Abstract
Osteoclasts are terminally differentiated cells that attach to bone and secrete proteases to degrade the bone matrix. The primary protease responsible for the degradation of the organic component of the bone matrix is Cathepsin K, which was largely thought to be unique to osteoclasts. Given its apparent selective expression in osteoclasts, the Cathepsin K promoter has been engineered to drive the expression of Cre recombinase in mice and has been the most relevant tool for generating osteoclast-specific gene loss. In an effort to understand the role of the ARF tumor suppressor in osteoclasts, we crossed Arf fl/fl mice to CtskCre/+ mice, which unexpectedly resulted in the germline loss of Arf. We subsequently confirmed Cre activity in gametes by generating CtskCre/+; Rosa+ mice. These results raise significant concerns regarding in vivo bone phenotypes created using CtskCre/+ mice and warrant further investigation into the role of Cathepsin K in gametes as well as alternative tools for studying osteoclast-specific gene loss in vivo.
Introduction
Osteoclasts are bone-resorbing cells derived from the monocyte/macrophage family of the hematopoietic lineage [1]. Osteoclasts work in conjunction with osteoblasts, which are the bone-forming cells, to maintain a steady state of bone turnover. Osteoclasts tightly attach to bone in order to create an acidic milieu, called the resorption lacunae, that is necessary for the degradation of both the inorganic and organic components of bone matrix. Within the resorption lacunae, osteoclasts secrete proteases that degrade the organic component of bone once the inorganic component is demineralized by the secreted acid. Cathepsin K is a lysosomal cysteine protease of the papain family and is the primary protease responsible for the degradation of type I collagen [2], [3], [4]. In both humans and mice, Cathepsin K is highly expressed in osteoclasts. However, in humans, Cathepsin K has also been detected in heart, liver, and lung tissues [5]. During mouse development, Cathepsin K mRNA expression is highest in musculoskeletal tissues including bone, cartilage, and skeletal muscle and is predominantly expressed in osteoclasts [6]. Given the apparent selective expression of Cathepsin K in osteoclasts, a knockin mouse was created using the endogenous germline Cathepsin K (Ctsk) promoter to drive the expression of Cre recombinase solely in osteoclasts. In partially replacing the Ctsk locus with Cre, one allele of Ctsk is lost; however, Ctsk+/− mice are capable of maintaining normal bone turnover [7]. Furthermore, expression of Cre mRNA in CtskCre/+ mice is thought to be unique to bone, with Cre activity reported only in osteoclasts [8]. This system is important to the field of bone biology as it is the most relevant tool for assessing osteoclast-specific gene alterations in vivo.
In our initial study, we wanted to assess the role of the ARF tumor suppressor in osteoclasts. ARF is one product of the Cdkn2a locus, a site of frequent mutations in human cancer. While Arf shares two of its three exons with INK4a at the Cdkn2a locus, it is read in an alternative reading frame, such that it bears no functional homology to that of INK4a [9]. Mice that are void of Arf (but retain wild-type INK4a) begin developing tumors (fibrosarcomas and lymphomas) as early as 8 weeks of age and, after one year, 80% of Arf−/− mice succumb to spontaneous tumor development [10]. Together, the human and mouse data overwhelmingly places ARF as a key regulator of tumorigenesis and warrants an intensive characterization of ARF’s functions. We have previously shown that basal ARF suppresses protein synthesis in mitotic cells [11]. To further characterize the mitotic-independent growth suppressive functions of ARF, we wanted to know if ARF plays a role in the growth and function of non-dividing cells. To this end, we are characterizing the role of ARF in osteoclasts. To assess osteoclast-specific Arf loss in vivo, we crossed Arffl/fl mice with CtskCre/+ mice. Here, we report the unanticipated finding that Cathepsin K-driven Cre expression causes germline deletion of floxed alleles in mice.
Results
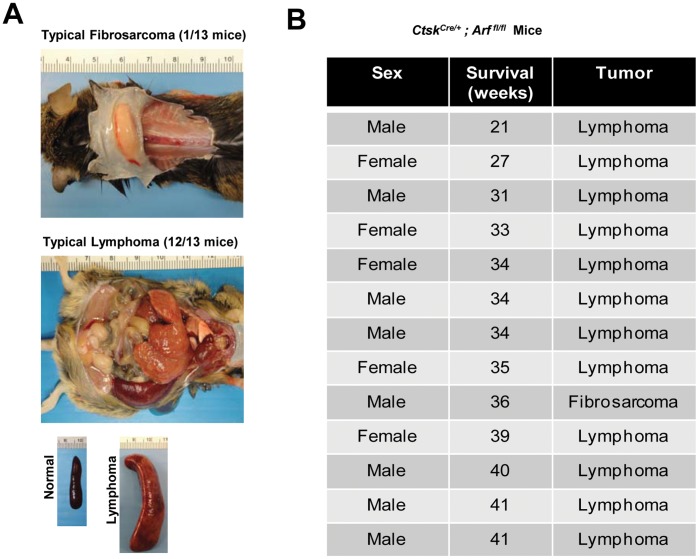
To study osteoclast-specific Arf loss in vivo, we crossed CtskCre/+ mice with mice containing floxed Cdkn2a exon1β, which is unique to ARF [12]. Resultant litters of CtskCre/+; Arf fl/fl mice developed spontaneous fibrosarcomas and lymphomas with 100% penetrance (13/13 mice) (Fig. 1A,B), consistent with tumor formation observed in traditional Arf −/− mice [10]. A primer set used to detect the presence of the 5′ loxP site (Fig. 2A, primer pair Lox F/Lox R-1) in tail DNA of Arffl/fl mice was unable to amplify a product after the mice had been bred with CtskCre/+, suggesting the loss of template (Fig. 2B, far left panel). We therefore reasoned that exon1β of Arf was not present in the tail DNA. To test this, we used two different primers sets that each contained a forward and reverse complementary sequence to a region of DNA outside of the loxP sites (Fig. 2A, primer pairs Lox F/Arf R and Lox F/Lox R-2). Both sets of primers resulted in products that suggested the loss of exon1β (Fig. 2B, right two panels). To further test for germline Arf loss, we exploited the fact that the testis is the only tissue in which we and others have been able to detect ARF protein by IHC [12]. Testes from mice, in which exon1β of Arf had seemingly been excised, were analyzed by IHC for ARF protein expression. While we detected ARF in the testis of wild-type mice, we were unable to detect ARF protein expression in mice showing loss of exon1β at the DNA level (Fig. 2C). Together, these results suggest that one copy of Cre under the control of the Ctsk promoter is sufficient to cause non-specific recombination and result in germline Arf loss.
Figure 1. Crossing Arffl/fl mice with CtsKCre/+ mice results in spontaneous tumor formation.
(A) CtskCre/+; Arffl/fl mice display both fibrosarcomas (top image) and lymphomas with splenomegaly (middle and bottom images). (B) Spontaneous tumor development occurred in all CtskCre/+; Arffl/fl mice.
Figure 2. Crossing Arf fl/fl mice with CtsKCre/+ mice results in germline Arf loss.
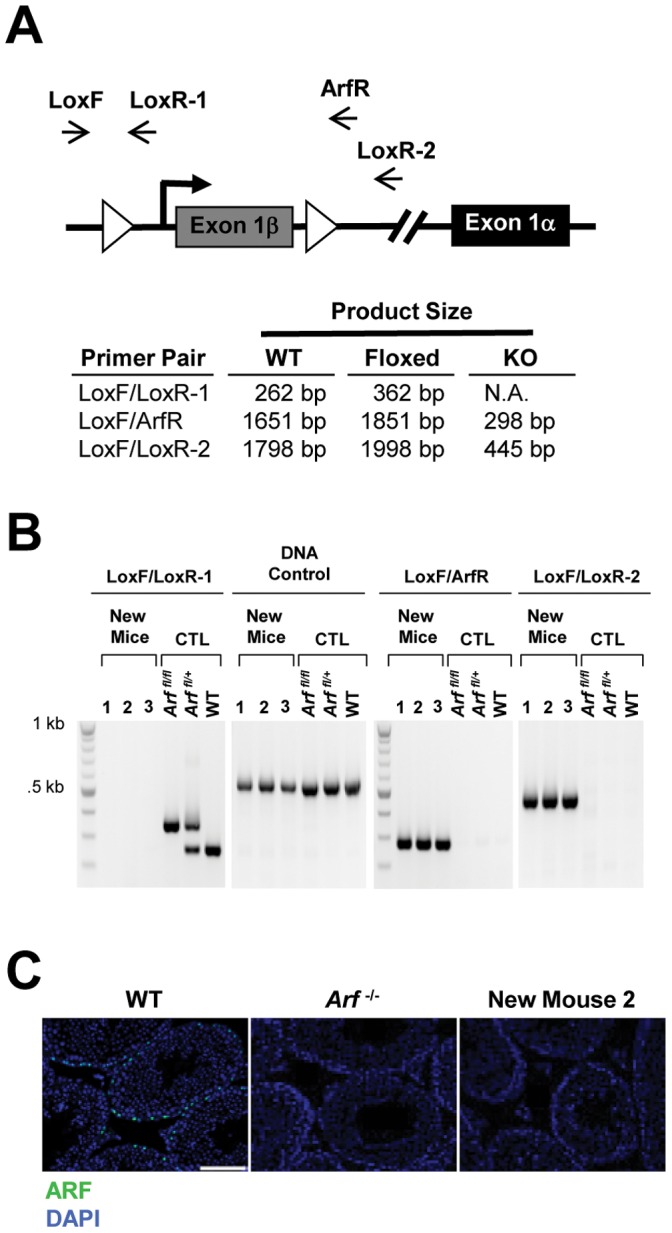
(A) Three sets of primer pairs were designed to detect the presence of floxed exon 1beta, which is unique to Arf. (B) PCR products were not detected upon genotyping new mice for the presence of the 5′ loxP site (far left panel). This cannot be attributed to an absence of DNA (middle left panel). Controls are mice that were never crossed with CtskCre/+ mice. Two sets of primer pairs were used to detect the presence of exon 1beta. Each set detects a product indicating loss of exon 1beta (product size labeled as “KO” in table). (C) Immunofluorescent staining for ARF in testis tissues indicates the loss of exon 1beta at the protein level (scale bar = 100 µM).
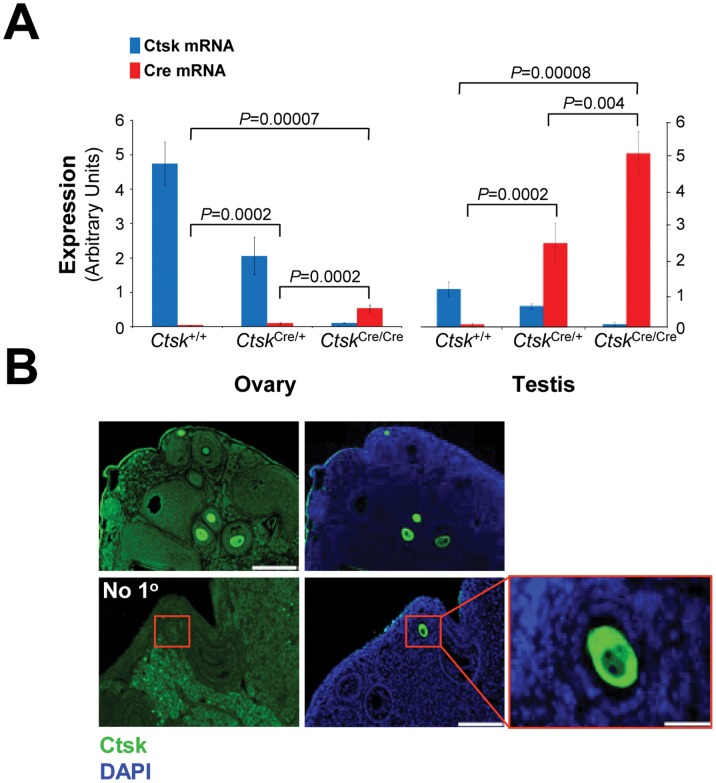
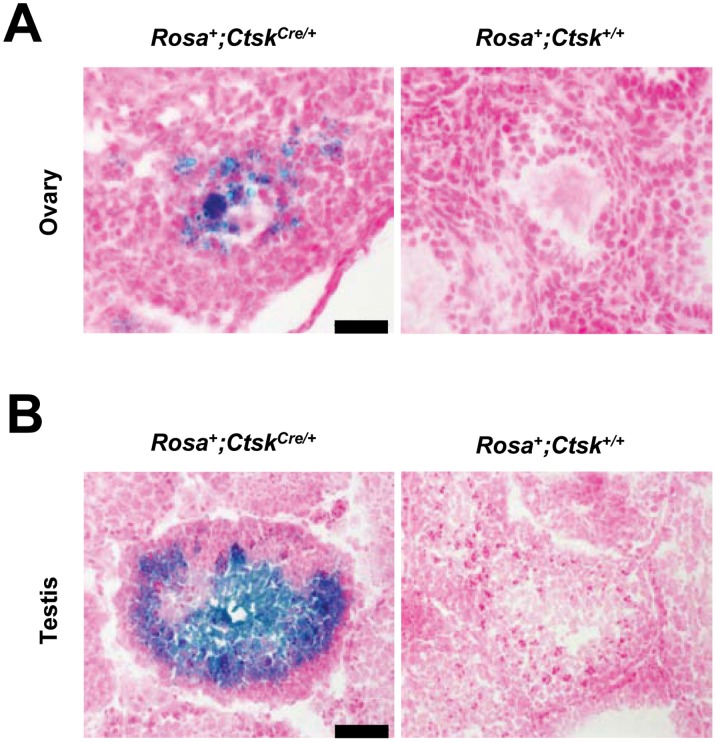
In light of our unexpected finding that Cathepsin K-driven Cre caused germline Arf loss when bred with Arf fl/fl mice, we sought to assess the reproductive tissues of CtskCre/+ mice. We first investigated the presence of Cre and Cathepsin K mRNAs in ovary and testis tissues by qRT-PCR. In ovaries of 10-week-old wild-type mice, we detected Cathepsin K mRNA. Cathepsin K mRNA decreased as Ctsk was replaced by Cre. Accordingly, we detected increasing amounts of Cre mRNA as Cathepsin K mRNA decreased (Fig. 3A, left). Similar results for Cathepsin K mRNA were obtained using testes RNA from 8-week-old wild-type mice, albeit at much lower levels relative to that observed in ovaries. Importantly, we again detected a dose-dependent decrease in Cathepsin K mRNA as Cre mRNA levels increased in testes (Fig. 3A, right). Given the striking presence of Cathepsin K mRNA in ovaries, we analyzed ovary tissues from 16-week-old mice for the presence of Cathepsin K by immunohistochemistry (IHC). Our results indicate that Cathepsin K is expressed at the protein level in ovaries and is primarily localized to oocytes within the developing follicles (Fig. 3B). Furthermore, we noted that follicles expressing Cathepsin K were primarily those in the early stages of maturation. As a control, adjacent tissue sections were stained without primary antibody. Sections incubated without antibody were negative for Cathepsin K in positive areas of the adjacent section that was incubated with the primary antibody (Fig. 3B). These results indicate that Cathepsin K is present in mouse ovaries and may be expressed at a specific stage during follicle development. Finally, we wanted to confirm the presence of Cre in the reproductive tissues by assaying for recombinase activity. To confirm Cre activity, we crossed CtskCre/+ mice with Rosa+ reporter mice. Ovary and testis tissues from Rosa+; CtskCre/+ mice were analyzed for Cre activity by LacZ staining. In ovaries, Cre activity was primarily detected in the oocyte and granulosa cells (Fig. 4A). In testes, abundant staining was evident in spermatozoa (Fig. 4B). This data demonstrates that Cathepsin K-driven Cre activity is present in gametes of both female and male mice. Of note, only one Cre allele was necessary for DNA excision at the Rosa locus. As a whole, this data demonstrates that crossing mice containing a floxed gene with CtskCre/+ mice can result in germ line loss of the floxed gene.
Figure 3. Cre is expressed in the gametes of CtskCre/+ mice.
(A) Real-time PCR was used to quantify the presence of Cathepsin K mRNA (blue) and Cre mRNA (red) in ovary (left) and testis (right) for all indicated genotypes (n = 3). Data are represented as means ± SD. A two-tailed t-test was used to generate indicated p values. (B) Ovaries from WT mice analyzed by IHC for Cathepsin K. Top panels, scale bar = 200 µM. Bottom, left panel shows staining without primary antibody. An adjacent section to the control (bottom, middle) was incubated with primary antibody (scale bar = 100 µM). Red box indicates positively-stained oocyte (bottom, right scale bar = 20 µM).
Figure 4. Cre expression in CtskCre/+ mouse gametes is verified with Rosa+ mice.
Reproductive organs of indicated genotypes were analyzed for Cre activity by LacZ staining. (A) In ovaries of Rosa+; CtskCre/+ mice, Cre activity was detected in oocytes and cells surrounding the developing oocytes (left panel). Controls were negative for LacZ staining (right panel). Scale bar = 50 µM. (B) In testes of Rosa+; CtskCre/+ mice, Cre activity was detected primarily in spermatozoa (left panel). Controls were negative for LacZ staining (right panel). Scale bar = 100 µM.
Discussion
Together, our results demonstrate that Cathepsin K is expressed in gametes, and this expression can result in germline excision of a floxed allele when bred with CtskCre/+ mice. Within the ovary, we observed Cathepsin K protein expression primarily in oocytes. Data from Chiu et al. showing expression of Cathepsin K mRNA in mouse ovaries supports this finding [13]. Expression of Cathepsin K has also been noted in human ovary samples [14]. While there is no published role for Cathepsin K in ovaries, our data showing that Cathepsin K may be expressed during early stages of oocyte development suggests a possible role for Cathepsin K during oocyte maturation. Moreover, other Cathepsins have also been shown to be expressed during specific stages of follicle development in teleosts [15]. In testis, Cre activity was noted within seminiferous tubules and was largely localized to mature spermatozoa. To support this finding, when creating transgenic mice that express Cre driven by the Ctsk promoter, Chiu et al. published the expression of Cre expression in the testis of multiple mouse lines [13].
Upon crossing CtskCre/+ mice with Arf fl/fl mice, we generated germline Arf loss as demonstrated by PCR, IHC for ARF in testis, and disease phenotypes that mimic traditional Arf-null mice [10]. Unfortunately, these findings place into question previous data that has been generated using mice containing Cre driven by the Ctsk promoter to create osteoclast-specific knockouts. Furthermore, our data may help explain the unexpected results that others have generated when using CtskCre/+ mice to generate osteoclast-specific gene ablation [16]. We are not suggesting that all animal models with Cre under the control the Ctsk promoter will result in a genomic knockout as neither every follicle nor every seminiferous tubule was positive for Cre activity. However, our data clearly indicate that careful testing is necessary when using this mouse model to ensure that the intended gene excision has occurred only in osteoclasts. Finally, these results warrant future investigation of an alternative tool to study osteoclast-specific gene loss in vivo and, in light of novel inhibitors intended to target Cathepsin K for therapy [17], a better understanding of the role of Cathepsin K in gametes.
Materials and Methods
Generation of CtskCre/+; Rosa+ and CtskCre/+; Arf fl/fl Mice
All animals were used in protocols that were reviewed and approved by the Washington University Animal Studies Committee. CtskCre/+ mice (generously provided by S. Kato) and the Rosa + reporter mice have been described previously [8], [18]. Cathepsin K-Cre “knockin” heterozygous mice (CtskCre/ +; maintained on a C57BL/6 background) were crossed with mice heterozygous for an R26R allele, where a constitutively active chromosomal gene was manipulated to insert the lacZ gene such that the β-galactosidase protein is produced only following the removal of a “stuffer” fragment flanked by loxP sites (Rosa+; maintained on a C57BL/6 background). CtskCre/+ mice were also crossed with mice homozygous for a floxed allele of Arf where exon1β is flanked by loxP sites (Arf fl/fl; maintained on a mixed background of C57BL/6 and 129SvJae, a kind gift from C. Sherr, St. Jude Children’s Research Hospital). Ctsk wild-type and “knockin” alleles were detected using primers Ctsk-P1 5′-TTATTCCTTCCGCCAGGATG-3′, Ctsk-P2 5′-TTGCTGTTATACTGCTTCTG-3′ and Ctsk-P3 5′-TAGTTTTTACTGCCAGACCG-3′. When used together in a PCR reaction, a wild-type allele generates a 135 bp fragment whereas a “knockin” allele produces a 300 bp fragment. Arf wild-type and floxed alleles were determined by using primers SpeLxF-30 5′-TTGCTACTTTACTGCAGCCAGACCACTAGG-3′ and SpeLxR-30 5′-CTCGGAGATTGAGAAAGCGGGAAGTCAAGC-3′ in which the wild-type allele generates a 260 bp product and the floxed allele generates a 360 bp product. The presence of the R26R allele was assessed by amplification of a 320 bp fragment using primers R26Rfwd 5′-AAAGTCGCTCTGAGTTGTTAT-3′ and R26Rrev 5′-GCGAAGAGTTTGTCCTCAACC-3′.
Ethics
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Animal Studies Committee of Washington University (Permit Number: 20100268).
Tissue Harvest and Histology
Testis and ovary tissues were taken from mice that were cardiac perfused with formalin, then fixed using a rapid microwave fixation. Fixed tissues were PBS washed then processed through graded alcohols and xylenes then embedded in paraffin. 5- µm tissue sections were immunostained. Alternatively, frozen testis and ovary tissues were prepared from sucrose perfused mice, embedded in OCT over liquid nitrogen, sectioned at 5- µm and LacZ stained.
Immunostaining
All sections were deparaffinized, rehydrated, washed in PBS, and blocked with serum-free Protein block (Dako) for 30 min at room temperature. All immunostaining required antigen retrieval which was performed in a food steamer using a 1x Reveal decloaker buffer (pH6.0) (Biocare Medical). Antibodies for the following markers were diluted in Antibody diluent (Dako) and applied overnight at 4°C: rat anti-p19ARF (1∶400, Abcam), and rabbit anti-Cathepsin K (1∶200, Abcam). A secondary antibody conjugated with Alexa Fluor 488 was placed on tissue sections for 1 hr at room temperature (1∶300, Invitrogen). Nuclei were counterstained using Slow Fade Gold Antifade reagent with 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen).
Quantitative Real-time PCR
Ovary and testis tissues were isolated, flash-frozen in liquid nitrogen, and homogenized in RNA-Solv (Omega Bio-Tek). The SuperScript III first-strand synthesis system (Invitrogen) was used to generate first strand cDNA. Real-time PCR was performed with iQ SYBR Green Supermix (Bio-Rad) on an iCycler thermal cycler (Bio-Rad).
LacZ Staining
Frozen sections were air-dried for 30 min at room temperature and fixed in 2% paraformaldehyde/0.125% glutaraldehyde in 1x PBS pH 7.4 for 5 min. Sections were washed sequentially in 2mM MgCl2/PBS, 2mM MgCl2, 0.02% Nonidet P40, 0.01% deoxycholate in PBS, and LacZ staining buffer (5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 2 mM MgCl2, 1x PBS), and then incubated in LacZ staining buffer supplemented with 1 mg/ml X-Gal overnight at 37°C with shaking. Nuclei were counterstained with Nuclear Fast Red (Dako).
Microscopy and Imaging
Gross pathological images were captured using a Sony Cyber-shot 12.1 megapixel digital camera equipped with a Carl Zeiss 5x optical zoom wide (28 mm) lens. Microscopy images for histology were obtained with a BX61 microscope (Olympus America) using the following objectives: UPlan Apochromatic 20X/NA 0.70, and UPlan Apochromatic 40X/NA 0.85. Tissue sections stained with LacZ were mounted with Krystalon (EMD). Histological microscopy images were obtained with a DP70 color Bayer mosaic digital camera, Peltier device cooled to −10°C (Olympus America). These images were captured with MicroSuite Biological Suite version 5 software (Olympus Soft Imaging Solutions) and resized and formatted with Adobe Photoshop CS3 software (Adobe Systems Incorporated). Fluorescence microscopy images were obtained with an Eclipse 90i microscope (Nikon) using the following objectives: Plan Apochromatic 10x/NA 0.45, Plan Apochromatic 20x/NA 0.75, and Plan Apochromatic Oil 100x/NA 1.40. Tissue sections for fluorescence microscopy images were mounted with SlowFade Gold Antifade reagent with DAPI (Invitrogen). Images were obtained using a CoolSnap HQ2 monochrome digital camera, Peltier cooled to −30°C (Photometrics). Fluorescence images were captured with MetaMorph version 7.6 software (MDS Analytical Technologies) and resized and formatted with Adobe Photoshop CS3 software.
Statistical Analysis
Data were analyzed using Excel (Microsoft Office). Results are expressed as the mean ±SD, and statistical significance was determined using a two-tailed t-test.
Acknowledgments
The authors would like to thank the members of the Weber laboratory, especially Chan Lee and Michael Benjamin, for their advice and technical assistance and Chuck Sherr, Steve Teitelbaum, Katherine Weilbaecher, Loren Michel, Mark Sands, Roberta Faccio, and Michelle Hurchla for insightful comments and reagents.
Funding Statement
This work was funded by National Institutes of Health grant R01CA120436. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Coccia PF, Krivit W, Cervenka J, Clawson C, Kersey JH, et al. (1980) Successful bone-marrow transplantation for infantile malignant osteopetrosis. N Engl J Med 302: 701–708. [DOI] [PubMed] [Google Scholar]
- 2. Zaidi M, Troen B, Moonga BS, Abe E (2001) Cathepsin K, osteoclastic resorption, and osteoporosis therapy. J Bone Miner Res 16: 1747–1749. [DOI] [PubMed] [Google Scholar]
- 3. Troen BR (2004) The role of cathepsin K in normal bone resorption. Drug News Perspect 17: 19–28. [DOI] [PubMed] [Google Scholar]
- 4. Georges S, Ruiz Velasco C, Trichet V, Fortun Y, Heymann D, et al. (2009) Proteases and bone remodelling. Cytokine Growth Factor Rev 20: 29–41. [DOI] [PubMed] [Google Scholar]
- 5. Littlewood-Evans A, Kokubo T, Ishibashi O, Inaoka T, Wlodarski B, et al. (1997) Localization of cathepsin K in human osteoclasts by in situ hybridization and immunohistochemistry. Bone 20: 81–86. [DOI] [PubMed] [Google Scholar]
- 6. Rantakokko J, Aro HT, Savontaus M, Vuorio E (1996) Mouse cathepsin K: cDNA cloning and predominant expression of the gene in osteoclasts, and in some hypertrophying chondrocytes during mouse development. FEBS Lett 393: 307–313. [DOI] [PubMed] [Google Scholar]
- 7. Saftig P, Hunziker E, Wehmeyer O, Jones S, Boyde A, et al. (1998) Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc Natl Acad Sci U S A 95: 13453–13458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, et al. (2007) Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell 130: 811–823. [DOI] [PubMed] [Google Scholar]
- 9. Quelle DE, Zindy F, Ashmun RA, Sherr CJ (1995) Alternative reading frames of the INK4a tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell 83: 993–1000. [DOI] [PubMed] [Google Scholar]
- 10. Kamijo T, Bodner S, van de Kamp E, Randle DH, Sherr CJ (1999) Tumor spectrum in ARF-deficient mice. Cancer Res 59: 2217–2222. [PubMed] [Google Scholar]
- 11. Apicelli AJ, Maggi LB Jr, Hirbe AC, Miceli AP, Olanich ME, et al. (2008) A non-tumor suppressor role for basal p19ARF in maintaining nucleolar structure and function. Mol Cell Biol 28: 1068–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gromley A, Churchman ML, Zindy F, Sherr CJ (2009) Transient expression of the Arf tumor suppressor during male germ cell and eye development in Arf-Cre reporter mice. Proc Natl Acad Sci U S A 106: 6285–6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chiu WS, McManus JF, Notini AJ, Cassady AI, Zajac JD, et al. (2004) Transgenic mice that express Cre recombinase in osteoclasts. Genesis 39: 178–185. [DOI] [PubMed] [Google Scholar]
- 14. Bromme D, Okamoto K (1995) Human cathepsin O2, a novel cysteine protease highly expressed in osteoclastomas and ovary molecular cloning, sequencing and tissue distribution. Biol Chem Hoppe Seyler 376: 379–384. [DOI] [PubMed] [Google Scholar]
- 15. Fabra M, Cerda J (2004) Ovarian cysteine proteinases in the teleost Fundulus heteroclitus: molecular cloning and gene expression during vitellogenesis and oocyte maturation. Mol Reprod Dev 67: 282–294. [DOI] [PubMed] [Google Scholar]
- 16. Qin A, Cheng TS, Lin Z, Pavlos NJ, Jiang Q, et al. Versatile roles of V-ATPases accessory subunit Ac45 in osteoclast formation and function. PLoS One 6: e27155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stoch SA, Wagner JA (2008) Cathepsin K inhibitors: a novel target for osteoporosis therapy. Clin Pharmacol Ther 83: 172–176. [DOI] [PubMed] [Google Scholar]
- 18. Soriano P (1999) Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21: 70–71. [DOI] [PubMed] [Google Scholar]