Abstract
Phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) regulates activities of numerous ion channels including inwardly rectifying potassium (Kir) channels, KCNQ, TRP, and voltage-gated calcium channels. Several studies suggest that voltage-gated potassium (KV) channels might be regulated by PI(4,5)P2. Wide expression of KV channels in different cells suggests that such regulation could have broad physiological consequences. To study regulation of KV channels by PI(4,5)P2, we have coexpressed several of them in tsA-201 cells with a G protein–coupled receptor (M1R), a voltage-sensitive lipid 5-phosphatase (Dr-VSP), or an engineered fusion protein carrying both lipid 4-phosphatase and 5-phosphatase activity (pseudojanin). These tools deplete PI(4,5)P2 with application of muscarinic agonists, depolarization, or rapamycin, respectively. PI(4,5)P2 at the plasma membrane was monitored by Förster resonance energy transfer (FRET) from PH probes of PLCδ1 simultaneously with whole-cell recordings. Activation of Dr-VSP or recruitment of pseudojanin inhibited KV7.1, KV7.2/7.3, and Kir2.1 channel current by 90–95%. Activation of M1R inhibited KV7.2/7.3 current similarly. With these tools, we tested for potential PI(4,5)P2 regulation of activity of KV1.1/KVβ1.1, KV1.3, KV1.4, and KV1.5/KVβ1.3, KV2.1, KV3.4, KV4.2, KV4.3 (with different KChIPs and DPP6-s), and hERG/KCNE2. Interestingly, we found a substantial removal of inactivation for KV1.1/KVβ1.1 and KV3.4, resulting in up-regulation of current density upon activation of M1R but no changes in activity upon activating only VSP or pseudojanin. The other channels tested except possibly hERG showed no alteration in activity in any of the assays we used. In conclusion, a depletion of PI(4,5)P2 at the plasma membrane by enzymes does not seem to influence activity of most tested KV channels, whereas it does strongly inhibit members of the KV7 and Kir families.
INTRODUCTION
Voltage-gated potassium (KV) channels are essential for repolarization of action potentials in neurons and cardiac, skeletal, and smooth muscle (Hille, 2001; Oliver et al., 2004; Pongs and Schwarz, 2010). Dysfunction of KV channels can lead to severe disease phenotypes ranging from forms of epilepsy to cardiac arrhythmias (Peters et al., 2005; Brown and Passmore, 2009; Charpentier et al., 2010). Because of their important role in governing cell excitability, KV channel activities are tightly controlled. Several modulatory mechanisms have been described. They include phosphorylation and dephosphorylation (Covarrubias et al., 1994; Martens et al., 1999), binding of calcium ions or of calcium-binding proteins like calmodulin (Gamper et al., 2005), binding of ATP (Seino, 1999), and translocation of channels into different cellular compartments by removal from the cell surface (Hicke, 1999). Over the last years, phospholipids have emerged as additional modulators of ion channels including KV channels, especially the low-abundance plasma membrane phosphoinositide, phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2; Hilgemann and Ball, 1996; Hilgemann et al., 2001; Suh and Hille, 2002, 2008; Oliver et al., 2004; Falkenburger et al., 2010a,b; Logothetis et al., 2010; Suh et al., 2010). Here we investigate the PI(4,5)P2 sensitivity of KV channels.
PI(4,5)P2 is localized to the cytoplasmic leaflet of the plasma membrane where it regulates ion channel and transporter activity and plays a role in cellular processes like exo- and endocytosis (Czech, 2000; Hille, 2001; Oliver et al., 2004; Di Paolo and De Camilli, 2006; Pongs and Schwarz, 2010). PI(4,5)P2 can regulate ion channels by binding directly within the channel structure and modulating their gating (Peters et al., 2005; Brown and Passmore, 2009; Charpentier et al., 2010; Hansen et al., 2011; Whorton and MacKinnon, 2011), and it also is the precursor for the generation of second messengers like diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3) through the cleavage of PI(4,5)P2 by phospholipase C (PLC; Covarrubias et al., 1994; Martens et al., 1999; Rhee, 2001). DAG and IP3 in turn activate enzymes like PKC and increase intracellular Ca2+ levels, both known modulators of ion channel activity (Covarrubias et al., 1994; Martens et al., 1999; Gamper et al., 2005; Nilius et al., 2005). Therefore, regulation of ion channel activity by PI(4,5)P2 breakdown can be through direct loss of a cofactor for channel gating or through secondary modifications.
Direct regulation of KV channels by PI(4,5)P2 has so far been reported for some KV1, KV3, KV7, and KV11 family members (Seino, 1999; Bian et al., 2001, 2004; Suh and Hille, 2002; Zhang et al., 2003; Oliver et al., 2004; Winks et al., 2005; Li et al., 2005; Decher et al., 2008). For example, quite dramatic changes of gating kinetics of exogenously expressed KV1.1/KVβ1.1, KV1.5/KVβ1.3, and KV3.4 channels by PI(4,5)P2 were seen in membrane patches excised from oocytes of Xenopus laevis. Addition of PI(4,5)P2 to the cytoplasmic face led to a strong increase in current amplitudes through a near elimination of the normal rapid channel inactivation gating (Hicke, 1999; Oliver et al., 2004; Decher et al., 2008). Similar experiments with excised patches from oocytes or Chinese hamster ovary (CHO) cells expressing KV7 (KCNQ) channels showed a PI(4,5)P2 requirement for channel opening (Hilgemann and Ball, 1996; Hilgemann et al., 2001; Suh and Hille, 2002, 2008; Zhang et al., 2003; Oliver et al., 2004; Li et al., 2005; Falkenburger et al., 2010a,b; Logothetis et al., 2010; Suh et al., 2010), but for KV7 channels, much additional evidence comes from another approach, namely by manipulating enzymes that make or destroy PI(4,5)P2 in intact cells. The initial experiments on KV7 channels used PLC and inhibitors of lipid kinases (Suh and Hille, 2002), and subsequent work exploited PI(4,5)P2 phosphatases either by dimerization to a membrane anchor (Suh et al., 2006) or by voltage activation of a voltage-sensitive phosphatase (Murata and Okamura, 2007; Falkenburger et al., 2010b). We consider that by preserving the cellular environment and manipulating enzymes, such experiments explore a more physiological distribution and concentration range of PI(4,5)P2 levels. Of the KV channels, only KV7.2 and KV7.3 have been studied by this style of experiment. In this study, we express members of other families of KV channels together with enzymes in tsA-201 cells, allowing us to manipulate PI(4,5)P2 levels either by a change in membrane potential or by application of agonists like oxotremorine methiodide (Oxo-M) and rapamycin as we recorded KV channel activity using whole-cell recording.
MATERIALS AND METHODS
Cell culture and plasmids
tsA-201 cells were cultured at 37°C and 5% CO2 in DMEM (Invitrogen) supplemented with 10% FBS (PAA) and 0.2% penicillin/streptomycin (Invitrogen). Cells were plated at a density of 50–70% in 35-mm dishes 1 d before transfection. Transfection was performed with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s specifications with 0.2–1.0 µg DNA per 35-mm dish. Cells were plated onto poly-l-lysine–coated glass chips 14–18 h before experiments. In brief, cells were treated with 400 µl 0.05% trypsin (Invitrogen) for 40 s before adding 1 ml DMEM with FBS. The cell suspension was spun down for 1 min at 250 g and resuspended in 1 ml FBS–supplemented DMEM. 200-µl cell suspension was added to a 35-mm plastic dish with coated glass chips and incubated for 14–18 h at 37°C and 5% CO2.
The following plasmids were given to us: human eCFP-PH(PLCδ1) and eYFP-PH(PLCδ1) from K. Jalink (The Netherlands Cancer Institute, Amsterdam, Netherlands); Dr-VSP-IRES-GFP (Dr-VSP) of zebrafish (Danio rerio) from Y. Okamura (Osaka University, Osaka, Japan); LDR (Lyn11-targeted FRB)-CFP from T. Balla (National Institutes of Health, Bethesda, MD); the phosphatase construct FKBP (FK506-binding protein)-Inp54p from T. Inoue (Johns Hopkins University School of Medicine, Baltimore, MD); M1R (M1 muscarinic receptor)-YFP from N. Nathanson (University of Washington, Seattle, WA); rat KV1.1, rat KV1.4, and rat KVβ1.1 from J. Trimmer (University of California, Davis, Davis, CA); human KV1.3, human KV1.5, human KVβ1.3, and human KV2.1 as well as hERG from O. Pongs (University of Hamburg, Hamburg, Germany); human KCNE2 from G. Abbott (Weill Cornell Medical College, New York, NY); rat KV3.4 from J. Surmeier (Northwestern University, Chicago, IL); human KV4.2, KV4.3, and human KChIP1, KChIP2, and DPP6-s from B. Rudy (New York University School of Medicine, New York, NY); hKV7.1-GFP and hKCNE1 from M. Shapiro (University of Texas, San Antonio, TX); human KV7.2 and human KV7.3 from D. McKinnon (State University of New York, Stony Brook, NY) and T. Jentsch (Max Delbrück Center for Molecular Medicine Berlin-Buch, Berlin, Germany); and human Kir2.1 from D. Logothetis (Virginia Commonwealth University, Richmond, VA). We also used a new dual phosphatase, pseudojanin to deplete both PI(4,5)P2 and PI(4)P (Lindner et al., 2011; Hammond et al., 2012). In brief, the construct was based around a previously described expression construct (Varnai et al., 2006) consisting of mRFP and FKBP, to which the Saccharomyces cerevisiae Sac1p phosphatase (GenBank/EMBL/DDBJ accession no. NM_001179777; residues 2–517) and the INPP5E 5-phosphatase domains (GenBank accession no. NM_019892; residues 214–644; with the C-terminal prenylation motif destroyed by mutagenesis) were inserted separated by a flexible linker (GGTARGAAA[GAG]2R). Pseudojanin-YFP was generated by replacing mRFP with YFP using NheI and NotI. “Dark” Dr-VSP (without IRES-GFP) was generated by subcloning the Dr-VSP cassette into pcDNA3.0 (Falkenburger et al., 2010b).
Electrophysiology
Whole-cell recordings were made with an EPC9 patch-clamp amplifier (HEKA) at a sampling rate of 10 kHz. Patch electrodes had a DC resistance between 1 and 3 MΩ when filled with intracellular solution for whole-cell recordings. Series resistance was compensated by 75% after compensation of fast and slow capacitance. For recordings of KV channels, bath solution (Ringer’s) consisted of 160 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 8 mM glucose, and 10 mM HEPES at pH 7.4. Pipette solution contained 175 mM KCl, 5 mM MgCl2, 0.1 mM K4-BAPTA, 3 mM Na2ATP, 0.1 mM Na3GTP, and 5 mM HEPES at pH 7.4. For recordings of Kir2.1 channels, bath solution consisted of 135 mM NaCl, 5 mM KCl, 1 mM MgCl2, 10 mM glucose, and 10 mM HEPES at pH 7.3. Pipette solution contained 140 mM KCl, 2 mM MgCl2, 0.1 mM K4-BAPTA, 3 mM Na2ATP, 0.1 mM Na3GTP, and 10 mM HEPES at pH 7.3. PMA was dissolved in DMSO at a concentration of 10 mM. A final concentration of 100 nM in Ringer’s solution was achieved by dilution of PMA stock solution in Ringer’s solution. Voltage protocols are given in figures and legends. Recordings were performed at room temperature (22–25°C).
Photometric measurements of calcium and Förster resonance energy transfer (FRET)
FRET between CFP and YFP was measured as previously described (Falkenburger et al., 2010a) and reported as the corrected fluorescence emission ratio FRETr. For intracellular Ca2+ measurements, 100 µM Fura-4F (Invitrogen) was added to the pipette solution and dialyzed into cells via the whole-cell pipette. Optical recordings were started 5 min after establishing whole-cell configuration to allow equilibration of the dye into the cells. Fura-4F was excited at 340 and 380 nm, and changes in Ca2+ concentrations are reported as the ratio F340/F380 of fluorescence emission intensities recorded with a 535/30-nm emission filter.
Confocal laser microscopy
All experiments were performed at room temperature on an LSM710 microscope (Carl Zeiss). Cells were superfused with Ringer’s solution throughout the experiments.
Data analysis and statistics
Data analysis used Igor Pro (WaveMetrics) and Excel (Microsoft). Statistical data are given as mean ± SEM unless otherwise stated. Student’s t test was used to test for statistical significance. P-values of <0.05 were considered significant.
Online supplemental material
Figs. S1–S6 provide current traces of KV7.2/KV7.3 channels in response to activation of M1R (Fig. S1), current traces of endogenous KV channels in tsA-201 cells (Fig. S2), current traces of Kir2.1 and KV7.2/KV7.3 channels in response to activation of M1R (Fig. S3), FRETr traces of PH probes in response to an activation of Dr-VSP combined with simultaneous whole-cell recording of KV1.5/KVβ1.3 current (Fig. S4), line scan measurements of PH probe fluorescence in cells before and after activation of M1R, Dr-VSP, and pseudojanin (Fig. S5), and current traces of KV4.3 channels alone or with coexpressed auxiliary subunits before and after activation of Dr-VSP (Fig. S6). Online supplemental material is available at http://www.jgp.org/cgi/content/full/jgp.201210806/DC1.
RESULTS
Modulation of KV7 channels by PI(4,5)P2 depletion at the plasma membrane
Unexpectedly, many of the KV channels we studied proved to be insensitive to enzymatic depletion of PI(4,5)P2. Because of such negative results, we begin with control experiments. They verify the effectiveness of our tools for manipulating phosphoinositides in tsA-201 cells using two families of potassium channels already known to be PI(4,5)P2 sensitive. First, we and others have shown a strong PI(4,5)P2 requirement of KV7.2 (KCNQ2)/KV7.3 (KCNQ3) channel currents. Decreases in PI(4,5)P2 lead to a strong inhibition of KV7.2/KV7.3-mediated currents (Suh and Hille, 2002; Zhang et al., 2003; Li et al., 2005; Winks et al., 2005; Falkenburger et al., 2010b).
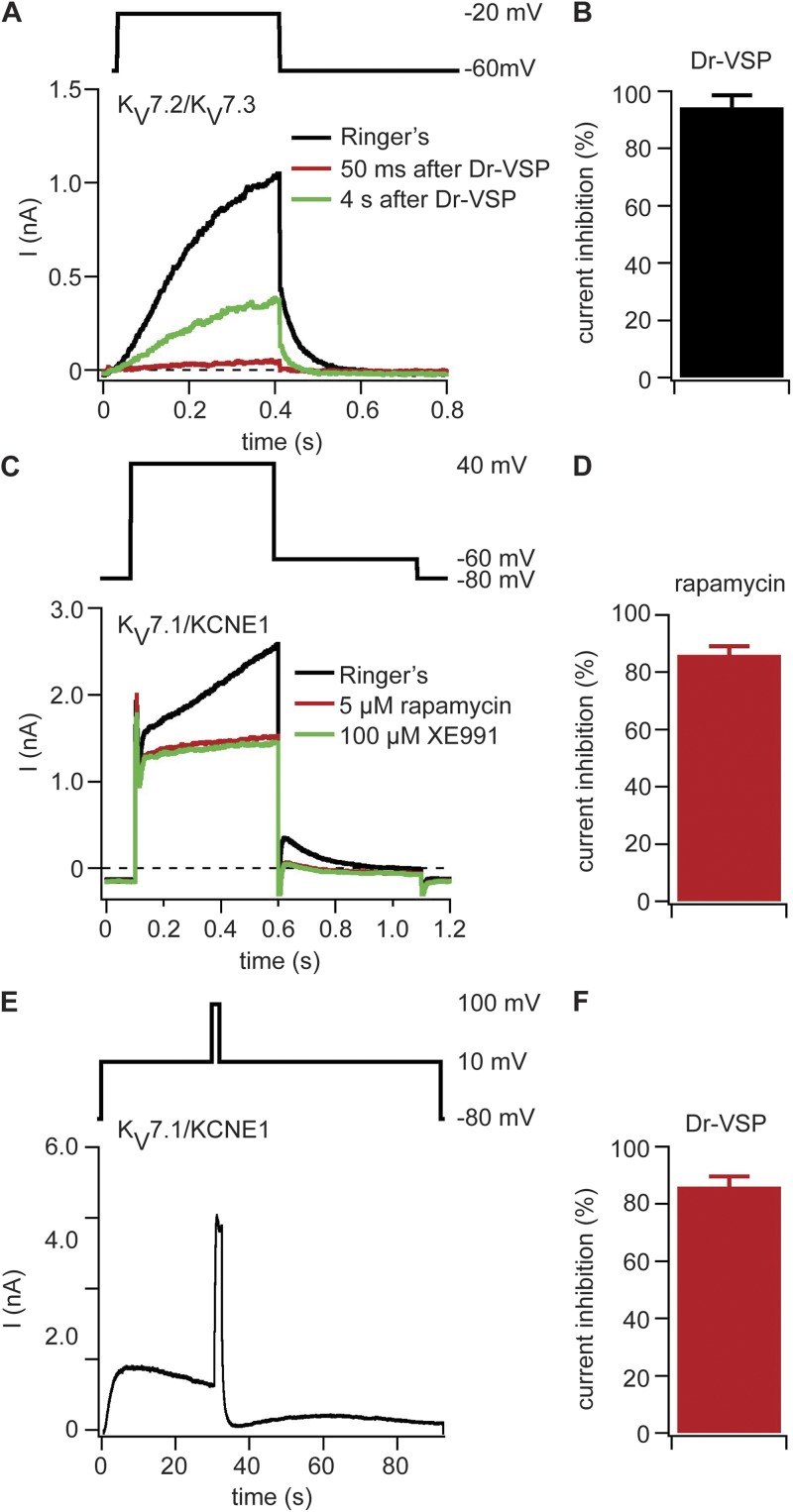
Fig. 1 A shows typical potassium outward current in KV7.2/KV7.3 channels. The channels activate during a test pulse to −20 mV from a holding potential of −60 mV and deactivate with a characteristic tail current upon stepping back to −60 mV. These channels were coexpressed with a voltage-sensitive lipid 5-phosphatase (Dr-VSP; Murata et al., 2005; Murata and Okamura, 2007; Okamura et al., 2009). The phosphatase dephosphorylates PI(4,5)P2 on the 5 position to give PI(4)P during large depolarization. Activation of Dr-VSP by a 2-s test pulse to 100 mV led to an immediate 94 ± 4% (n = 10) inhibition of the current (Fig. 1, A and B) followed by partial recovery at 4 s and typically a nearly complete recovery by 20 s as PI(4,5)P2 levels returned to their original state. Fig. S1 shows a comparable inhibition of KV7.2/KV7.3 channels upon activation of coexpressed M1 muscarinic receptors (M1R), as in previous work (Shapiro et al., 2000). Thus, activation of Dr-VSP or of M1R depletes PI(4,5)P2 sufficiently to elicit a nearly full but reversible drop in KV7.2/KV7.3 channel activity.
Figure 1.
PI(4,5)P2 depletion at the plasma membrane inhibits KV7.x channels. (A) Currents with coexpression of KV7.2, KV7.3, and Dr-VSP in tsA-201 cells. The black current trace was recorded before Dr-VSP activation, the red trace was recorded 50 ms after Dr-VSP activation by a 2-s pulse to 100 mV, and the green trace was recorded 4 s after Dr-VSP activation. Test pulse protocol is shown above the traces. (B) Reduction of KV7.2/KV7.3-mediated tail currents by activation of Dr-VSP (n = 10). (C) Currents traces recorded from a cell expressing KV7.1, KCNE1, Ins-5-P-FKBP-CFP, and LDR-CFP. Traces are shown before application of 5 µM rapamycin (black), after 60 s of rapamycin application (red), and after application of 100 µM XE991 (green). (D) Rapamycin-induced inhibition of XE991-sensitive current (n = 5). (E) Current trace of a cell expressing KV7.1, KCNE1, and Dr-VSP. Currents were recorded at 10 mV, Dr-VSP was activated after 30 s with a 2-s long test pulse to 100 mV, and then the membrane potential was returned to 10 mV. At the end of the recording, XE991 was applied to determine the amount of KV7.1-mediated current (not depicted). (F) Inhibition of XE991-sensitive current by Dr-VSP activation (n = 6). (B, D, and F) Error bars represent ±SEM.
After testing the familiar KV7.2/KV7.3 channel, we tried the closely related KV7.1, which has not been studied previously by the voltage-sensitive phosphatase method, together with its β subunit KCNE1. Their coexpression reconstitutes most characteristics of the IKs current recorded in cardiac myocytes (Charpentier et al., 2010). Recent studies suggest regulation of this channel complex by PI(4,5)P2 (Loussouarn et al., 2003; Park et al., 2005; Charpentier et al., 2010; Piron et al., 2010; Li et al., 2011). We expressed KV7.1 and KCNE1 together with a rapamycin-translocatable lipid 5-phosphatase (FKBP-Inp54p) and the membrane anchor LDR-CFP. The FKBP and LDR domains become tightly (irreversibly) dimerized when membrane-permeable rapamycin is added; recruitment of the lipid phosphatase to the plasma membrane dephosphorylates PI(4,5)P2 on the 5 position to PI(4)P (Inoue et al., 2005). The KV7.1/KCNE1 channel activates very slowly and requires a large depolarization, so we used a 500-ms test pulse to 40 mV from a holding potential of −80 mV and measured outward tail currents at −60 mV (Fig. 1 C). Upon application of rapamycin, the slowly activating outward currents and the tail currents were nearly eliminated (Fig. 1 C). We applied 100 µM XE991, a KV7 channel inhibitor, to block any residual KV7.1/KCNE1-mediated current and found the remaining current to be almost all XE991 insensitive (Fig. 1 C). On average, rapamycin-induced depletion of PI(4,5)P2 suppressed the KV7.1/KCNE1-mediated currents by 86 ± 3% (n = 5; Fig. 1 D). We considered the XE991-insensitive current to represent endogenous K+ current of the tsA-201 cells. A similar XE991-insensitive current was present in mock-transfected cells (Fig. S2). It activates mainly with large depolarizations positive to 0 mV.
Does transient PI(4,5)P2 depletion by activation of Dr-VSP also inhibit KV7.1/KCNE1 channels? We coexpressed these proteins, activated channels by a long test pulse to 10 mV, and recorded the resulting potassium outward current. After 30 s, we activated Dr-VSP by a 2-s test pulse to 100 mV and stepped back to the 10 mV level for 60 s more (Fig. 1 E). The KV7.1/KCNE1-mediated outward current was initially decreased by 86 ± 4% (n = 6; Fig. 1 F). At the end of the test pulse, XE991 was applied to correct for endogenous currents. The KV7.1/KCNE1-mediated currents recovered modestly after the Dr-VSP–activating test pulse was turned off, presumably reflecting partial recovery of PI(4,5)P2 levels after the transient depletion overlaid with slow accumulating channel inactivation at 10 mV (Fig. 1 E). Thus, both Inp54p recruitment to the plasma membrane and activation of Dr-VSP led to extensive inhibition of KV7.1/KCNE1 channel activity, confirming direct and strong regulation of this channel by PI(4,5)P2. We score the effects of PI(4,5)P2 depletion on KV7 channels as strong inhibition in summary Table 1.
Table 1.
M1 receptor and phosphatase effects on KV channel
| α subunit | Auxiliary subunit | M1R | Dr-VSP | Pseudojanin or FKBP-Inp54p (in case of KV7.1) |
| KV1.1 | KVβ1.1 | Slowing of inactivationa | No effect | No effect |
| KV1.3 | None | No effect | ND | No effect |
| KV1.4 | None | No effect | No effect | No effect |
| KV1.5 | ± KVβ1.3 | No effect | No effect | No effect |
| KV2.1 | None | ∼22% decrease | No effect | No effect |
| KV3.4 | None | Slowing of inactivationa | No effect | No effect |
| KV4.2/KV4.3 | None | No effect | No effect | ND |
| KV4.2/KV4.3 | KChIP1 | ND | No effect | ND |
| KV4.2/KV4.3 | KChIP1 + DPP6-s | ND | No effect | ND |
| KV4.2/KV4.3 | KChIP2 | ND | No effect | ND |
| KV7.1 (KCNQ1) | KCNE1 | ND | Strong inhibition | Strong inhibition |
| KV7.2/7.3 (KCNQ2/Q3) | None | Strong inhibitiona | Strong inhibitiona | Strong inhibition |
| KV11.1 (hERG) | None | ND | No effect | ND |
| KV11.1 (hERG) | KCNE2 | ∼30% decrease | No effect | No effect |
| Kir2.1 | None | No effect | ∼45% decrease | Strong inhibition |
These effects duplicate published work, see Discussion.
Kir2.1 channels are inhibited by activation of Dr-VSP or pseudojanin
The second potassium channel family whose PI(4,5)P2 requirement is well established is the inwardly rectifying Kir family. The Logothetis laboratory compared dose–response studies for restoration of channel activity by solutions of water-soluble diC8-PI(4,5)P2 applied to excised membrane patches containing KV7.2/KV7.3 (apparent Kd = 87 µM; Zhang et al., 2003; Li et al., 2005) or containing Kir2.1 (apparent Kd = 5 µM; Rohács et al., 2003). Comparing the midpoints of these curves suggests that Kir2.1 channels have a 17-fold higher PI(4,5)P2 affinity. Can our methods deplete PI(4,5)P2 enough to turn off such a high-affinity channel? We addressed this question by expressing Kir2.1 together either with Dr-VSP, M1R, or with the new translocatable dual phosphatase tool pseudojanin and LDR. Pseudojanin can be recruited to the plasma membrane by addition of rapamycin to cells expressing LDR where it converts PI(4,5)P2 in two steps all the way to PI, depleting both PI(4,5)P2 and PI(4)P (Hammond et al., 2012).
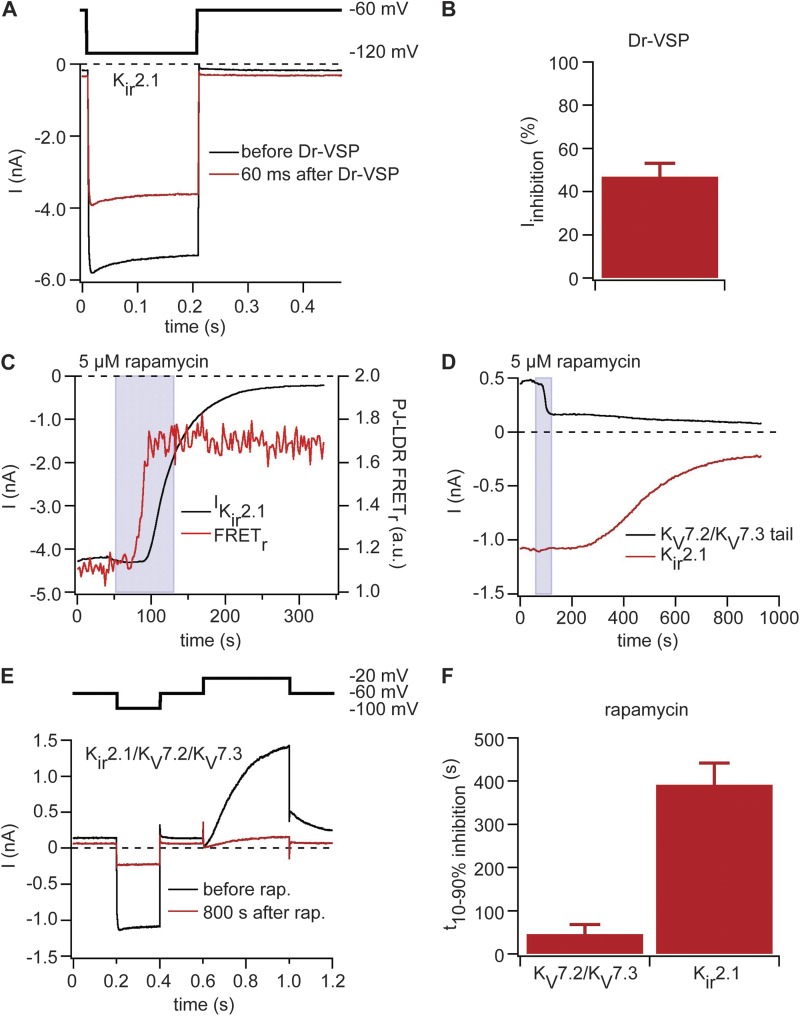
In the first experiments, we expressed Kir2.1 together with Dr-VSP. Test pulses to −120 mV from a holding potential of −60 mV elicited inwardly rectifying potassium currents in this channel. Dr-VSP was activated by a 2-s depolarization to 100 mV. It reduced Kir2.1-mediated currents by 47 ± 6% (n = 5; Fig. 2, A and B). The amount of inhibition is less than it was for KV7 channels, consistent with the concept that a higher PI(4,5)P2 affinity would reduce the ability of our tools to inhibit the channel. Nevertheless, even for this channel with very high PI(4,5)P2 affinity, a short depletion by Dr-VSP is able to inhibit half the current.
Figure 2.
Dr-VSP and pseudojanin can inhibit PI(4,5)P2-sensitive Kir2.1 channels. (A) Currents in Kir2.1 channels coexpressed with Dr-VSP. Black indicates current trace before activation of Dr-VSP, and red indicates current trace 60 ms after activation of Dr-VSP by a 2-s pulse to 100 mV. Hyperpolarizing test pulse protocol is shown above the traces. (B) Inhibition of Kir2.1-mediated currents by activation of Dr-VSP (n = 5). (C) Currents with coexpression of Kir2.1, pseudojanin (PJ)-YFP, and LDR-CFP recorded with the protocol shown in A. Black trace shows steady-state current over time, and the red trace is FRET ratio between pseudojanin-YFP and LDR-CFP. (D) Same experiment as in C but with expression of KV7.2 and KV7.3 as well as Kir2.1. Black trace shows KV7.2/KV7.3-mediated tail currents, and red trace shows Kir2.1-mediated steady-state current over time. (E) Current traces and pulse protocol for the recording shown in D. Black trace shows currents at start of rapamycin (rap.) application (60 s), and red trace is at 900 s (end of recording). (F) Time needed to decrease either KV7.2/KV7.3 or Kir2.1-mediated currents from 90 to 10% original amplitude (n = 4). (B and F) Error bars represent ±SEM.
We hypothesized that pseudojanin’s dual phosphatases might be able to decrease Kir2.1 activity even further as it continuously dephosphorylates PI(4,5)P2 and PI(4)P once rapamycin is added. Thus, we expressed Kir2.1 together with pseudojanin-YFP and LDR-CFP and applied the same pulse protocol. The Kir2.1 currents were stable before the addition of rapamycin. After addition of rapamycin, successful translocation of pseudojanin to the plasma membrane was signaled by an increase in FRET between YFP (on pseudojanin) and CFP (on the LDR anchor; Fig. 2 C, red line) within 25 s. After 50 s, the Kir2.1 current began to decrease, and over several hundred seconds it gradually decayed nearly to zero (Fig. 2 C). Thus, tools like Dr-VSP and pseudojanin inhibit this potassium channel effectively despite its high apparent affinity for PI(4,5)P2.
Finally, to monitor the Kir and KV channel activities simultaneously in the same cell, we coexpressed Kir2.1 with KV7.2/KV7.3, pseudojanin-YFP, and LDR-CFP. The dual-pulse protocol comprised a first hyperpolarizing step to −100 mV to activate Kir2.1 and a brief step to −60 mV followed by a depolarization to −20 mV to activate KV7.2/KV7.3. As anticipated, the KV7.2/KV7.3 current was reduced quickly after addition of rapamycin, and Kir2.1 current was reduced much more slowly (Fig. 2 D). Current traces before and after addition of rapamycin are shown in Fig. 2 E. The time for current inhibition from 10 to 90% was 46 ± 22 s for KV7.2/KV7.3 and 392 ± 50 s (n = 4) for Kir2.1 (Fig. 2 F). This finding is consistent with different affinities of KV7.2/KV7.3 and Kir2.1 channels for PI(4,5)P2, resulting in different kinetics of channel inhibition as PI(4,5)P2 at the plasma membrane is declining.
Having observed that Dr-VSP inhibits KV7.2/KV7.3 channels almost completely but Kir2.1 channels only to about ∼47%, we asked whether activation of M1R would inhibit Kir2.1 channels. We followed the same strategy used for our pseudojanin experiments and expressed Kir2.1 together with KV7.2 and KV7.3 in the same cell. Current recordings were performed as before. Interestingly, we observed an inhibition of KV7.2/KV7.3 activity by 61 ± 6% (n = 5) after activation of M1R, but Kir2.1 activity remained unaffected (Fig. S3). We conclude that the activation of PLC is not enough to reduce PI(4,5)P2 levels below the level needed to keep Kir2.1 channels active. Presumably lipid kinases are continually supplying new PI(4,5)P2 molecules.
KV1.1/KVβ1.1 channel complexes are sensitive to activation of M1 muscarinic receptors but not to activation of VSP
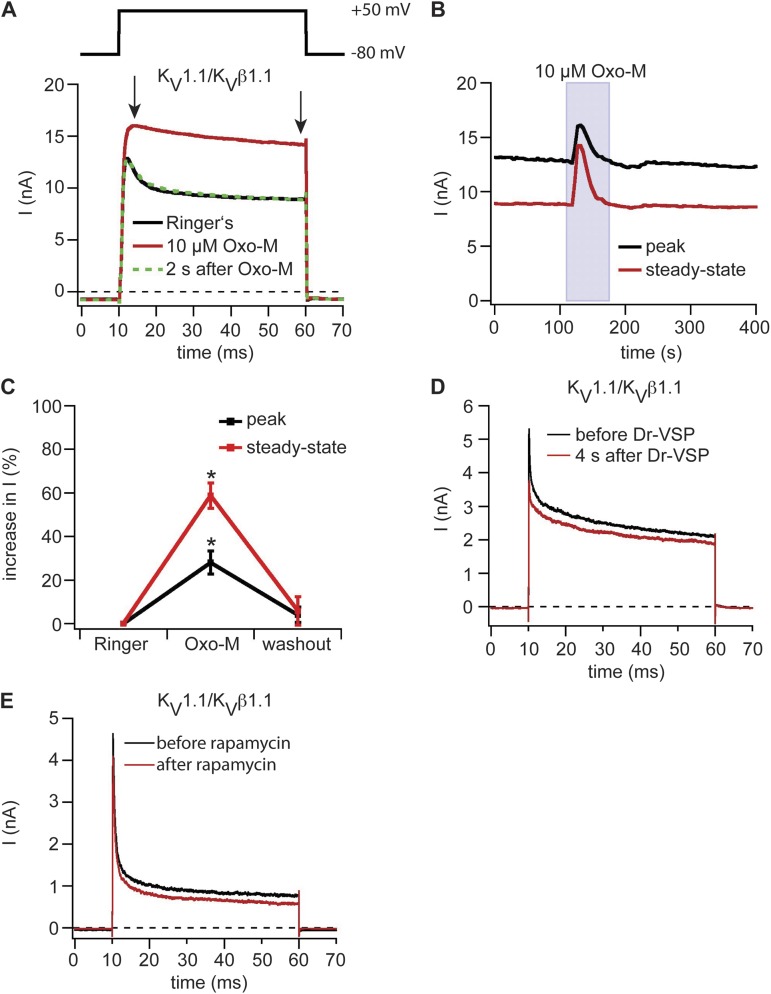
With these controls in hand, we proceeded to additional KV channels starting with the KV1 family. KV1.1 and the auxiliary KVβ1.1 subunit were coexpressed in tsA-201 cells together with M1R. A 50-ms depolarization to 50 mV from a holding potential of −80 mV elicited outward currents that inactivated rapidly, but only partially, to a sustained steady-state current (Fig. 3 A). Activation of M1R by 10 µM Oxo-M increased the currents with almost complete removal of fast inactivation (Fig. 3, A and B). Peak currents increased 28 ± 5%, and steady-state currents increased 59 ± 6% (n = 7; Fig. 3 C). Remarkably, the observed augmentation was transient, returning to the control amplitude even during continued Oxo-M application (Fig. 3, A and B).
Figure 3.
Modulation of KV1 family channels by depletion of PI(4,5)P2 at the plasma membrane. (A) Potassium outward currents in KV1.1/KVβ1.1 channel complexes coexpressed with M1R. Black indicates control current trace in Ringer’s solution, red indicates during application of 10 µM Oxo-M, and green indicates after washout of Oxo-M. Arrows indicate points at which peak and steady-state current amplitudes were measured. (B) Current amplitudes of peak and steady-state current of the cell shown in A over time. (C) Percent increase of peak and steady-state currents of KV1.1/KVβ1.1 channel complexes coexpressed with M1R upon stimulation with Oxo-M (n = 7). Error bars represent ±SEM. *, P < 0.05. (D) Current time course in a cell expressing KV1.1/KVβ1.1 channel complexes and Dr-VSP. Dr-VSP was activated by a 2-s pulse to 100 mV. Test pulse protocol as shown in A. (E) Same as D but with expression of pseudojanin-YFP and LDR-CFP instead of Dr-VSP and addition of 5 µM rapamycin.
Is the augmentation caused by depletion of PI(4,5)P2? This seems less plausible because the augmentation did not persist during application of Oxo-M despite continued PI(4,5)P2 depletion. Indeed two direct tests were negative. We observed no change in current amplitudes or inactivation when KV1.1/KVβ1.1 channel currents were tested by activation of Dr-VSP (Fig. 3 D). Similarly, there was no change of currents when pseudojanin was translocated to the plasma membrane in cells coexpressing KV1.1/KVβ1.1, YFP-tagged pseudojanin, and LDR-CFP (Fig. 3 E). Thus, depletion of PI(4,5)P2 is not likely to explain the strong augmentation of KV1.1/KVβ1.1 channel current by M1R activation; rather, one needs to consider other signaling pathways downstream of PI(4,5)P2 hydrolysis (see Discussion).
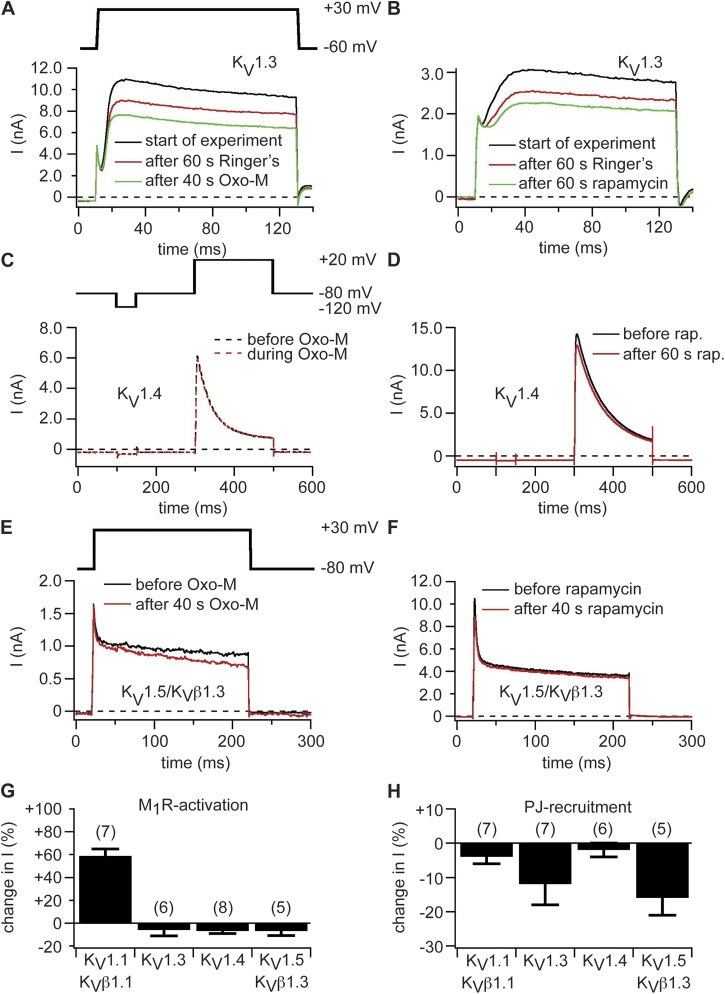
To test other members of the KV1 channel family, we expressed KV1.3, KV1.4, and KV1.5 channels (KV1.5 alone and with the β subunit KVβ1.3) together with Dr-VSP, pseudojanin, or M1R but did not observe any changes in current amplitudes or channel kinetics with any of these tools (Fig. 4). For example, Fig. 4 A shows representative traces of KV1.3 currents recorded with coexpressed M1R. We observed no significant effect after M1R activation. A certain amount of rundown of current was already present before activation of the receptor. Similarly, there was no response to translocation of pseudojanin (Fig. 4 B). Fig. 4 (C–F) shows traces of KV1.4 and KV1.5 currents. Again there is no alteration in the potassium currents after activation of M1R (Fig. 4, C and E) or translocation of pseudojanin (Fig. 4, D and F).
Figure 4.
KV1.3, KV1.4, and KV1.5 channels are insensitive to depletion of PI(4,5)P2 at the plasma membrane. (A) Current traces of KV1.3 channels coexpressed with M1R. Black indicates traces at start of experiment, red indicates after 60 s in Ringer’s, and green indicates traces after 40-s superfusion with 10 µM Oxo-M (100 s after start of experiment). (B) Currents in KV1.3 channels coexpressed with pseudojanin-YFP and LDR-CFP. Black indicates traces at start of experiment, red indicates after 60 s in Ringer’s, and green indicates traces after 60-s rapamycin application (120 s after start of experiment). Same pulse protocol as in A. (C) Currents in a cell expressing KV1.4 and M1R. Black indicates current trace in Ringer’s solution, and red indicates current trace during application of 10 µM Oxo-M. (D) KV1.4 channels coexpressed with pseudojanin-YFP and LDR-CFP. Black indicates current before application of rapamycin (rap.), and red indicates traces after 60 s of rapamycin application. (E and F) Currents in KV1.5 channels coexpressed with KVβ1.3 and M1R (E) or pseudojanin-YFP and LDR-CFP (F). Black indicates current before application of Oxo-M (E) or rapamycin (F), and red indicates current after 40-s application of Oxo-M (E) or rapamycin (F). (G and H) Percent changes in steady-state current amplitudes of KV1.x channels after activation of M1R (G) or recruitment of pseudojanin (PJ) to the plasma membrane (H). Numbers in parentheses indicate n numbers for individual experiments. Error bars represent ±SEM.
We performed simultaneous internal controls with FRET to show that PI(4,5)P2 depletion was occurring in each experiment with no modulation of potassium currents. For example, in Fig. S4, we coexpressed eCFP-PH(PLCδ1) and eYFP-PH(PLCδ1) as FRET reporters to monitor PI(4,5)P2 levels at the plasma membrane together with KV1.5/KVβ1.3 channels and Dr-VSP. Decreases in PI(4,5)P2 levels at the plasma membrane would be reported as a decrease in FRET interaction between CFP and YFP simultaneous with the patch-clamp measurements. Activation of Dr-VSP led to a substantial transient decrease in FRET ratio, indicating a transient decrease in PI(4,5)P2 at the plasma membrane, without significant change in channel activity (Fig. S4). The validity of the FRET method is confirmed by parallel experiments of translocation of PH probes performed with confocal microscopy in cells that did not express exogenous channels. We coexpressed YFP-tagged PH probes with M1R, Dr-VSP, or pseudojanin and performed line scan measurements with confocal laser microscopy before and after activation of these PI(4,5)P2-depleting enzymes. As expected, we observed a strong localization of PH probes at the plasma membrane before PI(4,5)P2 depletion and a reduction in YFP fluorescence at the plasma membrane of >70% after PI(4,5)P2 depletion with any of the enzymes we used (Fig. S5). As first reported by van der Wal et al. (2001), these experiments show that FRET and translocation methods give a similar picture about PI(4,5)P2 depletion. The confocal images (Fig. S5, A–F, insets) show that our three tools for PI(4,5)P2 depletion act uniformly along the membrane. They do not leave patches of unaltered PI(4,5)P2 behind. As a final internal control for PLC activation, we loaded cells with Fura-4F via the patch pipette while coexpressing KV channels and M1R to confirm that Ca2+ transients occurred during activation of M1R (see Fig. 8 D for example). With these internal controls, we feel confident in scoring the tested KV1 channels as not sensitive to PI(4,5)P2 depletion (Table 1).
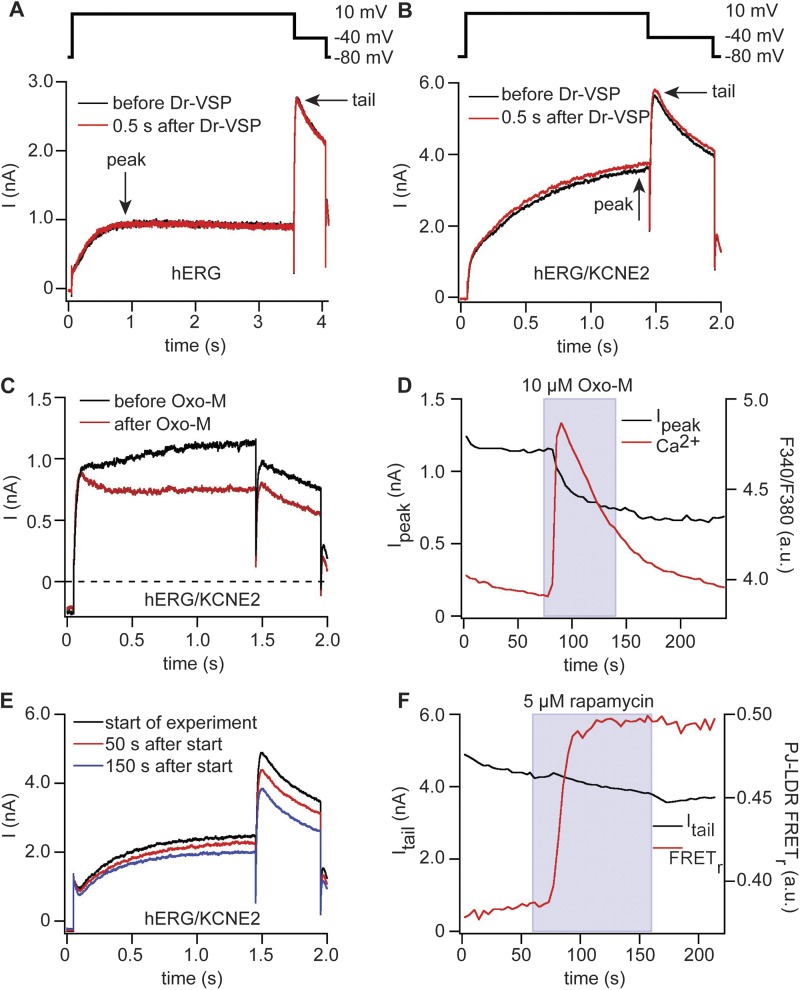
Figure 8.
hERG channels are sensitive to M1R activation but not to activation of Dr-VSP or pseudojanin. (A) Currents in hERG channels coexpressed with Dr-VSP. Black indicates before activation of Dr-VSP, and red indicates directly after activation of Dr-VSP. Arrows indicate points at which peak and tail current amplitudes were measured. (B) Same as in A but with additional coexpression of KCNE2 and modified pulse protocol. (C) Currents in hERG channels coexpressed with KCNE2 and M1R. Black indicates before application of 10 µM Oxo-M, and red indicates after application of Oxo-M. Pulse protocol as in B. (D) Time course of current amplitudes at 10 mV and Fura-4F ratio of cell in C. (E) Currents in a cell expressing hERG, KCNE2, pseudojanin-YFP, and LDR-CFP. Currents are shown at the beginning of the recordings (black), directly before application of rapamycin (50 s after start of experiment; red), and at the end of rapamycin application (150 s after start of experiment; blue). Pulse protocol as in B. (F) Time course of current amplitudes of tail currents at −40 mV of the experiment in E (black) and FRET ratio between pseudojanin (PJ)-YFP and the anchor LDR-CFP (red).
KV2.1 channels are not sensitive to PI(4,5)P2 depletion
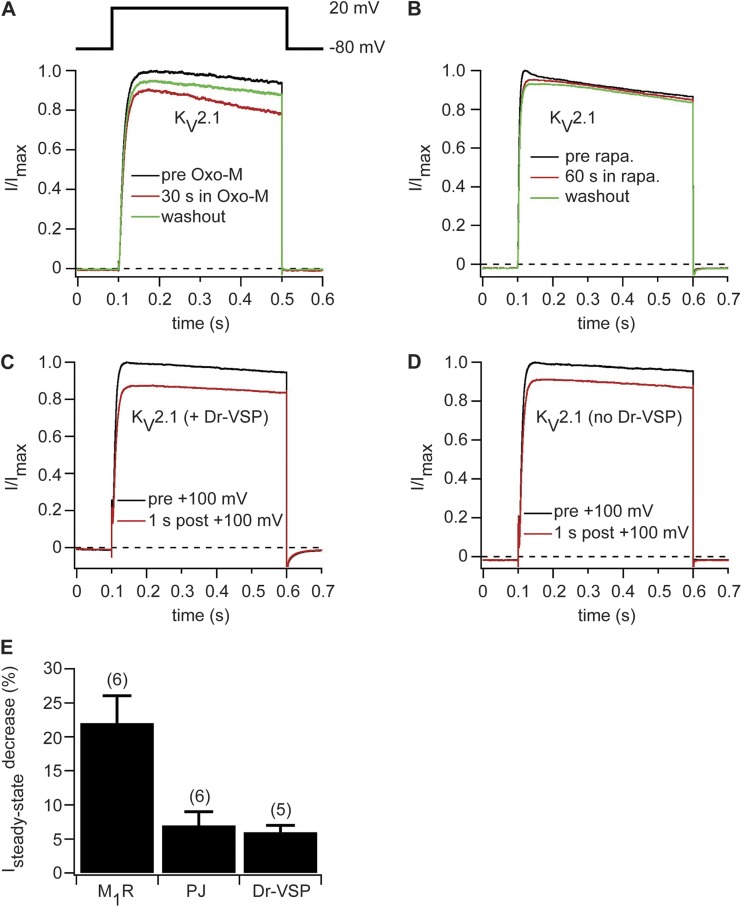
Next, we asked whether KV2.1 channels are sensitive to PI(4,5)P2 depletion. First, we expressed KV2.1 channels together with M1R. Currents were elicited by a depolarizing test pulse to 20 mV from a holding potential of −80 mV. Upon activation of M1R, we observed a 22 ± 4% (n = 6) decrease in steady-state current at the end of the depolarizing test pulse (Fig. 5, A and E). Upon washout of the agonist Oxo-M, steady-state currents increased again (Fig. 5 A). To test whether the decrease in current amplitude is caused by a direct modulation of KV2.1 channels by PI(4,5)P2 or a result of downstream signaling pathways after PI(4,5)P2 hydrolysis by PLC, we expressed KV2.1 channels together with either pseudojanin or Dr-VSP, which deplete PI(4,5)P2 without generating second messengers. Recruiting pseudojanin to the plasma membrane had no significant effect on KV2.1-mediated currents (Fig. 5, B and E). In another approach, we activated Dr-VSP by a 2-s depolarizing pulse to 100 mV and measured currents before and after activation of Dr-VSP. We observed a reduction of ∼25% in steady-state current amplitude when we tested for KV2.1-mediated currents 1 s after the Dr-VSP–activating pulse (Fig. 5 C). However, in control experiments on cells that did not express Dr-VSP but were subjected to the same depolarizing test pulse, we observed an almost identical reduction of KV2.1-mediated currents (Fig. 5 D). Thus, the reduction in current amplitude is likely caused by voltage-dependent inactivation of KV2.1 channels from the depolarizing pulse used to activate Dr-VSP. In summary, neither recruitment of pseudojanin to the plasma membrane nor activation of Dr-VSP led to a significant change in KV2.1-mediated currents (Fig. 5 E). We conclude that the observed small reduction of KV2.1-mediated currents after M1R activation is caused by signaling pathways downstream of PI(4,5)P2 hydrolysis by activation of PLC or is a result of a direct loss of PI(4,5)P2.
Figure 5.
KV2.1 channels are not sensitive to depletion of PI(4,5)P2 by activation of pseudojanin or Dr-VSP. (A) Normalized current traces recorded before (black), during (red), and after (green) application of 10 µM Oxo-M in a cell transiently transfected with KV2.1 and M1R. (B) Current traces before (black), after 60 s of application (red), and after washout of 5 µM rapamycin (rapa.) to a cell expressing KV2.1, pseudojanin-YFP, and LDR-CFP. (C) Normalized KV2.1-mediated current traces before (black) and 1 s after (red) a 2-s depolarizing pulse to 100 mV in cells coexpressing Dr-VSP. (D) Same as in C but without coexpression of Dr-VSP. (E) Decrease in KV2.1-mediated steady-state currents at 20 mV after activation of M1R or Dr-VSP or recruitment of pseudojanin (PJ) to the plasma membrane. Numbers in parentheses indicate numbers of individual experiments. Error bars represent ±SEM.
KV3.4 currents also can be increased and inactivation slowed upon M1R activation
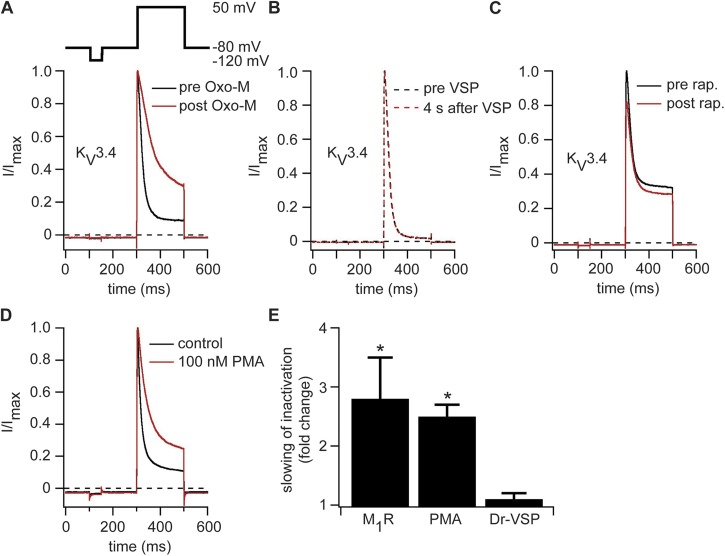
In a similar approach, we expressed KV3.4 channels together with M1R, Dr-VSP, or pseudojanin. Whole-cell recordings showed fast activating and inactivating outward potassium currents upon membrane depolarization as previously reported for these channels (Oliver et al., 2004). Activation of M1R by Oxo-M decreased the amount of N-type inactivation, leading to an increase in current amplitude at the end of the depolarizing test pulse (Fig. 6 A). The time constant for N-type inactivation was 44 ± 18 ms under control conditions and 97 ± 30 ms (n = 4) after application of Oxo-M, a 2.8 ± 0.7–fold slowing (Fig. 6 E). Despite the clear action of stimulating M1R, activating Dr-VSP or recruiting pseudojanin to the plasma membrane had no detected effect on KV3.4 current amplitudes (Fig. 6, B and C) or kinetics (Fig. 6 E). As was the case for KV1.1/KVβ1.1 channels, these experiments find no direct regulation of KV3.4 channels by depletion of PI(4,5)P2, but they do find a large effect of M1R, suggesting a possible role for the products of PI(4,5)P2 hydrolysis. Reports in the literature describe a decrease of inactivation of KV3.4 after phosphorylation of the N-terminal inactivation domain by PKC (Covarrubias et al., 1994; Ritter et al., 2012). Thus, the decrease of inactivation we observed after M1R activation could be the result of an activation of PKC by the DAG produced during PI(4,5)P2 hydrolysis by PLC. Indeed, incubating tsA-201 cells expressing KV3.4 for 15 min with 100 nM PMA to activate PKC produced a decrease in inactivation of KV3.4 much as we saw with M1R activation (Fig. 6 D). We measured a 2.5 ± 0.2–fold slowing (n = 5; Fig. 6 E), consistent with the notion that phosphorylation of KV3.4 by PKC underlies the effects of M1R activation.
Figure 6.
KV3.4 channels are modulated by activation of M1R but not by activation of Dr-VSP or pseudojanin. (A) Normalized current traces recorded before (black) and during (red) application of 10 µM Oxo-M in a cell transiently transfected with KV3.4 and M1R. (B) Same as in A but with expression of Dr-VSP and an activating pulse instead of M1R. (C) Current traces before (black) and after (red) application of 5 µM rapamycin (rap.) to a cell expressing KV3.4, pseudojanin-YFP, and LDR-CFP. (D) Representative normalized current traces of cells expressing KV3.4 recorded under control conditions (black) or after 15-min treatment with 100 nM PMA in Ringer’s solution (red). (E) Fold slowing of inactivation after activation of M1R, application of 100 nM PMA, or activation of Dr-VSP (n = 4 [M1R] or 5 [PMA and Dr-VSP]). Error bars represent ±SEM. *, P < 0.05.
Complexes of KV4.2 and KV4.3 channels with KChIPs and DPP6-s are not sensitive to PI(4,5)P2 depletion
KV4.2 and KV4.3 channels play an important role in the brain and heart (Amarillo et al., 2008; Levy et al., 2010). In most cell types, they are associated with β subunits called KChIPs that are located on the cytoplasmic side of the plasma membrane and influence KV4 channel current amplitude and inactivation kinetics (Pongs and Schwarz, 2010). They also can associate with another cofactor, DPP6-s, a protein that binds to KV4 channels from the extracellular side of the plasma membrane and exerts similar effects as KChIPs on channel activity (Nadal et al., 2003; Kim et al., 2008; Kaulin et al., 2009). Because of their wide-spread physiological roles, we assessed whether these channel complexes can be regulated directly by PI(4,5)P2. Accordingly, we expressed KV4.2 or KV4.3 channels together with Dr-VSP either alone or together with KChIPs and DPP6-s.
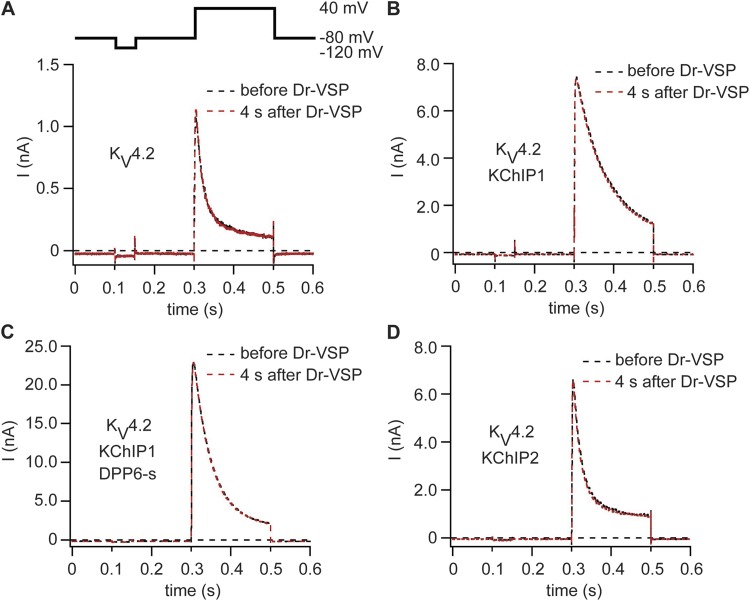
The pulse protocol was similar to that for recordings of KV3.4 channels (Fig. 7 A). Without any β subunits, KV4.2 and KV4.3 channels gave fast-activating currents that displayed fast inactivation as reported before (Birnbaum et al., 2004). Fig. 7 shows KV4.2 traces, and Fig. S6 shows KV4.3 traces. Activation of Dr-VSP had no detectable effect on current amplitudes or channel kinetics (Fig. 7 A). Coexpression with KChIP1 increased peak current amplitudes and slowed inactivation but did not introduce any sensitivity to Dr-VSP activation (Fig. 7 B). Biochemical experiments and recorded channel activities imply that under physiological conditions KV4 channels are simultaneously associated with KChIP1 and DPP6-s as auxiliary subunits (Pongs and Schwarz, 2010). We mimicked this situation by expressing KV4.2 together with KChIP1 and DPP6-s. This produced a dramatic increase in current amplitude as reported before for KV4 channels (Fig. 7 C; Nadal et al., 2003; Pongs and Schwarz, 2010) but no sensitivity to PI(4,5)P2 depletion. As a last configuration, we expressed KV4 channels together with KChIP2 and Dr-VSP. Again, activation of Dr-VSP led to no change of channel activity (Fig. 7 D). To check for a successful decrease of PI(4,5)P2 levels, all experiments included PH probes as FRET reporters to monitor PI(4,5)P2 levels at the plasma membrane. In all analyzed cells, activation of Dr-VSP led to a strong decrease in the FRET signal, indicative of a depletion of PI(4,5)P2 at the plasma membrane. Evidently, KV4 channels alone or together with their β subunits are not sensitive to a transient depletion of PI(4,5)P2 at the plasma membrane (Table 1).
Figure 7.
KV4.2 channels are insensitive to depletion of PI(4,5)P2 by Dr-VSP. (A) Current traces of a cell expressing KV4.2 and Dr-VSP. Black indicates before activation of Dr-VSP, and red indicates 4 s after activation of Dr-VSP. (B–D) Same protocol as in A but with coexpression of KChIP1 (B), KChIP1 and DPP6-s (C), or KChIP2 (D).
hERG channels are not sensitive to PI(4,5)P2 depletion
As a final KV family, we expressed hERG (KV11.1) channels together with Dr-VSP and PH probes to monitor PI(4,5)P2 levels. The hERG channel activity was assessed as tail currents at −40 mV after test pulses to 10 mV from a holding potential of −80 mV. Each activation of Dr-VSP led to a significant reduction in PH domain FRETr, verifying reduction of PI(4,5)P2 (not depicted), but there were no detectable changes in current amplitude or time course (Fig. 8 A). Repeating the experiment with coexpression of the β subunit KCNE2 did not change this negative result (Fig. 8 B). Next we tested whether activation of M1R modulates channel activity. With hERG and KCNE2, M1R activation led to a small decrease in both peak and tail currents (Fig. 8 C). The onset of the decrease in currents coincided with a rise of intracellular Ca2+ (Fig. 8 D), but, when the Ca2+ transient relaxed back to its original level, current amplitudes did not recover.
The depression of hERG current during M1R activation developed slowly over tens of seconds (Fig. 8 D). This suggested that our VSP experiment (Fig. 8, A and B) may have missed the appropriate time frame because we had looked for an effect only 0.5 s after VSP activation. We switched to recruiting pseudojanin to the plasma membrane to make a lasting depletion. FRET between the membrane anchor LDR-CFP and pseudojanin-YFP increased sharply as rapamycin was perfused (Fig. 8 F), but there was no significant effect on hERG current amplitudes or kinetics (Fig. 8, E and F). In the pseudojanin-expressing cells there was some current rundown starting even before application of rapamycin, which was not changed by recruiting pseudojanin to the plasma membrane (Fig. 8 F). Collectively, these findings support a small indirect regulation of channel activity by M1R activation but give no evidence for a direct modulation of hERG channels by PI(4,5)P2 depletion.
DISCUSSION
We have studied the PI(4,5)P2 sensitivity of nine KV channels, KV1.1, KV1.3, KV1.4, KV1.5, KV2.1, KV3.4, KV4.2, KV4.3, and KV11.1/KCNE (hERG), together with various additional auxiliary subunits (Table 1). When we depleted PI(4,5)P2 by activating Dr-VSP or recruiting pseudojanin to the plasma membrane, the observed properties of these expressed channels did not change. This result was quite unexpected because published experiments describe PI(4,5)P2 sensitivity of several of these channels, KV1.1 with KVβ1.1, KV1.4, KV1.5 with KVβ1.3 and KV3.4 (Oliver et al., 2004; Decher et al., 2008), as well as hERG (KV11.1; Bian et al., 2001, 2004; Bian and McDonald, 2007), especially in experiments with direct application of PI(4,5)P2 to large inside-out membrane patches from Xenopus oocytes. We must now consider possible sources of this apparent discrepancy. Is our use of “physiological” phosphatases in intact cells exploring the same range of PI(4,5)P2 as that with PI(4,5)P2 perfusion onto excised patches? Are different K+ channels localized to different plasma membrane subdomains where they experience different pools of PI(4,5)P2? Could superfusion of PI(4,5)P2 onto excised patches lead to unspecific side effects which might be an explanation for the differences between the published studies and our results? Could other lipids besides PI(4,5)P2 and PI(4)P be keeping KV channels active?
Depletion of PI(4,5)P2
Our results with activation of phosphatases were unambiguously negative, so it was essential to show that our tools actually depleted plasma membrane PI(4,5)P2. In many experiments, we recorded FRET from PH domains of PLCδ1 simultaneously with the current traces and found that PI(4,5)P2 was being depleted. We verified these results in line scan measurements of cells transfected with a YFP-tagged PH probe. We found a uniform localization of PH probes at the plasma membrane before PI(4,5)P2 depletion. Right after activation of Dr-VSP, M1R, or pseudojanin, we detected a strong decrease of YFP fluorescence at the plasma membrane and an obvious translocation of the PH probes into the cytoplasm. The PI(4,5)P2 depletion was uniform at the plasma membrane. It should be noted that because phosphatases do not generate IP3, the translocation of PH domains during phosphatase activation really reflects loss of free membrane PI(4,5)P2 rather than any gain of cytoplasmic IP3. In some experiments when activating M1R, we also validated activation of PLC by measuring the concomitant rises in Ca2+. The phosphatase tools, especially pseudojanin, were quite capable of inhibiting low-affinity KV7.2/7.3 channels, as is already well known (Suh et al., 2006; Murata and Okamura, 2007; Falkenburger et al., 2010b), as well as KV7.1/KCNE and high-affinity Kir2.1 channels by at least 90%. Even a short activation of Dr-VSP inhibited Kir2.1 by 45%. Activation of M1R was not sufficient to inhibit high-affinity Kir2.1 channels, whereas it strongly inhibited low-affinity KV7.2/KV7.3 channels. As both Dr-VSP and pseudojanin showed inhibition of Kir2.1 channels, we concluded that we would be able to detect a PI(4,5)P2 dependence of KV channels even if they have a high affinity to PI(4,5)P2 by using Dr-VSP and pseudojanin.
We can attempt to calibrate the membrane PI(4,5)P2 levels in terms of equivalent diC8-PI(4,5)P2 concentrations in solution. In these terms, the equivalent diC8-PI(4,5)P2 concentration of a resting cell would be near the apparent low-affinity Kd of 40–87 µM for KV7.2/7.3 channels (Zhang et al., 2003; Li et al., 2005; Telezhkin et al., 2012) so that these channels would be half activated. After pseudojanin activation, the equivalent concentration would be reduced considerably below the apparent Kd of 5 µM for Kir2.1 channels so that only 10% of these channels would be active (Rohács et al., 2003). Indeed, <1 µM diC8-PI(4,5)P2 suffices to support 10% Kir2.1 channel activity (Du et al., 2004). These parameters define the window of PI(4,5)P2 levels we have studied in terms of an equivalent diC8-PI(4,5)P2 scale.
Are other investigators exploring a different range of available PI(4,5)P2 than we are? It was when adding 10 µM brain PI(4,5)P2 to patches from Xenopus oocytes with KV1.1 with KVβ1.1, KV1.4, and KV1.5 with KVβ1.3, and KV3.4 channels that Oliver et al. (2004) and Decher et al. (2008) saw an extensive loss of fast inactivation. In inside-out patches excised into a medium without ATP, the PI(4,5)P2 pools might well gradually fall through irreversible phosphatase activity below the lowest levels we reach with pseudojanin in intact cells. In these conditions, Oliver et al. (2004) and Decher et al. (2008) reported inactivating gating kinetics much like those we and others see in whole-cell experiments with normal endogenous PI(4,5)P2. Consistent with our phosphatase results, they did not describe any rundown that accompanied gradual loss of endogenous PI(4,5)P2. When these authors then applied PI(4,5)P2, they used long-chain brain PI(4,5)P2 for which we unfortunately have no equivalent concentration calibration. At the reported 10-µM concentration, the phospholipid would have been suspended as micelles rather than in free solution. It made the currents become much larger and longer lasting. They report that PI(4)P, PI(4,5)P2, and PI(3,4,5)P3 were equally effective.
Could superfusion of excised patches with long-chain PI(4,5)P2 induce nonspecific effects? One possibility is that it leads to supraphysiological high concentrations of PI(4,5)P2 in the patch, above the concentration range we studied with our tools. At these elevated concentrations, PI(4,5)P2 might interact with domains in the channel proteins that would not normally couple to endogenous PI(4,5)P2. Another possible explanation for the apparent discrepancy between our results and those of Oliver et al. (2004) and Decher et al. (2008) could be that the nonspecific large cytoplasmic polyphosphoinositide-micelle effects are not meditated by incorporation into the membrane. Perhaps the micelles interact with extramembranous domains of the channel proteins like the N-terminal inactivation domains, thereby preventing these domains from interacting with the gate and conferring inactivation to the KV channels. This possibility seems plausible to us and would be an artifact.
Alternatively, can the differences be explained by postulating segregation of different channels into hypothetical lipid domains on the plasma membrane? One domain would contain KV7 channels, Kir2.1 channels, and PI(4,5)P2. It would also contain the M1Rs, PLC, PH domains, VSP, and the membrane anchor LDR-CFP so that activation of PLC or recruiting phosphatases to this domain would deplete PI(4,5)P2, inhibiting KV7 and Kir2.1 channel function and freeing almost all PH domains. This lipid domain would contain the great majority of the plasma membrane PI(4,5)P2 because, according to our chemical measurements in M1R-expressing CHO cells, 93% of the total cell PI(4,5)P2 can be cleaved in a 60-s treatment with carbachol (Horowitz et al., 2005). The other, presumably smaller, domain would harbor and segregate all the other KV channels we have tested. It would lack PI(4,5)P2 and not be influenced by the PI(4,5)P2 depletion. However, like the other domain, it would accept applied PI(4,5)P2, so that the KV channels would respond to that manipulation. We regard this, highly isolated, two-domain model as too contrived and implausible.
Our work did not reveal any lipid dependence of KV channels outside the KV7 family. Because our tests involved depleting only PI(4,5)P2 and PI(4)P, the possibility remains that KV channels can be kept active by lipids we did not deplete, although we know no reason to invoke a lipid requirement so far. There are examples in the literature. Thus, long-chain acyl-CoA esters, a metabolically active form of free fatty acids, activate KATP channels (Larsson et al., 1996; Bränström et al., 1997). Further, phosphatidic acid interacts with a variety of proteins (Kooijman and Burger, 2009) and activates, for instance, the bacterial mechanosensitive channel of large conductance (MscL; Powl et al., 2005a,b). Lastly, even KV7.2/KV7.3 channels can be kept active by phospholipids other than PI(4,5)P2 that have at least an acyl chain and a phosphate head group (Telezhkin et al., 2012). However, PI(4,5)P2 still has the highest affinity, and the natural abundance of any others is insufficient to keep the channels functioning after we recruit the 5-phosphatase to the plasma membrane.
Activation of Gq-coupled receptors and PKC
We also explored the sensitivity of some of the KV channels to activation of M1R, a Gq-coupled receptor. As expected, the KV7.2/7.3 currents were strongly depressed. Some other KV channels were not affected by M1R activation (KV1.3, KV1.4, and KV1.5 ± KVβ1.3), but the inactivation gating kinetics of two of the KV channels (KV1.1/KVβ1.1 and KV3.4) were strongly slowed. From our observations, we conclude that slowing of inactivation for KV1.1/KVβ1.1 and KV3.4 during PLC activation is not caused by the resulting depletion of PI(4,5)P2. Instead we consider it to be identical to slowing attributed by other authors to activation of PKC (Jonas and Kaczmarek, 1996; Levy et al., 1998; Winklhofer et al., 2003). For example, consider KVβ1.1, whose coexpression with KV1.1 makes fast inactivation by contributing an inactivation ball. This inactivation is slowed if PKC is activated pharmacologically or through a Gq-coupled metabotropic glutamate receptor; the slowing uses an indirect mechanism that may remove a phosphate at a PKA consensus site on KV1.1, Ser-446 (Jonas and Kaczmarek, 1996; Levy et al., 1998; Winklhofer et al., 2003). Although the mechanism is different for KV3.4 channels, the net effect of PKC is quite similar, a loss of inactivation (Covarrubias et al., 1994; Beck et al., 1998). Serines in the inactivation gate become phosphorylated, stopping inactivation. The effect can be mimicked by phosphomimetic mutations. Such receptor-induced loss of inactivation from KV3.4 channels has been seen in primary dorsal root ganglion neurons (Ritter et al., 2012). Similarly, we showed that stimulation of PKC with PMA closely mimics the decrease in inactivation we observed after M1R activation. This finding agrees with the published studies and reiterates the importance of DAG as a PKC-activating product downstream of PI(4,5)P2 hydrolysis after activation of Gq-coupled receptors.
In contrast, in Drosophila melanogaster, the shab channel (KV2 family) apparently is inhibited by PI(4,5)P2 in photoreceptors and in a fly S2 cell expression system (Krause et al., 2008). Photostimulation relieves the inhibition in a manner that requires PLC but not IP3 or a calcium rise and with the same rapid kinetics as phototransduction. Direct application of 40 µM diC8-PI(4,5)P2 to patches excised from S2 cells reversibly inhibits currents. These channels lack N-type fast inactivation so the rapid effects are not likely to involve the inactivation process. Rather they include a leftward shift of the conductance-voltage relation by light.
hERG channels
We come finally to hERG KV11.1 channels. Activating M1R reduced hERG current amplitude slightly; however, activating Dr-VSP yielded no detectable change. With pseudojanin, we did sometimes observe rundown of hERG current amplitude, but this rundown began before addition of rapamycin to recruit pseudojanin to the plasma membrane and was not accelerated by the rapamycin. The literature does suggest some PI(4,5)P2 dependence. In CHO cells, hERG current amplitude increases upon dialysis of 10 µM brain PI(4,5)P2 into cells via the patch pipette, and it decreases upon activation of Gq-coupled α1A-adrenergic receptors in a manner that is not blocked by PKC inhibitors or calcium chelators (Bian et al., 2001, 2004; Bian and McDonald, 2007). The effects are small changes of current amplitude and kinetic parameters. The small effects of α1A-adrenergic receptor stimulation resemble ours with M1R, but our experiments did not find evidence for regulation by PI(4,5)P2. We conclude that PI(4,5)P2 modulates hERG channels very modestly if at all and is not essential for hERG channel function. The current regulation through Gq-coupled GPCRs could well have some physiological effect on the cardiac action potential.
Use of heterologous expression systems
We performed all of our experiments in tsA-201 cells. This conferred certain advantages: We could record the activity of the KV channels of interest in isolation without interference from the many other ion channels that would be present in primary cells and might also be subject to modulation. In addition, we could readily express phosphoinositide-manipulating or reporting proteins like Dr-VSP and PH probes. There are also some disadvantages. Although they seem to have similar phosphoinositide metabolism, the tsA-201 cells may lack KV-interacting proteins present in primary cells that might govern their responses to PI(4,5)P2 depletion. In addition, the spatial organization of proteins in the plasma membrane might be different in tsA-201 cells, again affecting signaling pathways. Nonetheless, we are encouraged by the similarity of KCNQ channel modulation by receptors in tsA-201 cells and primary sympathetic neurons.
Conclusions
In summary, we confirm that members of the KV7 and Kir2 channel families have a strong PI(4,5)P2 requirement and that PI(4,5)P2 depletion reduces currents conducted by these channels nearly completely. In vivo, a graded reduction of these currents should occur during activation of Gq protein–coupled receptors that activate PLC in the plasma membrane. This situation is known for KV7.2/KV7.3-mediated IM (Shapiro et al., 1994; Suh and Hille, 2002; Brown et al., 2007; Hughes et al., 2007) and for N-type Ca2+ channels in sympathetic neurons (Shapiro et al., 1994; Suh et al., 2010) upon activation of endogenous M1 muscarinic receptors. Some KV channels, like KV1.1/KVβ1.1, KV3.4, and hERG, are modulated by apparently conventional second-messenger signaling pathways downstream of PI(4,5)P2 hydrolysis. Unexpectedly, many KV channels showed no change in properties when PI(4,5)P2 was manipulated by lipid phosphatases. We feel we have explored the range of free PI(4,5)P2 levels that might be experienced physiologically in the plasma membrane. Thus, any ability to modulate some of them with applications of brain PI(4,5)P2 as micelles may be unphysiological.
Supplementary Material
Acknowledgments
We would like to thank all colleagues who have generously provided us with plasmids (see Materials and methods). Byung-Chang Suh obtained preliminary results with muscarinic modulation of KV1.1/KVβ1.1, KV1.4, and KV3.4 that inspired this project. We are also thankful to Sharona E. Gordon, William N. Zagotta, Byung-Chang Suh, Eamonn J. Dickson, and Björn Falkenburger for comments on the manuscript, to all members of the Hille laboratory and many members of the Department of Physiology and Biophysics at the University of Washington for discussions and experimental advice, and to Lea M. Miller for technical help.
This study was supported by National Institutes of Health grant R01 NS08174 and the Alexander von Humboldt-Foundation (awards to M. Kruse).
Christopher Miller served as editor.
Footnotes
Abbreviations used in this paper:
- CHO
- Chinese hamster ovary
- DAG
- diacylglycerol
- FRET
- Förster resonance energy transfer
- IP3
- inositol 1,4,5-trisphosphate
- KV
- voltage-gated potassium
- Oxo-M
- oxotremorine methiodide
- PI(4,5)P2
- phosphatidylinositol 4,5-bisphosphate
- PLC
- phospholipase C
References
- Amarillo Y., De Santiago-Castillo J.A., Dougherty K., Maffie J., Kwon E., Covarrubias M., Rudy B. 2008. Ternary Kv4.2 channels recapitulate voltage-dependent inactivation kinetics of A-type K+ channels in cerebellar granule neurons. J. Physiol. 586:2093–2106 10.1113/jphysiol.2007.150540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck E.J., Sorensen R.G., Slater S.J., Covarrubias M. 1998. Interactions between multiple phosphorylation sites in the inactivation particle of a K+ channel. Insights into the molecular mechanism of protein kinase C action. J. Gen. Physiol. 112:71–84 10.1085/jgp.112.1.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian J.S., McDonald T.V. 2007. Phosphatidylinositol 4,5-bisphosphate interactions with the HERG K(+) channel. Pflugers Arch. 455:105–113 10.1007/s00424-007-0292-5 [DOI] [PubMed] [Google Scholar]
- Bian J., Cui J., McDonald T.V. 2001. HERG K(+) channel activity is regulated by changes in phosphatidyl inositol 4,5-bisphosphate. Circ. Res. 89:1168–1176 10.1161/hh2401.101375 [DOI] [PubMed] [Google Scholar]
- Bian J.S., Kagan A., McDonald T.V. 2004. Molecular analysis of PIP2 regulation of HERG and IKr. Am. J. Physiol. Heart Circ. Physiol. 287:H2154–H2163 10.1152/ajpheart.00120.2004 [DOI] [PubMed] [Google Scholar]
- Birnbaum S.G., Varga A.W., Yuan L.-L., Anderson A.E., Sweatt J.D., Schrader L.A. 2004. Structure and function of Kv4-family transient potassium channels. Physiol. Rev. 84:803–833 10.1152/physrev.00039.2003 [DOI] [PubMed] [Google Scholar]
- Bränström R., Corkey B.E., Berggren P.O., Larsson O. 1997. Evidence for a unique long chain acyl-CoA ester binding site on the ATP-regulated potassium channel in mouse pancreatic beta cells. J. Biol. Chem. 272:17390–17394 10.1074/jbc.272.28.17390 [DOI] [PubMed] [Google Scholar]
- Brown D.A., Passmore G.M. 2009. Neural KCNQ (Kv7) channels. Br. J. Pharmacol. 156:1185–1195 10.1111/j.1476-5381.2009.00111.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D.A., Hughes S.A., Marsh S.J., Tinker A. 2007. Regulation of M(Kv7.2/7.3) channels in neurons by PIP(2) and products of PIP(2) hydrolysis: significance for receptor-mediated inhibition. J. Physiol. 582:917–925 10.1113/jphysiol.2007.132498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier F., Mérot J., Loussouarn G., Baró I. 2010. Delayed rectifier K(+) currents and cardiac repolarization. J. Mol. Cell. Cardiol. 48:37–44 10.1016/j.yjmcc.2009.08.005 [DOI] [PubMed] [Google Scholar]
- Covarrubias M., Wei A., Salkoff L., Vyas T.B. 1994. Elimination of rapid potassium channel inactivation by phosphorylation of the inactivation gate. Neuron. 13:1403–1412 10.1016/0896-6273(94)90425-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech M.P. 2000. PIP2 and PIP3: complex roles at the cell surface. Cell. 100:603–606 10.1016/S0092-8674(00)80696-0 [DOI] [PubMed] [Google Scholar]
- Decher N., Gonzalez T., Streit A.K., Sachse F.B., Renigunta V., Soom M., Heinemann S.H., Daut J., Sanguinetti M.C. 2008. Structural determinants of Kvbeta1.3-induced channel inactivation: a hairpin modulated by PIP2. EMBO J. 27:3164–3174 10.1038/emboj.2008.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo G., De Camilli P. 2006. Phosphoinositides in cell regulation and membrane dynamics. Nature. 443:651–657 10.1038/nature05185 [DOI] [PubMed] [Google Scholar]
- Du X., Zhang H., Lopes C., Mirshahi T., Rohács T., Logothetis D.E. 2004. Characteristic interactions with phosphatidylinositol 4,5-bisphosphate determine regulation of kir channels by diverse modulators. J. Biol. Chem. 279:37271–37281 10.1074/jbc.M403413200 [DOI] [PubMed] [Google Scholar]
- Falkenburger B.H., Jensen J.B., Hille B. 2010a. Kinetics of M1 muscarinic receptor and G protein signaling to phospholipase C in living cells. J. Gen. Physiol. 135:81–97 10.1085/jgp.200910344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenburger B.H., Jensen J.B., Hille B. 2010b. Kinetics of PIP2 metabolism and KCNQ2/3 channel regulation studied with a voltage-sensitive phosphatase in living cells. J. Gen. Physiol. 135:99–114 10.1085/jgp.200910345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamper N., Li Y., Shapiro M.S. 2005. Structural requirements for differential sensitivity of KCNQ K+ channels to modulation by Ca2+/calmodulin. Mol. Biol. Cell. 16:3538–3551 10.1091/mbc.E04-09-0849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G.R.V., Fischer M.J., Anderson K.E., Holdich J., Koteci A., Balla T., Irvine R.F. 2012. PI4P and PI(4,5)P2 are essential but independent lipid determinants of membrane identity. Science. 10.1126/science.1222483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S.B., Tao X., MacKinnon R. 2011. Structural basis of PIP2 activation of the classical inward rectifier K+ channel Kir2.2. Nature. 477:495–498 10.1038/nature10370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicke L. 1999. Gettin’ down with ubiquitin: turning off cell-surface receptors, transporters and channels. Trends Cell Biol. 9:107–112 10.1016/S0962-8924(98)01491-3 [DOI] [PubMed] [Google Scholar]
- Hilgemann D.W., Ball R. 1996. Regulation of cardiac Na+,Ca2+ exchange and KATP potassium channels by PIP2. Science. 273:956–959 10.1126/science.273.5277.956 [DOI] [PubMed] [Google Scholar]
- Hilgemann D.W., Feng S., Nasuhoglu C. 2001. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci. STKE. 2001:re19 10.1126/stke.2001.111.re19 [DOI] [PubMed] [Google Scholar]
- Hille B. 2001. Ion Channels of Excitable Membranes. Third edition. Sinauer, Sunderland, MA: 814 pp [Google Scholar]
- Horowitz L.F., Hirdes W., Suh B.-C., Hilgemann D.W., Mackie K., Hille B. 2005. Phospholipase C in living cells: activation, inhibition, Ca2+ requirement, and regulation of M current. J. Gen. Physiol. 126:243–262 10.1085/jgp.200509309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S., Marsh S.J., Tinker A., Brown D.A. 2007. PIP(2)-dependent inhibition of M-type (Kv7.2/7.3) potassium channels: direct on-line assessment of PIP2 depletion by Gq-coupled receptors in single living neurons. Pflugers Arch. 455:115–124 10.1007/s00424-007-0259-6 [DOI] [PubMed] [Google Scholar]
- Inoue T., Heo W.D., Grimley J.S., Wandless T.J., Meyer T. 2005. An inducible translocation strategy to rapidly activate and inhibit small GTPase signaling pathways. Nat. Methods. 2:415–418 10.1038/nmeth763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas E.A., Kaczmarek L.K. 1996. Regulation of potassium channels by protein kinases. Curr. Opin. Neurobiol. 6:318–323 10.1016/S0959-4388(96)80114-0 [DOI] [PubMed] [Google Scholar]
- Kaulin Y.A., De Santiago-Castillo J.A., Rocha C.A., Nadal M.S., Rudy B., Covarrubias M. 2009. The dipeptidyl-peptidase-like protein DPP6 determines the unitary conductance of neuronal Kv4.2 channels. J. Neurosci. 29:3242–3251 10.1523/JNEUROSCI.4767-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Nadal M.S., Clemens A.M., Baron M., Jung S.C., Misumi Y., Rudy B., Hoffman D.A. 2008. Kv4 accessory protein DPPX (DPP6) is a critical regulator of membrane excitability in hippocampal CA1 pyramidal neurons. J. Neurophysiol. 100:1835–1847 10.1152/jn.90261.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooijman E.E., Burger K.N.J. 2009. Biophysics and function of phosphatidic acid: a molecular perspective. Biochim. Biophys. Acta. 1791:881–888 [DOI] [PubMed] [Google Scholar]
- Krause Y., Krause S., Huang J., Liu C.H., Hardie R.C., Weckström M. 2008. Light-dependent modulation of Shab channels via phosphoinositide depletion in Drosophila photoreceptors. Neuron. 59:596–607 10.1016/j.neuron.2008.07.009 [DOI] [PubMed] [Google Scholar]
- Larsson O., Deeney J.T., Bränström R., Berggren P.O., Corkey B.E. 1996. Activation of the ATP-sensitive K+ channel by long chain acyl-CoA. A role in modulation of pancreatic beta-cell glucose sensitivity. J. Biol. Chem. 271:10623–10626 10.1074/jbc.271.18.10623 [DOI] [PubMed] [Google Scholar]
- Levy D.I., Cepaitis E., Wanderling S., Toth P.T., Archer S.L., Goldstein S.A.N. 2010. The membrane protein MiRP3 regulates Kv4.2 channels in a KChIP-dependent manner. J. Physiol. 588:2657–2668 10.1113/jphysiol.2010.191395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M., Jing J., Chikvashvili D., Thornhill W.B., Lotan I. 1998. Activation of a metabotropic glutamate receptor and protein kinase C reduce the extent of inactivation of the K+ channel Kv1.1/Kvbeta1.1 via dephosphorylation of Kv1.1. J. Biol. Chem. 273:6495–6502 10.1074/jbc.273.11.6495 [DOI] [PubMed] [Google Scholar]
- Li Y., Gamper N., Hilgemann D.W., Shapiro M.S. 2005. Regulation of Kv7 (KCNQ) K+ channel open probability by phosphatidylinositol 4,5-bisphosphate. J. Neurosci. 25:9825–9835 10.1523/JNEUROSCI.2597-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zaydman M.A., Wu D., Shi J., Guan M., Virgin-Downey B., Cui J. 2011. KCNE1 enhances phosphatidylinositol 4,5-bisphosphate (PIP2) sensitivity of IKs to modulate channel activity. Proc. Natl. Acad. Sci. USA. 108:9095–9100 10.1073/pnas.1100872108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner M., Leitner M.G., Halaszovich C.R., Hammond G.R.V., Oliver D. 2011. Probing the regulation of TASK potassium channels by PI4,5P2 with switchable phosphoinositide phosphatases. J. Physiol. 589:3149–3162 10.1113/jphysiol.2011.208983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis D.E., Petrou V.I., Adney S.K., Mahajan R. 2010. Channelopathies linked to plasma membrane phosphoinositides. Pflugers Arch. 460:321–341 10.1007/s00424-010-0828-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loussouarn G., Park K.-H., Bellocq C., Baró I., Charpentier F., Escande D. 2003. Phosphatidylinositol-4,5-bisphosphate, PIP2, controls KCNQ1/KCNE1 voltage-gated potassium channels: a functional homology between voltage-gated and inward rectifier K+ channels. EMBO J. 22:5412–5421 10.1093/emboj/cdg526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens J.R., Kwak Y.G., Tamkun M.M. 1999. Modulation of Kv channel alpha/beta subunit interactions. Trends Cardiovasc. Med. 9:253–258 10.1016/S1050-1738(00)00037-2 [DOI] [PubMed] [Google Scholar]
- Murata Y., Okamura Y. 2007. Depolarization activates the phosphoinositide phosphatase Ci-VSP, as detected in Xenopus oocytes coexpressing sensors of PIP2. J. Physiol. 583:875–889 10.1113/jphysiol.2007.134775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y., Iwasaki H., Sasaki M., Inaba K., Okamura Y. 2005. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 435:1239–1243 10.1038/nature03650 [DOI] [PubMed] [Google Scholar]
- Nadal M.S., Ozaita A., Amarillo Y., Vega-Saenz de Miera E., Ma Y., Mo W., Goldberg E.M., Misumi Y., Ikehara Y., Neubert T.A., Rudy B. 2003. The CD26-related dipeptidyl aminopeptidase-like protein DPPX is a critical component of neuronal A-type K+ channels. Neuron. 37:449–461 10.1016/S0896-6273(02)01185-6 [DOI] [PubMed] [Google Scholar]
- Nilius B., Prenen J., Tang J., Wang C., Owsianik G., Janssens A., Voets T., Zhu M.X. 2005. Regulation of the Ca2+ sensitivity of the nonselective cation channel TRPM4. J. Biol. Chem. 280:6423–6433 10.1074/jbc.M411089200 [DOI] [PubMed] [Google Scholar]
- Okamura Y., Murata Y., Iwasaki H. 2009. Voltage-sensing phosphatase: actions and potentials. J. Physiol. 587:513–520 10.1113/jphysiol.2008.163097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D., Lien C.C., Soom M., Baukrowitz T., Jonas P., Fakler B. 2004. Functional conversion between A-type and delayed rectifier K+ channels by membrane lipids. Science. 304:265–270 10.1126/science.1094113 [DOI] [PubMed] [Google Scholar]
- Park K.-H., Piron J., Dahimene S., Mérot J., Baró I., Escande D., Loussouarn G. 2005. Impaired KCNQ1-KCNE1 and phosphatidylinositol-4,5-bisphosphate interaction underlies the long QT syndrome. Circ. Res. 96:730–739 10.1161/01.RES.0000161451.04649.a8 [DOI] [PubMed] [Google Scholar]
- Peters H.C., Hu H., Pongs O., Storm J.F., Isbrandt D. 2005. Conditional transgenic suppression of M channels in mouse brain reveals functions in neuronal excitability, resonance and behavior. Nat. Neurosci. 8:51–60 10.1038/nn1375 [DOI] [PubMed] [Google Scholar]
- Piron J., Choveau F.S., Amarouch M.Y., Rodriguez N., Charpentier F., Mérot J., Baró I., Loussouarn G. 2010. KCNE1-KCNQ1 osmoregulation by interaction of phosphatidylinositol-4,5-bisphosphate with Mg2+ and polyamines. J. Physiol. 588:3471–3483 10.1113/jphysiol.2010.195313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongs O., Schwarz J.R. 2010. Ancillary subunits associated with voltage-dependent K+ channels. Physiol. Rev. 90:755–796 10.1152/physrev.00020.2009 [DOI] [PubMed] [Google Scholar]
- Powl A.M., Carney J., Marius P., East J.M., Lee A.G. 2005a. Lipid interactions with bacterial channels: fluorescence studies. Biochem. Soc. Trans. 33:905–909 10.1042/BST20050905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powl A.M., East J.M., Lee A.G. 2005b. Heterogeneity in the binding of lipid molecules to the surface of a membrane protein: hot spots for anionic lipids on the mechanosensitive channel of large conductance MscL and effects on conformation. Biochemistry. 44:5873–5883 10.1021/bi047439e [DOI] [PubMed] [Google Scholar]
- Rhee S.G. 2001. Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem. 70:281–312 10.1146/annurev.biochem.70.1.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter D.M., Ho C., O’Leary M.E., Covarrubias M. 2012. Modulation of Kv3.4 channel N-type inactivation by protein kinase C shapes the action potential in dorsal root ganglion neurons. J. Physiol. 590:145–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohács T., Lopes C.M., Jin T., Ramdya P.P., Molnár Z., Logothetis D.E. 2003. Specificity of activation by phosphoinositides determines lipid regulation of Kir channels. Proc. Natl. Acad. Sci. USA. 100:745–750 10.1073/pnas.0236364100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino S. 1999. ATP-sensitive potassium channels: a model of heteromultimeric potassium channel/receptor assemblies. Annu. Rev. Physiol. 61:337–362 10.1146/annurev.physiol.61.1.337 [DOI] [PubMed] [Google Scholar]
- Shapiro M.S., Wollmuth L.P., Hille B. 1994. Angiotensin II inhibits calcium and M current channels in rat sympathetic neurons via G proteins. Neuron. 12:1319–1329 10.1016/0896-6273(94)90447-2 [DOI] [PubMed] [Google Scholar]
- Shapiro M.S., Roche J.P., Kaftan E.J., Cruzblanca H., Mackie K., Hille B. 2000. Reconstitution of muscarinic modulation of the KCNQ2/KCNQ3 K(+) channels that underlie the neuronal M current. J. Neurosci. 20:1710–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh B.C., Hille B. 2002. Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron. 35:507–520 10.1016/S0896-6273(02)00790-0 [DOI] [PubMed] [Google Scholar]
- Suh B.C., Hille B. 2008. PIP2 is a necessary cofactor for ion channel function: how and why? Annu. Rev. Biophys. 37:175–195 10.1146/annurev.biophys.37.032807.125859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh B.-C., Inoue T., Meyer T., Hille B. 2006. Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Science. 314:1454–1457 10.1126/science.1131163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh B.C., Leal K., Hille B. 2010. Modulation of high-voltage activated Ca(2+) channels by membrane phosphatidylinositol 4,5-bisphosphate. Neuron. 67:224–238 10.1016/j.neuron.2010.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telezhkin V., Reilly J.M., Thomas A.M., Tinker A., Brown D.A. 2012. Structural requirements of membrane phospholipids for M-type potassium channel activation and binding. J. Biol. Chem. 287:10001–10012 10.1074/jbc.M111.322552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wal J., Habets R., Várnai P., Balla T., Jalink K. 2001. Monitoring agonist-induced phospholipase C activation in live cells by fluorescence resonance energy transfer. J. Biol. Chem. 276:15337–15344 10.1074/jbc.M007194200 [DOI] [PubMed] [Google Scholar]
- Varnai P., Thyagarajan B., Rohács T., Balla T. 2006. Rapidly inducible changes in phosphatidylinositol 4,5-bisphosphate levels influence multiple regulatory functions of the lipid in intact living cells. J. Cell Biol. 175:377–382 10.1083/jcb.200607116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whorton M.R., MacKinnon R. 2011. Crystal structure of the mammalian GIRK2 K+ channel and gating regulation by G proteins, PIP2, and sodium. Cell. 147:199–208 10.1016/j.cell.2011.07.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklhofer M., Matthias K., Seifert G., Stocker M., Sewing S., Herget T., Steinhäuser C., Saaler-Reinhardt S. 2003. Analysis of phosphorylation-dependent modulation of Kv1.1 potassium channels. Neuropharmacology. 44:829–842 10.1016/S0028-3908(03)00070-4 [DOI] [PubMed] [Google Scholar]
- Winks J.S., Hughes S., Filippov A.K., Tatulian L., Abogadie F.C., Brown D.A., Marsh S.J. 2005. Relationship between membrane phosphatidylinositol-4,5-bisphosphate and receptor-mediated inhibition of native neuronal M channels. J. Neurosci. 25:3400–3413 10.1523/JNEUROSCI.3231-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Craciun L.C., Mirshahi T., Rohács T., Lopes C.M., Jin T., Logothetis D.E. 2003. PIP2 activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron. 37:963–975 10.1016/S0896-6273(03)00125-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.