Abstract
Mitochondrial aldehyde dehydrogenase-2 (ALDH2) has been characterized as an important mediator of endogenous cytoprotection in the heart. This study was designed to examine the role of ALDH2 knockout (KO) in the regulation of cardiac function after endoplasmic reticulum (ER) stress. Wild-type (WT) and ALDH2 KO mice were subjected to a tunicamycin challenge, and the echocardiographic property was examined. Protein levels of six items—78 kDa glucose-regulated protein (GRP78), phosphorylation of eukaryotic initiation factor 2 subunit α (p-eIF2α), CCAAT/enhancer-binding protein homologous protein (CHOP), phosphorylation of Akt, p47phox nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and 4-hydroxynonenal—were determined by using Western blot analysis. Cytotoxicity and apoptosis were estimated using 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide (MTT) assay and caspase-3 activity, respectively. ALDH2 deficiency exacerbated cardiac contractile dysfunction and promoted ER stress after ER stress induction, manifested by the changes of ejection fraction and fractional shortening. In vitro study revealed that tunicamycin significantly upregulated the levels of GRP78, p-eIF2α, CHOP, p47phox NADPH oxidase and 4-hydroxynonenal, which was exacerbated by ALDH2 knockdown and abolished by ALDH2 overexpression, respectively. Overexpression of ALDH2 abrogated tunicamycin-induced dephosphorylation Akt. Inhibition of phosphatidylinositol 3-kinase using LY294002 did not affect ALDH2-conferred protection against ER stress, although LY294002 reversed the antiapoptotic action of ALDH2 associated with p47phox NADPH oxidase. These results suggest a pivotal role of ALDH2 in the regulation of ER stress and ER stress–induced apoptosis. The protective role of ALDH2 against ER stress–induced cell death was probably mediated by Akt via a p47phox NADPH oxidase-dependent manner. These findings indicate the critical role of ALDH2 in the pathogenesis of ER stress in heart disease.
INTRODUCTION
The endoplasmic reticulum (ER) is a multifunctional intracellular organelle involved in multiprocesses within the cell, including synthesis and folding of secretory and membrane proteins, Ca2+ homeostasis and lipid biosynthesis (1–3). ER stress develops in response to a wide variety of cellular stressors, such as heat, hypoxia, metabolic starvation, angiotensin II, tumor necrosis factor-α and change in lipid metabolism (4). These stressors reduce the protein-folding capacity and disrupt the homeostatic functions of the ER, leading to the accumulation and aggregation of unfolded and misfolded protein in ER, a condition called “ER stress” (5). To date, it has been well established that three ER-resident transmembrane proteins are activated to initiate and mediate ER stress: pancreatic ER kinase-like ER kinase (PERK), activating transcription factor 6 (ATF6) and inositol-requiring enzyme 1 (IRE1). Compelling evidence indicates that ER stress–induced apoptosis is an important factor in the pathophysiological condition (5). Recently, ER stress received significant attention by the cardiovascular community, since it has been implicated in an array of human diseases including ischemia/reperfusion injury of the brain and heart, atherosclerosis, cardiac hypertrophy and heart failure (6,7). Thus, ER stress appears to be a candidate instigator for pathological cell death and functional change intimately involved in the maintenance of cardiovascular homeostasis, indicating an important role as a therapeutic target for the management of cardiovascular diseases (6).
Aldehyde dehydrogenase 2 (ALDH2) is emerging as a key enzyme of endogenous cytoprotection in the heart (8). It is involved in the detoxification of reactive aldehydes such as 4-hydroxy-2-nonenal (4-HNE) (8). Increasing evidence has revealed the beneficial role of ALDH2 against tissue and cellular injuries originated from alcohol, acetaldehyde and toxic aldehyde–induced reactive oxygen species formation (9–11). 4-HNE is a highly cytotoxic aldehyde that gets accumulated in the heart in response to ischemia/reperfusion insult, which in turn inactivates ALDH2 by protein adducts formation (12). Pharmacological enhancement of ALDH2 activity can protect the heart against ischemic injury (8). Transgenic overexpression of ALDH2 was found to prevent ethanol exposure–induced myocardial anomalies (10). To the contrary, inhibition of ALDH2 by oxidative stress leads to cardiac dysfunction in diabetes mellitus (13). These results further revealed a possible antioxidant property of this enzyme. Nevertheless, the association between ALDH2 and myocardial injury mediated by ER stress has not been elucidated.
On the basis of previous observations in the animal model challenged with tunicamycin (14), the aim of this study was to determine whether ALDH2 knockout (KO) triggers deterioration of cardiac function after ER stress induction in ALDH2 KO mice and whether ALDH2 overexpression may prevent tunica -mycin-induced ER stress and apoptosis.
MATERIALS AND METHODS
Animals
All experimental procedures were approved by the Animal Care and Use Committee at the Zhongshan Hospital. Male wild-type (WT) C57 BL/6 and ALDH2 KO mice (12–16 wks of age) were used for the study. Generation and characterization of the ALDH2 KO mice using gene targeting in embryonic stem cells were described in detail previously (15). Given that the ER environment can be perturbed with pharmacological agents through altered redox status, Ca2+ homeostasis and protein glycosylation in the ER, all mice were injected intraperitoneally with tunicamycin (3 mg/kg) (Sigma-Aldrich, St. Louis, MO, USA) to elicit ER stress for 48 h before assessment of mechanical properties (14).
Echocardiography
Echocardiographic acquisition and analysis were performed by an echocardiographer blinded to treatment groups. Anesthesia with spontaneous respiration was achieved with isoflurane. All images were taken at a heart rate >400 beats per minute to minimize effects of anesthesia, using a VisualSonics Vevo 770 echocardiography system (VisualSonic, Toronto, ON, Canada) and a 30-MHz transducer. The average of five consecutive cardiac cycles was used. Left ventricular end-diastolic dimension (LVEDD) and end-systolic dimension (LVESD) was measured by the M-mode method. Left ventricular fractional shortening was calculated as a percentage as follows: (LVEDD – LVESD)/LVEDD.
Cell Culture and Drug Treatment
Rat neonatal cardiomyocytes were isolated from 1- to 2-d-old Sprague Dawley rats by digestion with trypsin. Briefly, cardiac ventricular tissue was aseptically removed from neonatal rats, minced and then digested with 0.125% trypsin in Hank’s Balanced Salt Solution (Gibco; Life Technologies, Grand Island, NY, USA) at 37°C in a shaking water bath. The cells were released after the first digestion was discarded, whereas the cells from subsequent digestion were added to an equal volume of Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (Gibco; Life Technologies) until all cells were collected. Cells were pelleted by centrifugation at 1,000 rpm for 5 min and resuspended in DMEM supplemented 10% fetal bovine serum. The isolated cells were first plated in culture disks of 95% air and 5% CO2 at 37°C for 2 h to exclude nonmuscle cells. The suspended cells were then collected and plated at a density of 5 × 105 cells/cm2 and maintained under the same conditions as above. The cells were used for outlined experiments after 2–3 d later.
To induce ER stress, cardiomyocytes were incubated with tunicamycin (5 μg/mL) for 12 h. Cells were preincubated with N-acetyl-l-cysteine (NAC) (5 mmol/L) and LY294002 (20 μmol/L), respectively, for 1 h before adding tunicamycin. Cells were challenged by 4-hydroxynonenal at a concentration of 20 μmol/L to determine levels of ER stress markers.
Construction of Adenoviral Vectors and Infections
A mouse WT ALDH2 plasmid was constructed as described previously (16), provided by Shigeo Ohta (Nippon Medical School, Kawasaki, Japan). The pAd/CMV/V5-DEST vector containing the CMV promoter and C-terminal V5 epitope (Invitrogen, Grand Island, NY, USA) was used in the construction of adenoviral vectors. The coding sequence of ALDH2 or green fluorescence protein (GFP) was inserted into the pDONR™221 vector (Invitrogen), which was then recombined with pAd/CMV/V5-DEST vector according to the instruction of Gateway Technology (Invitrogen). The presence of ALDH2 in the pAd/CMV/V5-DEST vector was confirmed by DNA sequencing and restriction mapping. A vector containing GFP was used as a control for adenoviral vector infection. Cells were infected with adenoviruses for 24 h, after which the medium was replaced with a virus-free medium and cells were incubated for up to 24 h.
Knockdown of ALDH2 by RNA Interference
The ALDH2 small interfering ribonucleic acid (siRNA) used in this study was purchased from Qiagen (Hilden, Germany). The cells were transfected with ALDH2 siRNA or scrambled siRNA (Scr siRNA) using the Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s protocol. The transfection efficiency was monitored using a fluorescent oligonucleotide (Santa Cruz Biotechnology, Santa Cruz, CA, USA). After 24 h, cells were harvested and lysed with lysis buffer. The lysates were then subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with anti-ALDH2 antibody.
Western Immunoblot
Tissues or cultured cells were homogenized in extraction buffer containing 20 mmol/L Tris (pH 7.4), 150 mmol/L NaCl, 1 mmol/L ethylenediaminete-traacetic acid (EDTA), 1 mmol/L ethylene glycol-bis(β-aminoethyl ether)-N,N,N′N′-tetraacetic acid (EGTA), 1% Triton, 0.1% SDS and a protease and phosphatase inhibitor cocktail (1:100; Pierce). An equal amount of protein samples were separated by 10–12% SDS-PAGE and transferred to polyvinylidene difluoride membrane (0.22 μmol/L, Immobilon-P; Millipore, Billerica, MA, USA). Membranes were then blocked for 1 h in 5% bovine serum albumin in Tris-buffered saline containing 0.1% Tween 20 (TBS-T) before overnight incubation at 4°C with primary antibodies against anti–CCAAT/enhancer-binding protein homologous protein (anti-CHOP; 1:500; Cell Signaling Technology, Beverly, MA, USA), anti–78 kDa glucose-regulated protein (anti-GRP78; 1:1,000; Abcam, Cambridge, MA, USA), anti–phosphorylation of eukaryotic initiation factor 2 subunit α (anti-p-eIF2α; Ser52, 1:500; Santa Cruz Biotechnology), p47phox nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (1:500; Bioworld Technology, Minneapolis, MN, USA), anti-HNE Michael adducts (1:1,000; Calbiochem; EMD Millipore, Billerica, MA, USA) and anti-p-AKT (Ser473, 1:500; Cell Signaling Technology). The blots were then incubated for 1 h with horseradish peroxidase–conjugated secondary antibodies (1:4,000) and visualized by chemiluminescence (Pierce, Rockford, IL, USA). Protein levels were quantified by densitometry using Quantity One software (Bio-Rad, Hercules, CA, USA). The arbitrary units were normalized to GAPDH and expressed as fold-induction over control values.
MTT Assay
Cytotoxicity was estimated using the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide (MTT) assay. In brief, cardiomyocytes were plated in microtiter plates at a density of 4 × 104 cells/mL. MTT was added to each well at a final concentration of 0.5 mg/mL, and the plates were incubated for 4 h at 37°C. The formazan crystals in each well were dissolved in dimethyl sulfoxide. Formazan was quantified spectroscopically at 570 nm using a microplate reader. Cell survival was expressed as absorbance relative to that of untreated controls.
Caspase-3 Activity Assay
The caspase-3 activity assay is based on hydrolysis of the peptide substrate acetyl-Asp-Glu-Val-Asp p-nitroanilide (Ac-DEVD-pNA) by caspase-3, resulting in the release of p-nitroaniline (pNA) moiety. Cells were homogenized in ice-cold cell lysis buffer (50 mmol/L N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid [HEPES], 0.1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate [CHAPS], 1 mmol/L dithiothreitol, 0.1 mmol/L EDTA and 0.1% NP-40 (Pierce, Rockford, IL, USA). To detect the activity of caspase-3, cell lysate (10 μL) was added to 80 μL reaction buffer followed by an additional 10 μL of caspase-3 substrate (Ac-DEVD-pNA) and incubated at 37°C for 2 h. The samples were determined by SpectraMax M5 Multi-Mode Microplate Readers (Molecular Devices, Sunnyvale, CA, USA) at 405 nm. The fold-increase of caspase-3 activity was calculated by comparing the absorbance of p-NA from untreated controls with tunicamycin-treated samples. The fold-increase in caspase-3 activity was calculated by comparing the absorbance of p-nitroaniline from untreated controls with apoptotic samples.
TUNEL Assay
Transferase-mediated dUTP nick-end labeling (TUNEL) assay was performed using an in situ cell death detection kit (TMR Red, Roche Applied Science) according to the manufacturer’s instructions. Briefly, frozen sections (5 μm) were fixed and permeated. The TUNEL reaction mixture was added to the sections and incubated at 37°C for 60 min in a humidified chamber in the dark. Sections were rinsed three times in phosphate-buffered saline for 5 min each. The nuclei were labeled with 4′,6-diamidino-2-phenylindole (DAPI). After being embedded with antifade, samples were analyzed under a fluorescence microscope (Leica Qwin Plus, Buffalo Grove, IL, USA) using an excitation wavelength in the range of 520–560 nm (maximum 540 nm, green) and detection in the range of 570–620 nm (maximum 580 nm, red). The percentage of apoptotic cells was analyzed using the ImageJ software (National Institutes of Health, Bethesda, MD, USA, http://imagej.nih.gov/ij/).
Statistical Analysis
Data were expressed as mean ± standard error of the mean (SEM). Statistical analyses were performed using one-way analysis of variance with a Bonferroni multiple comparisons post hoc test for multiple group comparisons. P < 0.05 was considered statistically significant.
RESULTS
Effect of Tunicamycin Challenge in the ALDH2 KO and WT Mice
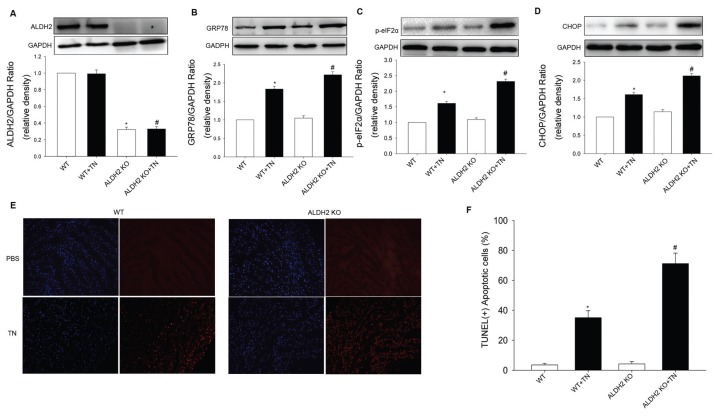
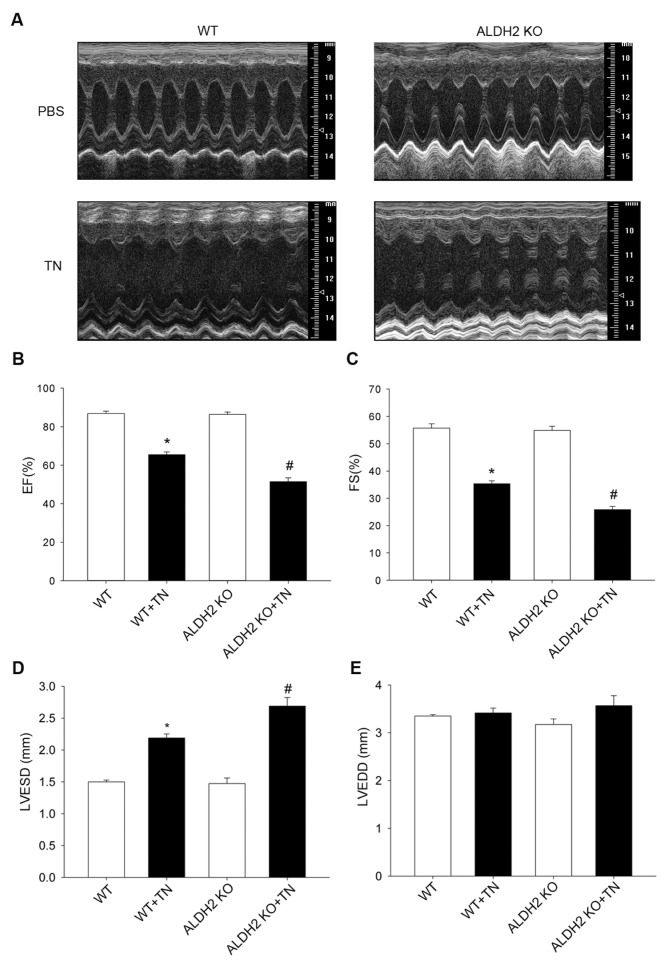
To elicit ER stress, mice were challenged with tunicamycin (3 mg/kg intra-peritoneally [IP]) before the assessment of echocardiographic properties. Western blot analysis confirmed the presence of ER stress manifested as the upregulated CHOP and GRP78 in myocardium after a tunicamycin challenge (3 mg/kg). These ER stress markers were also elevated in ALDH2 KO mice (Figures 1B–D). TUNEL assay revealed significantly greater apoptotic cell death after a tunicamycin challenge, with a more pronounced effect in ALDH2 KO mice (Figures 1E, F). In addition, our data depicted that tunicamycin overtly decreased ejection fraction and fractional shortening, indicating cardiac dysfunction after ER stress. We went on to test the effect of tunicamycin on cardiac function in ALDH2 KO mice and found that ER stress–induced cardiac dysfunction was further worsened in ALDH2 KO mice, as characterized by the alteration of ejection fraction, fractional shortening and LVESD. Last but not least, ADLH2 KO itself did not exert any significant effects on cardiac contractile function (Figure 2).
Figure 1.
Effect of tunicamycin (TN) challenge on ER stress in WT mice and ALDH2 KO mice. Protein levels in myocardium from WT mice and ALDH2 knockout mice with or without TN exposure were determined. (A) ALDH2. (B) GRP78. (C) p-eIF2α. (D) CHOP. (E) Apoptosis was detected using TUNEL assay. (F) Data depicted that ALDH2 KO significantly promoted apoptosis after a TN challenge, compared with WT mice. All values for given protein expression were normalized to that of GAPDH. The data (presented as percentages of control) are means ± SEM of three separate experiments. *p < 0.05 versus WT group; #p < 0.05 versus ALDH2 KO-TN group.
Figure 2.
Effect of tunicamycin (TN) on cardiac mechanical properties in WT mice and ALDH2 KO mice. An M-mode representation (A) is shown that clearly indicated the depressed cardiac mechanical properties. Ejection fraction (EF) (B) and fractional shortening (FS) (C) were decreased in ALDH2 KO mice compared with WT mice. LVESD (D) was increased in ALDH2 KO mice compared with WT mice after TN challenge. (E) There is no significantly statistical difference in LVEDD between groups. Data are mean ± SEM; n = 5 per group. *p < 0.05 versus WT group; #p < 0.05 versus ALDH2 KO-TN group.
Effect of ALDH2 on ER Stress Induced by Tunicamycin in Cardiomyocytes
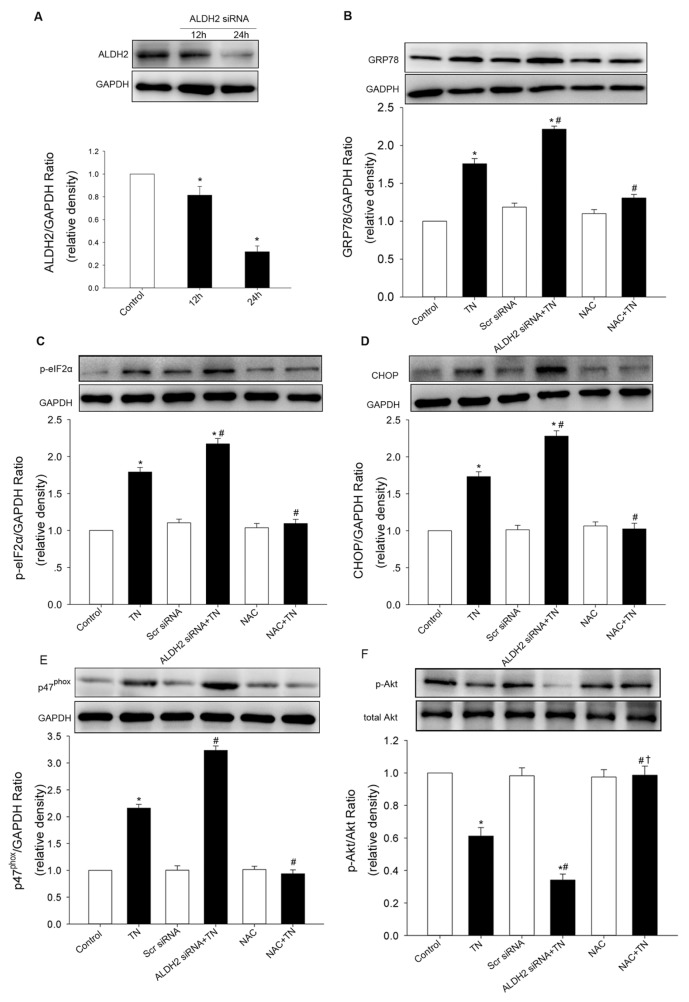
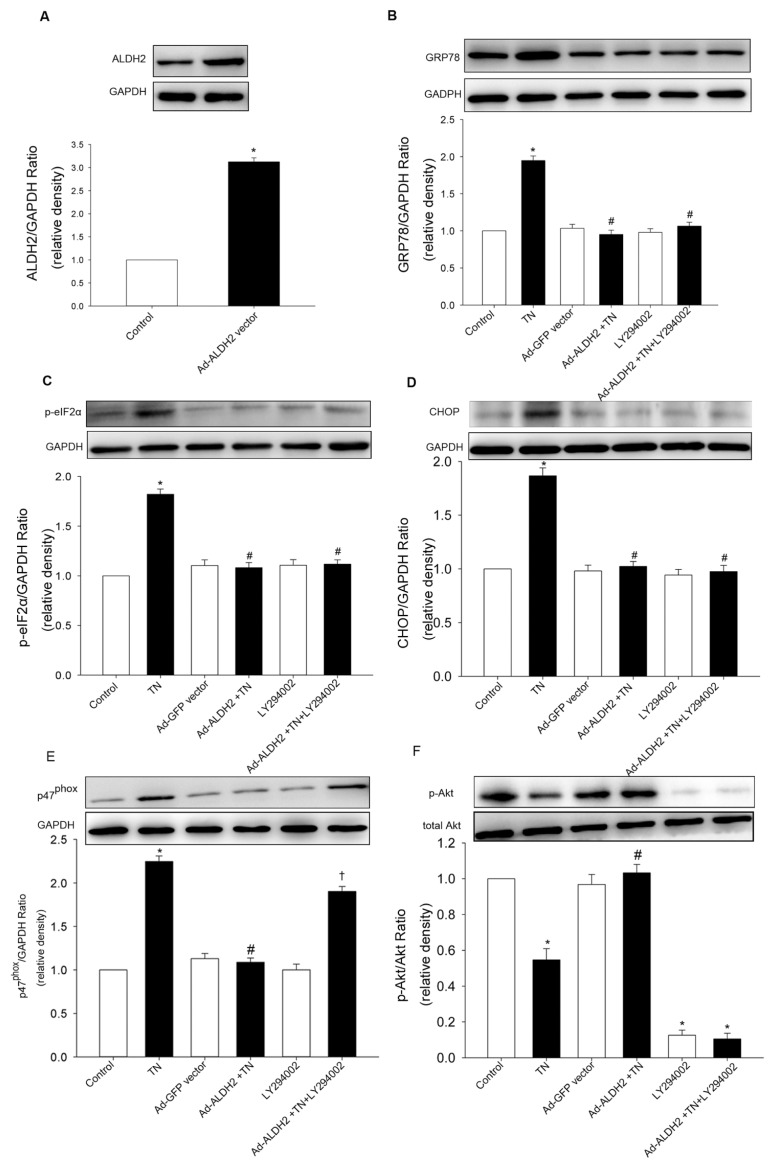
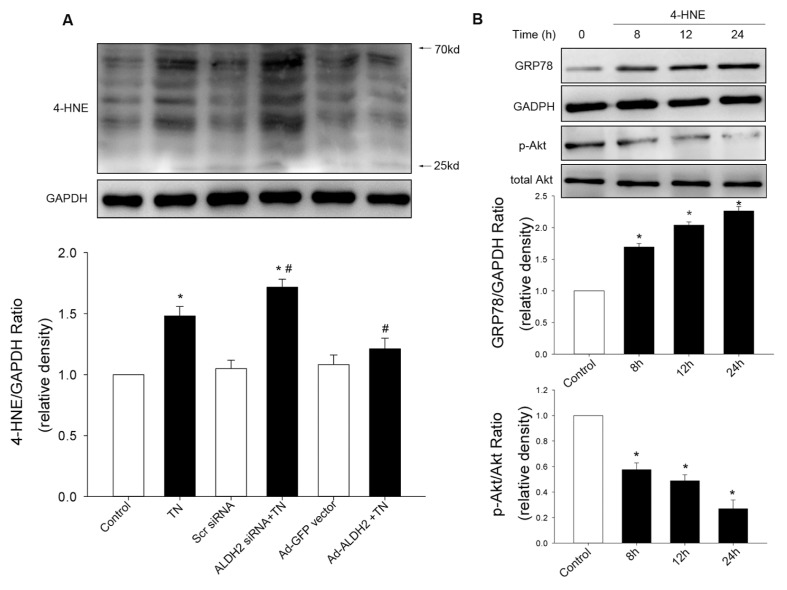
To confirm the effect of ALDH2 on ER stress, both cardiomyocytes transfected with an ALDH2 plasmid and cardiomyocytes treated with siRNA specific for ALDH2 were used, compared with cardiomyocytes transfected with the empty vector or scramble siRNA. Western blot analysis confirmed the efficiency of ALDH2 overexpression or knockdown. In this study, tunicamycin induced an overt increase in the protein levels of ER stress markers, which was exacerbated by ALDH2 knockdown and abolished by ALDH2 overexpression (Figures 3, 4). These data implicated beneficial cardio-protection of ALDH2 against ER stress induced by tunicamycin. Furthermore, the 4-HNE adducts were monitored using Western blot. As expected, 4-HNE protein adducts were found after tunicamycin treatment, the effect of which was obliterated by ALDH2 overexpression, while 4-HNE adducts were augmented by ALDH2 knockdown. In addition, we found that 4-HNE increased the levels of GRP78 and dephosphorylation of Akt in cardiomyocytes (Figure 5).
Figure 3.
Effect of ALDH2 knockdown on ER stress in cardiomyocytes. (A) ALDH2 expression was inhibited in cardiomyocytes transfected with siRNA specific for ALDH2 (ALDH2-siRNA). The expression of GRP78 (B), p-eIF2α (C) and CHOP (D) was elevated by ALDH2 siRNA transfection compared with the tunicamycin (TN) treatment group and control. (E) p47phox subunit was promoted by ALDH2 knockdown compared with the TN treatment group and control. (F) p-Akt was decreased by ALDH2 knockdown compared with the TN treatment group and control. NAC was used as a positive control. Scr siRNA indicates scrambled siRNA used as control, which did not affect the ER stress markers. All values for a given protein expression were normalized to that of GAPDH. The data (presented as percentages of control) are means ± SEM of three separate experiments. *p < 0.05 versus control; #p < 0.05 versus TN group; †p < 0.05 versus ALDH2 siRNA + TN group.
Figure 4.
Effect of ALDH2 overexpression on ER stress in cardiomyocytes. (A) ALDH2 expression was increased by ALDH2 adenoviral vector. Increased expression of GRP78 (B), p-eIF2 (C) and CHOP (D) were abrogated by ALDH2 overexpression. Effect of ALDH2 vector on ER stress was not affected by LY294002. (E) p47phox subunit upregulation by tunicamycin (TN) was mitigated by the ALDH2 vector compared with TN treatment group, effect of which was blocked by LY294002. (F) Depressed p-Akt by TN was activated by ALDH2 vector compared with the TN treatment group. ALDH2 vector and LY294002 alone did not increase ER stress markers and p47phox subunit. Ad-ALDH2, ALDH2 adenoviral vector. All values for given protein expression were normalized to that of GAPDH. The data (presented as percentages of control) are means ± SEM of three separate experiments. *p < 0.05 versus control; #p < 0.05 versus TN group; †p < 0.05 versus Ad-ALDH2 + TN group.
Figure 5.
Effect of ALDH2 on accumulation of 4-HNE protein adducts. 4-HNE protein adducts were determined after tunicamycin (TN) exposure. (A) 4-HNE adducts were accumulated by TN, which was mitigated by ALDH2 vector and aggravated by ALDH2 siRNA. (B) Time course of GRP78 expression and Akt phosphorylation in cardiomyocytes incubated with 4-HNE (20 μmol/L). All values for given protein expression were normalized to that of GAPDH. The data (presented as percentages of control) are means ± SEM of three separate experiments. *p < 0.05 versus control; #p < 0.05 versus TN group.
Effect of ALDH2 on Tunicamycin-Induced Change in p47phox NADPH Oxidase and Akt
Given that NADPH oxidase was implicated in ER-induced loss of cell survival (17), the role of NADPH oxidase (p47phox subunit) in the cytoprotection of ALDH2 after ER stress induction was evaluated. The protein levels of the p47phox subunit was significantly increased by tunicamycin and augmented by ALDH2 knockdown (Figure 3E). ALDH2 overexpression and treatment of NAC mitigated p47phox subunit upregulation induced by tunicamycin (Figure 4E). Additionally, phosphorylation of Akt was significantly dampened after ER stress induction in vitro, which was aggravated by ALDH2 knockdown (Figure 3F). In contrast, the depressed AKT activation was restored by ALDH2 overexpression and NAC treatment (Figure 4F). Total AKT was not affected by ER stress induction or ALDH2 overexpression. These data suggest that Akt signaling was implicated in the protection of ALDH2 against ER stress. Intriguingly, inhibition of phosphatidylinositol 3-kinase (PI3K) with LY294002 did not affect the decreased ER stress markers conferred by ALDH2, whereas it remarkably reversed the downregulation of p47phox elicited by ALDH2 (Figures 3, 4).
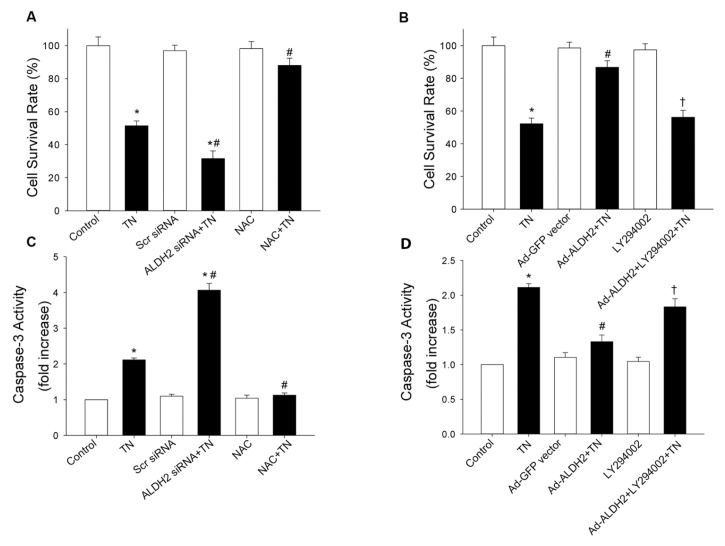
Effect of ALDH2 on Tunicamycin-Induced Apoptosis in Cardiomyocytes
Although Akt did not participate in ALDH2-mediated downregulation of ER stress, Akt was shown to play an important role in the maintenance of cell survival in diversified cells (18,19). Thus, the PI3K inhibitor (LY294002) was used to determine whether Akt was involved in cytoprotection of ALDH2 against apoptosis after ER stress induction. As expected, MTT assay depicted that tunicamycin-induced toxicity was promoted by siRNA silencing of ALDH2, while it was prevented by ALDH2 overexpression. Furthermore, apoptosis in cardiomyocytes was estimated by caspase-3 activity. Data revealed that tunicamycin significantly promoted caspase-3 activity, the effect of which was enhanced and attenuated, respectively, by ALDH2 silencing and ALDH2 overexpression. Interestingly, the antiapoptosis property of ALDH2, evaluated by MTT assay and caspase-3 activity, was abrogated by LY294002 (Figure 6). These results indicate that ALDH2-forced expression was associated with increased resistance of cardiomyocytes to tunicamycin-induced cytotoxicity and apoptosis, whereas ALDH2 silencing by siRNA potentiated tunicamycin-induced cell death through Akt signaling.
Figure 6.
Effect of ALDH2 on tunicamycin (TN)-induced apoptosis. (A) siRNA silencing of ALDH2 promoted TN-induced toxicity, evaluated by the MTT assay. (B) ALDH2 over-expression prevents cytotoxicity induced by TN, the effect of which was abrogated by LY294002. Cytotoxicity was evaluated by the MTT assay. (C) siRNA silencing of ALDH2 increased apoptosis in cardiomyocytes, determined by caspase-3 activity. (D) ALDH2 over-expression attenuated TN-induced elevation of caspase-3 activity, which was recovered by LY294002. The data (presented as percentages of control) are means ± SEM of three separate experiments. *p < 0.05 versus control; #p < 0.05 versus TN group; †p < 0.05 versus ALDH2 siRNA or Ad-ALDH2 + TN group.
DISCUSSION
The salient findings from the present study demonstrated that ALDH2 deficiency was susceptible to cardiotoxicity in response to ER stress induction, characterized by impaired cardiac function. This result is supported by our finding that ALDH2-forced expression was associated with downregulation of ER stress and the p47phox subunit, enhanced Akt activity and resistance of cardiomyocytes to ER stress–induced cytotoxicity and apoptosis. In addition, our data provided further support in that ALDH2 silencing using siRNA exacerbated ER stress and ER stress–induced cell death.
As an important pathological hallmark, ER stress has been implicated in the onset and development of cardiovascular diseases (6). Chronic activation of ER stress eventually induces an apoptotic response and cardiac anomalies (20). It is noteworthy that the most critical ER stress–induced apoptotic pathway is mediated through CHOP (20). Although the precise apoptotic mechanism mediated by CHOP is unknown, CHOP deficiency has been demonstrated to resist apoptotic stimulation from ER stress (21). Strikingly, as a member of the unfolded protein response, disulfide isomerase (PDI) was significantly increased to confer protection against ischemic damage (22). Tunicamycin was shown to increase the protein levels of PDI, which is necessary for appropriate protein folding and prevention of protein misfolding during stress (23). In this study, data reveal that ALDH2 significantly decreased the levels of ER stress markers and protected cells against apoptosis induced by ER stress. Given that cardiac dysfunction may result in part from loss of cardiomyocytes, increased apoptosis resulted in the deterioration of cardiac function defect in ALDH2 KO mice. These findings are in agreement with previous studies in that ALDH2 may serve as an important “mediator of endogenous survival signaling” against death stimuli (24).
4-HNE is one of the most abundant aldehydes formed during oxidation of polyunsaturated fatty and oxidative stress, and it is capable of modifying key enzymes by forming protein adducts to inhibit protein function (12). A recent study demonstrated that mechanical dysfunction of cardiomyocytes induced by 4-HNE was ablated by ALDH2 (25). Our results show that the 4-HNE adduct was accumulated after tunicamycin treatment. Additionally, 4-HNE was found to trigger upregulation of GRP78 and Akt inactivation in cardiomyocytes, in agreement with the role of 4-HNE on ER stress in vascular cells (26). Previous studies also reported that 4-HNE triggered suppression of Akt activity (27), compromised cardiomyocytes mechanical function and protein damage (25). ER stress induction was found to promote carbonyl formation (14,17), whereas carbonyl formation was also upregulated in response to 4-HNE exposure, which was attenuated by ALDH2 overexpression (25). Taken together, the above evidence suggests that the role of ALDH2 against ER stress, at least in part, was attributed to the detoxification of 4-HNE with ALDH2.
It is well known that PI3K-Akt signaling is a key mediator of cell survival in response to external stress (18,19). Akt was also implicated in the cardioprotection for cardiac myocyte survival (9,28). However, the role of Akt signaling in regulation of ER stress is controversial. Activation of Akt was found to rescue the cardiac mechanical function defect induced by ER stress, whereas it did not affect the protein levels of ER stress markers (14,17). Recent studies demonstrated that inhibition of ER stress by intermedin1–53 was blocked by the PI3K inhibitor (29). In the study, data depicted that Akt activity was dampened in response to a tunicamycin challenge, which was recovered by ALDH2. Nevertheless, PI3K inhibitor did not further aggravate protein levels of ER stress markers induced by tunicamycin, whereas Akt was much more inhibited. Interestingly, inhibition of PI3K did not affect the protection of ALDH2 against ER stress, whereas cell survival elicited by ALDH2 was blocked by inhibition of PI3K. These results suggested that the cytoprotection of ALDH2 was at least partially mediated by the PI3K-Akt pathway. However, further study is required to better elucidate the mechanism of ALDH2-regulated ER stress.
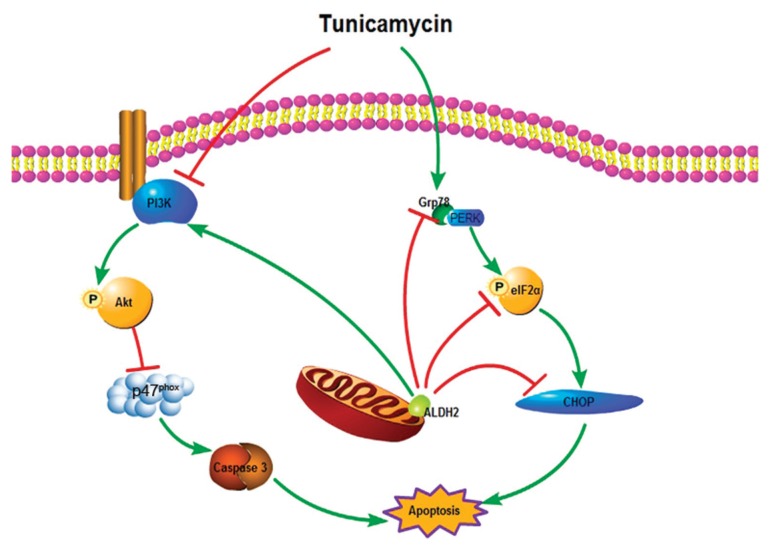
Although tremendous evidence indicates that ER stress is intimately inter -related with oxidative stress (30), the mechanisms that link ER stress and oxidative stress remain completely unclear. The NADPH oxidase is one of major sources of reactive oxygen species in the cardiovascular system, and the cross-talk between NADPH oxidase and mitochondrial reactive oxygen species generation is well established (31,32). NADPH oxidase was also found to link ER stress and oxidative stress in the setting of apoptosis (33). Moreover, it was demonstrated that thapsigargin treatment increased the levels of p47phox NADPH oxidase (17). Our results found that the levels of p47phox subunit were upregulated by tunicamycin in vitro, which was mitigated by ALDH2 overexpression. However, the inhibition of the p47phox subunit offered by ALDH2 was reversed by the PI3K inhibitor LY294002. Given that the p47phox subunit was involved in apoptosis induced by thapsigargin (17), it is plausible to speculate that p47phox subunit may be the downstream of Akt in the cytoprotection of ALDH2 (Figure 7).
Figure 7.
Schematic diagram of the protective effect of ALDH2 against ER stress.
CONCLUSION
In summary, our data suggest that ALDH2 is implicated in the regulation of ER stress and ER stress–induced apoptosis. The protective role of ALDH2 against cytotoxicity and cell death induced by ER stress induction is probably mediated by Akt signaling via p47phox NADPH oxidase. ALDH2 deficiency leads to further deterioration of cardiac dysfunction elicited by ER stress induction. These findings indicate a critical role of ALDH2 in the pathogenesis of ER stress in the heart.
The major limitation of this study is that transgenic mice of ALDH2 overexpression were unavailable to confirm the cardioprotection against ER stress–induced cardiac function defect.
ACKNOWLEDGMENTS
We thank Shigeo Ohta at Nippon Medical School, Japan, for providing the ALDH2 plasmids. This work was supported by the National Basic Research Program of China (2011CB503905), the National Science Fund for Distinguished Young Scholars (30725036) and the National Natural Science Foundation of China (30971250, 81100075).
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 2.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–30. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 3.Minamino T, Kitakaze M. ER stress in cardiovascular disease. J Mol Cell Cardiol. 2010;48:1105–10. doi: 10.1016/j.yjmcc.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 4.Minamino T, Komuro I, Kitakaze M. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ Res. 2010;107:1071–82. doi: 10.1161/CIRCRESAHA.110.227819. [DOI] [PubMed] [Google Scholar]
- 5.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–5. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groenendyk J, Sreenivasaiah PK, Kim Do H, Agellon LB, Michalak M. Biology of endoplasmic reticulum stress in the heart. Circ Res. 2010;107:1185–97. doi: 10.1161/CIRCRESAHA.110.227033. [DOI] [PubMed] [Google Scholar]
- 7.Tabas I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ Res. 2010;107:839–50. doi: 10.1161/CIRCRESAHA.110.224766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen CH, et al. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–5. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma H, Li J, Gao F, Ren J. Aldehyde dehydrogenase 2 ameliorates acute cardiac toxicity of ethanol: role of protein phosphatase and fork-head transcription factor. J Am Coll Cardiol. 2009;54:2187–96. doi: 10.1016/j.jacc.2009.04.100. [DOI] [PubMed] [Google Scholar]
- 10.Ma H, et al. Aldehyde dehydrogenase 2 knockout accentuates ethanol-induced cardiac depression: role of protein phosphatases. J Mol Cell Cardiol. 2010;49:322–9. doi: 10.1016/j.yjmcc.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li SY, et al. Attenuation of acetaldehyde-induced cell injury by overexpression of aldehyde dehydrogenase-2 (ALDH2) transgene in human cardiac myocytes: role of MAP kinase signaling. J Mol Cell Cardiol. 2006;40:283–94. doi: 10.1016/j.yjmcc.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Chen CH, Sun L, Mochly-Rosen D. Mitochondrial aldehyde dehydrogenase and cardiac diseases. Cardiovasc Res. 2010;88:51–7. doi: 10.1093/cvr/cvq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, et al. Inhibition of aldehyde dehydrogenase 2 by oxidative stress is associated with cardiac dysfunction in diabetic rats. Mol Med. 2011;17:172–9. doi: 10.2119/molmed.2010.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Xia Z, La Cour KH, Ren J. Activation of Akt rescues endoplasmic reticulum stress-impaired murine cardiac contractile function via glycogen synthase kinase-3beta-mediated suppression of mitochondrial permeation pore opening. Antioxid Redox Signal. 2011;15:2407–24. doi: 10.1089/ars.2010.3751. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Kitagawa K, et al. Aldehyde dehydrogenase (ALDH) 2 associates with oxidation of methoxyacetaldehyde; in vitro analysis with liver subcellular fraction derived from human and Aldh2 gene targeting mouse. FEBS Lett. 2000;476:306–11. doi: 10.1016/s0014-5793(00)01710-5. [DOI] [PubMed] [Google Scholar]
- 16.Ohsawa I, Nishimaki K, Yasuda C, Kamino K, Ohta S. Deficiency in a mitochondrial aldehyde dehydrogenase increases vulnerability to oxidative stress in PC12 cells. J Neurochem. 2003;84:1110–7. doi: 10.1046/j.1471-4159.2003.01619.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Ren J. Thapsigargin triggers cardiac contractile dysfunction via NADPH oxidase-mediated mitochondrial dysfunction: role of Akt dephosphorylation. Free Radic Biol Med. 2011;51:2172–84. doi: 10.1016/j.freeradbiomed.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–27. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 19.Brunet A, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 20.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–93. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 21.Zinszner H, et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–95. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Severino A, et al. Identification of protein disulfide isomerase as a cardiomyocyte survival factor in ischemic cardiomyopathy. J Am Coll Cardiol. 2007;50:1029–37. doi: 10.1016/j.jacc.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Toldo S, et al. Altered oxido-reductive state in the diabetic heart: loss of cardioprotection due to protein disulfide isomerase. Mol Med. 2011;17:1012–21. doi: 10.2119/molmed.2011.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Budas GR, Disatnik MH, Mochly-Rosen D. Aldehyde dehydrogenase 2 in cardiac protection: a new therapeutic target? Trends Cardiovasc Med. 2009;19:158–64. doi: 10.1016/j.tcm.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma H, Guo R, Yu L, Zhang Y, Ren J. Aldehyde dehydrogenase 2 (ALDH2) rescues myocardial ischaemia/reperfusion injury: role of autophagy paradox and toxic aldehyde. Eur Heart J. 2011;32:1025–38. doi: 10.1093/eurheartj/ehq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanson M, et al. Oxidized low-density lipoproteins trigger endoplasmic reticulum stress in vascular cells: prevention by oxygen-regulated protein 150 expression. Circ Res. 2009;104:328–36. doi: 10.1161/CIRCRESAHA.108.183749. [DOI] [PubMed] [Google Scholar]
- 27.Liu W, et al. Protein phosphatase 2A-linked and -unlinked caspase-dependent pathways for downregulation of Akt kinase triggered by 4-hydroxynonenal. Cell Death Differ. 2003;10:772–81. doi: 10.1038/sj.cdd.4401238. [DOI] [PubMed] [Google Scholar]
- 28.Clerk A, et al. Regulation of cardiac myocyte cell death. Pharmacol Ther. 2003;97:223–61. doi: 10.1016/s0163-7258(02)00339-x. [DOI] [PubMed] [Google Scholar]
- 29.Teng X, et al. Inhibition of endoplasmic reticulum stress by intermedin1–53 protects against myocardial injury through a PI3 kinase-Akt signaling pathway. J Mol Med (Berl) 2011;89:1195–205. doi: 10.1007/s00109-011-0808-5. [DOI] [PubMed] [Google Scholar]
- 30.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–93. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 31.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 32.Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim Biophys Acta. 2010;1797:897–906. doi: 10.1016/j.bbabio.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 33.Li G, Scull C, Ozcan L, Tabas I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J Cell Biol. 2010;191:1113–25. doi: 10.1083/jcb.201006121. [DOI] [PMC free article] [PubMed] [Google Scholar]