Abstract
A series of novel indomethacin analogues with carbaboranes as three-dimensional substitutes for the chlorophenyl ring have been prepared. Their cyclooxygenase (COX) inhibition and enzyme selectivity has been tested and compared to the corresponding adamantyl analogues. Surprisingly, only the ortho-carbaborane derivatives were active compounds. Preliminary biological studies gave an interesting insight into the validity of employing carbaboranes as pharmacophores.
Keywords: Carbaborane, Carborane, Cyclooxygenase, Indomethacin, Nonsteroidal anti-inflammatory drugs
1. Introduction
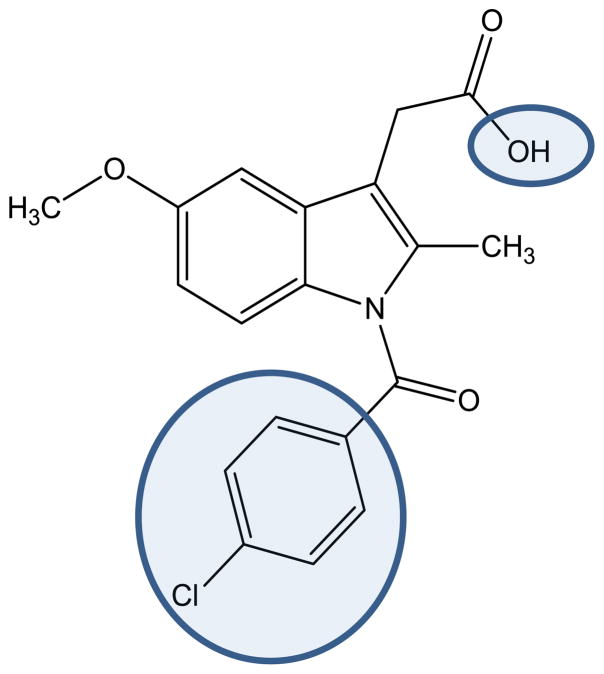
Indomethacin 2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl-1H-indol-3-yl}acetic acid is an indole-based nonsteroidal anti-inflammatory drug (NSAID). It is administered as an analgesic and anti-inflammatory agent to treat fever, edema and pain.1,2 Indomethacin’s therapeutic effects arise from the inhibition of cyclooxygenase (COX) enzymes and blockade of prostaglandin synthesis.3,4 COX exists as two different isoforms, COX-1 and COX-2. COX-1 is the so-called “housekeeping” enzyme, because it is constitutively expressed whereas COX-2 is highly inducible at sites of inflammations.5–9 COX-2 expression is highly elevated in cancer cells.10 Therefore, the COX-2 variant emerged as favored drug target.11 Classical NSAIDs, such as indomethacin (IC50 values of 0.05 μM for COX-1 and 0.75 μM for COX-2), inhibit both enzyme variants.12 Since the active site of COX-2 is approximately 25% larger than that of COX-1, a size-extension of classical NSAIDs successfully yielded inhibitors with a COX-2 selectivity.13–15 Indomethacin offers two possible positions in the periphery of the indole core for the introduction of bulky substituents in order to induce the desired selectivity (Figure 1).16–19
Fig. 1.
Indomethacin with two possible modification sites (highlighted) to obtain COX-2 selectivity.
The carboxylic acid function allows for various changes, which have already extensively been studied.12,20 Transformations of the chlorophenyl ring are synthetically more challenging, but revealed also very potent inhibitors.16,21 The presence of two additional chlorine substituents meta to the already present chlorine atom at the para position created a highly selective COX-2 inhibitor.16 The chlorine residues increased both size and electron deficiency of the benzoyl group. These two effects are probably responsible for the introduction of COX-2 selectivity.
The common modifications at the phenyl ring, however, allow only for a two-dimensional extension of the disc-shaped structure. To increase the volume three-dimensionally, different spherical substituents are required. Therefore, we selected the organic adamantyl moiety and the inorganic dicarba-closo-dodecaborane(12) (carbaborane) clusters. The latter seemed very promising, because they combine both bulkiness and electron deficiency.22,23
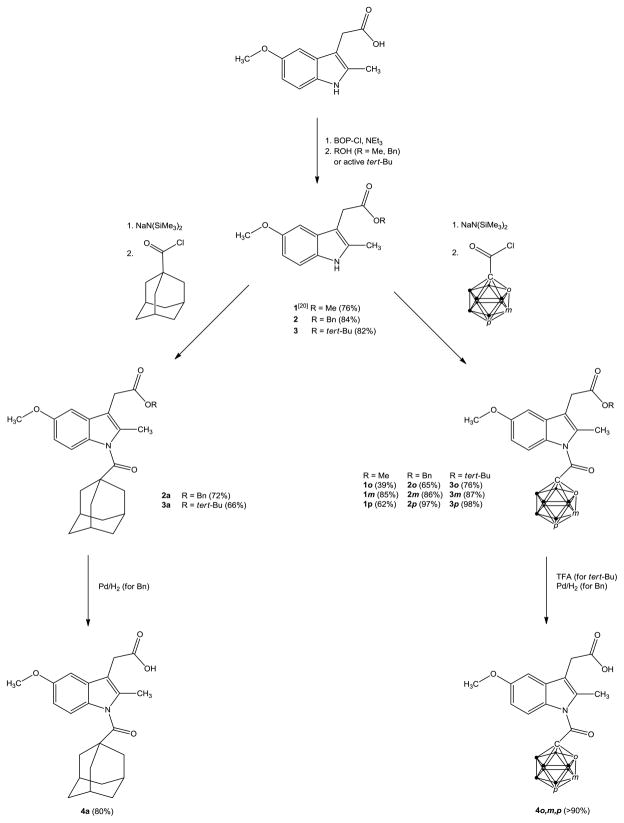
Carbaboranes are icosahedral clusters of ten BH units and two CH vertices with the latter organized either in ortho (o), meta (m), or para fashion (p, Scheme 1).24–26 The clusters occupy a volume similar to adamantane and are a little larger than a rotating benzene ring. Because of the delocalization of the cluster electrons, carbaboranes are also described as three-dimensional benzene analogues. The size of the carbaborane isomers is virtually the same, their intrinsic electronic properties, however, differ. Most characteristically is the electron-withdrawing effect which decreases in the order ortho- to para-carbaborane.27,28 The aim of this study was therefore to synthesize all three cluster analogues of indomethacin and to determine whether carbaboranes can be used as surrogates for the chlorophenyl ring to induce COX-2 selectivity.
Scheme 1.
Synthesis of the modified indomethacin analogues (● = BH, o, m, p = o, m, or p-CH).
2. Results
2.1 Chemistry
The carbaborane analogues of indomethacin (4o - p) could successfully be prepared in very good overall yields (Scheme 1).
The most challenging step was the choice of a proper protecting ester group, which should easily be introduced and later easily removed. Firstly, the methyl ester 1 was chosen, because it is already available.20 Transformation into the carbaborane analogues 1o-p afterwards was also successful, but surprisingly, deprotection later on failed completely. Thus, thiols,29 BBr3,30 Me3SnOH31,32 and different lipases33 were either inactive, cleaved preferably the methoxy ether group or eliminated the carbaborane substituent. Thus, the benzyl (2) and tert-butyl (3) esters were prepared, because of different deprotection protocols.
The benzyl ester could be obtained via N,N′-bis(2-oxo-3-oxazolidinyl)phosphinic chloride (BOP-Cl) activation in analogy to 1.34 Reduction of the amount of alcohol from four to one equivalent was found to be sufficient and even crucial to facilitate purification (separation from unreacted benzyl alcohol). The use of BOP-Cl failed to provide 3 in good yield (<20%). The same was observed when adamantanol was used. The nucleophilic attack of tertiary alcohols at the BOP-coupled and thus activated acid is probably sterically too hindered to guarantee high conversions in the presence of the free secondary indole nitrogen group. An alternative strategy, use of tert-butyl acetoacetate in combination with catalytic amounts of different acids, also failed, as well as the classic N,N′-diisopropylcarbodiimide (DIC) activation method.35 However, with CuCl and tert-butanol, DIC was transformed into 2-tert-butyl-1,3-diisopropylisourea (active tert-Bu), which finally produced the ester in satisfying yield (Scheme 1).36
A convenient method to introduce the carbaborane substituent at the indole nitrogen is salt elimination. The carbaboranyl chlorides were obtained from the unsubstituted clusters in excellent yields by slightly modified literature procedures via the corresponding carboxylic acids.37 A screening of different bases revealed NaN(SiMe3)2 as the base of choice. NaN(SiMe3)2 was better than NaH and superior to different lithium bases tested. Abandonment of aqueous workup after the reaction additionally increased the yields.
The methyl ester could not selectively be deprotected, cleavage of the benzyl and tert-butyl ester, however, gave the target compounds in excellent yields. The tert-butyl group could easily be removed using trifluoroacetic acid (TFA), the benzyl group could reductively be eliminated with Pd/H2.38 In the case of the carbaboranyl analogues, the tert-butyl strategy is preferred. It is easy to perform and did not harm the amide bond, which was partly cleaved under reducing conditions in the case of the ortho isomer. To deprotect the adamantyl-substituted analogue, the benzyl strategy is required, because TFA also eliminates the adamantyl substituent.
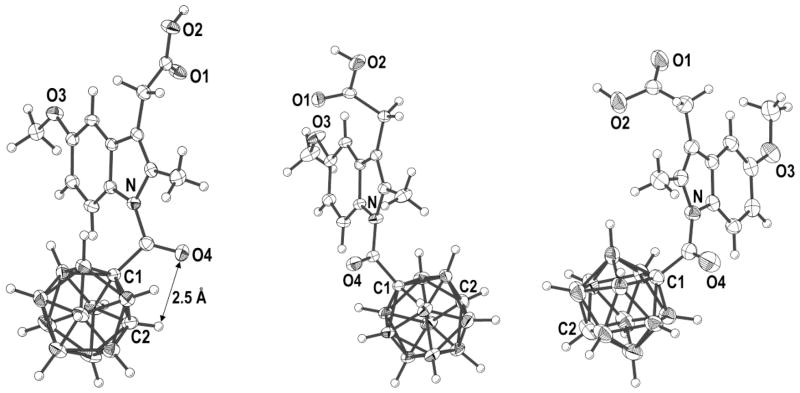
All carbaboranyl analogues (4o, m, p) were isolated as solids and recrystallized for X-ray structure determination (Figure 2).
Fig. 2.
ORTEP of 4o, m, p with selected atoms labeled, thermal ellipsoids are drawn at 50% probability. 4p crystallized with disordered n-pentane, 4o and 4m crystallized solvent-free.
As esterification of the carboxylic acid in indomethacin induces COX-2 selectivity, the esters (1o-3p) represent also potential drug candidates. To study the impact of the cluster in place of the chorophenyl ring, COX inhibition of these esters was also tested and compared to the corresponding indomethacin esters (OH in Figure 1 replaced by OMe (1i, 84%), OBn (2i, 88%) or O(tert-Bu) (3i, 56% yield). The latter were synthesized using BOP-Cl for activation of the carboxylic acid.39
2.2. COX-Inhibition Studies
To select the most promising drug candidates for COX inhibition, all compounds were initially screened at 25 μM concentration in a radioactivity assay measuring the conversion of [14C]-arachidonic acid (AA) to [14C]-prostaglandins. The assay monitors the change in [14C]-AA in the absence/presence of inhibitor relative to a control. Surprisingly, only the ortho-carbaborane derivatives (o series) revealed highly active compounds, whereas all compounds containing meta-, para-carbaboranyl and adamantyl substituents (m, p, and a series) were inactive (Table 1).
Table 1.
IC50 values as determined in a radioactivity assay. All other compounds (meta-, para-, adamantyl series, benzyl and tert-butyl ester of indomethacin) were inactive in concentrations as high as 25 μM. Data are representative of an average of at least two independent experiments.
| Compound | IC50 [μM]
|
|
|---|---|---|
| COX-1 | COX-2 | |
| Indomethacin (1) | 0.027 | 0.127 |
| Indomethacin methyl ester (1i)39 | > 25 | 0.250 |
| Indoborin (4o) | > 25 | 3.680 |
| Indoborin methyl ester (1o) | 12.800 | 0.084 |
| Indoborin benzyl ester (2o) | 15.050 | 11.600 |
| Indoborin tert-butyl ester (1o) | > 25 | 1.410 |
The ortho (o) series additionally revealed the desired selectivity shift in favor of COX-2. The ortho-carbaborane analogue of indomethacin 1-(1-carboxy-1,2-dicarba-closo-dodecaboran(12)yl)-5-methoxy-2-methyl-1H-indole-3-acetic acid (4o) (indoborin), inhibited COX-2 in the low micromolar range (IC50 (COX-2) = 3.68 μM) and was inactive against COX-1 at concentrations lower than 25 μM. Surprisingly, methyl ester 1o, which proved difficult to be deprotected, was the best COX-2 inhibitor acting in the nanomolar range (IC50 (COX-2) = 84 nM) while inhibiting COX-1 in only micromolar concentrations (IC50 (COX-1) = 12.8 μM).
Hence, ortho-carbaborane emerged as magnificent surrogate for the chlorophenyl ring of indomethacin to induce COX-2 selectivity in contrast to the other bulky substituents, such as meta-, para-carbaborane and adamantyl. As all carbaboranes differ only negligibly in their volumes, the unique electronic properties of ortho-carbaborane seem to be the reason for inducing both COX inhibition in general and in particular COX-2 selectivity. The presence of an additional ester group in the ortho-carbaboranyl analogues could further fine-tune the COX selectivity profile. The best COX-2 inhibitor tested was 1o with the small methyl ester group. 1o and the aromatic benzyl ester 2o also inhibited COX-1, but in the case of 1o the IC50 value was three orders of magnitude lower for COX-2. The bulky tert-butyl ester 3o and the free acid 4o exclusively inhibited COX-2 and not COX-1 in the relevant concentration range. All esters of the o series were better inhibitors than the corresponding indomethacin esters with the chlorophenyl substituent.
We had previously investigated carbaboranyl esters of indomethacin. This approach only succeeded to lower COX-1 inhibition, but failed to induce a clear COX-2 selectivity. Interestingly, these studies also showed ortho-carbaborane directly attached to the carboxylato group as the only active inhibitor.40
3. Summary and conclusion
In conclusion, carbaboranes are unique and yet underrepresented pharmacophores.23d Their versatile chemistry allows the integration of the clusters into various drug candidates in high-yielding procedures. The results of the indomethacin derivatives indicate that the syntheses of all cluster analogues are rather similar, whereas the resulting biochemical profile clearly depends on the carbaborane isomer. The COX inhibition potential of meta- and para-carbaborane was similar to the adamantyl analogue, which can therefore be regarded as being determined by geometric features only. The pronounced activity of the strong electron-withdrawing ortho-carbaborane derivatives, however, suggests that this isomer acts via its functionality. A detailed investigation (co-crystallization of 1o-4o with COX-2 and corresponding modeling studies) to understand the unexpected activity of the ortho-carbaborane derivatives is currently underway.
4. Experimental
4.1. Chemistry
4.1.1. General
All reactions were carried out under nitrogen atmosphere by using standard Schlenk techniques. The solvents were purified by a Solvent Purification System SPS-800 Series. All alcohols were distilled prior usage. Carbaboranes are commercially available from Katchem (Czech Republic). 5-Methoxy-2-methyl-1H-indole-3-acetic acid is commercially available (a facile synthesis is given in the see supplementary data). 5-Methoxy-2-methyl-1H-indole-3-acetic acid methyl ester (1) was prepared according to the literature.20 Flash chromatography was carried out on Merck SilicaGel 60 (0.035-0.070 mm). Merck Silica 60 F254 was used for thin-layer chromatography (TLC). The TLC plates were developed with palladium(II) chloride methanol solution. The infrared spectra were recorded on a Perkin-Elmer System 2000 FT-IR spectrometer as KBr pellets. The 1H, 13C, and 11B NMR spectra were recorded at 25 °C on an AVANCE DRX 400 spectrometer (Bruker). The chemical shifts for the 1H, 13C, and 11B NMR spectra are reported in parts per million (ppm) at 400.13, 100.63, and 128.38 MHz, respectively, with tetramethylsilane as standard for the first two and BF3(OEt2) as external standard for 11B NMR. Proton-coupled 13C NMR spectra were recorded for carbaborane-containing compounds; 2JCH were not always resolved. The number of boron atoms and 1JBH could not always be determined unambiguously due to broad and overlapping signals. Elemental analyses were recorded on a VARIO EL (Heraeus). The melting points were determined in capillaries (GALLENKAMP) and represent uncorrected values. The crystals for crystal structure determination were obtained from concentrated solutions at room temperature. The crystallographic data were collected on a CCD Oxford Xcalibur S diffractometer (λ(Mo-Kα) = 0.71073 Å) in ωand Φ scan mode. Semi-empirical from equivalents absorption corrections were carried out with SCALE3 ABSPACK and the structures were solved with direct methods.41,42 Structure refinement was carried out with SHELXL-97.43 CCDC 808548–808550 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
4.1.2. Syntheses
4.1.2.1. Esters of 5-methoxy-2-methyl-1H-indole-3-acetic acid
5-Methoxy-2-methyl-1H-indole-3-acetic acid benzyl ester (2)
5-Methoxy-2-methyl-1H-indole-3-acetic acid (4.00 g, 18.26 mmol) and BOP-Cl (4.65 g, 18.26 mmol) were suspended in dichloromethane (1 mL) and NEt3 (5.0 mL, 35.90 mmol) was slowly added and the mixture stirred for 10 min. Benzyl alcohol (1.9 mL, 18.26 mmol) was added and the mixture stirred for 12 h. Dichloromethane (200 mL) was added and the reaction mixture was washed with water (2 × 200 mL) and brine solution (100 mL). The organic layer was concentrated and purified by column chromatography with a mixture of hexanes (80–100 °C) and ethyl acetate (2:1) for elution. The solvent was removed under reduced pressure to yield the corresponding ester as yellow solid. Yield: 4.74 g (84%); elemental analysis calcd. (%) for C19H19NO3: C 73.77; H 6.19; found: C 73.13; H 6.19; m.p.: 55–56 °C; ESI MS (+) (CH3OH/CHCl3): m/z: 326.1 (100%, [M+OH]+); 1H NMR (CDCl3, ppm): 7.75 (s, br, 1H, NHindole), 7.29-7.23 (vbr, 5H, CHphenyl, together with solvent residual peak), 7.06 (d, 3JHH = 8 Hz, 1H, CHindole), 6.96 (d, 4JHH = 2 Hz, 1H, CHindole), 6.75 (dd, 3JHH = 8 Hz, 4JHH = 2 Hz, 1H, CHindole), 5.10 (s, 2H, CH2 benzyl), 3.76 (s, 3H, OCH3), 3.69 (s, 2H, CH2), 2.29 (s, 3H, CH3); 13C{1H} NMR (CDCl3, ppm): 172.0 (COO), 154.0 (CindoleO), 136.0 (Caromat), 133.7 (Caromat), 130.1 (Caromat), 128.8 (Caromat), 128.5 (CHphenyl), 128.1 (CaromatH), 128.0 (CHphenyl), 111.0 (CaromatH), 111.0 (CaromatH), 104.1 (Caromat), 100.2 (CaromatH), 66.5 (CH2 benzyl), 55.8 (OCH3), 30.5 (CH2), 11.7 (CH3); IR (selected, KBr, cm−1): ν̃ = 3400 (s, ν(N–H)), 3033 (w), 2938 (w), 1729 (s, ν(C=O)).
5-Methoxy-2-methyl-1H-indole-3-acetic acid tert-butyl esters (3)
DIC (12.1 mL, 77.8 mmol) was added to a mixture of CuCl (140 mg, 1.4 mmol) and tert-butanol (29.9 mL, 312.6 mmol) and the mixture was stirred at room temperature for 12 h. The volatile components were removed under reduced pressure. The solid was dissolved in THF (25 mL) and 5-methoxy-2-methyl-1H-indole-3-acetic acid (2) (7.0 g, 31.9 mmol) was added and the mixture stirred at room temperature for 2h. The solvent was evaporated and the solid was purified by column chromatography with a mixture of hexanes (80–100 °C) and ethyl acetate (3:1). The solvent was removed under reduced pressure to yield a white solid. Yield: 7.2 g (82%); elemental analysis calcd. (%) for C16H21NO3: C 69.80; H 7.69; found: C 69.57; H 7.67; m.p.: 110–111 °C; ESI MS (−) (CH3COCH3): m/z: 274.0 (100%, [M–H]);−1H NMR (CDCl3, ppm): 7.75 (s, vbr, NHindole), 7.14 (d, 3JHH = 8 Hz, 1H, CHindole), 7.00 (d, 4JHH = 2 Hz, 1H, CHindole), 6.76 (dd, 3JHH = 8 Hz, 4JHH = 2 Hz, 1H, CHindole), 3.85 (s, 3H, OCH3), 3.54 (s, 2H, CH2), 2.39 (s, 3H, CH3), 1.43 (s, 9H, C(CH3)3); 13C{1H} NMR (CDCl3, ppm): 171.4 (COO), 154.0 (CindoleO), 133.5 (Cindole), 130.2 (Cindole), 129.1 (Cindole), 110.9 (CindoleH), 110.9 (CindoleH), 105.0 (Cindole), 100.6 (CindoleH), 80.5 (C(CH3)3), 55.9 (OCH3), 31.9 (CH2), 28.1 (C(CH3)3), 11.9 (CH3); IR (selected, KBr, cm−1): ν̃ = 3342 (s, ν(N–H)), 3005 (w), 2980 (w), 2965 (w), 2914 (w), 2837 (w), 2047 (w), 1722 (s, ν(C=O)).
4.1.2.2. Substitutions at the indole NH group
General procedure
NaN(SiMe3)2 (1.1 eq., in toluene) was added at −78 °C to the ester (1 eq.) dissolved in toluene (1.5 mL/100 mg) and the mixture was stirred for 1 h at room temperature. The corresponding carbaboranyl carbonyl chloride (1.3 eq., stock solution in toluene) was added and the mixture stirred additionally for 12 h. The solvent was evaporated and the solid was purified by column chromatography with a mixture of hexanes (80–100 °C) and ethyl acetate (3:1). The solvent was removed under reduced pressure to yield the corresponding esters as slightly yellow solids.
1-(1-Carboxy-1,2-dicarba-closo-dodecaboran(12)yl)-5-methoxy-2-methyl-1H-indole-3-acetic acid methyl ester (1o)
Yield: 0.10 g (39% from 0.15 g 1); elemental analysis calcd. (%) for C16H25B10NO4: C 47.64; H 6.25; found: C 47.98; H 6.31; ESI MS (+) (CH3COCH3): m/z: 442.1 (100%, [M+K]+), 404.3 (75%, [M+H]+); m.p.: 126–127 °C, 1H NMR (CDCl3, ppm): 7.32 (d, 3JHH = 8 Hz, 1H, CHindole), 6.95 (d, 4JHH = 2 Hz, 1H, CHindole), 6.85 (dd, 3JHH = 8 Hz, 4JHH = 2 Hz, 1H, CHindole), 4.36 (s, 1H, CclusterH), 3.86 (s, 3H, OCH3), 3.69 (s, 3H, COOCH3), 3.63 (s, 2H, CH2), 3.30-1.40 (m, vbr, 10H, C2H10B10), 2.31 (s, 3H, CH3); 11B NMR (CDCl3, ppm): −1.3 (d, 1JBH = 154 Hz, C2H10B10), −2.5 (d, 1JBH = 141 Hz, C2H10B10), −8.5 (d, 1JBH = 192 Hz, C2H10B10), −12.0 (vbr, C2H10B10), −13.1 (vbr, C2H10B10); 13C NMR (CDCl3, ppm): 171.1 (s, CO), 166.6 (s, CO), 156.0 (s, CindoleO), 135.1 (m, vbr, Cindole), 130.1 (m, vbr, Cindole), 130.0 (m, vbr, Cindole), 113.2 (d, 1JCH = 161 Hz, CindoleH), 112.0 (dd, 1JCH = 161 Hz, 2JCH = 5 Hz, CindoleH), 111.3 (Cindole), 101.5 (dd, 1JCH = 161 Hz, 2JCH = 5 Hz, CindoleH), 74.6 (vbr, Ccluster), 59.2 (d, vbr, 1JCH = 201 Hz, CclusterH), 55.8 (q, 1JCH = 141 Hz, OCH3), 52.2 (q, 1JCH = 141 Hz, OCH3), 30.2 (t, 1JCH = 131 Hz, CH2), 11.4 (q, 1JCH = 131 Hz, CH3); IR (selected, KBr, cm−1): ν̃ = 3055 (w), 2955 (w), 2918 (w), 2602 (s, ν(B–H)), 2580 (s, ν(B–H)), 1743 (s, ν(C=O), 1712 (s, ν(C=O)).
1-(1-Carboxy-1,7-dicarba-closo-dodecaboran(12)yl)-5-methoxy-2-methyl-1H-indole-3-acetic acid methyl ester (1m)
Yield: 1.06 g (85% from 0.72 g 1); elemental analysis calcd. (%) for C16H25B10NO4: C 47.64; H 6.25; found: C 47.35; H 6.21; m.p.: 103–104 °C; ESI MS (+) (CH3COCH3): m/z: 442.1 (48%, [M+K]+), 404.2 (100%, [M+H]+); 1H NMR (CDCl3, ppm): 7.25 (d, 3JHH = 8 Hz, 1H, CHindole), 6.93 (d, 4JHH = 2 Hz, 1H, CHindole), 6.82 (dd, 1JHH = 8 Hz, 4JHH = 2 Hz, 1H, CHindole), 3.85 (s, 3H, OCH3), 3.68 (s, 3H, COOCH3), 3.62 (s, 2H, CH2), 3.06 (s, 1H, CclusterH), 3.30-1.40 (m, vbr, 10H, C2H10B10), 2.27 (s, 3H, CH3); 11B NMR (CDCl3, ppm): −4.7 (d, 1JBH = 154 Hz, 1B, C2H10B10), −5.8 (d, 1JBH = 128 Hz, 1B, C2H10B10), −10.4 (d, 1JBH = 141 Hz, 4B, C2H10B10), −13.1 (d, 1JBH = 169 Hz, 2B, C2H10B10), −15.2 (d, 1JBH = 192 Hz, 2B, C2H10B10); 13C NMR (CDCl3, ppm): 171.3 (m, vbr, CO), 167.3 (s, CO), 155.5 (m, vbr, CindoleO), 134.9 (q, 2JCH = 6 Hz, CindoleCH3), 130.4 (t, 2JCH = 10 Hz, Cindole), 129.5 (m, vbr, Cindole), 112.8 (d, 1JCH = 161 Hz, CindoleH), 111.8 (dd, 1JCH = 161 Hz, 2JCH = 5 Hz, CindoleH), 109.9 (m, vbr, Cindole), 101.1 (dd, 1JCH = 161 Hz, 2JCH = 5 Hz, CindoleH), 78.1 (vbr, Ccluster), 55.8 (q, 1JCH = 141 Hz, OCH3), 55.0 (d, vbr, 1JCH = 181 Hz, CclusterH), 52.1 (q, 1JCH = 141 Hz, OCH3), 30.2 (t, 1JCH = 131 Hz, CH2), 11.3 (q, 1JCH = 131 Hz, CH3); IR (selected, KBr, cm−1): ν̃ = 3047 (s, ν(C–H)), 3025 (w), 2953 (s, ν(C–H)), 2903 (w), 2836 (w), 2663 (m, ν(B–H)), 2611 (s, ν(B–H)), 1728 (s, ν(C=O)),.
1-(1-Carboxy-1,12-dicarba-closo-dodecaboran(12)yl)-5-methoxy-2-methyl-1H-indole-3-acetic acid methyl ester (1p)
Yield: 0.16 g (62% from 0.15 g 1); elemental analysis calcd. (%) for C16H25B10NO4: C 47.64; H 6.25; found: C 47.83; H 6.19; m.p.: 115–116 °C; ESI MS (+) (CH3COCH3): m/z: 442.1 (34%, [M+K]+), 404.2 (100%, [M+H]+); 1H NMR (CDCl3, ppm): 7.13 (d, 3JHH = 8 Hz, 1H, CHindole), 6.91 (d, 4JHH = 2 Hz, 1H, CHindole), 6.80 (dd, 3JHH = 8 Hz, 4JHH = 2 Hz, 1H, CHindole), 3.84 (s, 3H, OCH3), 3.67 (s, 3H, COOCH3), 3.59 (s, 2H, CH2), 3.50-1.50 (m, vbr, 10H, C2H10B10), 2.91 (s, 1H, CclusterH), 2.18 (s, 3H, CH3); 11B NMR (CDCl3, ppm): −13.0 (d, 1JBH = 180 Hz, 5B, C2H10B10), −15.1 (d, 1JBH = 167 Hz, 5B, C2H10B10); 13C NMR (CDCl3, ppm): 171.4 (m, vbr, CO), 168.0 (s, CO), 155.4 (m, vbr, CindoleO), 134.8 (m, vbr, Cindole), 130.5 (m, vbr, Cindole), 129.2 (m, vbr, Cindole), 112.7 (d, 1JCH = 161 Hz, CindoleH), 111.7 (dd, 1JCH = 161 Hz, 2JCH = 5 Hz, CindoleH), 109.4 (m, vbr, Cindole), 101.0 (dd, 1JCH = 161 Hz, 2JCH = 5 Hz, CindoleH), 85.3 (vbr, Ccluster), 63.8 (d, vbr, 1JCH = 181 Hz, CclusterH), 55.8 (q, 1JCH = 141 Hz, OCH3), 52.1 (q, 1JCH = 141 Hz, OCH3), 30.2 (t, 1JCH = 131 Hz, CH2), 11.1 (q, 1JCH = 131 Hz, CH3); IR (selected, KBr, cm −1): ν̃ = 3047 (s, ν(C–H)), 2997 (w), 2950 (w), 2605 (s, ν(B–H)), 1729 (s, ν(C=O)).
1-(1-Carboxy-1,2-dicarba-closo-dodecaboran(12)yl)-5-methoxy-2-methyl-1H-indole-3-acetic acid benzyl ester (2o)
Yield: 0.20 g (65% from 0.20 g 2); elemental analysis calcd. (%) for C22H29B10NO4: C 55.10; H 6.09; found: C 55.15; H 6.11; m.p.: 53–54 °C; ESI MS (−) (CH3COCH3): m/z: 375.3 (100%, [M–CH3–CH2C6H5] −); 1H NMR (CDCl3, ppm): 7.38-7.26 (m, vbr, 6H, CHaromat together with solvent residual peak), 6.92 (d, 4JHH = 2 Hz, 1H, CHindole), 6.85 (dd, 3JHH = 8 Hz, 4JHH = 2 Hz, 1H, CHindole), 5.11 (s, 2H, CH2 benzyl), 4.36 (s, 1H, CclusterH), 3.78 (s, 3H, OCH3), 3.67 (s, 2H, CH2), 3.36-1.49 (m, vbr, 10H, C2H10B10), 2.29 (s, 3H, CH3); 11B NMR (CDCl3, ppm): −1.2 (d, 1JBH = 128 Hz, 1B, C2H10B10), −2.5 (d, 1JBH = 154 Hz, 1B, C2H10B10), −8.5 (d, 1JBH = 154 Hz, 2B, C2H10B10), −11.9 (vbr, 3B, C2H10B10), −13.0 (vbr, 3B, C2H10B10); 13C NMR (CDCl3, ppm): 170.4 (s, vbr, CO), 166.6 (s, vbr, CO), 156.0 (s, vbr, CindoleO), 135.6 (s, vbr, Caromat), 135.1 (s, vbr, Caromat), 130.1 (s, vbr, Caromat), 130.0 (s, vbr, Caromat), 128.6 (m, vbr, CHphenyl), 128.3 (m, vbr, CHphenyl), 128.1 (m, vbr, CHphenyl), 113.2 (d, 1JCH = 161 Hz, CindoleH), 112.3 (dd, 1JCH = 161 Hz, 2JCH = 5 Hz, CindoleH), 111.2 (s, vbr, Caromat), 101.4 (dd, 1JCH = 161 Hz, 2JCH = 5 Hz, CindoleH), 74.6 (s, vbr, Ccluster), 66.8 (tt, 1JCH = 151 Hz, 3JCH = 4 Hz, OCH2), 59.2 (d, 1JCH = 201 Hz, CclusterH), 55.7 (q, 1JCH = 141 Hz, OCH3), 30.5 (t, 1JCH = 131 Hz, CH2), 11.4 (q, 1JCH = 131 Hz, CH3); IR (selected, KBr, cm−1): ν̃ = 3431 (w), 3064 (w), 2928 (w), 2834 (w), 2586 (m, ν(B–H)), 1733 (s, ν(C=O)).
1-(1-Carboxy-1,7-dicarba-closo-dodecaboran(12)yl)-5-methoxy-2-methyl-1H-indole-3-acetic acid benzyl ester (2m)
Yield: 0.53 g (86% from 0.40 g 2); elemental analysis calcd. (%) for C22H29B10NO4: C 55.10; H 6.09; found: C 54.70; H 6.13; m.p.: 57–58 °C; ESI MS (+) (CH3COCH3/CHCl3/Na+): m/z: 537.1 (100%, [M+CH3COCH3]+); 1H NMR (CDCl3, ppm): 7.31-7.26 (vbr, 6H, CHaromat together with solvent residual peak), 6.91 (s, 1H, CHaromat), 6.82 (d, 1JHH = 8 Hz, 1H, CHaromat), 5.11 (s, 2H, CH2 benzyl), 3.77 (s, 3H, OCH3), 3.66 (s, 2H, CH2), 3.05 (s, 1H, CclusterH), 3.50-1.50 (m, vbr, 10H, C2H10B10), 2.26 (s, 3H, CH3); 11B NMR (CDCl3, ppm): −4.8 (vbr, 1B, C2H10B10), −5.8 (vbr, 1B, C2H10B10), −10.5 (d, 1JBH = 154 Hz, 4B, C2H10B10), −13.1 (d, 1JBH = 180 Hz, 2B, C2H10B10), −15.3 (vbr, 2B, C2H10B10); 13C NMR (CDCl3, ppm): 170.7 (s, vbr, CO), 167.3 (s, CO), 155.5 (s, vbr, CindoleO), 135.7 (s, Caromat), 134.9 (s, Caromat), 130.4 (s, vbr, Caromat), 129.4 (s, vbr, Caromat), 128.5 (m, vbr, CHphenyl), 128.2 (m, vbr, CHphenyl), 128.0 (m, vbr, CHphenyl), 112.8 (d, 1JCH = 161 Hz, CindoleH), 112.0 (dd, 1JCH = 161 Hz, 2JCH = 5 Hz, CindoleH), 109.8 (s, vbr, Caromat), 101.0 (dd, 1JCH = 161 Hz, 2JCH = 5 Hz, CindoleH), 78.1 (s, Ccluster), 66.7 (t, 1JCH = 151 Hz, OCH2), 55.7 (q, 1JCH = 141 Hz, OCH3), 55.0 (d, 1JCH = 181 Hz, CclusterH), 30.5 (t, 1JCH = 131 Hz, CH2), 11.3 (q, 1JCH = 131 Hz, CH3); IR (selected, KBr, cm−): ν̃ = 3061 (w), 2996 (w), 2925 (w), 2834 (w), 2609 (s, ν(B–H)), 1733 (s, ν(C=O)).
1-(1-Carboxy-1,12-dicarba-closo-dodecaboran(12)yl)-5-methoxy-2-methyl-1H-indole-3-acetic acid benzyl ester (2p)
Yield: 0.30 g (97% from 0.20 g 2); elemental analysis calcd. (%) for C22H29B10NO4: C 55.10; H 6.09; found: C 54.70; H 6.13; m.p.: 77–78 °C; ESI MS (+) (CH3COCH3): m/z: 537.3 (100%, [M+CH3COCH3]+); 1H NMR (CDCl3, ppm): 7.31-7.26 (vbr, 5H, CHphenyl together with solvent residual peak), 7.13 (d, 1H, 3JCH = 8 Hz, CHindole), 6.88 (d, 4JHH = 2 Hz, 1H, CHindole), 6.79 (dd, 1H, 3JCH = 8 Hz, 4JHH = 2 Hz, CHindole), 5.11 (s, 2H, CH2 benzyl), 3.76 (s, 3H, OCH3), 3.63 (s, 2H, CH2), 3.33-1.49 (m, vbr, 10H, C2H10B10), 2.90 (s, 1H, CclusterH), 2.16 (s, 3H, CH3); 11B NMR (CDCl3, ppm): −13.0 (d, 1JBH = 154 Hz, 5B, C2H10B10), −15.1 (d, 1JBH = 167 Hz, 5B, C2H10B10); 13C NMR (CDCl3, ppm): 170.7 (s, vbr, CO), 167.9 (s, CO), 155.3 (s, vbr, CindoleO), 135.8 (s, vbr, Caromat), 134.8 (s, vbr, Caromat), 130.5 (s, vbr, Caromat), 129.1 (s, vbr, Caromat), 128.5 (s, vbr, CHphenyl), 128.2 (s, vbr, CHphenyl), 128.0 (s, vbr, CHphenyl), 112.7 (d, 1JCH = 161 Hz, CindoleH), 111.9 (dd, 1JCH = 161 Hz, 2JCH = 5 Hz, CindoleH), 109.3 (s, vbr, Cindole), 100.8 (dd, 1JCH = 161 Hz, 2JCH = 5 Hz, CindoleH), 85.3 (s, vbr, Ccluster), 66.6 (tt, 1JCH = 141 Hz, 3JCH = 5 Hz, OCH2), 63.8 (d, 1JCH = 181 Hz, CHcluster), 55.7 (q, 1JCH = 141 Hz, OCH3), 30.5 (t, 1JCH = 131 Hz, CH2), 11.2 (q, 1JCH = 131 Hz, CH3); IR (selected, KBr, cm−1): ν̃ = 3060 (w), 2929 (w), 2617 (s, ν(B–H)), 1727 (s, ν(C=O)).
1-(1-Adamantyl)-5-methoxy-2-methyl-1H-indole-3-acetic acid benzyl ester (2a)
Yield: 0.55 g (72% from 0.50 g 2) elemental analysis calcd. (%) for C30H33NO4: C 76.41; H 7.05; found: C 76.19; H 7.05; m.p.: 108–109 °C; ESI MS (+) (CH3COCH3/Na+): m/z: 494.3 (100%, [M+Na]+); 1H NMR (CDCl3, ppm): 7.29-7.26 (vbr, 5H, CHphenyl together with solvent residual peak), 7.14 (d, 3JHH = 8 Hz, 1H, CHindole), 6.94 (d, 4JHH = 2 Hz, 1H, CHindole), 6.79 (dd, 3JHH = 8 Hz, 4JHH = 2 Hz, 1H, CHindole), 5.11 (s, 2H, CH2 benzyl), 3.77 (s, 3H, OCH3), 3.70 (s, 2H, CH2), 2.31 (s, 3H, CH3), 2.05 (s, vbr, 9H, CH/CH2 adamantyl), 1.72 (s, vbr, 6H, CH2 adamantyl); 13C{1H} NMR (CDCl3, ppm): 186.0 (CO), 171.2 (CO), 154.8 (CindoleO), 135.9 (Caromat), 134.4 (Caromat), 130.2 (Caromat), 129.3 (Caromat), 128.5 (CHphenyl), 128.2 (CHphenyl), 128.0 (CHphenyl), 112.6 (CindoleH), 111.5 (CindoleH), 107.7 (Caromat), 100.6 (CindoleH), 66.6 (OCH2), 55.7 (OCH3), 46.6 (Cadamantyl), 39.3 (CH2 adamantyl), 36.2 (CH2 adamantyl), 30.6 (CH2), 28.0 (CH adamantyl), 11.8 (CH3); IR (selected, KBr, cm−1): ν̃ = 3091 (w), 3062 (w), 3032 (w), 3005 (w), 2907 (s, ν(C–H)), 2850 (m), 2680 (w), 2658 (w), 2346 (w), 2067 (w), 1958 (w), 1879 (w), 1854 (w), 1831 (w), 1802 (w), 1734 (s, ν(C=O)), 1709 (s, ν(C=O)).
1-(1-Carboxy-1,2-dicarba-closo-dodecaboran(12)yl)-5-methoxy-2-methyl-1H-indole-3-acetic acid tert-butyl ester (3o)
Yield: 0.37 g (76% from 0.30 g 3); elemental analysis calcd. (%) for C19H31B10NO4: C 51.22; H 7.01; found: C 51.54; H 6.99; m.p.: 109–110 °C; ESI MS (+) (CH3COCH3): m/z: 485.3 (100%, [M+K]+); 1H NMR (CDCl3, ppm): 7.31 (d, 3JHH = 8 Hz, 1H, CHindole), 6.95 (d, 4JHH = 2 Hz, 1H, CHindole), 6.85 (dd, 3JHH = 8 Hz, 4JHH = 2 Hz, 1H, CHindole), 4.37 (s, 1H, CclusterH), 3.85 (s, 3H, OCH3), 3.52 (s, 2H, CH2), 3.20-1.50 (m, vbr, 10H, C2H10B10), 2.31 (s, 3H, CH3), 1.41 (s, 9H, C(CH3)3); 11B NMR (CDCl3, ppm): −1.2 (d, 1JBH = 180 Hz, 1B, C2H10B10), −2.6 (d, 1B, C2H10B10), −8.5 (d, 1JBH = 154 Hz, 2B, C2H10B10), −11.9 (vbr, 3B, C2H10B10), −13.1 (vbr, 3B, C2H10B10); 13C NMR (CDCl3, ppm): 169.9 (s, vbr, CO), 166.5 (s, CO), 155.9 (s, vbr, CindoleO), 134.8 (s, vbr, Cindole), 130.2 (s, vbr, Cindole), 130.1 (s, vbr, Cindole), 113.2 (d, 1JCH = 161 Hz, CindoleH), 112.1 (s, vbr, Cindole), 112.0 (dd, 1JCH = 161 Hz, 2JCH = 5 Hz, CindoleH), 101.5 (dd, 1JCH = 161 Hz, 2JCH = 5 Hz, CindoleH), 81.2 (s, vbr, C(CH3)3), 74.6 (s, vbr, Ccluster), 59.3 (d, 1JCH = 191 Hz, CclusterH), 55.7 (q, 1JCH = 151 Hz, OCH3), 31.9 (t, 1JCH = 131 Hz, CH2), 28.0 (q, vbr, 1JCH = 131 Hz, C(CH3)3), 11.4 (q, 1JCH = 131 Hz, CH3); IR (selected, KBr, cm−1): ν̃ = 3080 (w), 3004 (w), 2978 (w), 2930 (w), 2831 (w), 2656 (w), 2574 (m, ν(B–H)), 1731 (s, ν(C=O)), 1719 (s, ν(C=O)).
1-(1-Carboxy-1,7-dicarba-closo-dodecaboran(12)yl)-5-methoxy-2-methyl-1H-indole-3-acetic acid tert-butyl ester (3m)
Yield: 0.14 g (87% from 0.10 g 3); elemental analysis calcd. (%) for C19H31B10NO4: C 51.22; H 7.01; found: C 51.41; H 7.03; m.p.: 150–151 °C; ESI MS (+) (CH3COCH3/Na+): m/z: 503.1 (100%, [M+CH3COCH3]+), 468.1 (29%, [M+Na]+) 1H NMR (CDCl3, ppm): 7.24 (d, 3JHH = 8 Hz, 1H, CHindole), 6.94 (d, 4JHH = 2 Hz, 1H, CHindole), 6.82 (dd, 3JHH = 8 Hz, 4JHH = 2 Hz, 1H, CHindole), 3.85 (s, 3H, OCH3), 3.51 (s, 2H, CH2), 3.06 (s, 1H, CclusterH), 3.30-1.40 (m, vbr, 10H, C2H10B10), 2.27 (s, 3H, CH3), 1.41 (s, 9H, C(CH3)3); 11B NMR (CDCl3, ppm): −4.7 (d, 1JBH = 154 Hz, 1B, C2H10B10), −5.9 (d, 1B, C2H10B10), −10.5 (d, 1JBH = 141 Hz, 4B, C2H10B10), −13.1 (d, 1JBH = 180 Hz, 2B, C2H10B10), −15.2 (d, 1JBH = 180 Hz, 2B, C2H10B10); 13C NMR (CDCl3, ppm): 170.2 (s, CO), 167.3 (s, CO), 155.5 (s, vbr, CindoleO), 134.6 (m, vbr, Cindole), 130.4 (m, vbr, Cindole), 129.6 (m, vbr, Cindole), 112.8 (d, 1JCH = 161 Hz, CindoleH), 111.7 (dd, 1JCH = 161 Hz, 2JCH = 6 Hz, CindoleH), 110.7 (m, vbr, Cindole), 101.1 (dd, 1JCH = 161 Hz, 2JCH = 6 Hz, CindoleH), 81.0 (m, vbr, C(CH3)3), 78.2 (vbr, Ccluster), 55.7 (q, 1JCH = 141 Hz, OCH3), 55.0 (d, vbr, 1JCH = 181 Hz, CclusterH), 31.9 (t, 1JCH = 121 Hz, CH2), 28.0 (q, 1JCH = 131 Hz, C(CH3)3), 11.3 (q, 1JCH = 131 Hz, CH3); IR (selected, KBr, cm−1): ν̃ = 3056 (m, ν(C–H)), 2978 (s, ν(C–H)), 2931 (s, ν(C–H)), 2834 (m), 2609 (s, ν(B–H)), 2066 (m), 1847 (m), 1728 (s, ν(C=O)).
1-(1-Carboxy-1,12-dicarba-closo-dodecaboran(12)yl)-5-methoxy-2-methyl-1H-indole-3-acetic acid tert-butyl ester (3p)
Yield: 0.16 g (99% from 0.10 g 3); elemental analysis calcd. (%) for C19H31B10NO4: C 51.22; H 7.01; found: C 51.14; H 7.01; m.p.: 56–57 °C; ESI MS (+) (CH3COCH3): m/z: 503.3 (100%, [M+CH3COCH3]+); 1H NMR (CDCl3, ppm): 7.12 (d, 3JHH = 8 Hz, 1H, CHindole), 6.91 (d, 4JHH = 2 Hz, 1H, CHindole), 6.79 (d, 3JHH = 8 Hz, 4JHH = 2 Hz, 1H, CHindole), 3.84 (s, 3H, OCH3), 3.48 (s, 2H, CH2), 3.35-1.14 (m, vbr, 10H, C2H10B10), 2.89 (s, 1H, CclusterH), 2.18 (s, 3H, CH3), 1.41 (s, 9H, C(CH3)3); 11B NMR (CDCl3, ppm): −13.0 (d, 1JBH = 154 Hz, 5B, C2H10B10), −15.1 (d, 1JBH = 180 Hz, 5B, C2H10B10); 13C NMR (CDCl3, ppm): 170.2 (s, vbr, CO), 167.9 (s, CO), 155.3 (s, vbr, CindoleO), 134.5 (s, vbr, Cindole), 130.5 (s, vbr, Cindole), 129.3 (s, vbr, Cindole), 112.7 (d, 1JCH = 161 Hz, CindoleH), 111.6 (dd, 1JCH = 161 Hz, 2JCH = 6 Hz, CindoleH), 110.2 (s, vbr, Cindole), 100.9 (dd, 1JCH = 161 Hz, 2JCH = 6 Hz, CindoleH), 85.4 (s, vbr, Ccluster), 80.9 (s, vbr, C(CH3)3), 63.8 (d, 1JCH = 181 Hz, CclusterH), 55.7 (q, 1JCH = 141 Hz, OCH3), 31.9 (t, 1JCH = 121 Hz, CH2), 28.0 (q, vbr, 1JCH = 131 Hz, C(CH3)3), 11.2 (q, 1JCH = 131 Hz, CH3); IR (selected, KBr, cm−1): ν̃ = 3059 (w), 2925 (m, ν(C–H)), 2617 (s, ν(B–H)), 1728 (s, ν(C=O)).
1-(1-Adamantyl)-5-methoxy-2-methyl-1H-indole-3-acetic acid tert-butyl ester (3a)
Yield: 0.16 g (67% from 0.15 g 3); elemental analysis calcd. (%) for C27H35NO4: C 74.11; H 8.06; found: C 74.12; H 8.07; m.p.: 54–55 °C; ESI MS (+) (CH3COCH3/Na+): m/z: 495.1 (100%, [M+ CH3COCH3]+); 1H NMR (CDCl3, ppm): 7.14 (d, 3JHH = 8 Hz, 1H, CHindole), 6.97 (d, 4JHH = 2 Hz, 1H, CHindole), 6.79 (dd, 3JHH = 8 Hz, 4JHH = 2 Hz, 1H, CHindole), 3.85 (s, 3H, OCH3), 3.54 (s, 2H, CH2), 2.32 (s, 3H, CH3), 2.05 (s, vbr, 9H, CH/CH2 adamantyl), 1.72 (s, vbr, 6H, CH2 adamantyl), 1.41 (s, 9H, C(CH3)3); 13C{1H} NMR (CDCl3, ppm): 185.9 (CO), 170.7 (CO), 154.7 (CindoleO), 134.1 (Cindole), 130.2 (Cindole), 129.2 (Cindole), 112.5 (CindoleH), 111.3 (CindoleH), 108.6 (Cindole), 100.8 (CindoleH), 80.7 (C(CH3)3), 55.7 (OCH3), 46.7 (Cadamantyl), 39.3 (CH2 adamantyl), 36.2 (CH2 adamantyl), 31.9 (CH2), 28.1 (C(CH3)3), 28.0 (CH adamantyl), 11.9 (CH3); IR (selected, KBr, cm−1): ν̃ = 2985 (m, ν(C–H)), 2934 (m, ν(C–H)), 2854 (m), 2362 (w), 2344 (w), 1720 (m, ν(C=O)).
4.1.2.3. Cleavage of the tert-butyl ester group
General procedure for 3o, m, p
The respective tert-butyl ester 3 was dissolved in dichloromethane (1.5 mL/100 mg ester) and then TFA (0.5 mL/100 mg ester) was added and the solution stirred for 3 h at room temperature. Dichloromethane and excess of TFA were removed under reduced pressure. Recrystallization from dichloromethane yielded the products 4 as white solids.
1-(1-Carboxy-1,2-dicarba-closo-dodecaboran(12)yl)-5-methoxy-2-methyl-1H-indole-3-acetic acid (4o)
Yield: 0.14 g (92% from 0.16 g 3o); elemental analysis calcd. (%) for C15H23B10NO4: C 46.26; H 5.95; found: C 46.04; H 6.00; m.p.: 190–191 °C; ESI MS (−) (CH3COCH3): m/z: 777.6 (100%, [2M–H]−); 1H NMR (CDCl3, ppm): 7.31 (d, 3JHH = 8 Hz, 1H, CHindole), 6.93 (d, 4JHH = 2 Hz, 1H, CHindole), 6.86 (dd, 3JHH = 8 Hz, 4JHH = 2 Hz, 1H, CHindole), 4.35 (s, 1H, CclusterH), 3.84 (s, 3H, OCH3), 3.65 (s, 2H, CH2), 3.37-1.50 (m, vbr, 10H, C2H10B10), 2.30 (s, 3H, CH3); 11B NMR (CDCl3, ppm): −2.4 (d, 1JBH = 141 Hz, C2H10B10), −8.5 (d, 1JBH = 141 Hz, C2H10B10), −12.0 (vbr, C2H10B10), −12.6 (vbr, C2H10B10); 13C NMR (CDCl3, ppm): 175.9 (s, vbr, CO), 166.6 (s, CO), 156.0 (s, vbr, CindoleO), 135.3 (s, vbr, Cindole), 130.1 (s, vbr, Cindole), 129.9 (s, vbr, Cindole), 113.3 (d, 1JCH = 161 Hz, CindoleH), 112.2 (dd, 1JCH = 161 Hz, 2JCH = 5 Hz, CindoleH), 110.5 (s, Cindole), 101.4 (dd, 1JCH = 161 Hz, 2JCH = 5 Hz, CindoleH), 74.5 (m, vbr, Ccluster), 59.2 (d, vbr, 1JCH = 191 Hz, CclusterH), 55.8 (q, 1JCH = 141 Hz, OCH3), 29.9 (t, 1JCH = 131 Hz, CH2), 11.3 (q, 1JCH = 131 Hz, CH3); IR (selected, KBr, cm−1): ν̃ = 3397 (w), 3068 (w), 3049 (m), 3009 (w), 2949 (m), 2913 (m), 2844 (w), 2729 (w), 2622 (m, ν(B–H)), 2578 (s, ν(B–H)), 1720 (s, ν(C=O)), 1706 (s, ν(C=O)). Structural data for 4o obtained from dichloromethane at 4 °C as yellow prisms: C15H23B10NO4, Mr = 389.44, monoclinic, space group P21/c, a = 1078.97(6), b = 1681.67(7), c = 1200.15(7) pm, β = 112.316(7)°, T = 130 K, V = 2.015(1) nm3, Z = 4, ρcalcd = 1.284 Mg m−3, μ = 0.080 mm−1, 3.04 < θ < 30.51°, R = 0.0473, wR = 0.0722, GOF = 0.726.
1-(1-Carboxy-1,7-dicarba-closo-dodecaboran(12)yl)-5-methoxy-2-methyl-1H-indole-3-acetic acid (4m)
Yield: 0.12 g (98% from 0.14 g 3m); elemental analysis calcd. (%) for C15H23B10NO4: C 46.26; H 5.95; found: C 46.20; H 5.96; m.p.: 154–156 °C; ESI MS (−) (CH3COCH3/NH3): m/z: 777.5 (100%, [2M–H]−), 388.2 (35%, [M–H]−), 344.3 (81%, [M–CO2H]−); 1H NMR (CDCl3, ppm): 7.25 (d, 3JHH = 8 Hz, 1H, CHindole), 6.91 (d, 4JHH = 2 Hz, 1H, CHindole), 6.82 (dd, 3JHH = 8 Hz, 4JHH = 2 Hz, 1H, CHindole), 3.83 (s, 3H, OCH3), 3.63 (s, 2H, CH2), 3.48-1.51 (m, vbr, 10H, C2H10B10), 3.06 (s, 1H, CclusterH), 2.26 (s, 3H, CH3); 11B NMR (CDCl3, ppm): −4.7 (vbr, 2B, C2H10B10), −10.4 (d, 1JBH = 141 Hz, 4B, C2H10B10), −13.1 (d, 1JBH = 180 Hz, 2B, C2H10B10), −15.2 (d, 1JBH = 180 Hz, 2B, C2H10B10); 13C NMR (CDCl3, ppm): 176.8 (s, vbr, CO), 167.3 (s, CO), 155.6 (s, vbr, CindoleO), 135.1 (s, vbr, Cindole), 130.4 (s, vbr, Cindole), 129.3 (s, vbr, Cindole), 112.9 (d, 1JCH = 161 Hz, CindoleH), 111.9 (dd, 1JCH = 161 Hz, 2JCH = 5 Hz, CindoleH), 109.1 (s, vbr, Cindole), 101.0 (d, 1JCH = 161 Hz, 2JCH = 5 Hz, CindoleH), 78.0 (s, vbr, Ccluster), 55.8 (q, 1JCH = 141 Hz, OCH3), 55.0 (d, 1JCH = 181 Hz, CclusterH), 30.1 (t, 1JCH = 131 Hz, CH2), 11.3 (q, 1JCH = 131 Hz, CH3); IR (selected, KBr, cm−1): ν̃ = 3228 (m), 3057 (m, ν(C–H)), 2962 (w), 2839 (w), 2611 (s, ν(B–H)), 1718 (s, ν(C=O)). Structural data for 4m obtained from n-pentane at 25 °C as yellow prisms: C15H23B10NO4, Mr = 389.44, monoclinic, space group P21/c, a = 863.2(5), b = 2180.5(5), c = 1081.7(5) pm, β = 103.147(5)°, T = 130 K, V = 1.983(2) nm3, Z = 4, ρcalcd = 1.301 Mg m−3, μ = 0.081 mm−1, 2.60 < θ < 30.51°, R = 0.0434, wR = 0.1115, GOF = 0.942.
1-(1-Carboxy-1,12-dicarba-closo-dodecaboran(12)yl)-5-methoxy-2-methyl-1H-indole-3-acetic acid (4p)
Yield: 0.21 g (> 99% from 0.24 g 3p); elemental analysis calcd. (%) for C15H23B10NO4: C 46.26; H 5.95; found: C 46.04; H 5.91; m.p.: 93–95 °C; ESI MS (−) (CH3COCH3): m/z: 777.5 (100%, [2M–H]−); 1H NMR (CDCl3, ppm): 7.13 (d, 3JHH = 8 Hz, 1H, CHindole), 6.89 (d, 4JHH = 2 Hz, 1H, CHindole), 6.80 (d, 3JHH = 8 Hz, 4JHH = 2 Hz, 1H, CHindole), 3.82 (s, 3H, OCH3), 3.60 (s, 2H, CH2), 3.36-1.47 (m, vbr, 10H, C2H10B10), 2.90 (s, 1H, CclusterH), 2.17 (s, 3H, CH3); 11B NMR (CDCl3, ppm): −13.0 (d, 1JBH = 154 Hz, 5B, C2H10B10), −15.1 (d, 1JBH = 181 Hz, 5B, C2H10B10); 13C NMR (CDCl3, ppm): 176.8 (s, vbr, CO), 167.9 (s, CO), 155.4 (s, vbr, CindoleO), 135.0 (s, vbr, Cindole), 130.5 (s, vbr, Cindole), 129.0 (s, vbr, Cindole), 112.7 (d, 1JCH = 151 Hz, CindoleH), 111.8 (dd, 1JCH = 161 Hz, 2JCH = 5 Hz, CindoleH), 108.6 (s, vbr, Cindole), 100.8 (dd, 1JCH = 161 Hz, 2JCH = 5 Hz, CindoleH), 85.2 (s, vbr, Ccluster), 63.9 (d, 1JCH = 181 Hz, CclusterH), 55.8 (q, 1JCH = 141 Hz, OCH3), 30.0 (t, 1JCH = 131 Hz, CH2), 11.1 (q, JCH = 131 Hz, CH3); IR (selected, KBr, cm−1): ν̃ = 3430 (w), 3061 (w), 3010 (w), 2926 (w), 2834 (w), 2621 (s, ν(B–H)), 1709 (s, ν(C=O)). Structural data for 4p obtained from n-pentane at 25 °C as yellow prisms: C15H23B10NO4·0.5(C5H12) Mr = 425.25, monoclinic, space group C2/c, a = 2464.7(1), b = 813.58(4), c = 2306.98(8) pm, β = 94.791(6)°, T = 130 K, V = 4.6098(4) nm3, Z = 8, ρcalcd = 1.226 Mg m−3, μ = 0.075 mm−1, 2.46 < θ < 25.35°, R = 0.0483, wR = 0.1116, GOF = 0.898.
4.1.2.4. Cleavage of the benzyl ester group
General procedure for 2a, m, p
The respective benzyl ester 2 was dissolved in THF (3.5 mL/100 mg ester) and Pd (5 mol% on carbon) was added. The mixture was stirred for 24 h under H2 atmosphere (10 bar) at room temperature. The solution was separated from the catalyst (either by normal filtration or through silica with hexanes/ethyl acetate) and concentrated in vacuo.
1-(1-Adamantyl)-5-methoxy-2-methyl-1H-indole-3-acetic acid (4a)
Yield: 0.13 g (80% from 0.20 g 2a); elemental analysis calcd. (%) for C23H27NO4: C 72.42; H 7.13; found: C 71.76; H 7.16; m.p.: 99–100 °C; ESI MS (−) (CH3COCH3/NH3): m/z: 380.1 (100%, [M–H]−); 1H NMR (CDCl3, ppm): 7.14 (d, 3JHH = 8 Hz, 1H, CHindole), 6.95 (d, 4JHH = 2 Hz, 1H, CHindole), 6.79 (dd, 3JHH = 8 Hz, 4JHH = 2 Hz, 1H, CHindole), 3.84 (s, 3H, OCH3), 3.65 (s, 2H, CH2), 2.31 (s, 3H, CH3), 2.05 (s, vbr, 9H, CH/CH2 adamantyl), 1.72 (s, vbr, 6H, CH2 adamantyl); 13C{1H} NMR (CDCl3, ppm): 185.9 (CO), 176.8 (CO), 154.8 (CindoleO), 134.6 (Cindole), 130.2 (Cindole), 128.9 (Cindole), 112.6 (CindoleH), 111.4 (CindoleH), 107.0 (Cindole), 100.5 (CindoleH), 55.8 (OCH3), 46.6 (Cadamantyl), 39.3 (CH2 adamantyl), 36.2 (CH2 adamantyl), 30.1 (CH2), 28.0 (CH adamantyl), 11.7 (CH3); IR (selected, KBr, cm−1): ν̃ = 3420 (m), 2907 (s, ν(C–H)), 2851 (m), 1707 (s, ν(C=O)), 1688 (s, ν(C=O)).
4.2. COX Inhibition Studies (Determination of IC50 values)
Concentration-dependent inhibition reactions were performed by pre-incubating the inhibitor and enzyme for 17 min at 25 °C, followed by 3 min at 37 °C prior to the addition of 50 μM [14C]-AA for 30 sec at 37 °C. All assays were terminated and analyzed for substrate consumption by TLC as previously described.44 All inhibitor concentrations for 50% enzyme activity (IC50) were determined graphically using Prism and were the average of at least two independent determinations.
Supplementary Material
Acknowledgments
This work was supported by the Studienstiftung des Deutschen Volkes (doctoral grant for M.S.), the Graduate School of Excellence “Building with Molecules and Nano-objects (BuildMoNa)” funded by the Deutsche Forschungsgemeinschaft and by a research grant from the National Institutes of Health (CA89450).
Footnotes
Supplementary data associated with this article (synthesis of 5-Methoxy-2-methyl-1H-indole-3-acetic acid, 1,2-, 1,7-, and 1,12-dicarba-closo-dodecaborane(12)-1-carbonyl chloride and esters of indomethacin (1i-3i) and full IR data) can be found in the online version at doi:xxxx.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hart FD, Boardman PL. Br Med J. 1963;2:965. doi: 10.1136/bmj.2.5363.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kistenmacher TJ, Marsh RE. J Am Chem Soc. 1972;94:1340. doi: 10.1021/ja00759a047. [DOI] [PubMed] [Google Scholar]
- 3.Vane JR. Nat New Biol. 1971;231:232. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 4.Ferreira SH, Moncada S, Vane JR. Nat New Biol. 1971;231:237. doi: 10.1038/newbio231237a0. [DOI] [PubMed] [Google Scholar]
- 5.Simmons DL, Botting RM, Hla T. Pharmacol Rev. 2004;56:387. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell JA, Warner TD. Nat Rev Drug Discov. 2006;5:75. doi: 10.1038/nrd1929. [DOI] [PubMed] [Google Scholar]
- 7.Fitzpatrick FA. Curr Pharm Des. 2004;10:577. doi: 10.2174/1381612043453144. [DOI] [PubMed] [Google Scholar]
- 8.Hinz B, Brune K. J Pharmacol Exp Ther. 2002;300:367. doi: 10.1124/jpet.300.2.367. [DOI] [PubMed] [Google Scholar]
- 9.Chandrasekharan NV, Simmons DL. Genome Biol. 2004;5:241. doi: 10.1186/gb-2004-5-9-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh N, Chaki R, Mandal V, Mandal SC. Pharmacol Rep. 2010;62:233. doi: 10.1016/s1734-1140(10)70262-0. [DOI] [PubMed] [Google Scholar]
- 11.Williams CS, Mann M, DuBois RN. Oncogene. 1999;18:7908. doi: 10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- 12.Kalgutkar AS, Marnett AB, Crews BC, Remmel RP, Marnett LJ. J Med Chem. 2000;43:2860. doi: 10.1021/jm000004e. [DOI] [PubMed] [Google Scholar]
- 13.Pouplana R, Lozano JJ, Ruiz J. J Mol Graph Model. 2002;20:329. doi: 10.1016/s1093-3263(01)00133-4. [DOI] [PubMed] [Google Scholar]
- 14.Kurumbail RG, Stevens AM, Gierse JK, McDonald JJ, Stegeman RA, Pak JY, Gildehaus D, Miyashiro JM, Penning TD, Seibert K, Isakson PC, Stallings WC. Nature. 1996;384:644. doi: 10.1038/384644a0. [DOI] [PubMed] [Google Scholar]
- 15.Michaux C, Charlier C. Mini Rev Med Chem. 2004;4:603. doi: 10.2174/1389557043403756. [DOI] [PubMed] [Google Scholar]
- 16.Black WC, Bayly C, Belley M, Chan CC, Charleson S, Denis D, Gauthier JY, Gordon R, Guay D, Kargman S, Lau CK, Leblanc Y, Mancini J, Ouellet M, Percival D, Roy P, Skorey K, Tagari P, Vickers P, Wong E, Xu L, Prasit P. Bioorg Med Chem Lett. 1996;6:725. [Google Scholar]
- 17.Olgen S, Akaho E, Nebioglu D. Eur J Med Chem. 2001;36:747. doi: 10.1016/s0223-5234(01)01258-2. [DOI] [PubMed] [Google Scholar]
- 18.Maguire AR, Plunkett SJ, Papot S, Clynes M, O’Connor R, Touhey S. Bioorg Med Chem. 2001;9:745. doi: 10.1016/s0968-0896(00)00292-3. [DOI] [PubMed] [Google Scholar]
- 19.Touhey S, O’Connor R, Plunkett S, Maguire A, Clynes M. Eur J Cancer. 2002;38:1661. doi: 10.1016/s0959-8049(02)00128-4. [DOI] [PubMed] [Google Scholar]
- 20.Kalgutkar AS, Crews BC, Saleh S, Prudhomme D, Marnett LJ. Bioorg Med Chem. 2005;13:6810. doi: 10.1016/j.bmc.2005.07.073. [DOI] [PubMed] [Google Scholar]
- 21.Loll PJ, Picot D, Ekabo O, Garavito RM. Biochemistry. 1996;35:7330. doi: 10.1021/bi952776w. [DOI] [PubMed] [Google Scholar]
- 22.Hermansson K, Wojcik M, Sjoberg S. Inorg Chem. 1999;38:6039. doi: 10.1021/ic990381l. [DOI] [PubMed] [Google Scholar]
- 23.(a) Schleyer PvR, Najafian K. Inorg Chem. 1998;37:3454. doi: 10.1021/ic980110v. [DOI] [PubMed] [Google Scholar]; (b) Scholz M, Bensdorf K, Gust R, Hey-Hawkins E. ChemMedChem. 2009;4:746. doi: 10.1002/cmdc.200900072. [DOI] [PubMed] [Google Scholar]; (c) Scholz M, Kaluerovi GN, Kommera H, Paschke R, Will J, Sheldrick WS, Hey-Hawkins E. Eur J Med Chem. 2011;46:1131. doi: 10.1016/j.ejmech.2011.01.030. [DOI] [PubMed] [Google Scholar]; (d) Scholz M, Hey-Hawkins E. Chem Rev. 2011;111:7035. doi: 10.1021/cr200038x. [DOI] [PubMed] [Google Scholar]
- 24.Bregadze VI. Chem Rev. 1992;92:209. [Google Scholar]
- 25.Valliant JF, Guenther KJ, King AS, Morel P, Schaffer P, Sogbein OO, Stephenson KA. Coord Chem Rev. 2002;232:173. [Google Scholar]
- 26.Armstrong AF, Valliant JF. Dalton Trans. 2007:4240. doi: 10.1039/b709843j. [DOI] [PubMed] [Google Scholar]
- 27.Hawthorne MF, Berry TE, Wegner PA. J Am Chem Soc. 1965;87:4746. doi: 10.1021/ja00949a014. [DOI] [PubMed] [Google Scholar]
- 28.Kalinin V, Ol’shevskaya V. Russ Chem Bull. 2008;57:815. [Google Scholar]
- 29.Sheehan JC, Daves GD. J Org Chem. 1964;29:2006. [Google Scholar]
- 30.Felix AM. J Org Chem. 1974;39:1427. [Google Scholar]
- 31.Salomon CJ, Mata EG, Mascaretti OA. J Org Chem. 1994;59:7259. [Google Scholar]
- 32.Nicolaou KC, Estrada AA, Zak M, Lee SH, Safina BS. Angew Chem Int Ed. 2005;44:1378. doi: 10.1002/anie.200462207. [DOI] [PubMed] [Google Scholar]
- 33.Candida Antarctica A, Candida Antarctica B, Rhizomucor Miehei Lipozyme 1, Candida Rugosa, Thermomyces Lanuginosa Lipolase 1, Candida Cylindracea, Pseudomonas Cepacia Amano PS2, Pseudomonas Fluorescens Amano AK2, Rhizopus Oryzae Amano F-AP152, Mucor Javanicus Amano M2, on different solid supports.
- 34.Diago-Meseguer J, Palomo-Coll AL, Fernández-Lizarbe JR, Zugaza-Bilbao A. Synthesis. 1980:547. [Google Scholar]
- 35.Taber DF, Gerstenhaber DA, Zhao X. Tetrahedron Lett. 2006;47:3065. [Google Scholar]
- 36.Gibson FS, Park MS, Rapoport H. J Org Chem. 1994;59:7503. [Google Scholar]
- 37.Kasar RA, Knudsen GM, Kahl SB. Inorg Chem. 1999;38:2936. doi: 10.1021/ic990037o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kocienski PJ. Protecting Groups. Thieme Medical Publishers; New York: 1994. [Google Scholar]
- 39.Hess S, Teubert U, Ortwein J, Eger K. Eur J Pharm Sci. 2001;14:301. doi: 10.1016/s0928-0987(01)00198-1. [DOI] [PubMed] [Google Scholar]
- 40.Scholz M, Blobaum AL, Marnett LJ, Hey-Hawkins E. Bioorg Med Chem. 2011;19:3242. doi: 10.1016/j.bmc.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Altomare A, Cascarano G, Giacovazzo C, Guagliardi A. J Appl Crystallogr. 1993;26:343. [Google Scholar]
- 42.SCALE3 ABSPACK: Empirical absorption correction, C. S.; package, O. D. L. Oxford (UK): 2006. [Google Scholar]
- 43.Sheldrick GM. SHELXL-97, Program for the Refinement of Crystal Structures. University of Göttingen; Göttingen (Germany): 1997. [Google Scholar]
- 44.Kalgutkar AS, Crews BC, Rowlinson SW, Marnett AB, Kozak KR, Remmel RP, Marnett LJ. Proc Natl Acad Sci USA. 2000;97:925. doi: 10.1073/pnas.97.2.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.