Abstract
Supplemental choline during early stages of development can result in long-lasting improvements to memory function. In addition, pre- or postnatal choline has been shown to be protective against some of the adverse effects of early alcohol exposure. The present experiment examined whether supplemental choline given to rats would protect against the effects of post-training alcohol administration on trace fear conditioning. Post-training alcohol exposure in adolescent rats results in poor performance in this hippocampus-dependent task, although delay conditioning is unaffected. Here, rats were given an s.c. injection of either saline or choline chloride daily on postnatal days (PD) 15-26. On PD 30 subjects were trained in a trace fear conditioning procedure. For the next three days animals were administered 2.5 g/kg ethanol or water control, and CS-elicited freezing was measured on PD 34. Results indicated that post-training alcohol disrupted the expression of trace conditioning and that supplemental choline on PD 15-26 was protective against this effect. That is, choline-treated animals subsequently given post-training ethanol performed as well as animals not given ethanol. These results indicate that supplemental choline given during the periweaning period protects against ethanol-induced impairments in a hippocampus-dependent learning task. Findings contribute to the growing literature showing improvements in learning and memory in subjects given extra dietary choline during critical periods of brain development.
Keywords: choline, hippocampus, frontal cortex, fear conditioning, alcohol, adolescence
Choline is an essential nutrient that has been shown to be particularly important during early brain development (Meck & Williams, 2003; Zeisel, 2004). It not only serves as a precursor to the neurotransmitter acetylcholine, which can itself have neurotrophic effects, but also is involved in the formation of major constituents of cell membranes and intracellular signaling chemicals. Extra dietary choline, given pre- and/or postnatally to rats, can result in improved learning and memory. Supplementation with choline can speed up the normal ontogeny of spatial memory (Mellott, Williams, Meck & Blusztajn, 2004), can lead to superior spatial (Meck, Smith & Williams, 1989; Tees & Mohammadi, 1999) and temporal (Meck & Williams, 1997a) memory performance in adults, and can mitigate aspects of cognitive decline in rodents that result from normal aging processes (Glenn et al., 2008; Meck & Williams, 1997b).
Extra dietary choline given pre- or postnatally can also be protective against the adverse effects of alcohol in rodent models of fetal alcohol exposure. Several studies now show that choline supplementation can attenuate or even reverse many of the negative effects of alcohol exposure on learning and memory processes. For example, deficits in spatial learning in the Morris water maze (Ryan, Williams & Thomas, 2008), visual discrimination learning (Thomas, La Fiette, Quinn & Riley, 2000), reversal learning (Thomas, Garrison & O'Neill, 2004) and trace conditioning (Thomas & Tran, 2011; Wagner & Hunt, 2006) caused by pre- or neonatal ethanol exposure can be significantly reduced by extra choline given during or even after the ethanol exposure period. The extent to which supplemental choline given during critical periods of brain development can protect against other adverse events has not received as much attention. However, choline supplementation has been shown to protect against some of the effects of prenatal nicotine in monkeys (Slotkin et al., 2008), seizure-induced impairments in spatial memory (Yang et al., 2000) and hippocampal neural degeneration in rats (Wong-Goodrich, Glenn, Mellott, Liu, Blusztajn & Williams, 2008), and can protect against NMDA antagonist-mediated excitotoxicity in some brain regions (Guo-Ross et al., 2002). Further, reports have shown improved behavioral outcomes in transgenic mouse models of Down (Moon et al., 2010) and Rett (Nag & Berger-Sweeney, 2007) syndromes by perinatal choline supplementation.
Hunt, Levillain, Spector and Kostelnik (2009) reported that post-training exposure to alcohol in adolescent rats produces impairments in the expression of a previously-acquired hippocampal learning task, trace fear conditioning. However, delay fear conditioning, a non-hippocampal variant of the procedure, was unaffected by post-training ethanol. Binge-like alcohol exposure is known to produce profound changes in hippocampal memory, whether that alcohol exposure occurs during the neonatal period (Goodlett & Johnson, 1997), adolescence (Osborne & Butler, 1983) or adulthood (Obernier, White, Swartzwelder & Crews, 2002). Such exposure can result in changes in cell numbers and densities in hippocampal regions (Bonthius & West, 1990; Crews, Braun, Hoplight, Switzer & Knapp, 2000), as well as changes in hippocampal neurogenesis (Crews, Mdzinarishvili, Kim, He & Nixon, 2006; Hamilton et al., 2011). Ethanol can also interfere with induction of LTP, especially in the adolescent hippocampus (Pyapali, Turner, Wilson & Swartzwelder, 1999). Thus, binge-like ethanol exposure during many different developmental periods can produce significant disruptions in hippocampal anatomy and function.
Given that extra dietary choline can attenuate or perhaps even prevent ethanol-induced behavioral abnormalities in models of fetal alcohol exposure (Ryan et al., 2008; Thomas, Garrison & O'Neill, 2004; Wagner & Hunt, 2006), and may be protective against other toxic events (e.g. Guo-Ross et al., 2002; Slotkin et al., 2008), the present study was conducted to examine whether supplemental choline would also be protective against the impairing effects of post-training ethanol exposure during adolescence, as reported by Hunt et al. (2009). Choline was administered daily on postnatal days (PD) 15-26, a period sensitive to some memory-enhancing effects of choline (Meck, Williams, Cermak & Blusztajn, 2008). Animals were trained in a trace conditioning procedure on PD 30. Ethanol was given for three days after training and subjects were tested for CS-elicited freezing on PD 34. Delay conditioning was not employed since this type of learning is unaffected by post-training ethanol exposure (Hunt et al., 2009).
Methods
Subjects
The subjects were 48 Sprague-Dawley rats derived from 6 litters (24 male, 24 female), born and reared in the animal vivarium at the College of William & Mary. Male and female breeders (Charles River Laboratories, Wilmington, MA) were maintained together in 50.8 × 40.6 × 21.6 cm clear polycarbonate cages. Cages with equipped with stainless steel lids that accommodated high-protein rat chow (LabDiet Formula 5008; 2000 ppm choline) and glass water bottles. Pine shavings served as bedding material. Cages were checked daily for pups, and the day of birth was designated as PD 0. Litters were culled to 8 pups on PD 2. Pups were weaned from the home cage on PD 21, at which time they were group housed with siblings in identical polycarbonate cages for the duration of the experiment. The vivarium was maintained on a 14:10 h light:dark cycle, with light onset at 0600 h. All procedures occurred during the light phase of the cycle and were approved by the Institutional Animal Care and Use Committee of the College of William & Mary.
Apparatus
Training occurred in two identical 38.0 × 26.0 × 22.0 cm modified Skinner boxes. The two shorter walls were made of aluminum and the two longer walls and top were made of clear Plexiglas. The floor was constructed of 5 mm stainless steel bars, spaced 1.5 cm apart, connected to a constant current shock generator. The unconditioned stimulus (US) was a 1-s, 0.5mA shock delivered through the grid floor. The CS was produced by a 25-W white bulb that was located behind one of the longer clear walls. The CS was 10 s in duration and flashed at a rate of two times per second. The conditioning chambers were housed within sound insulated shells. A 4-W red bulb was mounted on an inside wall of the shell and provided low-level illumination throughout the training sessions. A PC computer was used to interface Coulbourn Instruments (Allentown, PA) software and hardware, and controlled all stimulus presentations.
Testing occurred in a novel context located in a different room in the laboratory. The two test chambers were 29.0 × 21.5 × 46.5 cm and were made of clear Plexiglas. The top and bottom of each chamber was open. The bottom 11 cm of each chamber was constructed of horizontally arranged 5 mm stainless steel rods spaced 1.5 cm apart. The chambers rested on Plexiglas floors covered with brown paper. The chambers were housed in sound-attenuating shells, each with a 7-W white bulb mounted on an inner wall that provided low-level illumination throughout the test session. The CS used for test was identical to that used during training. Test sessions were videotaped using Sony videocameras (Model CCD-TRV67).
Procedure
The eight pups from each litter were pseudorandomly assigned to one of four treatment groups comprising a 2 (saline vs. choline) × 2 (water vs. ethanol) factorial design. One male and one female pup per litter were assigned to each group. Each group had n = 12 animals, with equal numbers of males and females in each.
Choline administration
Choline was administered via subcutaneous injection, once daily, beginning on PD 15 and continuing through PD 26. Meck et al. (2008) have reported that this period of postnatal development encompasses a sensitive period for choline's beneficial effects on some brain functions. Choline chloride (Sigma, St. Louis, MO) was dissolved in saline to produce an 18.8 mg/ml solution and was injected in a constant volume of 0.1 ml per subject per day. This procedure was based on one used by Thomas, O'Neill and Dominguez (2004). Control animals were injected with an equal volume of the saline vehicle. Injections were achieved using 30 gauge needles attached to 1 cc syringes.
Trace fear conditioning
Animals were trained on PD 30 in a single 30 min session. Subjects were given 5 CS-US pairings, with inter-trial intervals ranging from 200-300 s. Each trial consisted of a 10 s presentation of the CS followed, 10 s later, by onset of the US. Thus, the trace interval was 10 s. Training sessions began with a 5 min adaptation period and subjects were removed from the training context 5 min after the last US.
Post-training ethanol administration
Procedures used were based on those described by Hunt et al. (2009). Ethanol was administered via oral gavage once daily on PD 31-33. The dose was 2.5 g/kg per day of a 20% v/v solution dissolved in tap water. Controls were given an equal volume of the tap water vehicle. Intubations were achieved using polyethylene tubing (PE-50; Intramedic, Becton Dickinson and Co., Sparks, MD) attached to 5 ml syringes.
Testing
On PD 34, approximately 24 h after the final ethanol dose, all animals were tested for CS-elicited freezing and the test session was videotaped. After a 5 min adaptation period, subjects were given 5 CS alone trials (no shocks were given during test), separated by 60-120 s intervals. Videotaped records of the test session were subsequently scored for freezing behavior by an observer blind to the experimental treatment of the animals. Freezing was defined as the absence of all visible movements except those required for respiration (Fanselow, 1980). Freezing was scored for each test trial using a time sampling procedure. For 10 s prior to CS onset (pre-CS), and for the 10 s of the CS, animals were briefly observed at 2 s intervals and a judgment was made as to whether the animal was freezing or not. The percentage of intervals scored as freezing during each epoch was calculated (0-100%).
Results
Body weights
Body weights were recorded on the first and last day of choline administration, PD 15 and 26. A mixed-factor ANOVA was used to assess the effects of choline on body weights, with choline and sex serving as between groups factors, and day as a within group factor. The analysis yielded main effects of day [F (1, 44) = 4024.18, p < .001] and sex [F (1, 44) = 10.08, p < .01] as well as a Day × Sex interaction [F (1, 44) = 12.33, p < .001]. There was no effect of choline on body weights. On PD 15 males and females weighed the same (M = 42.39 +/- 0.71 g), but by PD 26 the males weighed more than the females (Mmales = 94.04 +/- 1.30 g; Mfemales = 87.07 +/- 1.40 g).
Weights recorded during the ethanol administration procedure (PD 31-33) were also analyzed using mixed-factor ANOVA. The analysis yielded significant main effects of sex [F (1, 40) = 26.21, p < .001] and day [F (1, 80) = 584.46, p < .001], and a Sex × Day interaction [F (2, 80) = 17.69, p < .001]. There was no effect of ethanol on body weights. Females overall weighed less than males and also gained less weight from PD 31 through 33.
Pre-CS freezing
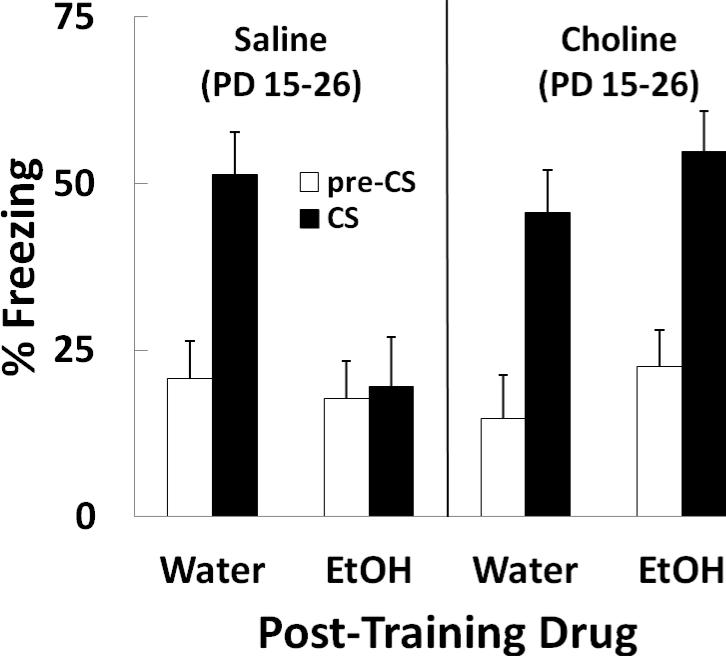
Percent pre-CS freezing was averaged across the 5 test trials and analyzed using a 2 (choline) × 2 (ethanol) × 2 (sex) between groups ANOVA. The analysis yielded no significant effects or interactions. Freezing prior to CS onset was relatively low and was equivalent across groups. Levels of pre-CS freezing are shown in Figure 1 (white bars).
Figure 1.
Mean (+/- SEM) % freezing during a baseline (pre-CS) period and during the light CS. Data are the % of intervals scored as freezing using a time-sampling procedure. Subjects were given s.c. saline or choline injections on postnatal days (PD) 15-26. On PD 30 subjects were trained using a trace fear conditioning procedure and were subsequently given i.g. water or 2.5 g/kg ethanol (EtOH) for three days. Subjects were tested for freezing on PD 34, approximately 24 h after the final ethanol exposure. Pre-CS freezing was equivalent in all groups. Post-training ethanol impaired trace conditioned responding (group Saline-EtOH), and supplemental choline prevented this effect (group Choline-EtOH).
CS freezing
Percent freezing during the CS are also shown in Figure 1 (black bars). CS freezing was averaged across the 5 test trials and also analyzed using a 2 × 2 × 2 ANOVA that was followed by Newman-Keuls post hoc tests (p < .05). The ANOVA yielded a main effect of choline [F (1, 38) = 4.14, p < .05] and a Choline × Ethanol interaction [F (1, 38) = 8.78, p < .01]. There were no effects of sex on CS freezing. Post-training ethanol exposure resulted in impaired the expression of trace conditioning, evident in the difference in CS freezing between groups Saline-Water and Saline-Ethanol. However, prior supplemental choline injections prevented this impairment by ethanol. Group Choline-Ethanol did not differ from group Choline-Water and responded with more CS freezing than group Saline-Ethanol.
Discussion
Results of this experiment demonstrate that supplemental choline given during the period surrounding weaning, PD 15-26, is protective against memory impairments induced by post-training ethanol exposure in adolescent rats. The present findings replicate those of a previous study from this lab (Hunt et al., 2009) that ethanol exposure occurring after training induces deficits in performance in a trace fear conditioning task. Hunt et al. (2009) also reported that post-training ethanol had no effect on the expression of delay fear conditioning, a non-hippocampal version of fear conditioning. The present results further indicate that animals given supplemental choline injections on PD 15-26 failed to exhibit the ethanol-induced impairment in trace conditioning. Thus, supplemental choline is protective against the effects of adolescent ethanol exposure on hippocampus-dependent learning.
The effects of post-training ethanol on the expression of trace fear does not appear to result from acute ethanol withdrawal effects. Hunt et al. (2009, Experiment 3) trained subjects in this trace procedure on PD 30, gave ethanol on PD 32-34 and tested subjects on PD 35. In this case, no ethanol-induced impairment in trace fear was observed. In this procedure, the animals were also tested 24 h after a three-day ethanol administration regimen. It is also possible that the choline supplementation protocol affected ethanol metabolism, such that blood-alcohol levels (BALs) were not as high in choline-supplemented rats. Although BALs were not recorded in this experiment, Thomas and colleagues (e.g. Thomas, Garrison & O'Neill, 2004; Thomas & Tran, 2011) have reported that neonatal choline supplementation does not affect peak blood-alcohol levels. Although Thomas’ results suggest that extra choline does not affect ethanol metabolism, the subjects in those experiments were much younger than those in the present study. Measuring BALs with the current choline and ethanol treatment protocols would be necessary to directly assess this possibility.
Acquisition in trace conditioning procedures is known to depend on intact hippocampal and prefrontal cortical functioning. Involvement of the hippocampus has been demonstrated in work employing both pre- (McEchron, Bouwmeester, Tseng, Weiss & Disterhoft, 1998) and post-training (Quinn, Oommen, Morrison & Fanselow, 2002) hippocampal lesions, and pharmacological blockade of muscarinic cholinergic receptors (Anagnostaras, Maren, Sage, Goodrich & Fanselow, 1999; Hunt & Richardson, 2007) or NMDA receptors (Quinn, Loya, Ma & Fanselow, 2005). The frontal cortex, in particular the medical prefrontal cortex (mPFC) also appears important for trace conditioning. Cells within this region exhibit bouts of activity during the trace interval in a fear conditioning procedure (Gilmartin & McEchron, 2005). Pre-training lesions of the mPFC also block acquisition of trace conditioning (Guimarais, Gregorio, Cruz, Guyon & Moita, 2011), although lesions made shortly after training are reported to have no effect (Quinn, Ma, Tinsley, Koch & Fanselow, 2008). Importantly, in all of these instances where trace conditioning is significantly disrupted, delay conditioning is typically spared. These findings argue for specific roles for the hippocampus and prefrontal cortex in trace learning, as opposed to fear conditioning more generally. As described previously, it is possible that post-training ethanol produces effects on trace conditioning because of its disruption in hippocampal and/or prefrontal function.
How choline is having its protective effects against ethanol amnesia is unclear. Supplemental choline treatments lead to improvements in cholinergic functioning, including an enhancement of depolarization-evoked ACh release in hippocampus and frontal cortex (Napoli, Blusztajn & Mellott, 2008) and decreases in AChE and ChAT activity in these same regions (Cermak, Holler, Jackson & Blusztajn, 1998). Choline supplementation has also been found to increase muscarinic receptor binding and levels of neurotrophic factors (e.g. BDNF, NGF) in hippocampus and frontal cortex (Meck et al., 1989; Napoli et al., 2008; Sandstrom, Loy & Williams, 2002). Choline also increases neurogenesis in the dentate gyrus (Glenn, Gibson, Kirby, Mellott, Blusztajn & Williams, 2007) that is important for trace conditioning (Shors, Miesegaes, Beylin, Zhao, Rydel & Gould, 2001) and lowers the threshold for LTP induction in the hippocampus (Pyapali, Turner, Williams, Meck & Swartzwelder, 1998). This suggests enhanced and more efficient cholinergic transmission in these brain regions that are important to trace fear conditioning following choline supplementation treatment.
Flesher, Butt and Kinney-Hurd (2011) reported increased acetylcholine release in hippocampus and mPFC in an appetitive trace conditioning procedure that was not observed in delay conditioning. Experience-dependent increases in acetylcholine release appears necessary for encoding, while decreases in release have been implicated in consolidation (Micheau & Marighetto, 2011). Further, hippocampal muscarinic receptors are involved in NMDA receptor-dependent synaptic plasticity (Sanchez et al., 2009; Scheiderer et al., 2008). As described previously, ethanol may have many different effects that compromise trace fear conditioning, either within the hippocampus, the frontal cortex, or both. Of interest is that many of the processes that are negatively affected by ethanol are similar to those known to be enhanced by early choline supplementation (e.g. NMDA receptor-dependent synaptic plasticity, hippocampal neurogenesis).
While the studies cited above employed prenatal or pre+postnatal choline administration, it is possible that long-term enhancements in cholinergic transmission could result from choline supplementation limited to the postnatal period. Along with the substantial postnatal development of the hippocampus (Altman & Bayer, 1975) and frontal cortex (Crews, He & Hodge, 2007), the basal forebrain cholinergic system undergoes considerable postnatal maturation during the first 4 weeks of life. Changes occurring during this time include increases in cholinergic cell body size and dendritic branching (Gould, Farris & Butcher, 1989), increased levels of ACh and ChAT (Coyle & Yamamura, 1976), and increases in the high-affinity choline uptake system and AChE activity (Abreu-Villaca, Filgueiras & Manhaes, 2011; Villalobos, Rios & Barbosa, 2001). Muscarinic receptor binding also develops during this period, achieving adult-like levels by about PD 28 (Coyle & Yamamura, 1976). Extra choline during this postnatal period may produce a cholinergic system that is more resistant to some adverse events (Meck et al., 1989; 2008; Napoli et al., 2008), although this has not been evaluated explicitly. The present results suggest that in the normally-developing animal, post-training ethanol exposure using the procedures described here is sufficient to impair some aspect of processing that result in deficits in the expression of trace fear, but that postnatal supplementation with choline yields a system that is protected in some way against the memory-impairing effects of high doses of ethanol.
A large amount of research has now shown that choline supplementation can produce benefits to brain function, but its limits need also be identified. For example, choline seems to have no beneficial effect on motor coordination deficits (Thomas, O'Neill & Dominguez, 2004) or impaired delay eyeblink conditioning (Thomas & Tran, 2011) caused by neonatal alcohol exposure, even though the choline treatment procedures were the same as those reported to successfully reverse other behavioral impairments resulting from alcohol exposure (cf. trace eyeblink conditioning; Thomas & Tran, 2011). In the present experiment, the choline treatment did not improve freezing in the Choline-Water group. This lack of effect could be the result of the training procedures producing asymptotic learning in the adolescent animal (see also Barnet & Hunt, 2005). Examining any potential enhancements in hippocampal learning processes by supplemental choline in otherwise untreated animals may be informative as to its neural basis (Mellott et al., 2004). This could easily be accomplished within the present paradigm by variations in the training procedure employed or increasing the complexity of the task with the use of longer trace intervals (Chowdhury, Quinn & Fanselow, 2005). Also, whether the benefits of periweaning choline like those reported here would extend to situations involving higher doses of ethanol, ethanol given for longer durations, or ethanol given at other times relative to learning (Crews et al., 2000; Yttri, Burk & Hunt, 2004) has not been evaluated. Nonetheless, the present experiment does indicate that extra dietary choline during this particular sensitive period of development affords at least some protection against ethanol's memory-impairing effects in adolescent rats.
Acknowledgments
This research was supported by NIH grant AA015343
References
- Abreu-Villaca Y, Filgueiras CC, Manhaes AC. Developmental aspects of the cholinergic system. Behavioural Brain Research. 2011;221:367–378. doi: 10.1016/j.bbr.2009.12.049. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer S. Postnatal development of the hippocampal dentate gyrus under normal and experimental conditions. In: Isaacson RL, Pribram KH, editors. The hippocampus: structure and development. Vol. 1. Plenum; New York: 1975. pp. 95–122. [Google Scholar]
- Anagnostaras SG, Maren S, Sage JR, Goodrich S, Fanselow MS. Scopolamine and Pavlovian fear conditioning in rats: Dose-effect analysis. Neuropsychopharmacology. 1999;21:731–744. doi: 10.1016/S0893-133X(99)00083-4. [DOI] [PubMed] [Google Scholar]
- Barnet RC, Hunt PS. Trace and long-delay fear conditioning in the developing rat. Learning and Behavior. 2005;33:437–443. doi: 10.3758/bf03193182. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, West JR. Alcohol-induced neuronal loss in developing rats: Increased brain damage with binge exposure. Alcoholism: Clinical and Experimental Research. 1990;14:107–118. doi: 10.1111/j.1530-0277.1990.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Cermak JM, Holler T, Jackson DA, Blusztajn JK. Prenatal availability of choline modifies development of the hippocampal cholinergic system. FASEB Journal. 1998;12:349–357. doi: 10.1096/fasebj.12.3.349. [DOI] [PubMed] [Google Scholar]
- Chowdhury N, Quinn JJ, Fanselow MS. Dorsal hippocampus involvement in trace fear conditioning with long, but not short, trace intervals in mice. Behavioral Neuroscience. 2005;119:1396–1402. doi: 10.1037/0735-7044.119.5.1396. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Yamamura HI. Neurochemical aspects of the ontogenesis of cholinergic neurons in the rat brain. Brain Research. 1976;118:429–440. doi: 10.1016/0006-8993(76)90310-3. [DOI] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcoholism: Clinical and Experimental Research. 2000;24:1712–1723. [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: A critical period of vulnerability for addiction. Pharmacology, Biochemistry and Behavior. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience. 2006;137:437–445. doi: 10.1016/j.neuroscience.2005.08.090. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Conditional and unconditional components of post-shock freezing. Pavlovian Journal of Biological Sciences. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Flesher MM, Butt AE, Kinney-Hurd BL. Differential acetylcholine release in the prefrontal cortex and hippocampus during Pavlovian trace and delay conditioning. Neurobiology of Learning and Memory. 2011;96:181–191. doi: 10.1016/j.nlm.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, McEchron MD. Single neurons in the medial prefrontal cortex of the rat exhibit tonic and phasic coding during trace fear conditioning. Behavioral Neuroscience. 2005;119:1496–1510. doi: 10.1037/0735-7044.119.6.1496. [DOI] [PubMed] [Google Scholar]
- Glenn MJ, Gibson EM, Kirby ED, Mellott TJ, Blusztajn JK, Williams CL. Prenatal choline availability modulates hippocampal neurogenesis and neurogenic responses to enriching experiences in adult female rats. European Journal of Neuroscience. 2007;25:2473–2482. doi: 10.1111/j.1460-9568.2007.05505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn MJ, Kirby ED, Gibson EM, Wong-Goodrich SJ, Mellott TJ, Blusztajn JK, Williams CL. Age-related declines in exploratory behavior and markers of hippocampal plasticity are attenuated by prenatal choline supplementation in rats. Brain Research. 2008;1237:110–123. doi: 10.1016/j.brainres.2008.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlett CR, Johnson TB. Neonatal binge ethanol exposure using intubation: Timing and dose effects on place learning. Neurotoxicology and Teratology. 1997;19:435–446. doi: 10.1016/s0892-0362(97)00062-7. [DOI] [PubMed] [Google Scholar]
- Gould E, Farris W, Butcher LL. Basal forebrain neurons undergo somatal and dendritic remodeling during postnatal development: A single-section Golgi and choline acetyltransferase analysis. Developmental Brain Research. 1989;46:297–302. doi: 10.1016/0165-3806(89)90293-9. [DOI] [PubMed] [Google Scholar]
- Guimarais M, Gregorio A, Cruz A, Guyon N, Moita MA. Time determines the neural circuit underlying associative fear learning. Frontiers in Behavioral Neuroscience. 2011 Dec 27;5 doi: 10.3389/fnbeh.2011.00089. Article 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo-Ross SX, Clark S, Montoya DAC, Jones KH, Obernier J, Shetty AK, White AM, Blusztajn JK, Wilson WA, Swartzwelder HS. Prenatal choline supplementation protects against postnatal neurotoxicity. Journal of Neuroscience. 2002;22:RC195. doi: 10.1523/JNEUROSCI.22-01-j0005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton GF, Murawski NJ, St. Cyr SA, Jablonski SA, Schiffino FL, Stanton ME, Klintsova AY. Neonatal alcohol exposure disrupts hippocampal neurogenesis and contextual fear conditioning in adult rats. Brain Research. 2011;1412:88–101. doi: 10.1016/j.brainres.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PS, Levillain ME, Spector BM, Kostelnik LA. Post-training ethanol disrupts trace conditioned fear in rats: Effects of timing of ethanol, dose and trace interval duration. Neurobiology of Learning and Memory. 2009;91:73–80. doi: 10.1016/j.nlm.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PS, Richardson R. Pharmacological dissociation of trace and long-delay fear conditioning in young rats. Neurobiology of Learning and Memory. 2007;87:86–92. doi: 10.1016/j.nlm.2006.06.003. [DOI] [PubMed] [Google Scholar]
- McEchron M, Bouwmeester H, Tseng W, Weiss C, Disterhoft J. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus. 1998;8:638–646. doi: 10.1002/(SICI)1098-1063(1998)8:6<638::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Meck WH, Smith RA, Williams CL. Organizational changes in cholinergic activity and enhanced visuospatial memory as a function of choline administered prenatally or postnatally or both. Behavioral Neuroscience. 1989;103:1234–1241. doi: 10.1037//0735-7044.103.6.1234. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Characterization of the facilitative effects of perinatal choline supplementation on timing and temporal memory. Neuroreport. 1997a;8:2831–2835. doi: 10.1097/00001756-199709080-00005. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Simultaneous temporal processing is sensitive to prenatal choline availability in mature and aged rats. Neuroreport. 1997b;8:3045–3053. doi: 10.1097/00001756-199709290-00009. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: Implications for memory and attentional processing across the lifespan. Neuroscience and Biobehavioral Reviews. 2003;27:385–399. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL, Cermak JM, Blusztajn JK. Developmental periods of choline sensitivity provide an ontogenetic mechanism for regulating memory capacity and age-related dementia. Frontiers in Integrative Neuroscience. 2008;1:1–11. doi: 10.3389/neuro.07.007.2007. Article 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellott TJ, Williams CL, Meck WH, Blusztajn JK. Prenatal choline supplementation advances hippocampal development and enhanced MAPK and CREB activation. FASEB Journal. 2004;18:545–547. doi: 10.1096/fj.03-0877fje. [DOI] [PubMed] [Google Scholar]
- Micheau J, Marighetto A. Acetylcholine and memory: A long, complex and chaotic but still living relationship. Behavioural Brain Research. 2011;221:424–429. doi: 10.1016/j.bbr.2010.11.052. [DOI] [PubMed] [Google Scholar]
- Moon J, Chen M, Gandhy SU, Strawderman M, Levitsky DA, Maclean KN, Strupp BJ. Perinatal choline supplementation improves cognitive functioning and emotion regulation in the Ts65Dn mouse model of Down syndrome. Behavioral Neuroscience. 2010;124:346–361. doi: 10.1037/a0019590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag N, Berger-Sweeney JE. Postnatal dietary choline supplementation alters behavior in a mouse model of Rett syndrome. Neurobiology of Disease. 2007;26:473–480. doi: 10.1016/j.nbd.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Napoli I, Blusztajn JK, Mellott TJ. Prenatal choline supplementation in rats increases the expression of IGF2 and its receptor IGF2R and enhances IGF2-induced acetylcholine release in hippocampus and frontal cortex. Brain Research. 2008;1237:124–135. doi: 10.1016/j.brainres.2008.08.046. [DOI] [PubMed] [Google Scholar]
- Obernier JA, White AM, Swartzwelder HS, Crews FT. Cognitive deficits and CNS damage after a 4-day binge ethanol exposure in rats. Pharmacology, Biochemistry and Behavior. 2002;72:521–532. doi: 10.1016/s0091-3057(02)00715-3. [DOI] [PubMed] [Google Scholar]
- Osborne GL, Butler AC. Enduring effects of periadolescent alcohol exposure on passive avoidance performance in rats. Physiological Psychology. 1983;11:205–208. [Google Scholar]
- Pyapali GK, Turner DA, Williams CL, Meck WH, Swartzwelder HS. Prenatal dietary choline supplementation decreases the threshold for induction of long-term potentiation in young adult rats. Journal of Neurophysiology. 1998;79:1790–1796. doi: 10.1152/jn.1998.79.4.1790. [DOI] [PubMed] [Google Scholar]
- Pyapali GK, Turner DA, Wilson WA, Swartzwelder HS. Age and dose-dependent effects of ethanol on the induction of hippocampal long-term potentiation. Alcohol. 1999;19:107–111. doi: 10.1016/s0741-8329(99)00021-x. [DOI] [PubMed] [Google Scholar]
- Quinn J, Loya F, Ma Q, Faneslow M. Dorsal hippocampus NMDA receptors differentially mediate trace and contextual fear conditioning. Hippocampus. 2005;15:665–674. doi: 10.1002/hipo.20088. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Ma QD, Tinsley MR, Koch C, Fanselow MS. Inverse temporal contributions of the dorsal hippocampus and medial prefrontal cortex to the expression of long-term fear memories. Learning and Memory. 2008;15:368–372. doi: 10.1101/lm.813608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JJ, Oommen SS, Morrison GE, Fanselow MS. Post-training excitotoxic lesions of the dorsal hippocampus attenuate forward trace, backward trace, and delay fear conditioning in a temporally specific manner. Hippocampus. 2002;12:495–504. doi: 10.1002/hipo.10029. [DOI] [PubMed] [Google Scholar]
- Ryan SH, Williams JK, Thomas JD. Choline supplementation attenuates learning deficits associated with neonatal alcohol exposure in the rat: Effects of varying the timing of choline administration. Brain Research. 2008;1237:91–100. doi: 10.1016/j.brainres.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez G, de Oliveira Alvares L, Oberholzer MV, Genro B, Quillfeldt J, Costa da Costa J, Cervanañsky C, Jerusalinsky D, Kornisiuk E. M4 muscarinic receptors are involved in modulation of neurotransmission at synapses of Schaffer collaterals on CA1 hippocampal neurons in rats. Journal of Neuroscience Research. 2009;87:691–700. doi: 10.1002/jnr.21876. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Loy R, Williams CL. Prenatal choline supplementation increases NGF levels in the hippocampus and frontal cortex of young and adult rats. Brain Research. 2002;947:9–16. doi: 10.1016/s0006-8993(02)02900-1. [DOI] [PubMed] [Google Scholar]
- Scheiderer CL, Smith CC, McCutchen E, McCoy PA, Thacker EE, Kolasa K, Dobrunz LE, McMahon LL. Coactivation of M1 muscarinic and α1 adrenergic receptors stimulates extracellular signal-regulated protein kinase and induces long-term depression at CA3-CA1 synapses in rat hippocampus. Journal of Neuroscience. 2008;28:5350–5358. doi: 10.1523/JNEUROSCI.5058-06.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ, Qiao D, Aldridge JE, Tate CA, Cousins MM, Proskocil BJ, Sekhon HS, Clark JA, Lupo SL, Spindel ER. Effects of prenatal nicotine exposure on primate brain development and attempted amelioration with supplemental choline or Vitamin C: Neurotransmitter receptors, cell signaling and cell development markers in fetal brain regions of rhesus monkeys. Neuropsychopharmacology. 2008;30:129–144. doi: 10.1038/sj.npp.1300544. [DOI] [PubMed] [Google Scholar]
- Tees RC, Mohammadi E. The effects of neonatal choline dietary supplementation on adult spatial and configural learning and memory in rats. Developmental Psychobiology. 1999;35:226–240. [PubMed] [Google Scholar]
- Thomas JD, Garrison M, O'Neill TM. Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicology and Teratology. 2004;26:35–45. doi: 10.1016/j.ntt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Thomas JD, La Fiette MH, Quinn VRE, Riley EP. Neonatal choline supplementation ameliorates the effects of prenatal alcohol exposure on a discrimination learning task in rats. Neurotoxicology and Teratology. 2000;22:703–711. doi: 10.1016/s0892-0362(00)00097-0. [DOI] [PubMed] [Google Scholar]
- Thomas JD, O'Neill TM, Dominguez HD. Perinatal choline supplementation does not mitigate motor coordination deficits associated with neonatal alcohol exposure in rats. Neurotoxicology and Teratology. 2004;26:223–229. doi: 10.1016/j.ntt.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Tran TD. Choline supplementation mitigates trace, but not delay, eyeblink conditioning deficits in rats exposed to alcohol during development. Hippocampus. 2011 doi: 10.1002/hipo.20925. DOI: 10.1002/hippo.20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos J, Rios O, Barbosa M. Postnatal development of cholinergic system in mouse basal forebrain: acetylcholinesterase histochemistry and choline-acetyltransferase immunoreactivity. International Journal fo Developmental Neuroscience. 2001;19:495–502. doi: 10.1016/s0736-5748(01)00034-x. [DOI] [PubMed] [Google Scholar]
- Wagner AF, Hunt PS. Impaired trace fear conditioning following neonatal ethanol: Reversal by choline. Behavioral Neuroscience. 2006;120:482–487. doi: 10.1037/0735-7044.120.2.482. [DOI] [PubMed] [Google Scholar]
- Wong-Goodrich SJ, Mellott TJ, Glenn MJ, Blusztajn JK, Williams CL. Prenatal choline supplementation attenuates neuropathological response to status epilepticus in the adult rat hippocampus. Neurobiology of Disease. 2008;30:255–269. doi: 10.1016/j.nbd.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Liu Z, Cermak JM, Tandon P, Sarkisian MR, Stafstrom CE, Neill JC, Blusztajn JK, Holmes GL. Protective effects of prenatal choline supplementation on seizure-induced memory impairment. Journal of Neuroscience. 2000;20:RC109. doi: 10.1523/JNEUROSCI.20-22-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yttri EA, Burk JA, Hunt PS. Intermittent ethanol exposure in adolescent rats: Dose-dependent impairment in trace conditioning. Alcoholism: Clinical and Experimental Research. 2004;28:1433–1436. doi: 10.1097/01.alc.0000147657.51745.a7. [DOI] [PubMed] [Google Scholar]
- Zeisel SH. Nutritional importance of choline for brain development. Journal of the American College of Nutrition. 2004;23:621S–626S. doi: 10.1080/07315724.2004.10719433. [DOI] [PubMed] [Google Scholar]