Abstract
Objective
Overweight and obesity are epidemic in populations with serious mental illnesses. We developed and pilot tested a behavioral weight loss intervention appropriately tailored for persons with serious mental disorders.
Methods
We conducted a single arm pilot study in two psychiatric rehabilitation day programs in Maryland and enrolled 63 overweight or obese adults. The six-month intervention provided group and individual weight management and group physical activity classes. The primary outcome was weight change from baseline to 6 months.
Results
Sixty-four percent of those potentially eligible at the centers enrolled. The mean age was 43.7 years; 56% were women, 49% were white and over half had a schizophrenia or schizoaffective disorder. One third had hypertension and one fifth had diabetes. Fifty-two (82%) completed the study; others were discharged from psychiatric centers before study completion. Average attendance across all weight management sessions was 70% (87% on days participants attended the center) and 59% for physical activity classes (74% on days participants attended the center). From a baseline mean of 210.9 lbs (SD 43.9), average weight loss for 52 participants was 4.5 pounds (SD 12.8) (p<0.014). On average, participants lost 1.9% of body weight. Mean waist circumference change was 3.1 cm (SD 5.6). Participants on average increased the distance on the six minute walk test by eight percent.
Conclusion
This pilot documents the feasibility and preliminary efficacy of a behavioral weight loss intervention in adults with serious mental illness who were attendees at psychiatric rehabilitation centers. The results may have implications for developing weight loss interventions in other institutional settings such as schools or nursing homes.
Keywords: weight loss intervention, obesity, mental illness, schizophrenia, bipolar disorder
Introduction
Persons with serious mental illness comprise a vulnerable population with a high prevalence of overweight and obesity and extremely high mortality -- three times higher than the general population (1, 2). These individuals, with mental health conditions such as schizophrenia, bipolar disorder and disabling depression, die primarily of cardiovascular disease. The high prevalence of overweight and obesity and constitute a public health epidemic in persons with serious mental illness, and likely contribute to the increased cardiovascular risk and observed mortality rates in this population (3).
Weight problems are multifactorial in persons with serious mental illness, due to lifestyle factors such as physical inactivity and unhealthy diet as well as psychotropic medications. Persons with serious mental illness are less physically active than the general population, and are more likely to report little or no physical activity (4, 5). Lack of affordable, safe places to exercise, and negative affective states such as sadness or depression likely are important barriers to regular physical activity. Some evidence also shows that persons with chronic mental illness have unhealthy diets compared to the general population with higher fat and lower fruit and vegetable intake, other reports show similar types of food but with higher overall caloric intake (6–8). Drugs within most classes of psychotropic medications, including antipsychotics, antidepressants and mood stabilizers, lead to weight gain (9). In particular, persons taking certain second generation antipsychotics are at risk for substantial weight increases; in the case of olanzapine, up to 0.9 kg per month (10). Weight gain from medications may be caused in part through increased appetite (11). Most with serious mental illness will require long-term pharmacologic therapy to control mental health symptoms and enable functioning in the community. Given the long-term need for and risks of psychotropic medications in persons with serious mental illness, interventions to decrease obesity and cardiovascular risk in this population are needed urgently.
Behavioral weight loss interventions in persons with chronic mental illness pose particular challenges. Persons with serious mental illness are likely to have competing demands such as active mental health symptoms, issues with housing, substance abuse or other social issues taking precedence over behavioral change for weight loss. Moreover, cognitive deficits are highly prevalent and, when present, contribute to difficulties in performing activities of daily living and sustaining employment (12). In view of these considerations, traditional behavioral Interventions that are effective in the general population should be tailored to the needs of persons with serious mental illness.
Evidence from small studies emphasizing primarily dietary counseling suggests that selected persons with serious mental illness can benefit from short-term weight loss interventions (3). Few weight loss interventions in this population have incorporated physical activity (13–17). Most studies focus on persons with specific diagnoses (e.g., schizophrenia) and/or those taking a specific, often single antipsychotic medication (13, 18–21). Studies generally have been in inpatient settings or recruit from outpatient mental health centers (21–23).
We propose that psychiatric rehabilitation programs are opportune and efficient settings for implementing behavioral weight loss interventions. Psychiatric rehabilitation programs are outpatient venues where persons with serious mental illness attend up to several days a week and receive vocational and other services to help to optimize functioning. Rehabilitation programs are usually housed in their own buildings including commons room, classrooms and commercial kitchen. Thus, the physical rehabilitation program environment is conducive to and also provides a natural congregation of individuals with serious mental illness for on-site weight loss interventions. Furthermore, most rehabilitation programs serve persons with a range of diagnoses, thus interventions targeted to these settings could generalize to broad populations with serious mental illness, not only schizophrenia.
The objective of this study was to test the feasibility and preliminary efficacy of a six month behavioral weight loss intervention in a psychiatric rehabilitation setting.
Subjects and Methods
Study Setting and Design
We conducted a single arm pilot study in two psychiatric rehabilitation day programs in Maryland. One center was urban, and one was suburban. The centers operated three mornings per week. We recruited participants and performed the intervention first at the urban site and then the suburban site. We recruited study participants face-to-face at the centers by presenting the study to all program attendees at regular mental health consumer meetings. We also discussed consumers’ potential eligibility with rehabilitation program staff. The study was approved by the Johns Hopkins University School of Medicine Institutional Review Board and by the institutional review board of the rehabilitation program. We report this non-controlled intervention study using guidelines from Transparent Reporting of Evaluations with Non-Randomized Designs (24).
Study Population
Mental health consumers ages 18 and older attending the psychiatric rehabilitation programs who were overweight or obese, with a body mass index at least 25 kg/m2 comprised the target study population. Given the high prevalence of cardiovascular risk factors in persons with serious mental illness, we chose to include persons meeting BMI criteria for either overweight or obesity. To attend the rehabilitation programs, mental health consumers had to have a diagnosis of a serious mental illness. The other study inclusion criterion included plans to remain at the rehabilitation program for at least six months.
Medical exclusion criteria included any contraindications to weight loss such as active malignancy, anorexia nervosa or end stage liver disease. Other medical exclusion criteria were individuals who could not walk for two minutes continuously, a myocardial infarction in the past six months or pregnancy.
In addition, study exclusion criteria included active substance abuse, a substance abuse treatment schedule incompatible with the intervention or persons considered unlikely to be able to participate in a group setting. We met with rehabilitation staff to discuss eligibility, including if a consumer was known to be leaving the program in six months, substance abuse issues, or if he or she was considered unlikely to be able to participate in a group setting. For example, if a consumer demonstrated excessive somnolence or extreme agitation, the staff may have recommended that he or she would not be a good candidate for participation in the group. We wanted to approximate as best as possible a real-world intervention that could be implemented in a psychiatric rehabilitation program. At the same time, this was a pilot study to test preliminary efficacy, and we wanted to enroll subjects likely able to participate in the intervention. We did not exclude potential participants based on mental health symptom scores or cognitive status (see Questionnaires).
Informed consent
All participants provided written informed consent using procedures reviewed and approved by the Johns Hopkins Institutional Review Boards. Separate consents were obtained for the screening and intervention phases of the study. The consent form was read to potential participants. They were also given time to read the form and to ask any questions. Then, a study staff member administered an evaluation of ability to give consent to the potential participant before the screening consent form was signed. The evaluation included having potential participants answer questions about the goal of the study, what they would be asked to do if they decided to join the study, what risks may be involved if they joined the study and who to contact if they wanted to withdraw from the study. If a participant was deemed not able to give consent, he/she could not participate in the study.
Weight Loss Intervention
The six-month weight loss intervention included three components: weight management counseling sessions; group physical activity sessions; and education for kitchen staff to provide healthier on-site meals. We based the intervention on the PREMIER Study, which incorporated social cognitive theory and behavioral self management in a lifestyle intervention to reduce blood pressure (25). Many of the behavioral strategies used in PREMIER have parallels in a psychiatric rehabilitation conceptual framework such as environmental and social supportive measures, skills training in modeling healthy eating behaviors, and rewards. We tailored the intervention to the cognitive needs of persons with serious mental illness attending psychiatric rehabilitation programs.
Weight management sessions consisted of one 45 minute group weight loss session per week, and one individual session with the interventionist every six weeks. In addition, participants were asked to weigh-in three times a week at the center assisted by a study staff member. A registered dietician led some of the weight management sessions, and a health educator led others. Both had bachelor’s level degrees. The group weight management sessions fit in to the regular schedule of classes offered by the rehabilitation programs. To adapt the weight management sessions to the cognitive needs of the population, classes emphasized repetition of concepts, materials written at a 5th–8th grade reading level and use of flip charts and handouts to minimize memory requirements. Session content included topics such as increasing fruit and vegetable consumption, decreasing portion sizes, and choosing healthy foods and low-calorie beverages. To emphasize rehearsing of behavioral skills, a technique shown to be successful in interventions for schizophrenia, sessions included hands-on activities such as measuring portions, tasting healthy foods and a trip to the local grocery store. Instead of using detailed food diaries, we modified self-monitoring materials to limit memory needs. Participants were encouraged to self-report food intake using a food tracker where they checked off when they ate categories of certain foods (e.g., servings of fruits and vegetables). Participants received a sticker on a chart each time they weighed-in during the intervention regardless of the weight itself. For each five stickers they selected a prize from a grab bag assembled from a dollar-store. The incentives were often related to nutrition or physical activity (e.g., measuring cups, athletic socks) or were other personal products (e.g., flashlight, gloves, shampoo).
Group physical activity classes were held on-site at the rehabilitation programs in all-purpose rooms. Classes were 45 minutes in length and emphasized moderate intensity physical activity in an aerobic dance type format. The classes provided both physical activity and the opportunity to rehearse exercise skills, as many of the participants had not recently participated in a structured exercise program. The exercise leaders were group exercise instructors from the community.
The intervention also included advice to rehabilitation programs to serve healthier meals. In both rehabilitation programs, lay kitchen staff with no formal nutrition training prepared meals in commercial kitchens. Study dieticians met first with program directors to introduce the intervention. Subsequently, dieticians met with kitchen staff between 2–4 times over the 6 months to assist kitchen staff with making changes to menus. They reviewed menus of breakfast and lunch served at the facilities and recommended basic changes such as decreasing sugar content of beverages and baking instead of frying foods.
Measures
Physical Measures
Measurements were collected at baseline and six months. Weight, waist circumference, accelerometry, blood pressure, six-minute walk and fasting serum measurements were obtained by trained and certified data collectors using standardized protocols. Weight was measured using a calibrated scale using the average of two measurements with participants in indoor clothes without shoes. Height was measured using a calibrated, wall-mounted stadiometer. Waist circumference was measured with an anthropometric tape in a 1 centimeter plane above the navel. Blood pressure measurements were determined by the OMRON 907 device which records blood pressure using an oscillometric technique (26). Appropriate cuff size was identified at baseline with re-measurement at follow-up. Physical activity was assessed with the RT3 (Stayhealthy, Inc), a triaxial accelerometer. The RT3 captures movement, or acceleration, from three planes resulting in vector magnitude for each minute of monitoring. This vector magnitude is summarized into activity counts that are used to determine intensity of activity performed during that time period. Participants were asked to wear the accelerometers during waking hours on their right hip for 4 days, including 1 weekend day. The RT3 has been shown to be a reliable and valid physical activity measure (27). Previous work showed that activity counts from the RT3 had strong correlation with steady state oxygen consumption during treadmill walking at different speeds (r=.79) and during non-regulated physical activity, r=.89 (27). Three blood pressure measurements at one visit were obtained on the right arm of participants after they rested quietly in the seated position for at least five minutes. Participants completed a six-minute walk test where they walked on a predefined course for six minutes and distance traveled was measured in feet. The six-minute walk is commonly used as a measure of functional capacity among deconditioned populations, and has been shown to be a reliable and valid test for obese adults (28). The Homeostasis Model Assessment-Insulin Resistance Index (HOMA IR) was calculated as fasting plasma insulin concentration in uU/ml x fasting plasma glucose concentration in mmol/L divided by 22.5 (29).
Questionnaires
Interviewers administered all instruments. We assessed smoking status and medical problems (e.g., hypertension) from a medical history questionnaire, and also categorized participants as having hypertension or diabetes based on medication use. Health status was assessed by the SF-36 (30). We assessed quality of life with the Lehman Quality of Life Short Form TL30S, an instrument validated for use in persons with schizophrenia (31). This measure assesses quality of life domains on 7-point Likert scales from 1 (terrible) to 7 (delighted). We assessed depressive symptoms with the Center for Epidemologic Studies Depression Scale (CES-D) (scores range 0 to 60). (32). Intake of nutrients and food groups was measured by the Block Diet Screeners (33, 34). We measured self efficacy with the General Self Efficacy Scale which uses a 4-point Likert scale. (scores range 10 to 40) (35). We assessed self efficacy for changes in diet and exercise habits with modified scales from the Eating Habits Confidence and Exercise Confidence instruments (scores range from 1 lowest to 5 highest confidence) (36). We also used a modified version of the Binge Eating Scale with scores ranging from 0 to 36; scores of 13–21 represented moderate binge eating and scores 22 and above represented binge eating (37). At baseline, we measured mental health symptoms with the Symptoms Checklist-90 General Symptom Index (GSI) (scores range 0 to 4) and cognitive status with the Repeatable Battery for Assessment of Neuropsychological Status (RBANS) (scores range 40 to 160) (38, 39, 40). We obtained psychiatric and substance abuse diagnoses and current medications from review of the rehabilitation center patient chart.
Statistical Analyses
The main hypothesis of change in weight from baseline to six months was tested using paired t-tests. We similarly assessed pre/post changes in waist circumference, blood pressure, six-minute walk and laboratory measurements. We analyzed accelerometry data for participants providing at least 6 hours of wear time for at least 3 weekdays and 1 weekend day. We also analyzed pre/post changes in fruit and vegetable and fat and fiber intake, self-efficacy, health status, quality of life and depression. Our primary analyses were based on all participants completing baseline and follow-up measurements regardless of their intervention attendance. We performed sensitivity analyses including all participants who we had an intervention weights for at least the first six week intervention period carrying the last weight forward and also one including all participants assuming that participants who did not have follow-up measurements remained at their baseline weight. The study was powered to detect a 4.4 lb. weight loss for 50 participants.
Results and Discussion
Results
Study population
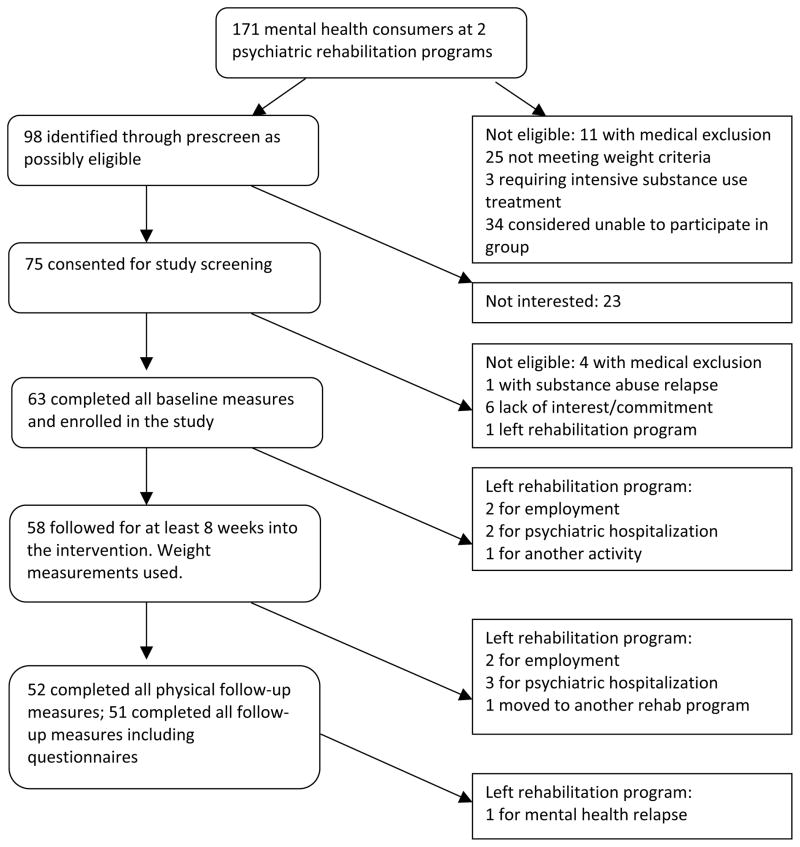
Of the 171 mental health consumers attending the two psychiatric rehabilitation programs, 98 were thought to be potentially eligible for the study after discussion with program staff (Figure 1). Of these, 75 consumers showed interest in the study and signed informed consent to be screened formally for eligibility and for baseline data collection. Sixty-three of these completed all baseline data collection and enrolled. The suburban site participants were younger and were predominantly White (Table 1). Of the 31 African Americans, 51% were women. About half had at least a high school education, and most had never been married. Mental health diagnoses reflected a population with serious mental illness in psychiatric rehabilitation programs with over half having a schizophrenia spectrum disorder. Participants on average took at least two psychotropic medications, with most taking an atypical antipsychotic. The mean RBANS total score reflected a population with cognitive impairment, as populations with schizophrenia have been shown to have an average score of about 70 (39). RBANS mean scores in the general population are 95. The CES-D and Symptom Checklist-90 General Symptom Index (GSI) showed high levels of mental health symptoms. A CES-D score of 16 or higher is associated with clinical depression. The SCL-90 GSI in a non-psychiatric population has a mean of 0.31 (41). Over half of participants smoked tobacco, one third had hypertension and a fifth had diabetes mellitus.
Figure 1.
Participant flow in weight loss intervention in psychiatric rehabilitation centers
Table 1.
Baseline characteristics of participants enrolled in weight loss intervention at psychiatric rehabilitation centers
| Urban site (n=32) | Suburban site (n=31) | All participants (n=63) | |
|---|---|---|---|
| Mean age in years (SD) | 45.7 (11.6) | 41.7 (11.3) | 43.7(10.8) |
| Women (%) | 59 | 52 | 56 |
| Race (%) | |||
| White | 16 | 84 | 49 |
| African-American | 84 | 13 | 49 |
| Asian | 0 | 3 | 2 |
| Education (%) | |||
| Not completing high school | 50 | 42 | 46 |
| High school graduate | 47 | 52 | 49 |
| College graduate | 3 | 6 | 5 |
| Marital Status (%) | |||
| Single-never married | 69 | 68 | 68 |
| Married | 0 | 6 | 3 |
| Previously married | 31 | 26 | 29 |
| Mental health diagnoses (%) | |||
| Schizophrenia | 42 | 26 | 34 |
| Schizoaffective | 9 | 32 | 20 |
| Other psychotic | 3 | 6 | 4 |
| Bipolar disorder | 18 | 29 | 23 |
| Depression | 24 | 16 | 20 |
| Anxiety disorder | 3 | 3 | 3 |
| Mental retardation (2° dx.) | 36 | 10 | 23 |
| Alcohol/substance abuse (2° dx) | 21 | 48 | 34 |
| Psychotropic medications (%) | |||
| Any antipsychotic | 85 | 99 | 88 |
| Atypical antipsychotic | 58 | 84 | 70 |
| >1 Atypical antipsychotic | 13 | 21 | 17 |
| Lithium/mood stabilizers | 27 | 39 | 33 |
| Antidepressant | 36 | 68 | 52 |
| Mean number psychotropics (SD) | 2.1 (1.1) | 2.8 (1.2) | 2.4 (1.2) |
| Mean RBANS (SD) | 59.9 (12.1) | 68.7 (14.7) | 64.2(14.2) |
| RBANS <70 (%) | 80 | 57 | 69 |
| Mean CES-Depression (SD) | 19.7 (11.2) | 24.3 (12.2) | 21.8 (12.3) |
| SCL-90 GSI | 0.9 (0.8) | 1.0 (0.7) | 1.0 (0.8) |
| Current smoker (%) | 55 | 63 | 59 |
| Hypertension (%) | 36 | 32 | 34 |
| Antihypertensive medication (%) | 34 | 23 | 29 |
| Diabetes mellitus (%) | 27 | 13 | 20 |
| Diabetes medication (%) | 21 | 6 | 14 |
| Cholesterol-lowering medication (%) | 19 | 10 | 14 |
Intervention attendance
Average attendance across all participants and all weight management classes was 87 percent for days that participants were present at the rehabilitation center and 70 percent overall, including days that participants did not come to the centers. Similarly, average physical activity class attendance was 74 percent for days participants were at the center and 59 percent overall. To be counted as attending physical activity class, participants had to exercise for at least 20 minutes of the 45 minute class.
Retention
Of the 63 participants, we obtained 6 month follow-up physical measurements on 52 and all measurements on 51 individuals, or 81 percent of our sample (Figure). The 11 participants without follow-up data left the rehabilitation centers before the end of the study. Five left for psychiatric hospitalizations, four left for employment, one moved to another day program and one moved to another daytime activity. One participant had a mental health relapse and left the center before we were able to complete all study follow-up. However, 58 participants completed at least eight weeks of the intervention, and we did record intervention weights for those participants.
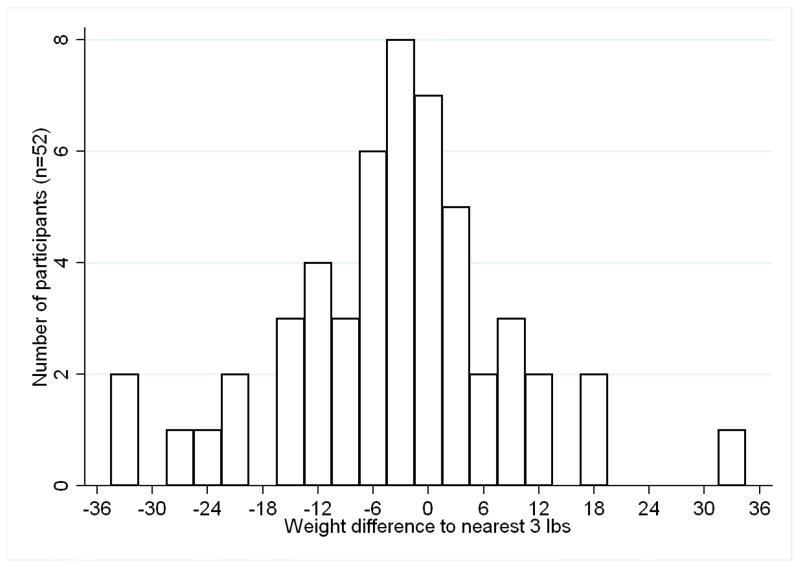
Weight loss
Differences in weight were achieved after the intervention (Table 2, Figure 2). For the 52 participants completing baseline and follow-up weights, mean weight loss was 4.5 pounds. On average, participants lost 1.9% of body weight. Figure 2 shows the distribution in weight loss with participants’ weight change in three pound increments. Mean weight loss was 11.6 pounds or 6.1 percent of body weight for the 52 percent of participants (n=27) who lost at least three pounds. Thirty-one percent of participants (n=16) lost at least 4 percent of their body weight. Body mass index decreased by 0.8 kg/m2. We found a non-significant relationship between higher initial BMI and greater weight loss (p<0.23). In sensitivity analyses, participants attending at least 8 weeks of the intervention (n=58 had mean weight loss of 4.8 pounds (S.D. 12.1) (p<0.01) (data not shown). Analyses including all enrolled participants assuming those not completing follow-up measurements remained at their baseline weight showed mean weight loss of 3.4 pounds (S.D. 11.3), (P<0.01).
Table 2.
Physical health measures before and after weight loss intervention in psychiatric rehabilitation centers (N=52)
| Baseline Mean (SD) | Follow-up Mean (SD) | Change Mean (SD) | P-value | |
|---|---|---|---|---|
| Weight (lbs) | 210.9 (43.9) | 206.4 (42.3) | −4.5 (12.8) | 0.014 |
| Body Mass Index (kg/m2) | 34.4 (7.4) | 33.6 (7.2) | −0.8 (2.1) | 0.008 |
| Waist circumference (cm) | 113.3 (15.1) | 110. (15.9) | −3.1 (5.6) | 0.0005 |
| 6 min fitness walk (ft) | 1358 (297) | 1463 (311) | +104 (180) | 0.0002 |
| Min/day moderate physical activity in bouts of >=10 min.a | 4.3 (10.3) | 12.7 (44.0) | +8.3 (35.3) | 0.04 |
| Cholesterol (mmol/L) | 4.63 (0.95) | 4.45 (0.96) | −0.18 (0.80) | 0.11 |
| LDL (mmol/L) | 2.70 (0.82) | 2.51 (0.76) | −0.18 (0.76) | 0.09 |
| HDL (mmol/L) | 1.18 (0.46) | 1.18 (0.44) | 0.00 (0.19) | 0.92 |
| Triglycerides (mmol/L) | 1.67 (0.94) | 1.72(1.11) | 0.05 (0.82) | 0.65 |
| Glucose (mmol/L) | 6.04 (2.98) | 5.87 (2.36) | −0.17 (0.76) | 0.41 |
| Insulin (pmol/L) | 115.83 (88.45) | 100.09 (95.57) | −14.02 (83.845) | 0.24 |
| HOMA-IR | 4.09 (3.56) | 3.81 (4.66) | −0.28 (3.94) | 0.61 |
| Systolic BP (mmHg) | 117.1 (11.7) | 117.3 (12.7) | 0.2 (11.9) | 0.91 |
| Diastolic BP (mmHg) | 69.8 (9.9) | 72.0 (11.3) | 2.2 (9.3) | 0.10 |
N=29 for those providing at least 6 hours of accelerometer data for 3 week days and 1 weekend day.
Figure 2.
Distribution of weight change after weight loss intervention in psychiatric rehabilitation centers
Secondary Outcomes
Waist circumference decreased in 75 percent of participants with mean waist circumference change of 3.1 cm overall. Sixty-nine percent of participants increased the distance they walked in 6 minutes, with mean increase in 6 minute walk of 8 percent overall. Daily minutes of moderate physical activity in bouts of at least ten minutes increased by 8 minutes, or the equivalent of 56 minutes per week.
Depression scores on the CES-D improved from baseline to follow-up (Table 3). Sixty-two percent of participants had a CES-D score of 16 or higher at baseline consistent with clinical depression, and 52% had a CES-D score of 16 or higher at follow-up (p<0.06) (data not shown). Health status scores showed non-significant decreases for General Health and Physical Functioning subscales and a non-significant increase for the Social Functioning subscale. Lehman Quality of Life scores showed no change overall.
Table 3.
Self-reported measures before and after weight loss intervention in psychiatric rehabilitation centers (N=51)
| Baseline Mean (SD) | Follow-up Mean (SD) | Change Mean (SD) | P-value | |
|---|---|---|---|---|
| General self-efficacy | 29.6 (6.0) | 29.7 (7.0) | 0.1 (5.2) | 0.46 |
| Eating self-efficacy | ||||
| Sticking to it | 3.9 (1.0) | 3.9 (1.0) | −0.05 (1.33) | 0.38 |
| Reducing calories | 4.1 (0.8) | 4.0 (0.9) | −0.1 (0.9) | 0.19 |
| Reducing salt | 3.2 (1.4) | 3.4 (1.6) | 0.2 (1.6) | 0.20 |
| Reducing fat | 4.0 (0.8) | 3.9 (1.0) | −0.2 (1.1) | 0.15 |
| Increasing fruit and vegetables | 4.3 (0.9) | 4.4 (1.0) | 0.1 (1.1) | 0.38 |
| Exercise self-efficacy | ||||
| Sticking to it | 3.5 (1.1) | 3.2 (1.2) | −0.3 (1.1) | 0.05 |
| Making time for exercise | 4.2 (1.0) | 4.0 (1.1) | −0.2 (1.1) | 0.12 |
| Binge Eating | 8.4 (6.5) | 7.1 (5.6) | −1.3 (1.1) | 0.27 |
| CES-Depression | 21.3 (12.5) | 18.4 (13.4) | −2.9 (7.9) | <0.01 |
| Health Status SF-36 | ||||
| General Health | 60.8 (23.9) | 59.9 (28.0) | −1.0 (19.0) | 0.36 |
| Physical functioning | 79.1 (19.0) | 78.2 (23.9) | −1.0 (17.9) | 0.35 |
| Social functioning | 73.3 (24.9) | 78 (25.8) | 4.8 (22.1) | 0.07 |
| Quality of life | ||||
| General life satisfaction | 4.9 (1.5) | 4.8 (1.7) | −0.1 (1.3) | 0.35 |
| Daily activities | 4.8 (1.7) | 5.1 (1.3) | 0.4 (2.0) | 0.10 |
| Social relationships | 4.6 (1.6) | 4.9 (1.6) | 0.3 (1.7) | 0.09 |
| Health | 4.6 (1.6) | 4.8 (1.7) | 0.2 (1.8) | 0.17 |
| Daily reported intake | ||||
| Total fat (g) | 94.4 (28.2) | 88.7 (24.2) | −5.7 (23.3) | 0.05 |
| Saturated fat (g) | 28.0 (10.1) | 25.9 (8.8) | −2.1 (8.6) | 0.05 |
| Percent fat | 35.0 (7.0) | 33.5 (6.0) | −1.4 (5.8) | 0.05 |
| Dietary cholesterol (mg) | 291.7 (92.7) | 273.1 (84.4) | −18.6 (75.8) | 0.05 |
| Fruit and vegetable servings | 4.0 (2.6) | 4.5 (2.7) | 0.5 (2.1) | 0.04 |
| Dietary fiber (g) | 11.9 (5.5) | 13.1 (6.0) | 1.1 (4.5) | 0.04 |
Other Measures
Reported daily intake of fat and dietary cholesterol decreased from before to after the intervention while reported fruit and vegetable servings and dietary fiber increased. The General Self-Efficacy and Eating Habits Self-Efficacy scales showed no change. The Sticking to Exercise scale of Exercise Self-Efficacy showed a small negative change. The modified Binge Eating Scale showed no significant change in mean score, although 24.5% at baseline had at least moderate binge eating and only 16.3% at follow-up had moderate binge eating (p<0.007). Total and LDL cholesterol, glucose and insulin levels and HOMA-IR showed non-significant decreases, while triglycerides and diastolic blood pressure showed non-significant small increases.
Possible Weight Loss Correlates
We did not find differences in weight change by race, gender, psychiatric diagnosis or by cognitive level (data not shown). In addition, we did not detect differences in weight change between participants receiving different types of psychotropic medications, including atypical antipsychotics and olanzapine. Although depression level improved after the intervention, change in weight was not related to change in depression levels. We also did not find relationships between self-efficacy constructs, attendance or reported dietary intake and weight change. In addition, eating habit self-efficacy was not related to reported diet or dietary change. Exercise self-efficacy was not associated with exercise attendance.
Discussion
This six month pilot study documented the feasibility and preliminary efficacy of a behavioral weight loss intervention in adults with serious mental illness in a psychiatric rehabilitation setting. Participants decreased their weight and their waist circumference, and they attained modest increases in physical fitness as measured by six-minute walk and in moderate intensity physical activity. Depression scores decreased as did the percentage of participants reporting at least moderate binge eating behavior after the intervention. The degree of weight loss attained should be associated with decreased blood pressure and cardiovascular disease risk (42, 43).
Rehabilitation center attendees showed interest in the weight loss intervention. Almost two-thirds of all potentially eligible consumers (and over one third of all consumers at the programs) were recruited, completed all screening and baseline measures and enrolled in the study. The weight loss intervention was well accepted by subjects as evidenced by their participation in weight management and exercise classes, and retention was high.
We saw some objective evidence that participants made lifestyle changes during the study. The distance traveled during the 6 minute walk test, a proxy for physical fitness, and physical activity measured by accelerometry both increased. These are consistent with physical activity class participation. Participants also reported less fat and dietary cholesterol intake and increased fruit, vegetable and dietary fiber daily.
This study adds to the growing body of evidence from small studies showing that persons with serious mental illness can benefit from short-term behavioral weight loss interventions (3). Most studies of weight loss interventions for those with chronic mental illness emphasize dietary change; fewer incorporate group physical activity, and of those, most consist of only weekly physical activity (13–17, 20). A recent study from Israel in long-term psychiatric inpatients with schizophrenia incorporated exercise five times per week and showed a 1.8 (SD 2.3) unit decrease in BMI at 3 months for the intervention compared to a 0.34 (SD 4.3) decrease in the comparison group (44). Studies focusing on nutritional interventions showed differences in weight in intervention groups compared to controls (loss or reduced gain) between 2 and 11 pounds. Littrell et. al. studied persons with schizophrenia or schizoaffective disorder taking olanzapine and showed 9 pounds of weight gain in controls and no weight loss in the active group after a 4 month educational intervention (21). Brar, et. al. showed a 1kg decrease in weight compared to control after a 14 week behavioral intervention in patients with schizophrenia or schizoaffective disorder switched from olanzapine to risperidone (19). In a 16 week behavioral intervention in 17 patients with schizophrenia taking only one atypical antipsychotic, Weber et.al. reported a 5.4 pound weight loss in the intervention group compared to 1.3 pounds loss in controls (23). A study by McKibbin of a 6-month behavioral weight loss intervention for persons with schizophrenia and diabetes showed a 5 pound weight loss in the intervention group and a 6 pound weight gain in controls (18).
Studies incorporating some physical activity component in the intervention, mainly once to twice weekly walking groups, with or without a control group, also showed a similar range of weight loss. Richardson et.al. performed a pre/post study including persons with a range of serious mental illnesses in an 18 week intervention (16). They reported only one-third of participants completed the study; of these, mean weight loss was 5.3 pounds. Ball et. al. studied persons taking a specific range of olanzapine dose and showed no overall difference in weight loss between intervention and control groups with 69% follow-up, although men in the intervention group did lose weight (13). Menza et.al. studied persons with schizophrenia or schizoaffective disorder taking atypical antipsychotics and showed a 6.6 pound weight loss in the intervention group and 7.0 pound gain in the control group with 65% follow-up at 12 months (15).
Most of the current literature highlighted above focuses on particular diagnoses and/or medications, reports results only for intervention completers or does not include a strong physical activity intervention component. Moreover, many do not follow guidelines for reporting randomized or non-randomized evaluations of behavioral interventions (24, 45).
Our study is one of the first to integrate weight management and physical activity classes into a psychiatric rehabilitation environment. The study population was ethnically diverse with a high burden of mental health symptoms and cognitive deficits. Unlike most previous weight loss studies for persons with serious mental illness, we included all interested and eligible rehabilitation center attendees, and did not limit to participants with only on one mental health diagnosis or class of antipsychotic medication.
In this lifestyle intervention study without a control group, weight changed the expected amount from before to after the intervention. Because adults typically gain weight over time, we may have expected net weight loss to be even greater in a controlled trial (18, 44). Average weight gain in populations with serious mental illness has not been reported but is likely higher than the general population. This information would be helpful in interpreting intervention results. We were also limited by sample size and by our lack of control group in detecting changes in instrument measures that may be related to weight loss. Although participants reported some dietary changes, we did not find these to be related to weight change. However, the dietary screeners capture less information about high-calorie beverages, which appeared to be one large calorie source that our participants decreased. We also did not find a statistical relationship between exercise participation and weight loss, although it is possible that the regular, moderate intensity physical activity in a previously sedentary group contributed to the intervention’s success. On-site physical activity classes may be particularly beneficial for a chronically mentally ill population as only participation is required, whereas dietary changes which rely on individual behavior may be more challenging.
Other measures that we thought might be either on the pathway to weight loss, such as self-efficacy, or associated with weight loss, like quality of life or health status, did not change after the intervention. Self-efficacy can decrease when individuals realize how hard it is to make sustained change in behaviors. Although most of these measures have been used previously in persons with serious mental illness, it is possible that our participants’ cognitive impairments may have limited the utility of some measures. At the same time, while not captured on standard instruments, we observed that participants often helped each other motivate to join in the exercise classes and believe that this type of social support made important contributions to intervention success.
Strengths of this pilot study include engaging an understudied, vulnerable population in a behavioral weight loss intervention. We obtained high rates of study enrollment, intervention participation and follow-up data collection. The small sample size and pre-post test design were appropriate for a pilot study, but the results need confirmation in larger, controlled studies. In addition, we had limited power for subgroup analyses.
This study has limitations. In addition to the lack of a comparison group, we did not specifically assess how well the participants understood the intervention or components. We also did not measure nutrition, weight management or physical activity knowledge. Moreover, while the participant selection process for the intervention would likely generalize to other psychiatric rehabilitation settings where staff know consumers well and work with them closely, it may not be best for mental health settings where this is not the case. In addition, we were not able to measure the effect of educating the kitchen staff to change the food environment on individual patient outcomes.
While these study results are relevant particularly for persons with chronic mental illness, the success of this pilot weight loss intervention has broader implications for populations with obesity in institutional settings. By appropriately tailoring the intervention and holding sessions at a time and familiar place where participants already attend regularly, we minimized barriers to group participation in a population with transportation, health and social barriers that make them generally poor candidates for traditional behavioral weight loss programs. Including exercise classes provided important opportunities for participants unlikely to reach moderate physical activity goals on their own. Day programs for elderly or disabled adults, prisons, schools or nursing homes may all be settings where similar programs to decrease obesity could be tested and implemented.
In summary, we tailored a behavioral weight loss intervention to the needs of persons with serious mental illness in psychiatric rehabilitation programs. Over the course of six-months, this intervention appeared feasible and effective in participants with a broad range of psychiatric and cognitive impairments. Randomized controlled studies of comprehensive weight loss interventions incorporating weight management and physical activity for persons with serious mental illness are needed to provide evidence of successful interventions that can be disseminated to ameliorate the health burden of obesity in this vulnerable population.
Acknowledgments
Funding was received from NIMH Grant R34070368.
Footnotes
Conflict of Interest
The authors declare no conflict of interest. Drs. Daumit, Crum and Appel have received NIH funding for their work. This study was funded by the NIMH, although the NIMH was not involved in the study design, data collection or analysis or decision to publish.
References
- 1.Colton CP, Ronald W, Manderscheid P. Congruencies in Increased Mortality Rates, Years of Potential Life Lost, and Causes of Death Among Public Mental Health Clients in Eight States. Preventing Chronic Disease. 2006 Apr;3(2):1–14. [PMC free article] [PubMed] [Google Scholar]
- 2.Brown S. Excess mortality of schizophrenia. A meta-analysis. Br J Psychiatry. 1997;171:502–8. doi: 10.1192/bjp.171.6.502. [DOI] [PubMed] [Google Scholar]
- 3.Allison D, Newcomer JW, Dunn Andrea L, PhD, Blumenthal James A, PhD, Fabricatore Anthony N, Gail P, Daumit L, MD, Cope Mark B, PhD, Riley William T, PhD, Betty Vreeland M, APRN, Hibbeln Joseph R, MD, Alpert Jonathan E., MD, PhD Obesity Among Those with Mental Disorders: A National Institute of Mental Health Meeting Report. [review and special article] Am J Prev Med. 2009 Apr;36(4):341–50. doi: 10.1016/j.amepre.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 4.Daumit GL, Goldberg RW, Anthony C, Dickerson F, Brown CH, Kreyenbuhl J, et al. Physical activity patterns in adults with serious mental illness. J Nerv Ment Dis. 2005 Oct;193(10):641–6. doi: 10.1097/01.nmd.0000180737.85895.60. [DOI] [PubMed] [Google Scholar]
- 5.Lindamer LA, McKibbin C, Norman GJ, Jordan L, Harrison K, Abeyesinhe S, et al. Assessment of physical activity in middle-aged and older adults with schizophrenia. Schizophr Res. 2008 Sep;104(1–3):294–301. doi: 10.1016/j.schres.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amani R. Is dietary pattern of schizophrenia patients different from healthy subjects? BMC Psychiatry. 2007;7:15. doi: 10.1186/1471-244X-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCreadie RG. Diet, smoking and cardiovascular risk in people with schizophrenia: descriptive study. Br J Psychiatry. 2003 Dec;183:534–9. doi: 10.1192/bjp.183.6.534. [DOI] [PubMed] [Google Scholar]
- 8.Strassnig M, Brar JS, Ganguli R. Nutritional assessment of patients with schizophrenia: a preliminary study. Schizophr Bull. 2003;29(2):393–7. doi: 10.1093/oxfordjournals.schbul.a007013. [DOI] [PubMed] [Google Scholar]
- 9.Sachs GS, Guille C. Weight gain associated with use of psychotropic medications. J Clin Psychiatry. 1999;60(Suppl 21):16–9. [PubMed] [Google Scholar]
- 10.Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005 Sep 22;353(12):1209–23. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 11.McIntyre RS, Mancini DA, Basile VS. Mechanisms of antipsychotic-induced weight gain. J Clin Psychiatry. 2001;62 (Suppl 23):23–29. [PubMed] [Google Scholar]
- 12.Mueser KT, McGurk SR. Schizophrenia. Lancet. 2004 Jun 19;363(9426):2063–72. doi: 10.1016/S0140-6736(04)16458-1. [DOI] [PubMed] [Google Scholar]
- 13.Ball MP, Coons VB, Buchanan RW. A program for treating olanzapine-related weight gain. Psychiatr Serv. 2001;52(7):967–9. doi: 10.1176/appi.ps.52.7.967. [DOI] [PubMed] [Google Scholar]
- 14.Kalarchian MA, Marcus MD, Levine MD, Haas GL, Greeno CG, Weissfeld LA, et al. Behavioral treatment of obesity in patients taking antipsychotic medications. J Clin Psychiatry. 2005 Aug;66(8):1058–63. doi: 10.4088/jcp.v66n0815. [DOI] [PubMed] [Google Scholar]
- 15.Menza M, Vreeland B, Minsky S, Gara M, Radler DR, Sakowitz M. Managing atypical antipsychotic-associated weight gain: 12–month data on a multimodal weight control program. J Clin Psychiatry. 2004 Apr;65(4):471–7. [PubMed] [Google Scholar]
- 16.Richardson CR, Avripas SA, Neal DL, Marcus SM. Increasing lifestyle physical activity in patients with depression or other serious mental illness. J Psychiatr Pract. 2005 Nov;11(6):379–88. doi: 10.1097/00131746-200511000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Vreeland B, Minsky S, Menza M, Rigassio Radler D, Roemheld-Hamm B, Stern R. A program for managing weight gain associated with atypical antipsychotics. Psychiatr Serv. 2003 Aug;54(8):1155–7. doi: 10.1176/appi.ps.54.8.1155. [DOI] [PubMed] [Google Scholar]
- 18.McKibbin CL, Patterson TL, Norman G, Patrick K, Jin H, Roesch S, et al. A lifestyle intervention for older schizophrenia patients with diabetes mellitus: A randomized controlled trial. Schizophr Res. 2006 Sep;86(1–3):36–44. doi: 10.1016/j.schres.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Brar JS, Ganguli R, Pandina G, Turkoz I, Berry S, Mahmoud R. Effects of Behavioral Therapy on Weight Loss in Overweight and Obese Patients with Schizophrenia or Schizpaffective Disorder. J Clin Psychiatry. 2005;66:205–12. doi: 10.4088/jcp.v66n0208. [DOI] [PubMed] [Google Scholar]
- 20.Chen CK, Chen YC, Huang YS. Effects of a 10-week weight control program on obese patients with schizophrenia or schizoaffective disorder: a 12-month follow up. Psychiatry Clin Neurosci. 2009 Feb;63(1):17–22. doi: 10.1111/j.1440-1819.2008.01886.x. [DOI] [PubMed] [Google Scholar]
- 21.Littrell KH, Hilligoss NM, Kirshner CD, Petty RG, Johnson CG. The effects of an educational intervention on antipsychotic-induced weight gain. J Nurs Scholarsh. 2003;35(3):237–41. doi: 10.1111/j.1547-5069.2003.00237.x. [DOI] [PubMed] [Google Scholar]
- 22.Lindenmayer JP, Khan A, Wance D, Maccabee N, Kaushik S, Kaushik S. Outcome evaluation of a structured educational wellness program in patients with serious mental illness. J Clin Psychiatry. 2009 Sep 22; doi: 10.4088/JCP.08m04740yel. [DOI] [PubMed] [Google Scholar]
- 23.Weber M, Wyne K. A cognitive/behavioral group intervention for weight loss in patients treated with atypical antipsychotics. Schizophr Res. 2006 Mar;83(1):95–101. doi: 10.1016/j.schres.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Des Jarlais DC, Lyles C, Crepaz N. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. m J Public Health. 2004 Mar;94(3):361–6. doi: 10.2105/ajph.94.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Appel L, Champagne C, Harsha D, Cooper LS, Obarzanek E, Elmer PJ, et al. Effects of Comprehensive Lifestyle Modification on Blood Pressure Control: Main results of the PREMIER Clinical Trial. Writing Group of the PREMIER Collaborative Research Group. JAMA. 2003;289:2083–93. doi: 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- 26.White WB, Anwar YA. Evaluation of the overall efficacy of the Omron office digital blood pressure HEM-907 monitor in adults. Blood Press Monit. 2001 Apr;6(2):107–10. doi: 10.1097/00126097-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Rowlands AV, Thomas PW, Eston RG, Topping R. Validation of the RT3 triaxial accelerometer for the assessment of physical activity. Med Sci Sports Exerc. 2004 Mar;36(3):518–24. doi: 10.1249/01.mss.0000117158.14542.e7. [DOI] [PubMed] [Google Scholar]
- 28.Larsson UE, Reynisdottir S. The six-minute walk test in outpatients with obesity: reproducibility and known group validity. Physiother Res Int. 2008 Jun;13(2):84–93. doi: 10.1002/pri.398. [DOI] [PubMed] [Google Scholar]
- 29.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 30.Russo J, Trujillo CA, Wingerson D, Decker K, Ries R, Wetzler H, et al. The MOS 36-Item Short Form Health Survey: reliability, validity, and preliminary findings in schizophrenic outpatients. Med Care. 1998 May;36(5):752–6. doi: 10.1097/00005650-199805000-00015. [DOI] [PubMed] [Google Scholar]
- 31.Lehman AF. Measurement of quality of life among persons with severe and persistent mental disorders. Soc Psychiatry Psychiatr Epidemiol. 1996;31:78–88. doi: 10.1007/BF00801903. [DOI] [PubMed] [Google Scholar]
- 32.Radloff L. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 33.Block G, Clifford C, Naughton MD, Henderson M, McAdams M. A brief dietary screen for high fat intake. Journal of Nutrition Education. 1989;21(5):199–207. [Google Scholar]
- 34.Block G, Gillespie C, Rosenbaum EH, Jenson C. A rapid food screener to assess fat and fruit and vegetable intake. Am J Prev Med. 2000 May;18(4):284–8. doi: 10.1016/s0749-3797(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 35.Schwarzer R. Measurement of Perceived Self-Efficacy: Psychometric Scales for cross-cultural research. Berlin: Berlin, Forschung and der Freien Universitat; 1993. [Google Scholar]
- 36.Sallis JF, Pinski RB, Grossman RM, Patterson TL, Nader PR. The development of self efficacy scales for health-related diet and exercise behaviors. Health Education Research. 1988;3(3):283–92. [Google Scholar]
- 37.Gormally J, Black S, Daston S, Rardin D. The assessment of binge eating severity among obese persons. Addict Behav. 1982;7(1):47–55. doi: 10.1016/0306-4603(82)90024-7. [DOI] [PubMed] [Google Scholar]
- 38.Derogatis LR, Lipman RS, Covi L. SCL-90: an outpatient psychiatric rating scale--preliminary report. Psychopharmacol Bull. 1973 Jan;9(1):13–28. [PubMed] [Google Scholar]
- 39.Dickerson F, Boronow JJ, Stallings C, Origoni AE, Cole SK, Yolken RH. Cognitive functioning in schizophrenia and bipolar disorder: comparison of performance on the Repeatable Battery for the Assessment of Neuropsychological Status. Psychiatry Res. 2004 Nov 30;129(1):45–53. doi: 10.1016/j.psychres.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Gold JM, Qeern C, Iannone VN, Buchanan RW. Repeatable battery for the assessment of neuropsychological status as a screening test in schizophrenia I: sensitivity, reliability, and validity. Am J Psychiatry. 1999;156(12):1944–1950. doi: 10.1176/ajp.156.12.1944. [DOI] [PubMed] [Google Scholar]
- 41.Derogatis LR. SCL-90-R Administration, Scoring and Procedures Manual. 3. 1994. [Google Scholar]
- 42.Stamler R, Stamler J, Gosch FC, Civinelli J, Fishman J, McKeever P, et al. Primary prevention of hypertension by nutritional-hygienic means, Final report of a randomized, controlled trial. JAMA. 1989 Oct 6;262(13):1801–7. [PubMed] [Google Scholar]
- 43.Stevens VJ, Obarzanek E, Cook NR, Lee IM, Appel LJ, Smith West D, et al. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med. 2001 Jan 2;134(1):1–11. doi: 10.7326/0003-4819-134-1-200101020-00007. [DOI] [PubMed] [Google Scholar]
- 44.Melamed Y, Stein-Reisner O, Gelkopf M, Levi G, Sivan T, Ilievici G, et al. Multi-modal weight control intervention for people with persistent mental disorders. Psychiatr Rehabil J. 2008 Winter;31(3):194–200. doi: 10.2975/31.3.2008.194.200. [DOI] [PubMed] [Google Scholar]
- 45.Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA. 2001 Apr 18;285(15):1987–1991. doi: 10.1001/jama.285.15.1987. [DOI] [PubMed] [Google Scholar]