Abstract
Background
Culex pipiens L. is the most widespread mosquito vector in temperate regions including North Africa. Cx. pipiens has two recognized forms or biotypes; pipiens and molestus are morphologically indistinguishable with distinct behavior and physiology that may influence their vectorial status. In our study, we prospected for the different forms of Cx. pipiens in Morocco.
Methods
Cx. pipiens larvae were collected in 9 sites throughout Morocco during summer 2010 and reared until imago stage. Cx. pipiens was identified using diagnostic primers designed for the flanking region of microsatellite CQ11.
Results
We established the presence of both forms of Cx. pipiens and their hybrids in Morocco.
Conclusions
Molecular identification provides the first evidence of the presence of Cx. pipiens form molestus in Morocco and hybrids between pipiens and molestus forms in North Africa. The epidemiological implications of our findings are discussed.
Keywords: Culex pipiens complex, Microsatellite, Molecular taxonomy, Morocco, North Africa
Background
The Culex pipiens complex includes several species; Cx. pipiens pipiens Linnaeus, 1758 and Cx. pipiens quinquefasciatus Say, 1823 are the most ubiquitous mosquitoes in temperate and tropical regions, respectively. Cx. p. pipiens has two distinct forms or biotypes: form pipiens and form molestus which are morphologically indistinguishable and differ in physiology and behavior. Cx. pipiens form pipiens is subjected to diapause (heterodynamic), is anautogeneous (only lays eggs after a blood-meal), and eurygamous (unable to mate in confined spaces). On the other hand, Cx. pipiens form molestus Forskal, 1775 does not diapause (homodynamic), is autogeneous (lays first batch of eggs without taking a blood-meal) and stenogamous (mates in confined spaces) [1,2]. In addition, the biotypes molestus and pipiens occupy distinct habitats in Russia and the northeastern United States. Indeed, molestus form occurs in underground areas in urban settings while pipiens form lives aboveground [3,4]. In Europe, sympatric occurrence of both biotypes has been observed in aboveground habitats as well as in underground habitats [5-7]. The two forms did not seem to be genetically isolated and were reported to hybridize in the United States and Europe [6-8]. They have different trophic preferences: pipiens biting mainly birds and molestus feeding on mammals, whereas hybrids exhibit an opportunistic behavior and can readily feed on both hosts. These feeding patterns are thought to influence the transmission of avian and mammalian pathogens.
In North Africa, Cx. p. pipiens is a competent vector of several pathogens infecting animals and humans including West Nile virus [9], Rift Valley Fever virus [10-12] and filarial worms [13-16]. Based on morphological characters, behavioral and reproductive specializations, the mosquito Cx. p. pipiens was described in the North African region [17-25]. Nevertheless, these classical characters present limited value. Therefore, our study aims to identify members of the Cx. pipiens complex present in Morocco based on a molecular identification.
Methods
Mosquitoes were collected as larvae using the “dipping” sampling method during summer 2010 from three Moroccan regions (Figure 1). A total of 9 sites were classified according to the habitat (urban, suburban or rural) and the type of breeding site (aboveground or underground). Fourth instar larvae were used for morphological identification [26] and reared until imago stage at 28 ± 1°C with 80% relative humidity and a 16 h:8 h photoperiod. Emerged adults were conserved at −20°C for subsequent molecular characterization.
Figure 1.
Localization of the collection sites in Morocco.
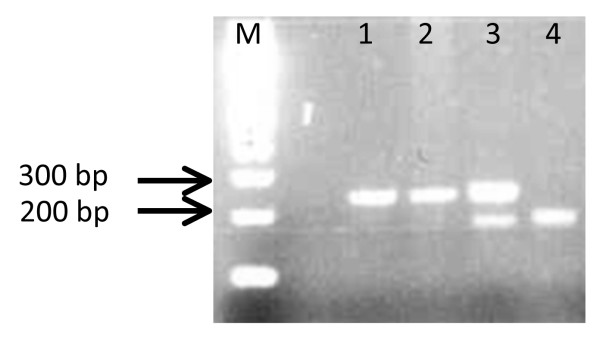
DNA extraction from F0 individuals was performed using the method of DNAzol as described in the manufacturer’s protocol. Specimens were identified as belonging to the Culex pipiens complex using a multiplex PCR assay described in Bahnck and Fonseca (2006) [27]. The locus CQ11 was used to distinguish between the two forms of Cx. pipiens. The DNA fragment size amplified varied between pipiens and molestus allowing us to distinguish the two forms in a single PCR reaction (Figure 2). Specimens of Cx. pipiens molestus from Japan were used as control.
Figure 2.
Example of PCR amplification of the flanking region of the CQ11 microsatellite ofCulex pipienscollected in an underground site in Casablanca (Morocco). DNA was extracted from individual mosquitoes and identified by PCR amplification of the flanking region of the CQ11 microsatellite. Lane M: 100-bp size marker; Lane 1: control Cx. pipiens form molestus from Japan; Lane 2: molestus form; Lane 3: pipiens form; Lane 4: hybrid form.
Results and Discussion
A total of 214 adults were characterized by PCR and frequencies of different forms are represented in Table 1. Overall, 52.3% of adults tested were homozygous for the 200 bp fragment which is characteristic of the pipiens form, 22% were homozygous for the 250 bp fragment identifying the molestus form and the remaining (25.7%) corresponded to hybrids.
Table 1.
Frequency of forms of theCulex pipienscomplex in Morocco
| City |
Habitat |
Breeding site |
Pipiens |
Molestus |
Hybrids |
|---|---|---|---|---|---|
| (Ground) | form (%) | form (%) | (%) | ||
| Tanger |
Urban |
Above |
69.6 (16) |
8.7 (2) |
21.7 (5) |
| |
Sub-urban |
Above |
52.2 (12) |
34.8 (8) |
13 (3) |
| |
Rural |
Above |
62.5 (15) |
8.3 (2) |
29.2 (7) |
| Casablanca/ |
Urban |
Above |
31 (9) |
17.2 (5) |
51.8(15) |
| Mohammedia |
Urban |
Under |
25 (8) |
59.4 (19) |
15.6 (5) |
| |
Sub-urban |
Above |
53.6 (15) |
17.8 (5) |
28.6 (8) |
| Marrakech |
Sub-urban |
Above |
60.9 (14) |
8.7 (2) |
30.4 (7) |
| |
Rural |
Above |
78.3 (18) |
4.3 (1) |
17.4 (4) |
| Rural | Under | 55.6 (5) | 33.3 (3) | 11.1 (1) |
Cx.pipiens larvae were collected at various sites in Morocco, reared to adults and identified by PCR amplification of the flanking region of the CQ11 microsatellite. In brackets, number of tested mosquitoes.
This study provides the first molecular evidence for the presence of Cx. pipiens form molestus in Morocco and hybrids in North Africa.
The molestus form has been described as a distinct species, Cx. molestus Forskal, 1775 from autogeneous Egyptian specimens. Because Cx. pipiens form molestus is stenogamous and autogenous, it colonizes underground areas in urban settings [3] with limited geographic distribution throughout the world. In our study, Cx. pipiens form pipiens and form molestus were found in urban, suburban and rural habitats. Indistinctly, the two forms co-occur in aboveground and underground breeding sites. Sympatric distribution of the biotypes molestus and pipiens in surface breeding sites has been observed in southern Europe and the United States [5,6,8] and in underground breeding sites in North Europe [7].
Conclusions
Until now, hybrids were mainly reported in the United States [4,8] and South Europe [6]. Our findings corroborate the presence of hybrids in all breeding sites sampled. Hybrids between molestus and pipiens forms are considered of great epidemiological importance. They exhibit intermediate physiological and behavioral traits [28] and can readily feed on avian and mammalian hosts [8,29]. This opportunistic biting behavior will potentiate the role of Cx. pipiens as a bridge-vector for the transmission of pathogens such as West Nile virus, from birds (amplification hosts) to humans [8,30].
Competing interests
The authors declare that they have no competing interests.
Authors’contributions
FA carried out mosquito genotyping, contributed to the interpretation of results and drafted the manuscript. MT participated in the design of experiments. MS participated in the design of experiments and mosquito collections. ABF designed the experiments and drafted the manuscript. All authors read and approved the final manuscript.
Contributor Information
Fadila Amraoui, Email: amraoui_fadila@yahoo.fr.
Mhamed Tijane, Email: tijane@fsr.ac.ma.
Mhammed Sarih, Email: mhammed.sarih@pasteur.ma.
Anna-Bella Failloux, Email: anna-bella.failloux@pasteur.fr.
Acknowledgments
We thank Laurence Mousson for technical help. FA was supported by the “Division Internationale” of the Institut Pasteur. This work was funded by the Institut Pasteur (ACIP grant A-08-2009) and the European Commission Framework Program Seven Award “InfraVec”.
References
- Harbach RE, Harrison BA, Gad AM. Culex (Culex) molestus Forskal (Diptera: Culicidae): Neotype designation, description, variation, and taxonomic status. Proc Entomol Soc Wash. 1984;86:521–542. [Google Scholar]
- Harbach RE, Dahl C, White GB. Culex (Culex) pipiens Linnaeus (Diptera, Culicidae) - concepts, type designations, and description. Proc Entomol Soc Wash. 1985;87:1–24. [Google Scholar]
- Byrne K, Nichols RA. Culex pipiens in London Underground tunnels: differentiation between surface and subterranean populations. Heredity. 1999;82:-15. doi: 10.1038/sj.hdy.6884120. [DOI] [PubMed] [Google Scholar]
- Huang S, Molaei G, Andreadis TG. Genetic insights into the population structure of Culex pipiens (Diptera: Culicidae) in the Northeastern United States by using microsatellite analysis. AmJTrop Med Hyg. 2008;79:518–527. [PubMed] [Google Scholar]
- Chevillon C, Eritja R, Pasteur N, Raymond M. Commensalism, adaptation and gene flow: mosquitoes of the Culex pipiens complex in different habitats. Genet Res. 1995;66:147–157. doi: 10.1017/S0016672300034492. [DOI] [PubMed] [Google Scholar]
- Gomes B, Sousa CA, Novo MT, Freitas FB, Alves R, Côrte-Real AR, Salgueiro P, Donnelly MJ, Almeida AP, Pinto J. Asymmetric introgression between sympatric molestus and pipiens forms of Culex pipiens (Diptera: Culicidae) in the Comporta region, Portugal. BMC Evol Biol. 2009;9:262. doi: 10.1186/1471-2148-9-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusken CBEM, de Vries A, Buijs J, Braks MAH, den Hartog W, Scholte EJ. First evidence for presence of Culex pipiens biotype molestus in the Netherlands, and of hybrid biotype pipiens and molestus in northern Europe. J Vector Ecol. 2010;35:210–212. doi: 10.1111/j.1948-7134.2010.00080.x. [DOI] [PubMed] [Google Scholar]
- Fonseca DM, Keyghobadi N, Malcolm CA, Mehmet C, Schaffner F, Mogi M, Fleischer RC, Wilkerson RC. Emerging vectors in the Culex pipiens complex. Science. 2004;303:1535–1538. doi: 10.1126/science.1094247. [DOI] [PubMed] [Google Scholar]
- Krida G, Diancourt L, Bouattour A, Rhim A, Chermiti B, Failloux AB. Assessment of the risk of introduction to Tunisia of the Rift Valley fever virus by the mosquito Culex pipiens. Bull Soc Pathol Exot. 2011;104:250–259. doi: 10.1007/s13149-010-0122-4. [DOI] [PubMed] [Google Scholar]
- Hoogstraal H, Meegan JM, Khalil GM, Adham FK. The Rift Valley fever epizootic in Egypt 1977–78. 2. Ecological and entomological studies. Trans R Soc Trop Med Hyg. 1979;73:624–629. doi: 10.1016/0035-9203(79)90005-1. [DOI] [PubMed] [Google Scholar]
- Meegan JM, Khalil GM, Hoogstraal H, Adham FK. Experimental transmission and field isolation studies implicating Culex pipiens as a vector of Rift Valley fever virus in Egypt. AmJTrop Med Hyg. 1980;29:1405–1410. doi: 10.4269/ajtmh.1980.29.1405. [DOI] [PubMed] [Google Scholar]
- Moutailler S, Krida G, Schaffner F, Vazeille M, Failloux AB. Potential vectors of Rift Valley fever virus in the Mediterranean Region. Vector Borne Zoonot Dis. 2008;8:749–753. doi: 10.1089/vbz.2008.0009. [DOI] [PubMed] [Google Scholar]
- Harb M, Faris R, Gad AM, Hafez ON, Ramzi R, Buck AA. The resurgence of lymphatic filariasis in the Nile Delta. Bull WHO. 1993;71:49–54. [PMC free article] [PubMed] [Google Scholar]
- Krida G, Bouattour A, Rodhain F, Failloux AB. Variability among Tunisian populations of Culex pipiens: genetic structure and susceptibility to a filarial parasite, Brugia pahangi. Parasitol Res. 1998;84:139–142. doi: 10.1007/s004360050371. [DOI] [PubMed] [Google Scholar]
- Abdel-Hamid YM, Soliman MI, Allam KM. Spatial distribution and abundance of culicine mosquitoes in relation to the risk of filariasis transmission in El Sharqiya Governorate, Egypt. Egypt Acad J Biolog Sci. 2009;1:39–48. [Google Scholar]
- Abdel-Hamid YM, Soliman MI, kenawy MA. Mosquitoes (Diptera: Culicidae) in relation to the risk of disease transmission in El Ismailia governorate, Egypt. J Egypt Soc Parasitol. 2011;41:109–118. [PubMed] [Google Scholar]
- Roubaud E. Le pouvoir autogène chez le biotype nord-africain du moustique commun Culex pipiens (L.) Bull Soc Path Exot. 1939;36:172–175. [Google Scholar]
- Knight KL, Malek AA. A morphological and biological study of Culex pipiens in the Cairo area of Egypt. Bull Soc Fouad I Entomol. 1951;35:175–185. [Google Scholar]
- Gaud J. Notes biogéographiques sur les Culicidés du Maroc. Arch Inst Pasteur Maroc. 1953;4:443–490. [Google Scholar]
- Vermeil C. Nouvelle contribution à l’étude du complexe Culex pipiens en Tunisie. Bull Soc Pathol Exot. 1954;47:841–843. [PubMed] [Google Scholar]
- Rioux JA. Les culicidés du “midi” méditerranéen. Lechevalier, Paris; 1958. [Google Scholar]
- Senevet G, Andarelli L, Graells R. A propos de Culex pipiens en Algérie. Arch Inst Pasteur Algérie. 1958;36:70–74. [PubMed] [Google Scholar]
- Rioux JA, Juminer B, Kchouk M, Croset H. Présence du caractère autogène chez Culex pipiens pipiens L. dans un biotope épigé de l’Ile de Djerba. Arch Inst Pasteur Tunis. 1965;42:1–8. [Google Scholar]
- Pasteur N, Rioux JA, Guilvard E, Pech-Perières J. Nouvelle mention, pour le “Midi” méditerranéen, de populations naturelles anautogènes et sténogames de Culex pipiens pipiens L. Ann Parasitol Hum Comp. 1977;52:205–210. [PubMed] [Google Scholar]
- Himmi O, Dakki M, Trari B, El Agbani MA. Les Culicidae du Maroc: clés d’identification, avec données biologiques et écologiques. Trav Inst Sci, Série Zool Rabat. 1995;44:51. [Google Scholar]
- Brunhes J, Rhaim A, Geoffroy B, Angel G, Hervy JP. Les moustiques de l’Afrique méditerranéenne. Logiciel d’identification et d’enseignement. IRD & IPT, CD-Rom collection didactique, Éditions IRD, Montpellier, France; 2000. [Google Scholar]
- Bahnk CM, Fonseca DM. Rapid assay to identify the two genetic forms of Culex (Culex) pipiens L. (Diptera: Culicidae) and hybrid populations. AmJTrop Med Hyg. 2006;75:251–255. [PubMed] [Google Scholar]
- Spielman A. Structure and seasonality of Nearctic Culex pipiens populations. Ann NY Acad Sci. 2001;951:220–234. doi: 10.1111/j.1749-6632.2001.tb02699.x. [DOI] [PubMed] [Google Scholar]
- Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, Daszak P, Fonseca DM. Genetic influences on mosquito feeding behavior and the emergence of zoonotic pathogens. AmJTrop Med Hyg. 2007;77:667–671. [PubMed] [Google Scholar]
- Hamer GL, Kitron UD, Brawn JD, Loss SR, Ruiz MO, Goldberg TL, Walker ED. Culex pipiens (Diptera: Culicidae): a bridge vector of West Niles Virus to humans. J Med Entomol. 2008;45:125–128. doi: 10.1603/0022-2585(2008)45[125:CPDCAB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]