Abstract
In the literature of the past 30 years there are only some publications concerned with hair loss and hyperprolactinemia in women. Therefore, the relevance of hyperprolactinemia was evaluated in 40 women with diffuse alopecia.
Hair loss was assessed by clinical appearance and the pluck trichogram. 82.5% of the female patients had diffuse hair loss and 17.5% had androgenetic alopecia.
The highest prolactin values measured were 1390 ng/ml and 255 ng/ml. Six patients had values between 150–80.4 ng/ml and 10 between 79.1–51.7 ng/ml. All others had prolactin values below 50 ng/ml. Fifteen untreated patients with elevated prolactin levels could be followed up. Without any prolactin-inhibiting drugs, reductions and normalizations beside moderate fluctuations could be detected.
Thyroid-specific diagnostics showed in 95% of the patients a normal thyroid function. 2.5% had a slight hyperthyreoidism and 2.5% had a slight hypothyreoidism. No female patient had clinical signs of androgenization and the determined androgens testosterone, androstendione and dihydroepiandrostendione were in the normal range.
According to these results, moderate elevated prolactin levels in association with diffuse or androgenetic hair loss can be neglected as causative for the hair loss, because there is no evidence that they have an influence to the pattern, the extent or the duration of the hair loss. These results are supported by investigations of other authors who described only in high doses of prolactin an inhibiting effect on human hair follicles in vitro. Nevertheless, moderate constantly elevated prolactin levels should induce further diagnostics to exclude a prolactin-producing tumor of the pituitary gland.
Keywords: diffuse hair loss, hyperprolactinemia, women
Introduction
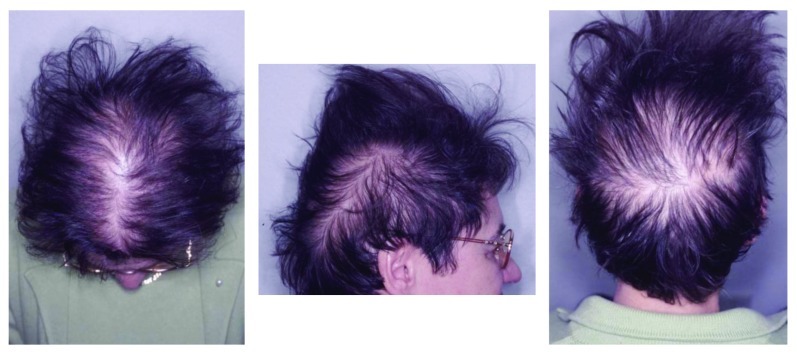
In the literature of the past 30 years there are only some publications concerning hair loss and hyperprolactinemia in women, although a causative relationship is discussed more often. The types of hair loss described with hyperprolactinemia are mainly from the androgenetic or diffuse type and connected with hormonal abnormalities, especially the androgens. Androgenetic alopecia in women is clearly classified1 and is caused by genetic inheritance, hormonal imbalances or by a combination of these parameters. Diffuse hair loss in women (Fig. 1), however can show clinically more different stages and the causes can be very different. Therefore, diffuse hair loss in women is always a challenge for every dermatologist. The possible causes and diseases which induce and imitate diffuse hair loss can be, for example, dermatological and internal diseases, endocrinological disorders, deficiency of iron, zinc or biotin, side effects of drugs or hair damage through cosmetic procedures.
Figure 1.
57-y-old female patient with diffuse hair loss and a moderate elevated prolactin level of 75.5 ng/ml. Diffuse hair loss is characterized by an uniform shedding of the hair in central, parietal and occipital region of the head.
Considering these facts, it made sense to evaluate the extent and relevance of hyperprolactinemia in women in with hair loss for daily clinical work.
Results
Distribution of age
Figure 2 shows the distribution of the patient’s age. Most of the patients with hyperprolactinemia were between 30 and 65 y with a mean of 45.8 y. But also younger and older patients can have elevated prolactin values, even when there is no more hormonal activity expected, as in higher age groups.
Figure 2.
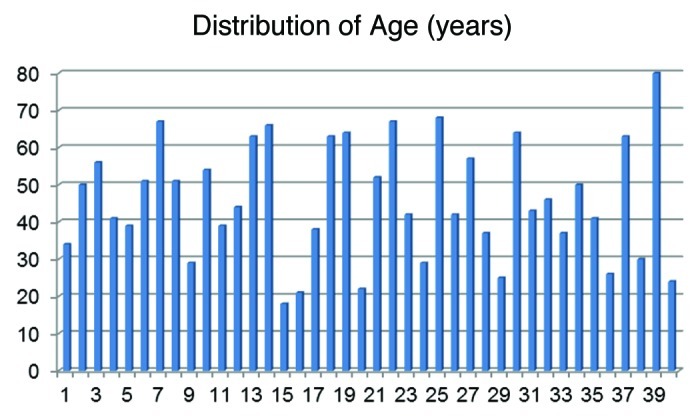
The age of the 40 patients with elevated prolactin levels ranged from 18–80 y (mean 45.8).
Classification of hair loss by clinical appearance
Twenty-nine patients (72.5%) suffered clinically from diffuse hair loss, six (15%) showed an androgenetic alopecia and five (12.5%) had a combination of diffuse and androgenetic alopecia.
Classification of hair loss by clinical appearance and pluck trichogram
The number of telogen hairs, as an additional marker for differentiation between androgenetic alopecia from the female pattern type and diffuse hair loss, allows a more exact classification. Patients who showed a diffuse shedding of their hair and an elevated telogen rate only in the frontal area were added to the androgenetic group. Those patients who had an elevated telogen rate in the frontal and occipital area were attributed to the group with diffuse hair loss. By this classification, 33 (82.5%) had diffuse hair loss and 7 (17.5%) an androgenetic alopecia. This demonstrates that in unclear clinical cases the trichogram allows a more accurate classification.
Number of anagen and telogen hairs
Figures 3and4 display the distribution of the anagen and telogen rate in the frontoparietal area of the scalp in percent (%) under consideration of the mean and the standard deviation. In average the anagen rate was 80.0% and the corresponding telogen rate 16.5%.
Figure 3.
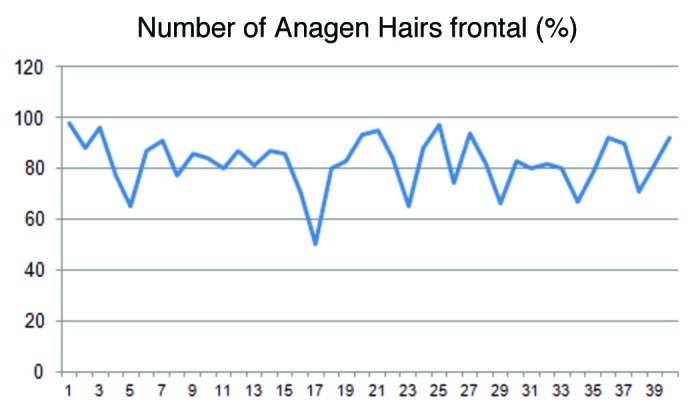
The illustration shows the fluctuation of the anagen hairs in the frontal region of the head. On average the anagen rate was 80.0%.
Figure 4.
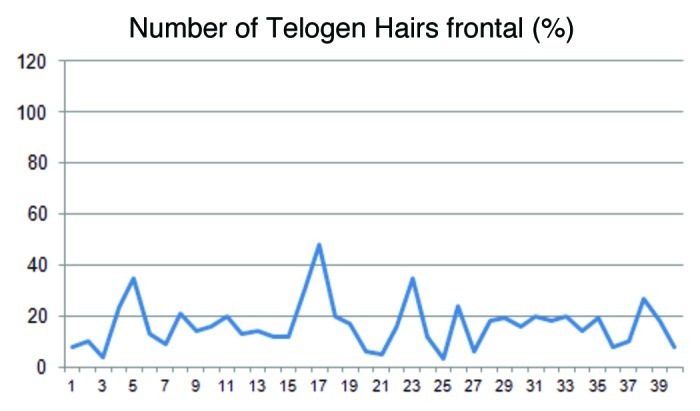
The percentage of the telogen hairs in the frontal region of the head varied from 4–48% (mean 16.5).
In the occipital area the anagen rate was similar to the frontal area with 82.0%, while the telogen was 15.6% (Figs. 5 and 6). These data demonstrate an almost similar hair loss over the whole scalp with a dominance of the diffuse hair loss pattern.
Figure 5.
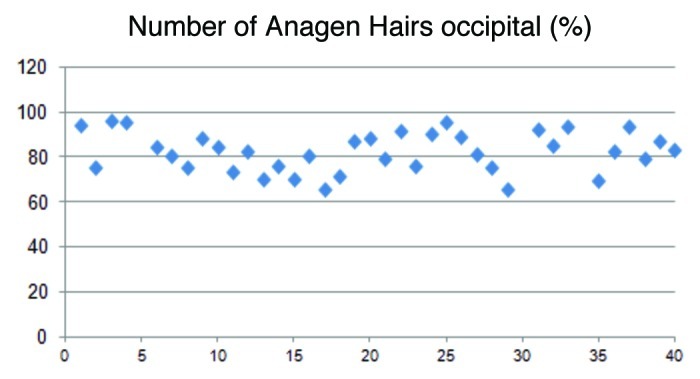
The illustration shows the fluctuation of the anagen hairs in the occipital region of the head. On average the anagen rate was 82.0%.
Figure 6.
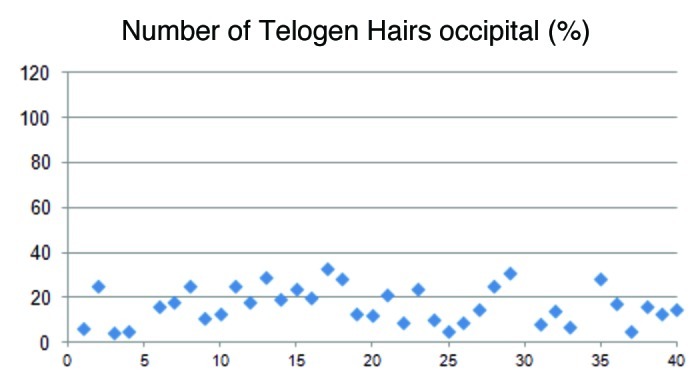
The percentage of telogen hairs in the occipital region of the head varied from 4–33% (mean 15.6).
Prolactin values
In Figure 7 all prolactin values are listed. The highest prolactin value was 1,390 ng/ml and the lowest 25.1 ng/ml. The second highest was 255 ng/ml. Six patients had values between 150 and 80.4 ng/ml and ten between 79.1 and 51.7 ng/ml. All others had prolactin values below 50 ng/ml and showed only moderate or slight elevations. Some patients with elevated levels received prolactin-inhibiting drugs by endocrinologists. In 15 patients the elevated prolactin level could be followed up without medication. Without any prolactin-inhibiting drugs, reductions and normalizations beside moderate fluctuations could also be diagnosed.
Figure 7.
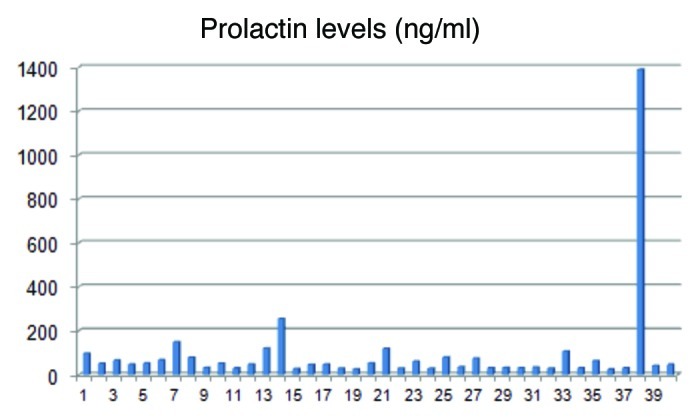
Prolactin levels of the 40 patients with diffuse hair loss. The prolactin values ranged from 25.1–1390 ng/ml. Most of the patients had values below 150 ng/ml.
Thyroid function
Ninety-five percent of the patients had a normal thyroid function, 2.5% showed a slight hyperthyreoidism and 2.5% had a slight hypothyreoidism. Additional diagnostics by ultra sound or scintigraphy revealed in 30% a goiter with no signs of malignancy.
Signs of androgenization
No clinical signs of androgenization could be detected and elevations of the androgens testosterone, androstendione and dihydroepiandrostendione could be excluded. Therefore there was no evidence for an ovarian or adrenal hyperandrogenemia in any female patient.
Discussion
Occasionally in female patients with diffuse hair loss an elevated prolactin level can be found. In these cases there is always the question about the relevance of this finding. Should only the elevated prolactin value be controlled or is a further diagnostic needed? Is this elevated prolactin level causative for the hair loss or is it only a side effect of the patient’s medication? To give help in the management with these questions this open retrospective study was performed because there is only one study2 which deals with elevated prolactin levels in female patients with mainly diffuse hair loss.
Classification and pattern of hair loss
Diffuse hair loss is characterized by a diffuse shedding of the scalp hair in the central, parietal and occipital area. In androgenetic alopecia or female pattern hair loss, hair diminishes predominantly on the crown. Due to Camacho-Martinez3 female alopecia with androgen increase is called female androgenetic alopecia and without androgen increase female pattern hair loss. The clinical picture of typical female androgenetic alopecia begins with a specific diffuse loss of hair from the parietal or frontovertical areas. In later stages both cases are typically characterized by an intact frontal hairline. Androgenetic alopecia from the male pattern type in women according to the classification of Hamilton4 is easy to diagnose. But sometimes it is difficult to distinct diffuse hair loss from female pattern hair loss in the beginning. While in diffuse hair loss telogen hairs are increased in the central and occipital part of the scalp, in androgenetic alopecia telogen hairs are only increased in the central area. Therefore patients were classified by their clinical appearance and by estimating the number of their anagen and telogen hairs in the frontal and occipital area. In contrast to the pull test which is very inaccurate the trichogram allows a good determination of the anagen and telogen hairs. Using this method five patients combining clinically a female androgenetic and diffuse pattern could be assigned either to the group with androgenetic or diffuse hair loss. Total, 82.5% of the patients had diffuse hair loss and 17.5% had female pattern hair loss with no signs of hyperandrogenism. Anagen and telogen rates can differ according to activity of the hair loss. In average the anagen rate was 80.0% in the frontoparietal and occipital region, whereas the corresponding telogen rate was frontoparietal 16.5% respectively 15.6% in the occipital region. Considering a normal range until 14% at both sides, in average a moderate telogen effluvium could be diagnosed.
According to these findings in every patient with androgenetic or diffuse hair loss a trichogram in the frontoparietal and occipital area should be performed. This procedure allows a more exact classification in questionable cases.
Definition of hyperprolactinemia
By definition hyperprolactinemia is diagnosed when the prolactin level is above the upper normal range. Normal prolactin levels vary between 2 and 25 ng/ml depending on age and the time of menstrual cycle. According to Trueb,5 values between 26–200 ng/ml lay in the so-called gray zone, whereas prolactin level > 200 ng/ml should be considered to be pathological. But this opinion is not always applicable because there are also patients with a prolactinoma and prolactin levels significantly below 100 ng/ml. Furthermore it has to be considered that prolactin secretion by the pituitary gland is not a constant, but a changing process depending on endogenous and exogenous influences. Therefore it is necessary to discuss such influences.
Hyperprolactinemia by diseases, endogenic and exogenic stimulants and drugs
First of all one has to exclude disorders of the hypothalamic-pituitary-axis as well as diseases which may cause hyperprolactinemia.6 Typical examples for psychiatric and neurogenic diseases which can induce hyperprolactinemia are depression, neuritis or irritations of the spinal cord. Disorders in the hypothalamic region and the pituitary stalk may be caused by encephalitis, sarcoidosis, traumatic injuries or chirurgical interventions. Furthermore, neoplasms like craniopharyngeoms and meningeoms or no prolactin-producing intra or supra cellular tumors have to be excluded. Regarding pituitary-specific diseases, a prolactinoma, cysts of the pituitary gland, hypophysitis or a status after radiation have to be taken into account. Hyperprolactinemia may also be the result of endocrinopathies like primary hypothyreoidism, secondary hypothalamic hyperthyreoidism, autoimmune diseases, acromegaly or hyperandrogenemia and others. But there are also endogenic substances from the central nervous system like thyroid-releasing hormone, endorphines, melatonin, gastric peptides and growth factors, which stimulate prolactin secretion. Moreover, sexual steroids like estrogen, manipulations or irritations of the breast, nutritional factors like protein-rich diets, alcohol or stress play an important role in this context. As the number of drugs is too long to list completely, only some important are exemplary mentioned. Important are drugs like antidepressants, tranquilizers, neuroleptics, antihypertensives, narcoleptics, tuberculostatic agents, antibiotics and antiandrogens like cyproterone acetate, which is frequently recommended by gynecologists in case of female androgenetic alopecia even when there is no hyperandrogenemia diagnosed.
Data from the literature concerning hair loss and hyperprolactinemia
In the literature of the past 30 years there are only five papers investigating hyperprolactinemia in connection with hair loss in women.2,7-10
In 1988 Orfanos and Hertel10 described functional hyperprolactinemia in five female patients seeking medical consultation for hair loss. With varying frequencies these patients suffered also from hypertrichosis, irregular menstrual bleeding, secondary amenorrhea, galactorrhea, seborrhea and persisting acne, while hormonal parameters including testosterone levels and thyroid gland function tests were unchanged. Trichogram investigations showed in two cases an increased telogen effluvium. Two patients had normal trichogram values and in one patient dystrophic hairs were dominant. In summary, it was suggested to evaluate prolactin levels together with androgens and thyroid function tests in patients with seborrhea, acne, hypertrichosis/hirsutism and alopecia, which represents on the whole the SAHA syndrome.
In the same year, Futterweit and colleagues2 published a paper about the prevalence of hyperandrogenism in 109 consecutive female patients with diffuse alopecia. The study also included an evaluation of associated hirsutism and/or menstrual dysfunction, plasma hormonal measurements, and ultrasonography of the ovaries. Only two patients had hyperprolactinemia caused by pituitary tumors. In 38.5% signs of hyperandrogenism were found by serological diagnostics but 64.2% had no clinical evidence of hirsutism or menstrual dysfunction.
Hypothyreoidism and hyperprolactinemia as a possible cause of androgenetic alopecia in the females were supposed also by Schmidt et al. in 1989.7 Thirty-one female patients suffering from androgenetic alopecia were examined by means of the thyroid release hormonal (TRH) test with regard to hypothyreoidism and hyperprolactinemia. In seven of the patients (23%) an increased TSH level after TRH stimulation was detected, indicating hypothyreoidism. Furthermore, nine of the patients (29%) also had increased prolactin levels after TRH stimulation, indicating hyperprolactinemia. Prolactin was especially focused on in this context because it is known to promote the suprarenal cortisol and androgen production. As thyroxin and prolactin interfere with the androgen metabolism, it was suggested that both conditions may contribute to the clinical picture of female pattern alopecia. These results were confirmed by further investigations of the group in a greater collective of patients and the multilayered hormonal influences of the androgen-stimulating effect of prolactin was supposed once more to play a role in female androgenic hair loss.9
Another paper by Schmidt et al.8 deals with hyperprolactinemia and hypophyseal hypothyroidism as cofactors in hirsutism. In view of the hormonal interactions between thyroxin, prolactin, androgens and thyroid hormones a TRH-stimulation test was performed in 38 female patients with androgenic hair loss and 27 with hirsutism and compared with the results in 45 female controls. Prior to stimulation, baseline concentrations of TSH were significantly elevated in hirsutism and in androgenic hair loss. In the case of prolactin, both baseline and stimulated levels were significantly highly elevated in hirsutism, while in androgenic hair loss only the stimulated levels were significantly elevated.
Besides these publications, Camacho-Martinez3 only describes possible hormonal disorders in connection with hair loss in women and their therapeutic options and Trueb5 supposes hyperprolacintemia may cause hair loss and hirsutism. Another publication by Olszewska et al.11 deals only with methods of hair loss evaluation in patients with endocrine disorders, without their own investigations.
In vitro investigations concerning the bioregulatory effects of prolactin (PRL) were performed by Foitzik et al. on organ-cultured human scalp hair follicles.12 They showed that human skin and normal human scalp hair follicles (HFS) express both prolactin and prolactin receptors. During the hair follicle transformation from growth (anagen) to regression (catagen), prolactin and prolactin receptor immunoreactivity appeared upregulated. Treatment of the hair follicles with high doses of prolactin resulted in a significant inhibition of hair shaft elongation and premature catagen development, along with reduced proliferation and increased apoptosis of hair bulb keratinocytes. From this data it was suggested that prolactin acts as an autocrine hair growth modulator with hair growth inhibitory effects. This may be an explanation for the former-described hair loss in patients with high prolactin levels, because only high doses of prolactin showed a significant inhibitory effect in vitro. While moderate elevated prolactin levels, like demonstrated in the current study, had no inhibitory effects on hair growth.
Thyroid function and signs of androgenization
In literature thyroid dysfunctions associated with hyperprolactinemia are described.7-9 Therefore in every patient TSH concentration was measured. Additionally, in 13 of 14 patients T3 and T4 concentrations were estimated. No correlation between prolactin levels and the thyroid specific parameters could be detected. Furthermore, 95% of the patients had a normal thyroid function, 2.5% showed a slight hyperthyreoidism and 2.5% had a slight hypothyreoidism. Diagnostics by ultra sound or scintigraphy revealed in 30% a goiter with no signs of malignancy. Finally it can be stated that the results of thyroid diagnostics are not specific for the discussed context but correspond to those in the normal population.
Similar conclusions also allow the results from the andogenetic diagnostics, because no clinical signs of androgenization or an increased level of testosterone, androstendione and dihydroepiandrostendione were found. Therefore it has to be assumed that androgenic hormonal abnormalities described in literature2,10 only occur in more severe endocrinological disorders like the polycystic ovary syndrome (PCOS), in combination with hirsutism or by severe dysfunctions of the hypothalamic-hypophyseal-axis. But on the other hand there are also patients with PCO and hyperprolactinemia with low mean serum testosterone concentrations.13
Relevance of elevated prolactin levels
In the study presented, normal prolactin values reach up to 25 ng/ml. The highest prolactin elevation had a 30-y-old female patient with 1,390 ng/ml. The second highest prolactin level was measured in a 66-y-old patient with 255 ng/ml, while the others showed only moderate or minimal elevations. In 15 patients a follow up of the prolactin level was possible because of further consultations in the praxis. Without any medical influences, reductions and normalizations beside moderate fluctuations could be detected. This demonstrates the instability of the prolactin secretion and the necessity for further controls in conspicuous cases. As shown in Figure 7, most of the patients had prolactin levels below 80 ng/ml. But one has to keep in mind also that moderate elevations of prolactin levels can be caused by a prolactinoma or a microprolactinoma. Like in other diseases, individual histories can demonstrate the difficulties to evaluate medical findings, especially when no static parameters but naturally fluctuating hormones are present. Therefore some interesting cases are discussed.
The female patient with the highest prolactin level of 1390 ng/ml had no prolactinoma and received bromocriptine medication for lowering the elevated prolactin level. She suffered from androgenetic alopecia from the female pattern type according to the classification of Ludwig. The trichogram showed a telogen rate of 27% in the frontoparietal and 16% in the occipital area. Furthermore there were no thyroid dysfunctions and no signs of androgenism or any antiandrogen drugs.
Exemplary for naturally fluctuating prolactin levels are the results of a 39-y-old female patient with diffuse hair loss and an initial prolactin level of 59.8 ng/ml. Prolactinoma was excluded by CT of the skull. As extracts of agnus castus are described to lower prolactin levels in moderate cases, the patient received 4 mg per day in the form of Agnucaston® tbl over 3 mo. Re-determination of prolactin confirmed an ongoing elevation of 47.5 ng/ml. Further controls without medication over the next 4 y showed a continuing decrease of prolactin with values of 19.0 to 15.0 and 5.1 ng/ml. The telogen rate decreased corresponding from 35% to 21%.
In another 63-y-old female patient with diffuse hair loss prolactin decreased from 120.9 ng/ml to 6.6 ng/ml within one year after discontinuing formoterol fumarate medication, a β2-receptor antagonist which was described for asthma. In this case prolactin normalization is likely due to the discontinuation of the medication. But telogen effluvium worsened from 14% to 30% in the frontoparietal area at the same time, while in the occipital area a slight reduction from 29% to 23% was detected. In this case an additional manifestation of an androgenetic hair loss has to be discussed.
A 67-y-old female patient with a prolactin level of 150 ng/ml received cabergolin by the endocrinologist. This potent dopamine agonist on D2 receptors has a direct inhibitory effect on pituitary lactotroph cells and is frequently used as a first-line agent in the management of prolactinomas. The prolactin level decreased under medication from 150 ng/ml to 18 and 26.4 ng/ml over a period of two years. Three weeks after discontinuation of medication, the prolactin level kept a low value of 2.9 ng/ml. A prolactinoma could be excluded by MRI prior to the medication.
A prolactinoma was only diagnosed in a 70-y-old female patient. She consulted the hair clinic because of persistent hair loss for 3 y. Clinically she had female pattern hair loss from the Ludwig type stadium one. The trichogram showed only a slight increase of 16% telogen hairs in frontoparietal area, whereas on the occipital a completely normal rate of 9% was found. The ferritin level was very low with 7.1 ng/ml. Other hair specific parameters including TSH were normal. Furthermore she took two drugs which are known to inhibit hair growth, carvedilol and omeprazole. According to these findings, hair loss was assumed to be caused primarily by iron deficiency and second by the drugs. Prolactin was not determined. In the following months the patient suffered from recurrent phases of dizziness and the neurologist recommended further diagnostics by MRI. By this diagnostics a prolactinoma was detected though prolactin was only moderatly increased to 60 ng/ml. This demonstrates clearly that also in patients with moderate elevations of prolactin a prolactinoma should be excluded, particularly when the prolactin level is constantly over 50 ng/ml.
Conclusion
Moderate elevated prolactin levels in association with diffuse or androgenetic hair loss can be neglected as causative for the hair loss because there is no evidence that they have an influence to the pattern, the extent or the duration of the hair loss. These results are supported by in vitro investigations of other authors who described only in high doses of prolactin an inhibiting effect on human hair follicles. Furthermore, it is very unlikely that moderate or slight elevations of the prolactin are accompanied by thyroid dysfunctions or elevated androgenic hormones. The described alopecias in association with hyperprolactinemias are most likely due to a severe hyperprolactinemias or other concomitant endocrinological and genetic disorders or syndroms, because they were mainly described in combination with the PCO syndrome, hirsutism or other signs of androgenization. In addition it is conceivable that some of the formerly described hyperprolactinemias were triggered by the conducted TRH tests or other exogenous or endogenous factors. Nevertheless, moderate constantly elevated prolactin levels should induce further diagnostics to exclude a prolactin-producing tumor of the pituitary gland.
Materials and Methods
Patients and types of hair loss
For the evaluation, 40 female patients in the age range of 18–80 y with hyperprolactinemia and hair loss from the diffuse and androgenetic type were enrolled in the study. The mean age of the patients was 45.8 y. The patients were recruited from the ambulant hair clinic over the past 15 y. Excluding criteria for the study were all other types of hair loss as well as pregnancy and lactation. Female pattern hair loss was diagnosed according to the classification of Ludwig.1 Typical for the Ludwig classification is a progressive shedding of the hair on the crown with a preserved hairline on the forehead. Diffuse hair loss is characterized by a diffuse shedding of the scalp hair in the central, parietal and occipital area (Fig. 1). But sometimes it is difficult to distinguish diffuse hair loss from female pattern hair loss in the beginning. Therefore, patients were classified by their clinical appearance and by estimating the number of anagen and telogen hairs in the frontoparietal and occipital area. This procedure allows a more correct classification in questionable cases. In the active stadium of female pattern hair loss the number of telogen hairs is only increased in the frontal area, while in diffuse hair loss the number of telogen hairs is increased in the frontal and occipital area.
Determination of anagen and telogen hairs
The number of anagen and telogen hairs was determined by pluck trichogram.14,15 This method is easy to perform, reliable and used for decades by dermatologists in Germany. In order to create optimum conditions for trichogram analysis and patients’ acceptance, the following points had to be observed. Since shampooing reduces the number of telogen hairs, which would falsify the results, it is important that the patients do not wash their hair for five days. A cosmetically good result can be achieved if, instead of a tuft of hair, only a narrow column of 50–100 hairs are pulled out in the frontoparietal region, on the contralateral side of the part, and occipital in height of the protuberantia occipitalis externa. For this, a column of hairs, approximately one inch in length was lifted with a tail comb and grasped with a rubber–faced surgical clamp. The hairs were pulled out with a sharp tug following the natural direction of hair growth. The column of hairs pulled out with their roots was placed on a slide and fixed with a transparent tape. After fixing, the surgical clamp was removed and the extending hairs were cut from the back of the slide. The specimen was lined and labeled. For examination under the light microscope the hair roots were embedded in 2–3 drops of tap water and covered with a large coverslip. Evaluation was performed under a binocular light microscope at a magnification of 40. The number of anagen and telogen hairs was counted in a meander-shaped method, section by section from left to right and the anagen/telogen ratio was figured out. In humans a normal hair cycle shows at least 80 percent of the hairs in the growing (anagen) phase and maximal 14 percent in the falling out (telogen) phase. Occasionally one can find some hairs in the transition phase from anagen to telogen, the so called catagen phase, which represents an apoptosis-driven regression. Otherwise there is also the possibility to find some broken off or dystrophic hairs. But to evaluate the hair loss only the anagen/telogen ratio is important.
In contrast to the unit area trichogram16 the mentioned pluck trichogram is less painful and delivers better cosmetic results, because there is only a small line left without hair. This small hair line can easily be covered by the remaining hair and after 4 mo every hair is regrown. For the unit area trichogram however a greater tuft of hairs has to be plucked out, which can be very painful, particularly when the hairs are firmly anchored. For the computer assisted digital phototrichogram17 an area of one square centimeter has to be shaved in the frontoparietal and occipital region of the scalp. This procedure can be a problem in long hair, because it takes several months until the hair has grown back. Furthermore the left hairs in the shaved area have to be black colored for measurement.
Serological Diagnostics
Prolactin level
Prolactin was determined serological in all patients, using the immunoassay method. The normal range of prolactin in women differs from age and the time of the menstrual cycle. In the follicular phase it ranges from 2.8–18.3 ng/ml, in the luteal phase from 4.4–25.0 ng/ml and in post menopause from 1.8–20.3 ng/ml. In slight and moderate elevations of prolactin no further diagnostics were performed and the patients were only monitored for the next year. Patients with higher elevations of the prolactin value were introduced to an endocrinologist for further diagnostics. Prolactinoma was excluded by X-ray, CT or magnetic resonance imaging (MRI) of the skull. As hyperprolactinemia can also be caused by many drugs and different diseases, all patient were be questioned for their individual vitae and medication. In 15 patients a follow up of the prolactin level was possible because of further consultations in the praxis.
Thyroid-specific parameters
Because hyperprolactinemia is sometimes described in connection with dysfunctions of the thyroid gland,7-9 all patient were additionally screened by measuring the thyroid-stimulating hormone (TSH). Additionally in 10 patients the trijodthyronine (T3) and in 13 patients the tetrajodthyronine (T4) levels were determined. In cases of pathological values the patients were presented to a specialist physician for nuclear medicine for further diagnostics.
Androgens
Some publications concern alopecia and hyperprolactinemia in combination with hirsutism, hypertrichosis, hyperandrogenism and the SAHA-syndrome.2,10 Especially the SAHA-syndrome, which is characterized by seborrhoe, acne, hirsutism/hypertrichosis and alopecia, indicates stronger hormonal imbalances. Therefore every patient was examined for these signs. Additionally total testosterone (n = 36), androstendione (n = 29) and dehydroepiandrosterone (n = 37) were determined in the serum.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/dermatoendocrinology/article/19472
References
- 1.Ludwig E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. 1977;97:247–54. doi: 10.1111/j.1365-2133.1977.tb15179.x. [DOI] [PubMed] [Google Scholar]
- 2.Futterweit W, Dunaif A, Yeh HC, Kingsley P. The prevalence of hyperandrogenism in 109 consecutive female patients with diffuse alopecia. J Am Acad Dermatol. 1988;19:831–6. doi: 10.1016/S0190-9622(88)70241-8. [DOI] [PubMed] [Google Scholar]
- 3.Camacho-Martínez FM. Hair loss in women. Semin Cutan Med Surg. 2009;28:19–32. doi: 10.1016/j.sder.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton JB. Patterned loss of hair in man; types and incidence. Ann N Y Acad Sci. 1951;53:708–28. doi: 10.1111/j.1749-6632.1951.tb31971.x. [DOI] [PubMed] [Google Scholar]
- 5.Trüeb RM. Hormone und Haarwachstum. Hautarzt. 2010;61:487–95. doi: 10.1007/s00105-009-1890-2. [DOI] [PubMed] [Google Scholar]
- 6.Bals-Pratsch M. Störungen des Prolaktinhaushalts. In: Leidenberger F, Strowitzki T, Ortmann O. Klinische Endokrinologie für Frauenärzte. Springer Heidelberg 2009; 4. Auflage: 349-369. [Google Scholar]
- 7.Schmidt JB, Schurz B, Huber J, Spona J. Hypothyreose und Hyperprolaktinämie als mögliche Ursache der androgenetischen Alopezie der Frau. Z Hautkr. 1989;64:9–12. [PubMed] [Google Scholar]
- 8.Schmidt JB, Lindmaier A, Spona J. Hyperprolaktinämie und hypophysäre Hypothyreose als Kofaktoren bei Hirsutismus und androgenem Haarausfall der Frau. Hautarzt. 1991;42:168–72. [PubMed] [Google Scholar]
- 9.Schmidt JB. Hormonal basis of male and female androgenic alopecia: clinical relevance. Skin Pharmacol. 1994;7:61–6. doi: 10.1159/000211275. [DOI] [PubMed] [Google Scholar]
- 10.Orfanos CE, Hertel H. Haarwachstumsstörung bei Hyperprolaktinämie. Z Hautkr. 1988;63:23–6. [PubMed] [Google Scholar]
- 11.Olszewska M, Warszawik O, Rakowska A, Słowińska M, Rudnicka L. Methods of hair loss evaluation in patients with endocrine disorders. Endokrynol Pol. 2010;61:406–11. [PubMed] [Google Scholar]
- 12.Foitzik K, Krause K, Conrad F, Nakamura M, Funk W, Paus R. Human scalp hair follicles are both a target and a source of prolactin, which serves as an autocrine and/or paracrine promotor of apoptosis-driven hair follicle regression. J Am Acad Pathol. 2006;168:748–56. doi: 10.2353/ajpath.2006.050468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conway GS, Honour JW, Jacobs HS. Heterogeneity of the polycystic ovary syndrome: clinical, endocrine and ultrasound features in 556 patients. Clin Endocrinol (Oxf) 1989;30:459–70. doi: 10.1111/j.1365-2265.1989.tb00446.x. [DOI] [PubMed] [Google Scholar]
- 14.Braun-Falco O, Heilgemeier GP. The trichogram. Structural and functional basis, performance and interpretation. In: Seminars in Dermatology, Volume 4, Number 1, Thieme-Stratton Inc. New York 1985: 40-52. [Google Scholar]
- 15.Lutz G. Das Trichogramm - Indikation, Durchführung und Interpretation. Der Deutsche Dermatologe. 2001;4:254–61. [Google Scholar]
- 16.Rushton H, James KC, Mortimer CH. The unit area trichogram in the assessment of androgen-dependent alopecia. Br J Dermatol. 1983;109:429–37. doi: 10.1111/j.1365-2133.1983.tb04617.x. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann R. TrichoScan: combining epiluminescence microscopy with digital image analysis for the measurement of hair growth in vivo. Eur J Dermatol. 2001;11:362–8. [PubMed] [Google Scholar]