Abstract
Optimal neuronal activity requires that supporting cells provide both efficient nutrient delivery and waste disposal. The incomplete processing of engulfed waste by their lysosomes can lead to accumulation of residual material and compromise their support of neurons. As most degradative lysosomal enzymes function best at an acidic pH, lysosomal alkalinization can impede enzyme activity and increase lipofuscin accumulation. We hypothesize that treatment to reacidify compromised lysosomes can enhance degradation. Here, we demonstrate that degradation of ingested photoreceptor outer segments by retinal pigmented epithelial (RPE) cells is increased by stimulation of D5 dopamine receptors. D1/D5 receptor agonists reacidified lysosomes in cells alkalinized by chloroquine or tamoxifen, with acidification dependent on protein kinase A. Knockdown with siRNA confirmed acidification was mediated by the D5 receptor. Exposure of cells to outer segments increased lipofuscin-like autofluorescence, but SKF 81297 reduced autofluorescence. Likewise, SKF 81297 increased the activity of lysosomal protease cathepsin D in situ. D5DR stimulation also acidified lysosomes of RPE cells from elderly ABCA4−/− mice, a model of recessive Stargardt’s retinal degeneration. In conclusion, D5 receptor stimulation lowers compromised lysosomal pH, enhancing degradation. The reduced accumulation of lipofuscin-like autofluorescence implies the D5 receptor stimulation may enable cells to better support adjacent neurons.
Keywords: Dopamine, lysosomal pH, autophagy, lipofuscin, retina
Introduction
Proper neuronal function depends upon the ongoing ability of adjacent cells to provide nutrients and process extruded material. In this respect, sustained photoreceptor function requires the regular delivery of visual cycle components and degradation of phagocytosed photoreceptor outer segments (POS) by the retinal pigmented epithelium (Strauss 2005). Lysosomes of the RPE cells are key organelles in this degradative pathway, and proper maintenance of the lysosomal environment is essential for the breakdown of material introduced through both the autophagy and phagocytic pathways (Kon & Cuervo 2010). Most lysosomal enzymes are pH sensitive with optimal activity between 4.0 and 5.0 (Hayase & Tappel 1970, Mego 1984). The sharp pH dependence of key lysosomal enzymes predicts that even a moderate elevation of pH will lower enzyme efficiency and reduce the clearance of material. The control of lysosomal pH (pHL) is complex, requiring balanced activities of vHATPase pumps, counter anion channels and pumps and calcium transporters (Ohkuma et al. 1982, Jentsch 2007, Patel & Docampo 2010). While lysosomogenesis requires the appropriate delivery of these transporters to the lysosomal membrane, it is likely that their activity, and thus the lysosomal pH, can be regulated on a rapid timescale in response to environmental changes.
We have previously determined that elevation of cytoplasmic cAMP restores the pHL of compromised RPE cells to more acidic levels (Liu et al. 2008). The reacidification is inhibited by membrane-permeable myristoylated PKI amide which specifically binds to the catalytic site of cAMP-activated protein kinase A (PKA), strongly implicating PKA. Importantly, cAMP- triggered reacidification was proven functionally relevant, as it increased the degradation of outer segments by cultured RPE cells (Liu et al. 2008).
As elevation of cytoplasmic cAMP can reacidify lysosomes and improve outer segment degradation, agonists that stimulate receptors coupled to the Gαs protein, which stimulates adenylyl cyclase to produce cAMP, could prove beneficial in the treatment of conditions characterized by excessive accumulation of partially degraded material such as lipofuscin. Dopamine acts at two families of receptors, D1-like (D1 and D5) and D2-like (D2, D3 and D4) (Beaulieu & Gainetdinov 2011) with the D1-like receptors coupled to Gαs/olf (Konig & Gratzel 1994, Sidhu et al. 1998) and D2-like receptors couples to Gαi. As dopamine receptors were identified on RPE cells (Versaux-Botteri et al. 1997) and D1-like receptor agonists are being tested for treatment of a variety of neural disorders (Cadet et al. 2010), we examined the role of D1-like dopamine receptors in the acidification of lysosomes in RPE cells.
Methods
ARPE-19 cells
ARPE-19 cells (ATCC) were grown to confluence in 25 cm2 culture flasks in a 1:1 mixture of Dulbecco’s modified Eagle medium (DMEM) and Ham’s F12 medium with 3 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and 2.5 mg/ml fungizone and/or 50 μg/ml gentamicin and 10% fetal bovine serum (all Invitrogen Corp).
Measurement of lysosomal pH from ARPE-19 cells
ARPE-19 cells were grown in black 96-well plates, rinsed 3x with isotonic solution (IS; (in mM) NaCl 105, KCl 5, HEPES Acid 6, Na HEPES 4, NaHCO3 5, mannitol 60, glucose 5, MgCl2 0.5, CaCl2 1.3) and incubated with 5 μM LysoSensor Yellow/Blue (Invitrogen Corp.) diluted with IS. Extensive trials have found the optimal response is obtained with 3 min dye loading and recorded within a 15 min post-incubation (Liu et al. 2008). Fluorescence was measured with a Fluoroskan 96-well plate reader (ThermoElectron Corp.). Lysosomal pH was determined from the ratio of light excited at 340nm vs. 380nm (>520 nM em) and calibrated by exposing cells to 10 μM H+/Na+ ionophore monensin and 20 μM H+/K+ ionophore nigericin in 20 MES, 110 KCl and 20 NaCl at pH 4.0–6.0 for 15 min.
siRNA Silencing of D1 or D5 receptors
DRD1 and DRD5 expression was silenced using manufacturer’s protocols. ARPE-19 cells were transfected with siRNAs specific for DRD1 receptor (s4283) or DRD5 receptor (s4291) purchased from Ambion. 70–80% confluent ARPE-19 grown in 25-cm2 flasks were transfected with siRNA using Amaxa Cell Line Nucleofector Kit V (VCA 1003, Lonza, N.J.). 1×106 cells were used per condition. Cells transfected with scrambled siRNA (Silencer negative control 1, catalog number 4611, Ambion, Austin, TX) served as a negative control. As an additional control, cells were mock-transfected using transfection reagent alone. The D1, D5 or scrambled siRNA were used at a final concentration of 300 nM. Lysosomal pH was determined 72 hrs after transfection.
Western blots
ARPE-19 cells were lysed in RIPA buffer (150 mM NaCl, 1.0% Triton X-100, 0.5% Na-Deoxycholate, 0.1% SDS, 50mM Tris, pH 8.0 and protease inhibitor cocktail) and centrifuged at 13000g for 10 min at 4°C. Protein concentrations were determined using the BCA kit (Pierce). Protein lysates were loaded in each lane in sample buffer (2% SDS, 10% glycerol, 0.001% bromophenol blue, and 0.05 M Tris-HCl, pH 6.8), separated on SDS–PAGE (Biorad) and transferred to PVDF membrane (Millipore). For identification of the dopamine receptors, 35 μg protein was run on a 10% gel, blots were blocked with 5% nonfat milk in PBS and incubated overnight with rabbit anti-D5DR (1:2000) or mouse anti-D1DR (1:1000, both Santa Cruz Biotechnology, CA). Mouse anti-β-actin was used as a control for normalizing (1:1000, Sigma). Visualization of the primary antibody was performed by incubating membranes with the corresponding peroxidase-conjugated secondary antibody (1:3000; GE Healthcare) for 1 hr. at room temperature. Finally, the blots were developed by enhanced chemiluminescence (ECL; Amersham) and captured on an ImageQuant LAS 400 image reader (GE Healthcare). Bands were quantified using the Alphaimager HP gel documentation system (ProteinSimple, Santa Clara, CA).
POS membrane preparation
Fresh bovine retinas were isolated in the light under sterile conditions as previously described (Boesze-Battaglia & Yeagle 1992). Thawed retinas were agitated in 30% (w/w) buffered sucrose solution (containing 5 mM HEPES pH 7.4, 65 mM NaCl, 2 mM MgCl2) followed by centrifugation in a Sorvall SS-34 rotor (7 min, 700 rpm, 4°C). The supernatant was diluted in two volumes of 10 mM HEPES pH 7.4 and further centrifuged (Sorvall SS-34 rotor, 20min, 17500 rpm, 4°C). The resulting pellet was then homogenized and layered on top of a discontinuous sucrose density gradient. Density gradient solutions of 36, 32, and 26% sucrose (w/w) were employed, and POS membranes were harvested from the 26%/32% sucrose solution interface (Papermaster & Dreyer 1974). POS prepared this way were washed in 3 volumes of 0.02M Tris buffer, pH 7.4 (Sorvall SS-34 rotor, 10min, 13000 rpm, 4°C). The pellet was resuspended in 2.5% (w/w) buffered sucrose solution and POS stored at −80°C.
Visualization of cellular autofluroscence
ARPE-19 cells were plated to confluence on 12 mm cover slips. The cells were then incubated without or with POS (106/ml) for 7 days. Culture medium and POS were renewed every alternate day during this time. After the final incubation, cells were washed to remove the non-internalized POS, and after waiting for a 2 hr “chase” period for remaining material to be internalized, cells were fixed with paraformaldehyde and stained with DAPI for 1 min to visualize the nuclei. For localization of POS-associated autofluorescence, cells exposed to the outer segments for 7 days were incubated in 5 μM LysoTracker Red DND-99 (Invitrogen Corp) in cell culture medium for 15 min. Cells were washed again before imaging with a Nikon A1 inverted confocal microscope. Images were acquired and processed with NIS-Elements software (Nikon Inc.).
Flow cytometry
ARPE-19 cells were grown to confluence in 6-well plates and incubated with POS (106/ml) for 2 hours (pulse); the cells were washed thoroughly to remove non-internalized POS followed by a 2 hours chase. Subsequently, the cells were incubated with and without 10 μM SKF 81297. Culture medium and POS were renewed every alternate day for 7 days. For flow cytometric quantification of lipofuscin-like autofluorescence, cells were repeatedly washed, detached with trypsin, and analyzed on one of two flow cytometers (FACS Calibur, BD Biosciences, Heidelberg, Germany or LRSII, BD Biosciences, Franklin Lakes, NJ) using the FITC channel (excitation laser wavelength, 488 nm; detection filter wavelength, 530/30 nm). Cell debris and cell clusters were identified and excluded from the run analysis using FTC and SSC. Over 10,000 gated events were recorded.
Assessment of degradative enzyme activity using BODIPY FL-pepstatin A probe
Cathepsin D activity was measured with the fluorescent probe BODIPY FL-pepstatin A (Invitrogen). The probe itself is synthesized by covalently conjugating the BODIPY (Boron dipyrromethene difluoride) fluorophore to pepstatin A, a potent and selective inhibitor of cathepsin D. As the probe binds to the active site of cathepsin D, fluorescence intensity provides a measure of the activity of cathepsin D. To quantify cathepsin D activity, cells were grown to confluence on black-walled, clear-bottomed 96-well plates until confluent, and then incubated for 48 hrs in either control culture medium, 10 μM CHQ in medium, or 10 μM CHQ + 10 μM SKF 81297. Cells were then incubated in 1 μM BODIPY probe at 37°C in the dark. After washing, fluorescence was quantified using a Fluoroskan plate reader at 485 nm/527 nm (ex/em). Background fluorescence was subtracted from the plates.
Isolation and measurement of lysosomal pH from fresh ABCA4−/− mouse RPE cells
ABCA4−/− mice were a kind gift from Dr. Gabriel Travis of the Jules Stein Eye Institute, UCLA. All mice were treated in accordance with University of Pennsylvania IACUC. Mice were reared at 5–15 lux and sacrificed with a CO2 overdose. Mouse eyes were isolated and processed as described (Liu et al. 2008). In brief, after enucleation, intact eyes were incubated in 2% dispase and 0.4 mg/ml collagenase IV for 45 min, rinsed and incubated in growth medium for 20 min (containing DMEM with 1x MEM + non-essential amino acids, 3 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and 2.5 mg/ml Fungizone and/or 50 μg/ml gentamicin, plus 10% fetal bovine serum; all Invitrogen Corp). In some experiments, the anterior segments and retinas were removed and the eyecup was rinsed with Versene (Dow Chemical) and incubated in 0.25% trypsin for 45 min. Sheets of RPE cells were separated from the choroid and triturated into single cells. Cells from 2–6 eyes were pooled, loaded with 2–5μM LysoSensor Yellow/Blue for 5 min at RT, rinsed and distributed into wells of 384 well UV Star plates (Greiner Bio-One, Monroe, NC) and measured as described above. Although eyes from ABCA4−/− mice were slightly autofluorescent, the signal from the dye was 100 fold greater, validating the measurements (Liu et al. 2008). Dopamine agonists were added to the bath 20 min before measurements were taken. Lysosomal pH was measured within 3 hrs post mortem. Due to the reduced number of cells, measurements from fresh RPE cells were not calibrated and are expressed as ratio of fluorescence excited at 340 vs. 380 nm and emitted >527 nm.
Materials
SKF 81297, A68930 and A77636 were obtained from Tocris Bioscience (#s 1447, 1534, and 1701, respectively). All other reagents were obtained from Sigma Chemical Corp. (St. Louis, MO) unless otherwise indicated.
Data Analysis
Data are reported as mean ± SEM. Statistical analysis used a 1-way ANOVA with appropriate post-hoc test. Results with p<0.05 were considered significant.
Results
D1/D5 receptor agonists acidify compromised lysosomes
Initial experiments examined the ability of D1-like receptor agonists to lower lysosomal pH in challenged ARPE-19 cells. Baseline pHL levels were typically in the range of 4.5 – 4.8. Tamoxifen increased lysosomal pH rapidly in various cell types, presumably through its actions as both a tertiary amine and by increasing proton permeability (Altan et al. 1999, Chen et al. 1999). Previous work has demonstrated that lysosomal alkalinization of RPE cells by tamoxifen is independent of the estrogen receptor (Liu et al. 2008). The pHL of cells exposed to 10 μM tamoxifen for 5 min rose significantly; while the absolute magnitude of the alkalinization varied, the pHL was usually in the range of 5.1–5.3. Chloroquine likewise alkalinized lysosomal pH.
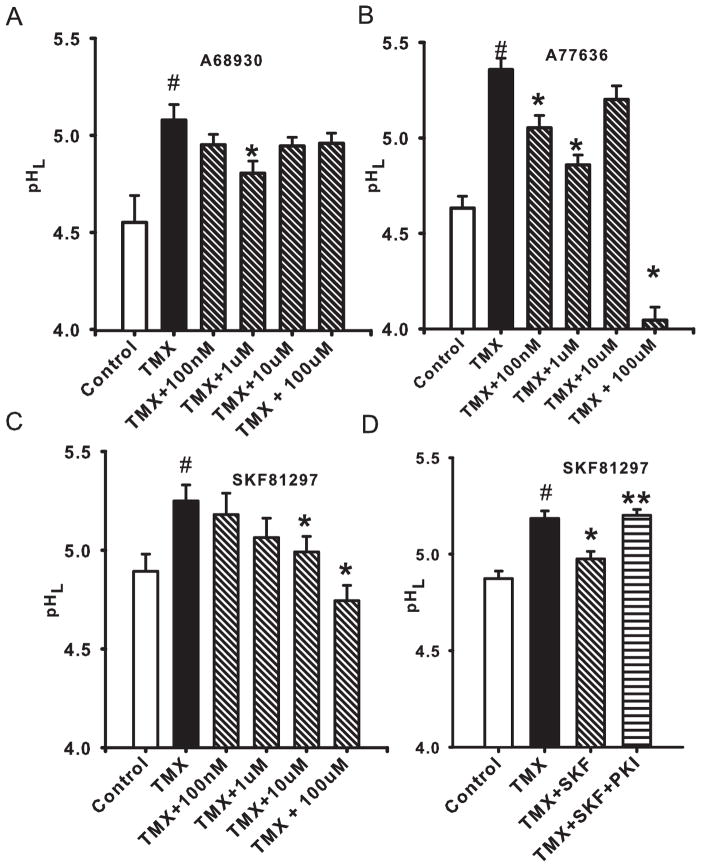
The D1-like receptor agonist A68930 led to a substantial acidification of lysosomes in cells exposed to tamoxifen (Fig. 1A). The effect was rapid, with stable pH levels observed within 10 min of drug application. A reduction in lysosomal pH was observed with 1 μM, but did not increase with concentration, perhaps due to the ability of increasing levels to stimulate D2-like receptors (DeNinno et al. 1991). Other D1-like receptor agonists A77636 (Fig. 1B) and SKF 81297 (Fig. 1C) were also effective at rapidly acidifying lysosomes (Fig. 1C).
Figure 1. D1/D5 receptor agonists lower lysosomal pH in challenged ARPE-19.
A. The agonist A68930 helped restore lysosomal pH (pHL) in ARPE-19 cells challenged by 10 μM of the lysotropic agent tamoxifen (TMX). n=(14–40).
B. The D1/D5 agonist A77636 also reduced pHL in cells exposed to 10 μM TMX. (n=44).
C. A third D1/D5 agonist SKF 81297 also acidified the lysosomes of cells treated with 10 μM tamoxifen (n=20).
D. The myristoylated protein kinase inhibitor PKI (14–22) amide (100 μM), the cell-permeant inhibitor of protein kinase A (PKA), blocked the reacidifying effects of SKF 81297 (10 μM) on cells treated with TMX (10 μM), implying a role for PKA in restoring lysosomal pH (pHL). (n=94). (# p<0.05 vs. control; * p<0.05 vs. TMX, ** p<0.05 vs. SKF 81297).
While all three D1/D5 receptor agonists displayed at least some efficacy in restoring lysosomal pH, additional experiments were performed using SKF 81297 as it displayed a relatively high selectivity for D1/D5 receptors over D2-like receptors (Andersen & Jansen 1990) and it gave the most consistent results in our trials. The ability of SKF 81297 to acidify compromised lysosomes was inhibited by myristoylated protein kinase inhibitor PKI (14–22) amide (100 μM), the cell-permeant inhibitor of protein kinase A (PKA) (Fig. 1E). PKI blocked the effects of SKF 81297 by 78% (n=53), strongly implicating PKA in the acidification of lysosomes by SKF 81297. This is consistent with the ability of cell-permeant cAMP to acidify compromised lysosomes, and with the involvement of PKA in this general activation (Liu et al., 2008). In addition to its effects on tamoxifen-treated cells, SKF 81297 was also effective at reversing the alkalinization produced by chloroquine, reducing lysosomal pH from 5.60 ±0.14 to 5.11 ±0.09 (n=24, p<0.005). However, SKF 81297 had no effect on the baseline lysosomal pH of cells treated with neither tamoxifen nor chloroquine (n=10; p=0.99)
Acidifying effect of single dose of SKF 81297 is sustained
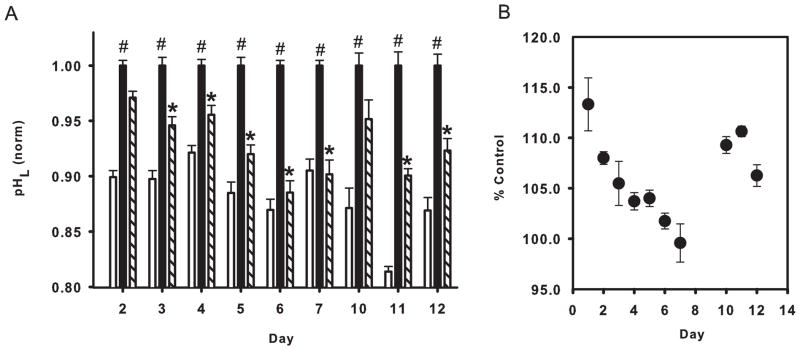
While the experiments above strongly suggested that stimulation of D1-like receptors restored the lysosomal pH in compromised RPE cells, they were all conducted over the course of several hours. To determine whether D1-like receptor stimulation could induce a sustained restoration of lysosomal pH in compromised RPE cells, agonist SKF 81297 was added to chloroquine treated cells, as chloroquine has been reported to induce prolonged effects in RPE cells in vivo (Peters et al. 2006). Confluent cells were treated with chloroquine in the presence and absence of SKF 81297 (both at 10 μM) on day 0, and the lysosomal pH was measured over the next 12 days. Medium was not changed for control or treatment wells. Measurements were performed in 7 trials, each measuring lysosomal pH on different combination of days. While absolute levels varied somewhat with both plating and measurement day, trends were clearly evident. Extended exposure to 10 μM chloroquine induced a relatively constant elevation in lysosomal pH. In contrast, it was apparent that the acidifying effect of SKF 81297 changed with exposure duration (Fig. 2A). SKF 81297 lowered pHL more effectively with increased exposure time. Remarkably, exposure of compromised cells to SKF 81297 completely restored the lysosomal pH to baseline levels at day 7 (Fig. 2B). Although the magnitude of the acidification was reduced, SKF 81297 still produced a significant acidification up to 12 days, the last day examined. No difference between treated and control cells could be discerned visually. Thus a single dose of SKF 81297 produced a cumulative reacidification of compromised cells.
Figure 2. Long term restoration of lysosomal pH.
A. The D1/D5 agonist SKF 81297 (10 μM) restored lysosomal pH (pHL) for multiple days in compromised ARPE-19 cells. Cells were treated with 10 μM chloroquine in the presence (hash bar) or absence (black bar) of 10 μM SKF 81297 on day zero; solution remained on cells but was not refreshed. pH levels were normalized to the mean value in chloroquine for each day’s measurements to compensate for variation across trials. *p <0.05 vs. CHQ; n=16–40 from 2–5 trials. (B) The relative effectiveness of SKF 81297 to restore lysosomal pH. Lysosomal pH in the presence of 10 μM SKF 81297 expressed as a percentage of the control pH in the same plate on the same day. The effectiveness of a single dose of SKF 81297 peaked 7 days after treatment, producing a near-complete restoration of lysosomal pH. Calculated from mean of 2–5 trials derived from 16–40 measurements.
Molecular identification of D5 receptor subtype
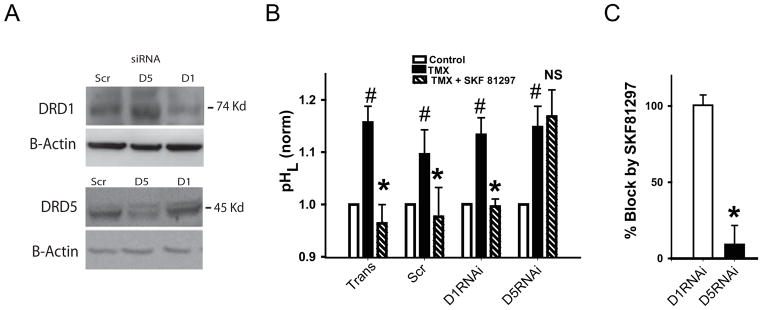
As available pharmacological tools are currently unable to distinguish between the D1 and D5 receptors with reasonable specificity, molecular approaches were used to determine which receptor was responsible for the lysosomal acidification by SKF 81297. Western blots confirmed that an antibody against the D1DR detected a band at expected size of 74 kD. The intensity of the band was reduced by siRNA against the D1DR (Fig. 3A). Likewise, an antibody against the D5DR detected a band at the expected size of 45 kD, with the intensity of the band reduced by siRNA against the D5DR. When normalized to actin and quantified to levels in scrambled siRNA, the band intensity of D1DR was decreased to 57% by siRNA against D1DR while siRNA against the D5DR increased expression to 162% of scrambled levels. Levels of D5DR were decreased by siRNA against D5DR to 75%, with siRNA against the D1DR leading to 106% of scrambled levels.
Figure 3. Stimulation of D5 receptor restores lysosomal acidity.
A. Western blots confirm specificity of the gene knockdown, as siRNA against the D1 receptor reduced expression of the D1 receptor (DRD1) but not the D5 receptor (D5DR, top panel). Likewise siRNA against D5 receptor reduced expression of the DRD5 but not the D1DR. Scr = scrambled RNA. β-actin staining was used to normalize levels 72 hrs post transfection.
B. RNAi knockdown of D5 receptor but not D1 receptor removed acidification by 10 μM D1/D5 agonist SKF 81297. TransCon = transfection control; Scramb = scrambled RNAi, D1RNAi = RNAi against D1 receptor, D5RNAi = RNAi against D5 receptor. Paired t test *p<0.05, Tmx vs. Tmx+SKF 81297, n=6 plates, 2–4 wells each. Data were normalized to the mean control in each set to account for variation between each separate set of transfections.
C. Quantification of effect of receptor knockdown. The magnitude of the acidification by 10 μM SKF 81297 was defined as % reacidification = 100*(TMX-(TMX+SKF))/(TMX-Control). The % reacidification was unaffected when cells were transfected with siRNA against D1DR. However siRNA against the D5DR reduced the % reacidification to only 10%. This strongly implicates the D5 receptor in the reacidification by SKF 81297. Paired t-test, * p=0.006 vs. D1RNAi, n=4.
As these siRNA probes were able to selectively reduce expression of the receptor target protein, their effect on the ability of SKF 81297 to restore acidity was tested. The baseline pH did not differ between cells transfected with scrambled siRNA, D1DR siRNA, D5DR siRNA or transcription controls in 7 separate transfection experiments (p 0.74, 0.68 and 0.53 vs. scrambled, respectively; transfection itself had a slight alkalinizing effect). To control for variations that occurred between trials, pH values were normalized to the mean value for scrambled control for each experiment, but still there was no difference in baseline levels (p>0.22). However, significant differences were observed when the ability of SKF 81297 to acidify the lysosomes of compromised cells was examined. Tamoxifen produced a similar alkalinization of lysosomes in all cells. SKF 81297 acidified the lysosomes of cells transfected with scrambled siRNA or exposed to transfection medium. While SKF 81297 likewise acidified the lysosomes of cells transfected with D1DR siRNA, the drug had little effect on lysosomal pH in cells exposed to D5DR siRNA (Fig. 3B). When the % reacidification of the effect of tamoxifen was calculated, SKF 81297 blocked 100.4 ± 9.1% of the alkalinizing effects of tamoxifen in the presence of D1DR siRNA, while it blocked only 10.4 ± 19.1 % of the alkalinization in the presence of D5DR siRNA (p=0.006, Fig. 3C). This strongly suggested that the response was mediated by the D5 dopamine receptor.
Of note, while the immunoblots suggest an increase in D1DR expression with D5DR siRNA knockdown, there was no evidence of an effect on a physiological level as baseline lysosomal pH did not differ significantly between cells treated with D1DR siRNA or D5DR siRNA, and as mentioned the effect of SKF 81297 was decreased, not increased, by D5DR siRNA. This further supports the role for the D5DR in lysosomal acidification.
D5 stimulation enhances degradative activity of RPE lysosomes
Degradative lysosomal enzymes are pH sensitive, acting optimally over a relatively narrow range of acidic values. As such, conditions which elevate lysosomal pH are predicted to reduce rates of degradation while treatments to reacidify lysosomes are predicted to enhance degradation. RPE lysosomes are required to degrade photoreceptor outer segments phagocytosed daily (Kevany & Palczewski 2010). As such, the effect of lysosomal pH manipulation of outer segment degradation was tested.
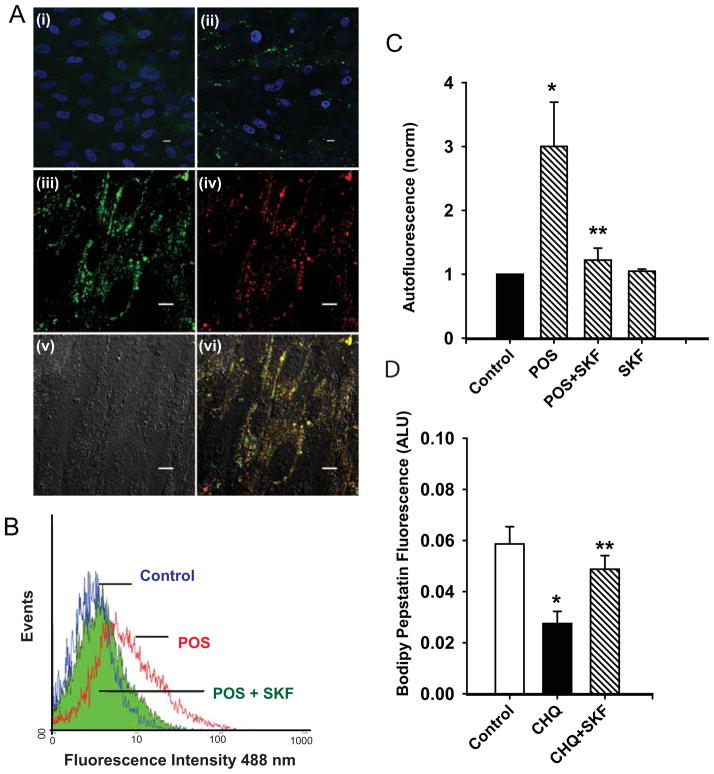
Initial experiments were designed to confirm that outer segments were internalized to the lysosomes. Unlabeled photoreceptor outer segments were fed to confluent ARPE-19 cells for 2 hrs and then medium was returned. This procedure was repeated every other day for 7 days. On the final day, cells were maintained in outer segment-free medium for 2 hrs to ensure sufficient time for binding, phagocytosis and trafficking. While cells not exposed to POS displayed little autofluorescence, cells exposed to POS displayed clear spots of autofluorescence when excited at 488 nm (Fig. 4Ai–ii). As this pattern of autofluorescence suggests organelle staining, costaining with LysoTracker Red was examined. The punctate pattern of autofluorescent staining from outer segments overlapped with the pattern for LysoTracker Red, indicating that most of the autofluorescence was restricted to lysosome-like organelles at this point (Fig. 4Aiii–vi).
Figure 4. D5 agonists reduce levels of photoreceptor outer segment autofluorescence.
A. Cultured ARPE-19 cells were examined by confocal fluorescence microscopy following 7 days of incubation without (i) or with (ii) unlabeled photoreceptor outer segments (POS). Lipofuscin-like cellular autofluorescence (green) was detected in panel ii using a fluorescein filter set (ex 480 nm, em 535 nm). Nuclei were visualized by DAPI staining blue. Scale bar = 10 μm. Autofluorescence associated with POS incubation (iii, pseudocolored green), and the signal from LysoTracker Red (ex 540 nm, em >570) showed considerable overlap (vi), implying the majority of POS were in acidic organelles 2 hrs after outer segments were removed from the bath. (v – DIC image). Scale bar = 10 μm.
B. SKF 81297 reduced the autofluorescence from internalized POS. Cells were fed POS for three hours, washed, and two hours chase period were allowed for outer segment delivery to the lysosomes. At this point, 10 μM SKF 81297 was added to the cells (adding the drug after the two hour interval ensured effects were restricted to outer segment digestion and did not alter binding or phagocytosis). This two stage treatment was repeated every 1–2 days for one week, with a total of three treatments. Cells were dissociated and the autofluorescence excited at 488 nm was determined using flow cytometry. Compared to control cells (blue), exposure to POS shifted the fluorescence to the right (red), indicating an increased fluorescence. Treatment with SKF 81297 shifted the curve back to the left (green) as autofluorescence was reduced.
C. Quantification of autofluorescence reduction by SKF 81297. The mean autofluorescence was increased by incubation with POS but restored to low levels by SKF 81297. SKF 81297 alone did not alter autofluorescence levels. Bars represent the mean ± SEM fluorescence in each sample and are representative of results in three separate experiments. Data were normalized to peak levels in untreated cells to control for variation between trials. * p <0.05 vs. control; ** p<0.05 vs. POS.
D. Bodipy-pepstatin A binding is improved by SKF 81297. While binding of the probe was reduced by treating cells with 10 μM chloroquine for 48 hrs, concurrent exposure to 10 μM SKF 81297 restored fluorescence. These results are consistent with chloroquine decreasing activity of pH sensitive lysosomal enzyme cathepsin D, and of SKF 81297 restoring enzyme activity.
Having established that photoreceptor outer segments were delivered to lysosomes within two hours, the autofluorescence was quantified and the ability of SKF 81297 to alter this autofluorescence was calculated. The cells were fed photoreceptor outer segments for 2 hrs, kept in outer segment-free medium for 2 hrs to allow for internalization. After 2 hrs, cells were fed 10 μM SKF 81297 for 19 hrs, at which point the outer segment feeding was resumed. This complex “pulse-chase” protocol was followed to ensure that drug treatment did not interfere with POS binding or internalization.
Treatment with photoreceptor outer segments substantially increased cellular autofluorescence, while treatment with SKF 81297 clearly decreased autofluorescence (Fig. 4B). In 5 trials, treatment with outer segments raised autofluorescence over 3-fold, while exposure to SKF 81297 reduced autofluorescence by 73 ± 12% (Fig. 4C). This implies that stimulation of the D5 receptor can enhance digestion of photoreceptor outer segments.
To provide additional evidence that stimulation of the D5 receptor increased lysosomal activity, the binding of the fluorescent Bodipy- pepstatin A to cells was assessed. Pepstatin A inhibits the lysosomal protease cathepsin D, and thus fluorescence is indicative of cathepsin D activity in situ. Incubation of cells with 10 μM of chloroquine significantly decreased the fluorescence, as expected. However, coincubation of SKF 81297 with the chloroquine substantially increased the pepstatin A fluorescence (Fig. 4D). These results are consistent with the ability of lysosomal alkalinization by chloroquine to decrease the activity of cathepsin D and the reacidification of lysosomes by SKF 81297 to restore activity. Together with the ability of SKF 81297 to reduce the autofluorescence associated with photoreceptor outer segments, these findings strongly suggest that SKF 81297 can increase the activity of degradative lysosomal enzymes in compromised cells.
Stimulation of D5 receptors acidifies lysosomes from RPE cells of ABCA4−/− mice
While the ability of D5 receptor stimulation to restore lysosomal pH in cells exposed to chloroquine or tamoxifen suggests general relevance, exposure to N-retinylidene-N-retinylethanolamine (A2E) may be a more direct challenge to RPE cells. A2E elevates the lysosomal pH of cultured RPE cells (Holz et al. 1999, Liu et al. 2008). The ABCA4−/− mouse model of recessive Stargardt’s disease is characterized by excessive accumulation of A2E (Mata et al. 2001) and the lysosomal pH of these mice is elevated as compared with age-matched controls (Liu et al. 2008). Given the potential importance for RPE pathophysiology, the ability of D5 receptor stimulation to lower lysosomal pH in RPE cells from ABCA4−/− mice was examined.
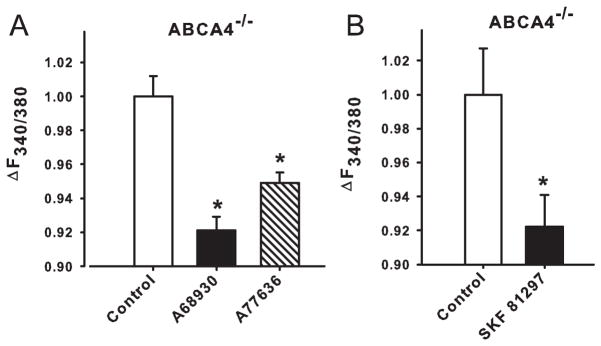
RPE cells were freshly isolated from ABCA4−/− mice and lysosomal pH was measured in vitro. Exposure of RPE cells from 11 month old mice to 1 μM A68930 or 1 μM A77636 decreased lysosomal pH (Fig. 5A). Interestingly, these drugs had no significant effect on the lysosomal pH of 5 month old ABCA4−/− mice. Additional experiments demonstrated that SKF81297 also reduced the signal from 12 month old ABCA4−/− mice (Fig. 5B).
Figure 5. Acidification of RPE lysosomes from ABCA4−/− mice.
A. Simulation of dopamine D1-like receptors by A68930 (1 μM) and A77636 (1 μM) decreased pHL of RPE cells freshly isolated from 11-month-old ABCA4−/− mice. *p<0.01 vs. untreated ABCA4−/−. n=8 measurements.
B. In a separate set of experiments, SKF 81297 (50 μM) also reduced the lysosomal pH in RPE cells freshly isolated from 12 month-old ABCA4−/− mice. *p<0.05 vs. untreated ABCA4−/−. n=3.
Discussion
Changes in lysosomal pH have direct and indirect actions on activity of degradative lysosomal enzymes. This study has demonstrated that in RPE cells, stimulation of the D5 dopamine receptor can enhance degradation and may be of benefit in conditions where lysosomal pH is increased. Several lines of evidence support this conclusion. Three different D1/D5 receptor agonists, A68930, A77636 and SKF 81297 all acidified compromised lysosomes in RPE cells (Fig. 1). Such reacidification occurred in lysosomes alkalinized by either tamoxifen or chloroquine. The acidification was dependent upon the actions of PKA, consistent with pathways identified previously (Liu et al. 2008). Of note, a single dose of agonist SKF 81297 was sufficient to acidify lysosomes for 12 days, with complete restoration found maximally 5–7 days after treatment (Fig. 2). Knockdown of the D5 receptor reduced the acidification by SKF 81297 while knockdown of the D1 receptor did not (Fig. 3), implying the D5 receptor was responsible. SKF 81297 increased the degradation of photoreceptor outer segments and reduced their lipofuscin – like autofluorescence and the activity of cathepsin D (Fig. 4), supporting a link between lysosomal acidification and increased activity of degradative enzymes. Finally, stimulation of the receptor lowered lysosomal pH of RPE cells from aged ABCA4−/− mice (Fig. 5), indicating that the pathways linking the D5 receptor to lysosomal acidification were maintained even in compromised RPE cells from “middle aged” mice. Overall, these findings demonstrate a clear link between stimulation of the D5 receptor, reduction of lysosomal pH and improved degradation by lysosomal enzymes.
Receptor pharmacology
Analysis of individual dopamine receptors is complicated by the lack of specificity demonstrated by many of the pharmacological tools, and D1 and D5 (D1b) receptors share over 80% homology (Beaulieu & Gainetdinov 2011). Selective reduction of the D1 and D5 receptors using molecular approaches demonstrated that the acidification of lysosomes in RPE cells was mediated by D5 receptors. While cultured bovine RPE cells were reported to contain predominantly D5 receptors (Versaux-Botteri et al. 1997), the presence of bands in the present study suggests cultured human ARPE-19 cells contain both D1 and D5 receptors. While receptor expression may be coordinated, it is clear that only the D5 receptor mediated lysosomal reacidification in these cells.
The agonists A77636 and A68930 are generally characterized as D1-like receptor agonists. A68930 acts at D1 receptors with an EC50 of 2.9 nM, and at D2 receptors with an EC50 of 3.8 μM (DeNinno et al. 1991). A77636 acts at D1-like receptors with a Ki = 39.8 nM, and at D2-like receptors with a Ki >10 μM, however (Kebabian et al. 1992). Enhanced stimulation of D2 receptors at higher concentrations may complicate effects of A68930 on lysosomal acidification; as D2 receptors are coupled to Gi proteins stimulation would work against an acidification. However, the relative selectivity of A68930 at the D1 vs. D5 receptor may also contribute to the response (Nergårdh et al. 2005). Although SKF 81297 is reported to act more selectively at D1 receptors (Beaulieu & Gainetdinov 2011), present results argue that it is also an effective agonist at D5 receptors. It should be noted that although the majority of experiments in this study were performed with SKF 81297, this does not rule out possible beneficial effects from other D1/D5 agonists. The oral availability of A77636 may be of interest in this regard (Kebabian et al. 1992).
Mechanisms of action
The ability of D5 receptor stimulation to lower lysosomal pH is most likely related to an elevation of cAMP levels. We have previously demonstrated that increasing cytoplasmic cAMP, either directly or via G-protein coupled receptors, lowers lysosomal pH in RPE cells (Liu et al. 2008). Figure 1D demonstrates that the acidifying actions of the agonist SKF 81297 are inhibited by PKI (14–22) amide, strongly suggesting that PKA is required for lysosomal acidification. Preliminary data suggest that the PKA-activated Cl− channel CFTR contributes to the PKA-dependent acidification of RPE lysosomes (Mitchell et al. 2008). Phosphorylation by PKA was recently demonstrated to enhance insertion of the vHATPase into the plasma membrane of proton-secreting kidney cells, enhancing secretion (Alzamora et al. 2010). It is possible that an analogous mechanism occurs on lysosomal membranes, and PKA also acts by enhancing expression of proton pumps in lysosomal membranes, although this remains to be determined. The inability of SKF 81279 to decrease baseline lysosomal pH is consistent with data indicating cAMP exhorts an acidification of greater magnitude from cells with alkalinized lysosomes than from baseline (Liu et al., unpublished observations). This suggests a model where the cAMP increase following D5DR stimulation effect the regulation of lysosomal pH but not its baseline maintenance.
It is important to note that stimulation of the D5 receptor effectively reacidified RPE cells, overriding the alkalinization caused by either tamoxifen or chloroquine. Further, receptor stimulation reacidified RPE cells from ABCA4−/− mice, where excess A2E is likely to increase lysosomal pH (Holz et al. 1999, Mata et al. 2000, Bergmann et al. 2004). Overall, this implies that the ability of D5 receptor stimulation to reacidify lysosomes is not specific for a particular type of alkalinizing insult. In other words, the lysosomal reacidification is mediated via a general mechanism that may be effective against a range of insults.
Physiological Implications
Stimulation of the D5 receptor in RPE cells by SKF 81297 induced several responses that suggest the approach may be worth further consideration. A single exposure to 10 μM SKF 81297 lowered lysosomal pH in chloroquine-treated ARPE -19 cells for 12 days. The restoration of acidity was cumulative, with the pH equal to control levels after 7 days. Importantly, the autofluorescence excited at 488 nm was substantially increased in cells fed outer segments, consistent with a lipofuscin-like accumulation. However, treatment with SKF 81297 decreased this autofluorescence by 54±4%. Not only does the improved clearance by SKF 81297 reinforce the relationship between lysosomal pH and degradative enzyme activity, but it also provides crucial functional evidence that this approach can improve the clearance of outer segments by these cells. It is important to stress that the pulse chase approach to feeding cells outer segments ensured that outer segments were predominantly within lysosomes before cells were treated with SKF 81297, implying the actions were specifically due to changes in lysosomal pH and not the binding or internalization stages. As such, the approach also applies to material delivered through autophagic pathways to the lysosomes. Experiments with Bodipy-pepstatin provide additional support for this link, and stress that the reacidification induced by SKF-81297 occurs over a relevant pH values. Like many lysosomal enzymes, the activity of Cathepsin D is sharply dependent of the pH of the surrounding milieu, with activity falling by 80% once the pH has risen to only 5.3 (Barrett 1977). These experiments demonstrate that the functional effects of SKF-81297 on compromised RPE cells are substantial and likely to improve degradation of compromised lysosomes in RPE cells.
The ability of D5 receptor stimulation to enhance outer segment degradation in RPE cells with alkalinized lysosomes may have implications for patients with macular degenerations such as Stargardt’s disease, for the lysosomal pH was increased in RPE cells from the ABCA4−/− mouse model of the disease (Liu et al. 2008). As such, the ability of receptor agonists to acidify lysosomes from RPE cells taken from older ABCA4−/− mice is important, for it implies that the mechanisms necessary to mediate receptor driven reacidification of lysosomes are still functioning even though the lysosomes in the cells have been distressed for an extended period. The lysosomal pH increased with age in these mice (Liu et al. 2008), consistent with the enhanced accumulation of A2E with age (Mata et al. 2000). The negligible effect of D5DR agonists in younger mice with near-normal lysosomal pH may be related to the increased magnitude of acidification induced by cAMP when given to cells with alkalinized lysosomes. This is also supported by the observation that SFK 81297 had no effect on cells that had not been treated with an alkalinizing agent. This makes the treatment of impaired tissue with D5 agonists ideally suited, as the lysosomal pH of any healthy cells should be minimally affected.
The potential therapeutic usefulness of D5 receptor stimulation will be limited by the specificity of the actions. While dopamine contributes to numerous processes in the retina, D4 receptors mediate many of the effects of dopamine on retinal function including modulation of photoreceptor proteins (Nir et al. 2002, Pozdeyev et al. 2008). In the neural retina, as in the brain, there is a relative paucity of D5 receptors (Beaulieu & Gainetdinov 2011), with immunohistochemical identification suggesting localization is limited to a selection of amacrine cells and ganglion cells (Versaux-Botteri et al. 1997, Nguyen-Legros et al. 1999). In the RPE, the overall effects of D5 receptor stimulation of RPE are likely to be complex. Elevation of cAMP slows the rate of photoreceptor phagocytosis by RPE cells (Edwards & Flaherty 1986), and stimulation of D5 receptors may well reduce phagocytosis rates (Masri et al. 1996). As phagocytosis generally follows a circadian pattern, temporal control of the delivery of agonists may enable the effects on phagocytosis and lysosomal degradation to be separated in vivo as they were in vitro. In this regard, chronic treatment with 1-methyl-4-phenyl-1,2,3,6-tetrahydro-pyridine (MPTP) decreased the dopaminergic amacrine cells in the retina and significantly increased the number of highly fluorescent yellow lipofuscin granules in the RPE (Mariani et al. 1986). The lipofuscin associated with dopamine reduction displayed the same spectral profile as the lipofuscin in RPE cells from older animals. This suggests a model where by dopamine released from the amacrine cells normally keeps the lysosomal pH of RPE cells low and outer segment degradation running smoothly; the removal of this source of dopamine may lead to lysosomal alkalinization and accumulation of autofluorescent debris There are, of course, alternative explanations, but this model is consistent with the results in Figure 4, where by application of SKF 81297 substantially reduced the degree of autofluorescence in RPE cells. Overall, these findings suggest that D5 receptor stimulation may be a critical pathway to enhance degradation in RPE cells in vivo.
The control of lysosomal function in supportive cells may also have broader implications for neuronal-glial interactions. Recently, astrocytes were shown to actively phagocytose material extruded from the midst of axons (Nguyen et al. 2011). It remains to be seen whether alkalinization of astrocytic lysosomes can impede this novel function, or whether stimulation of the D5DR can enhance this process.
Acknowledgments
This work is supported by grants from the NIH EY013434 and EY015537 (CHM), EY017045 (AML), EY018705 (KBB), Vision Research Core Grant EY001583 (CHM, AML, KBB), Research to Prevent Blindness (AML), the Paul and Evanina Bell Mackall Foundation Trust (AML) and the Jody Sack fund (WL). The authors would like to thank Michael Boulton for advice on the FACS analysis of autofluorescent cells, Bruce Shenker at the UPenn SDM Flow Cytometry facility, and Gabriel Travis for the ABCA4−/− mice. The authors have IP associated with these findings but no financial assistance has been received.
Abbreviations
- AKAP
A-kinase anchoring proteins
- CHQ
chloroquine
- D5DR
D5-type dopamine receptor
- FACS
fluorescence activated cell sorter
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydro-pyridine
- PKA
protein kinase A
- PKI
protein kinase inhibitor
- pHL
lysosomal pH
- POS
photoreceptor outer segments
- PVDF
Polyvinylidene fluoride
- RPE
retinal pigmented epithelial
- TMX
tamoxifen
Footnotes
Portions of this work has been previously presented in abstract form (Guha et al. 2009, Guha et al. 2010).
References
- Altan N, Chen Y, Schindler M, Simon SM. Tamoxifen inhibits acidification in cells independent of the estrogen receptor. Proc Nat Acad Sci USA. 1999;96:4432–4437. doi: 10.1073/pnas.96.8.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzamora R, Thali RF, Gong F, et al. PKA regulates vacuolar H+-ATPase localization and activity via direct phosphorylation of the a subunit in kidney cells. J Biol Chem. 2010;285:24676–24685. doi: 10.1074/jbc.M110.106278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen PH, Jansen JA. Dopamine receptor agonists: selectivity and dopamine D1 receptor efficacy. Eur J Pharmacol. 1990;188:335–347. doi: 10.1016/0922-4106(90)90194-3. [DOI] [PubMed] [Google Scholar]
- Barrett A. Protinases in Mammalian Cells and Tissues. Elsiver/North-Hollard Biomedical press; New York: 1977. [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Bergmann M, Schutt F, Holz FG, Kopitz J. Inhibition of the ATP-driven proton pump in RPE lysosomes by the major lipofuscin fluorophore A2-E may contribute to the pathogenesis of age-related macular degeneration. FASEB J. 2004;18:562–564. doi: 10.1096/fj.03-0289fje. [DOI] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Yeagle PL. Rod outer segment disc membranes are capable of fusion. Invest Ophthalmol Vis Sci. 1992;33:484–493. [PubMed] [Google Scholar]
- Cadet JL, Jayanthi S, McCoy MT, Beauvais G, Cai NS. Dopamine D1 receptors, regulation of gene expression in the brain, and neurodegeneration. CNS Neurol Disord Drug Targets. 2010;9:526–538. doi: 10.2174/187152710793361496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Schindler M, Simon SM. A mechanism for tamoxifen-mediated inhibition of acidification. J Biol Chem. 1999;274:18364–18373. doi: 10.1074/jbc.274.26.18364. [DOI] [PubMed] [Google Scholar]
- DeNinno MP, Schoenleber R, MacKenzie R, et al. A68930: a potent agonist selective for the dopamine D1 receptor. Eur J Pharmacol. 1991;199:209–219. doi: 10.1016/0014-2999(91)90459-4. [DOI] [PubMed] [Google Scholar]
- Edwards RB, Flaherty PM. Association of changes in intracellular cyclic AMP with changes in phagocytosis in cultured rat pigment epithelium. Curr Eye Res. 1986;5:19–26. doi: 10.3109/02713688608995161. [DOI] [PubMed] [Google Scholar]
- Guha S, Lu W, Liu J, Laties AM, Mitchell CH. Stimulation of the D1-like dopamine receptor reacidifies the lysosomal pH of compromised retinal pigmented epithelial cells. ARVO E-Abstract 2009 [Google Scholar]
- Guha S, Tu LA, Baltazar G, Argall AJ, Laties AM, Mitchell CH. Reduction of lipofuscin-like autofluorescence in RPE cells by sustained D1/D5 receptor stimulation. ARVO E-Abstract 2010 [Google Scholar]
- Hayase K, Tappel AL. Specificity and other properties of lysosomal lipase of rat liver. J Biol Chem. 1970;245:169–175. [PubMed] [Google Scholar]
- Holz FG, Schutt F, Kopitz J, Eldred GE, Kruse FE, Volcker HE, Cantz M. Inhibition of lysosomal degradative functions in RPE cells by a retinoid component of lipofuscin. Invest Ophthalmol Vis Sci. 1999;40:737–743. [PubMed] [Google Scholar]
- Jentsch TJ. Chloride and the endosomal-lysosomal pathway: emerging roles of CLC chloride transporters. J Physiol. 2007;578:633–640. doi: 10.1113/jphysiol.2006.124719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebabian JW, Britton DR, DeNinno MP, Perner R, Smith L, Jenner P, Schoenleber R, Williams M. A-77636: a potent and selective dopamine D1 receptor agonist with antiparkinsonian activity in marmosets. Eur J Pharmacol. 1992;229:203–209. doi: 10.1016/0014-2999(92)90556-j. [DOI] [PubMed] [Google Scholar]
- Kevany BM, Palczewski K. Phagocytosis of retinal rod and cone photoreceptors. Physiology (Bethesda) 2010;25:8–15. doi: 10.1152/physiol.00038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon M, Cuervo AM. Chaperone-mediated autophagy in health and disease. FEBS Lett. 2010;584:1399–1404. doi: 10.1016/j.febslet.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig B, Gratzel M. Site of dopamine D1 receptor binding to Gs protein mapped with synthetic peptides. Biochim Biophys Acta. 1994;1223:261–266. doi: 10.1016/0167-4889(94)90235-6. [DOI] [PubMed] [Google Scholar]
- Liu J, Lu W, Reigada D, Nguyen J, Laties AM, Mitchell CH. Restoration of lysosomal pH in RPE cells from cultured human and ABCA4(−/−) mice: pharmacologic approaches and functional recovery. Invest Ophthalmol Vis Sci. 2008;49:772–780. doi: 10.1167/iovs.07-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani AP, Neff NH, Hadjiconstantinou M. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treatment decreases dopamine and increases lipofuscin in mouse retina. Neurosci Lett. 1986;72:221–226. doi: 10.1016/0304-3940(86)90084-4. [DOI] [PubMed] [Google Scholar]
- Masri H, Goureau O, Hecquet C, Simon A, Nguyen-Legros J. Dopamine slows phagocytosis of rods from bovine pigment epithelium in vitro trough D1 receptor. C R Acad Sci III. 1996;319:687–691. [PubMed] [Google Scholar]
- Mata NL, Tzekov RT, Liu X, Weng J, Birch DG, Travis GH. Delayed dark-adaptation and lipofuscin accumulation in abcr+/− mice: implications for involvement of ABCR in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2001;42:1685–1690. [PubMed] [Google Scholar]
- Mata NL, Weng J, Travis GH. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc Nat Acad Sci USA. 2000;97:7154–7159. doi: 10.1073/pnas.130110497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mego JL. Role of thiols, pH and cathepsin D in the lysosomal catabolism of serum albumin. Biochem J. 1984;218:775–783. doi: 10.1042/bj2180775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CH, Laties AM, Liu J. Regulation of lysosomal pH in RPE cells by CFTR. Exp Eye Res Suppl. 2008 Abstract. [Google Scholar]
- Nergårdh R, Oerther S, Fredholm BB. Differences between A 68930 and SKF 82958 could suggest synergistic roles of D1 and D5 receptors. Pharmacol Biochem Behav. 2005;82:495–505. doi: 10.1016/j.pbb.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Nguyen-Legros J, Versaux-Botteri C, Vernier P. Dopamine receptor localization in the mammalian retina. Mol Neurobiol. 1999;19:181–204. doi: 10.1007/BF02821713. [DOI] [PubMed] [Google Scholar]
- Nguyen JV, Soto I, Kim KY, et al. Myelination transition zone astrocytes are constitutively phagocytic and have synuclein dependent reactivity in glaucoma. Proc Natl Acad Sci U S A. 2011;108:1176–1181. doi: 10.1073/pnas.1013965108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir I, Harrison JM, Haque R, Low MJ, Grandy DK, Rubinstein M, Iuvone PM. Dysfunctional light-evoked regulation of cAMP in photoreceptors and abnormal retinal adaptation in mice lacking dopamine D4 receptors. J Neurosci. 2002;22:2063–2073. doi: 10.1523/JNEUROSCI.22-06-02063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma S, Moriyama Y, Takano T. Identification and characterization of a proton pump on lysosomes by fluorescein-isothiocyanate-dextran fluorescence. Proc Natl Acad Sci U S A. 1982;79:2758–2762. doi: 10.1073/pnas.79.9.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papermaster DS, Dreyer WJ. Rhodopsin content in the outer segment membranes of bovine and frog retinal rods. Biochemistry. 1974;13:2438–2444. doi: 10.1021/bi00708a031. [DOI] [PubMed] [Google Scholar]
- Patel S, Docampo R. Acidic calcium stores open for business: expanding the potential for intracellular Ca2+ signaling. Trends Cell Biol. 2010;20:277–286. doi: 10.1016/j.tcb.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S, Reinthal E, Blitgen-Heinecke P, Bartz-Schmidt KU, Schraermeyer U. Inhibition of lysosomal degradation in retinal pigment epithelium cells induces exocytosis of phagocytic residual material at the basolateral plasma membrane. Ophthal Res. 2006;38:83–88. doi: 10.1159/000090268. [DOI] [PubMed] [Google Scholar]
- Pozdeyev N, Tosini G, Li L, Ali F, Rozov S, Lee RH, Iuvone PM. Dopamine modulates diurnal and circadian rhythms of protein phosphorylation in photoreceptor cells of mouse retina. Eur J Neurosci. 2008;27:2691–2700. doi: 10.1111/j.1460-9568.2008.06224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu A, Kimura K, Uh M, White BH, Patel S. Multiple coupling of human D5 dopamine receptors to guanine nucleotide binding proteins Gs and Gz. J Neurochem. 1998;70:2459–2467. doi: 10.1046/j.1471-4159.1998.70062459.x. [DOI] [PubMed] [Google Scholar]
- Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- Versaux-Botteri C, Gibert JM, Nguyen-Legros J, Vernier P. Molecular identification of a dopamine D1b receptor in bovine retinal pigment epithelium. Neurosci Lett. 1997;237:9–12. doi: 10.1016/s0304-3940(97)00783-0. [DOI] [PubMed] [Google Scholar]