Abstract
Visceral leishmaniasis (VL) caused by the intracellular parasite Leishmania donovani accounts for an estimated 12 million cases of human infection. It is almost always associated with anemia, which severely complicates the disease course. However, the pathological processes leading to anemia in VL have thus far not been adequately characterized to date. In studying the glycosylation patterns of peripheral blood cells we found that the red blood cells (RBC) of VL patients (RBCVL) express eight 9-O-acetylated sialoglycoproteins (9-O-AcSGPs) that are not detected in the RBC of healthy individuals (RBCN). At the same time, the patients had high titers of anti-9-O-AcSGP IgG antibodies in their sera. These two conditions appear to be linked and related to the anemic state of the patients, as exposure of RBCVL but not RBCN to anti-9-O-AcSGPs antibodies purified from patient sera triggered a series of responses. These included calcium influx via the P/Q-type but not L-type channels, activation of calpain I, proteolysis of spectrin, enhanced oxidative stress, lipid peroxidation, externalization of phosphatidyl serine with enhanced erythrophagocytosis, enhanced membrane fragility and, finally, hemolysis. Taken together, this study suggests that the enhanced hemolysis is linked to an impairment of membrane integrity in RBCVL which is mediated by ligand-specific interaction of surface 9-O-AcSGPs. This affords a potential explanation for the structural and functional features of RBCVL which are involved in the hemolysis related to the anemia which develops in VL patients.
Introduction
Leishmania donovani, the causative organism of visceral leishmaniasis (VL), is an obligatory intracellular parasite that resides and proliferates within the hostile environment of host macrophages [1]. Approximately 12 million humans suffer from VL with an incidence of 0.5 million cases per year and increasing prevalence on the Indian subcontinent [1]. The clinical spectrum of VL ranges from asymptomatic infection to mortality, if untreated.
VL is usually associated with severe anemia which severely complicates the clinical courses and adds to the patients' suffering [2]. In general, the average life span of the erythrocytes of patients with VL (RBCVL) is significantly reduced [3]. Accordingly the hemoglobin content in blood of these patients is lower than in normal healthy individuals. Despite its profound impact on the patients' fate and chances for recovery, little is known about the pathological processes contributing to the hemolysis and anemia.
Sialic acids (SA) are 9-carbon acidic sugars that constitute a family of monosaccharide and terminal components of glycoproteins and glycolipids that play very crucial roles in intercellular communication and defense against different pathogens under various pathological conditions [4]–[7]. Earlier we have demonstrated exclusive presence of eight VL-associated 9-O-acetylated sialoglycoproteins (9-O-AcSGPs) on RBCVL not detected on erythrocytes of healthy individuals [8]–[11]. Also the white blood cells of VL patients display different derivatives of sialic acids [12]–[21]. Additionally, high titers of anti-9-O-AcSGP IgG were found in the patients' sera [18], [22] whereas only a fraction of the polyclonal IgG2 purified from normal human serum shows specificity for 9-O-AcSA [23]–[24].
The structural integrity of mammalian erythrocyte is supported by a complex network of different cytoskeleton proteins which comprises of five to seven spectrin subunits linked to actin filaments [25]. Structural and biochemical changes of RBC that cause alterations in the cytoskeleton proteins may lead to degradation of the cell. Modifications like glycation and oxidation of spectrin have been documented in diabetes mellitus and associated to erythrocyte membrane changes [26]–[27]. We recently could demonstrate glycosylation and proteolytic cleavage of spectrin in RBC of VL patients [11].
With the present study we addressed the possible contribution of 9-O-AcSGPs associated with RBCVL and of anti-9-O-AcSGP IgGVL antibodies in VL. We report that anti-9-O-AcSGP IgG purified from serum of VL patients trigger a series of pathological events in RBCVL including (1) altered membrane properties as indicated by increased osmotic fragility, hydrophobicity, morphological changes like vesiculation, cell shrinkage, (2) influx of extracellular calcium ion (Ca2+) into the cell through P/Q-type channel and elevation of intracellular Ca2+ level, (3) activation of calpain I caused by elevated cytosolic Ca2+ accompanied by enhanced fragmentation of human erythrocytic α-spectrin to a 60 kDa 9-O-AcSGP (SGP-60) fragment, (4) enhanced production of reactive oxygen species (ROS) and lipid peroxidation, and (5) externalization of phosphatidyl serine (PS) and erythrophagocytosis of sensitized RBCVL. This study thus suggests a mechanism of hemolysis in VL patients that may relate to their anemic state.
Materials and Methods
Clinical samples
The study involved clinically confirmed VL patients (Table 1, n = 40; 20 males, 20 females; median age: 30 years) admitted to the School of Tropical Medicine, Kolkata. The diagnosis of VL was based on WHO recommended microscopic demonstration of Leishmania sp. amastigotes in splenic aspirates [28]. Blood was sent to the Indian Institute of Chemical Biology where it was processed immediately and the diagnosis validated by two in-house techniques in which the increased presence of linkage-specific 9-O-AcSGPs on erythrocytes was quantified by an erythrocyte binding assay [9] and anti-9-O-AcSGP antibodies in serum or plasma were detected by ELISA [18], [29]. The serum was also checked for the level of parasite-specific antibodies by ELISA with parasite lysates as coating antigen [22]. The hematological parameters of the patients were indicative of anemia but no other blood cell disorder. Controls included normal healthy individuals from endemic (n = 20) and non-endemic areas (n = 20) of the median age 28 for age matched study. The Institutional Human Ethical Committee had approved the study and samples were taken with the consent of donors, patients.
Table 1. Clinical and laboratory features of patients with visceral leishmaniasis (VL).
| Parameters | PatientVL | Normal |
| Number | Male (n = 20), Female (n = 20) | endemic area (n = 20), non-endemic area (n = 20) |
| Age (yr) | 30±2.25 | 28±4.32 |
| Height (m) | 1.55±0.72 | 1.65±0.52 |
| Weight (kg) | 38.50±4.25 | 60±5.25 |
| Body Mass Index (BMI)a | 16±0.55 | 22±1.25 |
| Duration of illness (month) | 4.02±0.25 | Not applicable |
| RBC count | 0.92–2.5×106/µl | 4.5–6.2×106/µl |
| Leukocyte count (/mm3) | 3.48–3.55×103 | 5–11×103 |
| Hemoglobin concn. (g/dl) | 5.32±0.32 | 11.5±2.2 |
| Mean cell hemoglobin concn. (g/dl) | 30–31 g/dl | 30–36 g/dl |
| Hematocrit (%) | 35 | 41–53 |
| Reticulocyte count (%) | 4.25–5.05 | 0.5–2.5 |
| Spleen size (cm) | 10.72±1.06 | Not palpable |
| Splenic aspirate scoreb | 4.05±0.17 | Negative |
| Bilirubin (mg/dl) | 1.5–2.5 | 0.9–1 |
| Albumin (g/dl) | 3.0–3.5 | 3.5–5.5 |
| Aminotransferase | Normal | Normal |
| Alkalinephosphatase | Normal | Normal |
| RBC-ELISAc | 0.9–1.12 | 0.2–0.3 |
| BSM-ELISAd | 0.77–1.1 | 0.16–0.23 |
| Parasite-ELISAe | 1.1–1.7 | 0.22–0.32 |
BMI is weight in kilograms divided by height square in meter, normal BMI = 18.5–24.9.
Splenic aspirate score is taken to be 4 when the number of parasites per microscopic field ranges from 1–10.
RBC-ELISA refers to the antigen-ELISA as described elsewhere [9]. The presence of 9-O-AcSGPs on RBCVL was determined by exploiting the binding specificity of a lectin, Achatinin-H, which has a restricted specificity towards 9-O-acetylated sialic acids [62] and therefore used as coating antigen. Briefly, Achatinin-H was immobilized in a 96-well plate and allowed to bind with RBC in 4°C for overnight. After washing, bound RBC was lysed by double distilled water. The extent of binding was determined by using a chromogenic substrate 2,7-diamino fluorine dihydrochloride and measuring absorbance values at 620 nm.
Anti-9-O-AcSGP antibody was detected by using BSM as coating antigen as described elsewhere [22].
Parasite specific antibody was detected by using parasite lysate as coating antigen as described elsewhere [8].
Anti-9-O-AcSGP IgG and sensitization of erythrocytes
Antibodies (IgG) specific for 9-O-AcSGPs were affinity purified from pooled sera from three patients (anti-O-AcSGP IgGVL) and normal healthy (NHS) individuals (anti-O-AcSGP IgGNHS), respectively, as described by Pal et al. [24]. Briefly, the succession of purification steps was 33% ammonium sulfate fractionation, removal of galactose-binding antibodies by passage through asialo-BSM Sepharose 4B, purification of anti-O-AcSGP by BSM (bovine submandibular mucin) Sepharose 4B affinity chromatograpgy, and isolation of IgG by Protein G affinity chromatography. The yield of anti-9-O-AcSGP IgG purified from 12 ml VL sera was 588±25 µg (49 µg/ml serum) and from 20 ml normal control sera 240±19 µg (12 µg/ml serum). For sensitization with anti-O-AcSGP IgG, RBC (1×107) were suspended in Ringer solution (125 mM NaCl, 5 mM KCl, 1 mM MgSO4, 32 mM HEPES, 5 mM glucose, 5 mM CaCl2, pH 7.4) and incubated with the antibodies at 37°C for 30 min. As positive controls, RBC were incubated with calcium ionophore (A23187, 1.0 µM, Sigma) in Ringer solution.
Osmofragility of erythrocytes
RBC (1×107) were incubated in NaCl concentrations from 0.1 to 0.9 g % as indicated for 1 h at 37°C and extent of hemolysis measured spectrophotometrically at 412 nm. Likewise, erythrocytes in Ringer solution were incubated with anti-O-AcSGP IgG antibodies (6 µg/ml) or A23187 (1 µM) or buffer only for 30 min at 37°C. After centrifugation at for 3 min 4000 rpm, the extent of hemolysis in supernatant was determined spectrophotometrically at 412 nm. Hemolysis in distilled water was taken as 100% lysis. Percent hemolysis (%) was calculated as OD412 nm at the given condition/OD412 nm with 100% lysis×100 [20].
Hydrophobicity measurements
Hydrophobicity was detected before and after sensitization of RBC (1×107) with anti-9-O-AcSGP IgGVL antibodies (6 µg/ml) in phosphate buffered saline (PBS) at 37°C for 30 min. Sensitized erythrocytes were washed with PBS, suspended in PBS and loaded with ANS in PBS (5 µl, 1 mM) for 1 h at 37°C. The binding of ANS to hydrophobic sites on erythrocyte membrane was measured with a spectrofluorimeter (Perkin-Elmer, LS55, Exmax = 365 nm) as described elsewhere [20], [30]. Fluorescence emission spectra were recorded from 400 to 800 nm with excitation and emission band passes of 5 nm.
Scanning electron microscopy (SEM)
The morphology of RBC before and after sensitization with anti-9-O-AcSGP IgGVL antibodies (6 µg/ml) at 37°C for 30 min was done by SEM. Cells were fixed overnight with 2.5% glutaraldehyde in PBS followed by an overnight incubation with osmium tetroxide (1%), dehydration in an ethanol series, carbon dioxide by the critical point method, sputter coating with gold and examined with a SEM (Vegaii Lsu, Tescan, Czech Republic) [31]. Micrographs were taken at magnification of 18,000 and about 200 erythrocytes were counted to calculate the percentage of deformed cells.
Measurement of reactive oxygen species
RBC were incubated with 2′,7′-dichlorofluorescein diacetate (H2DCF-DA,100 µM) in PBS for 30 min at 37°C and sensitized with anti-9-O-AcSGP IgG. ROS generation was detected and quantified by measuring the fluorescence intensity at λex, = 485 nm and λem = 538 nm. As controls, ROS generation was determined after treatment of the RBC with N-acetyl cysteine (10 mM), a scavenger of ROS. The results are expressed as fold increase in comparison to untreated erythrocytes [32].
Lipid peroxidation
Lipid peroxidation of erythrocyte membrane was measured by the thiobarbituric acid (TBA) method in which malondialdehyde (MDA), a product of peroxidation reaction of polyunsaturated fatty acids and thioberbituric acid-reactive species (TBA-RS) is used as indicator [33]. In brief, RBC membrane proteins were precipitated in twice volume of trichloro acetic acid (10%) for 30 min at 37°C and centrifuged at 1000× g for 10 min at 4°C. The cleared supernatant was incubated with thiobarbituric acid (0.67% in 7.1% sodium sulphate) at 100°C for 25 min in a water bath. After centrifugation at 1000× g for 10 min the absorbance of the pink color reaction product of MDA with TBA was measured at 535 nm. Calibration standards were generated with 1,1,3,3-tetramethoxypropane. Results are expressed as fold increase in comparison with unsensitized RBC.
Phosphatidyl serine (PS) externalization
RBC (1×106) in annexin V-binding buffer (10 mM HEPES, pH 7.4; 140 mM NaCl, 2.5 mM CaCl2) were incubated with FITC-labeled annexin V in the dark for 15 min at room temperature and analyzed by flow cytometry. A23187 (1.0 µM) treated and unsensitized RBC served as positive and negative controls, respectively.
Erythrophagocytosis assay
RBC (2×106) with or without sensitization with 6.0 µg/ml anti-O-AcSGP IgG for 30 min at 37°C, were layered over macrophages adhered on cover slips and incubated at 37°C for 1 h. Non-adherent erythrocytes were removed by gentle washing with PBS and cell surface-bound erythrocytes lysed by treatment with Tris-NH4Cl (140 mM NH4Cl, 17 mM Tris-HCl, pH 7.6) for 5 min. The slides were stained with diaminobenzidine for erythrocytes and counterstained with Giemsa stain for macrophages. Phagocytosis was calculated as the percentage of macrophages that had ingested one or more erythrocytes [34].
Measurement of cytoplasmic Ca2+
Erythrocytes (2×107) were washed in Ringer solution, loaded with Fluo-3/AM (2 µM, Calbiochem, Germany) in Ringer solution for 10 min at 37°C in dark under gentle shaking and washed twice with the same buffer [35]. Fluo 3-loaded erythrocytes were sensitization with buffer only or varying concentrations of anti-9-O-AcSGP IgG (0–40 µg/ml) in Ringer solution for 30 min at 37°C, washed twice and resuspended in same buffer and analyzed by spectrofluorimetry (λex 506 nm, λem 530 nm) and flow cytometry. Time dependent increase of intracellular calcium ion was also carried out by incubating RBC with anti-9-O-AcSGP IgG (2.5 µg/ml) for different time point at 37°C followed by flow cytometry. Calibration was done at the end of each experiment. To determine the Ca2+ channel type, Fluo 3-loaded RBCVL (2×107) in Ringer solution containing Ca2+ (5 mM) were preincubated without or with the indicated doses of ω-agatoxin TK, a spider venom peptide (P/Q-type Ca2+ channel blocker, Calbiochem) or nifedipine (L-type Ca2+ channel blocker, Sigma) for 20 min at 37°C, washed, sensitized with anti-9-O-AcSGP IgG (2.5 µg/ml) as above and analyzed by flow cytometry [35]. The Ca2+ concentrations were calculated as [Ca2+] = Kd [(F−Fmin)/(Fmax−F)] where Kd is the dissociation constant of the Ca2+ Fluo-3 complex (864 nM at 37°C), F fluorescence of anti-9-O-AcSGP IgG-treated RBC, Fmax the maximal fluorescence intensity with A23187 treated, Fmin corresponds to minimum fluorescence intensity with A23187 and EGTA [36].
Electrophoresis and Western Blotting
RBC (2×107) were incubated or not with anti-9-O-AcSGP IgGVL (10 µg/ml) for 60 min at 37°C in Ringer solution, washed and lysed by sonication on ice. Cell lysates were centrifuged to remove debris, the proteins separated in SDS-PAGE (10% or 7.5%) and blotted onto nitrocellulose. The Western blots were developed separately with horse reddish-labeled anti-calpain I antibodies (1∶1000, Cell Signaling, USA) and rabbit anti-spectrin antibodies (1∶1000, Sigma, USA). Positive and negative controls were RBC treated with A23187 (1 µM) in Ringer solution for 30 min at 37°C in the absence or presence of EGTA (25 mM), respectively. For confirmation of specificity, RBC were preincubated (15 min, 37°C) or coincubated during sesitization with the calpain inhibitor I N-acetyl-leucyl-leucyl-norleucinal (ALLN; 200 µM, Sigma) in Ringer solution before sensitization or A23187 treatment. The blots were developed with diaminobenzidine and peroxide, scanned densitometrically and analyzed with the Quantity One software (BIO-RAD, USA).
Calpain I assays
Spectrin was purified separately from RBC as described by Ungewickell et al. [37] and confirmed by SDS-PAGE and Western blot analysis as above. Spectrin (3.0 µg) was digested with the indicated doses of active calpain I (Sigma) for 60 min at 24°C in reaction buffer (50 mM HEPES, pH 7.0, 50 mM NaCl, 1 mM NaN3, 1 mM CaCl2, 1 mM DTT), the reactions stopped with EGTA (15 mM final concentration) [38] and the reaction product analyzed by SDS-PAGE. For specificity control calpain I (10 µg/ml) was pre-incubated with ALLN (150 µM) for 15 min on ice prior to addition of reaction buffer containing purified spectrin. Gels were stained by Coomassie brilliant blue and band densities were compared by densitometric analysis.
Statistical analysis
Results are reported as mean ± SD. All statistical analyses were done using Excel software (Microsoft Co.). The one or two-tailed t test for significance was performed, P<0.05 was considered significant.
Results
Alterations in the membrane characteristics of RBC in VL
To test the stability of RBCVL in comparison to RBCN, the cells were incubated in NaCl solutions with at concentrations ranging from 0 to isoosmotic 0.9 g%, and the degree of hemolysis was determined spectrometrically. The osmofragility thus determined is an indicator of cell stability and alterations in membrane properties. RBCVL were osmotically more fragile than RBCN, as indicated by an approximately 3-fold enhancement of lysis at 0.5 g% NaCl (Fig. 1A).
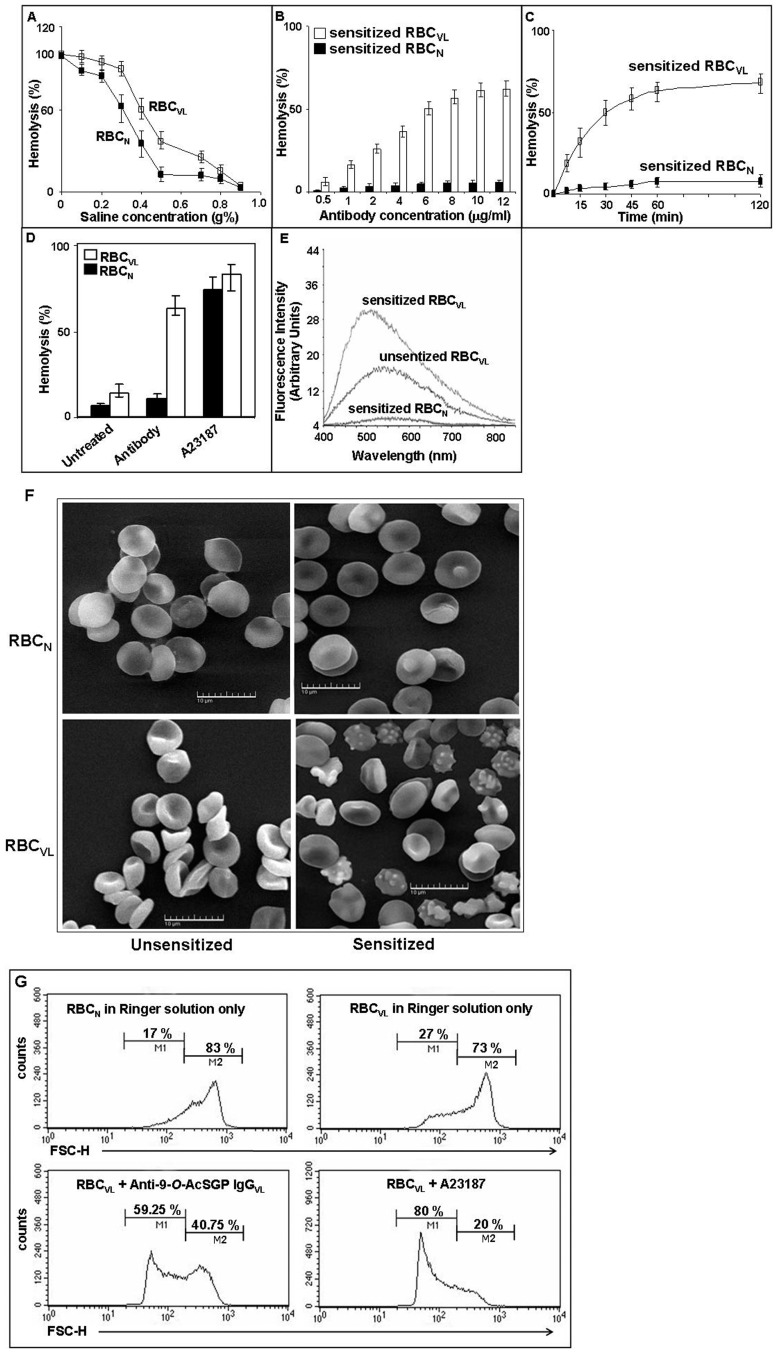
Figure 1. Altered membrane properties and reduced stability of RBCVL after sensitization with anti-9-O-AcSGP IgGVL antibodies.
A. The osmotic fragility of RBCVL (open square) compared to RBCN (filled square) was measured by degree of hemolysis with increasing osmotic stress by incubation in NaCl (0–0.9%) determined by spectrophotometry. Data are represented as % hemolysis, are means of triplicates ± SD and representative of three independent experiments. B. Sensitization of RBCVL for hemolysis with anti-9-O-AcSGP IgGVL antibodies. RBCVL (open bars) and RBCN (filled bars) were incubated for 30 min at 37°C with increasing concentration (0.5 µg/ml–12.0 µg/ml) of anti-9-O-AcSGP IgGVL and anti-9-O-AcSGP IgGNHS, respectively. The extent of hemolysis was then determined by spectrophotometry. The Spectrophotometric readings of RBCVL or RBCN in buffer under similar condition were taken as base values and subtracted from the experimental values. Data are expressed as means ± SD of three independent experiments. C. Kinetics of the hemolysis of RBCVL and RBCN after sensitization with anti-9-O-AcSGP IgGVL antibodies. RBCVL (open squares) and RBCN (filled squares) were incubated with equal (6.0 µg/ml) amounts of anti-9-O-AcSGP IgGVL and anti-9-O-AcSGP IgGNHS, respectively, for different time points (0–120 min). Base value was routinely subtracted from each experimental value as in Fig. 1B. Data are means ± SD of three independent experiments. D. Hemolysis of RBCN (filled bars) and RBCVL (open bars) after sensitization of RBCVL with anti-9-O-AcSGP IgGNHS or anti-9-O-AcSGP IgGVL (6.0 µg/ml), respectively, or incubation with the calcium ionophore A23187 (1 µM) in Ringer solution for 30 min. Data are means ± SD of three independent experiments. E. Enhanced membrane hydrophobicity of RBCVL after sensitization with anti-9-O-AcSGP IgGVL. The hydrophobicity was determined spectrofluorimetrically using ANS as indicator. RBCVL were incubated with anti-9-O-AcSGP IgGVL antibodies or PBS only for 30 min at 37°C. As controls, RBCN were incubated with anti-9-O-AcSGP IgGNHS. The cells were washed with PBS, loaded with ANS and incubated for 1 h at 37°C. The fluorescence emission spectra were recorded from 400 to 850 nm with excitation at 365 nm. The graphs are representatives of the results obtained from three independent experiments. F. Morphological changes of RBCVL after sensitization with anti-9-O-AcSGP IgGVL antibodies. RBCN and RBCVL were not sensitized or sensitized with anti-9-O-ACSGP IgGNHS or anti-9-O-AcSGP IgGVL antibodies, respectively, (6.0 µg/ml) for 30 min at 37°C, and then processed for SEM as described in Materials and Methods. Representative images from three independent experiments are shown. G. Decrease of RBCVL cell size after sensitization detected by low-angle scattering light flow cytometry (forward scatter, FCS). FCS was measured of RBCN (upper left panel) and of RBCVL without sensitization (upper right panel) or with sensitization with anti-9-O-AcSGP IgGVL (2.5 µg/ml) (lower left panel) or treatment with A23187 (1.0 µM) (lower right panel). Figure 1F is excluded from this article's CC-BY license. See the accompanying retraction notice for more information.
Both RBCVL and RBCN were sensitized for 30 min, at 37°C with increasing concentrations of anti-9-O-AcSGP IgGVL and anti-9-O-AcSGP IgGNHS, respectively. Sensitization of RBCVL with 6.0 or 10.0 µg/ml of anti-9-O-AcSGP IgGVL resulted in a high percentage of lysis, i.e. 50.25±2.0% and 61±3.0%, respectively (Fig. 1B). An increase in the lysis of sensitized RBCVL with 6.0 µg/ml of anti-9-O-AcSGP IgGVL was observed over time compared to sensitized RBCN (Fig. 1C). Sensitization of RBCVL using 6.0 µg/ml of anti-9-O-AcSGP IgGVL for 30 min at 37°C resulted in a 4.5 fold higher lysis than un-sensitized RBCVL i.e. 63±4.0% vs. 14±2.0% (Fig. 1D). Sensitized RBCVL displayed a 5.7-fold higher lysis compared to sensitized RBCN. A23187 in the presence of Ca2+ induced the maximum possible hemolysis of the erythrocytes.
RBCVL exhibited higher membrane hydrophobicity than RBCN, as indicated by increased fluorescence due to enhanced ANS binding. Sensitized RBCVL demonstrated a further increase in ANS binding and enhanced hydrophobicity compared to un-sensitized erythrocytes (Fig. 1E). A blue shift of the emission maxima from 544 to 500 nm in sensitized RBCVL suggested that sensitization increased the membrane hydrophobicity resulting in a greater number of accessible sites for the binding of ANS. Unsensitized RBCVL exhibited a higher hydrophobicity than sensitized RBCN (emission maxima at 560 nm). Unsensitized RBCN displayed only a negligible reading.
SEM revealed sensitized RBCVL to have a greater number of ultra structural morphological changes compared to un-sensitized RBCVL, suggesting a stressed condition in these erythrocytes (Fig. 1F). The presence of shrunken RBCVL, as reflected by morphometric analyses indicating membrane alterations due to a sensitization of the 9-O-AcSGPs. The sensitized RBCN did not exhibit any noteworthy alterations of the membrane, retaining a normal discoid shape.
Altered cell morphology in RBCVL
For further demonstration of changes in cell size, we used flow cytometry to monitor the forward light scattering of RBCVL before and after sensitization. The forward scattering data (FSC) showed there were only 27% un-sensitized RBCVL in M1 as compared to 73% cells in M2 (Fig. 1G). In contrast, sensitized RBCVL exhibited a higher percentage (59%) of cells in M1. RBCN exhibited only 17% cells in M1. A considerably enhanced (80%) percentage of cells were observed in the A23187-treated RBCVL.
Sensitized RBCVL exhibit enhanced ROS generation, lipid peroxidation and externalization of PS
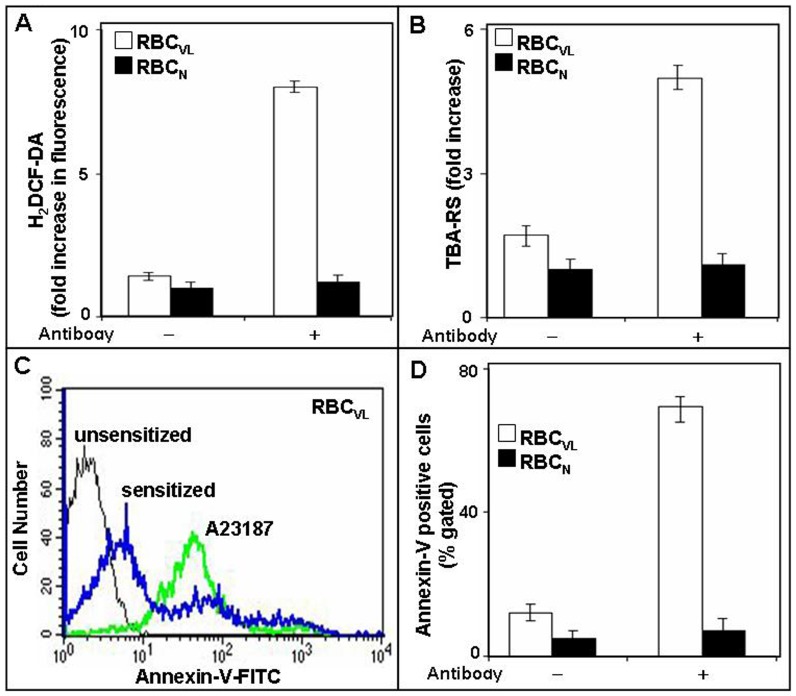
The generation of ROS is usually linked with membrane damage, specifically lipid-peroxidation, which may lead to membrane alterations such as PS externalization which are associated with cell death. As mature RBC is devoid of intracellular components such as nuclei and mitochondria, alterations may also result in these cells in a stressed condition. To address the question whether anti-9-O-AcSGP IgGVL is capable of inducing stress, we measured ROS generation, lipid peroxidation and PS-externalization in sensitized RBCVL. 5.7 and 3-fold higher levels of ROS and TBA-RS were observed in sensitized RBCVL compared to un-sensitized erythrocytes (Fig. 2A–B). RBCN displayed negligible ROS generation and insignificant lipid peroxidation. Sensitized RBCVL also showed enhanced annexin-V binding (32±5%) compared to un-sensitized (0.2±0.01%) erythrocytes, indicating a rapid externalization of PS, whereas sensitized and unsensitized RBCN demonstrated only a negligible percentage of annexin-V positive cells (Fig. 2C–D). A23187 treated RBCVL exhibited an increase in annexin-V positivity and served as a positive control.
Figure 2. Enhanced ROS, lipid peroxidation and externalization of phosphatidyl serine (PS) in RBCVL after sensitization with anti-9-O-AcSGP IgGVL.
A. Induction of ROS in sensitized RBCVL. Prior to sensitization with anti-9-O-AcSGP IgGVL or anti-9-O-AcSGP IgGNHS RBCVL and RBCN were incubated with the hydroperoxide indicator H2DCF-DA in PBS for 30 min at 37°C. Then the antibodies were added, the cell incubated as before and ROS generation determined by fluorimetry. As controls, ROS generation was determined after prior treatment of the RBC with N-acetyl cysteine, a quencher of hydroperoxides. The results are expressed as means ± S.D. (n = 5) of fold increases in comparison to the fluorescence levels detected with untreated erythrocytes. B. Increased lipid peroxidation in sensitized RBCVL. Lipid peroxidation of erythrocyte membrane was detected and quantified with thiobarbituric acid (TBA) and the TBA-reactive species (TBA-RS) measured with a spectrophotometer at 532 nm. The results are mean ± S.D of fold increase in comparison with unsensitized RBC of five independent measurments. C–D. Enhanced externalization of phosphatidylserine on sensitized RBCVL. Sensitized or unsensitized erythrocytes were incubated in annexin-V binding buffer with FITC-annexin-V and analyzed by flow cytometry. A23187-treated RBC in the presence of Ca2+ served as positive control. The results are shown as representative histogram of three independent experiments (C) and as comparative flow-cytometric analysis (D).
Sensitized RBCVL exhibit increased erythrophagocytosis
The exposure of PS on the outer leaflet of the plasma membrane is one of the signals that induce macrophages to bind and ingest apoptotic cells [39]. Sensitization of 9-O-AcSGPs on RBCVL by anti-O-AcSGP IgGVL antibodies resulted in an increase in phagocytosis of RBCVL compared to unsensitized, as evidenced by the increase in the percentage of positive macrophages that ingested one or more erythrocytes, from 3.0±1.0 to 52.0±5.0 (Table 2). The extent of phagocytosis may be dependent on the externalization of PS, as suggested by the good correlation (r = 0.92) with annexin-V positivity. In contrast, RBCN demonstrated negligible uptake by macrophages under identical conditions.
Table 2. Erythrophagocytosis Assay.
| RBCN | RBCVL | |
| a Group | bPositive macrophages (%) uptaking diseased erythrocytes | |
| RBC | 2±1 | 3±1 |
| Sensitized RBC | 3±2 | 52±5 |
RBCVL and RBCN (2×106), without or with sensitization by anti-O-AcSGP IgGVL and anti-O-AcSGP IgGNHS antibodies and processed for erythrophagocytosis assay as described in Materials and Methods. Results represent the mean ± S.D. of five separate determinations.
Positive macrophages (%) are the percentage of macrophages that ingested one or more erythrocytes and was used as the index of phagocytosis. Results represent the mean ± S.D. of five separate determinations.
Intracellular accumulation of Ca2+ in sensitized RBCVL
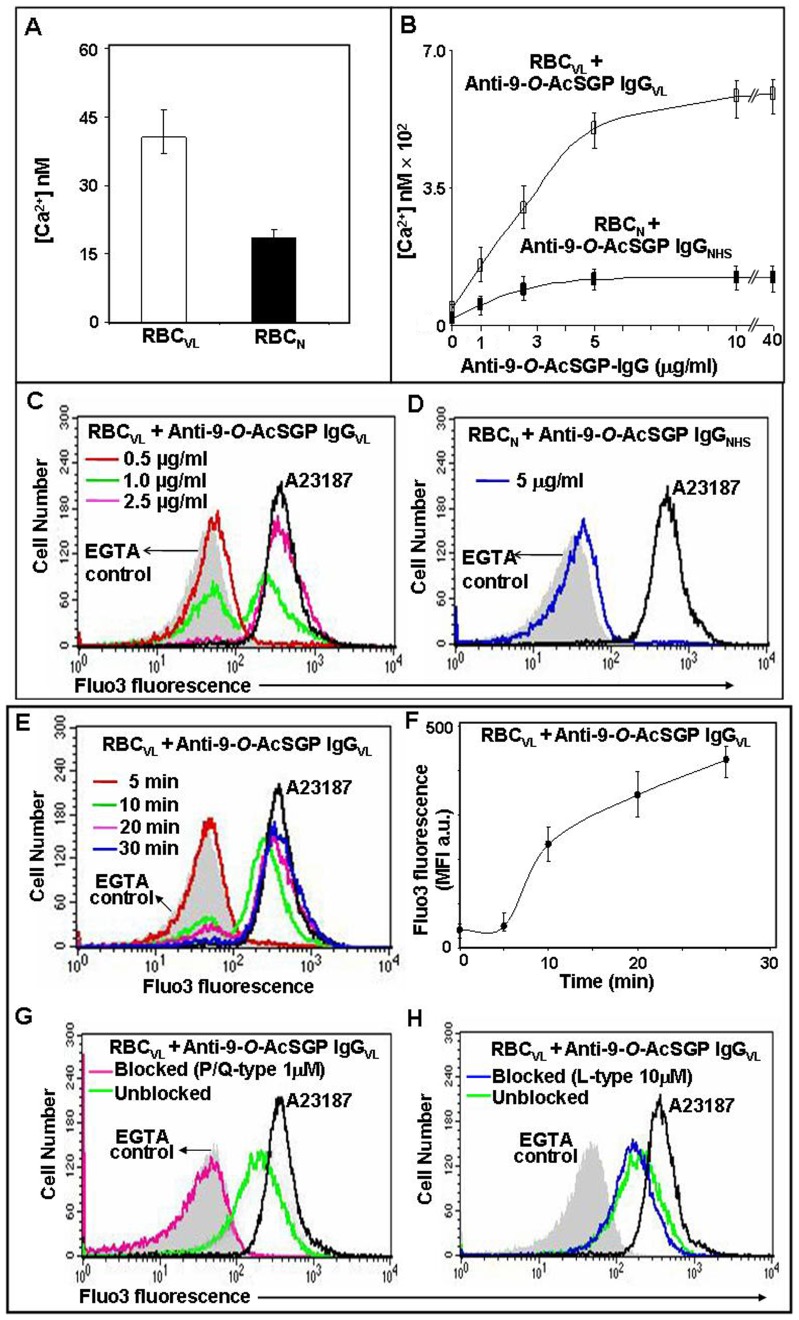
Spectrofluorimetric data suggested that the cytosolic Ca2+ ion content of un-sensitized RBCVL is slightly higher than RBCN, suggesting a stressed condition in VL (Fig. 3A). However, the sensitization of 9-O-AcSGPs on Fluo-3-loaded RBCVL showed a significant increase in fluorescence with increasing concentrations of anti-9-O-AcSGP IgGVL antibodies, indicating an enhanced Ca2+ influx as compared to the un-sensitized erythrocytes (Fig. 3B).
Figure 3. Calcium influx into RBC upon sensitization with anti-9-O-AcSGP IgGVL antibodies.
For all experiments, erythrocytes were washed and loaded with the Ca2+-indicator fluorochrome Fluo-3/AM in Ringer solution. A23187-treated Fluo-3/AM-loaded RBCVL served as positive controls (black line) and A23187-treated cells in the presence of EGTA as negative controls (gray background). A. Fluo-3/AM loaded RBCVL and RBCN (2×107) were left unsensitized or sensitized with varying concentrations of anti-9-O-AcSGP IgGVL or anti-9-O-AcSGP IgGNHS, respectively, (0–40 µg/ml) in Ringer solution for 30 min at 37°C. After washing the cells twice with same solution the fluorescence intensities of the Ca2+-Fluo-3 complexes was determined by spectrofluorimetry and the levels of intracellular calcium calculated as described in Materials and Methods. B. Cells treated as in A with 0.5 µg/ml (red line), 1.0 µg/ml (green line) and 2.5 µg/ml (pink line) anti-9-O-AcSGP IgGVL were analyzed by flow cytometry to determine the fraction of responding RBC. C. Similarly, RBCN were analyzed without and with sensitization with 5 µg/ml anti-9-O-AcSGP IgGNHS (blue line). D–E. Time dependent increase of intracellular Ca2+ in RBCVL after sensitization with anti-9-O-AcSGP IgGVL (2.5 µg/ml) for 5 min (red line), 10 min (green line), 20 min (pink line) and 30 min (blue line) at 37°C. Cells were washed and analyzed by flow cytometry. D. Histogram representation; E. Mean fluorescence intensities calculated from D. F–G. Inhibition of the Ca2+ influx into RBC sensitized with anti-9-O-AcSGP IgGVL. Prior to sensitization with anti-9-O-AcSGP-IgGVL (2.5 µg/ml), the cells were incubated without (green line) or with the P/Q-type channel blocker ω-agatoxin TK (1 µM, pink line, F), or without (green line) or with the L-type channel blocker nifedipine (10 µM, blue line, G) and analyzed by flow cytometry.
Sensitized Fluo-3-loaded RBCVL also displayed enhanced fluorescence with an increasing concentration of anti-9-O-AcSGP IgGVL antibodies as compared to un-sensitized erythrocytes, as determined by flow cytometry (Fig. 3C). It is noteworthy that not all of the cells responded equally to stimulation at the lower doses of the antibody (1.0 µg/ml) compared to 2.5 µg/ml, at which almost all of the cells showed higher fluorescence. In contrast, Fluo-3-loaded sensitized RBCN displayed only a minimal increase in fluorescence, even at higher doses, as compared to un-sensitized RBCN, suggesting the absence of 9-O-AcSGPs (Fig. 3B, 3D). However, a lack of signaling for other, undetermined reasons cannot be ruled out.
A time dependent increase in intracellular Ca2+ was observed in sensitized RBCVL (Fig. 3E–F). Almost all of the cells exhibited higher Fluo-3 fluorescence after 30 min of stimulation. As expected, Fluo-3-loaded RBCVL and RBCN incubated with A23187 exhibited maximum fluorescence and were used as positive control, which effect was decreased in the presence of EGTA, confirming the assay specificity.
P/Q-type channel mediated Influx of Ca2+ ions
In order to understand the Ca2+ influx pathway of sensitized RBCVL, we used different concentrations (0.01 µM, 0.05 µM, 0.1 µM, 0.5 µM, 1.0 µM and 2.0 µM) of ω-agatoxin TK, a P/Q-type Ca2+-ion channel blocker (Fig. 3G). At a concentration of 1 µM, ω-agatoxin TK strongly inhibited the influx of Ca2+ ions by reducing the MFI from 210 arbitrary units to the background value of 43 arbitrary units, suggesting the involvement of P/Q-type calcium channels in the Ca2+ ion influx in sensitized RBCVL. In contrast, nifedipine, an L-type channel blocker, even at a 10 µM concentration, did not inhibit the influx of Ca2+ (Fig. 3H). No inhibition could be detected at up to a 50 µM concentration of nifedipine.
Enhanced cytoplasmic Ca2+ activated calpain I in sensitized RBCVL
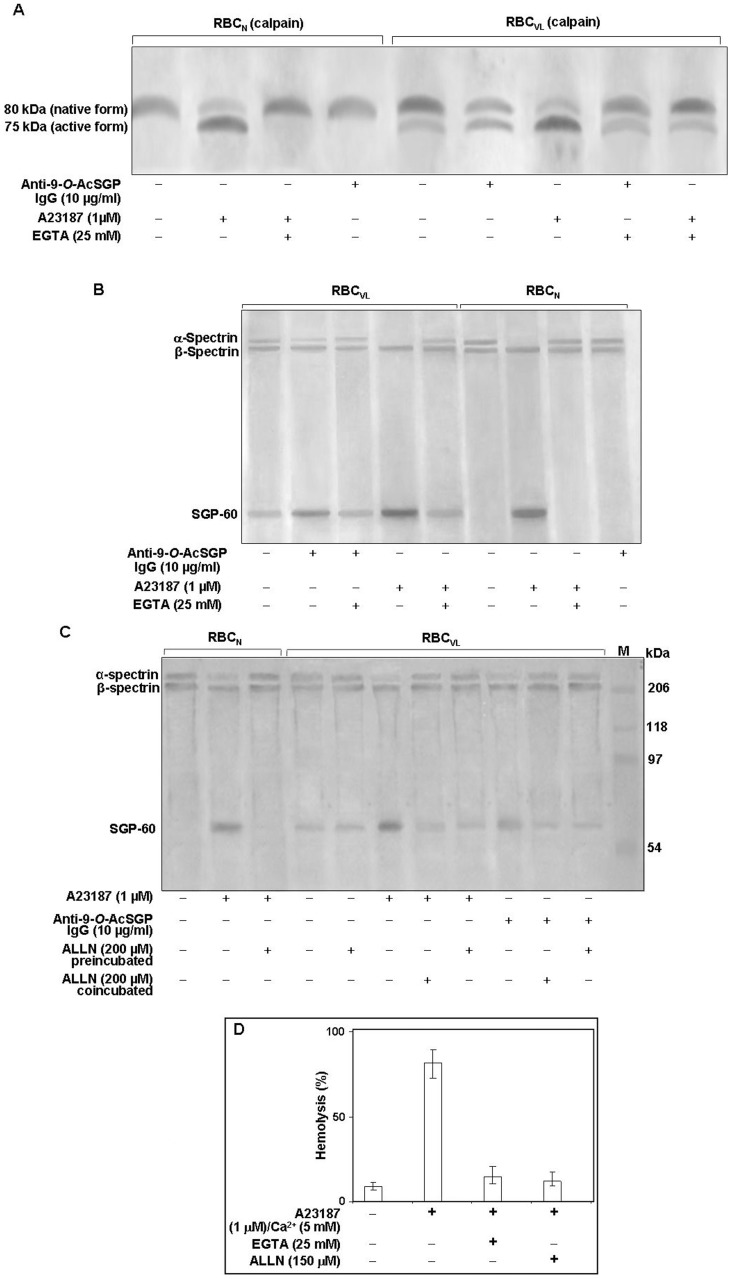
Increased intracellular Ca2+ activates the Ca2+-dependent protease calpain I [40]. Therefore, we investigated the involvement of enhanced Ca2+ in activating calpain I in sensitized/unsensitized RBCVL (Fig. 4A). Sensitized RBCVL displayed an approximate 2-fold increase of the 75 kDa active form of calpain I as a result of Ca2+-dependent autoproteolysis of the inactive membrane localized 80 kDa native form, indicating that an increased cytosolic Ca2+ level was sufficient for protease activation. It also suggested that the activation of calpain I was dependent on Ca2+ through sensitized 9-O-AcSGPs. A23187-treated RBCVL or RBCN produced an intense 75 kDa band. Unsensitized RBCVL inherently contain a less intense 75 kDa active form of calpain I which was completely absent in RBCN. Sensitization of RBCN with A23187 in the presence of EGTA resulted in only the native 80 kDa form.
Figure 4. Activation of calpain I in RBCVL or RBCN.
A. Cells (2×107) were incubated with or without anti-9-O-AcSGP IgGVL, anti-9-O-AcSGP IgGNHS (10 µg/ml) or the Ca2+ ionophore A23187 in the absence or presence of EGTA (25 mM) for 60 min at 37°C in Ringer solution, washed with same buffer and lysed by sonication on ice. After removal of cell debris by centrifugation the lysates were separated by SDS-PAGE, and calpain I was detected by Western blot analysis with an anti-calpain-I antibody. B. Enhanced degradation of α-spectrin in sensitized RBCVL. RBCVL and RBCN were suspended separately in Ringer solution and sensitized with anti-9-O-AcSGP IgGVL, anti-9-O-AcSGP IgGNHS or A23187 in the absence or presence of EGTA for 30 min at 37°C. The cells were then lysed as before and the lysates subjected to SDS-PAGE and Western blot analysis with a spectrin-specific antibody. C. Involvement of calpain I in the degradation of spectrin in RBCVL. RBCVL and RBCN were pre-incubated with the calpain I inhibitor ALLN in Ringer solution for 30 min at 37°C before sensitization or co-incubated together with anti-9-O-AcSGP IgGVL or A23187 as positive controls. The cells were processed as before and spectrin detected by Western blot after SDS-PAGE of the cellulaproteins. D. Dependence of the hemolysis of RBCN on the activation of calpain I. RBCN were incubated without or with A23187 and with A23187 plus EGTA or ALLN for 1 h at 37°C, and the degree of hemolysis determined spetrophotometrically as in Figure 1. The results are shown as representative bar graphs of three independent experiments.
Activated calpain I induced proteolysis of spectrinVL in sensitized RBCVL
Active calpain I cleaves cytoskeleton proteins such as spectrin [40]. The enhancement of the Ca2+ influxes upon sensitization suggests an underlying relationship of the 9-O-AcSGPs on RBCVL and the elevated level of calcium. A four-fold more intense band of the SGP-60 kDa protein and reduced intensity of the α-spectrin band in sensitized RBCVL suggests an enhanced fragmentation of spectrinVL compared to un-sensitized RBCVL (Fig. 4B). EGTA reduced the proteolysis of spectrinVL to SGP-60, indicating a role for calcium-dependant proteolysis in the fragmentation of spectrin. In support of this notion, A23187/Ca2+-treated RBCVL/RBCN exhibited an intense SGP-60 band and complete loss of the α-spectrin band, and effect which was reversed by the addition of EGTA (Fig. 4B). Similar treatment of RBCVL in the presence of ALLN did not exhibit any enhancement of the SGP-60 band (Fig. 4C). The SGP-60 band was absent in sensitized RBCN in the presence of ALLN (Fig. 4C).
Activated calpain I induced hemolysis in RBCN
The activation of calpain I caused a degradation of spectrin in RBC. Does this degradation lead to RBC hemolysis? We checked the percentage of hemolysis in A23187-treated RBCN (Fig. 4D). Approximately 85% of the RBC hemolysis was observed after the activation of calpain I by the A23187 treatment, suggesting a positive correlation of spectrin degradation with cells hemolysis. Calpain I-specific inhibitor (ALLN)-treated RBC or the chelation of cytosolic Ca2+ by EGTA reduced the percentage of hemolysis to 12–14%, confirming the assay specificity. RBCN exhibited only 8.4% hemolysis.
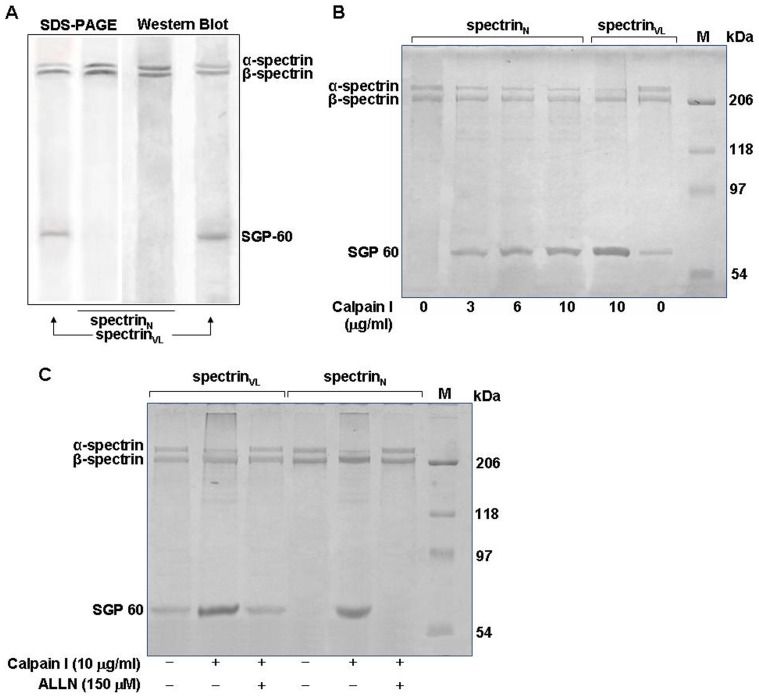
Activated calpain I cleaved purified spectrin
Purified spectrinVL showed bands of 280, 246 and 60 kDa (Fig. 5A). SGP-60 was not present in spectrinN. Purified spectrinVL digested with active calpain I displayed a similar enhancement of fragmented spectrinVL, as evidenced by the increased presence of the SGP-60 band and reduced intensity of α-spectrin compared to undigested spectrinVL, suggesting the presence of active calpain in the patient's erythrocytes may be responsible for such proteolysis (Fig. 5B). Similar treatment in the presence of ALLN exhibited a reduced intensity of the SGP-60 band, thus showing the specificity of the reaction (Fig. 5C). SpectrinN digested with active calpain I also displayed an increase in the intensity of SGP-60 and corresponding decrease in α-spectrin in a calpain I dose-dependent manner (Fig. 5B). The SGP-60 band was absent in both undigested spectrinN and in the case of treatment with active calpain I in the presence of ALLN (Fig. 5C).
Figure 5. Degradation of purified α-spectrin by activated calpain I.
A. Characterization of purified spectrins. SpectrinVL and spectrinN were purified from RBCVL and RBCN as described in Materials and Methods, and analyzed on SDS-PAGE (7.5%). The purified spectrins were Western blotted and detected with polyclonal rabbit anti-spectrin antibodies. B. Proteolysis of purified spectrinVL and spectrinN by active calpain I. SpectrinN and SpectrinVL (3.0 µg) were digested with different doses of active calpain I as indicated in reaction buffer, the reaction was stopped with EGTA and the products analyzed by SDS-PAGE. C. Inhibition of proteolysis by the calpain I inhibitor ALLN. Calpain I was preincubated with ALLN for 15 min on ice prior to addition of reaction buffer containing purified spectrinVL or spectrinN and processed as before.
Discussion
Studies reported by our group initially demonstrated the presence of 9-O-AcSGPs and anti-9-O-AcSGP antibodies in VL [22], [29]. Earlier we have reported the presence of only two 9-O-AcSGPs of molecular weight 36 kDa and 144 kDa on PBMCN respectively [41]–[43]. In contrast, several other distinct VL–associated newly induced 9-O-AcSGPs (19, 56, 65 kDa) were demonstrated on PBMCVL [21], [41], [44]. Interestingly, almost 40% of the membrane proteins present on the RBCVL were 9-O-acetylated, that were totally absent on RBCN which further signified their link with disease pathogenesis [8]–[11]. However, little progress has been made determining the extent of the contribution of 9-O-AcSGPs to the enhanced hemolysis of RBC in VL in the active disease state. Accordingly, our aim was to investigate the specific role of 9-O-AcSGPs in RBCVL hemolysis and their ligand-specific interaction with anti-9-O-AcSGP IgGVL. The major achievement of the study was to demonstrate the involvement of VL-associated 9-O-AcSGPs in triggering the altered cellular and membrane biochemical characteristics leading to the phagocytosis of these altered erythrocytes.
Sensitization of 9-O-AcSGP using anti-O-AcSGP IgGVL antibodies led to an alteration of the membrane characteristics, as evidenced by enhanced osmotic fragility and hydrophobicity, suggesting a mechanism for the membrane damage which developed in RBCVL in contrast with RBCN. Moreover, profound ultrastructural changes in morphology from the normal discoid shape and oxidative stress induced in the sensitized RBCVL indicate definite alterations in their membranes, suggesting a key role for 9-O-AcSGPs. Similar alterations in erythrocyte membrane organization have been documented in Fanconi's anemia [45] and acute childhood lymphoblastic leukemia [33].
The physiological concentration of anti-9-O-AcSGPs antibodies in normal serum against two 9-O-AcSGPs (36 kDa and 144 kDa) present on PBMCN is only 11–13 µg/ml [13], [18], [22], [46]. However, the total anti-9-O-AcSGPs (54 µg/ml) in VL-serum are developed against several 9-O-AcSGPs (112, 107, 103, 57, 51, and 48 kDa) newly induced both on PBMCVL and RBCVL. Therefore, it may be envisages that the enhanced anti-9-O-AcSGP antibody found in the VL serum is definitely different from the antibody present in normal human serum. Hence, affinity purified anti-9-O-AcSGPVL antibody used to sensitize 9-O-AcSGPs on RBCVL certainly has a distinct identity, specific and active. Accordingly, even a lower concentration (6 µg/ml) of anti-9-O-AcSGP IgGVL antibody used for sensitization is capable of inducing 5.7-fold higher degree of RBCVL hemolysis compared to sensitized RBCN. This observation signifies that even lower doses of the anti-9-O-AcSGP IgGVL antibody through the ligand-specific interaction could play an important role in hemolysis of RBCVL leading to anemia.
In contrast, no significant increase in the hemolysis (%) of RBCN was observed even at higher concentration of the anti-9-O-AcSGP IgGN antibody because of the negligible presence of 9-O-AcSGPs on normal cells. This further indicated that this change in red cell morphology due to this specific ligand-mediated interaction was VL-associated.
ROS are linked to cell death signaling in a variety of cell types. Loss of membrane PS asymmetry has been reported in human erythrocytes, sickle cell disease, thalassemia and diabetes [39], [47]–[48]. Increased oxidative stress in erythrocytes of Leishmania-infected hamsters has been reported [49]. A 6-fold higher ROS generation and a 4-fold increased lipid peroxidation in sensitized RBCVL suggested that the signaling through 9-O-AcSGPs was indeed VL-associated.
PS exposure on the outer leaflet of the cell membrane serves as a signal for the removal of apoptotic cells from the circulation [50]. Sensitized RBCVL demonstrated display an enhanced externalization of PS, an event which is reported to be correlated with membrane damage in other diseases [46], [47]–[48], [50]–[51]. A 17-fold higher erythrophagocytosis of sensitized RBCVL was demonstrated which indicated their efficient removal from the circulation, suggesting a probable cause for the anemia- associated VL patients.
Sensitization of RBCVL with anti-9-O-AcSGP IgGVL resulted in an increase in the cytosolic Ca2+ level. However, the uptake of Ca2+ was not equal in all of the cells, suggesting that the 9-O-AcSGP content of the cells is also not equal, with possibly a few cells having a higher number of 9-O-AcSGPs stimulated earlier at a lower dose of antibody and earlier time point. The increased cytosolic Ca2+ causes the activation of calpain I, which in turn meditated an enhanced proteolysis of spectrinVL. Hence, the possible mechanism of a destabilization of RBC by damaging cytoskeleton proteins which in turn leads to hemolysis has been established in VL, with a cell-specific role for 9-O-AcSGPs.
Calcium must be taken up from the extracellular compartment into the inside of the cell, as erythrocytes are devoid of any Ca2+ storage organelles such as the endoplasmic reticulum and mitochondria. We performed a channel-inhibition experiment in order to characterize the specific type of channel utilized in the course of ion influx. Inhibition of Ca2+ influx by ω-agatoxin TK confirmed the involvement of the P/Q-type channel. In contrast, even at higher doses, an L-type channel blocker (nifedipine) was unable to block the influx of Ca2+ during the sensitization process, clearly showing that this influx was not through the L-type channel.
The sensitization of RBCVL caused activation of the Ca2+ channel and along with an enhanced influx of the Ca2+ ion, which may have further caused the activation of the Na+/K+ ion channel [52]. Activation of the Na+/K+ ion channel opens up the Gardos channel followed by an efflux of water from the cells, as reflected by the shift of a significant population of sensitized cells towards a lower FSC. The RBCVL cell size was typically lower than RBCN, possibly due to the higher level of intracellular Ca2+ in the VL condition.
Spectrin, a cytoskeleton membrane protein which is crucial for the maintenance of the structural integrity of the cell, is thought to be a target of calpain-mediated proteolysis [53]. Supporting this idea, we observed increased cytosolic Ca2+ activated calpain I in sensitized RBCVL, as evidenced by the appearance of the active form mediating the proteolysis of spectrinVL. Calpain is activated by autoproteolysis and calpain I relocates from the cytoplasm to the inner surface of the plasma membrane, where it may cause damage to the cytoskeleton structure [40]. It degrades cytoskeletal proteins in neuronal cells during cerebral malaria, traumatic/post-traumatic neurodegeneration [54]–[57], aneurysmal subarachnoid hemorrhage [58] and spinal cord ischemia [59]. Calcium and phenylhydrazine-induced proteolysis of spectrin in rat and human erythrocytes has been documented [60]–[61].
The enhanced presence of SGP-60 in sensitized RBCVL which indicates the enhanced degradation of spectrin may be due to Ca2+-mediated proteolysis of cytoskeleton proteins destabilizing RBCVL. However, SGP-60 is already present in unsensitized RBCVL and its lack in RBCN suggested an association of degraded spectrin with the active disease state. The identification of SGP-60 as a fragment of erythrocytic α-I spectrin was confirmed by sequencing [11]. The fragmentation of purified spectrinVL/spectrinN by active calpain I displayed a similar pattern of spectrin-proteolysis as in RBCVL, suggesting an enhanced activation of calpain in RBCVL in vivo. Erythrocytes may have lost their membrane integrity as a consequence of spectrin degradation, as reflected in the enhanced hemolysis of the A23187-sensitized RBC. A higher degree of RBCN hemolysis may occur through the activation of calpain I, as chelating the cellular Ca2+ by EGTA suppressed the hemolysis. This was corroborated when a specific inhibitor of calpain I which blocks spectrin proteolysis (ALLN) was used, resulting in a decrease in the degree of hemolysis.
Taken together, the evidence suggests that 9-O-acetylated sialoglycoproteins have an important role in Ca2+ influx, activating calpain-I, which in turn cleaves spectrin, causing destabilization of RBCVL and ultimately their removal through phagocytosis by macrophages. Hence the study findings have yielded important insight into the pathophysiological role of 9-O-AcSGPs on RBCVL, including potential cell-biological mechanisms which result in anemia (Fig. S1).
Supporting Information
Overview of the proposed mechanism. A hypothetical model has been shown in describing the role of 9-O-AcSGPs for the hemolysis of erythrocytes in VL. The model highlights the possible events inside the RBCVL including activation of calpain I followed by spectrin degradation and phosphatidyl serine exposure after sensitization of 9-O-AcSGPs by anti-9-O-AcSGP IgGVL.
(TIF)
Acknowledgments
SS, AG and KB are Senior Research Fellows of the Council of Scientific and Industrial Research (CSIR) Govt. of India. We are thankful to Mr. A. Mallick, Dr. R. Bhadra, Mr. B. Das, IICB, Dr. R.B. Bhar and Mr. Pallab Dasgupta Instrumentation Science, Jadavpur for providing facility of SEM and their cooperation in this experiment. We are thankful to Dr. Kevin Boru (Pacific Edit, San Francisco, CA, USA) for English editing.
Funding Statement
This work received financial support from the CSIR-IICB, Department of Biotechnology (GAP235) (http://dbtindia.nic.in/index.asp), Indian Council of Medical Research (GAP266) (http://www.icmr.nic.in/), New Delhi, J.C. Bose Fellowship, Department of Science and Technology (P90807) (http://www.dst.gov.in/), Govt. of India. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Laurent T, Rijal S, Yardley V, Croft S, De Doncker S, et al. (2007) Epidemiological dynamics of antimonial resistance in Leishmania donovani: genotyping reveals a polyclonal population structure among naturally-resistant clinical isolates from Nepal. Infect Genet Evol 7: 206–212. [DOI] [PubMed] [Google Scholar]
- 2.Mahajan V, Marwaha RK (2007) Immune mediated hemolysis in visceral leishmaniasis. J Trop Pediatr 53: 284–286. [DOI] [PubMed] [Google Scholar]
- 3.Woodruff AW, Topley E, Knight R, Downie CG (1972) The anaemia of kala-azar. Br J Haematol 22: 319–29. [DOI] [PubMed] [Google Scholar]
- 4.Schauer R (2004) Sialic acids: fascinating sugars in higher animals and man. Zoology 107: 49–64. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh S, Bandyopadhyay S, Mukherjee K, Mallick A, Pal S, et al. (2007) O-acetylation of sialic acids is required for the survival of lymphoblasts in childhood acute lymphoblastic leukemia (ALL). Glycoconj J 24: 17–24. [DOI] [PubMed] [Google Scholar]
- 6.Mandal C, Chatterjee M, Sinha D (2000) Investigation of 9-O-acetylated sialoglycoconjugates in childhood acute lymphoblastic leukaemia. Br J Haematol 110: 801–812. [DOI] [PubMed] [Google Scholar]
- 7.Khatua B, Ghoshal A, Bhattacharya K, Mandal C, Saha B, et al. (2010) Sialic acids acquired by Pseudomonas aeruginosa are involved in reduced complement deposition and siglec mediated host-cell recognition. FEBS Lett 584: 555–561. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Sharma V, Chatterjee M, Mandal C, Sen S, Basu D (1998) Rapid diagnosis of Indian visceral leishmaniasis using achatininH, a 9-O-acetylated sialic acid binding lectin. Am J Trop Med Hyg 58: 551–554. [DOI] [PubMed] [Google Scholar]
- 9.Chava AK, Chatterjee M, Sundar S, Mandal C (2002) Development of an assay for quantification of linkage-specific O-acetylated sialoglycans on erythrocytes; its application in Indian visceral leishmaniasis. J Immunol Methods 270: 1–10. [DOI] [PubMed] [Google Scholar]
- 10.Chava AK, Chatterjee M, Sharma V, Sundar S, Mandal C (2004) Variable degree of alternative complement pathway-mediated hemolysis in Indian visceral leishmaniasis induced by differential expression of 9-O-acetylated sialoglycans. J Infect Dis 189: 1257–1264. [DOI] [PubMed] [Google Scholar]
- 11.Samanta S, Dutta D, Ghoshal A, Mukhopadhyay S, Saha B, et al. (2011) Glycosylation of Erythrocyte Spectrin and Its Modification in Visceral Leishmaniasis. PLoS ONE 6: e28169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghoshal A, Mukhopadhyay S, Gerwig GJ, Kamerling JP, Chatterjee M, et al. (2009) 9-O-acetylated sialic acids enhance entry of virulent Leishmania donovani promastigotes into macrophages. Parasitology 136: 159–173. [DOI] [PubMed] [Google Scholar]
- 13.Bandyopadhyay S, Chatterjee M, Das T, Bandyopadhyay S, Sundar S, et al. (2004) Antibodies directed against O-acetylated sialoglycoconjugates accelerate complement activation in Leishmania donovani promastigotes. J Infect Dis 190: 2010–2019. [DOI] [PubMed] [Google Scholar]
- 14.Ghoshal A, Mukhopadhyay S, Saha B, Mandal C (2009) 9-O-acetylated sialoglycoproteins are important immunomodulators in Indian visceral leishmaniasis. Clin Vaccine Immunol 16: 889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma V, Chatterjee M, Sen G, Chava AK, Mandal C (2000) Role of linkage specific 9-O-acetylated sialoglycoconjugates in activation of the alternate complement pathway in mammalian erythrocytes. Glycoconj J 17: 887–893. [DOI] [PubMed] [Google Scholar]
- 16.Chava AK, Chatterjee M, Mandal C (2005) Hand book of carbohydrate engineering. In: Taylor and Francis Group, book division, USA in Chapter 3. Yarema Kevin J, editor. 71–98. [Google Scholar]
- 17.Ghoshal A, Mukhopadhyay S, Mandal C (2008) Sialoglycotherapeutics in protozoal diseases. Mini Rev Med Chem 8: 358–369. [DOI] [PubMed] [Google Scholar]
- 18.Bandyopadhyay S, Chatterjee M, Pal S, Waller RF, Sundar S, et al. (2004) Purification, characterization of O-acetylated sialoglycoconjugates-specific IgM, and development of an enzyme-linked immunosorbent assay for diagnosis and follow-up of indian visceral leishmaniasis patients. Diagn Microbiol Infect Dis 50: 15–24. [DOI] [PubMed] [Google Scholar]
- 19.Chava AK, Bandyopadhyay S, Chatterjee M, Mandal C (2004) Sialoglycans in protozoal diseases: their detection, modes of acquisition and emerging biological roles. Glycoconj J 20: 199–206. [DOI] [PubMed] [Google Scholar]
- 20.Ansar W, Mukhopadhyay S, Habib SH, Basu S, Saha B, et al. (2009) Disease-associated glycosylated molecular variants of human C-reactive protein activate complement-mediated hemolysis of erythrocytes in tuberculosis and Indian visceral leishmaniasis. Glycoconj J 26: 1151–1169. [DOI] [PubMed] [Google Scholar]
- 21.Ghoshal A, Mandal C (2011) A Perspective on the Emergence of Sialic Acids as Potent Determinants Affecting Leishmania Biology. Glycoconj J 26: 675–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chatterjee M, Sharma V, Mandal C, Sundar S, Sen S (1998) Identification of antibodies directed against O-acetylated sialic acids in visceral leishmaniasis: its diagnostic and prognostic role. Glycoconj J 15: 1141–1147. [DOI] [PubMed] [Google Scholar]
- 23.Siebert HC, von der Lieth CW, Dong X, Reuter G, Schauer R, et al. (1996) Molecular dynamics-derived conformation and intramolecular interaction analysis of the N-acetyl-9-O-acetylneuraminic acid-containing ganglioside GD1a and NMR-based analysis of its binding to a human polyclonal immunoglobulin G fraction with selectivity for O-acetylated sialic acids. Glycobiology 6: 561–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pal S, Chatterjee M, Bhattacharyya DK, Bandhyopadhyay S, Mandal C (2000) Identification and purification of cytolytic antibodies directed aginst O-acetylated sialic acid in chilhood acute lymphoblastic leukemia. Glycobiology 10: 539–549. [DOI] [PubMed] [Google Scholar]
- 25.Bennett V, Baines AJ (2001) Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev 81: 1353–1392. [DOI] [PubMed] [Google Scholar]
- 26.Resmi H, Pekçetin Ç, Güner G (2001) Erythrocyte membrane and cytoskeletal protein glycation and oxidation in short-term diabetic rabbits. Clin Exp Med 1: 187–193. [DOI] [PubMed] [Google Scholar]
- 27.Starodubtseva MN, Kuznetsova TG, Yegorenkov NI, Cherenkevich SN (2008) Structural and mechanical characteristics of erythrocyte membranes in patients with type 2 diabetes mellitus. Bull Exp Biol Med 145: 99–103. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization (2010) Control of the leishmaniasis (Geneva). Tech Rep Ser 949: 186. [PubMed] [Google Scholar]
- 29.Ghoshal A, Mukhopadhyay S, Saha B, Mandal C (2009) 9-O-acetylated sialoglycoproteins are important immunomodulators in Indian visceral leishmaniasis. Clin Vaccine Immunol 16: 889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cardamone M, Puri NK (1992) Spectrofluorimetric assessment of the surface hydrophobicity of proteins. Biochem J 282: 589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukherjee K, Chowdhury S, Mondal S, Mandal C, Chandra S, et al. (2007) 9-O-acetylated GD3 triggers programmed cell death in mature erythrocytes. Biochem Biophys Res Commun 362: 651–657. [DOI] [PubMed] [Google Scholar]
- 32.Mandal D, Mazumder A, Das P, Kundu M, Basu J (2005) Fas-, caspase 8-, and caspase 3-dependent signaling regulates the activity of the aminophospholipid translocase and phosphatidylserine externalization in human erythrocytes. J Biol Chem 280: 39460–39467. [DOI] [PubMed] [Google Scholar]
- 33.Ghosh S, Bandyopadhyay S, Bhattacharya DK, Mandal C (2005) Altered erythrocyte membrane characteristics during anemia in childhood acute lymphoblastic leukemia. Ann Hematol 84: 76–84. [DOI] [PubMed] [Google Scholar]
- 34.Pradhan D, Williamson P, Schlegel RA (1994) Phosphatidylserine vesicles inhibit phagocytosis of erythrocytes with a symmetric transbilayer distribution of phospholipids. Mol Membr Biol 11: 181–187. [DOI] [PubMed] [Google Scholar]
- 35.Yang L, Andrews DA, Low PS (2000) Lysophosphatidic acid opens a Ca(++) channel in human erythrocytes. Blood 95: 2420–2425. [PubMed] [Google Scholar]
- 36.Tsien RY, Pozzan T, Rink TJ (1982) Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol 94: 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ungewickell E, Gratzer W (1978) Self-association of human spectrin. A thermodynamic and kinetic study. Eur J Biochem 88: 379–385. [DOI] [PubMed] [Google Scholar]
- 38.Hu RJ, Bennett V (1991) In vitro proteolysis of brain spectrin by calpain I inhibits association of spectrin with ankyrin-independent membrane binding site(s). J Biol Chem 266: 18200–18205. [PubMed] [Google Scholar]
- 39.Mandal D, Moitra PK, Saha S, Basu J (2002) Caspase3 regulates phosphatidylserine externalization and phagocytosis of oxidatively stressed erythrocytes. FEBS Lett 513: 184–188. [DOI] [PubMed] [Google Scholar]
- 40.Glaser T, Schwarz-Benmeir N, Barnoy S, Barak S, Eshhar Z, et al. (1994) Calpain (Ca(2+)-dependent thiol protease) in erythrocytes of young and old individuals. Proc Natl Acad Sci U S A 91: 7879–7883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bandyopadhyay S, Chatterjee M, Sundar S, Mandal C (2004) Identification of 9-O-acetylated sialoglycans on peripheral blood mononuclear cells in Indian visceral leishmaniasis. Glycoconj J 20: 531–536. [DOI] [PubMed] [Google Scholar]
- 42.Pal S, Ghosh S, Bandyopadhyay S, Mandal CN, Bandhyopadhyay S, et al. (2004) Differential expression of 9-O-acetylated sialoglycoconjugates on leukemic blasts: a potential tool for long-term monitoring of children with acute lymphoblastic leukaemia. Int J Cancer 111: 270–277. [DOI] [PubMed] [Google Scholar]
- 43.Pal S, Ghosh S, Mandal CN, Kohla G, Brossmer R, et al. (2004) Purification and characterization of 9-O-acetylated sialoglycoproteins from leukaemic cells and their potential as immunological tool for monitoring childhood acute lymphoblastic leukaemia. Glycobiology 14: 859–870. [DOI] [PubMed] [Google Scholar]
- 44.Sinha D, Chatterjee M, Mandal C (2000) O-acetylation of sialic acids- their detection, biological significance and alteration in diseases- a Review. Trends in Glycosci. Glycotechnol 12: 17–33. [Google Scholar]
- 45.Malorni W, Straface E, Pagano G, Monti D, Zatterale A, et al. (2000) Cytoskeleton alterations of erythrocytes from patients with Fanconi's anemia. FEBS Lett 468: 125–128. [DOI] [PubMed] [Google Scholar]
- 46.Ahmed H, Gabius HJ (1989) Purification and properties of a Ca2+-independent sialic acid-binding lectin from human placenta with preferential affinity to O-acetylsialic acids. J Biol Chem 264: 18673–18678. [PubMed] [Google Scholar]
- 47.de Jong K, Larkin SK, Styles LA, Bookchin RA, Kuypers FA (2001) Characterization of the phosphatidylserine-exposing subpopulation of sickle cells. Blood 98: 860–867. [DOI] [PubMed] [Google Scholar]
- 48.Borenstein-Ben Yashar V, Barenholz Y, Hy-Am E, Rachmilewitz EA, Eldor A (1993) Phosphatidylserine in the outer leaflet of red blood cells from beta-thalassemia patients may explain the chronic hypercoagulable state and thrombotic episodes. Am J Hematol 44: 63–65. [DOI] [PubMed] [Google Scholar]
- 49.Sen G, Mukhopadhayay R, Ghosal J, Biswas T (2001) Oxidative damage of erythrocytes: a possible mechanism for premature hemolysis in experimental visceral leishmaniasis. Ann Hematol 80: 32–37. [DOI] [PubMed] [Google Scholar]
- 50.Wilson MJ, Richter-Lowney K, Daleke D (1993) Hyperglycemia induces a loss of phospholipid asymmetry in human erythrocytes. Biochemistry 32: 11302–11310. [DOI] [PubMed] [Google Scholar]
- 51.Blumenfeld N, Zachowski A, Galacteros F, Beuzard Y, Devaux PF (1991) Transmembrane mobility of phospholipids in sickle erythrocytes: effect of deoxygenation on diffusion and asymmetry. Blood 77: 849–854. [PubMed] [Google Scholar]
- 52.Lijnen P, Echevaria-Vázquez D, Fagard R, Petrov V (1998) Protein Kinase C Induced Changes in Erythrocyte Na/H Exchange and Cytosolic Free Calcium in Humans. Am J Hypertens 11: 81–87. [DOI] [PubMed] [Google Scholar]
- 53.Croall DE, Morrow JS, DeMartino GN (1986) Limited proteolysis of the erythrocyte membrane skeleton by calcium-dependent proteinases. Biochim Biophys Acta 882: 287–296. [DOI] [PubMed] [Google Scholar]
- 54.Czogalla A, Sikorski AF (2005) Spectrin and calpain: a ‘target’ and a ‘sniper’ in the pathology of neuronal cells. Cell Mol Life Sci 62: 1913–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shukla M, Rajgopal Y, Babu PP (2006) Activation of calpains, calpastatin and spectrin cleavage in the brain during the pathology of fatal murine cerebral malaria. Neurochem Int 48: 108–113. [DOI] [PubMed] [Google Scholar]
- 56.Brophy GM, Pineda JA, Papa L, Lewis SB, Valadka AB, et al. (2009) alphaII-Spectrin breakdown product cerebrospinal fluid exposure metrics suggest differences in cellular injury mechanisms after severe traumatic brain injury. J Neurotrauma 26: 471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deng Y, Thompson BM, Gao X, Hall ED (2007) Temporal relationship of peroxynitrite-induced oxidative damage, calpain-mediated cytoskeletal degradation and neurodegeneration after traumatic brain injury. Exp Neurol 205: 154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lewis SB, Velat GJ, Miralia L, Papa L, Aikman JM, et al. (2007) Alpha-II spectrin breakdown products in aneurysmal subarachnoid hemorrhage: a novel biomarker of proteolytic injury. J Neurosurg 107: 792–796. [DOI] [PubMed] [Google Scholar]
- 59.Lee JC, Hwang IK, Yoo KY, Kim DS, Kim WK, et al. (2006) Degradation of spectrin via calpains in the ventral horn after transient spinal cord ischemia in rabbits. Neurochem Res 31: 989–998. [DOI] [PubMed] [Google Scholar]
- 60.Pant HC, Virmani M, Gallant PE (1983) Calcium-induced proteolysis of spectrin and band 3 protein in rat erythrocyte membranes. Biochem Biophys Res Commun 117: 372–377. [DOI] [PubMed] [Google Scholar]
- 61.Mortensen AM, Novak RF (1991) Enhanced proteolysis and changes in membrane-associated calpain following phenylhydrazine insult to human red cells. Toxicol Appl Pharmacol 110: 435–449. [DOI] [PubMed] [Google Scholar]
- 62.Sen G, Mandal C (1995) Carbohydrate Specficity and Characterization of the Combining site of AchatininH, A sialic acid binding lectin. Carbohydr res 268: 115–125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Overview of the proposed mechanism. A hypothetical model has been shown in describing the role of 9-O-AcSGPs for the hemolysis of erythrocytes in VL. The model highlights the possible events inside the RBCVL including activation of calpain I followed by spectrin degradation and phosphatidyl serine exposure after sensitization of 9-O-AcSGPs by anti-9-O-AcSGP IgGVL.
(TIF)