Abstract
The ATP-sensitive potassium (KATP) channel consisting of the inward rectifier Kir6.2 and SUR1 (sulfonylurea receptor 1) couples cell metabolism to membrane excitability and regulates insulin secretion. Inhibition by intracellular ATP is a hallmark feature of the channel. ATP sensitivity is conferred by Kir6.2 but enhanced by SUR1. The mechanism by which SUR1 increases channel ATP sensitivity is not understood. In this study, we report molecular interactions between SUR1 and Kir6.2 that markedly alter channel ATP sensitivity. Channels bearing an E203K mutation in SUR1 and a Q52E in Kir6.2 exhibit ATP sensitivity ∼100-fold higher than wild-type channels. Cross-linking of E203C in SUR1 and Q52C in Kir6.2 locks the channel in a closed state and is reversible by reducing agents, demonstrating close proximity of the two residues. Our results reveal that ATP sensitivity in KATP channels is a dynamic parameter dictated by interactions between SUR1 and Kir6.2.
INTRODUCTION
Heteromeric ATP-sensitive potassium (KATP) channels comprising inwardly rectifying potassium channels Kir6.x and sulfonylurea receptors SURx are molecular transducers that couple cell metabolism with cell excitability (Ashcroft, 1988; Aguilar-Bryan and Bryan, 1999; Nichols, 2006). The most extensively characterized are those formed by Kir6.2 and SUR1 (Inagaki et al., 1995), which express predominantly in pancreatic endocrine cells and neurons where they regulate hormone secretion and neuronal activity. Mutations in Kir6.2 and SUR1 that cause loss of channel function result in the insulin secretion disease congenital hyperinsulinism, whereas those causing gain of channel function lead to neonatal diabetes and in some cases developmental and neurological defects referred to as the DEND syndrome (Ashcroft, 2005).
A cardinal feature of KATP channels is their sensitivity to intracellular ATP (Ashcroft, 1988; Aguilar-Bryan and Bryan, 1999; Nichols, 2006). In inside-out membrane patches, ATP inhibits KATP currents with a half-maximal inhibitory concentration (IC50) of ∼10–20 µM (Aguilar-Bryan and Bryan, 1999; Nichols, 2006). Evidence to date indicates that ATP interacts with the pore subunit Kir6.2 to gate the channel closed (Tucker et al., 1997). Channels formed by Kir6.2 alone, made possible using a variant in which an endoplasmic reticulum retention signal in the distal C-terminal 35 aa is deleted, referred to as Kir6.2ΔC, to allow SUR1-independent surface expression (Zerangue et al., 1999), are still sensitive to ATP inhibition (Tucker et al., 1997). However, compared with channels formed by Kir6.2 (or Kir6.2ΔC) and SUR1, those formed by Kir6.2ΔC alone are significantly less sensitive to ATP (IC50 ∼140 µM; Tucker et al., 1997; Enkvetchakul et al., 2000; Babenko and Bryan, 2003). How SUR1 increases the ATP sensitivity of Kir6.2 remains a conundrum.
Another central feature of KATP channel gating is activation by membrane phosphoinositides, in particular phosphatidylinositol 4,5-bisphosphates (PIP2; Hilgemann and Ball, 1996; Fan and Makielski, 1997; Shyng and Nichols, 1998; Barrett-Jolley et al., 1999). PIP2 increases the open probability (Po) of the channel and renders it less sensitive to ATP inhibition (Baukrowitz et al., 1998; Shyng and Nichols, 1998). Like ATP, PIP2 is thought to interact directly with Kir6.2 to gate the channel (Baukrowitz et al., 1998; Shyng and Nichols, 1998; Wang et al., 2002). Moreover, as is the case for ATP, SUR1 greatly enhances channel sensitivity to PIP2 stimulation (Baukrowitz et al., 1998; Enkvetchakul et al., 2000). We have recently shown that this increased sensitivity to PIP2 results from stabilization of Kir6.2–PIP2 interactions via the N-terminal transmembrane domain (TMD) of SUR1 (aa 1–196; referred to as TMD0) and underlies the high Po and bursting pattern of single channel gating seen in wild-type (WT) channels as compared with Kir6.2ΔC channels (Pratt et al., 2009). Although TMD0 of SUR1 is sufficient to increase the sensitivity of Kir6.2 to PIP2, it does not confer the high ATP sensitivity observed in the Kir6.2/SUR1 channels. In fact, channels formed by TMD0-SUR1 and Kir6.2ΔC exhibit even more reduced ATP sensitivity than Kir6.2ΔC channels (Babenko and Bryan, 2003; Chan et al., 2003), suggesting the structural elements in SUR1 that endow the Kir6.2 channel with high ATP sensitivity lie outside TMD0.
In this study, we set out to identify Kir6.2-SUR1 intersubunit interactions that control channel ATP sensitivity. Using scanning mutagenesis and cysteine cross-linking approaches, we discovered a molecular interaction between SUR1 and Kir6.2 that profoundly impacts KATP channel response to ATP. We show that coexpression of a Kir6.2 with the mutation Q52E in the N-terminal domain and a SUR1 with the mutation E203K in the cytoplasmic region immediately following TMD0 yielded channels with ATP sensitivity nearly 100-fold higher than WT channels. This unprecedented high ATP sensitivity is a result of interactions between the two mutant residues, as cross-linking of the two residues mutated to cysteines stabilized channels in a closed state in an ATP-independent manner, unresponsive to PIP2 stimulation. Our results reveal an inter SUR1-Kir6.2 molecular coupling mechanism that controls channel sensitivity to ATP and PIP2. We propose that the interface between SUR1 and Kir6.2 near the plasma membrane where the two mutations reside is a key structural domain that determines ligand sensitivities in the heteromeric KATP channel complex.
MATERIALS AND METHODS
Molecular biology
Rat Kir6.2 cDNA constructs including the full-length WT subunit and a truncation mutant lacking the C-terminal 36 aa (Kir6.2ΔC) are in pCDNAI/Amp plasmid (Lin et al., 2008). Hamster SUR1 constructs are in pECE and include full-length subunits with or without an N-terminal FLAG epitope (DYKDDDDK; f-SUR1). The FLAG epitope does not change biochemical or functional properties of the channel (Cartier et al., 2001). Site-directed mutagenesis was performed using the QuikChange mutagenesis kit (Agilent Technologies), and mutations were confirmed by direct sequencing.
Immunoblotting
COSm6 cells were maintained in DMEM with 10% FBS and 1% penicillin/streptomycin. Cells ∼70% confluent on 35-mm dishes were transfected with 0.6 µg f-SUR1 and 0.4 µg Kir6.2 using FuGene6 (Roche). Cells were lysed 48–72 h after transfection in a buffer containing 20 mM HEPES, 5 mM EDTA, 150 mM NaCl, and 1% Triton-X with Complete protease inhibitors cocktail tablet, pH 7.0 (Roche; Yan et al., 2004, 2007). Protein concentrations were determined using the Lowry assay (Bio-Rad Laboratories). Equal amount of protein from each sample was separated by SDS-PAGE, transferred to nitrocellulose membrane, analyzed by an anti-SUR1 antibody raised against the C terminus of hamster SUR1 (KDSVFASFVRADK; Yan et al., 2007) followed by horseradish peroxidase–conjugated anti–rabbit secondary antibody (GE Healthcare), and visualized by chemiluminescence (Super Signal West Femto; Thermo Fisher Scientific). Densitometry was performed using ImageJ (National Institutes of Health).
Chemiluminescence assays
48 h after transfection, cells in 35-mm dishes were fixed with 2% paraformaldehyde for 30 min, preblocked in PBS/0.5% BSA for 30 min, incubated in 10 µg/ml M2 anti-FLAG antibody (Sigma-Aldrich) for 1 h, washed four times for 30 min in PBS/0.5% BSA, incubated in horseradish peroxidase–conjugated anti–mouse antibodies for 20 min, and washed again four times for 30 min in PBS/0.5% BSA. Chemiluminescence was quantified using a TD-20/20 luminometer (Turner Designs) after 10-s incubation in Power Signal ELISA Femto luminol solution (Thermo Fisher Scientific). Results of each experiment are the mean of two 35-mm dishes, and each data point shown is the mean of at least three experiments.
86Rb+ efflux assay
COSm6 cells were plated onto 6-well 35-mm culture dishes and transfected with WT SUR1 and WT or mutant rat Kir6.2 cDNA as described for immunoblotting. Cells were incubated for 24 h in culture medium containing 1 µCi/ml 86RbCl 2 d after transfection. Before measurement of 86Rb+ efflux, cells were incubated for 30 min at room temperature in Krebs-Ringer solution with metabolic inhibitors (2.5 µg/ml oligomycin and 1 mM 2-deoxy-d-glucose). At selected time points, the solution was aspirated from the cells and replaced with fresh solution. At the end of a 40-min period, cells were lysed. The 86Rb+ in the aspirated solution and the cell lysate was counted. The percentage efflux at each time point was calculated as the cumulative counts in the aspirated solution divided by the total counts from the solutions and the cell lysate (Shyng and Nichols, 1998).
Patch-clamp recordings
Voltage-clamp recordings from inside-out patches of transfected COSm6 cells were performed using an Axopatch 1D amplifier and pClamp9 acquisition software (Axon Inc.; Yan et al., 2004). Micropipettes were pulled from nonheparinized Kimble glass (Thermo Fisher Scientific) on a horizontal puller (Sutter Instrument) and had resistances of ∼1.5–2.0 MΩ. The bath and pipette solution (intracellular potassium solution [Kint]) was 140 mM KCl, 10 mM K-HEPES, and 1 mM K-EGTA, pH 7.4. 1 mM EDTA was added to Kint (Kint/EDTA) to prevent channel rundown (Lin et al., 2003). All currents were measured at −50 mV membrane potential (50 mV pipette potential) at room temperature, and inward currents are shown as upward deflections in all figures. When recording from cells expressing E128K mutant channels, the cells were pretreated with 300 µM tolbutamide overnight to augment expression, followed by a 2-h washout period. PIP2 (Sigma-Aldrich) was aliquoted in Kint at 5 mM, stored at −20°C until use, and then diluted in Kint/EDTA and sonicated for 15 min in ice water before use.
Po analysis
Intrinsic Po was estimated in two ways. For estimation by stationary noise analysis, short recordings (∼1 s) of macroscopic currents in Kint/EDTA or Kint/EDTA plus 1 mM ATP at −50 mV were used. Currents were sampled at >20 kHz and filtered at 5 kHz. Absolute mean current (I) and variance (σ2) in the absence of ATP were obtained by subtraction of the mean current and variance of ATP-inhibited patches. Single channel current (i) was assumed to be 3.6 pA at −50 mV in symmetrical Kint solution (corresponding to single channel conductance of 72 pS). Po was then calculated using the equation: Po = 1 − [σ2/(i × I)] (Sigworth, 1980; Shyng et al., 1997). For estimation by PIP2 response, each patch was treated with 5 µM PIP2 until the current reached a maximum; this max was assumed to represent near maximal Po of ∼0.97 (Enkvetchakul et al., 2000; Koster et al., 2008). Estimate of channel Po before PIP2 stimulation was then back-calculated using the equation Po = 0.97/(fold increase), where fold-increase = IPIP2/Icontrol, with Icontrol and IPIP2 being the current amplitude before and after PIP2 application, respectively.
Data analysis
Data are presented as means ± SEM. ATP inhibition dose–response data were fit with the Hill function (Irel = 1/(1 + ([ATP]/IC50)H)), where Irel is the current relative to maximal current in nucleotide-free Kint/EDTA solution, IC50 is the concentration of ATP that causes half-maximal inhibition, and H is the Hill coefficient. Statistical analysis was performed using independent two-population, two-tailed Student’s t test or one-way ANOVA with P < 0.05 considered statistically significant. Excel (Microsoft), Origin (OriginLab), and Prism (GraphPad Software) software were used to carry out data analysis.
Online supplemental material
Fig. S1 shows schematic representations of Kir6.2 and SUR1 proteins with details of ATP sensitivity screens performed. Fig. S2 shows that the Q52E-Kir mutation does not exert its effect on ATP sensitivity in the absence of SUR1. Fig. S3 shows that Q52C-Kir6.2//E203C-SUR1 channels have WT-like ATP sensitivity in the absence of cross-linking. Fig. S4 shows that control channels do not cross-link in the presence of an inhibitory concentration of ATP plus oxidizing agent; it also shows that Tris(2-carboxyethyl)phosphine (TCEP) is an effective reducing agent to reverse the cross-linking within Q52C-Kir6.2//E203C-SUR1 channels. Fig. S5 illustrates that Q52C-Kir6.2//E128C-SUR1 channels show no evidence of cross-linking. Fig. S6 shows MgADP stimulation of Q52E-Kir6.2 channels. Online supplemental material is available at http://www.jgp.org/cgi/content/full/jgp.201210803/DC1.
RESULTS
Mutation of Q52 to a negatively charged amino acid increases ATP sensitivity of SUR1/Kir6.2 channels
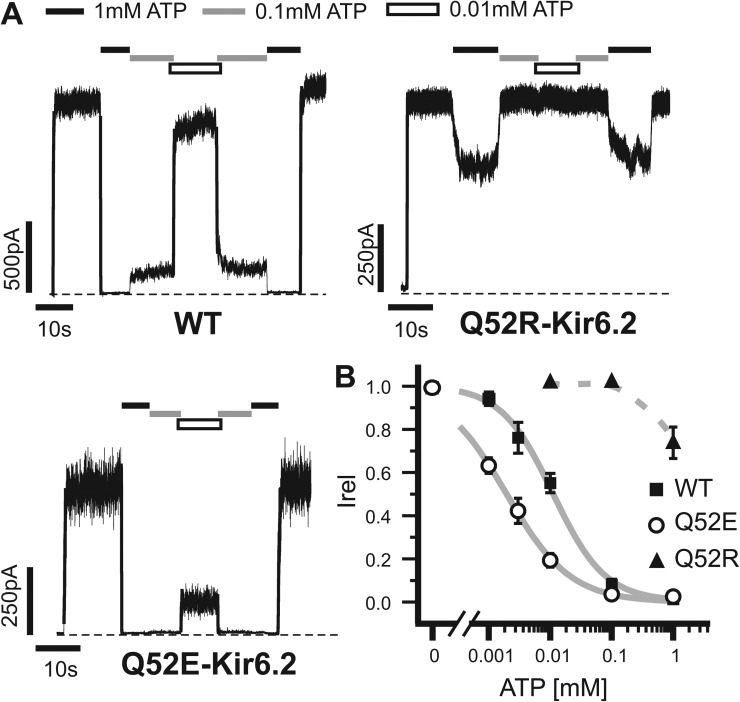
To identify Kir6.2 residues that might interact with SUR1 to modulate ATP sensitivity, we first conducted scanning mutagenesis of Kir6.2. We focused on Kir6.2 cytoplasmic domain residues in regions that are close to the proposed ATP-binding pocket and that are predicted to be surface exposed in the tetrameric structural model for potential interactions with SUR1 (Fig. S1, A for channel schematic and B for residues tested). These residues were mutated to glutamate or arginine, and ATP sensitivity of the resulting channels was measured. We observed altered ATP sensitivity in several mutations (Fig. S1 C). Most of them reduced channel ATP sensitivity just as numerous other mutations that have been reported before (Tucker et al., 1998; Enkvetchakul et al., 2000; Loussouarn et al., 2001; Cukras et al., 2002; Gloyn et al., 2004; Proks et al., 2004). However, Q52E stood out as the only mutation that significantly enhanced ATP sensitivity. The Q52E mutation caused a greater than fivefold increase in ATP sensitivity (IC50 of 2.0 ± 0.1 µM compared with the IC50 of 11 ± 1.0 µM for WT channels; Fig. 1). Interestingly, mutation of Q52 to a positively charged arginine (Q52R) is known to cause neonatal diabetes by increasing channel open state stability (Po; see Discussion) and decreasing channel ATP sensitivity (Fig. 1; Proks et al., 2004; Koster et al., 2005; Lin et al., 2006a; Tammaro et al., 2006). Q52R appears distinct from other ATP-insensitive mutations in that it has been proposed to cause ATP insensitivity in a SUR1-dependent way (Tammaro et al., 2006). In the absence of SUR1, Kir6.2ΔC channels harboring the Q52R mutation have ATP sensitivity indistinguishable from Kir6.2ΔC channels without the mutation (Tammaro et al., 2006). We therefore focused on this residue and tested whether the effect of Q52E on ATP sensitivity is also SUR1 dependent. Because in the absence of SUR1 the currents from the various Kir6.2ΔC channels were very small such that it was difficult to obtain an accurate IC50, we assessed ATP sensitivity by comparing the residual currents in 0.1 and 1 mM ATP (Fig. S2). The results show that in the absence of SUR1, ATP sensitivity of Q52E-Kir6.2ΔC channels is not significantly different from that of Kir6.2ΔC channels or Q52R-Kir6.2ΔC channels but is significantly lower than WT channels formed by Kir6.2 and SUR1. These findings suggest that mutation of Q52 to a negatively or positively charged amino acid may alter interactions with SUR1 to increase or decrease ATP sensitivity.
Figure 1.
ATP sensitivity of Q52-Kir6.2 mutants is charge dependent. (A) Representative traces from inside-out patch voltage-clamp recordings from COSm6 cells transfected with WT-SUR1 and WT-Kir6.2, Q52R-Kir6.2, or Q52E-Kir6.2 cDNA. Patches were exposed to various concentrations of ATP or nucleotide-free Kint/EDTA control solution. Dashed lines represent zero current. (B) Dose–response data illustrating the mean currents in several ATP concentrations relative to maximum in nucleotide-free solution. Best fit curves (solid lines) were generated using the Hill equation for WT and Q52E (IC50: 11 ± 1 µM and 2 ± 0 µM, respectively). Q52R dose–response data were fit manually (dashed line), with an estimated IC50 of ∼1 mM, similar to the value we reported previously (IC50 = 889 µM; Lin et al., 2006a). n = 3–10 for each data point. Error bars represent SEM, and some are smaller than the symbols.
A SUR1 mutation functionally interacts with Q52E of Kir6.2 to enhance ATP sensitivity
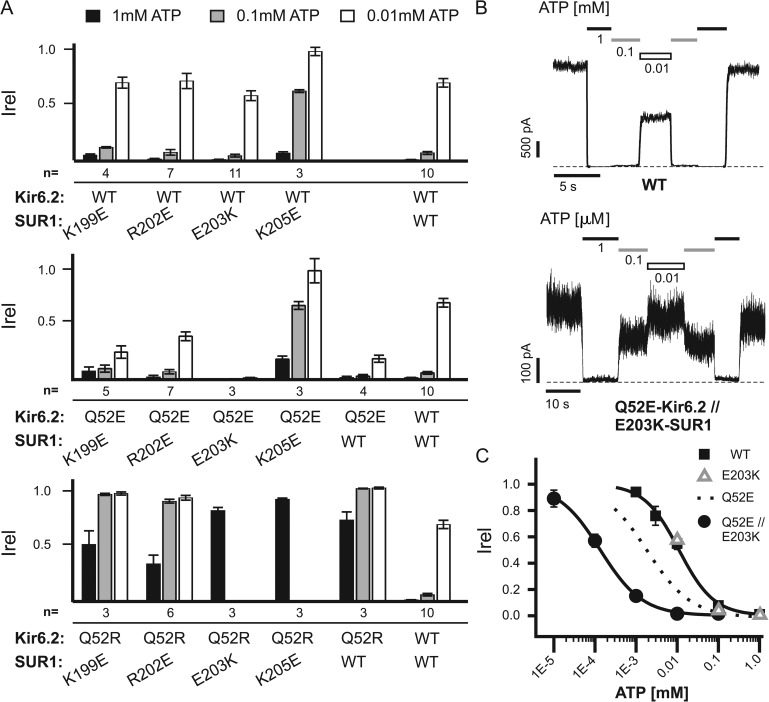
To identify a residue or residues in SUR1 that might interact with Q52E or Q52R to influence ATP sensitivity, we performed mutagenesis of SUR1, focusing on the following charged residues: K199, R202, E203, and K205. These residues are in a short stretch of cytosolic amino acids immediately following TMD0 and before the suggested amphipathic “sliding” helix in L0 (Fig. S1 A; Babenko, 2005). Sites targeted in the study were chosen based on a twofold rationale. First, Q52 resides adjacent to Kir6.2’s “slide” helix (also referred to as the “interfacial” helix [Hansen et al., 2011; Whorton and MacKinnon, 2011]), which has been proposed to lie near the sliding helix of SUR1 (Babenko, 2005), and thus, the screen was limited to residues adjacent to the SUR1 sliding helix (Fig. S1, A and B). Second, they are likely positioned close to the plasma membrane as Q52-Kir6.2 is based on homology models of Kir6.2 using closely related Kir channel crystal structures (Antcliff et al., 2005; Whorton and MacKinnon, 2011). These residues were mutated to oppositely charged amino acids (K199E, R202E, E203K, and K205E) and then coexpressed with WT-, Q52E-, or Q52R-Kir6.2. ATP sensitivity of the resulting channels was estimated by inside-out patch voltage-clamp recording in three different ATP concentrations (Fig. 2 A). When coexpressed with WT-Kir6.2, only K205E-SUR1 had an obvious effect on ATP sensitivity, causing reduced inhibition by ATP (hereinafter “//” is used to denote heteromeric Kir6.2 and SUR1 combinations and “/” is used to separate multiple mutations in a single SUR1 or Kir6.2 subunit). When coexpressed with Q52R-Kir6.2, none of the mutant SUR1s was a strong modifier of the low ATP sensitivity seen in Q52R-Kir6.2//WT-SUR1 channels. When coexpressed with Q52E-Kir6.2, the K199E- and R202E-SUR1 did not modify the effect of the Q52E-Kir6.2 mutation on ATP sensitivity. Combination of K205E-SUR1 and Q52E-Kir6.2 yielded channels with decreased ATP sensitivity close to that seen in WT-Kir6//K205E-SUR1 channels. However, the most striking change of ATP sensitivity indicative of functional interaction was seen in channels formed by Q52E-Kir6.2 and E203K-SUR1. The Q52E-Kir6.2//E203K-SUR1 channels exhibited a marked increase in sensitivity to ATP inhibition much greater than that caused by either mutation, with an IC50 of 140 ± 6 nM (or 0.14 µM), nearly 100-fold more sensitive than WT channels (Fig. 2, B and C). The result suggests that E203K of SUR1 interacts with Q52E of Kir6.2 either directly or indirectly to increase ATP sensitivity.
Figure 2.
Screening of SUR1 residues identifies the E203K mutation that when combined with Q52E in Kir6.2 gives rise to channels with extremely high ATP sensitivity. (A) Charged residues located near the SUR1 sliding helix (see Fig. S1, A and B) were mutated to the opposite charge and tested for their effects on ATP sensitivity with WT (top)-, Q52E (middle)-, and Q52R-Kir6.2 (bottom) using inside-out patch voltage-clamp recording. Current in 1, 0.1, and 0.01 mM ATP relative to nucleotide-free Kint/EDTA is shown with error bars representing SEM. Only 1 mM ATP was tested in the cases of E203K-SUR1//Q52R-Kir6.2 and K205E-SUR1//Q52R-Kir6.2 because of extremely low ATP sensitivity. The number of patches tested is given below each condition. (B) Representative traces from WT or Q52E-Kir6.2//E203K-SUR1 KATP channels exposed to various concentrations of ATP or nucleotide-free Kint/EDTA control solution; note difference in ATP concentrations used. Dashed lines represent zero current. (C) Dose–response data illustrating the mean currents in several ATP concentrations relative to maximum in nucleotide-free solution for WT and Q52E-Kir6.2//E203K-SUR1 KATP channels. Best fit curves were generated using the Hill equation (IC50: 11 ± 1 µM for WT and 140 ± 5 nM for Q52E-Kir6.2//E203K-SUR1). n = 3–9 for each data point. For comparison, the best fit curve for Q52E-Kir6.2 (see Fig. 1) and mean currents for the three ATP concentrations tested on E203K-SUR1 channels (see A) were also included. Error bars represent SEM, and some are smaller than the symbols.
Relationship between ATP sensitivity and intrinsic Po of mutant channels
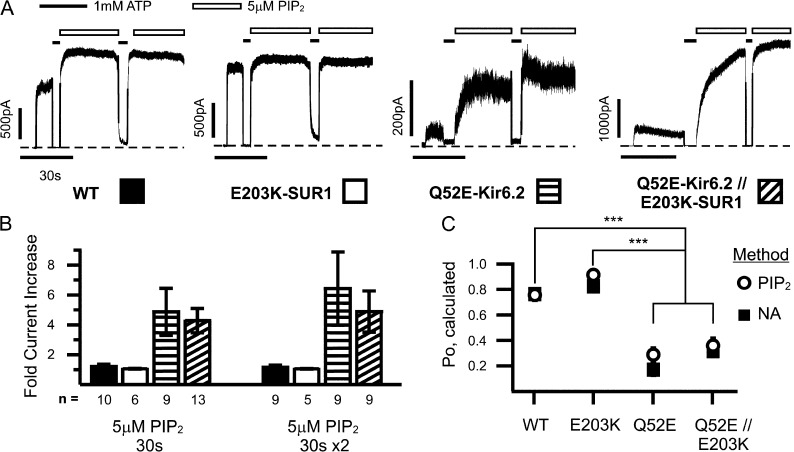
In KATP channels, it is well established that the apparent channel sensitivity to ATP is negatively correlated with channel intrinsic Po, the higher the Po, the lower the ATP sensitivity; intrinsic Po is in turn determined by channel interactions with PIP2, the stronger the interaction, the higher the Po (Enkvetchakul et al., 2000). Accordingly, given the ability to interact with PIP2, the Po of a channel can be pushed toward a maximum by increasing PIP2 concentrations in the membrane. This relationship can be used to estimate intrinsic Po, i.e., after saturating PIP2 treatment, the channel’s starting Po can be back-calculated by comparing post-PIP2 currents (Po assumed to be approaching maximum) to pre-PIP2 currents (Po unknown; see Materials and methods). An alternative method to estimate intrinsic Po from macroscopic currents is stationary noise analysis (Sigworth, 1980), which examines the means and rapid fluctuations in currents to approximate single channel properties as described in our previous studies (Lin et al., 2006b; Pratt et al., 2009). Both methods were used to determine whether increased ATP sensitivity seen in the Q52E-Kir6.2//WT-SUR1 and Q52E-Kir6.2//E203K-SUR1 channels was related to decreased channel Po (Fig. 3).
Figure 3.
Changes in intrinsic Po do not fully account for high Q52E-Kir6.2//E203K-SUR1 ATP sensitivity. (A) Representative traces from inside-out patch voltage-clamp recordings from COSm6 cell transfected with WT or mutant KATP channels exposed to 5 µM PIP2 twice for 30 s. Dashed lines represent zero current. (B) Mean fold increase in current after one or two 30-s treatments with PIP2 for WT or single or double mutant channels. The key to bar graph is indicated by the patterns shown below each condition in A. Number of patches tested is given below each condition. Error bars represent SEM. (C) Intrinsic Po was calculated by two indirect means (see Materials and methods), stationary noise analysis (NA) and PIP2 response method. Number in each dataset for PIP2 method is given in B (right); n for NA method is 19, 18, 17, and 19 for WT, E203K-SUR1, Q52E-Kir6.2, and Q52E-Kir6.2//E203K-SUR1, respectively. Error bars represent SEM, and some are smaller than the symbols. ***, P < 0.001 using one-way ANOVA with Tukey post-hoc test for NA and PIP2 methods.
WT and mutant channels in inside-out patches were exposed to 5 µM PIP2 for 30 s, followed by a 5-s exposure to 1 mM ATP and another 30-s exposure to PIP2 until the currents reached a maximum, as shown in Fig. 3 A. The fold increase of current from baseline was calculated (Fig. 3 B), with Q52E-Kir6.2//WT-SUR1 and Q52E-Kir6.2//E203K-SUR1 showing increased response compared with WT (4.4 ± 0.7, 3.7 ± 0.9 vs. 1.3 ± 0.1, respectively, after second exposure), whereas the E203K-SUR1 response was less than WT (1.1 ± 0.1). Intrinsic Po was estimated using these values (PIP2 method) as well as via noise analysis; estimates from the two methods are compared in Fig. 3 C. Q52E-Kir6.2//WT-SUR1 and Q52E-Kir6.2//E203K-SUR1 mutant channels had significantly reduced Po compared with WT (0.28 ± 0.05, 0.36 ± 0.06 vs. 0.76 ± 0.04 using the PIP2 method and 0.16 ± 0.04, 0.31 ± 0.04 vs. 0.76 ± 0.03 using noise analysis, respectively). Estimates of E203K Po are 0.92 ± 0.02 and 0.83 ± 0.02 using the PIP2 and noise analysis methods. The analyses revealed that although there is a 10-fold difference in IC50 between Q52E-Kir6.2//WT-SUR1 and Q52E-Kir6.2//E203K-SUR1, their estimated intrinsic Po values are not significantly different. This suggests that the unusually high ATP sensitivity of Q52E-Kir6.2//E203K-SUR1 can only be partially accounted for by changes in Po and/or channel–PIP2 interactions (see Discussion).
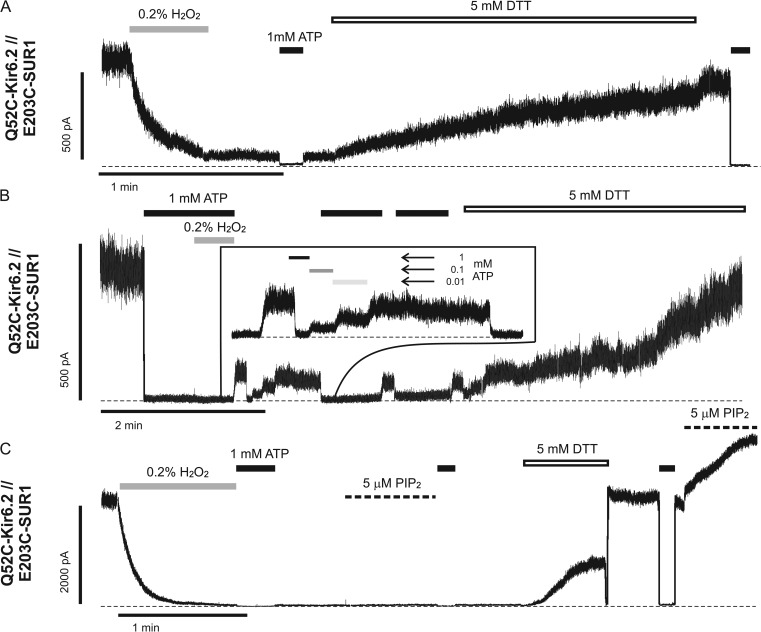
Cross-linking between Q52C of Kir6.2 and E203C of SUR1 stabilizes channels in a closed state
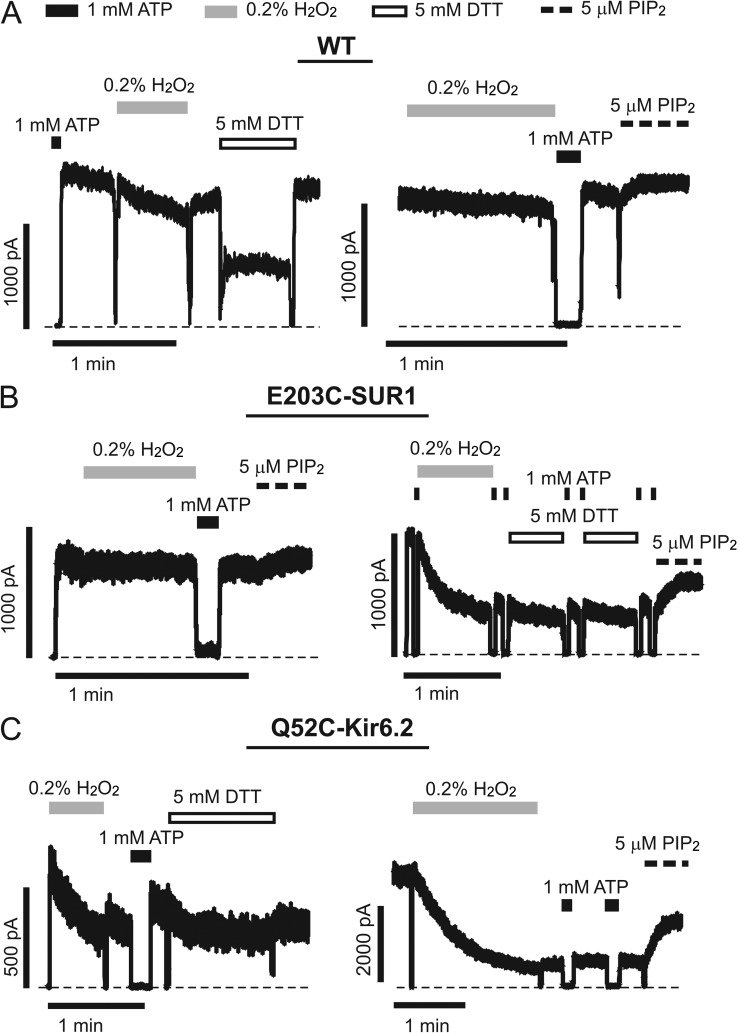
To test whether E203K of SUR1 and Q52E of Kir6.2 are in close proximity to interact directly, we substituted both residues with cysteines and performed cross-linking experiments. Thiol groups of cysteine residues can form disulfide bonds in the presence of an oxidizing agent (H2O2); this covalent interaction is reversible in the presence of a reducing agent (dithiothreitol [DTT] or TCEP). In inside-out patches, Q52C-Kir6.2//E203C-SUR1 channels exhibited ATP sensitivity indistinguishable from that of WT channels (Fig. S3). Strikingly, exposure to 0.2% H2O2 resulted in a rapid decline of channel activity to a residual current plateau (Fig. 4). Although too small to accurately make quantitative comparisons with pre-H2O2 treatment, these residual currents were still sensitive to ATP inhibition (Fig. 4, A and B; and Fig. S3). The residual currents could represent uncross-linked channels caused by incomplete oxidative reactions, cross-linked channels having very low Po, or both. Applications of 1 or 5 mM DTT gradually reversed the effect of H2O2, recovering channel activity to nearly the level seen before H2O2 exposure (Fig. 4); an alternative reducing agent, TCEP, also effectively recovered activity in these channels (Fig. S4). Note DTT at the concentrations used caused reversible channel inhibition as we have reported previously (Lin et al., 2003), partially masking its effect on reversing the H2O2-induced loss of channel activity. Upon returning to Kint/EDTA solution, the extent of recovery of channel activity by DTT became fully visible (Fig. 4). These observations indicate that cross-linking between Q52C-Kir6.2 and E203C-SUR1 causes the channel to enter an ATP-independent closed state. Addition of H2O2 to channels already closed by 1 mM ATP also induced cross-linking as indicated by the greatly reduced channel activity after washout of H2O2 and ATP and subsequent increase in activity with DTT (Fig. 4 B and Fig. S4). Because the Po of KATP channels in the absence of ATP (intrinsic Po) is dependent on channel interaction with PIP2, we asked whether the cross-linked channels could be activated by excess PIP2. As shown in Fig. 4 C (and Fig. S4), PIP2 failed to recover the activity of cross-linked Q52C-Kir6.2//E203C-SUR1 channels; however, after exposure to DTT, channel activity was readily increased by PIP2. These results indicate that cross-linking E203C of SUR1 with Q52C of Kir6.2 induces channel closure and stabilizes the channel in a closed state that cannot be activated by PIP2.
Figure 4.
Reversible cysteine cross-linking is observed between Q52C-Kir6.2 and E203C-SUR1. Representative traces of inside-out patch voltage-clamp recordings from COSm6 cell transfected with Q52C-Kir6.2 and E203C-SUR1. Patches were exposed to oxidizing agent (0.2% H2O2, gray lines), 1 mM ATP (black lines), reducing agent (5 mM DTT, open lines), or 5 µM PIP2 (thick dashed lines). Thin dashed lines represent zero current. (A) Current from Q52C-Kir6.2//E203C-SUR1 channels rapidly decreases to a plateau level in the presence of H2O2 and can be subsequently restored when DTT is applied. This pattern of activity suggests that the proximity of Q52C-Kir6.2 and E203C-SUR1 is close enough to allow intersubunit disulfide bond formation. (B) Cross-linking between Q52C-Kir6.2 and E203C-SUR1 can also occur from the ATP-bound, closed state as indicated by current decline with H2O2 in the presence of saturating ATP concentrations. This trace also shows that plateau current remains sensitive to ATP inhibition (inset; see also Fig. S3). (C) Cross-linking between Q52C-Kir6.2 and E203C-SUR1 locks channels in a PIP2-insensitive closed state. However, once cross-linking is reversed with reducing agent, PIP2-induced stimulation is also restored.
To exclude the possibility that H2O2 and DTT were acting on other endogenous cysteine residues, we performed control experiments on WT channels or channels containing only one cysteine mutation. For WT channels, the majority of the patches (9 out of a total of 12 patches) were little affected by H2O2 (Fig. 5 A). Occasionally we observed a decrease in current amplitude upon H2O2 exposure that differed from Q52C-Kir6.2//E203C-SUR1 channels in that it followed a much slower time course and was readily reversed upon returning to Kint/EDTA or exposure to PIP2 (Fig. 5 A, right). Similar findings were made with WT-Kir6.2//E203C-SUR1 channels (n = 5 with three patches showing little effect by H2O2 and two patches showing decreased currents that were recovered by PIP2; Fig. 5 B). The Q52C-Kir6.2//WT-SUR1 single cysteine mutant appeared more susceptible to H2O2-induced current decrease (12 out of 14 patches showed current decrease), but again the current was readily recovered by exposure to PIP2 (Fig. 5 C, right), suggesting the current decay was likely the result of rundown. Additionally, none of the three control channels showed evidence of cross-linking in the presence of 1 mM ATP (i.e., in the closed configuration; Fig. S4). In all three control channels, DTT exposure caused a reduction of activity that recovered to preexposure levels when channels were returned to the Kint/EDTA solution. The extent of this reduction was most pronounced in WT channels and less obvious in the single cysteine mutants for reasons we do not yet fully understand. Importantly, in no patches, whether preexposed to H2O2 or not, did we observe an increase in channel activity after DTT exposure, indicating that the effects of H2O2 and DTT on the three control channels were unrelated to cysteine cross-linking or cysteine modifications. The aforementioned results provide compelling evidence that in the Kir6.2//SUR1 channel complex, Q52 of Kir6.2 and E203 of SUR1 are in close proximity and cross-linking the two residues in the double cysteine mutant causes channel closure that can be reversed by DTT or TCEP.
Figure 5.
Cross-linking does not occur in WT, WT-Kir6.2//E203C-SUR1, or Q52C-Kir6.2//WT-SUR1 channels. (A–C) Representative traces of inside-out patch voltage-clamp recordings from COSm6 cell transfected with control WT (A), WT-Kir6.2//E203C-SUR1 (B), or Q52C-Kir6.2//WT-SUR1 (C) channels. Patches were exposed to oxidizing agent (0.2% H2O2, gray lines), 1 mM ATP (black lines), reducing agent (5 mM DTT, open lines), or 5 µM PIP2 (thick dashed lines). Thin dashed lines represent zero current. Two examples of each condition are shown. Occasional decreases in current were observed with H2O2 exposure, but always slower and less robust than compared with Q52C-Kir6.2//E203C-SUR1 channels (Fig. 4). Furthermore, control channels remain sensitive to PIP2 stimulation after H2O2 exposure, and in no case did DTT result in increased currents. Brief downward deflections seen in some of the traces are caused by solution exchange artifacts.
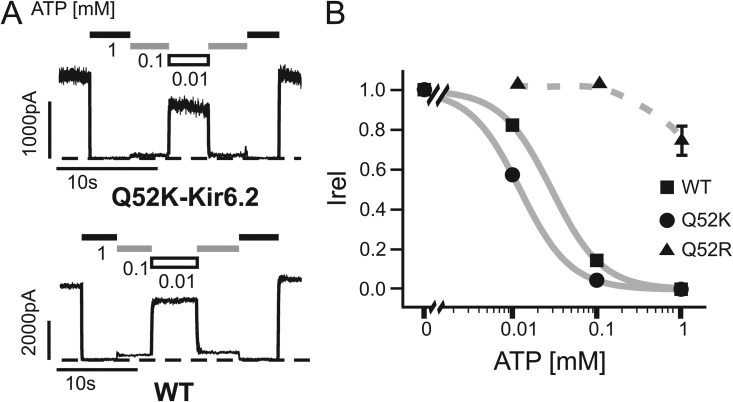
The Q52K-Kir6.2 and E203-SUR1 (WT-SUR1) charge pair does not recapitulate the high ATP sensitivity observed in Q52E-Kir6.2//E203K-SUR1 channels
Based on the high ATP sensitivity observed in the Q52E-Kir6.2//E203K-SUR1 channels and the evidence supporting physical proximity between the two residues, one might predict that reversing the charged amino acids at the two positions would also generate channels with high ATP sensitivity. If this were the case, why do the Q52R-Kir6.2//WT(E203)-SUR1 channels in which charge complementarity is preserved exhibit such low ATP sensitivity? To test whether the larger size of arginine is the culprit, we examined ATP sensitivity of channels formed by Q52K-Kir6.2 and WT(E203)-SUR1. Interestingly, the Q52K-Kir6.2//WT(E203)-SUR1 channel was much more sensitive to ATP inhibition compared with the Q52R-Kir6.2//WT(E203)-SUR1 channel; however, it was only approximately twofold more sensitive to ATP inhibition than WT (Fig. 6), in contrast to the near 100-fold increase in ATP sensitivity observed in Q52E-Kir6.2//E203K-SUR1 (Fig. 2). These results suggest that the specific interaction between Q52E-Kir6.2 and E203K-SUR1 that results in a super ATP-sensitive channel is likely dependent on the structural context surrounding the mutant amino acid pair.
Figure 6.
Q52K-Kir6.2//WT(E203)-SUR1 channels do not have extremely increased ATP sensitivity. (A) Representative traces of inside-out patch voltage-clamp recordings from COSm6 cell transfected with Q52K-Kir6.2//WT-SUR1 or WT channels. Patches were exposed to various concentrations of ATP or nucleotide-free Kint/EDTA control solution. Dashed lines represent zero current. (B) Dose–response data illustrating the mean currents in several ATP concentrations relative to maximum in nucleotide-free solution. Best fit curves (solid lines) were generated using the Hill equation for WT and Q52K-Kir6.2 (IC50: 29 ± 1 µM and 12 ± 0 µM, respectively). Note because only three ATP concentrations were tested, the IC50 values differ from data presented using more thorough analysis. Q52R dose–response data were fit manually (dashed line), with an estimated IC50 of ∼1 mM. n = 3–8 for each data point. Error bars represent SEM, and some are smaller than the symbols.
The effect of Q52-Kir6.2 mutation on ATP sensitivity is abrogated by a TMD0-SUR1 mutation E128K that uncouples SUR1 from Kir6.2
The profoundly reduced ATP sensitivity of the Q52R-Kir6.2//WT-SUR1 channel has been attributed to increased intrinsic channel open state stability (Proks et al., 2004). As the effect of Q52R is SUR1 dependent and open state stability (Po) correlates with the channel’s apparent affinity to PIP2, we considered the possibility that Q52R might enhance channel interaction with PIP2 in a SUR1-dependent manner. Our recent work has shown the TMD0 of SUR1 increases channel Po by stabilizing Kir6.2 in a PIP2-bound open state (Pratt et al., 2011). A mutation in the TMD0 of SUR1, E128K, uncouples SUR1 from Kir6.2, resulting in reduced Po and diminished PIP2 response that resemble those seen in channels formed by Kir6.2ΔC alone (Pratt et al., 2009, 2011). Interestingly, the WT-Kir6.2//E128K-SUR1 channels also have reduced ATP sensitivity close to that of Kir6.2ΔC channels, indicating that interactions between TMD0-SUR1 and Kir6.2 are required for full-length SUR1 to set the ATP sensitivity. We therefore tested how the E128K-SUR1 mutation affects the ability of Q52R-Kir6.2 to increase channel Po and reduce ATP sensitivity. Note because the E128K mutation impairs channel trafficking to the cell surface (Yan et al., 2007), in all experiments involving the use of E128K-SUR1, we treated cells with 300 µM tolbutamide (a KATP channel chaperone which binds reversibly to the channel) overnight to rescue the trafficking defect and increase surface expression of the mutant channels as we have shown previously (Pratt et al., 2009, 2011). Tolbutamide was then washed out for 2 h before recording to remove its channel inhibitory effect.
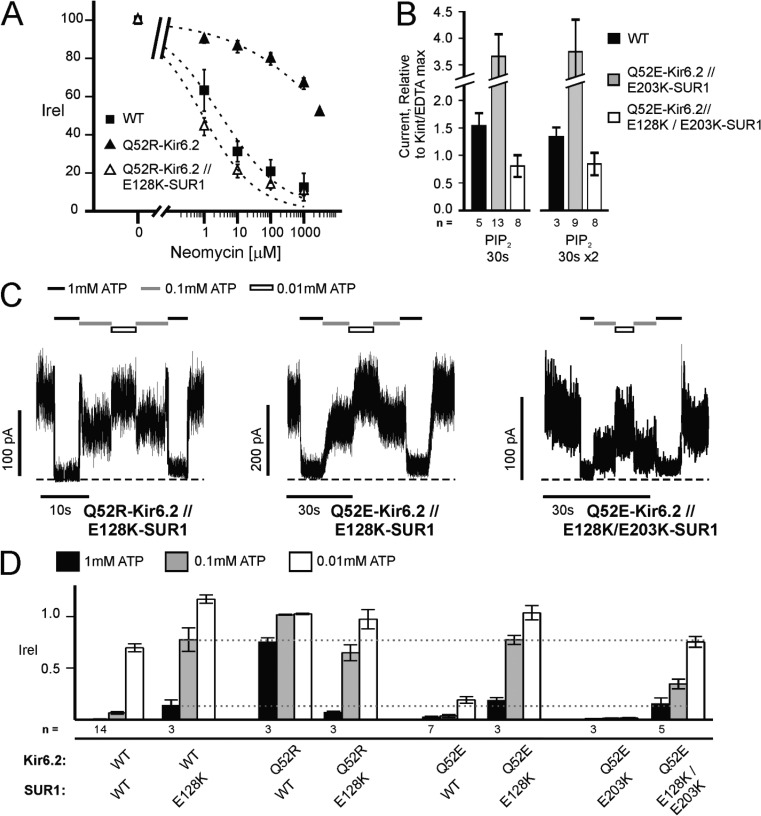
To first determine whether Q52R affects channel interactions with PIP2, channel activity after exposure to different concentrations of neomycin was recorded. Neomycin competes for PIP2 binding, thereby inhibiting channel activity, and has been used to indirectly assess the apparent affinity of Kir channels to PIP2 (Schulze et al., 2003). Neomycin dose–response curves showed that Q52R-Kir6.2//WT-SUR1 channels were indeed much less sensitive to neomycin inhibition (Fig. 7 A). However, when coexpressed with E128K-SUR1, Q52R-Kir6.2 failed to confer increased channel interaction with PIP2. In fact, Q52R-Kir6.2//E128K-SUR1 channels were even more sensitive to neomycin than WT channels, consistent with reduced Po expected from the E128K-SUR1 mutation. As expected, addition of the E128K mutation in SUR1 also diminished the ability of Q52E-Kir6.2//E203K-SUR1 channels to respond to PIP2 stimulation (Fig. 7 B; also see Fig. 3 [A and B] for comparison). Thus, the E128K mutation in SUR1 exerts a dominant effect over the Q52 mutations with regard to channel Po and PIP2 response.
Figure 7.
Phenotype of mutations at Q52 of Kir6.2 and/or E203 of SUR1 is abrogated by the E128K mutation in SUR1. (A) Q52R-Kir6.2 causes an increased apparent PIP2 affinity that is abrogated by E128K-SUR1. Apparent PIP2 affinity was indirectly assessed using neomycin (Fan and Makielski, 1997; Schulze et al., 2003). The increase in apparent PIP2 affinity (i.e., right-shifted neomycin dose–response) of Q52R-Kir6.2 relative to WT is abolished by the inclusion of E128K-SUR1. Dose–response curves (dotted lines) of currents inhibited by neomycin relative to control Kint/EDTA solution were calculated using the Hill equation (IC50 for WT, Q52R-Kir6.2, and Q52R-Kir6.2//E128K-SUR1 is 3.2 ± 1.3 µM, 5,935 ± 2,559 µM, and 0.9 ± 0.5 µM, respectively). Error bars represent SEM, and some error bars are smaller than the symbol; n = 4 for each data point. (B) E128K in SUR1 abolishes the ability of Q52E-Kir6.2//E203K-SUR1 channels to be stimulated by PIP2. The mean current increase after one or two 30-s exposures to 5 µM PIP2 relative to maximum control current (similar to Fig. 3, A and B) is shown. Error bars represent SEM. The number of patches tested is given below each condition. (C and D) Inhibition by ATP was tested in channels composed of E128K-SUR1 with Q52E-Kir6.2, Q52R-Kir6.2, or Q52E-Kir6.2//E203K-SUR1. (C) Representative inside-out patch voltage-clamp records. Patches were exposed to various concentrations of ATP or nucleotide-free Kint/EDTA control solution. Dashed lines represent zero current. (D) Averaged inhibition by 1, 0.1, and 0.01 mM ATP for various channel types with and without the E128K-SUR1 mutation. For comparison, the degree of inhibition by 1 and 0.1 mM ATP for E128K-SUR1 is highlighted by gray dotted lines. Error bars represent SEM. The number of patches tested is given below each condition.
Next, we measured ATP sensitivity of channels formed by coexpression of E128K-SUR1 and Q52R-Kir6.2. Rather than the very low ATP sensitivity seen in Q52R-Kir6.2//WT-SUR1 channels, Q52R-Kir6.2//E128K-SUR1 channels exhibited similar ATP sensitivity as WT-Kir6.2//E128K-SUR1 (Fig. 7, C and D). These results provide strong evidence that Q52R enhances channel interactions with PIP2 to increase channel Po and reduce ATP sensitivity and that these effects require functional coupling between TMD0-SUR1 and Kir6.2. We further tested whether Q52R-Kir6.2 might interact with E128-SUR1 to enhance channel–PIP2 interaction by probing the physical proximity between the two residues using the cysteine cross-linking approach. However, no change in channel activity was observed in Q52C-Kir6.2//E128C-SUR1 channels exposed to H2O2 or DTT (Fig. S5). Such negative results neither confirm nor refute the possibility that amino acids at position 128 of SUR1 and position 52 of Kir6.2 might interact chemically to affect channel gating. E128K-SUR1 also suppressed the effects of Q52E-Kir6.2 and Q52E-Kir6.2//E203K-SUR1 on ATP sensitivity. Current inhibition by ATP for Q52E-Kir6.2//E128K-SUR1 and Q52E-Kir6.2//E128K/E203K-SUR1 channels was closer to that of WT-Kir6.2//E128K-SUR1 (and Kir6.2ΔC channels [Pratt et al., 2009]) than that of either Q52E-Kir6.2//WT-SUR1 or Q52E-Kir6.2//E203K-SUR1 (Fig. 7, C and D). Collectively, the results show that functional uncoupling between TMD0-SUR1 and Kir6.2 caused by the E128K mutation precludes the molecular interactions between SUR1 and Kir6.2 necessary to define channel ATP sensitivity.
Channels formed by Q52E-Kir6.2 and E203K-SUR1 are not activated by metabolic inhibition
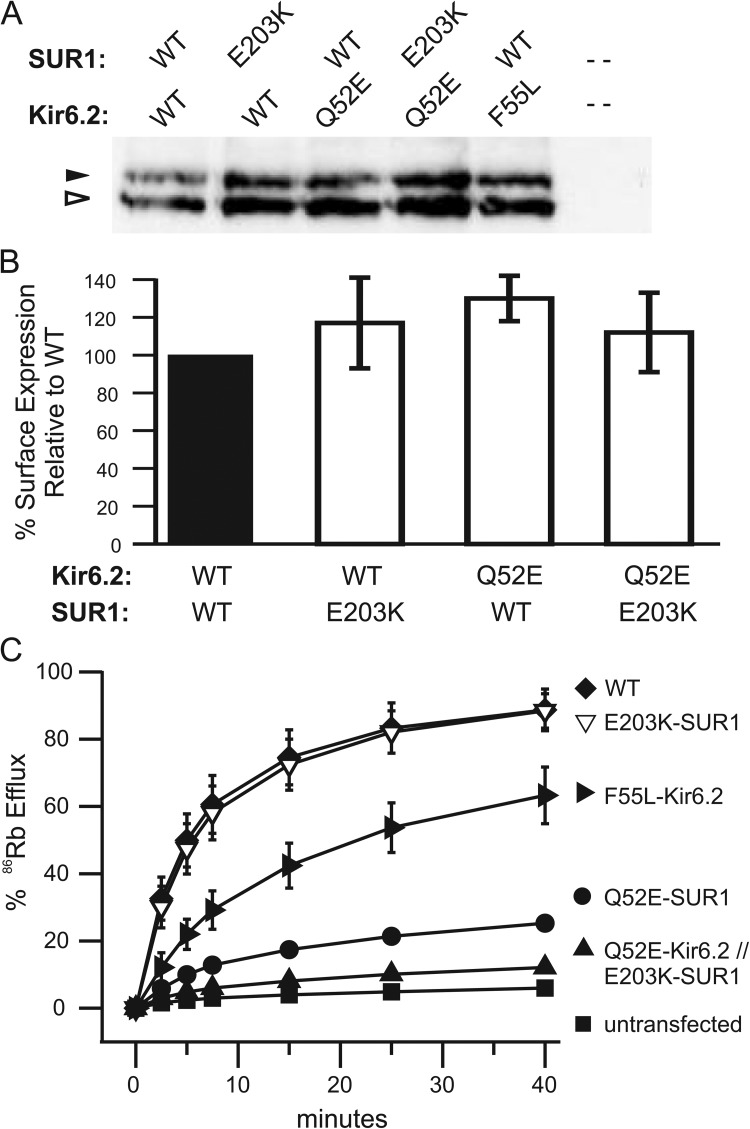
ATP inhibition and MgADP stimulation are two primary physiological mechanisms that regulate KATP channel activity in β cells. Although many gain-of-function mutations cause neonatal diabetes by decreasing channel sensitivity to ATP inhibition (Gloyn et al., 2004; Proks et al., 2004, 2006; Koster et al., 2005), no mutations identified to date in the opposite disease, congenital hyperinsulinism, have been shown to cause loss-of-function by increasing channel sensitivity to ATP. Rather, most congenital hyperinsulinism mutations reduce or abolish channel response to metabolic inhibition by compromising the ability of the channel to be stimulated by MgADP (Shyng et al., 1996; Shyng and Nichols, 1998; Pinney et al., 2008; Macmullen et al., 2011). To test whether hypersensitivity of channels to ATP inhibition will lead to lack of response to metabolic inhibition, we compared the activity of WT and mutant channels in intact cells treated with metabolic inhibitors using a 86Rb+ efflux assay (see Materials and methods; Shyng and Nichols, 1998). Because Q52E-Kir6.2//WT-SUR1 and Q52E-Kir6.2//E203K-SUR1 channels also show reduced intrinsic Po (Fig. 3 C), which could reduce efflux, we included the F55L-Kir6.2//WT-SUR1 mutant that exhibits ∼10-fold reduced intrinsic Po but normal nucleotide sensitivities and surface expression for comparison (Lin et al., 2006b). In WT channels, >90% efflux was observed over a 40-min period. In contrast, both Q52E-Kir6.2//WT-SUR1 and Q52E-Kir6.2//E203K-SUR1 mutants had greatly reduced activity close to untransfected control cells (Fig. 8 C), despite surface expression levels similar to WT (Fig. 8, A and B). The efflux activity of the two mutants correlated with their ATP sensitivity (Figs. 1 B and 2 C): the higher the ATP sensitivity, the lower the channel activity. The control F55L-Kir6.2//WT-SUR1 channel, although also showing reduced efflux relative to WT, was much more active in response to metabolic inhibition than the other two high ATP sensitivity mutants. Collectively, the results suggest that increased ATP sensitivity can lead to loss of KATP channel function under conditions that mimic low glucose such that, if expressed in β-cells, they are predicted to result in hyperinsulinemia and hypoglycemia.
Figure 8.
Increased ATP sensitivity in channels formed by E203K-SUR1 and Q52E-Kir6.2 compromise their ability to open in response to metabolic inhibition. (A and B) Processing and surface expression of KATP channels were assessed in COSm6 cells transfected with WT or mutant channels. (A) Western blot of SUR1 using anti-SUR1 antibody as described in Materials and methods. The complex-glycosylated, mature form of SUR1 (a proxy for surface expression) is indicated by the closed arrowhead, and the core-glycosylated, immature form is indicated by the open arrowhead. Mutations at Q52E-Kir6.2 and/or E203K-SUR1 do not disrupt protein trafficking. (B) KATP surface expression was quantified using chemiluminescence assays. Mutations at Q52E-Kir6.2 or E203K-SUR1 or both do not affect the number of channels at the cell surface. Control F55L-Kir6.2 channels also have WT-like surface expression (Lin et al., 2006b). Error bars represent SEM; n = 3. (C) Mean 86Rb+ efflux profile from COSm6 cells transiently expressing WT or mutant channel subunits. Efflux was measured in Ringer’s solution after metabolic inhibition (i.e., decreased intracellular ATP production). Channels composed of Q52E-Kir6.2 with and without E203K-SUR1 show significantly diminished efflux compared with controls, WT and F55L-Kir6.2; the latter control was included because it has decreased intrinsic Po (∼0.11) but normal ATP sensitivity and surface expression. Error bars represent SEM for n = 3 experiments, except F55L-Kir6.2, which represents the difference between n = 2 experiments. Some error bars are smaller than the symbols.
DISCUSSION
Intracellular ATP-induced closure is a defining property of KATP channels. A wealth of structure–function evidence supports the view that although the Kir6.2 tetramer harbors the ATP-binding sites for channel inhibition, the regulatory SUR subunit enhances sensitivity to this inhibition (Schwappach et al., 2000; Babenko and Bryan, 2003; Chan et al., 2003; Mikhailov et al., 2005). Despite this widely accepted view, the molecular mechanism underlying the effect of SUR1 on Kir6.2 ATP sensitivity is unknown. We show that ATP sensitivity of KATP channels can be increased by two orders of magnitude through engineered interactions between residues 52 of Kir6.2 and 203 of SUR1. The significance of our findings is manifold. First, to our knowledge, the Q52E-Kir6.2//E203K-SUR1 channel is the first mutant reported to have such a profound increase in ATP sensitivity. Second, the Q52C-Kir6.2//E203C-SUR1 cross-linking results provide direct physical and functional evidence for a molecular interaction between SUR1 and Kir6.2 that controls channel activity. Third, the study identifies a structural region at the SUR1-Kir6.2 interface near the plasma membrane that is critical for ATP and PIP2 gating. Finally, the study demonstrates that ATP sensitivity can be tuned bidirectionally by adjusting molecular interactions between SUR1 and Kir6.2 to impact channel function.
Many channel mutations alter apparent ATP sensitivity by modulating intrinsic Po (Enkvetchakul et al., 2000). We found the Po of Q52E-Kir6.2//E203K-SUR1 channels is indeed lower compared with WT channels (Fig. 3 C); however, decreased Po alone cannot account for the marked increase in ATP sensitivity of the channel because Q52E-Kir6.2//WT-SUR1 channels, despite having similarly reduced Po, are ∼15-fold less sensitive to ATP inhibition. Alternatively, these mutations could increase ATP sensitivity by stabilizing channels in an ATP-bound closed state either through direct contributions to ATP binding or allosteric effects. Q52 of Kir6.2 has not been implicated in ATP binding in the numerous mutagenesis studies published to date. Moreover, neither Q52R nor Q52E in Kir6.2 has an effect on ATP sensitivity of channels formed by Kir6.2ΔC alone (Fig. S2). Although it has been suggested that SUR1 increases channel sensitivity to ATP by participating in ATP binding (Babenko and Bryan, 2003; Babenko, 2005) direct evidence is lacking, and the single E203K mutation in SUR1 had little effect on channel ATP sensitivity. Thus, we consider direct contributions to ATP binding a less favorable explanation. In WT channels, ATP promotes closure, whereas PIP2 promotes opening of the ion-conducting pore. That cross-linking of E203C-SUR1 and Q52C-Kir6.2 induces channel closure in the absence of ATP and renders channels refractory to PIP2 stimulation demonstrates that closure of the Kir6.2 pore complex can be achieved by simply altering molecular interactions between SUR1 and Kir6.2 without inhibitory ligands. Together, the evidence led us to propose a scenario wherein the intersubunit interaction between E203K-SUR1 and Q52E-Kir6.2 allosterically stabilizes the channel complex in an ATP-bound closed state to increase the channel’s apparent ATP sensitivity.
Q52 resides in the N terminus of Kir6.2 near the amphipathic slide helix that parallels the plasma membrane. Structural modeling has placed Q52 on the outer surface of the Kir6.2 tetramer (Antcliff et al., 2005), suggesting the residue would be available for interaction with SUR1. E203 of SUR1 is located in the cytoplasmic loop known as ‘L0’ and more specifically, in a stretch of amino acids following the last transmembrane helix of TMD0 and before an amphipathic sliding helix proposed to lie close to the slide helix of Kir6.2 (Fig. S1; Babenko, 2005). It too is expected to be close to the plasma membrane. These predictions are consistent with our data, indicating close proximity of the two residues. Although Q52E-Kir6.2//E203K-SUR1 channels are highly sensitive to ATP inhibition, charge swap at the two positions as in Q52K-Kir6.2//WT(E203)-SUR1 channels does not recapitulate the same markedly increased ATP sensitivity. Even more striking, Q52R-Kir6.2//WT-SUR1 channels have the opposite phenotype and are highly insensitive to ATP. This directionality suggests that interactions between amino acid 203 of SUR1 and amino acid 52 of Kir6.2 are determined not only by the chemical nature of the two residues but also by their surrounding amino acids. Interestingly, the effect of Q52R-Kir6.2, Q52E-Kir6.2, or the combined Q52E-Kir6.2//E203K-SUR1 mutation on ATP sensitivity is dependent on another SUR1 residue, E128, in the short intracellular loop between transmembrane helices 3 and 4 of TMD0. TMD0-SUR1 confers Kir6.2 with the high intrinsic Po and bursting gating patterns observed in WT channels; however, it does not confer the hypersensitizing effect of SUR1 on ATP inhibition (Babenko and Bryan, 2003; Chan et al., 2003). We recently showed that a naturally occurring mutation at this position, E128K, disrupts functional coupling between TMD0-SUR1 and Kir6.2, leading to channels that exhibit reduced Po and reduced PIP2 sensitivity as seen in channels formed by Kir6.2ΔC alone (Pratt et al., 2009, 2011). In addition, E128K reduces channel ATP sensitivity to that seen in Kir6.2ΔC, suggesting the mutation not only prevents functional coupling between TMD0 and Kir6.2 with respect to Po but also between SUR1 structures downstream of TMD0 and Kir6.2 that control ATP sensitivity. Our observations that when E128K-SUR1 was combined with Q52R-Kir6.2, Q52E-Kir6.2, or Q52E-Kir6.2//E203K-SUR1, the resulting channels all had PIP2 and ATP sensitivity closer to that of WT-Kir6.2//E128K-SUR1 (or Kir6.2ΔC) further support this notion. Collectively, these findings point to a crucial role of TMD0, specifically E128, in coordinating the L0-Kir6.2 interface to regulate ATP sensitivity. Given that E128 is also predicted to lie close to the plasma membrane (Pratt et al., 2011), it may contribute to the chemical environment sensed by the ATP sensitivity control module. Although our attempts to probe the physical proximity between E128-SUR1 and Q52-Kir6.2 by cross-linking led to inconclusive results, it remains a possibility that the two residues interact chemically to influence gating. Short of a KATP crystal structure, detailed mutant cycle analysis may help clarify the relationship between these two residues.
Increased ATP sensitivity could render KATP channels less active under physiological conditions and lead to congenital hyperinsulinism, although no such mutation has yet been reported. Our results show deliberate channel mutations that increase sensitivity to ATP inhibition can indeed result in inability of the channel to be activated upon metabolic inhibition. Note we did consider the possibility that the reduced efflux might be caused by impaired response to MgADP. At least in the Q52E-Kir6.2//WT-SUR1 channels, MgADP response was still detectable, although it is difficult to compare the extent of MgADP stimulation between mutant and WT as they have different ATP sensitivity (Fig. S6). In the Q52E-Kir6.2//E203K-SUR1 channels, the ATP sensitivity is so high it was impossible to find a concentration of MgATP or MgADP within the physiological range that would antagonize the inhibitory effect of the nucleotides. Thus, even if the mutant channels were able to respond to MgADP, it was masked by ATP inhibition.
In summary, our study shows that KATP channel activity as determined by PIP2 and ATP sensitivity is a function of SUR1 and Kir6.2 interactions mediated by cytoplasmic domain interfaces near the membrane. Channel activities are known to be modulated by additional ligands including MgATP/ADP, diazoxide, and sulfonylureas. It is tempting to speculate that these stimulatory or inhibitory ligands might control channel activity by modulating SUR1-Kir6.2 cytoplasmic interface and thus altering channel sensitivity to PIP2 and ATP.
Supplementary Material
Acknowledgments
We thank Dr. Jeremy Bushman and Dr. Fang Wang for helpful discussions.
This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases to E.B. Pratt (F30DK081305) and S.-L. Shyng (DK066485-05).
Christopher Miller served as editor.
Footnotes
Abbreviations used in this paper:
- DTT
- dithiothreitol
- IC50
- half-maximal inhibitory concentration
- KATP
- ATP-sensitive potassium
- Kint
- intracellular potassium solution
- PIP2
- phosphatidylinositol 4,5-bisphosphate
- TCEP
- Tris(2-carboxyethyl)phosphine
- TMD
- transmembrane domain
- WT
- wild type
References
- Aguilar-Bryan L., Bryan J. 1999. Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr. Rev. 20:101–135 10.1210/er.20.2.101 [DOI] [PubMed] [Google Scholar]
- Antcliff J.F., Haider S., Proks P., Sansom M.S., Ashcroft F.M. 2005. Functional analysis of a structural model of the ATP-binding site of the KATP channel Kir6.2 subunit. EMBO J. 24:229–239 10.1038/sj.emboj.7600487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F.M. 1988. Adenosine 5′-triphosphate-sensitive potassium channels. Annu. Rev. Neurosci. 11:97–118 10.1146/annurev.ne.11.030188.000525 [DOI] [PubMed] [Google Scholar]
- Ashcroft F.M. 2005. ATP-sensitive potassium channelopathies: focus on insulin secretion. J. Clin. Invest. 115:2047–2058 10.1172/JCI25495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babenko A.P. 2005. K(ATP) channels “vingt ans après”: ATG to PDB to Mechanism. J. Mol. Cell. Cardiol. 39:79–98 10.1016/j.yjmcc.2004.12.004 [DOI] [PubMed] [Google Scholar]
- Babenko A.P., Bryan J. 2003. Sur domains that associate with and gate KATP pores define a novel gatekeeper. J. Biol. Chem. 278:41577–41580 10.1074/jbc.C300363200 [DOI] [PubMed] [Google Scholar]
- Barrett-Jolley R., Dart C., Standen N.B. 1999. Direct block of native and cloned (Kir2.1) inward rectifier K+ channels by chloroethylclonidine. Br. J. Pharmacol. 128:760–766 10.1038/sj.bjp.0702819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baukrowitz T., Schulte U., Oliver D., Herlitze S., Krauter T., Tucker S.J., Ruppersberg J.P., Fakler B. 1998. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 282:1141–1144 10.1126/science.282.5391.1141 [DOI] [PubMed] [Google Scholar]
- Cartier E.A., Conti L.R., Vandenberg C.A., Shyng S.L. 2001. Defective trafficking and function of KATP channels caused by a sulfonylurea receptor 1 mutation associated with persistent hyperinsulinemic hypoglycemia of infancy. Proc. Natl. Acad. Sci. USA. 98:2882–2887 10.1073/pnas.051499698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.W., Zhang H., Logothetis D.E. 2003. N-terminal transmembrane domain of the SUR controls trafficking and gating of Kir6 channel subunits. EMBO J. 22:3833–3843 10.1093/emboj/cdg376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukras C.A., Jeliazkova I., Nichols C.G. 2002. The role of NH2-terminal positive charges in the activity of inward rectifier KATP channels. J. Gen. Physiol. 120:437–446 10.1085/jgp.20028621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enkvetchakul D., Loussouarn G., Makhina E., Shyng S.L., Nichols C.G. 2000. The kinetic and physical basis of K(ATP) channel gating: toward a unified molecular understanding. Biophys. J. 78:2334–2348 10.1016/S0006-3495(00)76779-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z., Makielski J.C. 1997. Anionic phospholipids activate ATP-sensitive potassium channels. J. Biol. Chem. 272:5388–5395 10.1074/jbc.272.9.5388 [DOI] [PubMed] [Google Scholar]
- Gloyn A.L., Pearson E.R., Antcliff J.F., Proks P., Bruining G.J., Slingerland A.S., Howard N., Srinivasan S., Silva J.M., Molnes J., et al. 2004. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N. Engl. J. Med. 350:1838–1849 10.1056/NEJMoa032922 [DOI] [PubMed] [Google Scholar]
- Hansen S.B., Tao X., MacKinnon R. 2011. Structural basis of PIP2 activation of the classical inward rectifier K+ channel Kir2.2. Nature. 477:495–498 10.1038/nature10370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann D.W., Ball R. 1996. Regulation of cardiac Na+,Ca2+ exchange and KATP potassium channels by PIP2. Science. 273:956–959 10.1126/science.273.5277.956 [DOI] [PubMed] [Google Scholar]
- Inagaki N., Gonoi T., Clement J.P., IV, Namba N., Inazawa J., Gonzalez G., Aguilar-Bryan L., Seino S., Bryan J. 1995. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 270:1166–1170 10.1126/science.270.5239.1166 [DOI] [PubMed] [Google Scholar]
- Koster J.C., Remedi M.S., Dao C., Nichols C.G. 2005. ATP and sulfonylurea sensitivity of mutant ATP-sensitive K+ channels in neonatal diabetes: implications for pharmacogenomic therapy. Diabetes. 54:2645–2654 10.2337/diabetes.54.9.2645 [DOI] [PubMed] [Google Scholar]
- Koster J.C., Kurata H.T., Enkvetchakul D., Nichols C.G. 2008. DEND mutation in Kir6.2 (KCNJ11) reveals a flexible N-terminal region critical for ATP-sensing of the KATP channel. Biophys. J. 95:4689–4697 10.1529/biophysj.108.138685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.W., Lin Y.W., Yan F.F., Casey J., Kochhar M., Pratt E.B., Shyng S.L. 2006a. Kir6.2 mutations associated with neonatal diabetes reduce expression of ATP-sensitive K+ channels: implications in disease mechanism and sulfonylurea therapy. Diabetes. 55:1738–1746 10.2337/db05-1571 [DOI] [PubMed] [Google Scholar]
- Lin Y.W., Jia T., Weinsoft A.M., Shyng S.L. 2003. Stabilization of the activity of ATP-sensitive potassium channels by ion pairs formed between adjacent Kir6.2 subunits. J. Gen. Physiol. 122:225–237 10.1085/jgp.200308822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.W., MacMullen C., Ganguly A., Stanley C.A., Shyng S.L. 2006b. A novel KCNJ11 mutation associated with congenital hyperinsulinism reduces the intrinsic open probability of beta-cell ATP-sensitive potassium channels. J. Biol. Chem. 281:3006–3012 10.1074/jbc.M511875200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.W., Bushman J.D., Yan F.F., Haidar S., MacMullen C., Ganguly A., Stanley C.A., Shyng S.L. 2008. Destabilization of ATP-sensitive potassium channel activity by novel KCNJ11 mutations identified in congenital hyperinsulinism. J. Biol. Chem. 283:9146–9156 10.1074/jbc.M708798200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loussouarn G., Pike L.J., Ashcroft F.M., Makhina E.N., Nichols C.G. 2001. Dynamic sensitivity of ATP-sensitive K(+) channels to ATP. J. Biol. Chem. 276:29098–29103 10.1074/jbc.M102365200 [DOI] [PubMed] [Google Scholar]
- Macmullen C.M., Zhou Q., Snider K.E., Tewson P.H., Becker S.A., Aziz A.R., Ganguly A., Shyng S.L., Stanley C.A. 2011. Diazoxide-unresponsive congenital hyperinsulinism in children with dominant mutations of the β-cell sulfonylurea receptor SUR1. Diabetes. 60:1797–1804 10.2337/db10-1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhailov M.V., Campbell J.D., de Wet H., Shimomura K., Zadek B., Collins R.F., Sansom M.S., Ford R.C., Ashcroft F.M. 2005. 3-D structural and functional characterization of the purified KATP channel complex Kir6.2-SUR1. EMBO J. 24:4166–4175 10.1038/sj.emboj.7600877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols C.G. 2006. KATP channels as molecular sensors of cellular metabolism. Nature. 440:470–476 10.1038/nature04711 [DOI] [PubMed] [Google Scholar]
- Pinney S.E., MacMullen C., Becker S., Lin Y.W., Hanna C., Thornton P., Ganguly A., Shyng S.L., Stanley C.A. 2008. Clinical characteristics and biochemical mechanisms of congenital hyperinsulinism associated with dominant KATP channel mutations. J. Clin. Invest. 118:2877–2886 10.1172/JCI35414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt E.B., Yan F.F., Gay J.W., Stanley C.A., Shyng S.L. 2009. Sulfonylurea receptor 1 mutations that cause opposite insulin secretion defects with chemical chaperone exposure. J. Biol. Chem. 284:7951–7959 10.1074/jbc.M807012200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt E.B., Tewson P., Bruederle C.E., Skach W.R., Shyng S.L. 2011. N-terminal transmembrane domain of SUR1 controls gating of Kir6.2 by modulating channel sensitivity to PIP2. J. Gen. Physiol. 137:299–314 10.1085/jgp.201010557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proks P., Antcliff J.F., Lippiat J., Gloyn A.L., Hattersley A.T., Ashcroft F.M. 2004. Molecular basis of Kir6.2 mutations associated with neonatal diabetes or neonatal diabetes plus neurological features. Proc. Natl. Acad. Sci. USA. 101:17539–17544 10.1073/pnas.0404756101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proks P., Arnold A.L., Bruining J., Girard C., Flanagan S.E., Larkin B., Colclough K., Hattersley A.T., Ashcroft F.M., Ellard S. 2006. A heterozygous activating mutation in the sulphonylurea receptor SUR1 (ABCC8) causes neonatal diabetes. Hum. Mol. Genet. 15:1793–1800 10.1093/hmg/ddl101 [DOI] [PubMed] [Google Scholar]
- Schulze D., Krauter T., Fritzenschaft H., Soom M., Baukrowitz T. 2003. Phosphatidylinositol 4,5-bisphosphate (PIP2) modulation of ATP and pH sensitivity in Kir channels. A tale of an active and a silent PIP2 site in the N terminus. J. Biol. Chem. 278:10500–10505 10.1074/jbc.M208413200 [DOI] [PubMed] [Google Scholar]
- Schwappach B., Zerangue N., Jan Y.N., Jan L.Y. 2000. Molecular basis for K(ATP) assembly: transmembrane interactions mediate association of a K+ channel with an ABC transporter. Neuron. 26:155–167 10.1016/S0896-6273(00)81146-0 [DOI] [PubMed] [Google Scholar]
- Shyng S.L., Nichols C.G. 1998. Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science. 282:1138–1141 10.1126/science.282.5391.1138 [DOI] [PubMed] [Google Scholar]
- Shyng S.L., Sha Q., Ferrigni T., Lopatin A.N., Nichols C.G. 1996. Depletion of intracellular polyamines relieves inward rectification of potassium channels. Proc. Natl. Acad. Sci. USA. 93:12014–12019 10.1073/pnas.93.21.12014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng S., Ferrigni T., Nichols C.G. 1997. Regulation of KATP channel activity by diazoxide and MgADP. Distinct functions of the two nucleotide binding folds of the sulfonylurea receptor. J. Gen. Physiol. 110:643–654 10.1085/jgp.110.6.643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F.J. 1980. The variance of sodium current fluctuations at the node of Ranvier. J. Physiol. 307:97–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammaro P., Proks P., Ashcroft F.M. 2006. Functional effects of naturally occurring KCNJ11 mutations causing neonatal diabetes on cloned cardiac KATP channels. J. Physiol. 571:3–14 10.1113/jphysiol.2005.099168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker S.J., Gribble F.M., Zhao C., Trapp S., Ashcroft F.M. 1997. Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature. 387:179–183 10.1038/387179a0 [DOI] [PubMed] [Google Scholar]
- Tucker S.J., Gribble F.M., Proks P., Trapp S., Ryder T.J., Haug T., Reimann F., Ashcroft F.M. 1998. Molecular determinants of KATP channel inhibition by ATP. EMBO J. 17:3290–3296 10.1093/emboj/17.12.3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Wang K., Wang W., Cui Y., Fan Z. 2002. Compromised ATP binding as a mechanism of phosphoinositide modulation of ATP-sensitive K+ channels. FEBS Lett. 532:177–182 10.1016/S0014-5793(02)03671-2 [DOI] [PubMed] [Google Scholar]
- Whorton M.R., MacKinnon R. 2011. Crystal structure of the mammalian GIRK2 K+ channel and gating regulation by G proteins, PIP2, and sodium. Cell. 147:199–208 10.1016/j.cell.2011.07.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan F., Lin C.W., Weisiger E., Cartier E.A., Taschenberger G., Shyng S.L. 2004. Sulfonylureas correct trafficking defects of ATP-sensitive potassium channels caused by mutations in the sulfonylurea receptor. J. Biol. Chem. 279:11096–11105 10.1074/jbc.M312810200 [DOI] [PubMed] [Google Scholar]
- Yan F.F., Lin Y.W., MacMullen C., Ganguly A., Stanley C.A., Shyng S.L. 2007. Congenital hyperinsulinism associated ABCC8 mutations that cause defective trafficking of ATP-sensitive K+ channels: identification and rescue. Diabetes. 56:2339–2348 10.2337/db07-0150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerangue N., Schwappach B., Jan Y.N., Jan L.Y. 1999. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron. 22:537–548 10.1016/S0896-6273(00)80708-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.