Abstract
Comment on: Jensen M, et al. Cell 2012; 149:173-87.
Keywords: C. elegans, Frizzled receptor, Ror receptor tyrosine kinase, Wnt signaling, acetylcholine, neuromuscular junction, neurotransmission, nicotine, synaptic plasticity, translocation
Wnts are evolutionarily conserved secreted glycoproteins that contribute to the patterning and development of many tissues in C. elegans, Drosophila and mammals. In the nervous system, Wnt signaling contributes to postmitotic cell fate, axon pathfinding, synaptogenesis and synaptic function.1,2 However, as we discuss here, the role of Wnt signaling is not limited to the development of the nervous system, as it also has an ongoing role in the modulation of synaptic strength by experience. Indeed, disrupted Wnt signaling has been implicated in a variety of neurological disorders, such as Alzheimer disease, schizophrenia and depression, all of which are associated with altered synaptic function.3
In all nervous systems, networks of interconnected neurons process information at specialized points of contact called synapses. At these specializations, neurotransmitter released from the presynaptic membrane of one neuron binds to receptors localized to the postsynaptic membrane of the apposed neuron. The strength of transmission between neurons is modulated by experience-dependent changes at synapses. These dynamic processes—especially changes in the number of functional receptors at the postsynaptic membrane—are believed to underlie learning and memory.4,5
Elegant genetic studies in Drosophila larvae and in C. elegans have demonstrated that Wnt signaling can modify the structure and function of developing synapses.6,7 In mammals, application of exogenous Wnts has diverse effects on developing neurons, including pre- and post-synaptic differentiation, changes in spine density and on the endocytosis and recycling of neurotransmitter receptors.8,9 Together, these studies raise the question of whether Wnt signaling continues in the mature nervous system to dynamically regulate synaptic function.
We had previously found that CAM-1, a Ror-family receptor tyrosine kinase (RTK), is selectively required for cholinergic signaling mediated by ACR-16, the C. elegans homolog of the vertebrate α7 nicotinic acetylcholine receptor (AChR).10 In cam-1 mutants, the decreased ACh-gated current at the neuromuscular junction (NMJ) appears to be secondary to reduced surface delivery of ACR-16/α7. Because a CAM-1/RTK variant that lacked the intracellular kinase domain was functional,10 we reasoned that CAM-1/RTK might contribute to a heteromeric cell-surface receptor that regulates synaptic delivery of ACR-16/α7.
Using a genetic approach to identify modifiers of cholinergic neurotransmission,11 we found that translocation was dependent on LIN-17 (Frizzled receptor), CWN-2 (Wnt ligand related to Wnt5a) and DSH-1 (disheveled, the intracellular mediator of Wnt signaling). In these mutants, synaptic connectivity and neuromuscular architecture were apparently normal; however, translocation of ACR-16/α7 to the postsynaptic membrane was defective, resulting in accumulation of the receptor in subsynaptic compartments. The importance of Wnt signaling in the mature nervous system was revealed using heat shock expression to rescue Wnt signaling in adult mutants, which restored synaptic translocation of ACR-16/α7.
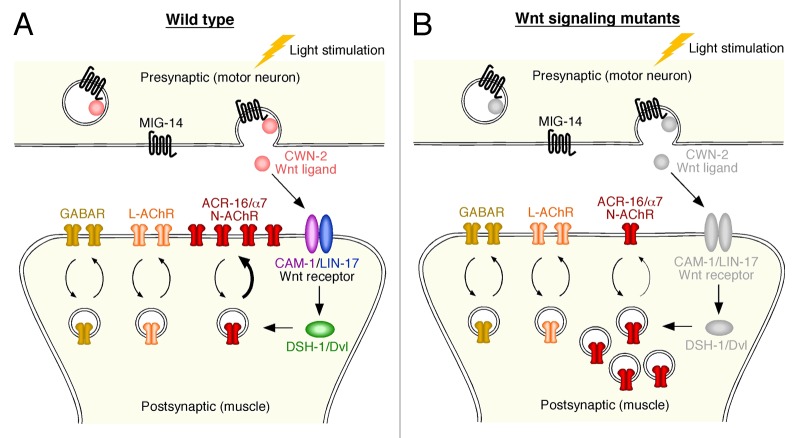
Our discovery that Wnt signaling functions in the adult nervous system led us to the hypothesis that it might have an ongoing role in regulating synaptic strength. To investigate this possibility, we developed a new optogenetic paradigm to study synaptic plasticity in C. elegans. Using light to stimulate motor neurons that expressed channelrhodopsin-2, we demonstrated that repeated activation of presynaptic neurons led to receptor translocation and increased ACR-16-mediated synaptic currents in postsynaptic muscle cells (Fig. 1A). These activity-dependent changes were abolished in Wnt signaling mutants (Fig. 1B). We determined that CWN-2/Wnt5a secreted from motor neurons was both necessary and sufficient for these activity-dependent changes in synaptic strength using tissue-specific rescue and knockout experiments.
Figure 1. Wnt signaling in the adult C. elegans nervous system is required for activity-dependent synaptic plasticity. (A) Motor neurons release CWN-2/Wnt5a, which binds to a novel heteromeric receptor composed of CAM-1/Ror receptor tyrosine kinase and LIN-17/Frizzled. Depending on motor neuron activity, MIG-14 mediated CWN-2 release can lead to a rapid, DSH-1/disheveled-dependent translocation of ACR-16/α7 nicotinic receptors to the synapse and an increase in receptor-mediated current. (B) NMJ morphology is intact in Wnt signaling mutants (cwn-2, cam-1, lin-17 or dsh-1), and other classes of neurotransmitter receptor (GABARs, levamisole AChRs) are normally localized, but postsynaptic ACR-16/α7 nicotinic AChRs are reduced with an associated increase in intracellular accumulations of these receptors.
Using bifluorescence complementation, we also found that the CWN-2/Wnt5a ligand signaled through a novel heteromeric receptor composed of the CAM-1/RTK and the LIN-17/Frizzled proteins. Presumably, dependence on a heteromeric receptor provides additional signaling specificity. For example, synaptic activity might be required to form the heteromer composed of CAM-1 and LIN-17. Alternatively, the heteromeric receptor might shunt signaling from the canonical Wnt signaling pathway to an alternate pathway, such as the PCP pathway, to cause rapid changes in receptor translocation and synaptic transmission. Activity-dependent changes in ACR-16/α7 were independent of new protein synthesis, indicating that signaling was not dependent on the canonical β-catenin-dependent pathway and that a novel signaling system is recruited for synaptic plasticity mediated by receptor translocation.
Dynamic translocation of receptors from subcellular compartments to the surface membrane is an essential and conserved feature found in diverse processes, including aquaporin-mediated fluid homeostasis and insulin-induced translocation of glucose transporters. Yet, we still have only a limited mechanistic understanding of how extracellular signals lead to precise changes in translocation of receptors. Studying these processes in neurons is particularly challenging given that the complex network of signaling molecules that surround neurons is disrupted in cultured cells. Thus, we argue that the NMJ in C. elegans provides an ideal platform for in vivo mechanistic studies of signaling-mediated receptor translocation. Furthermore, because the NMJ contains other classes of neurotransmitter receptors, which are not regulated by CWN-2 signaling and can serve as essential controls, we can use a systematic genetic approach to identify the gene products that regulate Wnt signaling-dependent receptor translocation. These experiments should shed light on evolutionarily conserved pathways for the control of synaptic plasticity and receptor homeostasis and could lead to new insights into learning, memory and disorders associated with defects in nervous system function.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/21138
References
- 1.van Amerongen R, et al. Development. 2009;136:3205–14. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- 2.Budnik V, et al. Curr Opin Neurobiol. 2011;21:151–9. doi: 10.1016/j.conb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korkut C, et al. Nat Rev Neurosci. 2009;10:627–34. doi: 10.1038/nrn2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kessels HW, et al. Neuron. 2009;61:340–50. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy MJ, et al. Neuron. 2011;69:856–75. doi: 10.1016/j.neuron.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korkut C, et al. Cell. 2009;139:393–404. doi: 10.1016/j.cell.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babu K, et al. Neuron. 2011;71:103–16. doi: 10.1016/j.neuron.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inestrosa NC, et al. Nat Rev Neurosci. 2010;11:77–86. doi: 10.1038/nrn2755. [DOI] [PubMed] [Google Scholar]
- 9.Ciani L, et al. Proc Natl Acad Sci USA. 2011;108:10732–7. doi: 10.1073/pnas.1018132108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francis MM, et al. Neuron. 2005;46:581–94. doi: 10.1016/j.neuron.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Jensen M, et al. Cell. 2012;149:173–87. doi: 10.1016/j.cell.2011.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]