Abstract
Hormone concentrations decline with aging. Up to now it was not clear, whether the decrease of hormone concentrations in blood samples are also present in cutaneous suction blister fluids, and whether skin from different anatomical sites shows different hormone concentrations.
Analysis of suction blister fluids and paired blood samples from young (mean 27.8 y) and old (mean 62.6 y) male subjects by UPLC-MS/MS showed that DHEA concentration in blood samples was age-dependently significantly reduced, but increased in suction blister fluids, while androstenedione behaved in an opposite manner to DHEA. Testosterone decreased age-dependently in blood samples and in suction blister fluids. Regarding skin sites, DHEA was lower in samples from upper back compared with samples from the forearm. In contrast, the concentrations of androstenedione and testosterone were higher in samples from upper back.
In vitro analyses showed that SZ95 sebocytes, but neither primary fibroblasts nor keratinocytes, were able to use DHEA as precursor for testosterone biosynthesis, which was confirmed by expression analysis of 3β-hydroxysteroiddehydrogenase in skin biopsies.
In conclusion, we show an inverse pattern of DHEA and androstenedione concentrations in blood vs. suction blister fluids, highlighting age-dependent changes of dermal testosterone biosynthesis, and a stronger metabolism in young skin. Furthermore, sebocytes play a central role in cutaneous androgen metabolism.
Introduction
Human skin is both, a hormone-sensitive organ and a peripheral tissue, able to synthesize hormones by itself.1,2 Testosterone is the most important androgen in males but it is also synthesized at significant levels in females at all ages.3 Testosterone can be converted to estradiol, which is an estrogen, synthesized by enzymatic aromatization in peripheral tissues.4,5 The concentration of estradiol in blood is much lower than that of testosterone.6,7 Both steroid hormones, testosterone and estradiol, are able to influence the appearance of human skin by inducing the synthesis of collagens, which preserve the structural integrity of the extracellular matrix and therefore help to maintain a youthful skin appearance characterized by a thick and elastic dermis without wrinkles.8-10
Testosterone is mainly synthesized by the gonads and to a lesser extent by the adrenals always using dehydroepiandrosterone (DHEA) as a precursor.11 As a consequence of age-dependent diminished metabolic productivity of steroidogenic organs, the levels of DHEA and testosterone in blood decline with advancing age.12,13 These age-dependent changes in hormone concentrations can aggravate the visible signs of skin aging such as wrinkles and sagging.9,10,14
For testosterone synthesis using DHEA as a precursor only two key enzymes are needed.15,16 The first step is the oxidative conversion of the hydroxyl group at the third carbon atom of DHEA by the 3β-hydroxysteroiddehydrogenase (3β-HSD), leading to the formation of androstenedione. Subsequently, the keto group at the 17th carbon atom of androstenedione is reduced by 17β-hydroxysteroiddehydrogenase (17β-HSD), resulting in the active testosterone. Aromatization of testosterone by aromatase (CYP19A1) leads to the generation of estradiol.17
It has been reported previously that significant amounts of testosterone can be derived from DHEA by local synthesis within human skin itself.18,19 Sebocytes seem to be important mediators in this process because the existence of all needed enzymes for testosterone metabolism has been confirmed.2,20-22
Up to now, the potential age dependence of testosterone biosynthesis in human skin in relation to its precursors, to the skin site and with respect to the age-dependent endocrine changes in plasma has not been investigated. To address this question, the concentrations of DHEA, androstenedione and testosterone were measured in blood and suction blister fluids of male volunteers. Suction blister fluid is mainly derived from the interstitial fluid of the skin. Its composition represents most of the components of serum at lower concentrations, but also locally generated metabolites.23,24 Because of this property, analysis of suction blister fluid composition is a powerful tool to determine local hormone concentrations in human skin, and to compare them with systemic hormone concentrations in parallel blood samples. Our data provide valuable new insights into the cutaneous mechanisms of sex-hormone metabolism.
Results
Influence of age on steroid hormone concentrations in blood and suction blister fluid of male subjects
To investigate age-dependent differences of steroid hormone concentrations in blood and suction blister fluid (SBF), an in vivo study was performed. The study was approved by the ethical committee of the University of Freiburg, Germany, and conducted in accordance to the Declaration of Helsinki. Concentrations of DHEA, androstenedione and testosterone in blood samples and SBF derived from the upper back of young (27.8 y) and old (62.6 y) male subjects were determined by UPLC-MS/MS after solid phase extraction.3
As shown in Figure 1, the concentrations of DHEA, androstenedione and testosterone were differently influenced by age. The concentration of DHEA in blood samples of young subjects (Median = 46.8 nM, interquartile range (IQR) = 39.8 nM) was significantly higher (p = 0.016) than in those derived from old subjects (Median = 21.6 nM, IQR = 7.4 nM) (Fig. 1A). On the contrary, DHEA concentration in SBF from young male subjects (median 9.0 nM, IQR = 16.9 nM) was less than half of the concentration of old male subjects (median 22.6 nM, IQR = 27.5 nM) (Fig. 1B). An inverse age pattern was seen for the androstenedione concentrations. The median androstenedione concentration was only 1.2 nM (IQR = 1.8 nM) in blood samples of young subjects (Fig. 1C), whereas a median concentration of 2.5 nM (IQR = 0.9) could be measured in the samples of old male subjects. In SBF of young male subjects the median androstenedione concentration was 1.2 nM (IQR = 1.1 nM), which was two fold higher than in samples of old male subjects (median = 0.6, IQR = 0.2 nM) (Fig. 1D).
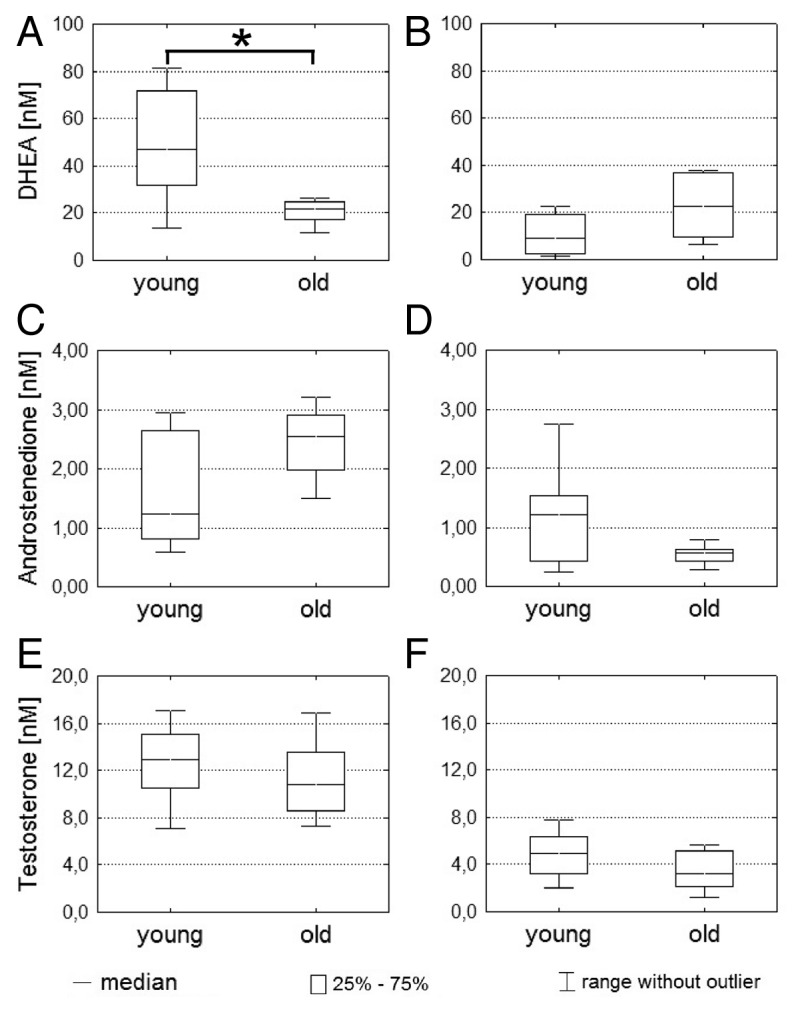
Figure 1. Determination of steroid hormone concentrations in blood and suction blister fluids. (A–F) Concentrations of DHEA, androstenedione and testosterone in blood and suction blister fluids of young (n = 8) and old male (n = 8) subjects were determined by UPLC-MS/MS after solid phase extraction. Blood concentrations of DHEA, androstenedione and testosterone are shown on the left side (A, C and E) and corresponding SBF concentrations on the right side (B, D and F). Statistical significance differences were marked with an asterisk (p < 0.05).
The concentration of testosterone in blood samples declined age-dependently from 12.9 nM (IQR = 4.5 nM) to 10.8 nM (IQR = 4.9 nM) (Fig. 1E). In SBF, testosterone concentration was age-dependently reduced by 35%, from 4.9 nM (IQR = 3.2 nM) to 3.2 nM (IQR = 3.0 nM) (Fig. 1F).
Influence of skin site on steroid hormone concentrations
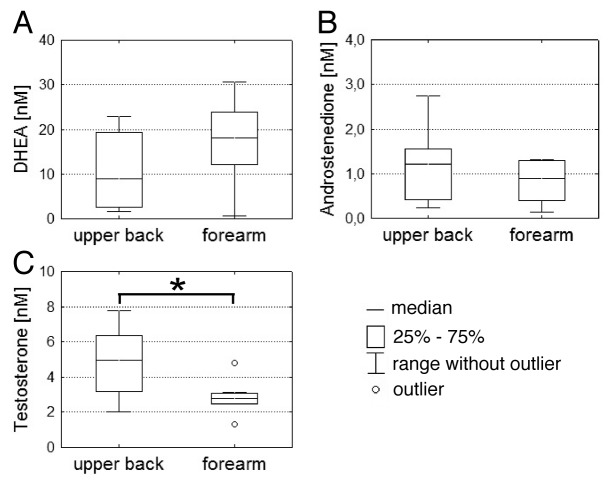
In order to assess the influence of skin site on dermal hormone levels, suction blisters of young male volunteers were collected from the upper back and the forearm of the same subject in parallel. In Figure 2, the concentrations of DHEA, androstenedione and testosterone are shown.
Figure 2. Steroid hormone concentrations in SBF from different skin sites. (A–C) SBF from upper back (n = 8) and forearm (n = 8) of young male subjects were generated in parallel. Concentrations of DHEA, androstenedione and testosterone were subsequently determined by UPLC-MS/MS after solid phase extraction. Statistical significance differences were marked with an asterisk (p < 0.05).
Depending on the skin site the concentrations of steroid hormones were differently altered. The concentrations of DHEA (Fig. 2A) were lower in SBF collected from the upper back compared with the forearm (median = 9.0 nM, IQR = 16.9 nM vs. 18.1 nM, IQR = 11.7 nM). In contrast, the concentrations of androstenedione (Fig. 2B) and testosterone (Fig. 2C) were higher in SBF from upper back compared with the forearm. For androstenedione there was only a slight concentration difference between SBF from the upper back (median 1.2 nM, IQR = 1.1 nM) and SBF from the forearm (0.9 nM, IQR = 0.9 nM), which was again not significantly different. Of note, the testosterone concentration in SBF from the upper back was 4.9 nM (IQR = 3.2 nM), which was significantly (p = 0.024) higher than in SBF from the forearm, where only 2.8 nM (IQR = 0.6 nM) of testosterone could be measured.
Testosterone metabolism in human skin cells
To investigate whether skin cells are able to synthesize androstenedione and testosterone from DHEA, primary fibroblasts, keratinocytes and the sebaceous gland cell line SZ95 were treated with 100 nM DHEA. After 24 h, supernatants of treated cells were collected and steroid hormones present in the supernatant were isolated by solid phase extraction and analyzed by UPLC-MS/MS.3
As shown in Table 1, only SZ95 sebocytes were able to use DHEA as precursor for synthesis of androstenedione and testosterone. In the supernatant of the SZ95 sebocytes, androstenedione accounts for 43.1% (+/− 4.0%) and testosterone for 31.5% (+/− 49.4%) of all detected steroid hormones, showing that the added precursor DHEA is used for generation of androstenedione and testosterone, with only 22.2% (+/− 13.0%) of DHEA being left after 24 h of hormone incubation.
Table 1. Relative steroid hormone concentrations in cell culture supernatants after DHEA treatment.
| DHEA | Androstenedione | Testosterone | |
|---|---|---|---|
|
SZ95-Sebocytes |
22,2% |
43,1% |
31,5% |
|
Fibroblasts |
94,4% |
0,2% |
0% |
| Keratinocytes | 98,0% | 0,4% | 0% |
Relative concentrations of DHEA, androstenedione and testosterone in supernatants from SZ95 sebocytes (n = 3), primary fibroblasts (n = 7) and primary keratinocytes (n = 4) 24 h after addition of DHEA. Sum of all detected steroid hormones were set to 100% for normalization of results.
Primary fibroblasts and keratinocytes did not use DHEA as precursor for generating the active androgens androstenedione and testosterone. Nearly the complete added amount (94.4% +/− 3.2% for fibroblasts and 98% +/− 0.7% for keratinocytes) of the initially added DHEA was still detectable after 24 h of incubation.
Expression of 3β-HSD type I enzyme in vitro
The initial step in generating testosterone using DHEA as precursor is the oxidative conversion of DHEA to androstenedione, which is catalyzed by the 3β-HSD enzyme. In contrast to the adrenals, where the 3β-HSD type II enzyme is expressed, the skin expresses the type I isoenzyme.25 Using qPCR-analysis, 3β-HSD type I transcription in SZ95 sebocytes could be confirmed. In contrast, neither primary fibroblasts nor keratinocytes showed any detectable signal for 3β-HSD type I mRNA transcription (data not shown).
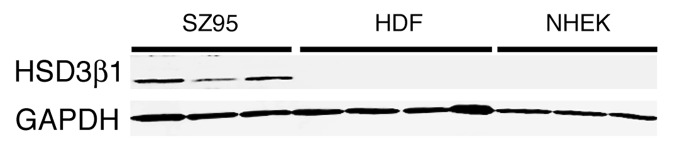
To confirm the qPCR-results, protein expression of the 3β-HSD type I enzyme was studied by western blot analysis. As shown in Figure 3, only the cell lysates of the SZ95 sebocytes showed a specific immunoreactivity for the 3β-HSD type I protein using a specific antibody. No immunoreactivity for 3β-HSD type I could be observed in cell lysates of primary fibroblasts and keratinocytes.
Figure 3. 3β-HSD type I expression in vitro. Confluent cultured SZ95 sebocytes (SZ95), primary dermal fibroblasts (HDF) and primary epidermal keratinocytes (NHEK) were harvested. Total protein fractions were isolated and subjected to western blot analysis using an anti 3β-HSD type I antibody. An anti-GAPDH antibody served as loading control.
Expression of 3β-HSD type I enzyme in vivo
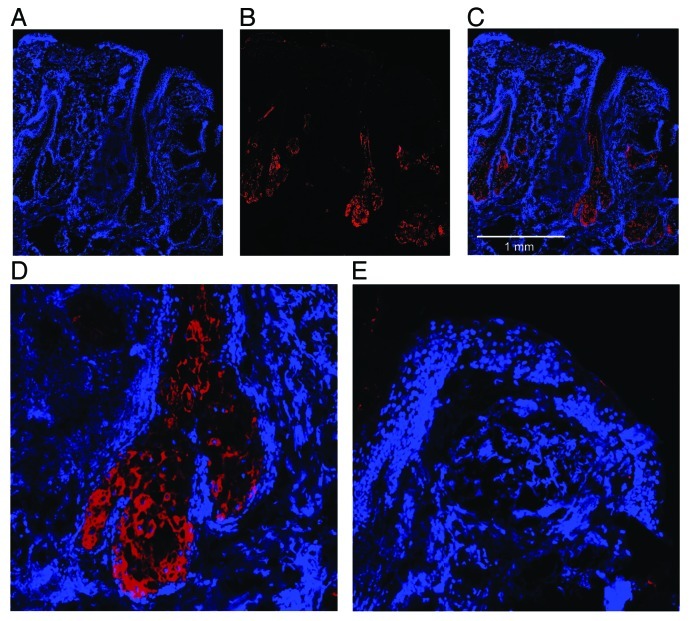
To confirm the role of sebocytes in cutaneous androstenedione metabolism from DHEA, 3β-HSD type I expression was visualized by cryosections of male scalp skin. A representative stain of 3β-HSD type I immunoreactivity is shown in Figure 4. 3β-HSD type I immunoreactivity was mainly limited to sebaceous glands. A weak staining was also noticeable in the lower parts of the hair follicle duct (Fig. 4B–C). However, the magnified views of a sebaceous gland (Fig. 4D) and the epidermis with upper parts of the hair follicle duct (Fig. 4E) confirmed that 3β-HSD type I expression is limited to sebaceous glands.
Figure 4. 3β-HSD type I expression in vivo. Skin biopsies of human scalp skin were cryosectioned. DAPI staining (A) was used to visualize histological structures. Positive 3β-HSD type I immunoreactivity was confirmed using an anti-3β-HSD type I antibody (B). A merge image (C) of DAPI staining and 3β-HSD type I immunoreactivity showed that the immunoreactivity was restricted to sebaceous glands and lower parts of the hair follicle duct, which is confirmed by a magnification of a sebaceous gland (D) and epidermis (E).
Discussion
Steroid hormone concentrations are influenced by age
Our in vivo study shows that the age-dependent concentrations of DHEA and androstenedione in SBF and blood samples behave in an opposite manner. We could confirm previous studies showing significantly decreasing DHEA and testosterone levels in blood samples during the aging process.26,27 The observed age-dependent decrease of DHEA levels in blood samples of old male subjects has been attributed to reduced systemic metabolism.11,28 In sharp contrast to the blood data, the dermal concentrations of DHEA are elevated in SBF of old male subjects compared with SBF of young male subjects.
Androstendione levels behave in an opposite manner since there is an age-dependent increase of androstendione in blood and a decrease in SBF. The percental change of dermal testosterone concentration between samples of young and old male subjects is even greater in SBF than in blood (35% vs. 16%), showing a higher local testosterone decline in skin of aged male subjects compared with blood.
The inverse age-dependent androgen patterns in blood vs. skin support the concept of an independent androgen metabolism in the skin undergoing separate skin-specific age-dependent changes. There seems to be an increased metabolism of DHEA to androstenedione and subsequently to the active downstream product testosterone in young skin vs. old skin. Therefore, young skin is able to use DHEA more efficiently for the synthesis of androgens than aged skin.
Steroid hormone concentrations are influenced by skin site
In order to analyze whether the cutaneous steroid hormone metabolism is also altered by topological features, such as the difference of sebaceous gland density, SBF from upper back and forearm were taken in parallel. Upper back and forearm were chosen, because these skin sites contain a different sebaceous gland distribution, with a higher density of sebaceous glands in the upper back, and a sebaceous gland scarcity in forearm skin.29,30
The analysis of steroid hormone concentrations shows that the dermal concentration of DHEA is lower in SBF from upper back compared with the forearm, but concentrations of the metabolic products of DHEA, androstenedione and testosterone, were higher in SBF from upper back. These two characteristic steroid hormone patterns indicate a higher metabolism from DHEA to androstenedione and testosterone in the upper back compared with the forearm. Because of the fact that all samples were taken in parallel, our data show that cutaneous hormone metabolism is clearly influenced by skin site, and not only by the concentrations of steroids in the blood. The observed differences in hormone concentrations between the upper back, which is rich in sebaceous glands, and the forearm with only sparse sebaceous glands, supports the published hypothesis, that sebocytes play a key role in the cutaneous androgen metabolism, and local androstenedione and testosterone allocation.20,31
Sebocytes generate testosterone using DHEA as a precursor
To analyze whether sebaceous glands have a role for the observed skin site-specific differences in generation of androstenedione and testosterone, the metabolism of testosterone using DHEA as precursor was analyzed in vitro. The experiments showed that only SZ95 sebocytes, but not primary fibroblasts or keratinocytes were able to use DHEA as a precursor for generating androstenedione and testosterone. In contrast, if androstenedione was used as testosterone precursor, generation of testosterone could be observed in the cell culture supernatants of SZ95 sebocytes, fibroblasts and keratinocytes (data not shown), confirming results indicating that 17β-HSD is expressed by all of these skin cell types.32
To investigate these findings further, the expression of the 3β-HSD type I enzyme was verified by qPCR-analysis and western blots. As anticipated, 3β-HSD type I enzyme was only detectable in samples of SZ95 sebocytes, but not in samples of primary fibroblasts and keratinocytes. To verify the in vivo function of sebaceous glands in cutaneous androstenedione generation, the localization of 3β-HSD type I enzyme was visualized by immunofluorescence stainings of facial skin biopsies. As predicted by qPCR-Analysis and western blots of cell cultures, 3β-HSD type I immunoreactivity was limited to sebaceous glands. The observed weak staining of lower parts of the hairfollicle duct could be due to the fact that sebocytes release their cytoplasm, containing 3β-HSD type I, into the duct after holocrine lysis.33,34 Hence, our data confirm the previous published in vitro results, that sebaceous glands are important in the initial step of cutaneous testosterone metabolism by generating androstenedione from DHEA.16,20,31
Human skin is an endocrine tissue and also a target of the produced hormones itself.1,2 In this respect, cutaneously produced testosterone could be one factor that induces sebocyte proliferation.35,36 Moreover, testosterone and also estradiol, which are generated by aromatization of testosterone in the skin, are able to promote collagen synthesis.10,37 Therefore, high cutaneous testosterone biosyntheses leading to maintenance of high cutaneous testosterone concentrations in vivo could help to preserve the integrity of the extracellular matrix.9,14
Conclusion
The concentration of DHEA and androstenedione in cutaneous samples and blood samples, with respect to the age of the subjects, behave in an opposite manner, which shows that the dermal metabolism of androgens is age-dependently modified. Moreover our data show that young skin is able to produce more testosterone than old skin. The observed site-specific differences support the hypothesis that sebaceous glands are likely to be modifiers in these processes by controlling the initial conversion of DHEA to androstenedione.
Materials and Methods
Determination of steroid hormone concentration
DHEA, androstenedione and testosterone were simultaneously measured by ultra perfomance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) according to previously published method.3 UPLC-MS/MS was performed by using a Quattro Premier/Xe mass spectrometer connected to an Acquity System (Waters Corporation). In brief: 0.1 ml serum, cell supernatant or suction blister fluid sample were mixed with 10 μl of internal standard, and extracted by solid phase extraction (SPE). As internal standard deuterium labeled 17-hydroxyprogesterone-d8 (Cambridge Isotope Laboratories, DLM-6598–0) and cortisol-d4 (Cambridge Isotope Laboratories, DLM-2218–0) were used. Calibrators and controls were prepared in steroid free serum for serum samples, and in DMEM medium (Gibco, 31053–028) for cell supernatant and suction blister fluids, and also extracted by SPE. Oasis MAX u-elution plates (Waters, WAT058943) were used for extraction and washed with 5% NH4OH solution (Merck, 105428) and 10% methanol (Fluka, 34966). All steroids were eluted with isopropanol (Fluka, 34965). The UPLC Quattro premier/Xe system (Waters Corporation) was operated in multiple reaction mode and all steroids were measured in the positive ion mode. For each steroid two different MRM transitions were determined (supplemental data). The total run time was 5 min. Quantification was linear from 0.1 to 200 nM (r2 > 0.992), and reproducible with an inter- and intra-assay coefficient < 15%. In addition the method was validated for suction blister fluids. Steroid-free suction blister fluid was generated by stirring suction blister fluid from different volunteers two times with active charcoal and subsequent filtration. The limit of detection for the determined hormones was 0.1 nM for androstenedione and testosterone and 0.5 nM for DHEA.
Collection of SBF and blood samples
Participants of this study were grouped in collectives of young (27.8 y, SD 2.4 y) and old (62.6 y, SD 2.4 y) healthy subjects (eight subjects each). Participants who received hormone replacement therapy or suffer from hormone related diseases were excluded. The use of topical hormones was also not allowed. The Body Mass Indexes (BMI) of all subjects ranged between 20 and 30 kg/m2. Suction blisters were prepared on upper back of all subjects and on forearms of the young subjects.
Fasting blood samples were collected with a 2.7 ml Sarstedt EDTA-Monovette rapidly in a short time period in the morning, immediately before generating suction blisters as described before. Blood plasma was received according to manufactures instructions and stored at −80°C for later analysis. After formation of suction blisters, the liquids were collected with a 1 ml syringe (Terumo, BS-01T and NN-2719R) and stored by −80°C for later steroid hormone analysis. For interpretation of data median values and interquartile ranges (IQR) were determined using Statistica 8 (Statsoft). IQR is the difference between the third and first quartile (IQR = Q75% − Q25%) and also called middle 50.
This study was approved by the Freiburg ethics committee and performed following the Declaration of Helsinki. All subjects gave their written informed consent.
Cell culture experiments
For functional analysis of cell specific testosterone metabolism, SZ95 sebocytes (n = 3), primary fibroblasts (n = 7) and keratinocytes (n = 4) were seeded at 80.000 cells/well in 24-well dishes.38 For cultivation of SZ95 sebocytes, Sebomed (Biochrom, F8205), and for fibroblasts DMEM (PAA, E15–877) containing 10% charcoal stripped FCS (C-C-Pro, S-15-M) was used. Keratinocytes were cultured using KGM-2 Gold media (Lonza, 195769). After 48 h of cultivation, the medium of SZ95 sebocytes and fibroblasts was replaced with medium containing 100 nM DHEA (Sigma-Aldrich, D4000), 0.2 mM Glutamax (Invitrogen, 35050038) and only 0.2% FCS or with KGM-2 Gold containing 100 nM DHEA for cultivation of keratinocytes. Twenty-four hours later supernatants were collected and frozen at -80°C for later determination of steroid hormone concentrations. After determination of steroid hormone concentrations the amounts were summed up and set to 100%.
Western blot
Harvested cells were lysed using RIPA-Puffer (Santa Cruz, sc-24948) according to manufactures instructions. For western blot analysis, 40 μg of protein extract were applied to 4–15% gradient gels (Bio-Rad Laboratories, 345–0027) and separated. Proteins were transferred onto a PVDF-membrane (Millipore, IPFL00010) by wet blot.
For blocking, 5% skim milk (Merck, 1153630500) in TBS (Bio-Rad Laboratories, 170–6435) was used for 1 h with gently shaking. Primary antibodies against 3β-HSD type I (1/200, Abcam, ab55268) and GAPDH (1/1000, Santa Cruz, sc-25778) were diluted in TBS-T (0.1% Tween, Merck, 8221840500) with 5% skim milk and applied to the membrane over night at 4°C with gently shaking. Secondary antibodies (1/10.000, anti rabbit IR-Dye 680 and 1/5.000, anti mouse IR-Dye 800, LI-COR Bioscience, 926–32221 and 926–32210) were applied for 1 h at room temperature (RT) after washing membranes three times with TBS-T for 5 min. The detection of proteins was done by scanning the membrane using the LI-COR Odyssey two colors detection system.
Immunfluorescence staining
Facial samples of healthy donors were removed during routine surgery and provided for research purposes with the informed consent of the donors. Punch biopsies were taken with a punch biopsy needle (Stiefel, 60000000000150) and embedded with Shandon Cryomatrix (Thermo Scientific, 6769006). After freezing with dry ice, biopsies were cryosectioned into 8 μm slices and mounted on a slide. Slides were fixated for 10 min using acetone at -20°C and air-dried.
After washing the slides for three times with PBS, unspecific binding sites were blocked for 20 min at RT with 10% donkey sera in PBS. Primary antibody incubation specific for 3β-HSD type I (1/100, Abnova, pab7013) was performed at 4°C overnight using a wet chamber. On the next morning slides were washed three times with PBS. A Cy 5 labeled anti goat secondary antibody (Jackson Immunoresearch, 705-175-147) was diluted 1/200 in PBS and incubated for 1 h. After three washing steps 0.1 μg/ml DAPI (Invitrogen, D1306) was applied for 2 min. Additional three washings steps later slides were covered with CV Ultra mounting medium (Leica, 14070937891) and a coverslip (Leica, 14071135637). Mosaic images were taken with a Zeiss Axiovert microscope system.
Acknowledgments
We thank Sonja Wessel for technical assistance by taking of fluorescence microscopy images.
Glossary
Abbreviations:
- 3β-HSD
3β-hydroxysteroiddehydrogenase
- 17β-HSD
17β-hydroxysteroiddehydrogenase
- BMI
body mass index
- DHEA
dehydroepiandrosterone
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HDF
human dermal fibroblasts
- HSD3β1
3β-hydroxysteroiddehydrogenase Type 1
- IQR
interquartile range or middle fifty
- MRM
Multiple reactions monitoring
- NHEK
normal human epidermal keratinocytes
- nM
nanomol/L
- SBF
suction blister fluid
- SPE
solid phase extraction
- SZ95
SZ95 sebocytes
- UPLC-MS/MS
ultraperformance liquid chromatography quadrupole mass spectrometry
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/dermatoendocrinology/article/19201
References
- 1.Zouboulis CC. Human skin: an independent peripheral endocrine organ. Horm Res. 2000;54:230–42. doi: 10.1159/000053265. [DOI] [PubMed] [Google Scholar]
- 2.Zouboulis CC. The human skin as a hormone target and an endocrine gland. Hormones (Athens) 2004;3:9–26. doi: 10.14310/horm.2002.11109. [DOI] [PubMed] [Google Scholar]
- 3.Kulle AE, Riepe FG, Melchior D, Hiort O, Holterhus PM. A novel ultrapressure liquid chromatography tandem mass spectrometry method for the simultaneous determination of androstenedione, testosterone, and dihydrotestosterone in pediatric blood samples: age- and sex-specific reference data. J Clin Endocrinol Metab. 2010;95:2399–409. doi: 10.1210/jc.2009-1670. [DOI] [PubMed] [Google Scholar]
- 4.Longcope C, Baker S. Androgen and estrogen dynamics: relationships with age, weight, and menopausal status. J Clin Endocrinol Metab. 1993;76:601–4. doi: 10.1210/jc.76.3.601. [DOI] [PubMed] [Google Scholar]
- 5.Labrie F, Bélanger A, Luu-The V, Labrie C, Simard J, Cusan L, et al. DHEA and the intracrine formation of androgens and estrogens in peripheral target tissues: its role during aging. Steroids. 1998;63:322–8. doi: 10.1016/S0039-128X(98)00007-5. [DOI] [PubMed] [Google Scholar]
- 6.Dolomie-Fagour L, Gatta B, Nguyen TD, Corcuff JB. Bioavailable estradiol in man: relationship with age and testosterone. Clin Chim Acta. 2008;398:145–7. doi: 10.1016/j.cca.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Araujo AB, Travison TG, Bhasin S, Esche GR, Williams RE, Clark RV, et al. Association between testosterone and estradiol and age-related decline in physical function in a diverse sample of men. J Am Geriatr Soc. 2008;56:2000–8. doi: 10.1111/j.1532-5415.2008.01965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markova MS, Zeskand J, McEntee B, Rothstein J, Jimenez SA, Siracusa LD. A role for the androgen receptor in collagen content of the skin. J Invest Dermatol. 2004;123:1052–6. doi: 10.1111/j.0022-202X.2004.23494.x. [DOI] [PubMed] [Google Scholar]
- 9.Black MM, Shuster S, Bottoms E. Osteoporosis, skin collagen, and androgen. Br Med J. 1970;4:773–4. doi: 10.1136/bmj.4.5738.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brincat M, Moniz CF, Studd JW, Darby AJ, Magos A, Cooper D. Sex hormones and skin collagen content in postmenopausal women. Br Med J (Clin Res Ed) 1983;287:1337–8. doi: 10.1136/bmj.287.6402.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Labrie F, Bélanger A, Cusan L, Gomez JL, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab. 1997;82:2396–402. doi: 10.1210/jc.82.8.2396. [DOI] [PubMed] [Google Scholar]
- 12.Baulieu EE, Thomas G, Legrain S, Lahlou N, Roger M, Debuire B, et al. Dehydroepiandrosterone (DHEA), DHEA sulfate, and aging: contribution of the DHEAge Study to a sociobiomedical issue. Proc Natl Acad Sci U S A. 2000;97:4279–84. doi: 10.1073/pnas.97.8.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nawata H, Yanase T, Goto K, Okabe T, Nomura M, Ashida K, et al. Adrenopause. Horm Res. 2004;62(Suppl 3):110–4. doi: 10.1159/000080509. [DOI] [PubMed] [Google Scholar]
- 14.Shuster S, Black MM, McVitie E. The influence of age and sex on skin thickness, skin collagen and density. Br J Dermatol. 1975;93:639–43. doi: 10.1111/j.1365-2133.1975.tb05113.x. [DOI] [PubMed] [Google Scholar]
- 15.Pelletier G. Expression of steroidogenic enzymes and sex-steroid receptors in human prostate. Best Pract Res Clin Endocrinol Metab. 2008;22:223–8. doi: 10.1016/j.beem.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Luu-The V, Bélanger A, Labrie F. Androgen biosynthetic pathways in the human prostate. Best Pract Res Clin Endocrinol Metab. 2008;22:207–21. doi: 10.1016/j.beem.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 17.McTernan PG, Anwar A, Eggo MC, Barnett AH, Stewart PM, Kumar S. Gender differences in the regulation of P450 aromatase expression and activity in human adipose tissue. Int J Obes Relat Metab Disord. 2000;24:875–81. doi: 10.1038/sj.ijo.0801254. [DOI] [PubMed] [Google Scholar]
- 18.Zouboulis CC, Chen WC, Thornton MJ, Qin K, Rosenfield R. Sexual hormones in human skin. Horm Metab Res. 2007;39:85–95. doi: 10.1055/s-2007-961807. [DOI] [PubMed] [Google Scholar]
- 19.Labrie F, Luu-The V, Labrie C, Pelletier G, El-Alfy M. Intracrinology and the skin. Horm Res. 2000;54:218–29. doi: 10.1159/000053264. [DOI] [PubMed] [Google Scholar]
- 20.Fritsch M, Orfanos CE, Zouboulis CC. Sebocytes are the key regulators of androgen homeostasis in human skin. J Invest Dermatol. 2001;116:793–800. doi: 10.1046/j.1523-1747.2001.01312.x. [DOI] [PubMed] [Google Scholar]
- 21.Itami S, Takayasu S. Activity of 3 beta-hydroxysteroid dehydrogenase delta 4-5 isomerase in the human skin. Arch Dermatol Res. 1982;274:289–94. doi: 10.1007/BF00403732. [DOI] [PubMed] [Google Scholar]
- 22.Dumont M, Luu-The V, Dupont E, Pelletier G, Labrie F. Characterization, expression, and immunohistochemical localization of 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4 isomerase in human skin. J Invest Dermatol. 1992;99:415–21. doi: 10.1111/1523-1747.ep12616131. [DOI] [PubMed] [Google Scholar]
- 23.Volden G, Thorsrud AK, Bjørnson I, Jellum E. Biochemical composition of suction blister fluid determined by high resolution multicomponent analysis (capillary gas chromatography--mass spectrometry and two-dimensional electrophoresis) J Invest Dermatol. 1980;75:421–4. doi: 10.1111/1523-1747.ep12524077. [DOI] [PubMed] [Google Scholar]
- 24.Kool J, Reubsaet L, Wesseldijk F, Maravilha RT, Pinkse MW, D’Santos CS, et al. Suction blister fluid as potential body fluid for biomarker proteins. Proteomics. 2007;7:3638–50. doi: 10.1002/pmic.200600938. [DOI] [PubMed] [Google Scholar]
- 25.Rhéaume E, Lachance Y, Zhao HF, Breton N, Dumont M, de Launoit Y, et al. Structure and expression of a new complementary DNA encoding the almost exclusive 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4-isomerase in human adrenals and gonads. Mol Endocrinol. 1991;5:1147–57. doi: 10.1210/mend-5-8-1147. [DOI] [PubMed] [Google Scholar]
- 26.Stárka L, Dusková M, Hill M. Dihydrotestosterone and testosterone throughout the life span of Czech men. Neuro Endocrinol Lett. 2008;29:201–4. [PubMed] [Google Scholar]
- 27.Leifke E, Gorenoi V, Wichers C, Von Zur Mühlen A, Von Büren E, Brabant G. Age-related changes of serum sex hormones, insulin-like growth factor-1 and sex-hormone binding globulin levels in men: cross-sectional data from a healthy male cohort. Clin Endocrinol (Oxf) 2000;53:689–95. doi: 10.1046/j.1365-2265.2000.01159.x. [DOI] [PubMed] [Google Scholar]
- 28.Dharia S, Parker CR., Jr Adrenal androgens and aging. Semin Reprod Med. 2004;22:361–8. doi: 10.1055/s-2004-861552. [DOI] [PubMed] [Google Scholar]
- 29.Plewig G, Kligman AM, Jansen T. Acne and rosacea. Springer, 2000. [Google Scholar]
- 30.Greene RS, Downing DT, Pochi PE, Strauss JS. Anatomical variation in the amount and composition of human skin surface lipid. J Invest Dermatol. 1970;54:240–7. doi: 10.1111/1523-1747.ep12280318. [DOI] [PubMed] [Google Scholar]
- 31.Chen W, Tsai SJ, Sheu HM, Tsai JC, Zouboulis CC. Testosterone synthesized in cultured human SZ95 sebocytes derives mainly from dehydroepiandrosterone. Exp Dermatol. 2010;19:470–2. doi: 10.1111/j.1600-0625.2009.00996.x. [DOI] [PubMed] [Google Scholar]
- 32.Hikima T, Maibach HI. Gender differences of enzymatic activity and distribution of 17beta-hydroxysteroid dehydrogenase in human skin in vitro. Skin Pharmacol Physiol. 2007;20:168–74. doi: 10.1159/000101386. [DOI] [PubMed] [Google Scholar]
- 33.Miyake K, Ciletti N, Liao S, Rosenfield RL. Androgen receptor expression in the preputial gland and its sebocytes. J Invest Dermatol. 1994;103:721–5. doi: 10.1111/1523-1747.ep12398601. [DOI] [PubMed] [Google Scholar]
- 34.Schneider MR, Paus R. Sebocytes, multifaceted epithelial cells: lipid production and holocrine secretion. Int J Biochem Cell Biol. 2010;42:181–5. doi: 10.1016/j.biocel.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 35.Zouboulis CC, Xia L, Akamatsu H, Seltmann H, Fritsch M, Hornemann S, et al. The human sebocyte culture model provides new insights into development and management of seborrhoea and acne. Dermatology. 1998;196:21–31. doi: 10.1159/000017861. [DOI] [PubMed] [Google Scholar]
- 36.Abdel-Naser MB. Selective cultivation of normal human sebocytes in vitro; a simple modified technique for a better cell yield. Exp Dermatol. 2004;13:562–6. doi: 10.1111/j.0906-6705.2004.00187.x. [DOI] [PubMed] [Google Scholar]
- 37.Nelson LR, Bulun SE. Estrogen production and action. J Am Acad Dermatol. 2001;45(Suppl):S116–24. doi: 10.1067/mjd.2001.117432. [DOI] [PubMed] [Google Scholar]
- 38.Zouboulis CC, Seltmann H, Neitzel H, Orfanos CE. Establishment and characterization of an immortalized human sebaceous gland cell line (SZ95) J Invest Dermatol. 1999;113:1011–20. doi: 10.1046/j.1523-1747.1999.00771.x. [DOI] [PubMed] [Google Scholar]