Abstract
Pulsed electron spin resonance (ESR) dipolar spectroscopy (PDS) in combination with site-directed spin labeling is unique in providing nanometer- range distances and distributions in biological systems. To date, most of the pulsed ESR techniques require frozen solutions at cryogenic temperatures to reduce the rapid electron spin relaxation rate and to prevent averaging of electron-electron dipolar interaction due to the rapid molecular tumbling. To enable measurements in liquid solution, we are exploring a triarylmethyl (TAM)-based spin label with a relatively long relaxation time where the protein is immobilized by attachment to a solid support. In this preliminary study, TAM radicals were attached via disulfide linkages to substituted cysteine residues at positions 65 and 80 or 65 and 76 in T4 lysozyme immobilized on Sepharose. Interspin distances determined using double quantum coherence (DQC) in solution are close to those expected from models, and the narrow distance distribution in each case indicates that the TAM-based spin label is relatively localized.
Pulsed electron spin resonance (ESR) dipolar spectroscopy (PDS),1 which includes double electron-electron resonance (DEER)2,3 and double quantum coherence (DQC),4–6 is a rapidly expanding technology for measuring nanometer-scale distances and distance distributions between paramagnetic centers in biological systems via magnetic dipolar interactions. In most cases, a pair of paramagnetic nitroxides is introduced by site-directed spin labeling (SDSL) using cysteine substitution mutagenesis 7 or genetically encoded unnatural amino acids.8 Distance distributions between metal centers are also measured using PDS.9–12 The PDS technique has been especially useful in obtaining structural constraints in membrane proteins13–20 and large protein complexes21–26 where crystallographic data is lacking.
Both DEER and DQC are based on detection of an electron spin echo and successful application requires a phase memory time (Tm) of the paramagnetic center on the order of, or longer than, the dipolar evolution period.1 The strength of the magnetic dipolar interaction is proportional to (3cos2θ − 1)/r3 where θ is the angle between the static magnetic field and the interspin vector, and r is the interspin distance. The angular dependence of the interaction identifies the second requirement for application of PDS, namely immobilization of the protein so that the interaction is not averaged over angular space by rotational diffusion.
In the usual implementation of PDS, these requirements are met with the sample in frozen solution at 50 – 80 K where the short Tm’s of nitroxide and metal ion spin labels are increased and where rotational diffusion of the protein is prevented. However, the freezing requirement brings with it potential problems, including the use of a cryoprotectant that may influence the distribution of conformational substates.27 Although slow freezing does not appear to alter the distance between spin labeled side chains located in relatively rigid regions of a protein, it does appear to affect the distance distribution,28 and it may well influence the protein conformational equilibria. Motivated by these considerations, we are currently exploring possible strategies for using PDS at ambient temperature, and herein report a promising approach for further development.
To meet the above requirements for using PDS at ambient temperatures, a novel spin label based on a triarylmethyl (TAM) radical with a long Tm was synthesized, and rotational diffusion of the spin labeled protein was effectively eliminated by attachment to a solid support.27 In this communication, we demonstrate the potential of PDS for measuring distances in liquid solution using T4 lysozyme (T4L) as a model protein, followed by a discussion of possible improvements and additional advantages of this new spin label.
The TAM spin labeling reagent is based on the radical CT03,29,30 which has been widely applied in ESR spectroscopy and imaging due to its extremely narrow single resonance line. The relaxation times of several TAM-based radicals have been reported to be as long as 20 μs for T1 and up to 3 μs for Tm.31, 32
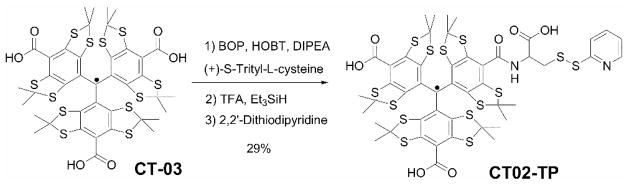
The synthesis of the TAM-based spin labeling reagent (CT02-TP) is shown in Scheme 1. The TAM radical CT-03 was conjugated with (+)-S-trityl-L-cysteine, followed by deprotection of the cysteine using TFA and triethylsilane. The resulting thiol reacted with 2, 2′-dithiodipyridine to afford the TAM spin label which was characterized by high resolution MS and ESR. Details of the synthesis are described in the Supporting Information (S.I.).
Scheme 1.
Synthesis of CT02-TP.
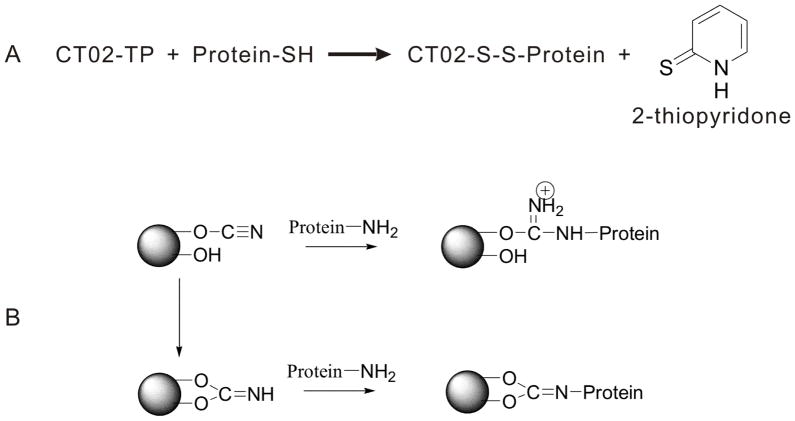
The proteins selected for reaction with CT02-TP were two double mutants of T4L, 65C/76C and 65C/80C. These sites are located in the rigid C helix of T4L. The reaction of CT02-TP with the cysteine residues in the protein forms a mixed disulfide bond with the production of 2-thiopyridone (Figure 1A) whose UV absorbance can be used to monitor the reaction.33 Initially, CT02-TP was reacted with the T4L derivatives in solution, but the doubly-labeled proteins showed limited solubility, forming precipitates during the reaction. To overcome this, the protein was attached to a solid support prior to reaction with CT02-TP. For this purpose, the free cysteines were firstly protected by reaction with 1-oxyl-3-methanesulfonylthiomethy-2,5-dihydro-2,2,5,5-tetramethyl-1H-pyrrole which generated the R1 nitroxide side chain often used in spin labeling.34 The R1 labeled T4L was then covalently immobilized on a cyanogenbromide activated sepharose as previously described (Figure 1B).27 The strong immobilization of T4L in this manner does not lead to its structural perturbation at the sites investigated.27 Unreacted cyanate esters or imidocarbonate sites on the activated sepharose were saturated with ethanolamine and DTT was added to cleave the R1 and regenerate active thiols. After removing DTT, CT02-TP was used to label the protein at a protein-to-label ratio of 1:3. Details of reactions and labeling efficiency are shown in the S.I. Standard spin echo decay experiments showed that the TAM radical attached to the proteins on the solid support had a Tm of about 0.7 μs at ambient temperature, an order of magnitude longer than that for a nitroxide under the same conditions.
Figure 1.
(A), reaction of CT02-TP with a protein SH. (B), immobilization of a protein using CNBr sepharose.
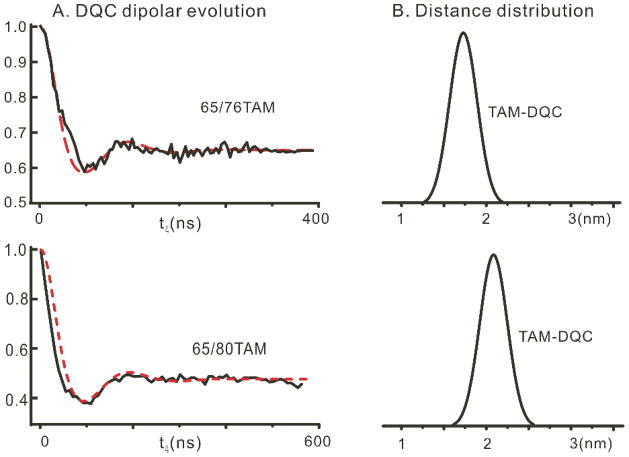
The DQC method is ideal for a single narrow resonance line,5 such as that of the TAM spin label. Samples with a protein concentration of ~500μM (typically 10 μL volume) were degassed and put into quartz tubes with both ends sealed to prevent the relaxation enhancement caused by oxygen. All DQC measurements were performed at 17.2 GHz using the facilities at ACERT (Cornell University); details of DQC experiments are in the S.I. The background-corrected DQC data and corresponding interspin distance distributions based on a Gaussian model for T4L 65/76 TAM and 65/80 TAM at 4 °C are shown in Figure 2; the mean distances are 1.8 nm and 2.1 nm, respectively, close to the corresponding Cα-Cα distances in the crystal structure of the protein (pdb 3lzm). We expect only a small deviation from the point-dipole approximation for TAM labels due to relatively small symmetric spin density delocalization, compared to some nitroxides.35
Figure 2.
(A), DQC evolution of TAM-labeled 65/76 (top panel) and 65/80 (lower panel) immobilized on Sepharose at 4 °C. The dashed curve shows a simulated DQC signal using a Gaussian model to represent the distance distribution. (B), Distance distribution functions obtained from the corresponding DQC signals in (A).
Although the experiments in Figure 2 were carried out at 4 °C for technical reasons having to do with dielectric loss from the sample, the Tm of the TAM radical changes little from 4 to 25 °C at both 9.5 GHz and 17.2 GHz.31 Therefore, the use of this new TAM spin label should enable DQC measurements in the physiological temperature range.
In summary, the data presented here demonstrate that PDS distance measurements can be made in liquid solution using a TAM-based spin label on an immobilized protein. Compared to a R1 nitroxide, the TAM spin label has additional advantages including the apparently high spatial localization and the lower power microwave pulses needed for DQC. A disadvantage relative to R1 is the physical size and the general hydrophobicity. However, it is on the same order of mass as the popular Alexa dyes used routinely in fluorescence spectroscopy, and if sites are confined to surface sites the size should not present a major problem.
For general use the TAM spin label needs further development to improve the reactivity and stability, reduce the hydrophobicity and further increase Tm. For example, titration of the reactive thiol content following the labeling reaction revealed that only ~ 65% of the cysteines reacted with CT02-TP (see S.I.). This could be due in part to steric constraints imposed by the solid support; because the attachment is via the abundant lysine residues distributed over the surface, attachment at some sites may occlude otherwise reactive cysteines. This potential problem can be solved by using unnatural amino acid- technology to provide a single specific site for attachment to the solid support.36 In addition, the reactivity toward cysteine can be improved by using a more reactive methanethiosulfonate derivative in place of the mixed TAM-thiopyridine disulfide in CT02-TP.
Finally, it will be necessary to increase the Tm to allow distance determination beyond 2.5 nm. The Tm of the free CT03 radical in 30% glycerol is ~ 2.5 μs at 4 °C.31 When attached to the protein on the solid support, this value drops to 0.7 μs. The shorter Tm of the TAM spin label on the protein is presumably due to a lower rotational diffusion rate of the label which modulates a weak anisotropic hyperfine coupling to the 14N in the amide linker and the anisotropic g-factor of the TAM spin. New TAM based labels are currently being synthesized to address these problems.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Health, grants NIH/NIGMS P41GM103521 and NIH/NCRR P41RR 016292 (JHF), HL38324, EB0890, and EB4900 (JLZ), NIH/NEI EY005216, NIH/NEI EY00331 and Jules Stein Professorship Endowment (WLH). We thank Dr. C.J. López, Dr. M.R. Fleissner and E.K. Brooks for providing mutants and essential training in molecular biology for ZY.
Footnotes
Supporting Information. Details of synthesis of the CT02-TP, preparation of protein samples, CW-ESR spectra and raw DQC data, as well as brief descriptions of the DQC method and conformational restriction and motion of the TAM label are included in the supporting information. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Borbat PP, Freed JH. Methods Enzymol. 2007;423:52. doi: 10.1016/S0076-6879(07)23003-4. [DOI] [PubMed] [Google Scholar]
- 2.Milov AD, Ponomarev AB, Tsvetkov YD. Chem Phys Lett. 1984;110:67. [Google Scholar]
- 3.Pannier M, Veit S, Godt A, Jeschke G, Spiess HW. J Magn Reson. 2000;142:331. doi: 10.1006/jmre.1999.1944. [DOI] [PubMed] [Google Scholar]
- 4.Saxena S, Freed J. J Chem Phys. 1997;107:1317. [Google Scholar]
- 5.Borbat PP, Mchaourab HS, Freed JH. J Am Chem Soc. 2002;124:5304. doi: 10.1021/ja020040y. [DOI] [PubMed] [Google Scholar]
- 6.Chiang Y-W, Borbat P, Freed JH. J Magn Reson. 2005;172:279. doi: 10.1016/j.jmr.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Todd AP, Cong J, Levinthal F, Levinthal C, Hubell WL. Proteins: Struct, Func, Bioinf. 1989;6:294. doi: 10.1002/prot.340060312. [DOI] [PubMed] [Google Scholar]
- 8.Fleissner MR, Brustad EM, Kálai T, Altenbach C, Cascio D, Peters FB, Hideg K, Peuker S, Schultz PG, Hubbell WL. Proc Natl Acad Sci USA. 2009;106:21637. doi: 10.1073/pnas.0912009106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becker J, Saxena S. Chem Phys Lett. 2005;414:248. [Google Scholar]
- 10.Raitsimring AM, Gunanathan C, Potapov A, Efremenko I, Martin JML, Milstein D, Goldfarb D. J Am Chem Soc. 2007;129:14138. doi: 10.1021/ja075544g. [DOI] [PubMed] [Google Scholar]
- 11.Roessler MM, King MS, Robinson AJ, Armstrong FA, Harmer J, Hirst J. Proc Natl Acad Sci USA. 2010;107:1930. doi: 10.1073/pnas.0908050107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Z, Kurpiewski MR, Ji M, Townsend JE, Mehta P, Jen-Jacobson L, Saxena S. Proc Natl Acad Sci USA Plus. 2012 doi: 10.1073/pnas.1200733109. Early Ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altenbach C, Kusnetzow AK, Ernst OP, Hofmann KP, Hubbell WL. Proc Natl Acad Sci USA. 2008;105:7439. doi: 10.1073/pnas.0802515105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joseph B, Jeschke G, Goetz BA, Locher KP, Bordignon E. J Biol Chem. 2011;286:41008. doi: 10.1074/jbc.M111.269472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicklisch SCT, Wunnicke D, Borovykh IV, Morbach S, Klare JP, Steinhoff HJ, Krämer R. Biochim Biophys Acta (BBA) - Biomembranes. 2011;1818:359. doi: 10.1016/j.bbamem.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 16.Feldmann EA, Ni S, Sahu ID, Mishler CH, Risser DD, Murakami JL, Tom SK, McCarrick RM, Lorigan GA, Tolbert BS, Callahan SM, Kennedy MA. Biochemistry. 2011;50:9212. doi: 10.1021/bi201226e. [DOI] [PubMed] [Google Scholar]
- 17.Kuo W, Herrick DZ, Cafiso DS. Biochemistry. 2011;50:2633. doi: 10.1021/bi200049c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swanson MA, Kathirvelu V, Majtan T, Frerman FE, Eaton GR, Eaton SS. Protein Sci. 2011;20:610. doi: 10.1002/pro.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smirnova I, Kasho V, Kaback HR. Biochemistry. 2011;50:9684. doi: 10.1021/bi2014294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Georgieva ER, Ramlall TF, Borbat PP, Freed JH, Eliezer D. J Biol Chem. 2010;285:28261. doi: 10.1074/jbc.M110.157214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhatnagar J, Borbat PP, Pollard AM, Bilwes AM, Freed JH, Crane BR. Biochemistry. 2010;49:3824. doi: 10.1021/bi100055m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stone KM, Townsend JE, Sarver J, Sapienza PJ, Saxena S, Jen-Jacobson L. Angew Chem, Int Ed. 2008;47:1. doi: 10.1002/anie.200803588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sen KI, Logan TM, Fajer PG. Biochemistry. 2007;46:11639. doi: 10.1021/bi700859p. [DOI] [PubMed] [Google Scholar]
- 24.Banham JE, Timmel CR, Abbott RJM, Lea SM, Jeschke G. Angew Chem, Int Ed. 2006;45:1058. doi: 10.1002/anie.200503720. [DOI] [PubMed] [Google Scholar]
- 25.Van Eps N, Preininger AM, Alexander N, Kaya AI, Meier S, Meiler J, Hamm HE, Hubbell WL. Proc Natl Acad Sci USA. 2011;108:9420. doi: 10.1073/pnas.1105810108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jao CC, Hegde BG, Gallop JL, Hegde PB, McMahon HT, Haworth IS, Langen R. J Biol Chem. 2010;285:20164. doi: 10.1074/jbc.M110.127811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.López CJ, Fleissner MR, Guo Z, Kusnetzow AK, Hubbell WL. Protein Sci. 2009;18:1637. doi: 10.1002/pro.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Georgieva ER, Roy AS, Grigoryants VM, Borbat PP, Earle KA, Scholes CP, Freed JH. J Magn Reson. 2012;216:69. doi: 10.1016/j.jmr.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dhimitruka I, Velayutham M, Bobko AA, Khramtsov VV, Villamena FA, Hadad CM, Zweier JL. Bioorg Med Chem Lett. 2007;17:6801. doi: 10.1016/j.bmcl.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Villamena FA, Sun J, Xu Y, Dhimitruka I, Zweier JL. J Org Chem. 2008;73:1490. doi: 10.1021/jo7022747. [DOI] [PubMed] [Google Scholar]
- 31.Owenius R, Eaton GR, Eaton SS. J Magn Reson. 2005;172:168. doi: 10.1016/j.jmr.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Fielding A, Carl P, Eaton G, Eaton S. Appl Magn Reson. 2005;28:231. [Google Scholar]
- 33.Grassetti DR, Murray JF., Jr Arch Biochem Biophys. 1967;119:41. doi: 10.1016/0003-9861(67)90426-2. [DOI] [PubMed] [Google Scholar]
- 34.Langen R, Cai K, Altenbach C, Khorana HG, Hubbell WL. Biochemistry. 1999;38:7918. doi: 10.1021/bi990010g. [DOI] [PubMed] [Google Scholar]
- 35.Riplinger C, Kao JPY, Rosen GM, Kathirvelu V, Eaton GR, Eaton SS, Kutateladze A, Neese F. J Am Chem Soc. 2009;131:10092. doi: 10.1021/ja901150j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seo MH, Han J, Jin Z, Lee D, Park HS, Kim HS. Anal Chem. 2011;83:2841. doi: 10.1021/ac103334b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.