Abstract
Objective
To explore academic outcomes in childhood-onset systemic lupus erythematosus (cSLE) and their relationship to variables such as demographic and socioeconomic status, neurocognitive functioning, behavioral/emotional adjustment, and cSLE disease status.
Methods
Forty pairs of children diagnosed with cSLE and healthy best-friend controls were rated by parents on a standardized scale of school competence. Information about participants’ demographic and socioeconomic status was obtained, along with measures of cSLE disease activity and damage. All participants received formal neurocognitive testing and were also rated on standardized scales of behavioral/emotional adjustment and executive functioning.
Results
Compared to healthy controls, school competence was rated as lower in the cSLE group, although the groups did not differ significantly on indices of cognitive, behavioral, emotional, or executive functioning. School competence ratings were correlated with reading and mathematics achievement test scores in both groups, and with ratings of mental self-regulation in the cSLE group. School competence ratings were correlated with measures of cSLE disease activity and treatment intensity.
Conclusion
cSLE is associated with inferior parent-rated academic outcomes compared to those noted in demographically-matched peers, despite similar neurocognitive function. The adverse academic outcomes which distinguish children with cSLE from their demographically-matched peers appear to be mediated by SLE disease activity and treatment.
Keywords: SLE, Children, cognition, NPSLE
INTRODUCTION
Systemic lupus erythematosus (SLE) is associated with significant morbidity, negatively affecting health-related quality of life (HRQoL) (1). Academic functioning represents a critical aspect of HRQoL in childhood and adolescence. However, it has attracted surprisingly limited research interest, despite concerns that children with SLE who perform poorly in school go on to meet fewer educational milestones (e.g., high school or college graduation), have less long-term occupational success, and experience higher rates of adult mental illness and substance abuse (2-6). The available data suggest that children with cSLE (childhood-onset systemic lupus erythematosus) are at risk for adverse academic outcomes (1,7,8). If cSLE affects academic functioning, then it is important to explore the mechanisms that underlie this effect and incorporate this knowledge into disease management.
In adults, adverse effects of SLE have been shown to impair cognitive and psychiatric functioning (9). Despite reports that cSLE is associated with cognitive deficits (10) and psychiatric morbidity (11,12), most previous studies have been limited to chart reviews lacking a well-matched control group. Conversely, a recent case-control study found the rate of neurocognitive dysfunction (NCD) in cSLE to be elevated relative to general population norms but comparable to that of controls who were closely matched on demographic variables (13). This methodologically-rigorous study raises questions regarding the contribution of cSLE disease and treatment factors to cognitive and psychiatric morbidity. It also highlights the importance of exploring other factors besides NCD that might contribute to lower school functioning in patients with cSLE.
The current study used a case-control design to achieve three goals: (1) determine whether individuals with cSLE have worse academic functioning than their peers of similar demographic and socioeconomic backgrounds; (2) investigate whether these matched groups differ with respect to cognitive, behavioral, and emotional functioning; and (3) explore demographic, cognitive, behavioral, emotional, and disease-related correlates of school functioning within samples of children with cSLE and unaffected peers.
MATERIALS & METHODS
Subjects
Forty children and adolescents with cSLE and 40 same sex best-friend controls of similar demographic characteristics participated in a study of functional and structural neuroimaging and cognitive functioning in cSLE, conducted at two tertiary pediatric rheumatology centers. Analyses of neuroimaging data from the study will be presented in a separate report, and are not discussed herein.
All participants spoke English as their primary language. This study was approved by the institutional review boards of both institutions and is in accordance with the ethical standards established in the 1964 Declaration of Helsinki. Prior to participation, the study was explained to each participant and their parent, and written informed consent obtained from parents of all participants. Written assent was also obtained from all participants over 11 years of age.
Childhood-onset SLE
Participants with cSLE fulfilled the updated American College of Rheumatology classification criteria prior to 17 years of age (14). To be eligible for participation, a patient had to be between the ages of 9 and 18 at the time of enrollment in the study. Patients with cSLE were excluded from participation if they had a history of comorbid conditions affecting their neurocognitive functioning prior to the diagnosis of cSLE, and if they had known structural brain abnormalities, neuropathies, or movement disorders.
Controls
Each index patient with cSLE was asked to identify a friend who was within one year of their age, of the same gender, and in the same school grade. This “best-friend approach” has been shown to result in good case-control matches on demographic variables (15). Controls had to be healthy, without known structural brain abnormalities or known NCD. No potential controls needed to be excluded from participation by these criteria.
Study assessment
In this cross-sectional study, participants were evaluated during a dedicated research visit lasting approximately 3 to 4 hours. Besides a physical examination and a review of systems, all study participants underwent thorough neurocognitive assessment. Data collected during this research visit are described below.
Demographics
Demographic information related to ethnicity, maternal education, number of parents in household, and family income was collected by parent report.
Disease Activity and Severity (cSLE subjects only)
Disease activity was measured using the SLEDAI-2K (Systemic Lupus Erythematosus Disease Activity Index) and the BILAG (British Isles Lupus Activity Group Index), while disease damage was assessed using the SDI (Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index) (16,17). The daily dose of oral prednisone (if prescribed) was recorded as a surrogate of treatment intensity.
Academic Functioning
Academic functioning was assessed via the School Competence scale of the Child Behavior Checklist (CBCL), a standardized questionnaire completed by parents of study participants (18). Academic functioning can be difficult to measure due to lack of consistent access to original school records, variations in grading schemes across schools and grade/age levels, and the fact that academic knowledge (assessed via formal testing) is only one of several contributors to classroom performance. Consistent with our goal of assessing classroom performance relative to norms on a common metric, the CBCL School Competence scale is comprised of standardized ratings of the child's functioning across multiple academic domains (e.g., reading/language arts, arithmetic), as well as implementation of academic interventions. Ratings on the CBCL School Competence scale are converted to a single T-score based on age- and sex-linked norms, with higher scores indicating better functioning.
Table 1 provides descriptions of the other psychometric information obtained from participants via parent questionnaires and formal neurocognitive testing, along with the functional domains they measure. Further details about these measures are presented below.
Table 1.
Descriptions of psychometric variables
| Name of Variable | Functional Domain | Population Norms (mean ± SD) | Valence (Higher Scores Reflect...) |
|---|---|---|---|
| Wechsler Abbreviated Scale of Intelligence (WASI): Full Scale IQ | General intelligence | 100 ± 15 | Better Functioning |
| Wechsler Intelligence Scales*: Working Memory Index | Working memory | 100 ± 15 | Better Functioning |
| Wechsler Intelligence Scales*: Processing Speed Index | Psychomotor speed | 100 ± 15 | Better Functioning |
| Wide Range Assessment of Memory and Learning-II (WRAML-II): Memory Screening Index | Verbal and visual memory | 100 ± 15 | Better Functioning |
| Woodcock-Johnson –III Tests of Achievement: Letter-Word Identification subtest | Reading decoding skills | 100 ± 15 | Better Functioning |
| Woodcock-Johnson –III Tests of Achievement: Calculation subtest | Arithmetic calculation skills | 100 ± 15 | Better Functioning |
| Conners Continuous Performance Test – II (CPT-II): Omissions | Attention | 50 ± 10 | Worse Functioning |
| Conners Continuous Performance Test – II (CPT-II): Commissions | Attention/impulsivity | 50 ± 10 | Worse Functioning |
| Conners Continuous Performance Test – II (CPT-II): Hit Reaction Time Standard Error | Attention | 50 ± 10 | Worse Functioning |
| Child Behavior Checklist (CBCL): Anxiety/Depression Scale | Parent-reported symptoms of depression and anxiety | 50 ± 10 | Worse Functioning |
| Child Behavior Checklist (CBCL): Externalizing Problems | Parent-reported symptoms of externalizing behavior (e.g., aggression, conduct problems) | 50 ± 10 | Worse Functioning |
| Child Behavior Checklist (CBCL): Total Problems | Overall parent-report behavior and emotion symptoms | 50 ± 10 | Worse Functioning |
| Children's Depression Inventory: Total Score | Child's report of depression symptoms | 50 ± 10 | Worse Functioning |
| Behavior Rating Inventory of Executive Function (BRIEF): Behavioral Regulation Index | Parent-reported behavioral self-regulation (e.g., impulse control, emotional control) | 50 ± 10 | Worse Functioning |
| Behavior Rating Inventory of Executive Function (BRIEF): Metacognition Index | Parent-reported mental self-regulation (e.g., organization, planning, self-initiation) | 50 ± 10 | Worse Functioning |
| Behavior Rating Inventory of Executive Function (BRIEF): Global Executive Composite | Overall self-regulation | 50 ± 10 | Worse Functioning |
See the text for citations related to each measure.
Wechsler Intelligence Scales = Wechsler Intelligence Scale for Children, 4th Ed (<17 years) and Wechsler Adult Intelligence Scale, 4th Ed (17+ years).
Behavioral and Emotional Functioning
Participants’ behavioral and emotional functioning was obtained by parent report on the CBCL (18). Three CBCL indices were studied. The Externalizing Problems index assesses delinquent and aggressive behaviors. The Anxious/Depressed subscale focuses on mood symptoms and was chosen over the broader Internalizing Index because the latter includes physical symptoms that could reflect legitimate medical concerns, rather than mood (19). The Total Problems index is an overall composite of behavioral and emotional concerns.
The Children's Depression Inventory was completed by participants as a self-report measure of depressive symptomatology (20).
Executive Functioning in Daily Life
Because executive functioning (e.g., behavior regulation, metacognitive skills such as planning and organization) can be very difficult to validly assess using formal one-on-one neuropsychological tests, parents completed the Behavior Rating Inventory of Executive Functioning (BRIEF) (21), a standardized questionnaire. The Behavioral Regulation, Metacognition, and Global Executive Composite scales of the BRIEF were considered in this study.
Formal Neurocognitive Testing
All participants underwent formal neurocognitive testing performed by a trained psychometrician, using a standardized neuropsychological battery for cSLE with details provided elsewhere (22). In brief, the battery consisted of the Wechsler Abbreviated Scale of Intelligence (WASI) (23) which is a well-validated measure of overall intelligence (Full Scale IQ); Working Memory and Processing Speed subscales of the age-appropriate Wechsler Intelligence Scales (24,25) which measure working memory and psychomotor speed, respectively; Wide Range Assessment of Memory and Learning-2 (WRAML-2) (26), from which the Memory Screening Index summarizes an individual's ability to learn and recall new verbal and visual information; selected subtests from the Woodcock-Johnson–III Tests of Achievement (27) which assessed basic reading/decoding ability (WJ-III Letter-Word Identification) and written arithmetic skills (WJ-III Calculation); and the Conners’ Continuous Performance Test–II (28) which assesses test-takers’ ability to sustain attention (CPT-II Omissions, CPT-II Mean Hit Reaction Time Standard Error) and inhibit impulsive responses (CPT-II Commissions) during a long, boring task. Age-normed scores are available for all instruments (22).
Statistical analysis
Primary analyses used paired-sample t-tests to compare means between the cSLE patients and their demographically matched best-friends (controls); and Pearson's correlation coefficients (r's) to assess relationships between continuous variables. In addition, nonparametric Wilcoxon's signed rank tests and Spearman's correlation coefficients were used as supplementary analyses for paired-sample t- tests and Pearson's r's, respectively. Multivariate analyses, including mixed effect models and partial correlation coefficients, were used to compare means and assess relationships after adjusting for social-demographic characteristics. Only results from primary analyses are presented in the paper, as there were no important discrepancies between nonparametric and parametric analyses. For categorical variables, associations with cSLE/control group membership were assessed using logistical models after adjusting for within-pair correlations using a GEE method. For numerical variables in the cSLE group, their means were compared to those of population norms using z-tests. Strength of correlation was considered “very strong”, “strong”, “moderate”, “weak”, and “poor” if the magnitude of r was 0.9~1, 0.7~0.9, 0.5~0.7, 0.3~0.5 and 0~0.3 respectively (29). Statistical computations were performed using a SAS 9.3 software (SAS, Cary, NC) package. P-values <0.05 were considered statistically significant.
RESULTS
Demographics of the study participants and disease information about the cSLE group are provided in Table 2. Patients with cSLE were somewhat older than the best-friend controls but otherwise the groups were closely matched on major demographic indices. The mean disease duration of the cSLE group was about 2 years, with mild to moderate disease activity and disease-related damage. Prednisone therapy was used in 31 (77.5%) of the cSLE patients.
Table 2.
Demographics of study population*
| Variable | Category | cSLE (n=40) | Controls (n=40) | p-value |
|---|---|---|---|---|
| Age at enrollment (years) | 14.8 ± 2.3 | 13.9 ± 3.2 | 0.03 | |
| Gender | Female | 85.0% | 85.0% | 1.0 |
| Ethnicity | White | 30.0% | 32.5% | 0.98 |
| Black | 45.0% | 47.5% | ||
| Hispanic | 17.5% | 15.0% | ||
| Asian and other | 7.5% | 5% | ||
| Grade Level | Elementary School (4-6) | 20.0% | 20.0% | 1.0 |
| Middle School (7-8) | 17.5% | 17.5% | ||
| High School (9-12) | 62.5% | 62.5% | ||
| Maternal education level | No High School Diploma | 7.5% | 10.0% | 0.7 |
| Completed High School Diploma | 30.0% | 37.5% | ||
| Education Beyond High School | 62.5% | 52.5% | ||
| Family Income | < $25,000 | 20.0% | 15.8% | 0.81 |
| $26-$50,000 | 35.0% | 34.2% | ||
| $51-$75,000 | 20.0% | 28.9% | ||
| >$75,000 | 25.0% | 21.1% | ||
| cSLE Duration (months) | 23.7 ± 23.1 | |||
| Physician assessment of disease activity† | 2.4 ± 2.0 | |||
| Disease activity (SLEDAI-2k)‡ | 4.9 ± 4.4 | |||
| Disease activity (BILAG)§ | 3.0 ± 3.8 | |||
| Disease damage (SDI)∥ | 0.4 ± 0.8 | |||
| On Prednisone therapy | 77.5% | |||
| Prednisone daily dose [mg] (N=31) | 19.8 ± 17.4 | |||
Except where indicated otherwise, values are mean ± SD; cSLE = childhood-onset systemic lupus erythematosus.
Measured on categorical Likert scale with 0 = inactive cSLE; 10 = very active cSLE.
Systemic Lupus Disease Activity Index; range 0 – 104; 0 = inactive cSLE.
British Isles Lupus Activity Group Index; A=9; B= 3; C= 1; D or E= 0; lower scores indicate lower cSLE activity.
Systemic Lupus Collaborating Clinics/American College of Rheumatology damage index.
Academic functioning of cSLE and control groups
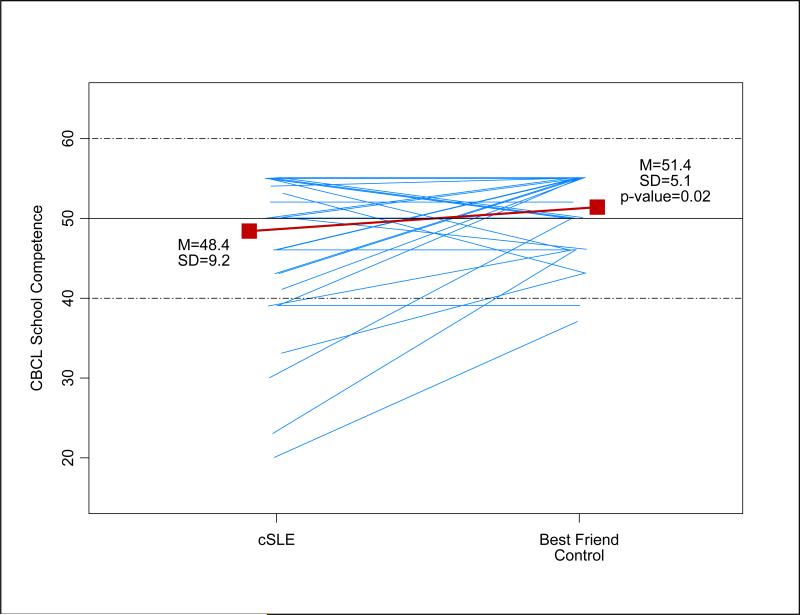
Parent-rated school competence, as measured by the CBCL, was significantly lower in the cSLE group than the control group (mean ± SD: 48.4 ± 9.2 versus 51.4 ± 5.1, p = 0.02). Figure 1 presents a plot of the CBCL School Competence T-Score ratings for each cSLE best-friend control pair. Although the mean score for both groups fell within the normal range, greater variability is apparent in the cSLE group.
Figure 1. Plots of CBCL School Competence t -scores between cSLE's and paired best friends (controls).
Each blue line represents a cSLE-best friend pair; a red solid square represents the sample mean; the horizontal solid line represents the normative mean; and two doted lines represent ±1 normative standard deviation (SD) from the normative mean.
Comparisons between cSLE and control subjects on indices of cognitive, behavioral, emotional, and executive function
As shown in Table 3, the cSLE and demographically-matched control groups were not significantly different in their performance during formal neurocognitive testing or on measures of behavioral, emotional, or executive functioning (CBCL, BRIEF, CDI). Conversely, compared to published norms, the cSLE group had significantly weaker scores on the Wechsler Working Memory and Processing Speed Indexes, and significantly better scores than published norms on the CBCL Externalizing Problems and BRIEF Behavior Regulation indexes, and on the CDI Total Score of overall depressive symptoms.
Table 3.
Group comparisons for cognitive, behavioral, emotional, and executive functioning indices*
| p-value |
||||
|---|---|---|---|---|
| Variable | cSLE | Controls | cSLE vs Controls† | cSLE vs Population Norms‡ |
| WASI Full Scale IQ | 101.0 ± 11.6 | 98.6 ± 12.6 | 0.24 | 0.62 |
| Wechsler Working Memory Index | 90.4 ± 20.0 | 94.3 ± 13.3 | 0.27 | <0.01 |
| Wechsler Processing Speed Index | 94.3 ± 21.2 | 100.0 ± 13.0 | 0.09 | 0.02 |
| WRAML-II Screening Index | 102.8 ± 14.1 | 100.2 ± 14.0 | 0.37 | 0.23 |
| WJ-III Letter-Word Identification | 96.9 ± 10.4 | 96.3 ± 10.6 | 0.74 | 0.20 |
| WJ-III Calculation | 96.8 ± 14.5 | 94.2 ± 15.6 | 0.31 | 0.20 |
| CPT-II Omissions | 52.6 ± 13.8 | 55.8 ± 17.3 | 0.37 | 0.10 |
| CPT-II Commissions | 48.5 ± 7.4 | 50.4 ± 10.3 | 0.35 | 0.34 |
| CPT-II Hit Reaction Time Standard Error | 48.6 ± 10.7 | 49.6 ± 10.0 | 0.64 | 0.36 |
| CBCL Anxiety/Depression Scale | 52.2 ± 4.0 | 52.5 ± 4.0 | 0.71 | 0.17 |
| CBCL Externalizing Problems | 46.0 ± 8.0 | 47.1 ± 8.6 | 0.49 | 0.01 |
| CBCL Total Problems | 48.3 ± 10.0 | 47.2 ± 8.3 | 0.54 | 0.28 |
| CDI Total Score | 43.8 ± 7.7 | 44.3 ± 8.0 | 0.76 | <0.01 |
| BRIEF Behavioral Regulation Index | 46.4 ± 7.0 | 47.6 ± 7.6 | 0.44 | 0.02 |
| BRIEF Metacognition Index | 49.0 ± 10.0 | 48.0 ± 7.4 | 0.59 | 0.54 |
| BRIEF Global Executive Composite | 47.8 ± 8.5 | 47.6 ± 7.2 | 0.88 | 0.16 |
All values are mean ± SD; cSLE = childhood-onset systemic lupus erythematosus.
Paired-samples t-test
Single-sample z-test
WASI = Wechsler Abbreviated Scale of Intelligence; Wechsler = Wechsler Intelligence Scale for Children, 4th Ed (<17 years) and Wechsler Adult Intelligence Scale, 4th Ed (17+ years); WRAML-II = Wide Range Assessment of Memory and Learning, 2nd Ed; WJ-III = Woodcock-Johnson Tests of Achievement, 3rd Ed; CPT-II = Conners’ Continuous Performance Test, 2nd Ed. BRIEF = Behavior Rating Inventory of Executive Function; CBCL = Child Behavior Checklist; CDI = Children's Depression Inventory. See Table 1 and text for further detail about psychometric variables.
Correlates of academic functioning
Table 4 summarizes the associations between CBCL School Competence ratings and the behavioral, emotional, executive and cognitive functioning of participants in the cSLE and matched control groups. Importantly, correlations of these variables with school competence did not differ significantly between the two groups. Variables that were associated with school performance in the cSLE group were, for the most part, similarly strongly associated in the control group. Not surprisingly, CBCL School Competence ratings were moderately correlated with performance on Woodcock-Johnson Achievement subtests measuring reading decoding and math calculation skills. Interestingly, while we observed expected weak correlations for the overall sample between School Competence ratings and the Wechsler Working Memory and Processing Speed indices, School Competence was not significantly correlated with measures of overall intelligence, sustained attention, or impulse control. Significant correlations were found, however, between School Competence ratings and the BRIEF Metacognition index and Global Executive Composite, within the cSLE group and in the overall sample.
Table 4.
Psychometric correlates of school functioning in cSLE, controls, and entire study population
| Variable | cSLE | Controls | Combined Sample |
|---|---|---|---|
| WASI Full Scale IQ | 0.17 | 0.31 | 0.19 |
| Wechsler Working Memory Index | 0.27 | 0.23 | 0.27* |
| Wechsler Processing Speed Index | 0.20 | 0.31 | 0.25* |
| WRAML-II Screening Index | 0.04 | 0.07 | 0.04 |
| WJ-III Letter-Word Identification | 0.48** | 0.33* | 0.40** |
| WJ-III Calculation | 0.42** | 0.37* | 0.37* |
| CPT-II Omissions | -0.02 | -0.06 | -0.01 |
| CPT-II Commissions | -0.10 | -0.23 | -0.13 |
| CPT-II Hit Reaction Time Standard Error | -0.04 | 0.05 | 0.00 |
| CBCL Anxiety/Depression Scale | -0.24 | -0.30 | -0.23* |
| CBCL Externalizing Problems | -0.14 | -0.07 | -0.09 |
| CBCL Total Problems | -0.26 | -0.25 | -0.26* |
| CDI Total Score | -0.17 | -0.31 | -0.20 |
| BRIEF Behavioral Regulation Index | -0.16 | -0.28 | -0.17 |
| BRIEF Metacognition Index | -0.54** | -0.19 | -0.44** |
| BRIEF Global Executive Composite | -0.46** | -0.28 | -0.40** |
p < .05
p < .005
All values are Pearson correlations; cSLE = childhood-onset systemic lupus erythematosus.
Although some of the correlations superficially differed across the cSLE and Control groups, none of these differences were statistically significant (see text).
See legend for Table 3 for variable names.
In addition to the measures collected for all participants, we assessed the relationship between CBCL School Competence and measures of cSLE activity, damage and treatment intensity. As shown in Table 5, School Competence was significantly related to disease activity as indicated by both the SLEDAI and BILAG indices, and also to higher prednisone doses.
Table 5.
Disease-related correlates of school functioning in cSLE.
| Variable | |
|---|---|
| SLE duration [months] | 0.10 |
| Physician assessment of disease activity | -0.18 |
| Disease activity (SLEDAI) | -0.55** |
| Disease activity (BILAG) | -0.54** |
| Disease damage (SDI) | -0.22 |
| Prednisone daily dose [mg] | -0.40* |
All values are Pearson correlations
p < .05
p < .005
cSLE = childhood-onset systemic lupus erythematosus, SLEDAI = Systemic Lupus Disease Activity Index; BILAG = British Isles Lupus Activity Group Index; SDI = Systemic Lupus Collaborating Clinics/American College of Rheumatology Damage index.
DISCUSSION
We found that children with SLE have significantly inferior academic outcomes than their peers, despite indistinguishable performance levels on neuropsychological tests and equivalent ratings of externalizing behavior, mood, and executive functioning. These academic outcome findings are in line with our previous research (1), which revealed parent-and self-report ratings of school functioning on the PedsQL™ to be significantly lower in cSLE than a normal national comparison sample (30). Our current results extend those findings to demonstrate an effect even when comparing patients with cSLE to demographically similar peers.
Given the importance of scholastic performance for children and adolescents, it is important to understand the mechanism by which cSLE might impair school functioning. Our findings indicate that disease activity and treatment intensity are significant correlates of poor school competence in patients with cSLE. Furthermore, cognitive variables that were associated with school competence in healthy controls had similar associations in children with cSLE: reading skill, mathematics skill, short-term attention/working memory, and mental processing speed. Importantly, although participants with cSLE scored differently than published norms on several outcome measures, this appears to be due to their demographic differences from the normative sample. The cSLE group did not significantly differ from demographically-matched controls on any cognitive, behavioral, emotional, or executive functioning measure. Thus, while poor school performance in any individual may relate to neuropsychological disturbance, we did not find evidence that such disturbances are the systematic mechanism by which cSLE results in diminished school performance. Based on the associations observed in our cross-sectional analyses, we can entertain two possible explanations for the difference in the academic functioning between children and adolescents with cSLE and their peers.
The most obvious explanation to consider is related to the adverse effect that cSLE-related symptoms and signs may have upon school participation. In our study, we clearly demonstrated that cSLE disease activity and prednisone treatment dose are related to worse academic outcomes. Although school attendance was not specifically measured in our study, it would make sense that cSLE patients with more active disease requiring more intense medication regimens are more likely to be absent from school than their less severely-affected counterparts. Earlier reports and our own registry data indicate an increased number of missed days of school in cSLE (31). This raises the possibility of a disruptive effect of cSLE as a chronic illness with acute episodes that interfere with children's functioning at school and in other performance contexts.
Second, although we did not identify significant differences between cSLE and the best-friend controls on formal neurocognitive measures, one cannot entirely exclude the possibility that cSLE disease activity or its treatment exerts a modest disruptive effect upon cognitive functioning, impacting school performance as a result. While the current sample of 40 children with SLE and 40 matched case-controls is the largest sample to date, our study might have been underpowered to detect subtle neurocognitive effects of cSLE on the performance of standardized tests such as the Wechsler Processing Speed Index.
The patients included in this study were mostly female, with mild/moderate disease activity approximately two years after diagnosis (32), and they reflected the diversity of SES commonly seen in cSLE (13,33). The high incidence of cSLE among U.S. ethnic and racial minorities makes a direct comparison to normative U.S. populations unfitting. Hence, we used a matched-control design to carefully select an appropriate comparison group that would enable us to better separate the impact of cSLE as a disease from that of socio-demographic effects. We are convinced that we achieved this, based upon the extremely similar demographic composition of the two groups. There was a statistically significant difference in age across the two groups, but because all of our major outcome measures were age-normed, the small age difference would not be expected to impact our results. This assumption is supported by our exploratory analyses which adjusted for age and found no substantive change in the findings reported.
Even though the current parent ratings and test results are within the average range, on a few measures both groups showed a trend to deviate from reference norms. For example, a deviation of 0.64 SD was noted in the cSLE group on the Wechsler Working Memory Index. While such differences may seem relatively small on an individual basis, their impact within a population such as cSLE can be substantial. A shift of -0.64 SD of the distribution of scores in the cSLE group corresponds to a 248% increase in cSLE Working Memory performances falling in the deficient range. Thus, even seemingly modest differences in mean scores can result in dramatically increased numbers of children who exceed psychometric thresholds for clinical deviance. Given that cSLE disproportionately affects children of minority and lower socioeconomic status, this impact may be especially apparent.
Our failure to identify disproportionate levels of NCD in cSLE subjects relative to case-controls is consistent with the recent report of Williams et al 2011 (13) where the frequency of NCD was similar in cSLE compared to demographically-matched peers. The replication of this finding highlights the role of demographic and socioeconomic factors in cSLE, and the importance of considering how these confounding factors affect patients’ HRQoL. In particular, these findings stress a key weakness of other studies that rely exclusively upon comparisons against published norms. If the sample being studied is demographically dissimilar to the normative group, findings may be misleading.
Limitations of the present study are acknowledged. First, our definition of academic outcome was based on a single measure (School Competence ratings) from a single informant source (parents). Ideally, a study of academic competence would benefit from the inclusion of additional indicators of academic outcome. Unfortunately, a definition of academic competence that is comprehensive, multi-informant based, objective, and consistent across educational settings is not currently available. While future investigators may consider more objective measures of academic functioning (e.g., copies of report cards), we caution that such an approach is not a panacea, as differences in grading standards across teachers, schools, and curricula would remain. Similarly, academic skills assessed in an artificial, office-based setting (i.e., individual achievement testing) at best approximate actual classroom performance, and tests of basic reading and math skills would not necessarily be sensitive to the impact of a disease with onset in middle to late childhood, when the focus of learning turns towards higher-level skills. Second, although this study assessed a broad range of cognitive, behavioral, and emotional functioning, we cannot rule out the possibility of constructs or measures that might be more sensitive to cSLE. Third, though the current sample represents the largest group yet of children and adolescents with SLE to be prospectively studied, the statistical power of our analyses was nevertheless limited by its size. Future multicenter investigations may address this shortcoming. Our experience with “best friend” case-control methodology, however, taught us that this approach can be extremely challenging to implement. Another limitation of the present study, that is more easily addressed, was our failure to obtain systematic information about school disruption. Future studies of academic outcome in cSLE should quantify the disruptive effect of cSLE upon school participation, using records of school attendance/absence and medical history of patients’ hospitalizations and day treatment visits.
The current investigation carries several implications for clinical care in cSLE. First, because our data suggest that disease-related factors may significantly impede patients’ academic progress, they highlight the importance of minimizing such disruption by optimizing patient care (e.g., minimizing missed school) and maximizing treatment adherence. Second, although we did not demonstrate systematic deficits of neurocognitive functioning in cSLE relative to demographically-matched peers, this does not mean that such deficits are absent in individual patients. Monitoring for such deficits continues to be an important component of routine patient care, even if the deficits are not clearly related to the disease process. Finally, the current study underscores the importance of considering socioeconomic/demographic variables in both research and clinical care of cSLE and other chronic diseases that are disproportionately seen in minority populations.
In summary, our results indicate that children and adolescents with SLE do in fact experience poorer academic outcomes than demographically-matched healthy peers, and that cSLE disease severity and treatment intensity are associated with school competence. However, neither neurocognitive functioning nor ratings of behavioral, emotional, and executive functioning appear to be the mechanism by which cSLE impacts school competence.
SIGNIFICANCE & INNOVATION.
Innovation
This study found significantly inferior academic outcomes in children and adolescents with systemic lupus erythematosus (cSLE) than in demographically-matched controls.
Elevated cSLE disease activity is significantly associated with inferior academic outcomes, though we did not find clear evidence that neurocognitive deficits mediate this relationship. Disruption of school attendance due to cSLE and its treatment may significantly impede patients’ academic functioning.
Significance
Management of cSLE should consider the disruptive effect of illness and treatment upon school attendance and performance, emphasizing the importance of minimizing such disruption by optimizing patient care and treatment adherence.
ACKNOWLEDGEMENT
We would like to thank Meredith Amaya, Allison Clarke, Kate Dahl, Antoinette Dezzutti, Donna Diedenhofer, April German, Lev Gottlieb, Jennifer Heil, Jennifer Keller, Andrew Phillips, Michal Rischall, Rebecca Wasserman Lieb, Lisa Welcome, and Mariah Wells for their assistance with neuropsychological testing, and Erin Thomas for her assistance in coordinating and scheduling participants. We would also like to thank Dr. M. Douglas Ris for his assistance in the initial conceptualization of the larger project.
A special thanks to Mrs. Elaine Holtkamp for her administrative support of the study and assistance with the manuscript.
Grant Support: This study is supported by the NIAMS Clinical Research Center P60-AR047884. This publication was also supported by an Institutional Clinical and Translational Science Award, NIH/NCRR Grant Number 5UL1RR026314-03. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Ying had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Zelko, Beebe, Brunner, Klein-Gitelman, Ying.
Acquisition of data. Zelko, Baker, Nelson, Ali, Cedeno, Dina, Klein-Gitelman, Brunner, Beebe.
Analysis and interpretation of data. Ying, Zelko, Beebe, Klein-Gitelman, Brunner.
REFERENCES
- 1.Hinze CH, Suzuki M, Klein-Gitelman M, et al. Neutrophil gelatinase-associated lipocalin is a predictor of the course of global and renal childhood-onset systemic lupus erythematosus disease activity. Arthritis Rheum. 2009 Sep;60(9):2772–2781. doi: 10.1002/art.24751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iramaneerat C. Predicting academic achievement in the medical school with high school grades. Journal of the Medical Association of Thailand = Chotmaihet thangphaet. 2006 Sep;89(9):1497–1505. [PubMed] [Google Scholar]
- 3.Dubow EF, Huesmann LR, Boxer P, Pulkkinen L, Kokko K. Middle childhood and adolescent contextual and personal predictors of adult educational and occupational outcomes: a mediational model in two countries. Developmental psychology. 2006 Sep;42(5):937–949. doi: 10.1037/0012-1649.42.5.937. [DOI] [PubMed] [Google Scholar]
- 4.Crum RM, Juon HS, Green KM, Robertson J, Fothergill K, Ensminger M. Educational achievement and early school behavior as predictors of alcohol-use disorders: 35-year follow-up of the Woodlawn Study. Journal of studies on alcohol. 2006 Jan;67(1):75–85. doi: 10.15288/jsa.2006.67.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Locke TF, Newcomb MD. Adolescent predictors of young adult and adult alcohol involvement and dysphoria in a prospective community sample of women. Prev Sci. 2004 Sep;5(3):151–168. doi: 10.1023/b:prev.0000037639.78352.3c. [DOI] [PubMed] [Google Scholar]
- 6.Rosenbaum JE. Beyond college for all. Sage; New York: 2001. [Google Scholar]
- 7.Moorthy LN, Peterson MG, Harrison MJ, Onel KB, Lehman TJ. Quality of life in children with systemic lupus erythematosus: a review. Lupus. 2007;16(8):663–669. doi: 10.1177/0961203307077539. [DOI] [PubMed] [Google Scholar]
- 8.Moorthy LN, Peterson MG, Hassett A, Baratelli M, Lehman TJ. Impact of lupus on school attendance and performance. Lupus. 2010 Apr;19(5):620–627. doi: 10.1177/0961203309355810. [DOI] [PubMed] [Google Scholar]
- 9.Kozora E, Arciniegas DB, Filley CM, et al. Cognition, MRS neurometabolites, and MRI volumetrics in non-neuropsychiatric systemic lupus erythematosus: preliminary data. Cogn Behav Neurol. 2005 Sep;18(3):159–162. doi: 10.1097/01.wnn.0000181543.05064.4b. [DOI] [PubMed] [Google Scholar]
- 10.Muscal E, Brey RL. Neurologic manifestations of systemic lupus erythematosus in children and adults. Neurol Clin. 2010 Feb;28(1):61–73. doi: 10.1016/j.ncl.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harel L, Sandborg C, Lee T, von Scheven E. Neuropsychiatric manifestations in pediatric systemic lupus erythematosus and association with antiphospholipid antibodies. J Rheumatol. 2006 Sep;33(9):1873–1877. [PubMed] [Google Scholar]
- 12.Sibbitt WL, Jr., Brandt JR, Johnson CR, et al. The incidence and prevalence of neuropsychiatric syndromes in pediatric onset systemic lupus erythematosus. J Rheumatol. 2002 Jul;29(7):1536–1542. [PubMed] [Google Scholar]
- 13.Williams TS, Aranow C, Ross GS, et al. Neurocognitive impairment in childhood-onset systemic lupus erythematosus: measurement issues in diagnosis. Arthritis Care Res (Hoboken) 2011 Aug;63(8):1178–1187. doi: 10.1002/acr.20489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997 Sep;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 15.Reiter-Purtill J, Gerhardt CA, Vannatta K, Passo MH, Noll RB. A controlled longitudinal study of the social functioning of children with juvenile rheumatoid arthritis. J Pediatr Psychol. 2003 Jan-Feb;28(1):17–28. doi: 10.1093/jpepsy/28.1.17. [DOI] [PubMed] [Google Scholar]
- 16.Brunner HI, Feldman BM, Bombardier C, Silverman ED. Sensitivity of the Systemic Lupus Erythematosus Disease Activity Index, British Isles Lupus Assessment Group Index, and Systemic Lupus Activity Measure in the evaluation of clinical change in childhood-onset systemic lupus erythematosus. Arthritis Rheum. 1999;42(7):1354–1360. doi: 10.1002/1529-0131(199907)42:7<1354::AID-ANR8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Brunner H, Silverman E, To T, Bombardier C, Feldman BM. Risk Factors for Damage in Childhood-Onset Systemic Lupus Erythematosus: Cumulative Disease Activity and Medication Use Predict Disease Damage. Arthritis Rheum. 2002;45:436–444. doi: 10.1002/art.10072. [DOI] [PubMed] [Google Scholar]
- 18.Achenbach TM, Ruffle TM. The Child Behavior Checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatr Rev. 2000 Aug;21(8):265–271. doi: 10.1542/pir.21-8-265. [DOI] [PubMed] [Google Scholar]
- 19.Perrin EC, Stein REK, Drotar D. Cautions in Using the Child-Behavior Checklist - Observations Based on Research About Children with a Chronic Illness. Journal of Pediatric Psychology. 1991 Aug;16(4):411–421. doi: 10.1093/jpepsy/16.4.411. [DOI] [PubMed] [Google Scholar]
- 20.Kovacs M. The Children's Depression, Inventory (CDI). Psychopharmacol Bull. 1985;21(4):995–998. [PubMed] [Google Scholar]
- 21.Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior rating inventory of executive function. Child Neuropsychol. 2000 Sep;6(3):235–238. doi: 10.1076/chin.6.3.235.3152. [DOI] [PubMed] [Google Scholar]
- 22.Ross GS, Zelko F, Klein-Gitelman M, et al. A proposed framework to standardize the neurocognitive assessment of patients with pediatric systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2010 Jul;62(7):1029–1033. doi: 10.1002/acr.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wechsler D. Wechsler abbreviated scale of intelligence : WASI. Psychological Corporation, Harcourt Brace; San Antonio [u.a.]: 1999. [Google Scholar]
- 24.Wechlser D. Wechsler Intelligence Scale for Children. Fourth Edition Harcourt Assessment, Inc; San Antonio, TX: 2003. [Google Scholar]
- 25.Wechsler D. Wechsler Adult Intelligence Scale. Fourth Edition NCS Pearson; San Antonio, TX: 2008. [Google Scholar]
- 26.Adams W, Sheslow D. WRAML2 : Wide Range Assessment of Memory and Learning : administration and technical manual. 2nd ed. Wide Range; Wilmington, DE: 2003. [Google Scholar]
- 27.Woodcock RW, Johnson MB, Mather N. Woodcock-Johnson Tests of Cognitive Ability : standard and supplemental batteries : WJ-R Woodcock-Johnson Psycho-Educational Battery-Revised. DLM Teaching Resources; Allen, Tex.: 1990. [Google Scholar]
- 28.Conners C. Conners’ Continuous Performance Test II. N. Tonawanda, NY: 2004. [Google Scholar]
- 29.Rubin A. Statistics for evidence-based practice and evaluation. 3rd Ed. Cengage - Brooks/Cole; Belmont, CA: 2012. [Google Scholar]
- 30.Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care. 1999;37(2):126–139. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Moorthy LN, Peterson MG, Baratelli MJ, Hassett AL, Lehman TJ. Preliminary cross-cultural adaptation of a new pediatric health-related quality of life scale in children with systemic lupus erythematosus: an international effort. Lupus. 2010 Jan;19(1):83–88. doi: 10.1177/0961203309345770. [DOI] [PubMed] [Google Scholar]
- 32.Mina R, Brunner HI. Pediatric lupus--are there differences in presentation, genetics, response to therapy, and damage accrual compared with adult lupus? Rheum Dis Clin North Am. 2010 Feb;36(1):53–80. vii–viii. doi: 10.1016/j.rdc.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Son MF, Hersh AO, Brunner HI, Eberhard BA, von Scheven E, Investigators C. Socioeconomic Status Is Associated with Disability in Pediatric Systemic Lupus Erythematosus.. Paper presented at: American College of Rheumatology Annual Meeting; Chicago. 2011. [Google Scholar]