Abstract
Comment on: Liu J, et al. Cell Cycle 2012; 11:2643-9.
Repair of DNA double-strand breaks (DSB) is essential for cell survival. Two major pathways, the highly accurate homologous recombination (HR) repair and the more error-prone non-homologous end-joining (NHEJ) pathway, are engaged in these repair processes, and their relative contribution is dependent on the cell cycle. Phosphorylation events triggered by DNA damage response and checkpoint kinases, including ATM, ATR, DNA-PK, CHK1 and CHK2, are important signaling mechanisms during DSB repair. However, in order for DSB repair to proceed in a coordinated manner and to be completed, the phosphorylation of key proteins in the pathways needs to be reversed at specific steps by protein dephosphorylation. The phosphatases PP1, PP2, PP4, PP6 and PPM1D have so far been implicated in homologous recombination repair of DSBs.1 Some of the substrates that are dephosphorylated by these phosphatases are replication protein A2 (RPA2), a single-stranded DNA binding protein and gamma-H2AX, a phosphorylated specialized histone, both proteins serving as platforms for the repair reactions.1,2
It has been less clear which protein phosphatases are involved in the nonhomologous end-joining pathway. In this issue of Cell Cycle, Liu et al. identified a PP4 phosphatase complex as an important component of NHEJ.3 Using a GFP-based in vivo NHEJ assay and a random plasmid integration assay, the authors show that depletion of PP4, by targeting either its catalytic subunit PP4C or its regulatory subunit PP4R2 compromises NHEJ. Following immunoprecipitation of PP4R2, the authors conducted mass spectrometric analysis of proteins associating with this subunit. Besides other components of the PP4 phosphatase complex, they identified the protein KAP1 as a strongly enriched binding partner. KAP1 is a scaffold protein functioning as a transcriptional repressor in most experimental systems, owing to its ability to associate with epigenetic modulators including histone H3 lysine 9 methyltransferases, histone deacetylases and heterochromatin protein 1 (HP1).4 KAP1 interacts with the sequence-specific KRAB zinc finger proteins and other transcription factors, facilitating its binding to specific compartments of the genome.
DSB repair needs to operate effectively throughout the entire nucleus, both within the more easily accessible active chromatin and within compacted, heterochromatinized loci. Earlier studies had indicated that KAP1 plays an important role as a phosphorylation target during the DNA damage response and in DSB processing in heterochromatin.5-7 Serine 824, which is near the C-terminal bromodomain of KAP1, becomes phosphorylated by ATM upon treatment of cells with DNA damaging agents that produce DSBs. Liu et al. now show that depletion of PP4C leads to an increase of KAP1 serine-824 phosphorylation, and that PP4 can dephosphorylate KAP1 in vitro. Similar results were reported recently by Lee et al.8 Liu et al. also found that KAP1 promotes NHEJ in vivo, and that it seems to be in the same pathway with PP4, although the effect of PP4 depletion on NHEJ efficiency was stronger, suggesting that this phosphatase also dephosphorylates other proteins involved in this repair pathway.3 The current data suggest that appropriate phosphorylation of KAP1 may enable DSB repair, whereas hyper- or hypo-phosphorylation could be detrimental to the repair process. Interestingly, KAP1 has been hypothesized to prevent homologous recombination at certain loci. Chromatin-immunoprecipitation studies have suggested that prevalent genomic targets of KAP1 are near the 3′ ends of many zinc finger genes,4 although it has not been possible to identify a transcriptional role for KAP1 at these binding sites. These ZNF genes contain blocks of high sequence homology that could be lost during recombination processes. Future studies will need to determine if unphosphorylated KAP1 indeed functions as a sequence-specific repressor of homologous recombination in the absence of DNA damage.
The study by Liu et al. provides important initial insights into the role of the PP4 phosphatase complex in this major DSB repair process.3 One target of the phosphatase has been identified as the co-repressor KAP1, the phosphorylation of which is thought to promote relaxation of inaccessible chromatin. Besides having a direct role at DNA damage sites, KAP1 phosphorylation and dephosphorylation are also thought to have a transcriptional role in the DNA damage response.9 Future studies will determine the functional details of the KAP1 phosphorylation-dephosphorylation cycle (Fig. 1) in the NHEJ pathway of DNA strand break repair.
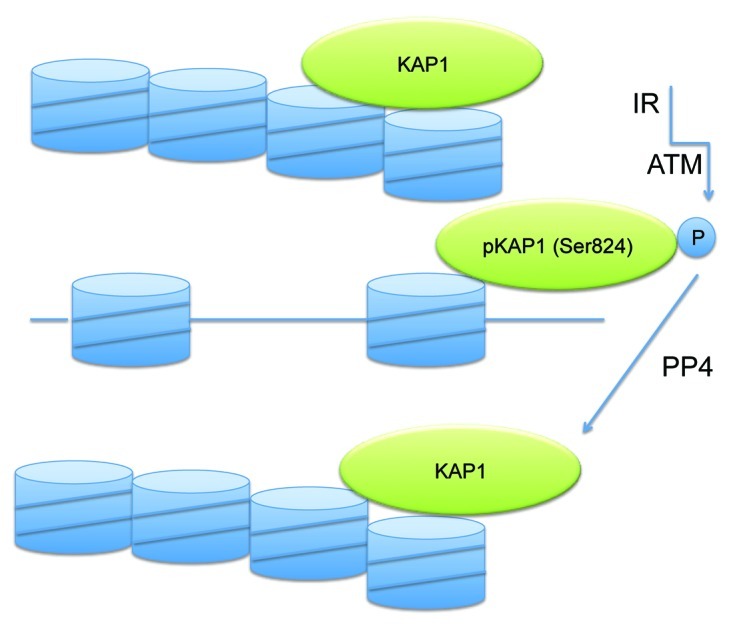
Figure 1. Schematic diagram of KAP1 phosphorylation and dephosphorylation. ATM-mediated phosphorylation of KAP1 on serine 824 leads to transient relaxation of chromatin allowing double-strand break repair processes to operate more easily within inactive chromatin domains. KAP1 phosphorylation is reversed by the PP4 phosphatase complex before completion of the repair process.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/21234
References
- 1.Lee DH, et al. Trends Biochem Sci. 2011;36:569–77. doi: 10.1016/j.tibs.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chowdhury D, et al. Mol Cell. 2008;31:33–46. doi: 10.1016/j.molcel.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J, et al. Cell Cycle. 2012;11:2643–9. doi: 10.4161/cc.20957. [DOI] [PubMed] [Google Scholar]
- 4.Iyengar S, et al. J Biol Chem. 2011;286:26267–76. doi: 10.1074/jbc.R111.252569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noon AT, et al. Nat Cell Biol. 2010;12:177–84. doi: 10.1038/ncb2017. [DOI] [PubMed] [Google Scholar]
- 6.White DE, et al. Cancer Res. 2006;66:11594–9. doi: 10.1158/0008-5472.CAN-06-4138. [DOI] [PubMed] [Google Scholar]
- 7.Ziv Y, et al. Nat Cell Biol. 2006;8:870–6. doi: 10.1038/ncb1446. [DOI] [PubMed] [Google Scholar]
- 8.Lee DH, et al. EMBO J. 2012;31:2403–15. doi: 10.1038/emboj.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, et al. J Biol Chem. 2007;282:36177–89. doi: 10.1074/jbc.M706912200. [DOI] [PubMed] [Google Scholar]


