Abstract
Cancer cell invasion and metastasis are the most important adverse prognostic factors for pancreatic cancer. Identification of biomarkers associated with outcome of pancreatic cancer may provide new approaches and targets for anticancer therapy. The aim of this study is to examine the relationship between the expression of RhoT1, Smad4 and p16 and metastasis and survival in patients with pancreatic cancer. The analysis showed that the high cytoplasmic expression levels of RhoT1, Smad4 and p16 in pancreatic cancer tissues had significantly negative correlation with lymph node metastasis (LNM) (P = 0.017, P = 0.032, P = 0.042, respectively). However, no significant association was observed between perineural invasion (PNI) and the expression of above three proteins (all P>0.05). Additionally, the survival analysis showed that the low expression levels of RhoT1 and Smad4 were significantly associated with worse survival (P = 0.034, P = 0.047, respectively). In conclusion, these results indicated that the low-expression levels of RhoT1 and Smad4 were significantly associated with LNM and shorter survival. RhoT1 may be considered as a potential novel marker for predicting the outcome in patients with pancreatic cancer.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the predominant form of pancreatic cancer, which ranks fourth in cancer-related causes of death [1]. It was estimated that 43140 Americans were diagnosed and 36800 patients died of pancreatic cancer in the United States in 2010 [2]. Despite extensive clinical and scientific efforts, the prognosis of this exceptionally lethal disease has not improved significantly over the past decades [2], [3]. The median survival after diagnosis is 3–6 months without treatment, and resectional surgery and adjuvant treatment increase median survival to around 23 months [4].
Numerous studies have found that RAS regulates the growth and metastasis of pancreatic cancer cells. The Rho family of GTPases is a subfamily of the Ras superfamily. Rho GTPases have been reported to contribute to most steps of cancer initiation and progression including the acquisition of unlimited proliferation potential, survival and evasion from apoptosis, tissue invasion and the establishment of metastases [5], [6], [7], [8], [9], [10], [11], [12]. The Rho family of GTPases contains 20 members. Most of what we know about the role of Rho GTPases in cancer cell invasion comes from the studies of the prototypic members RhoA, RhoB and RhoC, Rac1 and Cdc42. However, little is known about roles of other less-characterized family members in cancer. Some previous studies have revealed that RhoA and RhoC expression are frequently increased in human cancers, while RhoB is often down-regulated. RhoT1 belongs to the mitochondrial Rho GTPase family [13]. However, RhoT1 protein is classified as atypical GTPases because it is not regulated as the other classical GTPases [14], [15]. So far little is known about the role of RhoT1 in cancer progression. In a previous study, we have identified a total of 1276 genes that are differentially expressed in PDAC. Among them, 691 genes are up-regulated and 585 genes are down-regulated genes, including RhoT1, Smad4 and p16 [16]. Therefore, we are interested in knowing whether the RhoT1 is similarly involved in the development of cancer.
In addition, previous studies have reported that mutation of Smad4 is identified in approximately 50% of pancreatic adenocarcinomas [17]. A number of studies have shown that loss of Smad4 is generally observed in pancreatic carcinogenesis, and inactivation of Smad4 is associated with poor prognosis in pancreatic cancer [18], [19]. Similarly, it has been demonstrated that loss of p16 expression is observed in most pancreatic tumor [20], [21], and constitutes a key event in the multistep process of pancreatic ductal cell transformation. However, the significance of p16 and Smad4 inactivation for complex and tissue-specific aspects of pancreatic cancer progression, such as angiogenesis and metastasis, is less understood [22]. Also, there are relatively few studies that have examined the possible role of Smad4 and p16 in the progression of LNM and PNI in pancreatic cancer.
Cancer cell invasion and metastasis are critical steps in pancreatic cancer progression, and are the main causes of poor survival in pancreatic cancer. Predicting invasion and metastasis for patients with pancreatic cancer may provide important insights into the pancreatic cancer progression and prognosis. Identification of biomarkers associated with outcome of pancreatic cancer can provide new approaches and targets for anticancer therapy. Therefore, we have carried out this study to examine the expression of RhoT1, Smad4 and p16 in pancreatic cancer, and to analyze whether the expression patterns of RhoT1, Smad4 and p16 are correlated with metastatic potential and are predictive of clinical outcome in patients with pancreatic cancer.
Results
Patient Characteristics
As shown in Table 1, the sample consisted of 162 patients with a diagnosis of pancreatic cancer (102 men and 60 women). The mean age at diagnosis was 59 year-old (range 34 to 85 year-old). The median tumor size was 4 cm (range 0.5 to 14 cm). Most tumors (117/162, 72%) were well differentiated, 8 (8%) were moderately differentiated, and 31 (19%) were poorly differentiated. 80 (49.4%) tumors were categorized as American Joint Committee on Cancer (AJCC) stage I, 80 as (49.4%) stage II, none as stage III, and 2 (1.2%) as stage IV. 83 (51.2%) of 162 patients had PNI. 65 (40.1%) of 162 patients had LNM, The median number of lymph nodes (LNs) examined was 7. The median number of LNs assessed in the node-positive patients was 10 compared with a median of 6 lymph nodes in the node-negative patients (P = 0.007).
Table 1. Clinicopathological characteristics of the pancreatic cancer.
| Characteristics | No. of Patients | % |
| Age (years) | ||
| median | 59 | |
| range | 34–85 | |
| Gender | ||
| female | 60 | 37 |
| Male | 102 | 63 |
| Stage | ||
| stage I | 80 | 49.4 |
| stage II | 80 | 49.4 |
| stage III | 0 | 0 |
| stage IV | 2 | 1.2 |
| Lymph node status | ||
| negative | 97 | 59.9 |
| positive | 65 | 40.1 |
| Lymph node ratio (LNR) | ||
| LNR = 0 | 97 | 59.9 |
| LNR>0, <0.5 | 50 | 30.9 |
| LNR≧0.5 | 15 | 9.2 |
| Perineural invasion | ||
| negative | 79 | 48.8 |
| positive | 83 | 51.2 |
| Tumor size | ||
| median (cm) | 4 | |
| range (cm) | 0.5–14 | |
| Tumor differentiation | ||
| well | 117 | 72.2 |
| moderate | 14 | 8.7 |
| poor | 31 | 19.1 |
Associations between the Various Clinicopathological Factors and the Presence of LNM and PNI
Associations between the various clinicopathological factors and the presence of LNM and PNI were analyzed to identify the risk factors of LNM and PNI (Table 2). These factors included: gender, age (<60 years or ≧60 years), location of tumor (head or body and rear), long diameter of tumor (<4.0 cm or ≧4.0 cm), tumor differentiation (poor or moderate/well). Location of tumor was significantly associated with LNM (P = 0.03). There was no significant association between LNM and age, gender, long diameter of tumor and tumor differentiation (P>0.05 for each). In addition, no significant association was observed between PNI and age, gender, location of tumor, long diameter of tumor and tumor differentiation (all P>0.05).
Table 2. Associations between the various clinicopathological factors and the presence of lymph node metastasis and perineural invasion.
| Variables | lymph node metastasis | perineural invasion | ||||
| Positive | Negative | P value | Positive | Negative | P value | |
| n = 65 | n = 97 | n = 83 | n = 79 | |||
| Age (years) | ||||||
| <60 | 39 | 50 | 0.491 | 41 | 48 | 0.146 |
| ≧60 | 26 | 47 | 42 | 31 | ||
| Gender | ||||||
| female | 22 | 38 | 0.289 | 28 | 32 | 0.372 |
| male | 43 | 59 | 55 | 47 | ||
| Location | ||||||
| head | 53 | 64 | 0.03 | 59 | 58 | 0.74 |
| body/rear | 12 | 33 | 24 | 21 | ||
| Size | ||||||
| <4 | 33 | 41 | 0.287 | 38 | 36 | 0.978 |
| ≧4 | 32 | 56 | 45 | 43 | ||
| Differentiation | ||||||
| poor | 11 | 20 | 0.558 | 17 | 14 | 0.655 |
| moderate/well | 54 | 77 | 66 | 65 | ||
Expression of RhoT1, Smad4 and P16 in Pancreatic Cancer Tissues and Paracancerous Tissues
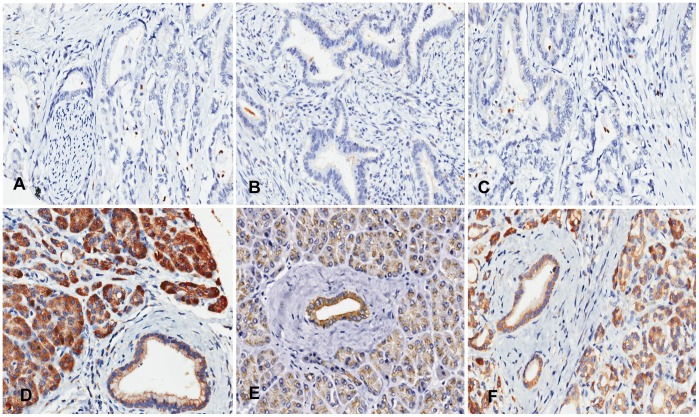
RhoT1 and p16 staining were localized to the cytoplasm (Figure 1). Although, Smad4 was expressed mainly in the cytoplasm (Figure 1), some Smad4 was seen in the nucleus. The cytoplasmic expression levels of RhoT1, Smad4 and p16 were lower in cancer tissues than paracancerous tissues (P<0.0001, P<0.0001, P = 0.002, respectively). There was a strong correlation between cytoplasmic and nuclear Smad4 expression (Spearman correlation coefficient = 0.321; P<0.0001). However, no significant difference was observed in nuclear Smad4 expression between cancer tissues and paracancerous tissues (P>0.05).
Figure 1. Panels A, B and C respectively show the negative expression of RhoT1, Smad4 and p16 in the cancer tissues.
Panels D, E and F respectively show the positive expression of RhoT1, Smad4 and p16 in the paracancerous tissues. All images were taken at 200× magnification.
Associations between the Expression Levels of RhoT1, Smad4 and P16 and the Clinicopathological Features
The analysis showed that high cytoplasmic expression level of RhoT1 was significantly negative correlation with LNM in cancer tissues (P = 0.003). The difference remained significant even after adjustment for age, gender, cancer location and long diameter of tumor (P = 0.017). Similarly, cytoplasmic expression levels of Smad4 and p16 in cancer tissues were also negatively correlated with LNM (P = 0.032, P = 0.042, respectively). However, there were no significant associations between PNI and the expression levels of three proteins (all P>0.05; Table 3). Since no significant difference was observed between cancer tissues and paracancerous tissues, nuclear expression of Smad4 was eliminated from further testing the association with the clinicopathological features. Logistic regression analysis showed that the cytoplasmic expression level of RhoT1 in cancer tissues acted as an independent risk factor for LNM (P = 0.042). However, according to the analysis, the cytoplasmic expression levels of Smad4 and p16 could not be used as independent risk factors for LNM (P>0.05). Additionally, the results showed that no significant association was observed between the cytoplasmic expression levels of RhoT1, Smad4, p16 and clinicopathological features, which including age, gender, tumor location, size, tumor differentiation, and AJCC stage (all P>0.05; Table 4).
Table 3. Associations between the cytoplasmic expression of RhoT1, Smad4, p16 and the presence of lymph node metastasis and perineural invasion.
| Variables | lymph node metastasis | perineural invasion | ||||
| Positive | Negative | P value | Positive | Negative | P value | |
| n = 65 | n = 97 | n = 83 | n = 79 | |||
| RhoT1 expression | ||||||
| high | 12 | 43 | 0.003 | 23 | 32 | 0.198 |
| low | 53 | 54 | 60 | 47 | ||
| Smad4 expression | ||||||
| high | 8 | 35 | 0.032 | 25 | 18 | 0.304 |
| low | 57 | 62 | 58 | 61 | ||
| p16 expression | ||||||
| high | 7 | 29 | 0.042 | 20 | 16 | 0.35 |
| Low | 58 | 68 | 63 | 63 | ||
Table 4. Associations between the cytoplasmic expression of RhoT1, Smad4, p16 and the clinicopathological features.
| Variables | RhoT1 expression | Smad4 expression | p16 expression | ||||||
| low | high | P value | low | high | P value | low | high | P value | |
| Age (years) | |||||||||
| <60 | 60 | 29 | 0.922 | 70 | 19 | 0.097 | 70 | 19 | 0.299 |
| ≧60 | 47 | 26 | 49 | 24 | 56 | 36 | |||
| Gender | |||||||||
| female | 36 | 24 | 0.660 | 38 | 22 | 0.382 | 48 | 12 | 0.897 |
| male | 71 | 31 | 81 | 21 | 78 | 24 | |||
| Location | |||||||||
| head | 80 | 37 | 0.876 | 88 | 29 | 0.744 | 94 | 23 | 0.468 |
| body/rear | 27 | 18 | 31 | 14 | 32 | 13 | |||
| Size | |||||||||
| <4 | 47 | 27 | 0.324 | 52 | 22 | 0.186 | 59 | 15 | 0.114 |
| ≧4 | 60 | 28 | 67 | 21 | 57 | 21 | |||
| Differentiation | |||||||||
| moderate/well | 81 | 45 | 0.278 | 94 | 37 | 0.808 | 102 | 29 | 0.149 |
| poor | 21 | 10 | 25 | 6 | 24 | 7 | |||
| AJCC stage | |||||||||
| stage I | 46 | 34 | 0.191 | 50 | 30 | 0.242 | 56 | 24 | 0.33 |
| stage II | 60 | 20 | 68 | 12 | 69 | 11 | |||
| stage IV | 1 | 1 | 1 | 1 | 1 | 1 | |||
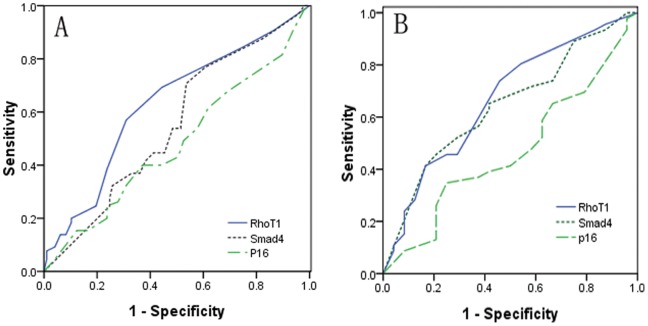
Additionally, the ROC curves were established to assess the potential diagnostic value of these proteins for predicting between specimens with LNM and specimens without LNM. The analysis discovered that the area under the ROC curve (AUC) for RhoT1 was 0.629 [95% confidence interval (CI) 0.541 to 0.718], the AUC for Smad4 was 0.551 (95% CI 0461 to 0.640) and the AUC for p16 was 0.480 (95% confidence interval 0.388 to 0.572). The statistical analysis indicated that only AUC value for RhoT1 was significantly greater than 0.5 (P = 0.005). At the cutoff of 75% (relative expression positive-rate), sensitivity of the RhoT1 was 69.2% and specificity was 55.7% (Figure 2–A).
Figure 2. A shows the Receiver operator characteristic (ROC) curve analysis using the expression of RhoT1, Smad4 and p16 for discriminating LNM (n = 162).
The area under the ROC curve (AUC) for RhoT1, Smad4 and p16 were 0.629, 0.551, and 0.480 respectively. B Shows the ROC curve analysis of the expression of RhoT1, Smad4 and p16 for overall survival (n = 70). The AUC values for three proteins were 0.667, 0.643, and 0.469, respectively.
Survival Analysis
The survival analysis showed that the survival rates after 5 years was 28.6%, median survival was 14 months. The analysis of survival showed LNM, tumor differentiation and stage (I v II) as being negatively significant predictors of pancreatic cancer OS (P = 0.01, P = 0.003, P = 0.036, respectively), and patients with an Lymph node ratio (LNR) >0 to <50% had a longer median survival (11 months) compared with patients who had an LNR ≧50% (8 months) (P = 0.035) (data not shown). In contrast, the evaluation of OS by age, group, gender, tumor location, tumor size, and PNI did not indicate any significant differences (P>0.05 for all). Two patients with stage IV were excluded from the survival analysis because the sample size was too small.
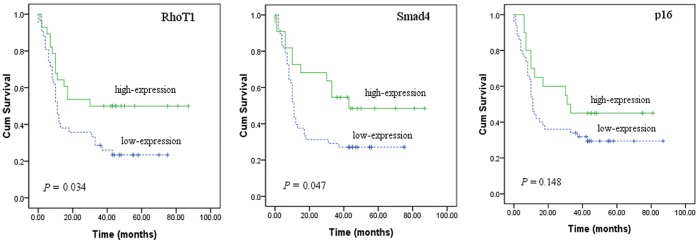
In addition, the 5-year survival rates were 23.4%, 27.1% and 50%, 48.5% in patients with low-expression and high-expression of RhoT1 and Smad4 respectively. Patients with high cytoplasmic expression levels of RhoT1 and Smad4 achieved better survival than patients with low-expression levels of RhoT1 and Smad4 (P = 0.034, P = 0.047, respectively; Figure 3). However, there was no statistically significant relationship in OS between patients with high or low expression of p16 (P = 0.148). The AUC values for RhoT1, Smad4 and p16 were 0.667 (95% CI 0.532 to 0.802), 0.643 (95% CI 0.509 to 0.777), and 0.469 (95% CI 0.328 to 0.610) respectively. Additionally, the analysis showed that AUC value for RhoT1 was significantly greater than 0.5 (P = 0.022). However, no significant difference was observed between areas of Smad4 and p16 in ROC analysis (P = 0.051, P = 0.674, respectively) (Figure 2–B).
Figure 3. Kaplan–Meier curves showing correlation between high or low expression of RhoT1, Smad4, p16 and overall survival in patients with pancreatic cancer (n = 70).
High expression levels of RhoT1 and Smad4 correlated with better survival (P = 0.034, P = 0.047, respectively; log rank test), no significant association was observed between the expression level of p16 and overall survival (P>0.05).
Table 5 shows the results of univariate Cox proportional hazards analysis for the major clinicopathologic features and for the cytoplasmic expression of RhoT1, Smad4 and p16 in pancreatic cancer tissues. The analysis showed that the variables with an increase in the risk of death included poor differentiation [hazard ratio (HR), 2.484; 95% CI, 1.329–4.642; P = 0.004], Higher stage (HR, 1.82; 95% CI, 1.01–3.28; P = 0.044) and LNM (HR, 2.09; 95% CI, 1.16–3.77; P = 0.014) and the low-expression of RhoT1 (HR, 1.92; 95% CI, 1.02–3.61; P = 0.042). Multivariate analysis by Cox regression and correction for all histopathologic features and RhoT1 expression revealed that tumor differentiation was an independent prognostic factor (P = 0.01), whereas tumor stage, LNM and RhoT1 expression were not statistically significant (P = 0.623, P = 0.101, P = 0.3, respectively) (Table 5).
Table 5. Univariate and multivariable Cox regression analysis of overall survival (n = 70).
| Features | Univariate analysis | Multivariable analysis | ||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Tumor features | ||||
| age (years),<60 v ≧ 60 | 1.38(0.77 to 2.46) | 0.273 | ||
| sex, female v male | 0.60(0.32 to 1.13) | 0.114 | ||
| tumor location, head v body/rear | 1.08(0.59 to 1.99) | 0.787 | ||
| tumor size, <4 v ≧ 4 | 1.41(0.79 to 2.52) | 0.239 | ||
| tumor differentiation, poor v moderate/well | 2.48(1.32 to 4.64) | 0.004 | 2.49(1.24 to 5.01) | 0.010 |
| AJCC stage, II v I | 1.82(1.01 to 3.28) | 0.044 | 1.29(0.46 to 3.66) | 0.623 |
| LNM, positive v negative | 2.09(1.16 to 3.77) | 0.014 | 2.35(0.84 to 6.57) | 0.101 |
| PNI, positive v negative | 1.57(0.88 to 2.82) | 0.124 | ||
| Expression of proteins, low v high | ||||
| RhoT1 | 1.92(1.02 to 3.61) | 0.042 | 1.43(0.72 to 2.84) | 0.300 |
| Smad4 | 1.94(0.98 to 3.84) | 0.056 | ||
| p16 | 1.62(0.82 to 3.20) | 0.162 | ||
Note: HR: Hazard ratio; LNM: lymph node metastasis; PNI: perineural Invasion.
Discussion
Current studies have reported that molecular markers can provide crucial information for understanding pancreatic cancer progression better. Comparing the protein expression of cancer specimens with different invasion and metastasis status may provide a clue for novel metastasis-associated factors. In the present study, we found that there were significant differences of the cytoplasmic expression of RhoT1, Smad4 and p16 between cancer and paracancerous tissues. These results implied that RhoT1, Smad4 and p16 were involved in the progression of pancreatic cancer. Furthermore, low cytoplasmic expressions of RhoT1 and Smad4 were associated with LNM and worse survival in patients with pancreatic cancer.
RhoT1 belongs to the mitochondrial Rho GTPase family and is first reported by Fransson et al in 2003 [23]. Previous studies showed that Rho-GTPases were involved in the regulation of a wide variety of cellular processes, and played important roles in carcinogenesis, cancer cell migration, invasion and metastasis. For example, the expression of RhoA and RhoC was often increased in human tumors, over-expression of RhoA and RhoC in tumors were associated with metastasis and invasion [24]. In contrast, RhoB was often down-regulated, low-expression of RhoB was inversely correlated with tumor aggressiveness [25], [26], [27], [28]. These results indicated that there were differences in the expression patterns of Rho family members in human cancer. In this study, we found that the cytoplasmic expression level of RhoT1 in cancer tissues was lower than that in paracancerous tissues, and was significantly decreased in patients with LNM compared with those without LNM (P = 0.017) after adjustment for age, gender, cancer location and long diameter of tumor. Like RhoB, high-expression of RhoT1 was negatively correlated with tumor aggressiveness. However, its exact molecular mechanism is largely unknown. Several previous studied have demonstrated that RhoT1 is implicated in regulation of mitochondrial homeostasis and apoptosis [15], [23], a possible mechanism might be that cancer cell is resistant to RhoT1-mediated apoptosis and allows to avoid apoptotic cell death, which results in the initiation and progression of cancer. To our knowledge, this is the first study demonstrating that the cytoplasmic expression of RhoT1 is involved in the progression of pancreatic cancer. Collectively, these results suggest that RhoT1 may be a novel tumor suppressor gene of pancreatic cancer, and the low cytoplasmic expression of RhoT1 may serve as a potential predictor for the tendency to metastasize. However, no significant association between RhoT1 and PNI was found in present study. This indicates that the differences in mechanisms may exist between LNM and PNI. Further studies are clearly needed to elucidate the mechanism of RhoT1 involved in pancreatic cancer.
Additionally, Smad4 is one of the most commonly inactivated genes in pancreatic cancer, and loss of p16 expression is observed in most pancreatic cancer [20], [21]. In this study, we found that the low expression of Smad4 was associated with LNM, which was consistent with the results of some previous studies [29], [30], [31]. Tanaka et al [32] reported that Loss of Smad4 protein expression and chromosome 18q deletion were distinctly associated with metastasis. In another study, Tanaka et al also found that the expression of Smad4 was weaker in the lymph node positive group compared with the negative group (P = 0.00075) [33]. The results indicated that Smad4 inactivation was an essential molecular event in the process of LNM. Moreover, we found that low expression of p16 was also correlated with LNM, which was consistent with following two studies. Zhi-jie Fu et al [34] reported that p16 expression in laryngeal squamous cell carcinoma (LSCC) was down-regulated with cervical LNM (P<0.05), the down-expression of p16 may be an important predictor for cervical LNM in patients of LSCC. Daniela et al [35] suggested that positive cytoplasmic p16 staining may be related to a lower metastatic potential of primary malignant melanoma. Thus, our study further confirmed that Smad4 and p16 played important role in the process of LNM in pancreatic cancer.
In present study, we further examined the correlation between the cytoplasmic expression levels of RhoT1, Smad4 and p16 and OS. We found that patients whose cancers had low cytoplasmic expression level of Smad4 had significantly worse survival than patients with high cytoplasmic expression of Smad4 (P = 0.047). This finding was consistent with some previous research [36], [37]. In addition, the possible correlation between the expression of some members of Rho GTPases and clinical outcome has previously been described. For example, Takao et al [38]suggested that Rac1 was involved in LNM of urothelial carcinoma of the upper urinary tract, and associated with a shorter disease-free survival time (P<0.01) and shorter OS (P<0.001). Similarly, another study [28] reported that the high-expression of RhoA and RhoC were associated not only with muscle invasion and LNM (P<0.001, P<0.05, respectively), but also with poorer survival in bladder cancer (P<0.0001). Inversely, high-expression of RhoB was correlated with better overall survival (P<0.05). However, so far few studies have investigated the relationship between the expression of RhoT1 and the outcome or prognosis for patients with pancreatic cancer. In this study, we found that high RhoT1 expression was inversely correlated with survival of patients with pancreatic cancer (P = 0.034). Moreover, the results of univariate Cox proportional hazards analysis for the cytoplasmic expression of RhoT1, Smad4 and p16 in pancreatic cancer tissues showed that the low-expression of RhoT1 was correlated with an increase in the risk of death (P = 0.042). Taken together, the present study suggests that RhoT1 may be considered as a potential prognostic biomarker for overall survival, and as a potential therapeutic target for intervention in patients with pancreatic cancer.
In conclusion, our findings highlight that low cytoplasmic expression levels of RhoT1 and Smad4 were significantly associated with increasing risk of LNM and poorer survival. To the best of our knowledge, this is the first report demonstrating that the expression of RhoT1 may potentially be used to predict the outcome of patients with pancreatic cancer. More studies with a larger number of cases are necessary to validate our findings.
Materials and Methods
Patient Population
The study was approved by the ethical committee of Biobank Center related hospitals. Samples with informed consent were collected between 1995 and 2009 from 162 patients who underwent pancreatic surgery, and were stored at Biobank Center of National Engineering Center for Biochip at Shanghai. 162 patients with both clinical data and adequate tissue for inclusion in this study were identified. Clinical information, included age, gender, presentation and pathologic findings included tumor size, stage, differentiation, perineural invasion and lymph node status, were obtained from original pathology reports. Pathologic staging was updated according to current American Joint Committee on Cancer guidelines. Since we collected and analyzed the data retrospectively, follow-up data were not available in all cases. With a cut-off date of December 2011, 92 patients in our study were lost to follow-up, 70 patients with pancreatic cancer were included in our final survival analysis. Overall survival was measured from time of definitive operation to death from pancreatic cancer. Until now, 46 of the 70 patients died.
Tissue Microarray Construction
Original formalin-fixed, paraffin-embedded specimens were used to construct a PDAC tissue microarray (FFPE TMA). Hematoxylin and Eosin (H&E)-stained standard slides from each tumor specimen were reviewed by a single pathologist (MR), who was blinded to specimen protein expression status. Representative tumor regions and its paracancerous nonmalignant pancreatic specimens (NMPs) were selected from each tissue block and 2 tissue cores (0.6 mm in diameter) were taken from each region using an automated tissue arrayer (Beecher Instruments, Sun Prarie, WI). Cores were transferred to individual recipient blocks. In all cases, cores were taken normal adjacent pancreas were also used as internal controls. Five-micron sections were cut from each recipient block. Sections were stained with H&E to confirm the presence of tumor within each core.
Immunohistochemistry and Scoring
This is the first manuscript based on our pancreatic TMA. TMA slides were deparaffinized, rehydrated through graded alcohol, washed with Tris-buffered saline, and processed using a streptavidin– biotin–peroxidase complex method. Antigen retrieval was performed by microwaveheating sections in 10 mm sodium citrate buffer (pH 6) for 10 minutes. After quenching of endogenous peroxidase activity and blocking of nonspecific binding, 3 antibodies (RhoT1, Santa company, polyclone antibody, expression in cytoplasm; Smad4, Abcam company, monoclone antibody, expression in cytoplasm and nucleus; p16, BD company, monoclone antibody, expression in cytoplasm) were added at a special dilution, 1∶15, 1∶15,1∶300 respectively, after which slides were incubated at 4°C overnight. The corresponding secondary biotinylated rabbit antibody was used at a special dilution for 30 minutes at 37°C. After further washing with Tris-buffered saline, sections were incubated with StrepABComplex/horseradish peroxidase (1∶100, DAKO) for 30 minutes at 37°C. Chromogenic immunolocalization was performed by exposure to 0.05% 3,3-diaminobenzidine tetrahydrochloride. Other cores containing PDAC served as positive controls for those genes expression. Normal serum was used in the place of primary antibody as a negative control. Slides were counterstained with hematoxylin before dehydration and mounting.
Immunohistochemical stains were scored semi-quantitatively according to the percentage and intensity of positive-staining epithelium cells (cytoplasmic and nuclear staining of epithelium cells were scored independently): 1, 0 points for no staining; 2, 1 point for <20%; 3, 2 points for 20–75%; 4, 3 points for >75%, as described previously [39]. Next, the average intensity of immunoreactivity was graded on a scale of 0 to 3 (0, none; 1, weak, 2, intermediate; and 3 strong). The total score was the product of the scores for the intensity and positive rate of staining (Staining index = intensity × positive rate; absent, 0; mild, 1–3; moderate, 4–6; and strong, 7–9). For data analysis, Staining index scored as either absent or mild were considered low-expression, either moderate or strong were considered high-expression. Slides were reviewed by 2 independent observers blinded to clinical and pathologic data. In cases of disagreement, a consensus was reached by joint review.
Statistical Analysis
The association between individual clinicopathological variables and LNM, between those proteins expression and LNM were statistically analyzed using the χ2 -test. Independent risk factors for LNM were determined using logistic regression analysis to identify those variables independently associated with metastasis. The optimal sensitivity and specificity of expression levels of three proteins were evaluated by receiver operating characteristic (ROC) curve analysis. The Kaplan-Meier method was used to estimate the survival function, and the log-rank test was used to examine statistical significance. Cox proportional hazards model was conducted to estimate hazard ratios for OS and to test for independent prognostic factors. All statistics were two-tailed with P value <0.05 considered statistically significant. Analyses were performed using the SPSS software package (version 17.0).
Funding Statement
HJ is supported by Key Discipline Construction Project of Pudong Health Bureau of Shanghai, China (Grant No: PWZxkq2010–05). HG is supported by China National 863 Project Foundation for Cancer Genomics (Pancreas Genomics) (Grant No: 1006AA02A302), respectively. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Li DH, Abbruzzese JL (2010) New Strategies in Pancreatic Cancer: Emerging Epidemiologic and Therapeutic Concepts. Clinical Cancer Research 16: 4313–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jemal A, Siegel R, Xu JQ, Ward E (2010) Cancer Statistics, 2010. Ca-a Cancer Journal for Clinicians 60: 277–300. [DOI] [PubMed] [Google Scholar]
- 3. Hidalgo M (2010) Pancreatic Cancer. New England Journal of Medicine 362: 1605–1617. [DOI] [PubMed] [Google Scholar]
- 4. Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, et al. (2010) Adjuvant Chemotherapy With Fluorouracil Plus Folinic Acid vs Gemcitabine Following Pancreatic Cancer Resection A Randomized Controlled Trial. Jama-Journal of the American Medical Association 304: 1073–1081. [DOI] [PubMed] [Google Scholar]
- 5. Vega FM, Ridley AJ (2008) Rho GTPases in cancer cell biology. Febs Letters 582: 2093–2101. [DOI] [PubMed] [Google Scholar]
- 6. del Pulgar TG, Benitah SA, Valeron PF, Espina C, Lacal JC (2005) Rho GTPase expression in tumourigenesis: evidence for a significant link. Bioessays 27: 602–613. [DOI] [PubMed] [Google Scholar]
- 7. Berthold J, Schenkova K, Rivero F (2008) Rho GTPases of the RhoBTB subfamily and tumorigenesis. Acta Pharmacologica Sinica 29: 285–295. [DOI] [PubMed] [Google Scholar]
- 8. Rathinam R, Berrier A, Alahari SK (2011) Role of Rho GTPases and their regulators in cancer progression. Frontiers in Bioscience-Landmark 16: 2561–2571. [DOI] [PubMed] [Google Scholar]
- 9. Whale A, Hashim FN, Fram S, Jones GE, Wells CM (2011) Signalling to cancer cell invasion through PAK family kinases. Frontiers in Bioscience-Landmark 16: 849–864. [DOI] [PubMed] [Google Scholar]
- 10. Stengel K, Zheng Y (2011) Cdc42 in oncogenic transformation, invasion, and tumorigenesis. Cellular Signalling 23: 1415–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sahai E, Marshall CJ (2002) RHO-GTPases and cancer. Nature Reviews Cancer 2: 133–+. [DOI] [PubMed] [Google Scholar]
- 12. Schmitz AAP, Govek EE, Bottner B, Van Aelst L (2000) Rho GTPases: Signaling, migration, and invasion. Experimental Cell Research 261: 1–12. [DOI] [PubMed] [Google Scholar]
- 13. Wennerberg K, Der CJ (2004) Rho-family GTPases: it’s not only Rac and Rho (and I like it). Journal of Cell Science 117: 1301–1312. [DOI] [PubMed] [Google Scholar]
- 14. Aspenstrom P, Ruusala A, Pacholsky D (2007) Taking Rho GTPases to the next level: The cellular functions of atypical Rho GTPases. Experimental Cell Research 313: 3673–3679. [DOI] [PubMed] [Google Scholar]
- 15. Fransson A, Ruusala A, Aspenstom P (2006) The atypical Rho GTPases Miro-1 and Miro-2 have essential roles in mitochondrial trafficking. Biochemical and Biophysical Research Communications 344: 500–510. [DOI] [PubMed] [Google Scholar]
- 16. Hua J, Xiao-ying S, Yi-dong H, Wen X, Lan Z, et al. (2009) Analysis of genomic expression profiles of pancreatic cancer. CHINESE JOURNAL OF PANCREATOLOGY 9: 187–189. [Google Scholar]
- 17. Wan M, Huang J, Jhala NC, Tytler EM, Yang L, et al. (2005) SCF(beta-TrCP1) controls Smad4 protein stability in pancreatic cancer cells. American Journal of Pathology 166: 1379–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Blackford A, Serrano OK, Wolfgang CL, Parmigiani G, Jones S, et al. (2009) SMAD4 Gene Mutations Are Associated with Poor Prognosis in Pancreatic Cancer. Clinical Cancer Research 15: 4674–4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao S, Ammanamanchi S, Brattain M, Cao L, Thangasamy A, et al. (2008) Smad4-dependent TGF-beta signaling suppresses RON receptor tyrosine kinase-dependent motility and invasion of pancreatic cancer cells. Journal of Biological Chemistry 283: 11293–11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gerdes B, Ramaswamy A, Ziegler A, Lang SA, Kersting M, et al. (2002) p16(INK4a) is a prognostic marker in resected ductal pancreatic cancer - An analysis of p16(INK4a), p53, MDM2, an Rb. Annals of Surgery 235: 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ohtsubo K, Watanabe H, Yamaguchi Y, Hu YX, Motoo Y, et al. (2003) Abnormalities of tumor suppressor gene p16 in pancreatic carcinoma: immunohistochemical and genetic findings compared with clinicopathological parameters. Journal of Gastroenterology 38: 663–671. [DOI] [PubMed] [Google Scholar]
- 22. Schulz P, Scholz A, Rexin A, Hauff P, Schirner M, et al. (2008) Inducible re-expression of p16 in an orthotopic mouse model of pancreatic cancer inhibits lymphangiogenesis and lymphatic metastasis. Br J Cancer 99: 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fransson A, Ruusala A, Aspenstrom P (2003) Atypical Rho GTPases have roles in mitochondrial homeostasis and apoptosis. Journal of Biological Chemistry 278: 6495–6502. [DOI] [PubMed] [Google Scholar]
- 24. Arpaia E, Blaser H, Quintela-Fandino M, Duncan G, Leong HS, et al. (2012) The interaction between caveolin-1 and Rho-GTPases promotes metastasis by controlling the expression of alpha5-integrin and the activation of Src, Ras and Erk. Oncogene 31: 884–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou J, Zhu Y, Zhang G, Liu N, Sun L, et al. (2011) A distinct role of RhoB in gastric cancer suppression. Int J Cancer 128: 1057–1068. [DOI] [PubMed] [Google Scholar]
- 26. Huang M, Prendergast GC (2006) RhoB in cancer suppression. Histol Histopathol 21: 213–218. [DOI] [PubMed] [Google Scholar]
- 27. Zhou JT, Zhu YJ, Zhang GY, Liu N, Sun LJ, et al. (2011) A distinct role of RhoB in gastric cancer suppression. International Journal of Cancer 128: 1057–1068. [DOI] [PubMed] [Google Scholar]
- 28. Kamai T, Tsujii T, Arai K, Takagi K, Asami H, et al. (2003) Significant association of Rho/ROCK pathway with invasion and metastasis of bladder cancer. Clinical Cancer Research 9: 2632–2641. [PubMed] [Google Scholar]
- 29. Liu F (2001) SMAD4/DPC4 and pancreatic cancer survival - Commentary re: M. Tascilar, et al., The SMAD4 protein and prognosis of pancreatic ductal adenocarcinoma. Clin. Cancer Res., 7: 4115–4121, 2001. Clinical Cancer Research 7: 3853–3856. [PubMed] [Google Scholar]
- 30. Biankin AV, Morey AL, Lee CS, Kench JG, Biankin SA, et al. (2002) DPC4/Smad4 expression and outcome in pancreatic ductal adenocarcinoma. Journal of Clinical Oncology 20: 4531–4542. [DOI] [PubMed] [Google Scholar]
- 31. Maitra A, Molberg K, Albores-Saavedra J, Lindberg G (2000) Loss of Dpc4 expression in colonic adenocarcinomas correlates with the presence of metastatic disease. American Journal of Pathology 157: 1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tanaka T, Watanabe T, Kazama Y, Tanaka J, Kanazawa T, et al. (2006) Chromosome 18q deletion and Smad4 protein inactivation correlate with liver metastasis: a study matched for T- and N-classification. British Journal of Cancer 95: 1562–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tanaka T, Watanabe T, Kazama Y, Tanaka J, Kanazawa T, et al. (2008) Loss of Smad4 protein expression and 18qLOH as molecular markers indicating lymph node metastasis in colorectal cancer - A study matched for tumor depth and pathology. Journal of Surgical Oncology 97: 69–73. [DOI] [PubMed] [Google Scholar]
- 34. Fu ZJ, Ma ZY, Wang QR, Lei DP, Wang R, et al. (2008) Overexpression of CyclinD1 and underexpression of p16 correlate with lymph node metastases in laryngeal squamous cell carcinoma in Chinese patients. Clinical & Experimental Metastasis 25: 887–892. [DOI] [PubMed] [Google Scholar]
- 35. Mihic-Probst D, Mnich CD, Oberholzer PA, Seifert B, Sasse B, et al. (2006) p16 expression in primary malignant melanoma is associated with prognosis and lymph node status. International Journal of Cancer 118: 2262–2268. [DOI] [PubMed] [Google Scholar]
- 36. He SM, Zhao ZW, Wang Y, Zhao JP, Wang L, et al. (2011) Reduced expression of SMAD4 in gliomas correlates with progression and survival of patients. J Exp Clin Cancer Res 30: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Blackford A, Serrano OK, Wolfgang CL, Parmigiani G, Jones S, et al. (2009) SMAD4 gene mutations are associated with poor prognosis in pancreatic cancer. Clinical Cancer Research 15: 4674–4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kamai T, Shirataki H, Nakanishi K, Furuya N, Kambara T, et al. (2010) Increased Rac1 activity and Pak1 overexpression are associated with lymphovascular invasion and lymph node metastasis of upper urinary tract cancer. Bmc Cancer 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ohuchida K, Mizumoto K, Ishikawa N, Fujii K, Konomi H, et al. (2005) The role of S100A6 in pancreatic cancer development and its clinical implication as a diagnostic marker and therapeutic target. Clinical Cancer Research 11: 7785–7793. [DOI] [PubMed] [Google Scholar]