profile was obtained in the non-fasting state and we were unable to differentiate these fasting and non-fasting samples.
Outcome measure
The primary outcome of interest, all-cause mortality, was ascertained from our EHR and linkage of our registry with the Social Security Death Index (SSDI). This Death Master File from the SSDI contains over several million records of deaths that have been reported to Social Security Administration and is updated monthly. We assumed a 90-day lag in death documentation, and therefore censored patients without reported deaths 90 days before SSDI data extraction. Data were extracted on April 2011 and censoring was assigned on January 2011. Patients were followed from their date of inclusion in the registry (date of second qualifying eGFR) until date of last Cleveland Clinic Health System encounter or SSDI data extraction on April 2011, whichever was last.
Statistical analysis
Baseline characteristics between (i) CKD patients with and without measured serum triglyceride levels and (ii) patients of age <65 years and ≥65 years were compared using chi-square and two-sample t-tests for categorical and continuous variables, respectively. Based on the American Heart Association's clinical criteria, CKD patients with measured outpatient serum triglyceride values in the year prior to being classified as CKD were further classified into three groups: <150 mg/dL (normal), 150–199 mg/dL (borderline-high) and ≥200 mg/dL (high and very high) [6]. Because there were only 239 patients in the very high category (≥500 mg/dL) and <200 of them had complete covariate data for the mortality model, we combined the high and very high serum triglycerides into one group (≥200 mg/dL). Associations of the baseline characteristics and these three groups were assessed using chi-square and analysis of variance tests for categorical and continuous variables, respectively.
The factors associated with serum triglycerides > 200 mg/dL (referent group <200 mg/dL) were examined using logistic regression analysis. Covariates were chosen a priori based on factors previously shown or thought to be related to both serum triglyceride levels and mortality. These included age, gender, race, body mass index (BMI), eGFR, diabetes, hypertension, coronary artery disease, heart failure, smoking and year of entry into our CKD registry. To evaluate whether survival among persons with CKD was associated with serum triglyceride levels, we used Kaplan–Meier plots and log-rank tests with entry into the CKD registry as the time of origin. Cox proportional hazards models were used to assess the association between the baseline serum triglyceride levels and all-cause mortality while adjusting for the covariates mentioned above and the presence of cerebrovascular disease, chronic obstructive pulmonary disease, malignancy, serum albumin, hemoglobin, LDL cholesterol, high-density lipoprotein (HDL) cholesterol and use of statins and fibrates at baseline. Due to non-linearity, serum albumin and hemoglobin were modeled using splines. We conducted a sensitivity analysis in which the association between time-averaged serum triglyceride levels (for patients who had multiple measurements up to 1 year after being classified as CKD) and all-cause mortality was examined.
We tested two-way interactions between serum triglyceride and the following covariates in the adjusted model: age, race, presence of diabetes and hypertension, eGFR, use of statins and fibrates and none of these interactions other than with age were significant. We fit the adjusted model using serum triglyceride categories and also using log-transformed continuous triglycerides values. Eight percent of the patients had missing covariate data; 5% and 4% of patients were missing hemoglobin and albumin data, respectively, and they were excluded from the multivariable model. To illustrate the interaction between serum triglyceride level and age, we estimated the log hazard of mortality for each patient in the adjusted model. We then plotted the log hazard of mortality against triglyceride values separately for each age group using cubic regression models.
All data analyses were conducted using Unix SAS version 9.2 (SAS Institute, Cary, NC), and graphs were created using R 2.12.2 (The R Foundation for Statistical Computing, Vienna, Austria). The CKD registry and this study were approved by the Cleveland Clinic Institutional Review Board.
Results
Baseline patient characteristics
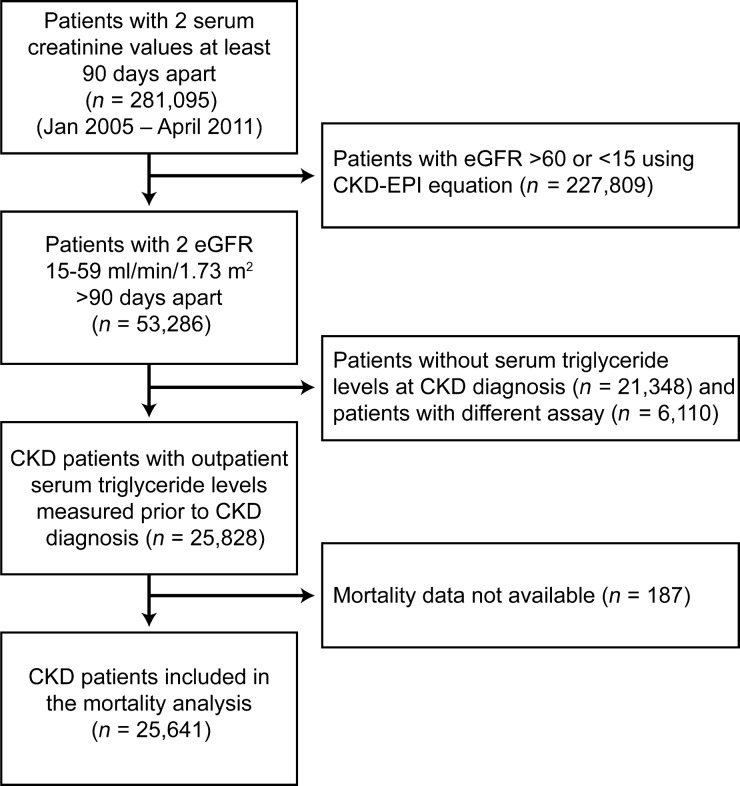
Of 53 286 patients in our CKD registry, 25 828 (48% ) patients had serum triglyceride levels measured at least once using a standard assay in the year prior to being classified as having CKD and were included in this analysis. Patients who had serum triglycerides measured using different assays [n = 6110 (11% )], had serum triglyceride measured after being classified as having CKD or did not have serum triglyceride measured in our health care system were excluded (Figure 1). The mean age of the study population was 71.6 ± 11.3 years with 53% being females and 12% African-Americans. Patients who had serum triglyceride levels measured were significantly different from those who lacked serum triglyceride data except for African-American race and HDL cholesterol (Table 1). Patients who were aged <65 years were significantly different from those aged ≥65 years in several demographic factors, presence of comorbid conditions and other laboratory parameters (Supplementary Table S1).
Fig. 1.
Flow chart showing how patients were selected for this analysis from the EHR-based registry.
Table 1.
Characteristics of Stage 3 and Stage 4 CKD patients with and without serum triglyceride levels measured before being classified as CKDa
| Variableb | Serum triglycerides not measured (n = 21 348) | Serum triglycerides <150 mg/dL (n = 15 961) | Serum triglycerides 150–199 mg/dL (n = 4793) | Serum triglycerides ≥200 mg/dL (n = 5074) |
|---|---|---|---|---|
| Age (mean ± SD)c | 72.9 ± 12.6 | 72.6 ± 11.0 | 71.4 ± 11.0 | 68.6 ± 11.8 |
| Female gender (%) | 55.4 | 52.6 | 55.5 | 52.2 |
| African-American race (%) | 12.8 | 14.4 | 9.5 | 7.7 |
| Mean eGFR (mL/min/1.73 m2) (mean ± SD)c | 45.8 ± 10.8 | 48.9 ± 9.7 | 48.0 ± 10.0 | 47.1 ± 10.5 |
| Stage of CKD | ||||
| 3a (eGFR 45–59 mL/min/1.73 m2) | 60.0 | 72.3 | 68.6 | 65.3 |
| 3b (eGFR 30–44 mL/min/1.73 m2) | 29.6 | 21.7 | 24.6 | 25.8 |
| 4 (eGFR 15–29 mL/min/1.73 m2) | 10.4 | 5.9 | 6.8 | 8.8 |
| BMI kg/m2 (mean ± SD) | 28.6 ± 6.5 | 28.8 ± 6.1 | 30.5 ± 6.3 | 31.2 ± 6.4 |
| BMI categories | ||||
| < 18.5 kg/m2 | 1.9 | 1.0 | 0.56 | 0.45 |
| 18.5–24.9 kg/m2 | 26.5 | 25.0 | 15.8 | 13.2 |
| 25–29.9 kg/m2 | 32.4 | 38.1 | 35.0 | 32.6 |
| ≥30 kg/m2 | 31.5 | 32.7 | 45.1 | 49.9 |
| Smoking (%) | 8.0 | 6.3 | 7.5 | 10.2 |
| Diabetes (%) | 11.2 | 27.4 | 33.5 | 39.2 |
| Hypertension (%) | 79.0 | 92.6 | 93.9 | 94.3 |
| Coronary artery disease (%) | 13.3 | 29.7 | 28.1 | 26.5 |
| Congestive heart failure (%) | 7.3 | 9.3 | 8.8 | 9.6 |
| Hyperlipidemia (%) | 57.0 | 89.4 | 93.9 | 95.7 |
| COPD (%) | 6.4 | 10.6 | 11.8 | 10.4 |
| Cerebrovascular disease (%) | 6.4 | 11.8 | 11.3 | 9.5 |
| Malignancy (%) | 31.8 | 17.4 | 17.2 | 16.5 |
| Statins use (%) | 67.8 | 72.5 | 71.8 | |
| Fibrates use (%) | 5.7 | 10.1 | 20.8 | |
| Hemoglobin (g/dL) (mean ± SD)c | 12.6 ± 1.9 | 13.0 ± 1.7 | 13.2 ± 1.7 | 13.2 ± 1.7 |
| Serum albumin (g/dL) (mean ± SD)c | 4.0 ± 0.53 | 4.2 ± 0.40 | 4.2 ± 0.38 | 4.2 ± 0.39 |
| Total cholesterol (mg/dL) (mean ± SD)c | 185.9 ± 43.2 | 173.3 ± 40.6 | 185.8 ± 40.3 | 205.1 ± 53.7 |
| HDL cholesterol (mg/dL) (mean ± SD) | 53.5 ± 18.2 | 57.7 ± 16.9 | 49.9 ± 13.2 | 45.0 ± 12.5 |
| LDL cholesterol (mg/dL) (mean ± SD)c | 103.9 ± 35.4 | 95.6 ± 33.2 | 101.5 ± 35.6 | 103.3 ± 44.6 |
aCOPD, chronic obstructive pulmonary disease; SD, standard deviation.
bChi-square test P <0.05 unless otherwise noted comparing patients with serum triglyceride measured versus all those not measured.
ct-test P <0.05 comparing patients with triglycerides measured versus all those not measured. African-American race and HDL level only variables not significantly different between measured group versus not.
Patients with serum triglyceride levels <150 mg/dL [n = 15 961 (62% )], 150–199 mg/dL [n = 4793 (18.5% )] and ≥200 mg/dL [n = 5074 (19.5% )] differed on demographics and comorbid conditions (Table 1). Patients with serum triglyceride <150 mg/dL had higher HDL and lower LDL levels than the patients with serum triglyceride 150–199 and ≥200 mg/dL. Use of statins and fibrates was 68 and 5.7% , respectively, among patients with serum triglyceride <150 mg/dL as compared to 72 and 21% among patients with serum triglyceride ≥200 mg/dL.
Factors associated with higher serum triglyceride levels (≥200 mg/dL)
In the multivariable analysis, patients of African-American descent, increasing age and eGFR were associated with lower odds of having higher serum triglyceride levels. Higher BMI categories, presence of diabetes and hypertension and smoking history were associated with higher odds of having higher serum triglyceride levels (Table 2).
Table 2.
Factors associated with high serum triglyceride levels (≥200 mg/dL) among Stage 3 and Stage 4 CKD patientsa
| Effect | aOR (95% CI) |
|---|---|
| Age (per 5 year increase) | 0.87 (0.86–0.89) |
| Male gender | 0.98 (0.91–1.04) |
| African-American race | 0.39 (0.35–0.44) |
| BMI | |
| < 18.5 versus 18.5–24.9 kg/m2 | 0.72 (0.46–1.1) |
| 25–29.9 versus 18.5–24.9 kg/m2 | 1.48 (1.34–1.63) |
| 30–34.9 versus 18.5–24.9 kg/m2 | 2.07 (1.86–2.29) |
| 35–39.9 versus 18.5–24.9 kg/m2 | 2.36 (2.09–2.67) |
| ≥40 versus 18.5–24.9 kg/m2 | 1.94 (1.68–2.24) |
| eGFR (per 5 mL/min/1.73 m2 increase) | 0.93 (0.92–0.95) |
| Diabetes | 1.43 (1.34–1.54) |
| Hypertension | 1.38 (1.20–1.58) |
| Coronary artery disease | 0.88 (0.82–0.95) |
| Congestive heart failure | 1.04 (0.93–1.17) |
| Smoking | 1.57 (1.41–1.76) |
aaOR: multivariate adjusted odds ratio.
Serum triglyceride and mortality
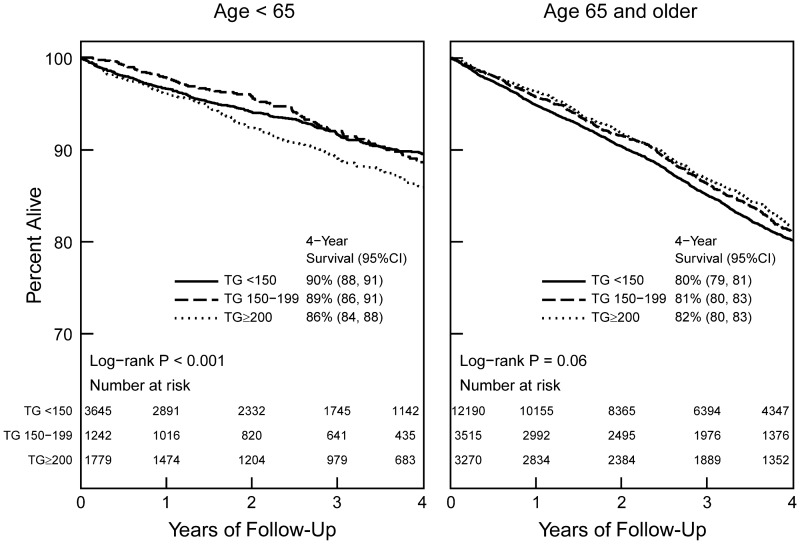
Among 25 641 patients with mortality information available, 3866 died during an average follow-up of 3.1 years. The Kaplan–Meier analysis showed no significant difference in all-cause mortality in the different serum triglyceride groups for patients aged ≥65 years (Figure 2). In the Cox proportional hazards model that included all patients, after adjusting for demographics, comorbid conditions, renal function, lipid parameters and anti-hyperlipidemic use, a serum triglyceride level of 150–199 mg/dL was not associated with death while serum triglyceride ≥200 mg/dL was associated with a 11% increased hazard for death (Table 3). When examined as a log-transformed continuous variable, serum triglyceride was not associated with mortality for the overall group [hazard ratio (HR) 1.06 per each log increase in triglyceride, 95% confidence interval (95% CI) 0.98–1.14].
Fig. 2.
Kaplan–Meier survival curve based on serum triglyceride levels among Stage 3 and Stage 4 CKD patients.
Table 3.
Associations between serum triglyceride levels (baseline values) and all-cause mortality among Stage 3 and Stage 4 CKD patients
| Unadjusted HR (95% CI) (n = 25 641) | Adjusted HRa (95% CI) (n = 23 480) | |
|---|---|---|
| Serum triglyceride categories | ||
| 150–199 versus <150 mg/dL | ||
| Overall | 0.93 (0.86–1.02) | 1.00 (0.92–1.10) |
| Age <65 years | 0.86 (0.68–1.09) | |
| Age ≥65 years | 1.03 (0.93–1.12) | |
| ≥200 versus <150 mg/dL | ||
| Overall | 0.93 (0.86–1.01) | 1.11 (1.01–1.22) |
| Age <65 years | 1.38 (1.15–1.65) | |
| Age ≥65 years | 0.97 (0.88–1.08) | |
aAdjusted for age, gender, race, BMI, eGFR, diabetes, hypertension, malignancy, congestive heart failure, cerebrovascular disease, coronary artery disease, chronic obstructive pulmonary disease, smoking status, hemoglobin, albumin, HDL and LDL levels, use of statins and use of fibrates.
Interaction with age
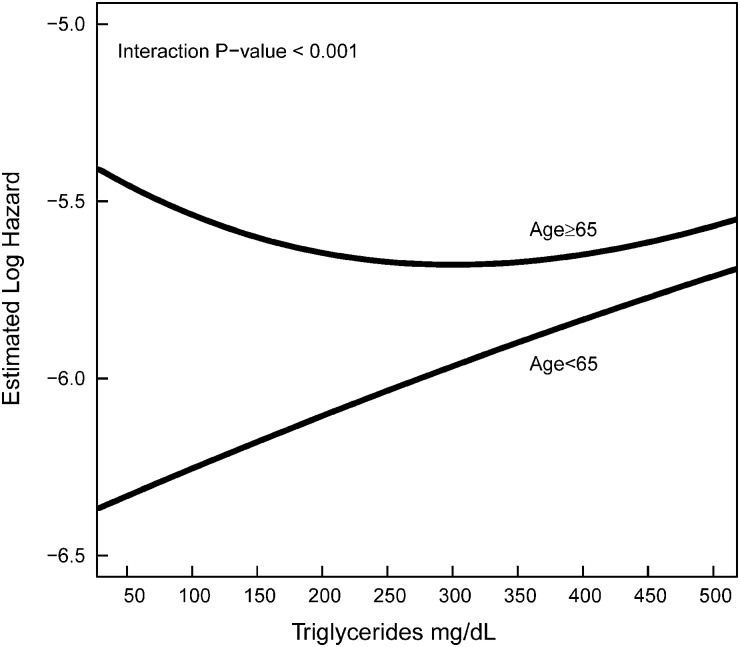
We found a significant interaction of age with serum triglyceride indicating that the association between serum triglyceride and all-cause mortality differs based on age (P <0.001). The increased mortality hazard associated with serum triglyceride levels of ≥200 mg/dL was present for patients aged <65 years but not for aged ≥65 years (Table 3). Similar results were found when serum triglycerides were examined as a continuous variable (log-transformed values) with patients aged <65 having a higher risk for death (HR 1.26, 95% CI 1.10–1.45) but not for patients aged ≥65 years (HR 0.96, 95% CI 0.89–1.04) (Figure 3).
Fig. 3.
Associations between all-cause mortality log-hazard and baseline serum triglyceride levels among aged <65 and ≥65 years.
Sensitivity analysis
In the time-averaged analysis, similar associations between serum triglyceride levels and all-cause mortality were noted for the overall group, aged <65 and ≥65 years, as the model that used baseline serum triglycerides only (Table 4).
Table 4.
Associations between time-averaged serum triglyceride levels (up to 1 year after the second eGFR <60 mL/min/1.73 m2) and all-cause mortality among Stage 3 and Stage 4 CKD patients
| Unadjusted HR (95% CI) N = 25 641 | Adjusted HRa (95% CI) N = 23 480 | |
|---|---|---|
| Serum triglyceride categories | ||
| 150–199 versus <150 mg/dL | ||
| Overall | 0.92 (0.85–1.005) | 0.99 (0.91–1.09) |
| Age <65 years | 0.95 (0.76–1.19) | |
| Age ≥65 years | 0.99 (0.89–1.09) | |
| ≥200 versus <150 mg/dL | ||
| Overall | 0.96 (0.88–1.04) | 1.09 (0.996–1.20) |
| Age <65 years | 1.26 (1.05–1.51) | |
| Age ≥65 years | 0.99 (0.89–1.10) | |
| Continuous variable | ||
| Log triglyceride | ||
| Overall | 0.91 (0.86–0.98) | 1.05 (0.97–1.14) |
| Age <65 years | 1.19 (1.02–1.39) | |
| Age ≥65 years | 0.96 (0.89–1.05) | |
aAdjusted for age, gender, race, BMI, eGFR, diabetes, hypertension, malignancy, congestive heart failure, cerebrovascular disease, coronary artery disease, chronic obstructive pulmonary disease, smoking status, hemoglobin, albumin, HDL and LDL levels, use of statins and fibrates.
Discussion
Despite the higher use of anti-hyperlipidemic agents, hypertriglyceridemia (38% ) is common among non-dialysis-dependent CKD patients. Factors such as being of African-American descent and increasing age were negatively associated with high serum triglyceride levels (≥200 mg/dL) while the presence of diabetes, obesity and hypertension were positively associated with high serum triglyceride levels (≥200 mg/dL). Serum triglyceride levels ≥200 mg/dL were independently associated with increased risk for all-cause mortality among non-dialysis-dependent CKD. This association was particularly prominent among CKD patients aged <65 years while patients aged ≥65 year do not incur the same risk for death with serum triglyceride levels ≥200 mg/dL.
As discussed, previous observational studies have examined the relationship between serum triglycerides, cardiovascular disease and mortality. In a non-diabetic cohort, Chawla et al. [11] reported no associations between all the individual lipid parameters (total cholesterol, LDL cholesterol, HDL cholesterol and serum triglycerides) and the composite end point of all-cause mortality, cardiovascular mortality and progression to kidney failure. This lack of association might be related to the small sample size of the study cohort. Muntner et al. [10] reported a 3-fold increased risk for coronary heart disease (RR 2.73, 95% CI 1.46–5.10) among patients with Stage 3 and Stage 4 CKD and serum triglyceride levels of ≥182 mg/dL in the ARIC study cohort. Recently, higher serum triglyceride levels have been associated with the development and progression of kidney disease, which might further contribute to the cardiovascular disease burden [15, 16]. Our study adds to the literature by including a divergent population and showing an association with all-cause mortality. In contrast to other studies, we report our results using clinically determined serum triglyceride categories and summarized results based on age.
The impact of different cardiovascular risk factors on cardiovascular disease might vary as age increases. Secondary analysis of the Honolulu Heart Program (3572 participants) showed that the effects of total cholesterol on coronary heart disease seemed to decline with advancing age. Higher BMI was associated with coronary heart disease in participants aged 45–54 years but not among who were 75–93 years in this cohort. The associations between smoking and coronary heart disease also weakened with age in the same cohort [17]. Similarly, we show that the effect of triglycerides on all-cause mortality also differs by age in CKD population. Even though a higher prevalence of some comorbid conditions among patients aged <65 years might explain these associations (Supplementary Table S1), this needs to be further studied. Whether the effects of other cardiovascular risk factors on cardiovascular disease and death differ between younger and elderly CKD patients is unclear and may be explored in future studies.
Observational studies have shown that higher LDL cholesterol levels are associated with cardiovascular disease and mortality among non-dialysis-dependent CKD [18]. Subsequently, subgroup analysis of the CKD population included in the large randomized controlled statin trials of the general population and their meta-analysis showed a reduction in cardiovascular disease burden with statin use [19, 20]. The recently published Study of Heart and Renal Protection (SHARP) trial reported a statistically significant reduction in atherosclerotic events (primary end point of the study) with the use of statins plus ezetimibe but not for all-cause mortality [21]. In a subgroup analysis based on serum triglyceride levels, beneficial effects of statins on atherosclerotic events were noted only for patients with serum triglyceride levels ≥2 mmol/L (175 mg/dL) [21]. Despite adequate reduction in LDL cholesterol levels, the rate of cardiovascular events was 15.1% in the treatment arm in SHARP trial. This data, along with ours and other available observational study evidence, suggest that other lipid parameters such as serum triglycerides may be an important modifiable risk factor amenable to intervention in CKD.
Patients with CKD are at risk for malnutrition. Among dialysis patients, prior studies have shown an inverse association between total cholesterol levels and mortality but this association was attributed to the cholesterol-lowering effect of systemic inflammation and malnutrition [22]. Kovesdy et al. [23] reported higher mortality for non-dialysis-dependent CKD patients with lower serum triglycerides ( < 115 mg/dL) compared to higher serum triglyceride levels (>258 mg/dL). However, this was attenuated after adjustment for case-mix and malnutrition-inflammation complex syndrome. We adjusted for widely used nutritional parameters such as serum albumin and BMI but lacked inflammatory markers such as high-sensitivity C-reactive protein.
This study has significant strengths that include utilizing a large number of Stage 3 and 4 CKD patients with lack of lost to follow-up compared to the prior studies. Our study population also has a significant proportion of African-American patients, female gender patients and patients with diabetes. An additional strength is the prior validation of our CKD registry and the included comorbid conditions using standard definitions in the literature. However, there are important limitations. Apart from being an observational study that is subject to residual confounding, the primary limitation of this retrospective analysis is its inability to adjust for residual confounding. In addition, we included patients with eGFR <60 mL/min/1.73 m2 while patients with an earlier stage of CKD (eGFR ≥60 mL/min/1.73 m2 with albuminuria and other structural abnormalities) were not included. Therefore, these results may not be applicable to patients with Stages 1–2 CKD.
Furthermore, over 50% of our eligible patients either had serum triglyceride measured using a different assay or did not have serum triglyceride levels measured at all rendering the possibility of an attrition bias. We might have used non-fasting serum triglycerides for some patients in this analysis. However, previous studies have confirmed the utility of non-fasting triglycerides in assessing cardiovascular risk and thus they are unlikely to have influenced our results [8, 24]. Even though we were able to adjust for several confounding variables including serum albumin levels, we lacked data relating to albuminuria and serum phosphorus, which are known risk factors for mortality in this population. In addition, we lacked details about control of diabetes, physical activity levels and medication compliance that may influence serum triglyceride levels. We also did not have cause-specific mortality data and hospitalization data for our patients.
In conclusion, serum triglyceride levels ≥200 mg/dL are associated with all-cause mortality among Stage 3 and Stage 4 CKD patients. This effect was prominent among CKD patients <65 years and not evident among patients aged over 65. Future studies should confirm these findings and examine cause-specific mortality in this high-risk population. Importantly, future clinical studies may explore whether lowering serum triglyceride levels will reduce cardiovascular disease burden and mortality among CKD patients aged <65 years.
Supplementary Material
Acknowledgements
Part of the work in this manuscript was presented as an abstract at the American Society of Nephrology annual meeting held in Philadelphia on 11 November 2011. The authors wish to thank Welf Saupe, Vicky Konig, Donna Rumley and John Sharp of Cleveland Clinic who helped in data extraction during the development of the registry.
Funding. S.D.N.: National Institutes of Health, the National Center for Research Resources, Multidisciplinary Clinical Research Career Development Program Grant number: RR024990 and DK094112-01. J.D.S.: National Institute of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK085185-01A1) and investigator initiated-grant support from PhRMA foundation, Genzyme and Roche Organ Transplant Research Foundation and DK094112-01. S.E.J.: NIH 1K23DK091363-01 and DK094112-01. J.V.N.: DK094112-01. M.J.S.: K24 DK078204. The creation of the registry was funded by an unrestricted grant from Amgen, Inc. to the Department of Nephrology and Hypertension Research and Education Fund.
The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of National Center for Research Resources (NCRR) or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview. The authors have no relevant financial interest in the study.
Conflict of interest statement. None declared.
References
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Attman PO, Samuelsson O. Dyslipidemia of kidney disease. Curr Opin Lipidol. 2009;20:293–299. doi: 10.1097/MOL.0b013e32832dd832. [DOI] [PubMed] [Google Scholar]
- 3.Keane WF, Tomassini JE, Neff DR. Lipid abnormalities in patients with chronic kidney disease. Contrib Nephrol. 2011;171:135–142. doi: 10.1159/000327317. [DOI] [PubMed] [Google Scholar]
- 4.Ritz E, Wanner C. Lipid abnormalities and cardiovascular risk in renal disease. J Am Soc Nephrol. 2008;19:1065–1070. doi: 10.1681/ASN.2007101128. [DOI] [PubMed] [Google Scholar]
- 5.Sarwar N, Sandhu MS, et al. Triglyceride Coronary Disease Genetics Consortium and Emerging Risk Factors Collaboration. Triglyceride-mediated pathways and coronary disease: collaborative analysis of 101 studies. Lancet. 2010;375:1634–1639. doi: 10.1016/S0140-6736(10)60545-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller M, Stone NJ, Ballantyne C, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2292–2333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 7.Labreuche J, Deplanque D, Touboul PJ, et al. Association between change in plasma triglyceride levels and risk of stroke and carotid atherosclerosis: systematic review and meta-regression analysis. Atherosclerosis. 2010;212:9–15. doi: 10.1016/j.atherosclerosis.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Varbo A, Nordestgaard BG, Tybjaerg-Hansen A, et al. Nonfasting triglycerides, cholesterol, and ischemic stroke in the general population. Ann Neurol. 2011;69:628–634. doi: 10.1002/ana.22384. [DOI] [PubMed] [Google Scholar]
- 9.Di Angelantonio E, Sarwar N, et al. Emerging Risk Factors Collaboration. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muntner P, He J, Astor BC, et al. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the atherosclerosis risk in communities study. J Am Soc Nephrol. 2005;16:529–538. doi: 10.1681/ASN.2004080656. [DOI] [PubMed] [Google Scholar]
- 11.Chawla V, Greene T, Beck GJ, et al. Hyperlipidemia and long-term outcomes in nondiabetic chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:1582–1587. doi: 10.2215/CJN.01450210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jun M, Foote C, Lv J, et al. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet. 2010;375:1875–1884. doi: 10.1016/S0140-6736(10)60656-3. [DOI] [PubMed] [Google Scholar]
- 13.Navaneethan SD, Jolly SE, Schold JD, et al. Development and validation of an electronic health record-based chronic kidney disease registry. Clin J Am Soc Nephrol. 2011;6:40–49. doi: 10.2215/CJN.04230510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee PH, Chang HY, Tung CW, et al. Hypertriglyceridemia: an independent risk factor of chronic kidney disease in Taiwanese adults. Am J Med Sci. 2009;338:185–189. doi: 10.1097/MAJ.0b013e3181a92804. [DOI] [PubMed] [Google Scholar]
- 16.Thomas G, Sehgal AR, Kashyap SR, et al. Metabolic syndrome and kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2011;6:2364–2373. doi: 10.2215/CJN.02180311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abbott RD, Curb JD, Rodriguez BL, et al. Age-related changes in risk factor effects on the incidence of coronary heart disease. Ann Epidemiol. 2002;12:173–181. doi: 10.1016/s1047-2797(01)00309-x. [DOI] [PubMed] [Google Scholar]
- 18.Seliger SL, Weiss NS, Gillen DL, et al. HMG-CoA reductase inhibitors are associated with reduced mortality in ESRD patients. Kidney Int. 2002;61:297–304. doi: 10.1046/j.1523-1755.2002.00109.x. [DOI] [PubMed] [Google Scholar]
- 19.Tonelli M, Isles C, Curhan GC, et al. Effect of pravastatin on cardiovascular events in people with chronic kidney disease. Circulation. 2004;110:1557–1563. doi: 10.1161/01.CIR.0000143892.84582.60. [DOI] [PubMed] [Google Scholar]
- 20.Navaneethan SD, Pansini F, Perkovic V, et al. HMG CoA reductase inhibitors (statins) for people with chronic kidney disease not requiring dialysis. Cochrane Database Syst Rev. 2009;2:CD007784. doi: 10.1002/14651858.CD007784. [DOI] [PubMed] [Google Scholar]
- 21.Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Coresh J, Eustace JA, et al. Association between cholesterol level and mortality in dialysis patients: role of inflammation and malnutrition. JAMA. 2004;291:451–459. doi: 10.1001/jama.291.4.451. [DOI] [PubMed] [Google Scholar]
- 23.Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Inverse association between lipid levels and mortality in men with chronic kidney disease who are not yet on dialysis: effects of case mix and the malnutrition-inflammation-cachexia syndrome. J Am Soc Nephrol. 2007;18:304–311. doi: 10.1681/ASN.2006060674. [DOI] [PubMed] [Google Scholar]
- 24.Mora S, Rifai N, Buring JE, et al. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation. 2008;118:993–1001. doi: 10.1161/CIRCULATIONAHA.108.777334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.