Abstract
To test the hypothesis that the antioxidant enzyme superoxide dismutase (SOD) mimetic TEMPOL improves arterial aging, young (Y, 4–6 mo) and old (O, 26–28 mo) male C57BL6 mice received regular or TEMPOL-supplemented (1mM) drinking water for 3 weeks (n=8/group). Aortic superoxide was 65% greater in O (p<0.05 vs. Y), which was normalized by TEMPOL. O had large elastic artery stiffening, as indicated by greater aortic pulse wave velocity (aPWV, 508 ± 22 vs. 418 ± 22 AU), which was associated with increased adventitial collagen I expression (p<0.05 vs. Y). TEMPOL reversed the age-associated increases in aPWV (434 ± 21 AU) and collagen in vivo, and SOD reversed increases in collagen I in adventitial fibroblasts from older rats in vitro. Isolated carotid arteries of O had impaired endothelial function as indicated by reduced acetylcholine-stimulated endothelium-dependent dilation (EDD) (75.6 ± 3.2 vs. 94.5 ± 2.0%) mediated by reduced nitric oxide (NO) bioavailability (L-NAME) associated with decreased endothelial NO synthase (eNOS) expression (p<0.05 vs. Y). TEMPOL restored EDD (94.5 ± 1.4%), NO bioavailability and eNOS in O. Nitrotyrosine and expression of NADPH oxidase were ~100–200% greater, and MnSOD was ~75% lower in O (p<0.05 vs. Y). TEMPOL normalized nitrotyrosine and NADPH oxidase in O, without affecting MnSOD. Aortic pro-inflammatory cytokines were greater in O (p<0.05 vs. Y) and normalized by TEMPOL. Short-term treatment of excessive superoxide with TEMPOL ameliorates large elastic artery stiffening and endothelial dysfunction with aging, and this is associated with normalization of arterial collagen I, eNOS, oxidative stress and inflammation.
Keywords: superoxide dismutase, inflammation, collagen, nitric oxide, glycation endproducts
Introduction
Advancing age is the major risk factor for cardiovascular diseases (CVD) and this is largely attributable to stiffening of the large elastic arteries and the development of vascular endothelial dysfunction (Lakatta 2003; Mitchell et al. 2010). Large elastic artery stiffening with aging is mediated in part by molecular changes to the arterial wall including increased collagen I deposition, reductions in elastin and post-translational modifications of these proteins involving the formation of advanced glycation end-products (AGEs) (Lakatta 2003). Vascular endothelial dysfunction develops with aging as a result of reductions in the nitric oxide (NO) bioavailability, as indicated by impaired NO-mediated endothelium-dependent dilation (Celermajer et al. 1994) (Taddei et al. 1995; d'Uscio et al. 1997). The latter may occur with or without reductions in endothelial NO synthase (eNOS) (Spier et al. 2004; Durrant et al. 2009; Rippe et al. 2010), the enzyme responsible for NO production in the vascular endothelium.
The primary mechanism believed to cause arterial dysfunction with aging is oxidative stress, a state in which bioavailability of reactive oxygen species exceeds the capacity of antioxidant defenses, leading to cellular dysfunction and damage. We recently showed increased superoxide production in aorta of old mice using direct measurement with spin trapping and electronic paramagnetic resonance spectroscopy (Rippe et al. 2010; Sindler et al. 2011) suggesting, along with earlier reports (Hamilton et al. 2001; Francia et al. 2004; Lesniewski et al. 2009; Sindler et al. 2009), that this is a key contributor to oxidative stress in arterial aging. Excessive superoxide may cause vascular aging in part by stimulating inflammation, as characterized by increased expression of pro-inflammatory cytokines (Zou et al. 2006; Csiszar et al. 2008; Ungvari et al. 2010; Lesniewski et al. 2011; Sindler et al. 2011). As such, therapies that reduce superoxide bioavailability have the potential to ameliorate arterial oxidative stress and inflammation, and improve vascular dysfunction with aging.
In the present study, we tested the hypothesis that short-term treatment with TEMPOL, a superoxide dismutase (SOD) mimetic that scavenges superoxide anion (Simonsen et al. 2009), would reduce arterial superoxide bioavailability, decrease large elastic artery stiffness, and improve NO-mediated vascular endothelial function in old mice. We also hypothesized that the improvements in arterial function in old mice would be associated with evidence of reduced oxidative stress and inflammation. Finally, we sought to gain preliminary insight into some of the molecular mechanisms by which TEMPOL treatment may mediate these changes.
Results
Animal characteristics
Animal characteristics of the groups (n=8/group) are shown in Table 1. Body mass, heart mass and carotid artery baseline diameter were greater in the old compared with the young control mice (p<0.05). Ex vivo carotid artery preconstriction to phenylephrine and arterial blood pressure, the latter measured in a separate cohort of animals (n=3–4/group), did not differ with age. TEMPOL treatment had no effects on any of the aforementioned variables in the young or old mice.
Table 1. Animal characteristics.
| YC | OC | YT | OT | |
|---|---|---|---|---|
| Body mass (g) | 28 ± 1 | 34 ± 1* | 29 ± 1 | 33 ± 1* |
| Heart mass (mg) | 146 ± 4 | 184 ± 4* | 143 ± 3 | 194 ± 8* |
| Carotid artery lumen diameter (µm) | 404 ± 5 | 455 ± 8* | 400 ± 4 | 454 ± 4* |
| Carotid artery constriction to phenylephrine | ||||
| Absolute (µm) | 339 ± 6 | 366 ± 7* | 331 ± 12 | 352 ± 10* |
| Relative (%) | 82 ± 1 | 81 ± 1 | 82 ± 2 | 80 ± 2 |
| Blood pressure (mmHg) | ||||
| Systolic | 100 ± 2 | 96 ± 2 | 99 ± 2 | 95 ± 4 |
| Diastolic | 70 ± 3 | 66 ± 4 | 70 ± 3 | 66 ± 4 |
Values are mean ± SEM.
p < 0.05 for OC vs. YC and YT, OT vs. YC and YT. YC, Young control; OC, Old control; YT, Young TEMPOL; OT, Old TEMPOL
TEMPOL treatment decreases arterial superoxide production in old mice
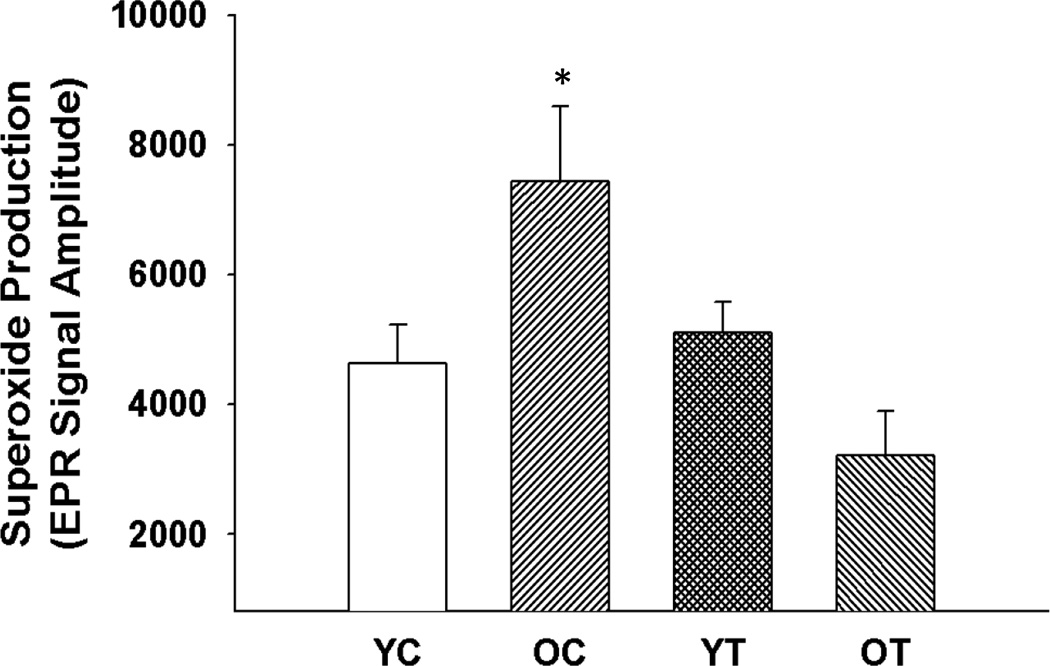
Aortic superoxide production was greater in the old compared with the young control mice (p<0.05, Figure 1). TEMPOL treatment normalized superoxide production in the old mice without affecting the young mice. These results indicate that TEMPOL is effective in lowering the excessive arterial superoxide production associated with aging.
Figure 1. Aortic superoxide production.
Mean electron paramagnetic resonance signal for superoxide in aortic rings from young and old control (YC and OC) and young and old TEMPOL-supplemented (YT and OT) mice. Values are mean ± SEM. (n = 4 – 8 per group) *p < 0.05 for OC vs. YC, YT and OT.
TEMPOL treatment reduces aortic pulse wave velocity in old mice
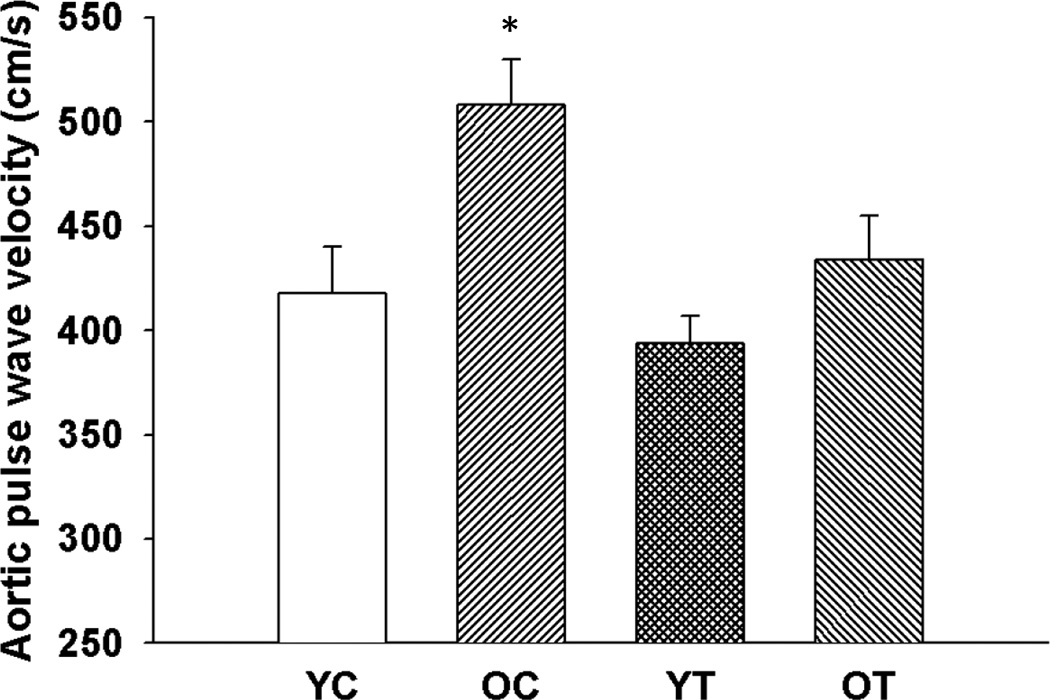
Aortic pulse wave velocity was greater in old compared with young control mice (p<0.05, Figure 2). TEMPOL normalized aortic pulse wave velocity in old mice without affecting young mice. These results indicate that superoxide-lowering therapy with TEMPOL reverses age-associated large elastic artery stiffening.
Figure 2. Large elastic artery stiffness.
Aortic pulse wave velocity in young and old control (YC and OC) and young and old TEMPOL-supplemented (YT and OT) mice. Values are mean ± SEM. (n = 5 – 7 per group) *p < 0.05 for OC vs. YC, YT and OT.
TEMPOL treatment reduces collagen I expression in old mice
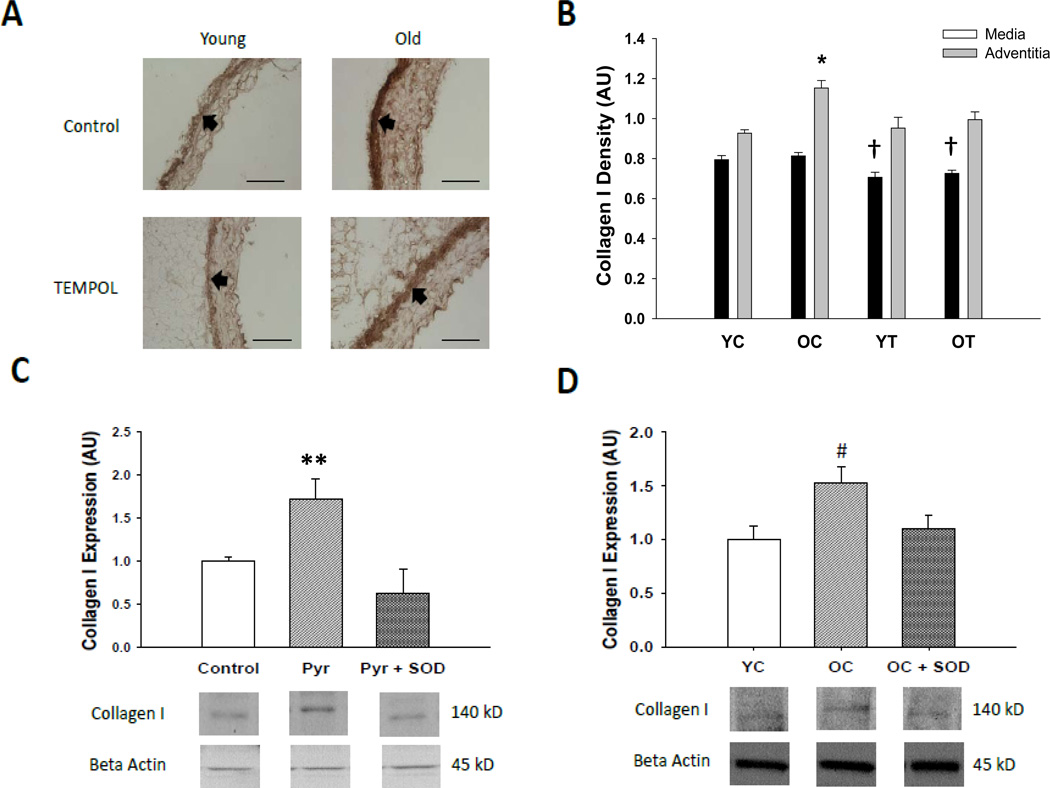
Collagen I was greater (p<0.05) in the adventitial layer of old compared with young control mice with no age-related changes observed within the medial layer (Figure 3A and B). TEMPOL treatment reversed the age-associated increase of collagen I in the adventitia, while also reducing expression in the media of young and old mice (p<0.05).
Figure 3. Collagen I protein expression.
(A) Representative immunohistochemistry images of collagen I in thoracic aortic sections from young and old control (YC and OC) and TEMPOL (YT and OT) supplemented mice. Arrows demarcate the medial-adventitial border; bar = 100 µm. (B) Collagen I protein in thoracic aortic sections of young and old control (YC and OC) and TEMPOL supplemented (YT and OT) mice. (C) Effects of pyrogallol (Pyr, 10 µM), polyethylene glycol conjugated superoxide dismutase (PEG-SOD, 20 U/mL) and the combination on collagen I adventitial fibroblasts from aorta of young rats. (D) Collagen I expression in adventitial fibroblasts from young and old control rats (YC and OC) and effects of PEG-SOD (20 U/mL) in fibroblasts from old rats (OC + SOD). Values are mean ± SEM. (n = 5 – 7 per group) * p < 0.05 for Adventitia OC vs. Adventitia YC, Adventitia YT and Adventitia OT. † p < 0.05 for Media YT vs. Media YC and Media OC, Media OT vs. Media YC and Media OC. ** p < 0.05 for Pyr vs. control, Pry + SOD. # p < 0.05 for OC vs. YC and OC + SOD.
Elastin was lower in the media, but not in the adventitia, of old compared with young control animals (p<0.05, Table 2). TEMPOL reduced elastin in the young animals and further reduced expression in the old mice (p<0.05).
Table 2. Elastin protein and advanced glycation endproducts in aorta.
| YC | OC | YT | OT | |
|---|---|---|---|---|
| Elastin | ||||
| Media (AU) | 0.681 ± 0.04 | 0.632 ± 0.02* | 0.632 ± 0.02† | 0.571 ± 0.01*† |
| Adventitia (AU) | 0.599 ± 0.02 | 0.596 ± 0.02 | 0.587 ± 0.01 | 0.582 ± 0.02 |
| AGEs | ||||
| Media (AU) | 0.618 ± 0.01 | 0.692 ± 0.03* | 0.642 ± 0.03 | 0.713 ± 0.07* |
| Adventitia (AU) | 0.718 ± 0.03 | 0.789 ± 0.01* | 0.645 ± 0.04 | 0.756 ± 0.04* |
Values are mean ± SEM.
p < 0.05 for OC vs. YC and YT, OT vs. YC and YT.
p < 0.05 for YT vs. YC and OC, OT vs. YC and OC. AGEs, advanced glycation end-products; YC, Young control; OC, Old control; YT, Young TEMPOL; OT, Old TEMPOL
Expression of AGEs was greater in the adventitial and medial layers of old compared with young control animals (p<0.05, Table 2). TEMPOL treatment had no effect on AGEs in either age group.
SOD treatment inhibits superoxide-induced collagen deposition in vitro
Because the only effect of TEMPOL on these proteins that could contribute to reductions in stiffness was the reduction in collagen I, additional experiments were performed in cultured rat aortic adventitial fibroblasts to further link SOD treatment to inhibition of superoxide-induced collagen deposition. Pyrogallol, a superoxide generator, increased collagen I protein in fibroblasts obtained from aorta of young rats by ~100% (p<0.05, Figure 3C). Treatment with PEG-SOD completely prevented the induction of collagen by superoxide in these cells. Adventitial fibroblasts cultured from aorta of old rats had greater collagen I protein compared with cells from young animals, and this was reversed with PEG-SOD treatment (p<0.05, Figure 3D). Collectively, these observations indicate that inhibiting superoxide bioavailability in vitro, as occurred in the arteries of the old mice with short-term TEMPOL treatment in vivo, prevents and reverses collagen I deposition in cells known to populate the adventitia, i.e., the arterial layer in which collagen expression was normalized by TEMPOL.
TEMPOL treatment ameliorates vascular endothelial dysfunction in old mice
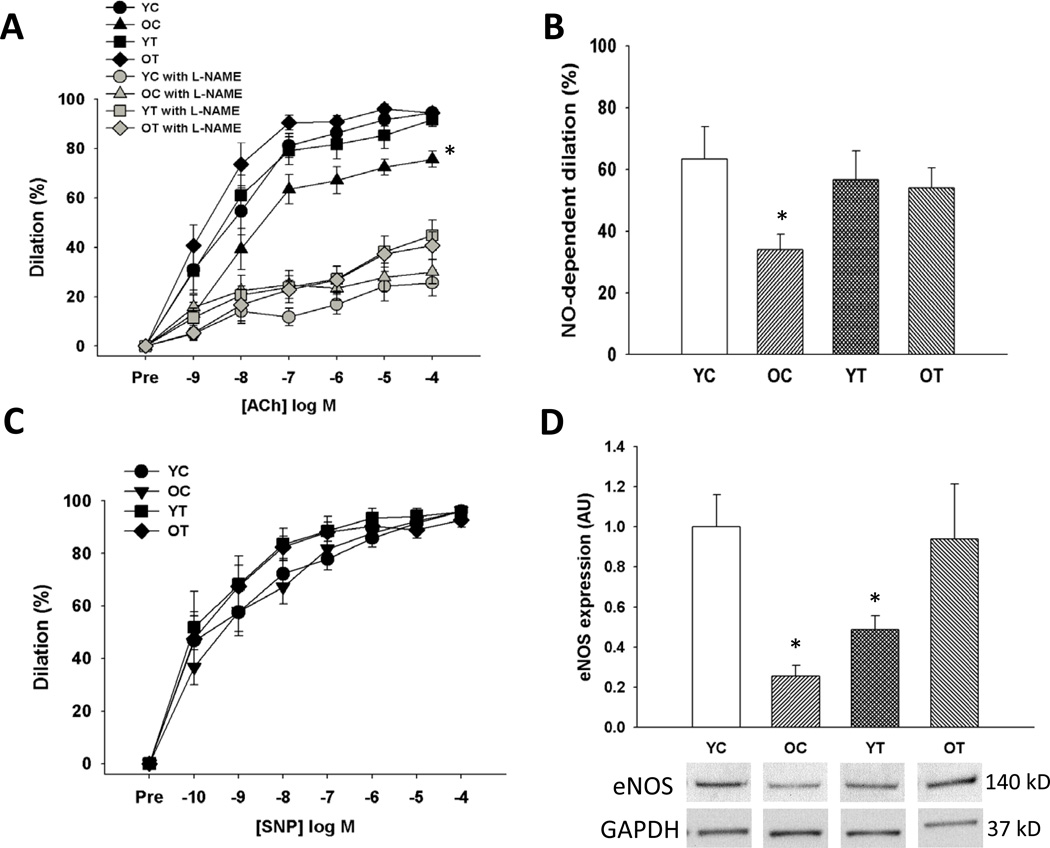
Old animals had lower endothelium-dependent dilation to acetylcholine compared with young control mice (p<0.05) due to a smaller NO influence, as indicated by a smaller reduction in endothelium-dependent dilation in the presence vs. absence of the NO inhibitor N-G-nitro-L-arginine methyl ester (L-NAME) (Figure 4 A and B). TEMPOL improved endothelium-dependent dilation to acetylcholine in old mice by restoring NO-mediated dilation, but had no effect in the young animals (Figure 4 A and B). Endothelium-independent dilation to sodium nitroprusside did not differ among the groups (Figure 4C). Old animals had lower eNOS protein in the aorta compared with young control animals (Figure 4D) and this was normalized by TEMPOL treatment. These data demonstrate that TEMPOL restores NO-mediated endothelium-dependent dilation and eNOS in old mice in the absence of improvements in vascular smooth muscle sensitivity to NO.
Figure 4. Nitric oxide (NO)-mediated endothelium-dependent dilation.
(A) Endothelium-dependent dilation to acetylcholine (ACh) alone and in the presence of the endothelial NO synthase (eNOS) inhibitor N-G-nitro-L-arginine methyl ester (L-NAME) in young and old control (YC and OC) and young and old TEMPOL-supplemented (YT and OT) mice. (B) NO-dependent dilation (max dilationACh – max dilationACh + L-NAME). (C) Endothelium-independent dilation to sodium nitroprusside (SNP). (D) Aortic protein expression of eNOS expressed relative to GAPDH and normalized to YC mean value. Representative western blot images below. Values are mean ± SEM. (n = 7–8 per group) *p < 0.05 for OC vs. YC, YT and OT.
TEMPOL treatment normalizes vascular oxidative stress and NADPH oxidase in old mice
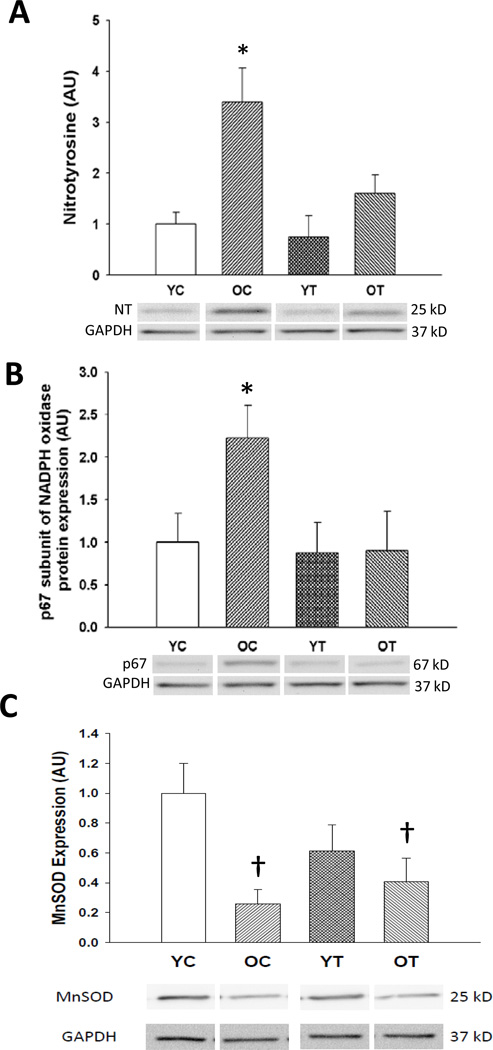
In aortas of old compared with young mice, nitrotyrosine, a marker of oxidative stress, was ~ 200% greater (p<0.05) (Figure 5A), and was associated with an ~100% increase in the p67 subunit of the oxidant enzyme nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase) (p<0.05) (Figure 5B). TEMPOL normalized aortic nitrotyrosine in old mice and reversed the age-associated increases in aortic NADPH oxidase p67 expression. Protein expression of the antioxidant enzyme manganese SOD was decreased in the aorta of old mice (p<0.05), but this was unaffected by TEMPOL treatment (Figure 5C). These results indicate the treatment with TEMPOL reverses vascular oxidative stress in old mice and that this is associated with normalization of expression of the oxidant enzyme NADPH oxidase.
Figure 5. Oxidative stress.
(A) Nitrotyrosine abundance, (B) p67 subunit of nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase (NADPH oxidase), and (C) manganese superoxide dismutase (MnSOD) in aorta of young and old control (YC and OC) and young and old TEMPOL-supplemented (YT and OT) mice. Data are expressed relative to GAPDH and normalized to YC mean value. Representative Western blot images below. Values are mean ± SEM. (n = 4–8 per group) * p < 0.05 for OC vs. YC, YT and OT. † p < 0.05 for OC vs. YC and OT vs. YC.
TEMPOL treatment ameliorates vascular inflammation in old mice
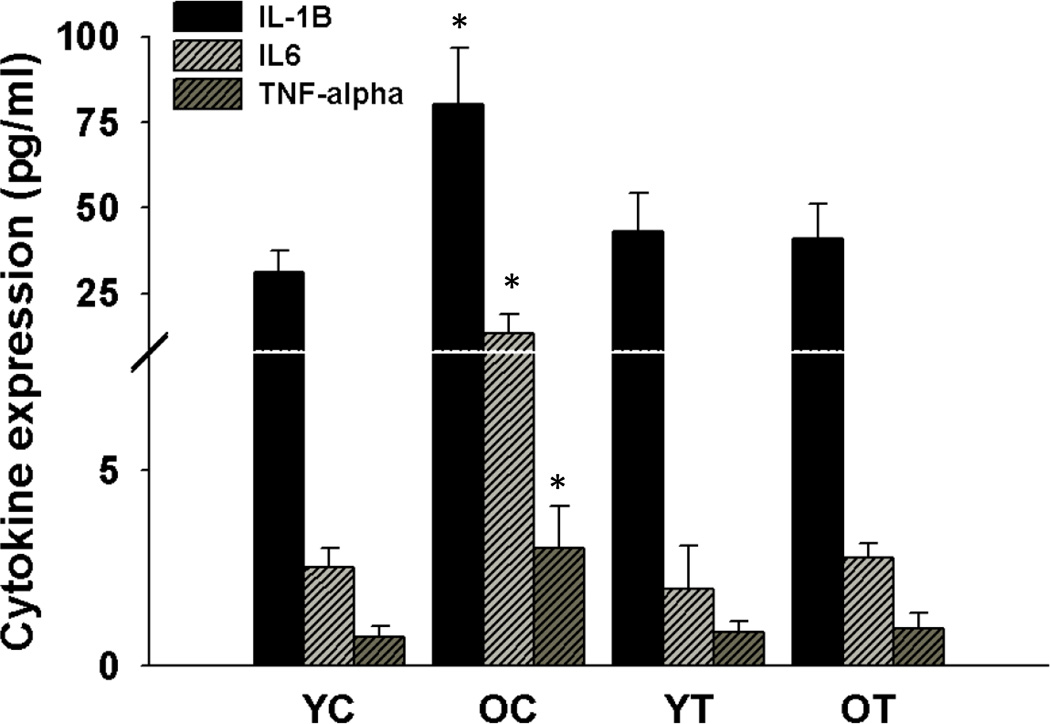
Protein expression of the pro-inflammatory cytokines interleukin-1 (IL-1), interleukin-6 (IL-6) and tumor necrosis factor α (TNFα) was increased in the aortas of old compared with young mice (p<0.05, Figure 6). TEMPOL normalized expression of these inflammatory cytokines in old mice, while having no effect in the young controls. These observations indicate that short-term suppression of superoxide with TEMPOL ameliorates arterial inflammation with aging.
Figure 6. Pro-inflammatory cytokines.
Interleukin-1β (IL-1β), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) in aorta from young and old control (YC and OC) and young and old TEMPOL-supplemented (YT and OT) mice. Values are mean ± SEM. (n = 7–8 per group) * p < 0.05 for OC vs. YC, YT and OT.
Discussion
Advancing age is the major risk factor for CVD due primarily to stiffening of the large elastic arteries and development of vascular endothelial dysfunction. Thus, treatments that can prevent or reverse these changes to arteries with aging hold great promise for preventing age-associated CVD. The novel finding of the present study is that short-term treatment with TEMPOL, an exogenous SOD mimetic, normalizes the age-associated increase in arterial superoxide production measured directly by spin trapping and electron paramagnetic resonance spectroscopy, and ameliorates large elastic artery stiffness and carotid artery endothelial dysfunction in old C57BL6 mice. These preclinical findings suggest that TEMPOL may have translational efficacy as a pharmacological intervention for treatment of arterial aging in humans. Our findings also provide preliminary insight into the mechanisms by which TEMPOL may exert these favorable effects on aging arteries, including inhibition of adventitial collagen I deposition, increasing NO bioavailability and reversing vascular oxidative stress and inflammation.
Large elastic artery stiffness
The present findings are consistent with previous work from our laboratory (Sindler et al. 2011) and others (Reddy et al. 2003) demonstrating increases in aortic pulse wave velocity with aging in mice, reflective of large elastic artery stiffening. Our results extend these earlier observations by demonstrating that short-term treatment with the SOD mimetic TEMPOL completely reverses this age-associated increase in aortic pulse wave velocity. These findings have important implications for aging in humans because elevated aortic pulse wave velocity recently has emerged as a strong independent risk factor for incident CVD in older adults (Mitchell et al. 2010). Thus, the present data provide critical preclinical evidence for assessing the efficacy of TEMPOL for treatment of large elastic artery stiffness in middle-aged and older adults.
Our results also provide insight into some of the mechanisms that may be involved. Arterial blood pressure influences aortic pulse wave velocity (Cavalcante et al. 2011). However, consistent with previous findings on normotensive animals (Schnackenberg & Wilcox 1999; Gaubert et al. 2007; Mariko et al. 2011), we observed no significant differences in blood pressure with aging or with TEMPOL, suggesting that blood pressure did not contribute to the increased aortic pulse wave velocity with aging or with the reductions with TEMPOL treatment in old mice. Rather, these data further suggest that changes to the arterial wall were involved.
Structural modifications to the arterial wall involving increased collagen I deposition, reduced elastin expression and cross-linking of these proteins by AGEs are thought to contribute to age-associated stiffening of large elastic arteries (Lakatta & Levy 2003) (Kass et al. 2001). As we reported recently (Fleenor et al. 2010), expression of collagen I, the major collagen in arteries (Diez 2007), was selectively increased within the arterial adventitia in old compared with young control animals, whereas elastin was reduced specifically in the medial layer with aging (Wang & Lakatta 2002; Csiszar et al. 2007; Fleenor et al. 2010). As observed previously (Qiu et al. 2007), abundance of AGEs was greater in the aortas of older animals and we extended these findings here by showing that this occurs in both the adventitial and medial layers. The only change in these potential mechanisms with TEMPOL that was directionally associated with the destiffening influence observed was a reduction in adventitial collagen I expression. Although consistent with previous observations that TEMPOL reduces elastin expression in vascular smooth muscle cells (Klemm et al. 2011), the TEMPOL-induced decreases in medial elastin found in the present study would, if anything, act to further increase stiffness in the aorta of the old animals, and no effects on AGEs were observed.
Because the results of our in vivo treatment suggested that TEMPOL may have reduced aortic adventitial collagen by reducing superoxide bioavailability, we sought to establish further evidence for this mechanism of action in vitro. We studied fibroblasts given their prevalence in the arterial adventitia and used PEG-SOD to directly scavenge superoxide (Lijnen et al. 2008). As we showed recently (Fleenor et al. 2010), superoxide generation produced by administration of the agent pyrogallol induced an ~100% increase in collagen I of fibroblasts cultured from aorta of young animals. This was completely prevented by administration of PEG-SOD. To determine if superoxide scavenging could reverse age-associated increases in collagen I expression, fibroblasts from aorta of old rats were treated with PEG-SOD and this normalized collagen I expression to levels observed in cells from young animals. Together, the results of these in vitro experiments support the idea that TEMPOL reduced arterial collagen I expression in old mice by inhibiting the actions of superoxide.
Vascular endothelial dysfunction
Consistent with previous reports from our laboratory (Rippe et al. 2010; Sindler et al. 2011), we found that endothelium-dependent dilation was impaired in the carotid arteries of old mice due to a reduction in the NO-mediated dilatory component, and that this was associated with a decrease in eNOS protein expression. The results of the present study extend these earlier findings by demonstrating that treatment of excessive superoxide with TEMPOL completely restores endothelium-dependent dilation in old mice by improving NO bioavailability and that this is associated with normalization of eNOS protein. TEMPOL treatment had no effect on endothelium-independent dilation, indicating that the improvements in endothelium-dependent dilation were mediated by increases in endothelial NO production. The data also are consistent with our previous work showing that acute ex vivo administration of TEMPOL to carotid arteries of old mice restores endothelium-dependent dilation (Durrant et al. 2009; Rippe et al. 2010; Lesniewski et al. 2011; Sindler et al. 2011). Most importantly, these results demonstrate for the first time that treatment of excessive age-associated superoxide may have therapeutic potential to prevent and/or reverse vascular endothelial dysfunction in middle-aged and older humans. This has substantial clinical implications as endothelial dysfunction is considered the primary antecedent to the development of age-related clinical cardiovascular disorders including coronary and peripheral artery disease, hypertension and stroke (Lakatta & Levy 2003).
Oxidative stress and inflammation
Our finding of increased arterial oxidative stress with aging, as indicated by marked increases in aortic nitrotyrosine staining in the old mice, is in agreement with previous reports from our laboratory (Rippe et al. 2010; Lesniewski et al. 2011; Sindler et al. 2011) and others (van der Loo et al. 2000; Csiszar et al. 2002; Yang et al. 2009). Nitrotyrosine is produced by post-translational nitration of tyrosine residues primarily by peroxynitrite, a byproduct of the reaction between superoxide and NO (Radi 2004). As we have reported previously, the greater arterial oxidative stress in our old animals was associated with increased superoxide production, which was, in turn, associated with increased expression of the superoxide-producing enzyme, NADPH oxidase (Durrant et al. 2009; Rippe et al. 2010), and reduced expression of the mitochondrial-expressed antioxidant enzyme, manganese SOD (Sindler et al. 2011). The present findings show that short-term treatment with TEMPOL normalizes aortic nitrotyrosine staining, superoxide and expression of the p67 subunit of NADPH oxidase 2 without influencing manganese SOD in old mice. Overall, these results are consistent with the concept that TEMPOL reverses age-associated arterial oxidative stress via normalization of superoxide bioavailability. Our data also show for the first time that TEMPOL reduces expression of the major superoxide-producing enzyme in vascular tissue, the p67 subunit of NADPH oxidase 2, and this could contribute to the reduction in superoxide concentrations observed.
Excessive superoxide and oxidative stress are believed to stimulate inflammation with aging (Csiszar et al. 2008; Ungvari et al. 2010). Consistent with previous reports by our laboratory (Lesniewski et al. 2011; Sindler et al. 2011) and others(Csiszar et al. 2007; Csiszar et al. 2004; Zou et al. 2006; Ungvari et al. 2007), expression of pro-inflammatory cytokines was increased in aorta of old mice in the present study. Here we extend these earlier observations by showing that 3 weeks of treatment with TEMPOL normalizes aortic pro-inflammatory cytokines in old mice. These results demonstrate novel anti-inflammatory effects of TEMPOL in arteries with aging, and suggest therapeutic potential of this compound for treatment of vascular inflammatory disorders including arterial aging.
Conclusions
Short-term treatment with the SOD mimetic TEMPOL normalized arterial superoxide production and ameliorated large elastic artery stiffness and endothelial dysfunction, while reversing oxidative stress and inflammation in old mice. These preclinical findings provide the experimental basis for translational studies aimed at determining the efficacy of TEMPOL in the prevention and treatment of arterial aging in humans.
Experimental procedures
Animals
Young (4–6 months) and old (26–28 months; ~50% survival) male C57BL6 mice were obtained from the National Institute on Aging rodent colony and were fed normal rodent chow ad libitum. After an acclimation period of 2 weeks, the young and old mice were divided into two subgroups: control animals continued on regular drinking water whereas the treated animals had TEMPOL supplemented at a dose (1mM) used previously in vivo (Schnackenberg & Wilcox 1999), (Yanes et al. 2005). Mice (8/group) were treated for a period of 3 weeks because we recently found that duration of treatment with different pharmacological agent to be effective in lowering superoxide and improving large elastic artery stiffness and EDD in old C57BL6 mice (Sindler et al. 2011). All mice were housed in an animal care facility at the University of Colorado at Boulder on a 12 h:12 h light-dark cycle. All animal procedures conformed to the Guide to the Care and Use of Laboratory Animals (NIH publication n. 85-23, revised 1996) and were approved by the UCB Animal Care and Use Committee.
Arterial superoxide production
Production of superoxide was measured by electron paramagnetic resonance (EPR) spectrometry as previously described (Rippe et al.; Sindler et al. 2011). Two-millimeter aortic rings were incubated for 60 min at 37° C in 200 µl of Krebs-HEPES buffer containing 0.55 mmol/L 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine (Alexis Biochemicals) and analyzed immediately on an MS300 X-band EPR spectrometer (Magnettech, Berlin, Germany).
Aortic pulse wave velocity
Aortic pulse wave velocity was measured as described previously (Kim et al. 2009; Sindler et al. 2011). Mice were anesthetized with 2% isoflurane and placed supine on a heating board with legs secured to ECG electrodes. Aortic velocity was measured with Doppler probes at the transverse aortic arch and abdominal aorta. Pre-ejection time, the time between the R-wave of the ECG to foot of the Doppler signal, was determined for each site. Aortic pulse wave velocity was calculated by dividing the distance between the transverse and abdominal probes by the difference in the thoracic and abdominal pre-ejection times.
Arterial Blood pressure
Systolic and diastolic blood pressure was assessed in a separate cohort of mice (n=3–4/group) using a CODA non-invasive tail-cuff system (Kent Scientific)(Daugherty et al. 2009). Mice were placed in restrainers on a warm pad. After 10 minutes, 5 acclimation cycles followed by 20 data collection cycles of blood pressure measurements were recorded. Blood pressure was acquired on 3 consecutive days and averaged. The inter-day coefficient of variation for systolic and diastolic blood pressure was 7 ± 1 and 11 ± 2 mmHg, respectively.
Immunohistochemistry
Immunohistochemistry was performed as previously described (Fleenor et al. 2010). Briefly, segments of thoracic aorta were excised and frozen in optimal cutting temperature compound (Fisher Scientific) in liquid nitrogen cooled isopentane. Sections (7 µm) were fixed with acetone and washed in Tris Buffer. All slides were stained in one batch with the Dako EnVision+ System-HRP-DAB kit according to the manufacturer’s protocol (Dako). Primary antibodies for collagen type I (1:4000, Millipore), alpha elastin (1:25, Abcam) and AGEs (1:200, GeneTex) were incubated for 1 hour at 4< C. The labeled polymer secondary was applied for 30 minutes and staining was visualized after a 2-minute exposure to diaminobenzidine. Slides were counterstained with hematoxylin to visualize the nuclei that were subsequently dehydrated and cover slipped. Digital photomicrographs were obtained using a Nikon Eclipse TS100 photomicroscope, and quantification was performed with Image-Pro Plus software (Media Cybernetics) as described previously (Fleenor et al. 2010)
Primary adventitial fibroblast cell culture
Male Sprague-Dawley rats (6 and 24 months of age) were obtained from the University of Colorado at Boulder breeding colony. Rats were euthanized with an overdose of carbon dioxide. Adventitial fibroblasts were isolated from the aorta as previously described (Pagano 1997) (Fleenor et al. 2010). To determine the effects of the superoxide generator pyrogallol (Sigma) and age on collagen production, cells were grown to 90–95% confluence and serum starved for 24 hours prior to experimentation. Cells from young animals were pretreated with polyethylene glycol-superoxide dismutase (PEG-SOD, 20 Units/mL) for 30 minutes followed by a 24 hour pyrogallol (10 µM) treatment. Fibroblasts from old rats were treated with or without PEG-SOD (20 Units/mL) for 24 hours. Western blots were performed as described previously (Fleenor et al. 2010; Sindler et al. 2011). Antibodies for western blots were collagen type I (1:2000, Millipore) and β-actin (1:1000, Cell Signaling).
Carotid artery vasodilatory responses
Endothelium-dependent and endothelium-independent dilation were determined ex vivo in isolated carotid arteries as previously described (Rippe et al. 2010; Sindler et al. 2011). Briefly, mice were anesthetized using isoflurane and euthanized by exsanguination via cardiac puncture. The carotid arteries were carefully excised, cannulated onto glass micropipettes and secured with nylon (11-0) suture in myograph chambers (DMT Inc.) containing buffered physiological saline solutions. The arteries were pressurized to 50 mmHg at 37° C and were allowed to equilibrate for 1 h. After submaximal preconstriction with phenylephrine (2 µmol/L), increases in luminal diameter in response to acetylcholine (ACh: 1 × 10−9 - 1 × 10−4 mol/L) with and without co-administration of the NO synthase inhibitor N-G-nitro-L-arginine methyl ester (L-NAME), (0.1 mmol/L, 30 min incubation) were determined. Endothelium-independent dilation was determined by vasodilation in response to sodium nitroprusside (SNP: 1 × 10−10 - 1 × 10−4 mol/L). All pharmacologic agents were added to the abluminal surface of the artery.
All dose response data are presented on a percent basis. Preconstriction was calculated as a percentage of maximal diameter according to the following formula:
Because of differences in maximal carotid artery diameter between young and old animals, vasodilator responses were recorded as actual diameters expressed as a percentage of maximal response according to the following formula:
Where Dm is maximal inner diameter at 50 mmHg, Ds is the steady-state inner diameter recorded after the addition of drug, and Db is the steady-state inner diameter following preconstriction before the first addition of drug.
NO-dependent dilation was determined from the maximal EDD in the absence or presence of L-NAME according to the following formula:
Protein expression
Aortas were used as a surrogate large elastic artery to provide sufficient tissue for analysis of protein expression by Western blot as described previously (Rippe et al. 2010; Sindler et al. 2011) (Lesniewski et al. 2009) (Lesniewski et al. 2011). The vessels were excised, cleared of surrounding tissues and frozen in liquid nitrogen before storage at −80° C. The tissue was pulverized over liquid nitrogen and homogenized in ice-cold RIPA lysis buffer containing protease and phosphatase inhibitors [Protease Inhibitor Cocktail Tablet (Roche, Indianapolis, IN, USA) and 0.01% phosphatase inhibitor cocktail (Sigma, St. Louis, MO, USA)]. Ten micrograms of protein was loaded on 4–12% polyacrylamide gels, separated by electrophoresis and transferred onto nitrocellulose membranes for Western blot analysis. Antibodies for Western blot analysis included anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH 1:1000, Cell Signaling), anti-nitrotyrosine (1:100, Abcam) (for 25 and 55 kD band), anti-p67 phox (1:1000, Cell Signaling), anti-MnSOD (1:2000, Stressgen), and anti-endothelial NO synthase (eNOS 1:500, BD Biosciences). Concentrations of the pro-inflammatory cytokines IL-1β, IL-6 and TNF-α were determined in aortic whole cell lysates by multiplex ELISA (Searchlight Mouse Inflammatory Cytokine Kit; Aushon Biosystems; Billerica, MA) as previously described (Sindler et al. 2011).
Statistics
Results are presented as mean ± SEM. Statistical analysis was performed with SPSS 17.0 software. For the ex vivo vasodilatory dose response, group differences were determined by repeated measures ANOVA. A two-way ANOVA was used to analyze pulse wave velocity and histology data. For maximal dilation, protein expression, superoxide production and animal characteristics comparisons between groups were made using ANOVA. Significance was determined using p<0.05.
Acknowledgements
We thank Kurt Marshall, Kate Howell, Nadia Claassen, Natasha Marvi, Hylke Sneider and Jason Eng for technical assistance. This work was supported by the National Institutes of Health (AG013038, AG000279, HL007822 and HL107120).
Footnotes
Author contributions
Amy L. Sindler, Bradley S. Fleenor, and Douglas R. Seals contributed to the conception, experimental design, interpretation of the data and writing of this manuscript. Amy L. Sindler, Bradley S. Fleenor and Melanie L. Zigler collected most of the data. All authors were involved in the preparation and the final approval of the paper.
References
- Cavalcante JL, Lima JAC, Redheuil A, Al-Mallah MH. Aortic Stiffness: Current Understanding and Future Directions. Journal of the American College of Cardiology. 2011;57:1511–1522. doi: 10.1016/j.jacc.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. Journal of the American College of Cardiology. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective Effects of Anti-Tumor Necrosis Factor-{alpha} Treatment in Aging. Am J Pathol. 2007;170:388–698. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-Induced Phenotypic Changes and Oxidative Stress Impair Coronary Arteriolar Function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiological Genomics. 2004;17:21–30. doi: 10.1152/physiolgenomics.00136.2003. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Wang M, Lakatta EG, Ungvari Z. Inflammation and endothelial dysfunction during aging: role of NF-{kappa}B. J Appl Physiol. 2008;105:1333–1341. doi: 10.1152/japplphysiol.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Uscio LV, Moreau P, Shaw S, Takase H, Barton M, Luscher TF. Effects of Chronic ETA-Receptor Blockade in Angiotensin II-Induced Hypertension. Hypertension. 1997;29:435–441. doi: 10.1161/01.hyp.29.1.435. [DOI] [PubMed] [Google Scholar]
- Daugherty A, Rateri D, Hong L, Balakrishnan A. Measuring blood pressure in mice using volume pressure recording, a tail-cuff method. Journal of Visualized Experiments. 2009;15:1291. doi: 10.3791/1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez J. Arterial stiffness and extracellular matrix. Advances in Cardiology. 2007;44:76–95. doi: 10.1159/000096722. [DOI] [PubMed] [Google Scholar]
- Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. The Journal of Physiology. 2009;587:3271–3285. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA, Seals DR. Arterial stiffening with ageing is associated with transforming growth factor-β1-related changes in adventitial collagen: reversal by aerobic exercise. The Journal of Physiology. 2010;588:3971–3982. doi: 10.1113/jphysiol.2010.194753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francia P, delli Gatti C, Bachschmid M, Martin-Padura I, Savoia C, Migliaccio E, Pelicci PG, Schiavoni M, Luscher TF, Volpe M, Cosentino F. Deletion of p66shc Gene Protects Against Age-Related Endothelial Dysfunction. Circulation. 2004;110:2889–2895. doi: 10.1161/01.CIR.0000147731.24444.4D. [DOI] [PubMed] [Google Scholar]
- Gaubert ML, Sigaudo-Roussel D, Tartas M, Berrut G, Saumet JL, Fromy Brr. Endothelium-derived hyperpolarizing factor as an in vivo back-up mechanism in the cutaneous microcirculation in old mice. The Journal of Physiology. 2007;585:617–626. doi: 10.1113/jphysiol.2007.143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Superoxide Excess in Hypertension and Aging : A Common Cause of Endothelial Dysfunction. Hypertension. 2001;37:529–534. doi: 10.1161/01.hyp.37.2.529. [DOI] [PubMed] [Google Scholar]
- Kass DA, Shapiro EP, Kawaguchi M, Capriotti AR, Scuteri A, deGroof RC, Lakatta EG. Improved Arterial Compliance by a Novel Advanced Glycation End-Product Crosslink Breaker. Circulation. 2001;104:1464–1470. doi: 10.1161/hc3801.097806. [DOI] [PubMed] [Google Scholar]
- Kim JH, Bugaj LJ, Oh YJ, Bivalacqua TJ, Ryoo S, Soucy KG, Santhanam L, Webb A, Camara A, Sikka G, Nyhan D, Shoukas AA, Ilies M, Christianson DW, Champion HC, Berkowitz DE. Arginase inhibition restores NOS coupling and reverses endothelial dysfunction and vascular stiffness in old rats. J Appl Physiol. 2009;107:1249–1257. doi: 10.1152/japplphysiol.91393.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm DJ, Majka SM, Crossno JT, Jr, Psilas JC, Reusch JEB, Garat CV. Reduction of Reactive Oxygen Species Prevents Hypoxia-induced CREB Depletion in Pulmonary Artery Smooth Muscle Cells. Journal of Cardiovascular Pharmacology. 2011;58:181–191. doi: 10.1097/FJC.0b013e31821f2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta EG. Arterial and Cardiac Aging: Major Shareholders in Cardiovascular Disease Enterprises: Part III: Cellular and Molecular Clues to Heart and Arterial Aging. Circulation. 2003;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and Cardiac Aging: Major Shareholders in Cardiovascular Disease Enterprises: Part I: Aging Arteries: A "Set Up" for Vascular Disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Lesniewski LA, Connell ML, Durrant JR, Folian BJ, Anderson MC, Donato AJ, Seals DR. B6D2F1 Mice Are a Suitable Model of Oxidative Stress-Mediated Impaired Endothelium-Dependent Dilation With Aging. J Gerontol A Biol Sci Med Sci. 2009;64A:9–20. doi: 10.1093/gerona/gln049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniewski LA, Durrant JR, Connell ML, Henson GD, Black AD, Donato AJ, Seals DR. Aerobic exercise reverses arterial inflammation with aging in mice. American Journal of Physiology - Heart and Circulatory Physiology. 2011;301:H1025–H1032. doi: 10.1152/ajpheart.01276.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijnen P, Petrov V, van Pelt J, Fagard R. Inhibition of Superoxide Dismutase Induces Collagen Production in Cardiac Fibroblasts. Am J Hypertens. 2008;21:1129–1136. doi: 10.1038/ajh.2008.242. [DOI] [PubMed] [Google Scholar]
- Mariko B, Pezet M, Escoubet B, Bouillot S, Andrieu J-P, Starcher B, Quaglino D, Jacob M-P, Huber P, Ramirez F, Faury G. Fibrillin-1 genetic deficiency leads to pathological ageing of arteries in mice. The Journal of Pathology. 2011;224:33–44. doi: 10.1002/path.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GF, Hwang S-J, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial Stiffness and Cardiovascular Events: The Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano P, Clark JK, Cifuentes-Pagano ME, Clark SM, Callis GM, Quinn MT. Localization of a constitutively active, phagocyte-like NADPH oxidase in rabbit aortic adventitia: Enhancement by angiotensin II. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:14483–14488. doi: 10.1073/pnas.94.26.14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H, Depre C, Ghosh K, Resuello RG, Natividad FF, Rossi F, Peppas A, Shen Y-T, Vatner DE, Vatner SF. Mechanism of Gender-Specific Differences in Aortic Stiffness With Aging in Nonhuman Primates. Circulation. 2007;116:669–676. doi: 10.1161/CIRCULATIONAHA.107.689208. [DOI] [PubMed] [Google Scholar]
- Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proceedings of the National Academy of Sciences. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AK, Li Y-H, Pham TT, Ochoa LN, Trevino MT, Hartley CJ, Michael LH, Entman ML, Taffet GE. Measurement of aortic input impedance in mice: effects of age on aortic stiffness. Am J Physiol Heart Circ Physiol. 2003;285:H1464–H1470. doi: 10.1152/ajpheart.00004.2003. [DOI] [PubMed] [Google Scholar]
- Rippe C, Lesniewski L, Connell M, LaRocca T, Donato A, Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell. 2010;9:304–312. doi: 10.1111/j.1474-9726.2010.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnackenberg CG, Wilcox CS. Two-Week Administration of Tempol Attenuates Both Hypertension and Renal Excretion of 8-Iso Prostaglandin F2α. Hypertension. 1999;33:424–428. doi: 10.1161/01.hyp.33.1.424. [DOI] [PubMed] [Google Scholar]
- Simonsen U, Christensen FH, Buus NH. The effect of tempol on endothelium-dependent vasodilatation and blood pressure. Pharmacology & Therapeutics. 2009;122:109–124. doi: 10.1016/j.pharmthera.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Sindler AL, Delp MD, Reyes R, Wu G, Muller-Delp JM. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. Journal of Physiology. 2009;587:3885–3897. doi: 10.1113/jphysiol.2009.172221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindler AL, Fleenor BS, Calvert JW, Marshall KD, Zigler ML, Lefer DJ, Seals DR. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell. 2011;10:429–437. doi: 10.1111/j.1474-9726.2011.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol. 2004;556:947–958. doi: 10.1113/jphysiol.2003.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and Endothelial Function in Normotensive Subjects and Patients With Essential Hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of Vascular Aging: New Perspectives. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2010;65:1028–1041. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-{kappa}B activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–H47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Lüscher TF. Enhanced Peroxynitrite Formation Is Associated with Vascular Aging. The Journal of Experimental Medicine. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Lakatta EG. Altered Regulation of Matrix Metalloproteinase-2 in Aortic Remodeling During Aging. Hypertension. 2002;39:865–873. doi: 10.1161/01.hyp.0000014506.13322.66. [DOI] [PubMed] [Google Scholar]
- Yanes L, Romero D, Iliescu R, Cucchiarelli VE, Fortepiani LA, Santacruz F, Bell W, Zhang H, Reckelhoff JF. Systemic arterial pressure response to two weeks of Tempol therapy in SHR: involvement of NO, the, RAS, oxidative stress. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2005;288:R903–R908. doi: 10.1152/ajpregu.00530.2004. [DOI] [PubMed] [Google Scholar]
- Yang Y-M, Huang A, Kaley G, Sun D. eNOS uncoupling and endothelial dysfunction in aged vessels. American Journal of Physiology - Heart and Circulatory Physiology. 2009;297:H1829–H1836. doi: 10.1152/ajpheart.00230.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Yoon S, Jung KJ, Kim CH, Son TG, Kim MS, Kim YJ, Lee J, Yu BP, Chung HY. Upregulation of aortic adhesion molecules during aging. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2006;61:232–244. doi: 10.1093/gerona/61.3.232. [DOI] [PubMed] [Google Scholar]