Abstract
Objectives
This review highlights current methods and strategies for accelerated in vitro drug release testing of extended release parenteral dosage forms such as polymeric microparticulate systems, lipid microparticulate systems, in situ depot-forming systems, and implants.
Key findings
Extended release parenteral dosage forms are typically designed to maintain the effective drug concentration over periods of weeks, months or even years. Consequently, “real-time” in vitro release tests for these dosage forms are often run over a long time period. Accelerated in vitro release methods can provide rapid evaluation and therefore are desirable for quality control purposes. To this end, different accelerated in vitro release methods using United States Pharmacopoeia (USP) apparatus have been developed. Different mechanisms of accelerating drug release from extended release parenteral dosage forms, along with the accelerated in vitro release testing methods currently employed are discussed.
Conclusions
Accelerated in vitro release testing methods with good discriminatory ability are critical for quality control of extended release parenteral products. Methods that can be used in the development of in vitro-in vivo correlation (IVIVC) are desirable, however for complex parenteral products this may not always be achievable.
Keywords: accelerated in vitro release testing, extended release parenteral dosage forms, USP apparatus, quality control
Introduction
Extended release parenteral dosage forms (such as polymeric microparticulate systems, lipid microparticulate systems, in situ depot-forming systems, and implants) have attracted extensive attention during the past decades.[1-6] Such systems can maintain effective drug concentrations over extended periods of time, minimize undesirable fluctuations in systemic drug concentration and reduce administration frequency, thus improving patience compliance.[7] Since MR parenteral dosage forms usually contain substantial amounts of potent therapeutic agents, dose dumping or unanticipated changes of in vivo drug release characteristics may lead to severe side effects.[8] Accordingly, it is essential to understand in vivo performance and have appropriate in vitro release testing methods that can mimic the in vivo performance of these systems.
In vitro release testing methods with good discriminatory ability are critical for quality control purposes and the development of methods that can be used in in vitro-in vivo correlation (IVIVC) are desirable to assist in formulation development and help reduce the regulatory burden of bioequivalence testing. It has been more than 100 years since the first dissolution test was introduced by Noyes and Whitney.[9] In 1970, the United State Pharmacopeia (USP) adopted the basket-stirred-flask test (USP apparatus 1) as the first official dissolution test method for solid oral dosage forms.[10] Since then, dissolution testing has become an essential quality control test. The dissolution test is referred to as an “in vitro drug release” test in the case of extended release parenteral dosages forms.[11] In general, compendial apparatus and methods should be used as a first approach in drug development. However, unlike conventional solid oral dosage forms, extended release parenteral dosage forms have very wide array of physicochemical and release characteristics. Considering the diversity of such systems, it is very challenging to develop regulations and standards. Consequently, at this time, there are no standard compendial method(s) for in vitro release testing of extended release parenteral release dosage forms.
Various in vitro release testing methods (such as sample-and-separate, dialysis sac, and continuous flow methods) have been used for extended release parenteral dosage forms.[12-16] Extended release parenteral dosage forms such as microspheres are typically designed to maintain the effective drug concentration over periods of weeks, months or even years. Therefore, “real-time” in vitro release studies for these dosage forms would require extended periods of time, which would impact the time to batch release of product and hence reduce the effective product shelf life. Over the past few years, accelerated in vitro release methods have received considerable attention in order to shorten the time required to study drug release.[17; 18] Parameters that can be utilized to achieve accelerated release include: temperature, solvent, ionic strength, pH, enzymes, surfactants as well as agitation rate.[19] However, such accelerated conditions may not only accelerate the rate of drug release but also change the mechanism of drug release.[20; 21] Therefore, it is very important to understand the drug release mechanism as well as how accelerated parameters may affect it.
Ideally, drug release from “real-time” and accelerated tests should follow the same release mechanism with a 1:1 correlation between the release profiles.[22] However, it is possible that the drug mechanism(s) may change since accelerated release tests are typically performed under extreme conditions (e.g. high temperatures as well as acidic or basic pH conditions). Nevertheless, “real-time” and accelerated release profiles should show a minimum of a rank order relationship between different formulations.[19] Accelerated release testing should be capable to serve as a discriminatory tool as long as all formulations experience similar changes and continue to exhibit performance characteristics that can be differentiated from each other. It is recommended that the specifications for accelerated release should include a determination of at least 80 % of the cumulative amount released for comparison with “real-time” studies.[23]
This review will summarize in vitro drug release mechanism(s) of commonly investigated extended release parenteral dosage forms, followed by a discussion of current accelerated in vitro release testing methods for these systems.
Mechanism(s) of in vitro drug release from extended release parenteral dosage forms
The drug release mechanisms from extended release parenteral dosage forms may vary depending on the characteristics of the dosage forms as well as the conditions and the methods used for in vitro release testing. Therefore, understanding the in vitro drug release mechanism can facilitate the development of a suitable accelerated in vitro release testing method.
Polymeric microparticulate systems
Polymeric microspheres are one of the most commonly investigated extended release parenteral dosage forms. These systems can be administrated via subcutaneous or intramuscular injection as well as by direct injection into the target site or tissue. Since microspheres cannot be retrieved following parenteral administration, the polymers used in formulation design should be biodegradable as well as biocompatible.[24] A variety of biodegradable polymers, including lactide/glycolide polymers (such as polylactic acid (PLA) and poly (lactic-co-glycolic acid) (PLGA)), poly-ε-caprolactone, polyanhydrides, polyortho esters, and albumin, have been utilized to prepare microparticles.[25; 26; 24; 27]
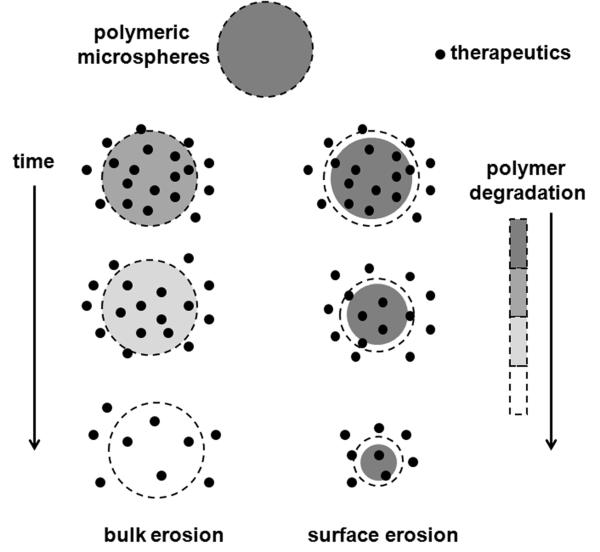
Based on the physicochemical characteristics of the polymer, drug release from polymeric microparticles may involve diffusion, polymer erosion or a combination thereof.[28; 29] It is known that diffusion governs the initial burst release phase, while polymer erosion is dominant in the later primary release phase.[28; 30; 31] Fick’s second law of diffusion can be used to elucidate the diffusion-controlled drug release phase.[32] In the case of the erosion-controlled release phase, drug release is mainly controlled by polymer erosion resulting from hydrolysis of the polymer chains. As shown in Figure 1, there are two different polymer erosion scenarios: surface (heterogeneous) and bulk (homogeneous) erosions.[33] In surface erosion, polymer (e.g. polyanhydrides or polyortho esters) erosion takes place at the external matrix boundary, which may result in a decreasing microsphere diameter.[29; 34] In bulk erosion, polymer (e.g. poly lactide/glycolide polymers) degradation takes place throughout the entire microsphere structure.[33] Several parameters including polymer properties (e.g. molecular weight, copolymer composition, and crystallinity),[35; 36] drug properties,[37] particle size,[38; 39] as well as release conditions (such as release media and agitation),[40] can affect drug release from polymeric microspheres.
Figure 1.
Commonly used in vitro release testing methods for polymeric microparticles include: sample-and-separate methods, continuous flow cell methods, and dialysis methods.[14]
Lipid microparticulate systems
Lipid microparticulate systems (such as oil suspensions, multivesicular liposomes, and lipid microparticles) can be injected subcutaneously, intramuscularly or intra-articularly to achieve sustained drug delivery.[41; 42] Lipid nanoparticles (small unilamellar liposomes as well as solid lipid nanoparticles) can also be injected intravenously. In general, the duration of release from lipid microparticulate systems is no longer than one week. This can be compared to polymeric microparticulate systems which can be programmed to release anywhere from a few days to years.[24] Although lipid microparticulate systems have been in clinical use for several decades in the field of schizophrenia and hormone replacement therapy,[43; 44; 42] there is some controversy over the drug release mechanism(s) from such systems.
DepoFoam™ is a lipid-based drug delivery system consisting of microscopic, spherical particles (10-20 μm) with hundreds of nonconcentric aqueous chambers. Drug release from DepoFoam™ is controlled by drug diffusion through the phospholipid bilayers.[45] In the case of oil suspensions, two drug release mechanisms have been proposed: i) the suspended drug dissolves in the oil phase prior to release into the aqueous phase via partitioning;[46; 47] and ii) the solid drug particles in the oil phase are transported via sedimentation to the oil-water interface or directly into the aqueous media where the drug undergoes dissolution/release.[48]
In vitro release testing methods used for the lipid-based microparticulate systems can be divided into three categories: i) lipophilic solution floating on the top of the release medium;[47] ii) dialysis techniques;[49; 41] and iii) continuous flow cell methods.[42] Different experimental setups can influence oil-water interfacial area, hydrodynamics (stirring conditions) and sink conditions, thus affecting drug release from such systems.[42]
In situ depot-forming systems
In situ depot-forming systems consist of a biodegradable carrier dissolved in a suitable solvent in which the drug is either dispersed or dissolved. Following parenteral administration (e.g. subcutaneous, intratumoral or intramuscular injection), a depot is formed at the site of injection to achieve sustained drug release over several days or months.[50] The mechanism of depot formation can be classified into:[51; 52] 1) thermoplastic pastes; 2) in situ cross-linked polymer systems; 3) in situ polymer precipitation; 4) thermally induced gelling systems; 5) organogels; and 6) hydrophobic fatty acid-based injectable pastes.
Drug release from in situ depot-forming systems is typically controlled by diffusion as well as a combined mechanism of diffusion/polymer erosion.[50; 53; 54] The hydrophobicity and concentration of the biodegradable carrier, the polar nature/water miscibility of the organic solvent as well as the aqueous solubility and loading of the drug affect the drug release rate.[24]
For in situ forming systems, most in vitro release tests have been performed using variants of the sample-and-separate methodology.[55] In addition, the dialysis method has also been used to evaluate in situ depot-forming formulations.[56] When performing an in vitro release test, the pre-gelled formulation is usually held in a special retainer to achieve a defined geometry or formulation-buffer interfacial area. Alternatively, the already formed formulation can be placed into the release medium using a syringe.[57; 58]
Implantable systems
Typically, implantable systems are inserted into specific body sites by minor surgical procedures or simple injection. Unlike the polymeric microparticulate systems, both biodegradable and non-biodegradable materials can be used to prepare implants. In the case of non-biodegradable implants, a second surgical procedure is required to remove the implants.[24]
The drug release mechanism from implants is complex and depends on various factors, including the type and amount of material used (e.g. biodegradable polymers, lipids or nonbiodegradable polymers), the properties of incorporated drug as well as the preparation techniques.[59] Similar to other biodegradable polymer-based dosage forms, drug release from polymeric implants is mainly controlled by diffusion or a combination of diffusion and polymer erosion.[5] Lipid (e.g. triglycerides, monoglycerides, and fatty acids) implants have been used as alternative carriers for sustained protein delivery to avoid the creation of acidic micro-climates associated with hydrolysis degradation of biodegradable polymers (e.g. PLGA).[60-62] It has been reported that drug release from lipid implants is typically controlled by diffusion.[61] In addition, the swelling process as well as the addition of release modifiers (e.g. PEG) may also play important roles on drug release from lipid implants.[63-65] Nonbiodegradable polymers or biodegradable elastomers were utilized along with osmotic pressure to achieve precise zero-order drug delivery kinetics.[66; 67] These osmotically controlled implants are different from other implantable systems that rely on diffusion or polymer erosion for sustained drug delivery.
To study the in vitro drug release from the implantable systems, the implants are usually placed into glass vials with or without agitation.[66; 61; 63] In addition, a flow-through apparatus has also been used to determine drug release from such systems.[68-70]
Parameters accelerating in vitro drug release
Based on a knowledge of the drug release mechanisms from the extended release parenteral dosage forms, it is known that several parameters (such as temperature, pH, surfactant, agitation rate, and presence of enzymes) can affect the drug release profile (e.g. hasten the rate of polymer hydration and degradation or enhance drug diffusion), thus accelerating drug release.[71-73]
Temperature
Elevated temperature has been widely used to accelerate drug release from the extended release parenteral dosage forms.[18; 22; 15; 74; 70] High temperature can increase molecular mobility. At temperatures above the polymer glass transition temperature (Tg), the increased polymer mobility results in significant acceleration of drug release via diffusion.[18; 22] Furthermore, high temperature can enhance hydration and degradation of polymers, thus accelerating erosion-controlled drug release.[22]
Ideally, accelerated tests should be predictive of “real-time” release tests.[19; 21] The Arrhenius equation (Equation 1) has been used to determine whether drug release rates at elevated temperatures can be used to predict “real-time” release.[75; 22; 70]
| (1) |
where k is zero-order release rate, A is a constant, Ea is the energy of activation, R is the gas constant (cal/deg mol) and T is the absolute temperature.
Taking the natural logarithm of the Arrhenius equation yields:
| (2) |
The rate constant (k) at different elevated temperatures can be calculated based on the release data. A plot of ln(k) versus 1/T gives a straight line, whose the slope is −Ea/2.303R, where Ea is the energy of activation.
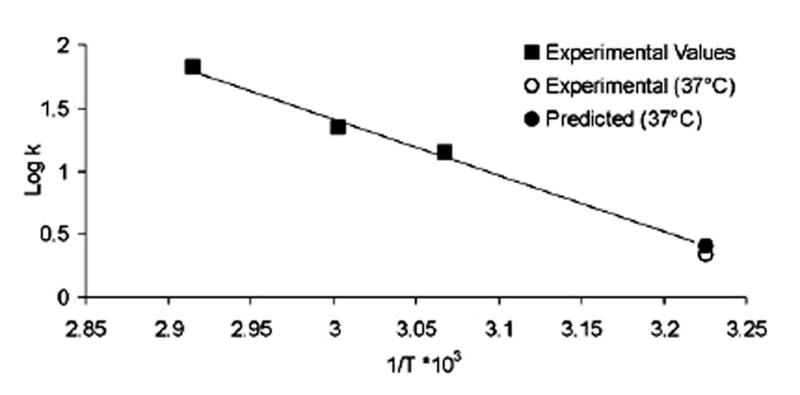
As shown in Figure 2, dexamethasone release rates from PLGA microspheres at elevated temperatures were successfully used to predict “real-time” release applying the Arrhenius equation.[22] The predicted “real-time” rate constant at 37°C (solid circle) was in agreement with the experimental value at 37°C (open circle).
Figure 2.
In the case of polymeric microparticles, the initial burst release phase that is governed by diffusion changed significantly at elevated temperature. However, elevated temperature accelerated testing failed to accurately predict the “real-time” burst release phase due to a combination of two competing factors.[76] Elevated temperature can increase polymer mobility, thus resulting in increased drug release via diffusion. Meanwhile the increased polymer mobility can cause microsphere surface morphology changes (e.g. pore closure), which in turn may decrease drug release.[22] Therefore, it is recommended that elevated accelerated release tests should be augmented by an initial “real-time” study that allows adequate assessment of any burst release phase.[23] In addition, it should be noted that high temperature may result in accelerated degradation of media components as well as the drug.[11]
pH
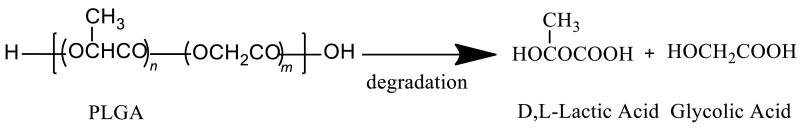
pH is another important parameter that can affect the hydrolysis kinetics of biodegradable polyesters, resulting in accelerated drug release from these systems (e.g. polymeric microparticulate systems or polymeric implants).[34] It is well known that PLGA is degraded by non-enzymatic hydrolysis of the ester backbone under physiological conditions (as shown in Figure 3). Accordingly, both acidic and basic conditions can accelerate degradation of such polymers. However, the polymer erosion mechanism appeared different under these two pH conditions.[77; 78] Under acidic conditions, PLGA erosion followed a bulk erosion profile that was similar to the degradation characteristics obtained at pH 7.4.[33] Whereas under basic conditions (pH>13), degradation appeared to occur by surface erosion.[33] It has also been demonstrated that morphology changes in the microspheres at acidic pH is considerably different from that at pH 7.4. This may be due to a more homogeneous degradation pattern at pH 2.4.[34]
Figure 3.
Although extreme pH conditions can hasten drug release, the acceleration of drug release is not as significant as that achieved at high temperatures. Additionally, extreme pH conditions may not be suitable for the drugs that are sensitive to these extreme pH conditions.
Release media
Accelerated drug release from the extended release parenteral dosage forms can also be achieved by adding surfactants or organic solvents into the release media. In the case of lipid implants, the presence of surfactants (e.g. Tween 20) in the release media can facilitate wetting and buffer penetration, and/or increase drug solubility in the media (via micelle solubilization), resulting in faster drug release.[61; 79] Moreover, some surfactants (e.g. 0.1% Tween 81) may interact with lipid matrix and induce the formation of cracks in the lipid matrix, thus accelerating drug release.[79]
Organic solvents (e.g. ethanol, acetonitrile) have been successfully used to achieve accelerated drug release.[80; 73] The addition of acetonitrile to the release media can increase the porosity of PLGA-based stent matrices and therefore result in accelerated drug release. This method has been used to discriminate different variables in the manufacturing process and a good correlation with “real-time” release was shown.[73]
Other parameters
Changing other parameters (such as the agitation conditions, and the interfacial area) that have an influence on the in vitro drug release characteristics can also accelerate drug release from extended release parenteral dosage forms.[81; 82] For example, the drug oil-water distribution coefficient is a key parameter influencing drug release from oil depot formulations. Accordingly, a rotating dialysis cell model that generates a high oil-buffer interfacial area can accelerate drug release from such formulations.[82]
Current in vitro accelerated release models
In contrast to oral and transdermal extended release dosage forms, no standard pharmacopeial or other regulatory method exists for in vitro drug release testing of extended release parenteral dosage forms. Moreover, the current USP apparatus, initially designed for in vitro release of oral and transdermal products, are not directly applicable for extended release parenteral dosage forms. For example, USP apparatus 1 (basket) and 2 (paddle) based on the sample-and-separate methodology typically require large volumes of release media, which are not suitable for many low dose parenteral formulations. USP apparatus 3 (reciprocating cylinder) was designed for bead-type delivery systems. USP apparatus 4 (flow through cell) was designed for extended release oral dosage forms and as such is more applicable for extended release parenteral dosage forms compared to the other compendial apparatus (refer below). USP apparatus 5 (paddle over disc) and 6 (cylinder) that were designed for transdermal formulations are not desirable for in vitro release testing of polymeric microparticulate formulations, since the microparticles cannot be easily retained in these apparatus. USP apparatus 7 (reciprocating disc) was designed for transdermal systems and non-disintegrating extended release oral dosage forms. USP apparatus 7 has been used for some extended release parenteral dosage forms such as drug-eluting stents.
Over the past few years, extensive efforts have been made to develop suitable in vitro release testing methods for extended release parenteral dosage forms.[14; 16; 65; 42] Since accelerated in vitro release testing can be used to rapidly assess and predict “real-time” drug release profiles, it is recommended that accelerated in vitro release testing should be developed as early as possible in the formulation development process.[19; 75; 68] The current in vitro accelerated release testing methods used for extended release parenteral dosage forms include sample-and-separate methods, continuous flow cell methods as well as dialysis methods.
Sample-and-separate methods
This method is the most widely used research method for polymeric microparticles and implants.[14; 61; 8] Conventionally, polymeric microparticles and implants are introduced into a vessel/vial containing release media and release is assessed over time. In the case of polymeric microparticles, centrifugation of the release media followed by sampling of the supernatant is widely used. In some cases, release media replacement may be necessary in order to maintain sink conditions or avoid drug degradation in release media.[83] Different experimental setups (such as size of container, agitation, and sampling methods) can affect the in vitro drug release profile.
Accelerated methods based on the sample-and-separate methodology at acidic pH (pH 4) and elevated temperature (50°C) were developed to accelerate leuprolide acetate release from PLGA depot formulations.[18] Complete release was achieved in 30-40 hours at 50°C compared to 42 days under “real-time” conditions. This method was able to differentiate different formulations and correlate well with “real-time” release at 37°C. USP apparatus 7 (reciprocating disk/stent holder) was utilized to develop an accelerated in vitro release method for biodegradable drug eluting stents. In this study, acetonitrile was added into the release medium to achieve over 80 % of Everolimus release within 24 h.[73]
Although the sample-and-separate methodology provides a direct and reasonably accurate assessment of in vitro drug release, there are some limitations associated with this method such as inadequate agitation, loss of microparticulates during sampling, and the use of vials/vessels of different dimensions that makes intra-laboratory comparison difficult.[81; 14]
Continuous flow cell methods
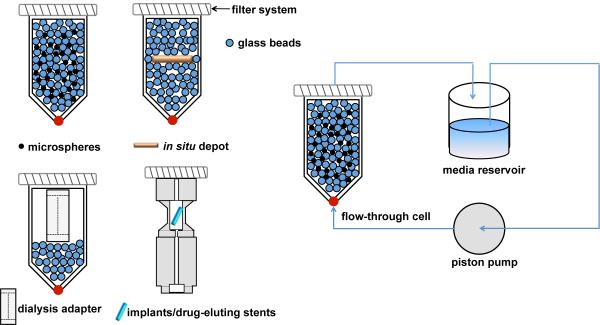
USP apparatus 4 (continuous flow cell method) was originally developed for extended release oral dosage forms. Modifications of the USP apparatus 4 have been used to assess drug release from extended release parenteral dosage forms such as microspheres,[81; 84; 85] liposomes,[86], drug-eluting stents and implants.[16; 69] As shown in Figure 4, parenteral dosage forms have been placed into flow through cells together with glass beads and/or adaptors. For example, microspheres have been loaded into flow through cells with glass beads to prevent microsphere aggregation and facilitate laminar flow of the release media throughout the cells.[81] A dialysis adaptor has been developed to hold nanoparticles such as liposomes within the flow through cells. The release media is circulated through the flow through cells and drug release is monitored from the effluent (open system) or the external media reservoir (closed system, Figure 4).
Figure 4.
USP apparatus 4 can simulate the in vivo environment such as subcutaneous tissue, since small volumes of media can be used and constant circulation can mimic the dynamic in vivo environment. In addition, the media volume used with the USP apparatus 4 can be modified to allow testing of various formulations, and this is particularly important for many low dose parenteral formulations. Elevated temperature and acidic pH accelerated release tests using USP apparatus 4 have been developed for rapid evaluation of microsphere formulations.[22; 15; 34]
Dialysis methods
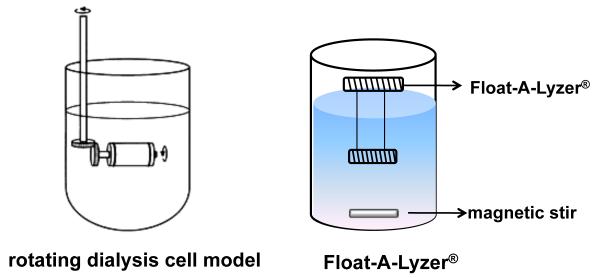
Dialysis methods appear to be an attractive option to study drug release from polymeric microparticles or in situ depot-forming systems. This method has been used to study drug release from oily parenteral depots,[87; 82] microspheres,[12; 40] liposomes,[88] and implants.[89] Among the dialysis methods, a rotating dialysis cell model and a Float-A-Lyzer® method have been used (Figure 5).[75; 90] Accelerated drug release testing using the Float-A-Lyzer® was performed at high temperatures to investigate leuprolide acetate release from PLGA microspheres.[75] A good correlation between the accelerated release profile and the “real-time” release data was obtained in this study.
Figure 5.
In order to develop suitable dialysis methods for in vitro release testing of the extended release parenteral dosage forms, the following parameters must be considered: agitation conditions; donor and acceptor cell volumes; and dialysis membrane molecular weight cutoff. It is recommended that the inside volume of the dialysis sac should be at least 6-10 fold less than that of the outer release media in order to provide a driving force for drug transport through the dialysis membrane.[14] Furthermore, if the drug binds to the dialysis membrane, this technique is not applicable.
There are several disadvantages associated with the dialysis methods such as: i) violation of sink conditions within the dialysis sacs; ii) lack of agitation within the sacs that can lead to aggregation and consequent change in release profiles; and iii) since this is non-compendial method different laboratories may use different setups which can result in different release profiles.
Mathematical models
Beside the experimental release testing methods as outlined above, a mathematical model is also desirable to evaluate whether accelerated test data are predictive of “real-time” release profiles. The use of the Weibull function in modeling drug release from extended release dosage forms (e.g. biodegradable microspheres) was recommended at a previous AAPS/FIP workshop.[11] The Weibull function assumes that the drug release is governed by polymer erosion coupled with minimal initial burst release as well as minimal diffusive release. This equation was used to model the drug release from PLGA microspheres (Equation 3) under accelerated and “real-time” testing conditions.[75]
| (3) |
where X is the percentage of drug released at time t and release is complete when Xinf is 100 %, α is a scale factor corresponding to the apparent rate constant, and β is a shape factor. The shape of the simulated curve can be characterized as exponential (β=1); sigmoid or S-shaped with an upward curvature followed by a turning point (β>1); or parabolic, with a higher initial slope and after that consistent with exponential (β<1).[91; 75; 29]
Considering that an initial burst release phase may occur for polymeric microspheres, the Weibull equation has been modified to include the initial burst release phase:[75]
| (4) |
where Xburst is the percentage of drug released in the burst phase.
Analysis of the goodness of fit (R2) of model parameters α and β at accelerated and “real-time” conditions can identify accelerated parameters to optimize appropriate accelerated conditions to correlate with “real-time” release. These will facilitate the rational design of accelerated in vitro drug release testing method to serve as a reliable quality control tool.
Although the Weibull function is considered to be one of the most powerful mathematical models for the description of drug release kinetics in either Euclidean or fractal spaces,[10] it is an empirical model that is not deduced based on kinetics and therefore cannot adequately characterize drug release kinetics.[91]
Conclusions
Extended release parenteral dosages forms have been successfully used for the treatment of a variety of diseases. In order to assure the performance and safety of these products, the development of a suitable in vitro release testing method is crucial. Over the past decades, extensive efforts have been made to understand drug release mechanism(s) from these formulations. However, due to their differing complexities, the in vitro drug release mechanism(s) from such systems (e.g. lipid microparticulate systems) are far from fully elucidated. Consequently, it is very challenging to develop suitable in vitro release testing methods for these systems. Modification of standardized technologies or conventional methods and corresponding method validation may be necessary in order to accommodate the special characteristics of these formulations.
“Real-time” in vitro release testing is necessary to gain a mechanistic understanding of drug release and to develop a good in vitro-in vivo correlation, whereas accelerated release testing for extended release parenteral dosage forms is essential for quality control purposes as well as to assist in formulation development. Ideally, “real-time” and accelerated tests should follow the same release mechanism. However, for quality control purposes an accelerated test that follows a different release mechanism may be acceptable as long as it can discriminate out of specification batches and meets other required criteria. In the case of dosage forms that show an initial burst release, it may be necessary to conduct an initial “real-time” study to assess the burst release as this may be lost in the accelerated test. It is also important that the accelerated test should mimic the physiological conditions at the site of administration to the extent possible.
Other factors that should be taken into consideration during the method development process include stability of release media components and of the drug, as well as the robustness of the test apparatus to withstand the applied extreme conditions.
Acknowledgements
The authors would like to thank the National Institutes of Health Grant (# R43 EB011886-01) and the US Army Medical Research (#W81XWH-07-1-0688) for financial support.
Footnotes
Declarations Conflict of interest The author(s) declare(s) that they have no conflict of interest to disclose.
References
- 1.Davis JM, et al. Depot antipsychotic drugs. Place in therapy. Drugs. 1994;5:741–773. doi: 10.2165/00003495-199447050-00004. [DOI] [PubMed] [Google Scholar]
- 2.Simone EA, et al. Polymeric carriers: role of geometry in drug delivery. Expert Opin Drug Deliv. 2008;12:1283–1300. doi: 10.1517/17425240802567846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu F, Jin T. Polymer-based sustained-release dosage forms for protein drugs, challenges, and recent advances. AAPS PharmSciTech. 2008;4:1218–1229. doi: 10.1208/s12249-008-9148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huynh NT, et al. Lipid nanocapsules: a new platform for nanomedicine. Int J Pharm. 2009;2:201–209. doi: 10.1016/j.ijpharm.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 5.Bhardwaj U, et al. PLGA/PVA hydrogel composites for long-term inflammation control following s.c. implantation. Int J Pharm. 2010;1-2:78–86. doi: 10.1016/j.ijpharm.2009.09.046. [DOI] [PubMed] [Google Scholar]
- 6.Huang Y, et al. In vitro and in vivo performance of a dual drug-eluting stent (DDES) Biomaterials. 2010;15:4382–4391. doi: 10.1016/j.biomaterials.2010.01.147. [DOI] [PubMed] [Google Scholar]
- 7.Martinez MN, et al. Breakout session summary from AAPS/CRS joint workshop on critical variables in the in vitro and in vivo performance of parenteral sustained release products. J Control Release. 2010;1:2–7. doi: 10.1016/j.jconrel.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 8.Larsen C, et al. Role of in vitro release models in formulation development and quality control of parenteral depots. Expert Opin Drug Deliv. 2009;12:1283–1295. doi: 10.1517/17425240903307431. [DOI] [PubMed] [Google Scholar]
- 9.Noyes AA, Whitney WR. The rate of solution of solid substances in their own solutions. J Am Chem Soc. 1897;930:934. [Google Scholar]
- 10.Dokoumetzidis A, Macheras P. A century of dissolution research: from Noyes and Whitney to the biopharmaceutics classification system. Int J Pharm. 2006;1-2:1–11. doi: 10.1016/j.ijpharm.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Siewert M, et al. FIP/AAPS guidelines to dissolution/in vitro release testing of novel/special dosage forms. AAPS PharmSciTech. 2003;1:E7. doi: 10.1208/pt040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Souza SS, DeLuca PP. Development of a dialysis in vitro release method for biodegradable microspheres. AAPS PharmSciTech. 2005;2:E323–328. doi: 10.1208/pt060242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong CM, et al. In vitro degradation and controlled release behavior of D,L-PLGA50 and PCL-b-D,L-PLGA50 copolymer microspheres. J Control Release. 2005;1:53–64. doi: 10.1016/j.jconrel.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 14.D’Souza SS, DeLuca PP. Methods to assess in vitro drug release from injectable polymeric particulate systems. Pharm Res. 2006;3:460–474. doi: 10.1007/s11095-005-9397-8. [DOI] [PubMed] [Google Scholar]
- 15.Iyer SS, et al. A ‘biorelevant’ approach to accelerated in vitro drug release testing of a biodegradable, naltrexone implant. Int J Pharm. 2007;1-2:119–125. doi: 10.1016/j.ijpharm.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 16.Neubert A, et al. Development of a vessel-simulating flow-through cell method for the in vitro evaluation of release and distribution from drug-eluting stents. J Control Release. 2008;1:2–8. doi: 10.1016/j.jconrel.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Aso Y, et al. Effect of temperature on mechanisms of drug release and matrix degradation of poly(d,l-lactide) microspheres. Journal of Controlled Release. 1994;1:33–39. [Google Scholar]
- 18.Shameem M, et al. A short-term (accelerated release) approach to evaluate peptide release from PLGA depot formulations. The AAPS Journal. 1999;3:1–6. doi: 10.1208/ps010307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burgess DJ, et al. Assuring quality and performance of sustained and controlled release parenterals: EUFEPS workshop report. AAPS J. 2004;1:100–111. doi: 10.1208/ps060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burgess DJ, et al. Assuring quality and performance of sustained and controlled release parenterals: workshop report. AAPS PharmSci. 2002;2:E7. doi: 10.1208/ps040205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez M, et al. In vitro and in vivo considerations associated with parenteral sustained release products: a review based upon information presented and points expressed at the 2007 Controlled Release Society Annual Meeting. J Control Release. 2008;2:79–87. doi: 10.1016/j.jconrel.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Zolnik BS, et al. Elevated temperature accelerated release testing of PLGA microspheres. J Control Release. 2006;3:293–300. doi: 10.1016/j.jconrel.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 23.Burgess DJ, et al. Assuring quality and performance of sustained and controlled release parenterals: AAPS workshop report, co-sponsored by FDA and USP. Pharm Res. 2002;11:1761–1768. doi: 10.1023/a:1020730102176. [DOI] [PubMed] [Google Scholar]
- 24.Shi Y, Li L. Current advances in sustained-release systems for parenteral drug delivery. Expert Opin Drug Del. 2005;6:1039–1058. doi: 10.1517/17425247.2.6.1039. [DOI] [PubMed] [Google Scholar]
- 25.Patil GV. Biopolymer albumin for diagnosis and in drug delivery. Drug Development Research. 2003;3:219–247. [Google Scholar]
- 26.Jain JP, et al. Role of polyanhydrides as localized drug carriers. Journal of Controlled Release. 2005;3:541–563. doi: 10.1016/j.jconrel.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 27.Zalfen AM, et al. Controlled release of drugs from multi-component biomaterials. Acta Biomater. 2008;6:1788–1796. doi: 10.1016/j.actbio.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 28.Faisant N, et al. PLGA-based microparticles: elucidation of mechanisms and a new, simple mathematical model quantifying drug release. Eur J Pharm Sci. 2002;4:355–366. doi: 10.1016/s0928-0987(02)00023-4. [DOI] [PubMed] [Google Scholar]
- 29.Arifin DY, et al. Mathematical modeling and simulation of drug release from microspheres: Implications to drug delivery systems. Advanced Drug Delivery Reviews. 2006;12-13:1274–1325. doi: 10.1016/j.addr.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Alexis F. Factors affecting the degradation and drug-release mechanism of poly(lactic acid) and poly[(lactic acid)-co-(glycolic acid)] Polym Int. 2005;1:36–46. [Google Scholar]
- 31.Kumar R, Palmieri MJ., Jr Points to consider when establishing drug product specifications for parenteral microspheres. AAPS J. 2010;1:27–32. doi: 10.1208/s12248-009-9156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koizumi T, Panomsuk SP. Release of medicaments from spherical matrices containing drug in suspension: Theoretical aspects. International Journal of Pharmaceutics. 1995;1:45–49. [Google Scholar]
- 33.von Burkersroda F, et al. Why degradable polymers undergo surface erosion or bulk erosion. Biomaterials. 2002;21:4221–4231. doi: 10.1016/s0142-9612(02)00170-9. [DOI] [PubMed] [Google Scholar]
- 34.Zolnik BS, Burgess DJ. Effect of acidic pH on PLGA microsphere degradation and release. J Control Release. 2007;3:338–344. doi: 10.1016/j.jconrel.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 35.Park TG. Degradation of poly(lactic-co-glycolic acid) microspheres: effect of copolymer composition. Biomaterials. 1995;15:1123–1130. doi: 10.1016/0142-9612(95)93575-x. [DOI] [PubMed] [Google Scholar]
- 36.Tracy MA, et al. Factors affecting the degradation rate of poly(lactide-co-glycolide) microspheres in vivo and in vitro. Biomaterials. 1999;11:1057–1062. doi: 10.1016/s0142-9612(99)00002-2. [DOI] [PubMed] [Google Scholar]
- 37.Sandor M, et al. Effect of protein molecular weight on release from micron-sized PLGA microspheres. J Control Release. 2001;3:297–311. doi: 10.1016/s0168-3659(01)00446-1. [DOI] [PubMed] [Google Scholar]
- 38.Siepmann J, et al. Effect of the size of biodegradable microparticles on drug release: experiment and theory. J Control Release. 2004;1:123–134. doi: 10.1016/j.jconrel.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 39.Berchane NS, et al. Effect of mean diameter and polydispersity of PLG microspheres on drug release: experiment and theory. Int J Pharm. 2007;1-2:118–126. doi: 10.1016/j.ijpharm.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 40.Faisant N, et al. Effects of the type of release medium on drug release from PLGA-based microparticles: experiment and theory. Int J Pharm. 2006;2:189–197. doi: 10.1016/j.ijpharm.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 41.Larsen SW, et al. On the mechanism of drug release from oil suspensions in vitro using local anesthetics as model drug compounds. European Journal of Pharmaceutical Sciences. 2008;1:37–44. doi: 10.1016/j.ejps.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Larsen S Weng, Larsen C. Critical factors influencing the in vivo performance of long-acting lipophilic solutions--impact on in vitro release method design. AAPS J. 2009;4:762–770. doi: 10.1208/s12248-009-9153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zuidema J, et al. Release and absorption rates of intramuscularly and subcutaneously injected pharmaceuticals (II) International Journal of Pharmaceutics. 1994;3:189–207. [Google Scholar]
- 44.Fredholt K, et al. Modification of in vitro drug release rate from oily parenteral depots using a formulation approach. European Journal of Pharmaceutical Sciences. 2000;3:231–237. doi: 10.1016/s0928-0987(00)00104-4. [DOI] [PubMed] [Google Scholar]
- 45.Mantripragada S. A lipid based depot (DepoFoam technology) for sustained release drug delivery. Prog Lipid Res. 2002;5:392–406. doi: 10.1016/s0163-7827(02)00004-8. [DOI] [PubMed] [Google Scholar]
- 46.Madan PL. Sustained-release drug delivery systems: Part V, parenteral products. Pharm Manuf. 1985;51:57. [Google Scholar]
- 47.Larsen SW, et al. In vitro assessment of drug release rates from oil depot formulations intended for intra-articular administration. Eur J Pharm Sci. 2006;5:348–354. doi: 10.1016/j.ejps.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 48.Crommelin DJA, De Blaey CJ. In vitro release studies on drugs suspended in non-polar media II. The release of paracetamol and chloramphenicol from suspensions in liquid paraffin. International Journal of Pharmaceutics. 1980;1:29–42. [Google Scholar]
- 49.Larsen DH, et al. Assessment of rate of drug release from oil vehicle using a rotating dialysis cell. European Journal of Pharmaceutical Sciences. 2000;3:223–229. doi: 10.1016/s0928-0987(00)00105-6. [DOI] [PubMed] [Google Scholar]
- 50.Zentner GM, et al. Biodegradable block copolymers for delivery of proteins and water-insoluble drugs. J Control Release. 2001;1-3:203–215. doi: 10.1016/s0168-3659(01)00276-0. [DOI] [PubMed] [Google Scholar]
- 51.Packhaeuser CB, et al. In situ forming parenteral drug delivery systems: an overview. Eur J Pharm Biopharm. 2004;2:445–455. doi: 10.1016/j.ejpb.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 52.Chitkara D, et al. Biodegradable injectable in situ depot-forming drug delivery systems. Macromol Biosci. 2006;12:977–990. doi: 10.1002/mabi.200600129. [DOI] [PubMed] [Google Scholar]
- 53.Wang L, et al. Drug release from injectable depots: two different in vitro mechanisms. J Control Release. 2004;2:207–216. doi: 10.1016/j.jconrel.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 54.McHugh AJ. The role of polymer membrane formation in sustained release drug delivery systems. J Control Release. 2005;1-3:211–221. doi: 10.1016/j.jconrel.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 55.Hyun H, et al. In vitro and in vivo release of albumin using a biodegradable MPEG-PCL diblock copolymer as an in situ gel-forming carrier. Biomacromolecules. 2007;4:1093–1100. doi: 10.1021/bm060991u. [DOI] [PubMed] [Google Scholar]
- 56.Kranz H, Bodmeier R. A novel in situ forming drug delivery system for controlled parenteral drug delivery. Int J Pharm. 2007;1-2:107–114. doi: 10.1016/j.ijpharm.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 57.Katakam M, et al. Controlled release of human growth hormone in rats following parenteral administration of poloxamer gels. Journal of Controlled Release. 1997;1:21–26. [Google Scholar]
- 58.Kempe S, et al. Do in situ forming PLG/NMP implants behave similar in vitro and in vivo? A non-invasive and quantitative EPR investigation on the mechanisms of the implant formation process. Journal of Controlled Release. 2008;3:220–225. doi: 10.1016/j.jconrel.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 59.Kreye F, et al. Lipid implants as drug delivery systems. Expert Opin Drug Del. 2008;3:291–307. doi: 10.1517/17425247.5.3.291. [DOI] [PubMed] [Google Scholar]
- 60.Mohl S, Winter G. Continuous release of rh-interferon [alpha]-2a from triglyceride matrices. Journal of Controlled Release. 2004;1:67–78. doi: 10.1016/j.jconrel.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 61.Guse C, et al. Drug release from lipid-based implants: elucidation of the underlying mass transport mechanisms. Int J Pharm. 2006;2:137–144. doi: 10.1016/j.ijpharm.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 62.Koennings S, et al. In vitro investigation of lipid implants as a controlled release system for interleukin-18. Int J Pharm. 2006;2:145–152. doi: 10.1016/j.ijpharm.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 63.Herrmann S, et al. Mechanisms controlling protein release from lipidic implants: effects of PEG addition. J Control Release. 2007;2:161–168. doi: 10.1016/j.jconrel.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 64.Lim Soo P, et al. Drug release mechanism of paclitaxel from a chitosan-lipid implant system: effect of swelling, degradation and morphology. Eur J Pharm Biopharm. 2008;1:149–157. doi: 10.1016/j.ejpb.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 65.Siepmann F, et al. A novel mathematical model quantifying drug release from lipid implants. J Control Release. 2008;3:233–240. doi: 10.1016/j.jconrel.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 66.Gu F, et al. Osmotic-driven release kinetics of bioactive therapeutic proteins from a biodegradable elastomer are linear, constant, similar, and adjustable. Pharm Res. 2006;4:782–789. doi: 10.1007/s11095-006-9750-6. [DOI] [PubMed] [Google Scholar]
- 67.Wright JC. Critical variables associated with nonbiodegradable osmotically controlled implants. AAPS J. 2010;3:437–442. doi: 10.1208/s12248-010-9199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iyer SS, et al. Profiling in vitro drug release from subcutaneous implants: a review of current status and potential implications on drug product development. Biopharm Drug Dispos. 2006;4:157–170. doi: 10.1002/bdd.493. [DOI] [PubMed] [Google Scholar]
- 69.Browne DC, Kieselmann K. Low-level drug release-rate testing of ocular implants using USP Apparatus 4 dissolution and HPLC end analysis. Dissolution Technol. 2010;1:12–14. [Google Scholar]
- 70.Shen J, Burgess DJ. Accelerated in vitro release testing of implantable PLGA microsphere/PVA hydrogel composite coatings. International journal of pharmaceutics. 2011 doi: 10.1016/j.ijpharm.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Makino K, et al. Preparation and in vitro degradation properties of polylactide microcapsules. Chem Pharm Bull (Tokyo) 1985;3:1195–1201. doi: 10.1248/cpb.33.1195. [DOI] [PubMed] [Google Scholar]
- 72.Duda JL, Zielinski JM. Free-volum theory. In: P., editor. Diffusion in Polymers. Marcel Dekker; New York: 1996. [Google Scholar]
- 73.Kamberi M, et al. A novel accelerated in vitro release method for biodegradable coating of drug eluting stents: Insight to the drug release mechanisms. Eur J Pharm Sci. 2009;3-4:217–222. doi: 10.1016/j.ejps.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 74.Rawat A, et al. Validation of USP apparatus 4 method for microsphere in vitro release testing using Risperdal Consta. International journal of pharmaceutics. 2011;2:198–205. doi: 10.1016/j.ijpharm.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 75.D’Souza SS, et al. A model-dependent approach to correlate accelerated with real-time release from biodegradable microspheres. AAPS PharmSciTech. 2005;4:E553–564. doi: 10.1208/pt060470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zolnik BS, et al. Elevated temperature accelerated release testing of PLGA microspheres. Journal of controlled release : official journal of the Controlled Release Society. 2006;3:293–300. doi: 10.1016/j.jconrel.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 77.Makino K, et al. Mechanism of hydrolytic degradation of poly(L-lactide) microcapsules: effects of pH, ionic strength and buffer concentration. J Microencapsul. 1986;3:203–212. doi: 10.3109/02652048609031574. [DOI] [PubMed] [Google Scholar]
- 78.de Jong SJ, et al. New insights into the hydrolytic degradation of poly(lactic acid): participation of the alcohol terminus. Polymer. 2001;7:2795–2802. [Google Scholar]
- 79.Koennings S, et al. Influence of wettability and surface activity on release behavior of hydrophilic substances from lipid matrices. J Control Release. 2007;2:173–181. doi: 10.1016/j.jconrel.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 80.Walden M, et al. The effect of ethanol on the release of opioids from oral prolonged-release preparations. Drug Dev Ind Pharm. 2007;10:1101–1111. doi: 10.1080/03639040701377292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zolnik BS, et al. Application of USP Apparatus 4 and in situ fiber optic analysis to microsphere release testing. Dissolution Technol. 2005;11:14. [Google Scholar]
- 82.Larsen SW, et al. Assessment of drug release from oil depot formulations using an in vitro model -- potential applicability in accelerated release testing. Drug Dev Ind Pharm. 2008;3:297–304. doi: 10.1080/03639040701655994. [DOI] [PubMed] [Google Scholar]
- 83.Murty SB, et al. Identification of chemically modified peptide from poly(D,L-lactide-co-glycolide) microspheres under in vitro release conditions. AAPS PharmSciTech. 2003;4:E50. doi: 10.1208/pt040450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Voisine JM, et al. In situ fiber optic method for long-term in vitro release testing of microspheres. Int J Pharm. 2008;1-2:206–211. doi: 10.1016/j.ijpharm.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 85.Rawat A, Burgess DJ. USP apparatus 4 method for in vitro release testing of protein loaded microspheres. Int J Pharm. 2011;1-2:178–184. doi: 10.1016/j.ijpharm.2011.02.057. [DOI] [PubMed] [Google Scholar]
- 86.Bhardwaj U, Burgess DJ. A novel USP apparatus 4 based release testing method for dispersed systems. Int J Pharm. 2010;1-2:287–294. doi: 10.1016/j.ijpharm.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 87.Schultz K, et al. Rotating dialysis cell as in vitro release method for oily parenteral depot solutions. Int J Pharm. 1997;2:163–169. doi: 10.1016/s0378-5173(97)00229-9. [DOI] [PubMed] [Google Scholar]
- 88.Saarinen-Savolainen P, et al. Method for evaluating drug release from liposomes in sink conditions. International Journal of Pharmaceutics. 1997;1:27–33. [Google Scholar]
- 89.Dash AK, et al. Development of an in vitro dissolution method using microdialysis sampling technique for implantable drug delivery systems. J Pharm Sci. 1999;10:1036–1040. doi: 10.1021/js980480g. [DOI] [PubMed] [Google Scholar]
- 90.Pedersen BT, et al. Characterization of the rotating dialysis cell as an in vitro model potentially useful for simulation of the pharmacokinetic fate of intra-articularly administered drugs. Eur J Pharm Sci. 2005;1:73–79. doi: 10.1016/j.ejps.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 91.Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;2:123–133. doi: 10.1016/s0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]