Abstract
The Religious Orders Study is a longitudinal clinical-pathologic cohort study of aging and Alzheimer’s disease (AD). In this manuscript, we summarize the study methods including the study design and describe the clinical evaluation, assessment of risk factors, collection of ante-mortem biological specimens, brain autopsy and collection of selected post-mortem data. The results: 1) review the relation of neuropathologic indices to clinical diagnoses and cognition proximate to death; 2) examine the relation of risk factors to clinical outcomes; 3) examine the relation of risk factors to measures of neuropathology; and 4) summarize additional study findings. We then discuss and contextualize the study findings.
Keywords: Religious Orders Study, Dementia, Alzheimer’s Disease, Cognitive Function, Epidemiology, Clinical-pathologic Study, Neuropathology, Cohort
INTRODUCTION
Careful clinical characterization followed by examination of neural tissues after death has been a fundamental approach to understanding neurologic diseases for more than two centuries. For much of the 19th century, post-mortem examination was limited to macroscopic evaluation. However, developments in optics, tissue fixation, and tissue staining ushered in the modern era of histopathologic examination. In the first decade of the 20th century, Professor Alzheimer combined the case report of a woman with progressive dementia with microscopic histopathologic examination of her brain and described the disease that currently bears his name [1]. For the next several decades, AD was considered a relatively rare progressive dementia of mid-life and was thought to be something quite distinct from the senility of old age. A series of clinical-pathologic studies in the late 1960’s, however, demonstrated that old age dementia was often associated with the same lesions described by Dr. Alzheimer and that AD was likely the most common cause of dementia in old age [2–5], and even present in brains of persons without dementia [4]. Within a few years, the significance of this observation was brought into focus in an editorial forecasting the increasing burden of AD associated with the aging population [6]. Recognition of the impending public health problem contributed to the formation of the Neurobiology of Aging Program within the nascent National Institute on Aging and the emergence of the AD Centers program. By the mid 1980s, consensus criteria for the clinical [7] and pathologic [8] diagnoses of AD were established, and standardized clinical and pathologic procedures were developed and being implemented nationwide [9–10]. The concept of definite AD as a clinical pathologic entity with progressive dementia documented during life and neocortical plaques and tangles documented at autopsy was solidified.
AD centers across the country became expert at enrolling subjects with typical AD and following them until death and obtaining brain autopsy [11–15]. However, the standard approach to obtaining specimens from persons without AD was to monitor the autopsy suite for brains from older persons, document the absence of AD pathology, and subsequently review medical records to ensure the absence of clinical evidence of obvious dementia [16–19]. Obtaining brain autopsies from persons without dementia clinically evaluated proximate to death was problematic. For example, between 1987 and 1995, the Consortium to Establish a Registry for AD (CERAD) which included 24 federally funded AD Centers from across the United States enrolled 1094 patients with AD and 463 controls into a longitudinal study of aging and AD that included brain donation. During this time period there were 202 autopsies out of 411 deaths among those with AD; by contrast, there were only 8 autopsies out of 25 deaths among those recruited without dementia [20]. Prior to 1993, there were very few reports of neuropathologic findings among persons prospectively evaluated and documented to be free of dementia proximate to death [21–23]. None of these studies included more than 20 persons without dementia. At that time, carefully examined clinical-pathologic single case studies of non-demented elderly were of interest [24]. In spite of these small numbers, it became evident that some older persons without dementia could have widespread AD pathology. This finding challenged the idea that there was a simple correspondence between the dichotomous clinical and pathologic diagnoses of AD. Thus, by the early 1990’s there was an emerging consensus that more information was needed regarding the neuropathologic changes associated with aging among persons without dementia.
In early 1992, we learned of a fascinating study being conducted by Drs. David Snowdon, Jim Mortimer, Bill Markesbery and colleagues with the School Sisters of Notre Dame [25]. The Nun Study was following several hundred sisters over age the age of 75 years, most of them without dementia, all of whom volunteered to brain donation at death. The organ donation effort was embedded within a well-conceived epidemiologic birth-cohort study. Building on the success of Nun Study, we approached a number of Religious communities to obtain support for the Rush Religious Orders Study. The proposal was submitted to the National Institute on Aging for funding as a Core of the Rush Alzheimer’s Disease Core Center in October 1992. It was funded in July 1993 and clinical evaluations commenced in January of 1994.
The Religious Orders Study had two overall goals intended to complement other ongoing work in the field, especially the Nun Study. One was to provide a source of brain tissue from well characterized comparable men and women with and without dementia for clinical-pathologic studies of aging and AD. The second was to obtain brain tissue from persons on whom risk factor information was obtained prior to the onset of dementia for studies linking risk factors to clinical and neuropathologic phenotypes. To accomplish these goals, the study enrolled participants without dementia and documented a wide range of potential risk factors for common chronic conditions of old age. Thus, the Religious Orders Study has all of the essential features of a cohort study of risk factors for incident AD and cognitive decline. In addition, all participants must agree to organ donation as a condition of entry. Over time, some participants develop dementia prior to death resulting in post-mortem material from comparable men and women with and without dementia.
METHODS
Study Design
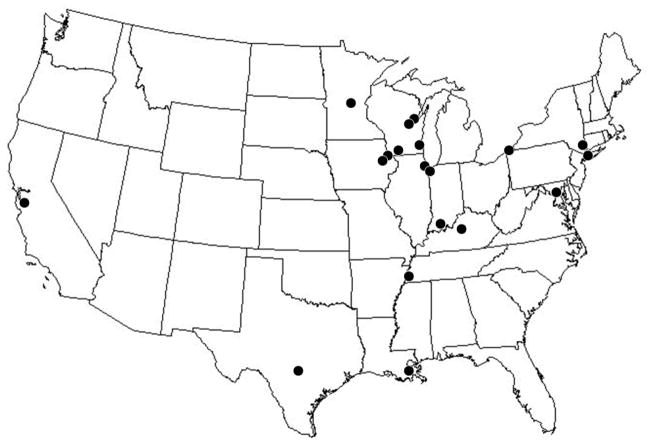
The Religious Orders Study enrolls Catholic nuns, priests and brothers, from more than 40 groups across the United States (Figure 1). Participants are without known dementia and agree to annual clinical evaluation and brain donation (some in the Chicago area also agree to donate, spinal cord, nerve, and muscle). Each subject signs a consent form and an Anatomical Gift Act. The study was approved by the Institutional Review Board of Rush University Medical Center.
Figure 1.
Major locations of Religious Orders Study participants.*
* Archdiocesan Priests, Chicago, IL, Dubuque, IA and Milwaukee, WI; Benedictine monks, Lisle, IL, and Collegeville, MN; Benedictine Sisters, Erie, PA; Benedictine Sisters of the Sacred Heart, Lisle, IL; Capuchins, Appleton, WI; Christian Brothers, Chicago, IL, and Memphis, TN; Diocesan Priests, Gary, IN; Dominicans, River Forest, IL; Felician Sisters, Chicago, IL; Franciscan Handmaids of Mary, New York, NY; Franciscans, Chicago, IL; Holy Spirit Missionary Sisters, Techny IL; Maryknolls, Los Altos, CA, and Maryknolls, NY; Norbertines, De Pere, WI; Oblate Sisters of Providence, Baltimore, MD; Passionists, Chicago, IL; Presentation Sisters, BVM, Dubuque, IA; Racine Dominicans, Racine, WI; Salestian Sisters of St. John Bosco, San Antonio, TX; Sagrado Corazón, San Antonio, TX; Servites, Chicago, IL; Sinsinawa Dominican Sisters, Chicago, IL, and Sinsinawa, WI; Sisters of Charity, BVM, Chicago, IL, and Dubuque IA; Sisters of the Holy Family, New Orleans, LA; Sisters of the Holy Family of Nazareth, Des Plaines, IL; Sisters of Mercy of the Americans, Chicago, IL, Aurora, IL, and Erie, Pa; Sisters of St. Benedict, St. Cloud, MN and St. Joseph, MN; Sisters of St. Casimir Chicago, IL; Sisters of St. Francis of Mary Immaculate, Joliet, IL; Sisters of St. Joseph of La Grange, LaGrange Park, IL; St. Teresa De Jesus, San Antonio, TX.; Society of Divine Word, Techny, IL; Trappists, Gethsemane, KY and Peosta, IA; and Wheaton Franciscan Sisters, Wheaton, IL.
The study primarily recruits persons living communally, including employed (e.g., Teaching Orders) and retired (e.g., Missionary Orders) persons. The study includes three predominantly African American communities in New York, Baltimore, and New Orleans, and enrolls Hispanic sisters primarily from communities in and around San Antonio. All data collection forms have been translated into Spanish. Working with religious communities offers a number of advantages. First, they are altruistic and have a history of participating in research projects from which they may derive little to no personal benefit. Second, they live communally and loss of contact with participants is rare, facilitating the high follow-up and autopsy rates required to ensure internal study validity. Third, their wishes for organ donation are likely to be honored by the Superior and biological family members are unlikely to interfere with the participants’ written preference. Finally, the participants have similar education, socioeconomic and life experiences for most of their adult lives. This allows for tighter control of these potentially confounding variables in analyses of incident AD and cognitive decline.
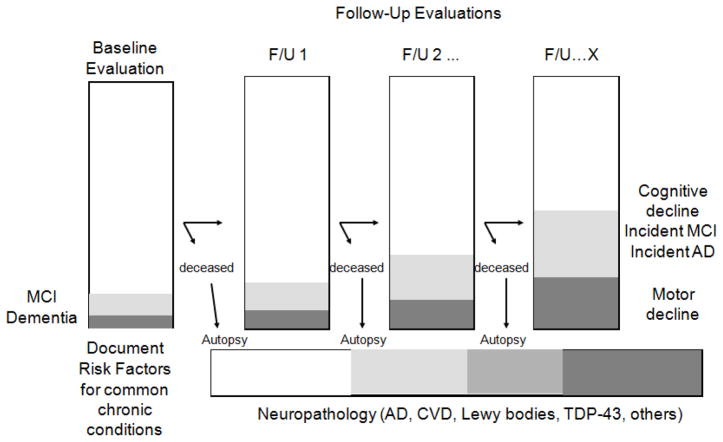
The study design (Figure 2) supports the following analyses in a single dataset: 1) the association of neurobiologic indices with AD, MCI, and cognition proximate to death and over multiple years prior to death; 2) the association of risk factors for incident AD, incident MCI, and cognitive decline; and 3) the modeling of neurobiologic pathways linking risk factors to clinical phenotypes. The collection of parkinsonian signs and other measures of motor function allow for similar analyses to be conducted with motor function and decline, and disability.
Figure 2.
Overall study design of the Religious Orders Study
Through October of 2011, the baseline evaluation has been completed on 1,162 persons. There have been 387 cases of incident MCI and 287 cases of incident dementia (follow-up rate 95%), and 539 autopsies (autopsy rate 95%).
Demographic Variables and Socioeconomic Status
Race and ethnicity are ascertained using the 1990 U. S. Census questions. Measures of socioeconomic status include education and occupation. Birth address was linked to 1920 census data to determine early-life socioeconomic status [26]. Other indicators of early life socioeconomic status include parental education and occupation.
Clinical Diagnoses of Dementia, AD, MCI and Other Medical Conditions
Medical conditions are documented by clinical evaluation or self-report. To reduce costs and enhance uniformity of diagnostic decisions over time and space, a decision tree designed to mimic expert clinical judgment was implemented by computer to inform several clinical diagnoses, including dementia and AD [27,28]. It combines data reduction techniques for the cognitive performance testing with a series of discrete clinical judgments made in series by a neuropsychologist and a clinician, to develop presumptive diagnoses of dementia and AD. An algorithm uses these decisions to provide diagnoses of MCI and amnestic MCI [29,30]. Persons without dementia or MCI are categorized as no cognitive impairment (NCI) [31]. The clinician is afforded an opportunity to override the decision tree diagnoses. The diagnoses of dementia and AD conform to the standard approach [7].
A similar decision tree approach also is employed to aid five other diagnoses including stroke, cognitive impairment due to stroke (vascular dementia), parkinsonism, Parkinson’s disease (PD), and depression as described [32–37]. Other less common causes of dementia in community-based studies (e.g., Lewy body disease) are made by contemporary standards [38].
Most of the remaining diagnoses are by self report, including cardiovascular risk factors (e.g., hypertension, diabetes) and conditions (e.g., myocardial infarction, congestive heart failure, claudication) [39,40]. Tobacco and alcohol use is documented. Musculoskeletal pain is recorded [41]. Systolic and diastolic blood pressure is measured directly [40]. All over the counter and prescription medications are recorded. In some cases, clinical diagnoses used in analyses are supported by medication use [39,40]. Thus, a diagnosis of diabetes was based on self report and medication use (e.g., insulin). Sensitivity analyses are performed to ensure findings are not driven by inconsistent information (e.g., diabetes limited to persons on medications). We are also collecting hemoglobin A1c on a subset of participants.
Cognitive Performance Tests
A battery of 21 cognitive performance tests is administered each year (Table 1) [9,42–55]. The Mini-Mental State Examination (MMSE) [40] is primarily used to describe the cohort. Eleven tests are used for diagnostic classification [29]. Nineteen tests assess a range of cognitive abilities and are used to construct a global composite measure of cognition and separate summary measures of five cognitive domains, including episodic memory, semantic memory, working memory, perceptual speed, and visuospatial ability. The composite measures are created by converting each test within each domain to a z-score and averaging the z-scores, as previously described [56]. Seven tests can be administered by telephone [57]. This version yields composites for episodic memory, semantic memory, and working memory, in addition to global cognition.
Table 1.
Cognitive performance tests and their classification.
| Test | Diagnosis | Composite | Telephone |
|---|---|---|---|
| Mini-Mental State Examination | Orientation | MMSE22 | |
| Logical Memory Ia | Episodic memory | ||
| Logical Memory IIa | Episodic memory | ||
| Immediate story recall | Episodic memory | Episodic memory | |
| Delayed story recall | Memory | Episodic memory | Episodic memory |
| Word List Memory (3 trials) | Episodic memory | ||
| Word List Recall | Memory | Episodic memory | |
| Word List Recognition | Memory | Episodic memory | |
| Complex Ideational Material | Language | ||
| Boston Naming Test | Language | Semantic memory | |
| Category Fluency (fruits, animals) | Language | Semantic memory | Semantic memory |
| Extended Range Vocabulary | Semantic memory | ||
| National Adult Reading test | Semantic memory | ||
| Digit Span Forward | Working memory | Working memory | |
| Digit Span Backward | Attention | Working memory | Working memory |
| Digit Ordering | Working memory | Working memory | |
| Alpha Span | Working memory | ||
| Symbol Digit | Attention | Perceptual speed | |
| Number Comparison | Perceptual speed | ||
| Judgment of Line Orientation | Visuospatial | Visuospatial ability | |
| Standard Progressive Matrices | Visuospatial | Visuospatial ability |
Motor Function and Structure
A detailed assessment of motor function is administered each year. The battery includes a modified version of the motor portion of the Unified Parkinson’s Disease Rating Scale (mUPDRS) which is used to assess four parkinsonian sign domains including bradykinesia, rigidity, parkinsonian gait, and tremor, in addition to a composite global measure of parkinsonism [58–60]. This is supplemented by upper (e.g., finger tapping, Purdue pegboard, grip strength) and lower (e.g., walking speed) extremity motor performance tests [40,61–64]. Body mass index (BMI) is calculated from measured height and weight and is used as a measure of muscle structure [65].
Self Report Activities of Daily Living
Self-report measures assess the ability to live independently and perform basic and instrumental activities of daily living, and mobility disability [66–69]. Gait speed of ≤0.4 m/s is also used as a measure of performance-based mobility disability [41].
Risk Factors of Interest
Experiential factors include current cognitive and physical activities [70]. Affect includes measures of depressive symptoms, and state measures of anxiety and anger [71,72]. Personality includes measures of neuroticism, extroversion, openness, agreeableness, and conscientiousness, and trait measures of anxiety and anger [73,74].
Ante-Mortem Biological Specimens
Currently, a blood sample is taken at baseline and aliquots of serum and plasma are stored in a −80°C freezer. Lymphocytes are extracted to provide a source of DNA for genetic studies. Serum and DNA is available from nearly all participants at baseline. Plasma, for which collection started substantially later, is only available from about half of the cohort. About 350 persons have serum and plasma collected annually, and a urine for several years.
Ante-Mortem Biological Specimen Data
DNA has been used to characterize apolipoprotein E allele status (APOE) [75], and more recently, it has been used generate genome-wide genotyping data generated on a Affymetrix 6.0 platform and imputed to 2.2 million single nucleotide polymorphisms (SNPs) with HapMAP [76]. We are in the process of imputing addition SNPs with the 1000 genomes project. We are also in the process of generating Methylome-wide data with an Illumina 450 Beadchip.
Serum, plasma and urine are being used in a variety of add-on projects [77–79]. We are currently in the process of collecting anti-phospholipid antibodies and related indices for studies of cerebrovascular disease.
Post-Mortem Biological Specimens
Participants reside in more than a dozen states across the United States (Figure 1). Staff is on-call 24 hours per day, seven days a week, to coordinate transportation of the body, communicate with prearranged groups who help in performing autopsies outside of Chicago, and to perform autopsies at the Rush Alzheimer’s Disease Center for local sites. The basic protocol is the same regardless of where the participant expires. The calvarium is opened, cerebrospinal fluid is removed, and the brain is removed and weighed. The brainstem and cerebellum are dissected from the hemispheres and one hemisphere and the cerebellum are cut into 1 cm slabs in a plexiglass jig. The 1 cm slabs are frozen in a −80°C freezer and the remainder of the brain is placed in 4% paraformaldehyde. Digital pictures of the hemispheres and slabs are obtained at the time of autopsy in Chicago and when the fixed hemisphere and frozen slabs are received from the outside autopsy groups. After paraformaldehyde fixation, the fixed hemisphere is cut into 1 cm slabs, photographed and tissue blocks are dissected from specified brain regions (including hippocampus, entorhinal cortex, anterior cingulate cortex, midfrontal cortex, superior frontal cortex, inferior temporal cortex, middle temporal cortex, inferior parietal cortex, primary occipital cortex, anterior basal ganglia, anterior thalamus, and midbrain) and paraffin embedded for subsequent histochemistry and immunocytochemistry. Gross features and pathology (e.g., atrophy, cerebrovascular disease) are described at the time of blocking, and blocks are obtained from all areas of visible pathology (e.g., infarctions, tumors) from direct observation or from the digital photographs. The remainder of the brain is transferred to a graded cryoprotectant solution (final solution of 20% glycerol and 2% DMSO in phosphate buffer) for long term storage at 4°C.
The protocol in Chicago is slightly more detailed as it also entails removal of spinal cord, muscle, and nerve on a subset, and post-mortem imaging on a subset.
Post-Mortem Data
The post-mortem neuropathologic evaluation includes a uniform structured assessment of AD pathology, cerebral infarcts, Lewy body disease, and other pathologies common in aging and dementia. The procedures follow those outlined by the pathologic dataset recommended by the National Alzheimer’s Disease Coordinating Center (NACC) [80]. Pathologic diagnoses of AD use NIA-Reagan and modified CERAD criteria, and the staging of neurofibrillary pathology uses Braak Staging [81–83]. The location, size, and age of each macroscopic infarct are recorded as described [35]. Microscopic infarctions are identified on H&E stained sections as is nigral degeneration; amyloid angiopathy is identified with amyloid immunostained sections; nigral, limbic, and neocortical Lewy bodies are identified on sections stained with α-synuclein [84–89].
Other post-mortem data are collected as part of separately funded projects. For example, counts of neuritic plaques diffuse plaques, and neurofibrillary tangles based on silver stain from five brain regions are used to create a global measure of AD pathology [75]. Amyloid load and the density of paired helical filament tau (PHFtau) are determined in eight brain regions and summarized [86]. More recently we have started to quantify TDP-43 [90].
Statistical Approaches
The study design allows for many different types of analyses that address a variety of issues related to the causes and consequences of common age related neurologic conditions, while controlling for potential confounding variables such as age, sex, and education, and others.
Cross-sectional analyses examining the relation of neuropathology to cognitive status proximate to death typically employ logistic regression for dichotomous outcomes (e.g., AD vs. no AD) and analysis of variance when examining difference across three diagnostic groups (e.g., NCI, MCI, and AD) with pairwise comparisons performed with the Tukey test [31]. Linear regression is used for continuous outcomes (e.g., level of cognition) [86]. We have also conducted a number of qualitative [75,86] and quantitative [91,92] mediation analyses which model a putative sequence of events using the cross sectional associations.
Longitudinal analyses examining the relation of risk factors to cognitive status typically employ Cox proportional hazards models for dichotomous outcomes (e.g., incident AD) [29,93]. More recently, we have used models for discrete (tied) data [94]. Other approaches such as accelerated failure models have been used when the assumptions of proportional hazards are not met [30]. Mixed models are used to examine the relation of risk factors to change in cognitive function over time [29,95]. In this approach individuals are assumed to follow the mean path of the group, conditional on a set of explanatory variables, except for random effects which cause the initial level of cognition to be higher or lower and the rate of change to be faster or slower. In situations for which the outcome has significant floor or ceiling effects that cannot be eliminated by data transformation, other approaches such as generalized estimating equations (GEE) have been used [96]. Change point models have also been used to characterize other paths of cognitive decline [97].
FINDINGS
From January of 1994 through October of 2011, 1,168 persons agreed to participate and the baseline clinical evaluation has been completed on 1,162. Of these, 806 (69.4%) are women, 1,023 (88.0%) are non-Hispanic white. Of 56 Hispanics, 8 are being tested in Spanish. The mean age is 75.7 years and the mean education is 18.1 years. 1,079 (92.9%) were without dementia following the baseline clinical evaluation.
The overall follow-up of survivors approaches 95%, and only 47 persons have withdrawn from the study since the first participants were evaluated in 1994. There have been 387 cases of incident MCI including 132 cases of incident amnestic MCI, and 287 cases of incident dementia including 273 cases of incident AD (with or without a coexisting condition), to date.
The autopsy rate approaches 95% with 539 autopsies of 574 deaths (93.9%). Of these, 343 (63.6%) are women, 511 (94.8%) are non-Hispanic white, the average age at death is 86.6 and the interval from last clinical evaluation to death is 9.0 months. The post-mortem interval is 8 hours 38 minutes. The MMSE proximate to death is 20.8 and the clinical diagnosis proximate to death includes 166 (31.0%) without cognitive impairment, 123 (23.0%) with MCI, 194 (36.3%) with probable AD, 38 (7.1%) with possible AD, and 14 (2.6%) with dementia due to another condition (four cases are currently under review). The diagnostic neuropathologic evaluation of the brain has been completed for the first 526 (97.6%).
Spinal cord, muscle and nerve have been obtained from 68 participants. Post-mortem brain imaging has been performed on 72 participants.
Relation of Common Neuropathologic Indices to Clinical AD, MCI, and Level of Cognition
Alzheimer’s Disease Pathology
About 90% of persons meeting clinical criteria for AD also met pathologic criteria for the disease [27]. However, about half of persons with MCI also met pathologic criteria for AD [31,98]. Further, about a third of those without cognitive impairment met pathologic criteria for AD; however, among this group of very high functioning individuals, the presence of AD pathology was associated with deficits in episodic memory [99].
Mesial temporal lobe amyloid and neurofibrillary pathology were related to episodic memory across the spectrum of diagnoses [100–103]. Similar findings were found when amyloid and tau were assessed biochemically [104]. Using immunohistochemistry, amyloid load and PHFtau tangles were correlated. In multivariate regression models, tangles mediated the association of amyloid with cognition [86]. These data are consistent with a sequence of pathologic events in AD which begins with amyloid deposition and subsequent tangle formation.
Cerebrovascular Disease
Persons with dementia were more likely to have cerebral infarcts, and those with MCI had intermediate number of infarcts, compared to those without cognitive impairment [31,35,98,105]. Interestingly, while infarcts were related to all cognitive abilities, in analyses controlling for AD pathology, they were most strongly related to perceptual speed [35]. Further, AD pathology and cerebral infarcts had an additive effect on the odds of dementia [105]. Microscopic infarctions were also related to odds of dementia even after controlling for macroscopic infarcts and AD pathology [88]. Finally, cerebral amyloid angiopathy was also related to level of cognition, even controlling for measures of AD pathology and cerebral infarcts [89].
Lewy Bodies
Lewy bodies were most common in persons with dementia and intermediate among those with MCI [31,98]. Persons with MCI and dementia were more likely to have neocortical Lewy bodies even after controlling for AD pathology and infarcts [98]. When assessed biochemically, alpha-synuclein, the principal component of Lewy bodies, was also related to cognition [106].
Mixed Pathologies
The combination of AD pathology with cerebral infarcts or Lewy bodies is so common that mixed pathologies are the most common cause of dementia. Interestingly, mixed pathology is also as common as “pure” AD pathology as a cause of probable AD. In addition mixed pathology accounts for a substantial proportion of cases of both amnestic and non-amnestic MCI [98]. Consistent with this, all three pathologies are strongly related to multiple cognitive abilities, including episodic memory [107].
Relation of Neuropathology to Cognitive Decline
The design of the Religious Orders Study allows us to examine the relation of neuropathology not only to level of cognition proximate to death, but also to change in cognition over multiple years prior to death. In one study, we examined the relation of neuropathology to change basic lexical knowledge (i.e., word reading and vocabulary) over time, a skill which is thought to be relatively preserved in mild dementia and is often used to estimate premorbid ability. We found that the level of AD pathology, macroscopic cerebral infarctions, and neocortical Lewy bodies were all associated with rate of decline of lexical knowledge [108]. In fact, all cognitive abilities seem to dedifferentiate as cognition declines [109].
We used change point models to examine the influence of death on the trajectory of cognitive change prior to death, a concept called terminal decline [97]. We found that for those who die, the rate of cognitive decline accelerates more than six fold about four years prior to death. Next we incorporated neuropathologic indices into these models to explore their association with terminal and presumably disease related decline and the slower preterminal decline that could represent “normal” aging [110]. Neurofibrillary tangles and neocortical Lewy bodies contributed to disease-related cognitive decline, but substantial disease-related decline was evident even in the absence of these lesions. Neurofibrillary tangles, neocortical Lewy bodies, and cerebral infarctions all contributed to age-related cognitive decline. In fact little age-related decline was evident in the absence of these lesions suggesting that much of age-related cognitive decline is the result of these three common neuropathologies.
Another approach was also used to examine the relation of neuropathology to cognitive decline [111]. Profile mixtures models identified three classes of decliners, a slow class (65%), a moderate class (27%), and a rapid class (8%). Amount of AD pathology, especially tangles, was strongly related to class membership, with more AD pathology being associated with the rapid class of decliners.
Relation of Risk Factors to Incident AD and MCI, and Cognitive Decline (Table 2)
Table 2.
Factors related to incident AD and MCI, and cognitive decline.
| Risk Factor | Incident AD | Cog Dec [1] | Incident MCI | Cog Dec [2] |
|---|---|---|---|---|
| Age | X | X | ||
| Cognitive activity | X | X | ||
| Depressive symptoms | X | X | ||
| Neuroticism | X | X | X | X |
| Conscientiousness | X | X | X | |
| MCI | X | NA | NA | NA |
| Parkinsonian signs | X | X | ||
| Grip strength | X | |||
| Decline in BMI | X | X | ||
| Diabetes | X | X | ||
| APOE ε4 | X | X | X | X |
| APOE ε2 | X | |||
| CR1 | X | X | ||
| PICALM | X | |||
| CEPT | X | X |
cognitive decline among persons without dementia at baseline
cognitive decline among persons without dementia or MCI at baseline
Age, Sex, and Education
Older age was associated with a more rapid rate of decline in all cognitive abilities [56]. Sex was not related to incident AD or to rate of cognitive decline [112]. Finally, education was not related to incident AD or rate of cognitive decline [70]. We also examined the influence of age, sex, and education on retest-effects, as this may have obscured associations with cognitive decline [113]. Adult cognitive activities also appeared to be of greater importance than years of education [114]. Although retest effects were substantial, they were not related to any of the demographic variables. Our index of early life socioeconomic status was related to level of cognition at baseline, but not to incident AD or cognitive decline [26].
Cognitive and Physical Activities
Participation in cognitively stimulating activities was associated with a reduced risk of AD and with a slower rate of cognitive decline [70]. The effect was rather selective for working memory and perceptual speed, two measures of processing resources. We also examined the relation of physical activity to risk of AD; although the point estimates were protective, the results were not significant [70].
Depressive Symptoms
Depressive symptoms were associated with an increased risk of AD and a more rapid rate of cognitive decline [36]. The effect was robust and cut across the full spectrum of cognitive domains. Because depressive symptoms may be an early sign of AD, we examined change in depressive symptoms during the prodromal phase of AD [115]. We did not find evidence of increasing depressive symptoms in the years immediately prior to the development of AD suggesting that depressive symptoms are unlikely to be a consequence of declining cognition in preclinical AD.
Neuroticism and Conscientiousness
Neuroticism was associated with an increased risk of AD and with a more rapid rate of cognitive decline among persons without dementia, and risk of MCI and change in cognition among persons without dementia or MCI [116,117]. Interestingly, the association was most robust for episodic memory. By contrast, conscientiousness was associated with a reduced risk of AD and MCI, and a slower rate of cognitive decline [118]. The effect was robust and cut across the full spectrum of cognitive domains.
Measures of Motor Function and Structure
Progression of parkinsonian signs was associated with a greater risk of AD [119]. The effect was most robust for gait and rigidity. However, each parkinsonian sign was associated with decline in all five cognitive domains. Stronger grip strength was also associated with a reduced risk of AD [63]. Body mass index (BMI) was used as a measure of muscle structure. Loss of BMI was associated with an increased risk of AD and a greater rate of cognitive decline [65]. The findings were similar in analyses that examined weight loss.
Medical Conditions and Medications
A preliminary study found that cerebrospinal fluid levels of vitamin E were related to measures of AD pathology [120]. Diabetes was associated with an increased risk of AD and a more rapid rate of cognitive decline [39]. The association was relatively selective for perceptual speed, the domain most strongly related to cerebral infarctions [35]. Neither measured systolic or diastolic blood pressure was related to risk of AD or cognitive decline [121]. Neither use of non-steroidal anti-inflammatories nor aspirin was associated with risk of AD or cognitive decline [122]. Statin use was not associated with risk of AD or cognitive decline [123].
Apolipoprotein E Allele Status (APOE) and Other Genetic Variants
The APOE ε4 allele was associated with a greater risk of AD and rate of cognitive decline among persons without dementia, and greater risk of MCI rate of cognitive decline among those without dementia or MCI [94,124]. It was also associated with risk of AD among persons with MCI [125]. By contrast, the ε2 allele was associated with a slower rate of cognitive decline among those without dementia [126]. Interestingly, the associations for both alleles were most robust for episodic memory. CR1 and PICALM were also associated with cognitive decline and AD risk [76]. An initial study case-control with cholesterol ester transfer protein (CETP) polymorphisms was null [127]. However, a follow-up found that the 1405V polymorphism was associated with cognitive decline, risk of AD, and with AD pathology [128].
We examined the association of of other single nucleotide polymorphisms (SNP) with AD, including SNPs in the genes for brain-derived neurotrophic factor, and hepatic lipase which were null [129,130]. By contrast, the 211 haplotype of the low density lipoprotein receptor gene was associated with AD [131]. We also reported that a SNP which appears to modulate the splicing efficiency of low-density lipoprotein receptor was associated with increased AD odds in males [132].
Genome-wide data is now contributing to a number of studies of cognitive decline, meta-analyses, and candidate SNP analyses [133–141].
Relation of Risk Factors to Neuropathologic Indices (Table 3)
Table 3.
Relation of risk factors for AD to neuropathologic indices.
| Risk Factor | Related to pathology | Modifies relation of pathology to cognition |
|---|---|---|
| APOE ε4 | AD, Infarcts | |
| APOE ε2 | AD | |
| CR1 | AD | |
| CEPT | AD | |
| Parkinsonian signs | AD | |
| PICALM | Unrelated | No effect modification |
| BMI | AD | |
| Diabetes | Infarcts | |
| Education | AD | |
| Sex | AD | AD |
| Conscientiousness | AD, Infarct | |
| Depressive symptoms | Unrelated | No effect modification |
| Neuroticism | Unrelated | No effect modification |
Association of Risk Factors with AD Pathology and Cerebral Infarctions
The APOE ε4 allele was associated with greater AD pathology and the ε2 allele was associated with less AD pathology [75,142,143]. AD pathology also mediated the association of the ε4 allele with clinical AD and level of cognition [15]. In other words, the cross sectional association between the ε4 allele and cognition was markedly reduced and no longer significant after accounting for AD pathology. Further, amyloid load mediated the association of the ε4 allele with level of cognition proximate to death, and to the presence of neurofibrillary tangles [143]. These data are consistent with a sequence of pathologic events in which APOE triggers a cascade of events eventually resulting in amyloid deposition and subsequent tangle formation. Similar findings were seen with CR1 suggesting that this polymorphism was also associated with AD in part through an association with AD pathology [76]. The PICALM SNP reported in the GWAS AD case-control studies was not associated with AD pathology [76]. However, a separate SNP identified in a yeast model of AD did associate with measures of AD pathology [141]. Both the ε4 allele and CR1 were also associated with amyloid angiopathy [144,145]. A number of other polymorphisms were related to AD pathology in a candidate gene study [146].
The APOE ε4 allele was also associated with macroscopic cerebral infarcts [144]. Further, in a series of mediation analyses, cerebral infarcts mediated the association of the ε4 allele with level of perceptual speed proximate to death [91,92]. Diabetes also was associated with macroscopic cerebral infarcts but not to measures of AD pathology [147].
Risk Factors that Modify the Relation of Neuropathology to Cognitive Function
These analyses formally examine whether the relation between a risk factor and cognition differs by the amount of pathology. For example, while years of formal education were not directly related to measures of AD pathology, education modified the association of AD pathology with cognition [148]. In other words, a unit of AD pathology had much less effect on cognition among persons with more years of education. In further analyses, the effect was more strongly associated with amyloid deposition than with tangle formation [149].
We found AD pathology was more strongly related to clinical AD and level of cognition among women compared to men [150]. There was also a small direct effect such that women had more tangles.
While conscientiousness was also associated with a reduced risk of AD and a slower rate of cognitive decline, it was not associated with measures of AD pathology or cerebral infarctions. However, both neuropathologic indices modified the relation of conscientiousness to cognition [118]. Unexpectedly, the effect was in the opposite direction to what was predicted. In this case, both AD pathology and cerebral infarcts had a stronger association (i.e., reduced cognition further) among persons with more conscientiousness.
Risk Factors Associated with AD and Cognition Independent of Neuropathologic Indices
Finally, a number of risk factors were neither directly related to measures of neuropathology nor did they modify the relation of neuropathology to cognition. These included depressive symptoms and neuroticism [116,151–153]. Interestingly, measures of psychological distress, based on a composite measure of depressive symptoms and the anxiety trait scale, were related to indices of dendrites and dendritic spines in the CA3 subfield of the hippocampus [154], a characteristic finding in animal models of chronic stress, and an area implicated in depression [155].
Selected Other Study Findings
Additional Studies of Mild Cognitive Impairment (MCI)
MCI was associated with an increased risk of developing AD and a greater rate of cognitive decline, especially episodic memory [29,30]. Persons with MCI had entorhinal cortex and hippocampal atrophy and disruption of parahippocampal white matter fibers [156]. They also had reductions of fractional anisotropy in posterior white matter regions [157]. Evidence of white matter changes were also found biochemically [158]. Finally, among persons with MCI, both baseline entorhinal cortex hippocampal volume and rate of change in entorhinal cortex and hippocampal volume are associated with risk of AD [159,160].
Relative to persons without cognitive impairment, persons with MCI have neuronal loss in layer II of the entorhinal cortex [161]. A number of studies examined the cholinergic system. Persons with MCI also had reduced neuron numbers in the cholinergic basal forebrain [162,163]. At the same time, there was an apparent upregulation of choline acetyltransferase activity in the hippocampus and frontal cortex, but not the primary visual cortex [164–1666]. While levels of nerve growth factors were maintained, it was not correlated with choline acetyltransferase activity [167]. Other neurotransmitter systems were investigated. The glutaminergic system was related to amyloid deposition and also appeared to be upregulated in MCI [168,169]. Changes in the GABAergic system were also described [170].
Relation of Factors to Motor Function, Disability, and Risk of Death
Diabetes was related to a more rapid change in parkinsonian signs, especially parkinsonian gait [171]. Change in parkinsonian signs, especially gait and rigidity was associated with an increased risk of death [37]. Systolic blood pressure was associated with a more rapid decline in lower extremity function assessed with quantitative measures of gait and balance [40]. While the effect persisted after controlling for vascular disease at baseline, it was attenuated when controlling for incident stroke suggesting that the effect might be mediated by cerebrovascular disease.
Parkinsonian signs, especially parkinsonian gait, were associated with a wide range of pathologies including nigral degeneration, nigral Lewy bodies, nigral neurofibrillary tangles, and cerebrovascular disease [87,172,173]. BMI was also associated with AD pathology and did not differ by dementia status [174].
Musculoskeletal pain was also associated with an elevated risk of mobility disability [41]. A greater number of joints affected was associated with a higher risk.
The personality traits of extraversion and conscientiousness were associated with a reduced risk of incident disability [175]. Psychological distress, depression, and suppressed anger were all associated with an increased risk of death whereas conscientiousness was associated with a reduced risk of death [176,177].
Studies of Implicit Memory
One class of implicit memory, repetition priming, is characterized by facilitation in speed or accuracy of processing an item as a result of prior exposure [178]. In contrast to episodic memory which show steady declines with age, measures of priming appear relatively stable over time, a finding that is not due to measurement instability [178,179]. Priming tasks known to draw on both visual-perceptual and conceptual processing were associated with slower rates of decline in activities of daily living, and visual-perceptual were associated with slower rates of progression of parkinsonian signs, especially parkinsonian gait [180,181]. Higher levels of neuropathology also related to lower levels of conceptual processing, or meaning-based but were not related to other priming tasks [182].
Other Neurobiologic Changes in Aging, MCI and AD
Mechanisms leading to cell death were also investigated including the role of nitric oxide-mediated neurodegeneration, and the role of caspase, Pin1, sirtuin 1, tissue transglutaminase, neprilysin, ryanodine receptor, and BDNF-TrkB signaling, in amyloid deposition, tangle formation, and neurodegeneration [183–195]. There also have been studies of oxidative stress, including astrocyte expression of heme oxygenase-1, oxysterol formation, and erythropoietin receptor expression [196–198]. Additional studies have examined inflammatory markers [199] and proteomics [200,201]. Tissue has been used to investigate the relation of amyloid deposits to amyloid imaging agents [202]. Genome-wide studies of aging and disability are also underway [203].
DISCUSSION
The Religious Orders Study has generated nearly 200 peer-reviewed publications to date on a wide range of issues related to aging and AD. The main findings can be summarized into four major headings: 1) continuum of cognition and neuropathology; 2) implications of mixed pathologies; 3) evidence of neural reserve; and 4) non-cognitive consequences of AD pathology.
Continuum of Cognition and Neuropathology from Normality to MCI to Dementia
Embedded within the US health care system is a categorical approach to disease. Patients, families, payers, and public health planners generally approach disease as something that is present or absent. However, many common chronic conditions of aging do not develop over minutes or hours or days such as a stroke or myocardial infarction. Rather, diseases such as osteoporosis and chronic obstructive pulmonary disease have an insidious onset that develops over years and possibly decades and the demarcation between the presence and absence of the disease is a matter of consensus criteria based on imperfect data. For some conditions, a designation intermediate between normality and disease is employed such as pre-hypertension and osteopenia. The situation is analogous for AD and MCI. Our data are most consistent with a continuum of cognitive change accompanied by a continuum of neuropathology with no precise clinical or neuropathologic cutpoint that distinguishes dementia from MCI or MCI from normality. In fact, using change point models, we recently found that about five to six years prior to the time a clinical diagnosis of AD is made, rate of cognitive decline sharply accelerates by about 15 fold [204]. In addition, MCI is also preceded by an accelerated rate of cognitive decline that begins nearly five years prior to diagnosis. Our findings are consistent with other studies that have addressed this issue [205–209]. These data suggest that much of cognitive decline among persons without dementia is not the result of a normative aging process but rather the result of the common neuropathologies that ultimately result in MCI and dementia. Further, these findings also suggest that there is unlikely to be qualitatively meaningful biologic distinctions between MCI and AD, regardless of the definition used. Our findings on AD contributed to the recent reconceptualization of AD as a condition that begins with an asymptomic pathophysiologic process that progresses into MCI due to AD, followed by end stage dementia due to AD [210–212].
Implications of Mixed Pathologies for the Prevention of AD
Many groups have found that mixed pathologies contribute to dementia [213–218]. A major goal of the Religious Orders Study is to understanding biologic pathways linking risk factors to clinical disease. It turns out that among the risk factors investigated to date, only APOE, CR1 and CEPT are strongly related to clinical AD through the AD pathology. And both APOE and CR1 have other effects such as associations with cerebral infarctions and amyloid angiopathy. By contrast, because cerebral infarcts increase the odds of clinical AD, risk factors for cerebral infarctions will also be risk factors for clinically diagnosed AD despite the absence of an association with AD pathology. Thus, we found that diabetes was likely related to clinical AD through an association with infarctions. However, the association is complex and other studies have reported a number of different findings [219–222]. These findings suggest that translating findings from epidemiologic studies to animal models should be done with caution [223]. Many animal models, such as transgenic models, are intended to investigate the role of amyloid deposition and tangle formation in the pathogenesis of AD. Further, the findings also suggest that association studies of pure AD will be biased since factors associated with co-morbidities will appear to be protective for AD. Our findings contributed to the development of new statement on the vascular contributions to cognitive impairment [224].
Evidence and Implications of Neural Reserve
Several experiential and psychological risk factors are related to clinical AD without evidence of having a direct effect on the accumulation of any of the three major neuropathologies linked to late life dementia. In some cases, the risk factor alters the relation of pathology to clinical outcomes. Others replicated our findings using both neuropathology and neuroimaging measures [225–228]. In other cases, such as depressive symptoms and psychological distress, the risk factor seems to have a separate but additive effect to the development of clinical disease. However, one study reported an association between depression and hippocampal AD pathology [229]. These findings suggest that there are unidentified neurobiologic pathways leading to cognitive decline. These could include neurodegeneration without a pathologic footprint. Alternatively, it may be that we are simply unable to identify the pathologic footprint based on current knowledge and abnormal proteins will be identified in the future. For example, in one preliminary study, variants in the RELN gene were associated with neuropathology among persons without dementia [140]. Together these data suggest that other neurobiologic indices must be important for the development of dementia [230]. Identifying these indices is important as they are potential new targets for therapeutic interventions. A variety of efforts are ongoing in the Religious Orders Study aimed at identifying additional therapeutic targets including epigenome-wide DNA methylation and histone acetylation studies, RNA expression studies, and proteomics.
Noncognitive Consequences of AD Pathology
We identified a number of motoric signs associated with risk of AD that are also related to AD pathology. Unlike APOE for which the temporal relation between the exposure and AD pathology is secure, and unlike diabetes for which there is ample evidence from other sources to suggest that it is a risk factor for cerebrovascular disease, it is difficult to imagine a scenario in which parkinsonian signs and BMI actually lead to the accumulation of AD pathology. Thus, a more parsimonious explanation is that these signs actually represent noncognitive clinical manifestations of AD pathology that are present prior to the time persons meet criteria for dementia. Our clinical findings have been observed in other cohort studies. Prior studies have reported a relation between parkinsonian signs and AD risk [231,232]. Further, prior clinical-pathologic studies have found a relation between nigral tangles and parkinsonian signs among persons with dementia [233–235]. However, we are unaware of prior studies that have examined these associations among persons without dementia. Clinical studies also show that low BMI and weight loss in old age is related to dementia risk [236–239]. We are not aware of other studies that have examined these associations with brain pathology.
Together, these data suggest that AD pathology is a cause of impaired motor function in older persons without dementia. For most neurologic diseases, the classic approach in neurology is to localize the lesions and then determine its etiology. Thus, for stroke and brain tumors, for example, the clinical manifestation depends largely on the anatomic location. However, this approach is not currently employed for neurodegenerative diseases such as AD and Parkinson’s disease (PD). These diseases are defined clinically and confirmed pathologically. There are many reasons related to the history of the field that has resulted in this development. However, it is much more likely that the location of AD pathology and Lewy bodies dictate the clinical expression of AD and PD and that loss of motor function can precede loss of cognition in AD, and likewise, loss of cognition can precede loss of motor function in PD.
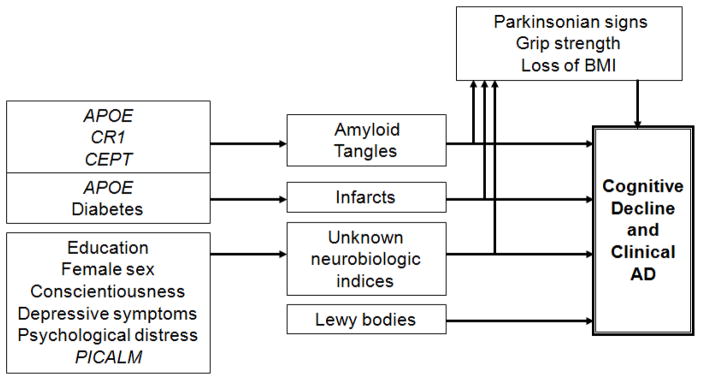
Overall Model Summarizing Findings (Figure 3)
Figure 3.
Overview of clinical pathologic relationships reported in the Religious Orders Study through March 2010.
The model highlights the important role of mixed pathology in the development of AD, the finding that some risk factors work through as yet unidentified biologic pathways, and that AD and other pathology can lead to non-cognitive manifestations which may be evident prior to the development of overt dementia. Our finding that only genetic risk factors are associated with AD pathology have lead us to consider a model whereby AD pathology is primarily under the control of genetic variation. By contrast, experiential and psychological factors may primarily affect how your brain responds to the accumulation of pathology, i.e., neural or cognitive reserve.
Future studies are aimed at identifying additional risk factors and novel biologic pathways. For example, a genome wide association study is already underway seeking to identify additional genetic variants associated with AD pathology, in addition to variants associated with cognitive decline and incident AD [203]. In addition, two epigenome wide association studies currently are underway to examine other mechanisms that may link experiential and psychological phenotypes to cognitive decline. These include an epigenome-wide association study with DNA methylation using the Illumina 450 and an epigenome-wide association study of histone acetylation (H3K9Ac) using chromatin immunoprecipiation followed by sequencing (ChIP-Seq). Finally, post-mortem imaging is now being used to further investigate vascular disease [240,241].
Strengths and Weakness of the Study
The study has many strengths. Individuals are documented to be free of dementia or free of MCI at baseline and there is a large sample size and a relatively long duration of follow-up with repeated measures of cognition and large number of persons with incident AD and incident MCI. The follow-up rates of survivors and the autopsy rates are very high reducing the bias that occurs when select persons drop out of longitudinal studies or when autopsy is not obtained. Availability of postmortem specimens and data allow examination of underlying biologic mechanisms of disease. Structured procedures ensure uniformity of diagnostic decisions across time and space. Examiners are blinded to previously collected data further reducing bias. In addition, the similarity of lifestyle adds further control of potentially confounding variables.
The study also has limitations. The truncated range of socioeconomic status limits our ability to investigate these variables. For example, we did not find a relation of education to AD risk which could be due to truncated range of education; although we did find a relation of cognitive activity to AD risk [70]. Most cardiovascular risk factors are limited to self report. There are a restricted number of racial and ethnic minority clergy communities limiting their numbers in the study. It is an observational study and inferences regarding causality must be made with caution. Although, for many psychological and experiential risk factors, the effect sizes are likely to be small and cumulative and beyond the resolution of randomized clinical trials. Finally, it is a volunteer cohort of catholic clergy who must agree to autopsy at study entry and participants are not representative of any particular population of older persons. Thus, similar types of studies will need to be done on cohorts with a much wider range of life experiences, in cohorts of racial and ethnic minorities, and in population based cohorts. In fact, the Religious Orders Study has data collection methods that are similar in many respects with other cohort studies, including the Memory and Aging Project and the Minority Aging Research Study, both the subject of a report in this volume. In addition, several risk factor associations have been replicated in the Chicago Health and Aging Project, a cohort study of incident AD in a geographically defined biracial population on the south side of Chicago [242]. These include late life cognitive activity, depressive symptoms, distress proneness, early life socioeconomic status, and use of anti-inflammatories [243–248].
Acknowledgments
This work is dedicated to the nuns, priests, and brothers participating in the Religious Orders Study.
We thank Tracy Colvin, MPH, Julie Bach, MSW, PhD, Karen Lowe-Graham, MS, George Hoganson, and Karen Skish, MS, PA(ASCP)MT, for study recruitment and coordination, and John Gibbons, MS and Greg Klein for data management.
Work presented here was supported by grants from the National Institute on Aging: P30AG10161, R01AG15819, R01AG24871, R01AG26147, R01AG26916, P01AG09466, P01AG14449; K08AG00849, K23AG23675, the Illinois Department of Public Health, the Elsie Heller Brain Bank Endowment Fund, and the Robert C. Borwell Chair of Neurological Sciences.
References
- 1.Amaducci LA, Rocca WA, Schoenberg BS. Origin of the distinction between Alzheimer’s disease and senile dementia: how history can clarify nosology. Neurology. 1986;36:1497–9. doi: 10.1212/wnl.36.11.1497. [DOI] [PubMed] [Google Scholar]
- 2.Roth M, Tomlinson BE, Blessed G. Correlation between scores for dementia and counts of ‘senile plaques’ in cerebral grey matter of elderly subjects. Nature. 1966;209:109–10. doi: 10.1038/209109a0. [DOI] [PubMed] [Google Scholar]
- 3.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 4.Tomlinson BE, Blessed G, Roth M. Observations on the brains of non-demented old people. J Neurol Sci. 1968;7:331–56. doi: 10.1016/0022-510x(68)90154-8. [DOI] [PubMed] [Google Scholar]
- 5.Tomlinson BE, Blessed G, Roth M. Observations on the brains of demented old people. J Neurol Sci. 1970;11:205–42. doi: 10.1016/0022-510x(70)90063-8. [DOI] [PubMed] [Google Scholar]
- 6.Katzman R. Editorial: The prevalence and malignancy of Alzheimer disease. A major killer. Arch Neurol. 1976;33:217–8. doi: 10.1001/archneur.1976.00500040001001. [DOI] [PubMed] [Google Scholar]
- 7.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 8.Khachaturian ZS. Diagnosis of Alzheimer’s disease. Arch Neurol. 1985;42:1097–105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- 9.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–65. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 10.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 11.Morris JC, McKeel DW, Jr, Fulling K, Torack RM, Berg L. Validation of clinical diagnostic criteria for Alzheimer’s disease. Ann Neurol. 1988;24:17–22. doi: 10.1002/ana.410240105. [DOI] [PubMed] [Google Scholar]
- 12.Hansen L, Salmon D, Galasko D, et al. The Lewy body variant of Alzheimer’s disease: a clinical and pathologic entity. Neurology. 1990;40:1–8. doi: 10.1212/wnl.40.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Lopez OL, Swihart AA, Becker JT, et al. Reliability of NINCDS-ADRDA clinical criteria for the diagnosis of Alzheimer’s disease. Neurology. 1990;40:1517–22. doi: 10.1212/wnl.40.10.1517. [DOI] [PubMed] [Google Scholar]
- 14.Kukull WA, Larson EB, Reifler BV, Lampe TH, Yerby MS, Hughes JP. The validity of 3 clinical diagnostic criteria for Alzheimer’s disease. Neurology. 1990;40:1364–1369. doi: 10.1212/wnl.40.9.1364. [DOI] [PubMed] [Google Scholar]
- 15.Gearing M, Mirra SS, Hedreen JC, Sumi SM, Hansen LA, Heyman A. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). X. Neuropathology confirmation of the clinical diagnosis of Alzheimer’s disease. Neurology. 1995;45:461–466. doi: 10.1212/wnl.45.3.461. [DOI] [PubMed] [Google Scholar]
- 16.Saper CB, German DC, White CL., 3rd Neuronal pathology in the nucleus basalis and associated cell groups in senile dementia of the Alzheimer’s type: possible role in cell loss. Neurology. 1985;35:1089–95. doi: 10.1212/wnl.35.8.1089. [DOI] [PubMed] [Google Scholar]
- 17.Honer WG, Dickson DW, Gleeson J, Davies P. Regional synaptic pathology in Alzheimer’s disease. Neurobiol Aging. 1992;13:375–82. doi: 10.1016/0197-4580(92)90111-a. [DOI] [PubMed] [Google Scholar]
- 18.McKee AC, Kosik KS, Kowall NW. Neuritic pathology and dementia in Alzheimer’s disease. Ann Neurol. 1991;30:156–65. doi: 10.1002/ana.410300206. [DOI] [PubMed] [Google Scholar]
- 19.Arriagada PV, Marzloff K, Hyman BT. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurology. 1992;42:1681–8. doi: 10.1212/wnl.42.9.1681. [DOI] [PubMed] [Google Scholar]
- 20. [accessed November 23, 2011]; http://cerad.mc.duke.edu/database.htm.
- 21.Katzman R, Terry R, DeTeresa R, et al. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988;23:138–44. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- 22.Crystal HA, Dickson DW, Sliwinski MJ, et al. Pathological markers associated with normal aging and dementia in the elderly. Ann Neurol. 1993;34:566–73. doi: 10.1002/ana.410340410. [DOI] [PubMed] [Google Scholar]
- 23.Berg L, McKeel DW, Jr, Miller JP, Baty J, Morris JC. Neuropathological indexes of Alzheimer’s disease in demented and nondemented persons aged 80 years and older. Arch Neurol. 1993;50:349–58. doi: 10.1001/archneur.1993.00540040011008. [DOI] [PubMed] [Google Scholar]
- 24.Hof PR, Bierer LM, Perl DP, et al. Evidence for early vulnerability of the medial and inferior aspects of the temporal lobe in an 82-year-old patient with preclinical signs of dementia. Regional and laminar distribution of neurofibrillary tangles and senile plaques. Arch Neurol. 1992;49:946–53. doi: 10.1001/archneur.1992.00530330070019. [DOI] [PubMed] [Google Scholar]
- 25.Snowdon DA. Aging and Alzheimer’s disease: lessons from the Nun Study. Gerontologist. 1997;37:150–6. doi: 10.1093/geront/37.2.150. [DOI] [PubMed] [Google Scholar]
- 26.Wilson RS, Scherr PA, Hoganson G, Bienias JL, Evans DA, Bennett DA. Early life socioeconomic status and late life risk of Alzheimer’s disease. Neuroepidemiology. 2005;25:8–14. doi: 10.1159/000085307. [DOI] [PubMed] [Google Scholar]
- 27.Bennett DA, Schneider JA, Aggarwal NT, et al. Decision rules guiding the clinical diagnosis of Alzheimer’s disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006;27:169–176. doi: 10.1159/000096129. [DOI] [PubMed] [Google Scholar]
- 28.Weir DR, Wallace RB, Langa KM, et al. Reducing case ascertainment costs in US population studies of Alzheimer’s disease, dementia, and cognitive impairment—Part 1. Alzheimer’s & Dementia. 2011;7:94–109. doi: 10.1016/j.jalz.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 30.Aggarwal NT, Wilson RS, Beck TL, Bienias JL, Bennett DA. Mild cognitive impairment in different functional domains and incident Alzheimer’s disease. Journal of Neurology, Neurosurgery, & Psychiatry. 2005;76:1479–1484. doi: 10.1136/jnnp.2004.053561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64:834–841. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- 32.Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: Diagnostic criteria for research studies - Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 33.Langston JW, Widner H, Goetz CGT, et al. Core Assessment Program for Intracerebral Transplantations (CAPIT) Movement Disord. 1992;7:2–13. doi: 10.1002/mds.870070103. [DOI] [PubMed] [Google Scholar]
- 34.Robins LN, Helzer JE, Ratcliff KS, Seyfried W. Validity of the Diagnostic Interview Schedule, II: DSM-III diagnoses. Psychol Med. 1982;12:855–870. doi: 10.1017/s0033291700049151. [DOI] [PubMed] [Google Scholar]
- 35.Schneider JA, Wilson RS, Cochran EJ, et al. Relation of cerebral infarctions to dementia and cognitive function in older person. Neurology. 2003;60:1082–1089. doi: 10.1212/01.wnl.0000055863.87435.b2. [DOI] [PubMed] [Google Scholar]
- 36.Wilson RS, Barnes LL, Mendes de Leon CF, et al. Depression, change in cognitive function, and risk of Alzheimer’s disease in older persons. Neurology. 2002;59:364–371. doi: 10.1212/wnl.59.3.364. [DOI] [PubMed] [Google Scholar]
- 37.Wilson RS, Schneider JA, Beckett LA, Evans DA, Bennett DA. Progression of gait disorder and rigidity and risk of death in older persons. Neurology. 2002;58:1815–1819. doi: 10.1212/wnl.58.12.1815. [DOI] [PubMed] [Google Scholar]
- 38.McKeith IG, Perry EK, Perry RH. Report of the second dementia with Lewy body international workshop: diagnosis and treatment. Consortium on Dementia with Lewy Bodies Neurology. 1999;53:902–905. doi: 10.1212/wnl.53.5.902. [DOI] [PubMed] [Google Scholar]
- 39.Arvanitakis Z, Bienias JL, Wilson RS, Evans DA, Bennett DA. Diabetes and risk of Alzheimer’s disease and decline in cognitive function. Archives of Neurology. 2004;61:661–666. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 40.Shah RC, Wilson RS, Bienias JL, Arvanitakis Z, Evans DA, Bennett DA. Blood pressure and lower limb function in older persons. Journal of Gerontology: Medical Sciences. 2006;61:839–843. doi: 10.1093/gerona/61.8.839. [DOI] [PubMed] [Google Scholar]
- 41.Shah RC, Buchman AS, Boyle PA, et al. Musculoskeletal pain is associated with increased risk of mobility disability in community dwelling elders. Journals of Gerontology: Medical Sciences. 2011;66:82–88. doi: 10.1093/gerona/glq187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: A practical method for grading the mental state of patients for the clinician. J Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 43.Goodlass H, Kaplan D. The assessment of aphasia and related disorders. 2. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 44.Wechsler D. Wechsler Memory Scale-Revised manual. New York: Psychological Corporation; 1987. [Google Scholar]
- 45.Albert MS, Smith LA, Scherr PA, et al. Use of brief cognitive tests to identify individuals in the community with clinically-diagnosed Alzheimer’s disease. Intl J Neurosci. 1991;57:167–178. doi: 10.3109/00207459109150691. [DOI] [PubMed] [Google Scholar]
- 46.Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 47.Ekstrom RB, French JW, Harman HH, Dermen D. Manual for kit of factor-referenced cognitive tests. Princeton: Educational Testing Service; 1976. [Google Scholar]
- 48.Nelson HE. National Adult Reading Test (NART): Test manual. Windsor, UK: NFER Nelson; 1982. [Google Scholar]
- 49.Blair JR, Spreen O. Predicting premorbid IQ: A revision of the National Adult Reading Test. The Clinical Neuropsychologist. 1989;3:129–136. [Google Scholar]
- 50.Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. Journal of Clinical and Experimental Neuropsychology. 1991;13:933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- 51.Cooper JA, Sagar HJ. Incidental and intentional recall in Parkinson’s disease: An account based on diminished attentional resources. J Clin Exper Neuropsychol. 1993;15:713–731. doi: 10.1080/01688639308402591. [DOI] [PubMed] [Google Scholar]
- 52.Craik FIM. A functional account of age differences in memory. In: Klix E, Hagendorf H, editors. Human memory and cognitive capabilities: Mechanisms and performances. Amsterdam: Elsevier Science; 1986. pp. 409–422. [Google Scholar]
- 53.Smith A. Symbol Digit Modalities Test manual-revised. Los Angeles: Western Psychological; 1984. [Google Scholar]
- 54.Benton AL, Sivan AB, Hamsher K, deS, Varney NR, Spreen O. Contributions to neuropsychological assessment. 2. New York: Oxford University Press; 1994. [Google Scholar]
- 55.Raven JC, Court JH, Raven J. Manual for Raven’s progressive matrices and vocabulary: Standard Progressive Matrices. 1992. Oxford: Oxford Psychologists Press; 1992. [Google Scholar]
- 56.Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychology and Aging Psych & Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- 57.Wilson RS, Bennett DA. Assessment of cognitive decline in old age with brief tests amenable to telephone administration. Neuroepidemiology. 2005;25:19–25. doi: 10.1159/000085309. [DOI] [PubMed] [Google Scholar]
- 58.Fahn S, Elton RL. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne DI3, editors. Recent developments in Parkinson’s disease. Vol. 2. Florham Park, NJ: MacMillan Healthcare Information; 1987. pp. 153–163. [Google Scholar]
- 59.Bennett DA, Shannon KM, Beckett LA, Goetz CG, Wilson RS. Metric properties of nurses’ ratings of parkinsonian signs with a modified Unified Parkinson’s Disease Rating Scale. Neurology. 1997;49:1580–1587. doi: 10.1212/wnl.49.6.1580. [DOI] [PubMed] [Google Scholar]
- 60.Bennett DA, Shannon KM, Beckett LA, Wilson RS. Dimensionality of parkinsonian signs in aging and Alzheimer’s disease. J Gerontol: Med Sci. 1999;54:M191–M196. doi: 10.1093/gerona/54.4.m191. [DOI] [PubMed] [Google Scholar]
- 61.Tiffin J, Asher EJ. The Purdue Pegboard: Norms and studies of reliability and validity. J Appl Psychol. 1948;32:234–247. doi: 10.1037/h0061266. [DOI] [PubMed] [Google Scholar]
- 62.Wilson RS, Bienias JL, Evans DA, Bennett DA. The Religious Orders Study: Overview and relation between change in cognitive and motor speed. Aging Neuropsych Cog. 2004;11:280–303. [Google Scholar]
- 63.Buchman AS, Wilson RS, Boyle PA, Bienias JL, Bennett DA. Grip strength and risk of incident AD. Neuroepidemiology. 2007;29:66–73. doi: 10.1159/000109498. [DOI] [PubMed] [Google Scholar]
- 64.Aggarwal NT, Wilson RS, Beck TL, Bienias JL, Bennett DA. Motor dysfunction in mild cognitive impairment and risk of incident Alzheimer’s disease. Archives of Neurology. 2006;63:1763–1769. doi: 10.1001/archneur.63.12.1763. [DOI] [PubMed] [Google Scholar]
- 65.Buchman AS, Wilson RS, Bienias JL, Shah R, Evans DA, Bennett DA. Change in body mass index (BMI) and risk of incident Alzheimer’s disease (AD) Neurology. 2005;65:892–898. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- 66.Katz S, Akpom C. A measure of primary sociobiological functions. Int J Health Serv. 1976;6:493–508. doi: 10.2190/UURL-2RYU-WRYD-EY3K. [DOI] [PubMed] [Google Scholar]
- 67.Rosow I, Breslau N. A Guttman health scale for the aged. J Gerontol. 1966;21:556–559. doi: 10.1093/geronj/21.4.556. [DOI] [PubMed] [Google Scholar]
- 68.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86. [PubMed] [Google Scholar]
- 69.Nagi SZ. An epidemiology of disability among adults in the United States. Milbank Mem Fund Q. 1976;54:439–468. [PubMed] [Google Scholar]
- 70.Wilson RS, Mendes de Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer’s disease. JAMA. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- 71.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D depression symptoms index. J Aging Hlth. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 72.Spielberger CD. Manual for the State-Trait Anxiety Inventory (form Y) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 73.Spielberger CD. State-Trait Anger Expression Inventory—revised research edition. Odessa, FL: Psychological Assessment Resources, Inc; 1991. [Google Scholar]
- 74.Costa PT, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) professional manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- 75.Bennett DA, Wilson RS, Schneider JA, et al. Apolipoprotein E4 allele, Alzheimer’s disease pathology, and the clinical expression of Alzheimer’s disease. Neurology. 2003;60:246–252. doi: 10.1212/01.wnl.0000042478.08543.f7. [DOI] [PubMed] [Google Scholar]
- 76.Chibnik LB, Shulman JM, Leurgans SE, et al. The Alzheimer’s susceptibility locus CR1 is associated with increased amyloid plaque burden and age-related cognitive decline. Annals of Neurology. 2011;69:560–569. doi: 10.1002/ana.22277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klaver AC, Coffey MP, Smith LM, Bennett DA, Loeffler DA. Power analysis to establish adequate group sizes for ELISA measurement of specific serum anti-Abeta antibodies in Alzheimer’s disease, mild cognitive impairment, and aged noncognitively impaired subjects. Journal of Neuroinflammation. 2011;8:93. doi: 10.1186/1742-2094-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mufson EJ, Leurgans S. Inability of plasma and urine F2A-isoprostane levels to differentiate mild cognitive impairment from Alzheimer’s disease. Neurodegener Dis. 2010;7:139–42. doi: 10.1159/000289224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lurain NS, Hanson BA, Martinson J, et al. The association of human cytomegalovirus (HCMV) markers of infection with cognitive impairment and Alzheimer’s Disease. 35th Annual International Herpesvirus Workshop; Salt Lake City, Utah. July 24, 2010. [Google Scholar]
- 80.Honig LS, Kukull W, Mayeux R. Atherosclerosis and AD: analysis of data from the US National Alzheimer’s Coordinating Center. Neurology. 2005;64:494–500. doi: 10.1212/01.WNL.0000150886.50187.30. [DOI] [PubMed] [Google Scholar]
- 81.The National Institute on Aging. Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- 82.Mirra SM, Hart MN, Terry RD. Making the Diagnosis of Alzheimer’s Disease. A Primer for Practicing Pathologists. Arch Pathol Lab Med. 1993;117:132–144. [PubMed] [Google Scholar]
- 83.Braak H, Braak E. Neuropathological staging of AD-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 84.Schneider JA, Bienias JL, Gilley DW, et al. Improved Detection of Nigral Pathology in AD. J Histochem Cytochem. 2002;50:99–106. doi: 10.1177/002215540205000111. [DOI] [PubMed] [Google Scholar]
- 85.Schneider JA, Bennett DA. Where vascular meets neurodegenerative disease. Stroke. 2010;41:S144–S146. doi: 10.1161/STROKEAHA.110.598326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load and with clinical Alzheimer’s disease and level of cognitive function. Arch Neurol. 2004;61:378–384. doi: 10.1001/archneur.61.3.378. [DOI] [PubMed] [Google Scholar]
- 87.Buchman AS, Shulman JM, Nag S, et al. Nigral pathology and parkinsonian signs in elders without Parkinson’s disease. Annals of Neurology. 2011 doi: 10.1002/ana.22588. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA. Microinfarct pathology, dementia, and cognitive systems. Stroke. 2011;42:722–7. doi: 10.1161/STROKEAHA.110.595082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Arvanitakis Z, Leurgans SE, Wang ZB, Wilson RS, Bennett DA, Schneider JA. Cerebral amyloid angiopathy pathology in older persons with and without dementia. Annals of Neurology. 2011;69:320–327. doi: 10.1002/ana.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tremblay C, St-Amour I, Schneider JA, Bennett DA, Calon F. Accumulation of TDP-43 in mild cognitive impairment and Alzheimer’s disease. Journal of Neuropathology and Experimental Neurology. 2011;70:788–798. doi: 10.1097/NEN.0b013e31822c62cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li Y, Schneider JA, Bennett DA. Estimation of the mediation effect with a binary mediator. Statistics in Medicine. 2007;26:3398–3414. doi: 10.1002/sim.2730. [DOI] [PubMed] [Google Scholar]
- 92.Li Y, Bienias JL, Bennett DA. Confounding in the estimation of mediation effects. Computational Statistics & Data Analysis. 2007;51:3173–3186. doi: 10.1016/j.csda.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cox DR. Regression models and life tables (with discussion) J Soc Stat Soc B. 1972;74:187–220. [Google Scholar]
- 94.Boyle PA, Buchman AS, Wilson RS, Kelly JF, Bennett DA. The ApoE e4 allele Is associated with incident mild cognitive impairment among community-dwelling older persons. Neuroepidemiology. 2010;34:43–49. doi: 10.1159/000256662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Laird N, Waire J. Random effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 96.Fleischman DA, Bienias JL, Bennett DA. Repetition priming and change in functional ability in older persons without dementia. Neuropsychology. 2009;23:98–104. doi: 10.1037/a0013994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wilson RS, Beckett LA, Bienias JL, Evans DA, Bennett DA. Terminal decline in cognitive function. Neurology. 2003;60:1782–1787. doi: 10.1212/01.wnl.0000068019.60901.c1. [DOI] [PubMed] [Google Scholar]
- 98.Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. Neuropathology of probable AD and amnestic and non-amnestic MCI. Annals of Neurology. 2009;66:200–208. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based clinical-pathologic studies. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 100.Ghoshal N, Garcia-Sierra F, Wuu J, et al. Tau conformational changes correspond to impairments of episodic memory in mild cognitive impairment and Alzheimer’s disease. Exp Neurol. 2002;177:475–93. doi: 10.1006/exnr.2002.8014. [DOI] [PubMed] [Google Scholar]
- 101.Mitchell TW, Nissanov J, Han LY, et al. Novel method to quantify neuropil threads in brains form elders with and without cognitive impairment. Journal of Histochemistry & Cytochemistry. 2000;48:1627–1638. doi: 10.1177/002215540004801206. [DOI] [PubMed] [Google Scholar]
- 102.Mitchell TW, Mufson EJ, Schneider JA, et al. Parahippocampal tau pathology in healthy aging, mild cognitive impairment, and early Alzheimer’s disease. Ann Neurol. 2002;51:182–9. doi: 10.1002/ana.10086. [DOI] [PubMed] [Google Scholar]
- 103.Mufson EJ, Chen EY, Cochran EJ, Beckett LA, Bennett DA, Kordower JH. Entorhinal cortex β-amyloid load in individuals with mild cognitive impairment. Experimental Neurology. 1999;158:469–490. doi: 10.1006/exnr.1999.7086. [DOI] [PubMed] [Google Scholar]
- 104.Tremblay C, Pilote M, Phivilay A, Emond V, Bennett DA, Calon F. Biochemical characterization of Aβ and tau pathologies in persons with mild cognitive impairment and Alzheimer disease. Journal of Alzheimer’s Disease. 2007;12:377–390. doi: 10.3233/jad-2007-12411. [DOI] [PubMed] [Google Scholar]
- 105.Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer’s disease pathology. Neurology. 2004;62:1148–1152. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- 106.Wang DS, Bennett DA, Mufson E, Cochran E, Dickson DW. Decreases in soluble alpha-synuclein in frontal cortex correlate with cognitive decline in the elderly. Neurosci Lett. 2004;359:104–8. doi: 10.1016/j.neulet.2004.01.044. [DOI] [PubMed] [Google Scholar]
- 107.Dowling NM, Farias ST, Reed BR, et al. Neuropathological associates of multiple cognitive functions in two community-based cohorts of older adults. Journal of the International Neuropsychological Society. 2011;17:602–614. doi: 10.1017/S1355617710001426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wilson RS, Krueger KR, Boyle PA, Bennett DA. Loss of basic lexical knowledge in old age. Journal of Neurology, Neurosurgery, & Psychiatry. 2011;82:369–372. doi: 10.1136/jnnp.2010.212589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wilson RS, Segawa E, Hizel LP, Boyle PA, Bennett DA. Terminal dedifferentiation of cognitive abilities. Neurology. doi: 10.1212/WNL.0b013e31824f7ff2. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wilson RS, Leurgans SE, Boyle PA, Schneider JA, Bennett DA. Neurodegenerative basis of cognitive decline in old age. Neurology. 2010;75:1070–8. doi: 10.1212/WNL.0b013e3181f39adc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hayden KM, Reed BR, Manly JJ, et al. Cognitive decline in the elderly: an analysis of population heterogeneity. Age and Aging. 2011;40:684–689. doi: 10.1093/ageing/afr101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Barnes LL, Wilson RS, Schneider JA, Bienias, Evans DA, Bennett DA. Cognitive decline in older men and women. Neurology. 2003;60:1777–1781. doi: 10.1212/01.wnl.0000065892.67099.2a. [DOI] [PubMed] [Google Scholar]
- 113.Wilson RS, Li Y, Bienias JL, Bennett DA. Cognitive decline in old age: Separating practice effects from the effects of growing older. Psychology & Aging. 2006;21:774–789. doi: 10.1037/0882-7974.21.4.774. [DOI] [PubMed] [Google Scholar]
- 114.Reed B, Dowling M, Farias ST, et al. Cognitive activities during adulthood are more important than education for building brain reserve. Journal of the International Neuropsychological Society. 2011;17:615–624. doi: 10.1017/S1355617711000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wilson RS, Arnold SE, Beck TL, Bienias JL, Bennett DA. Change in depressive symptoms during the prodromal phase of Alzheimer’s disease. Archives of General Psychiatry. 2008;65:439–45. doi: 10.1001/archpsyc.65.4.439. [DOI] [PubMed] [Google Scholar]
- 116.Wilson RS, Evans DA, Bienias JL, Mendes de Leon CF, Schneider JA, Bennett DA. Proneness to psychological distress and risk of Alzheimer’s disease. Neurology. 2003;61:1579–1585. doi: 10.1212/01.wnl.0000096167.56734.59. [DOI] [PubMed] [Google Scholar]
- 117.Wilson RS, Schneider JA, Boyle PA, Arnold SE, Tang Y, Bennett DA. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68:2085–2092. doi: 10.1212/01.wnl.0000264930.97061.82. [DOI] [PubMed] [Google Scholar]
- 118.Wilson RS, Schneider JA, Arnold SE, Bienias JL, Bennett DA. Conscientiousness and the incidence of Alzheimer’s disease and mild cognitive impairment. Archives of General Psychiatry. 2007;64:1204–12. doi: 10.1001/archpsyc.64.10.1204. [DOI] [PubMed] [Google Scholar]
- 119.Wilson RS, Schneider JA, Bienias JL, Evans DA, Bennett DA. Parkinsonian-like signs and risk of incident Alzheimer’s disease in older persons. Arch Neurol. 2003;60:539–544. doi: 10.1001/archneur.60.4.539. [DOI] [PubMed] [Google Scholar]
- 120.Hensley K, Barnes LL, Christov A, et al. Analysis of postmortem ventricular cerebrospinal fluid from patients with and without dementia indicates association of vitamin E with neuritic plaques and specific measures of cognitive performance. Journal of Alzheimer’s Disease. 2011;24:767–774. doi: 10.3233/JAD-2011-101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shah RC, Wilson RS, Bienias JL, Arvanitakis Z, Evans DA, Bennett DA. Effect of blood pressure on incident Alzheimer’s disease and change in global cognitive function in older persons. Neuroepidemiology. 2005;25:30–36. doi: 10.1159/000089235. [DOI] [PubMed] [Google Scholar]
- 122.Arvanitakis Z, Grodstein F, Bienias JL, et al. The relation of NSAIDs to incident AD, change in cognitive function, and AD pathology. Neurology. 2008;70:2219–25. doi: 10.1212/01.wnl.0000313813.48505.86. [DOI] [PubMed] [Google Scholar]
- 123.Arvanitakis Z, Schneider JA, Wilson RS, et al. Statins, incident AD, change in cognitive function, and neuropathology. Neurology. 2008:1795–1802. doi: 10.1212/01.wnl.0000288181.00826.63. [DOI] [PubMed] [Google Scholar]
- 124.Wilson RS, Schneider JA, Barnes LL, et al. Effect of apolipoprotein E ε4 allele status on decline in different cognitive systems and development of Alzheimer’s disease over a 6-year period. Arch Neurol. 2002;59:1154–1160. doi: 10.1001/archneur.59.7.1154. [DOI] [PubMed] [Google Scholar]
- 125.Aggarwal NT, Wilson RS, Bienias JL, Berry-Kravis E, Bennett DA. The Apolipoprotein E ε4 Allele and incident AD in person’s with Mild Cognitive Impairment. Neurocase. 2005;11:3–7. doi: 10.1080/13554790490903038. [DOI] [PubMed] [Google Scholar]
- 126.Wilson RS, Bienias JL, Berry-Kravis E, Evans DA, Bennett DA. The apolipoprotein E ε2 allele and decline in episodic memory. J Neurol Neurosurg Psychiat. 2002;73:672–677. doi: 10.1136/jnnp.73.6.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhu H, Gopalraj R, Kelly JF, Bennett DA, Estus S. Lack of genetic association of cholesterol ester transfer protein polymorphisms with late onset Alzheimer’s disease. Neurosci Let. 2005;381:36–41. doi: 10.1016/j.neulet.2005.01.078. [DOI] [PubMed] [Google Scholar]
- 128.Yu L, Shulman J, Chibnik L, et al. The CEPT I405V polymorphism is with cognitive in cognition and higher risk of incident Alzheimer’s disease. Aging Cell. doi: 10.1111/j.1474-9726.2011.00777.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bodner SM, Berrettini W, van der Lein V, et al. Genetic variation in the brain derived neurotrophic factor gene in Alzheimer’s disease. American Journal of Medical Genetics Neuropsychiatric Genetics. 2005;134B:1–5. doi: 10.1002/ajmg.b.30154. [DOI] [PubMed] [Google Scholar]
- 130.Zhu H, Taylor JW, Bennett DA, Younkin SG, Estus S. Lack of association of hepatic lipase polymorphisms with late onset Alzheimer’s disease. Neurobiology of Aging. 2008;29:793–4. doi: 10.1016/j.neurobiolaging.2006.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gopalraj RK, Zhu H, Kelly JF, Pulliam JF, Bennett DA, Estus S. Genetic association of low density lipoprotein receptor and Alzheimer’s Disease. Neurobiology of Aging. 2005;26:1–7. doi: 10.1016/j.neurobiolaging.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 132.Zou F, Gopalraj RK, Lok J, et al. Sex-dependent association of a common low density lipoprotein receptor polymorphism with RNA splicing efficiency in the brain and Alzheimer’s disease. Human Molecular Genetics. 2008;17:929–35. doi: 10.1093/hmg/ddm365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.De Jager PL, Shulman JM, Chibnik LB, et al. A genome-wide scan for common variants affecting rate of age-related cognitive decline. Neurobiology of Aging. doi: 10.1016/j.neurobiolaging.2011.09.033. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sweet RA, Bennett DA, Graff-Radford N, Mayeux R the National Institute on Aging Late-Onset Alzheimer’s Disease Genetics Study. Characteristics and familial aggregation of psychosis in Alzheimer disease in the National Institute on Aging Late-Onset Alzheimer Disease Family Study Group. Brain. 2010;133:1155–1162. doi: 10.1093/brain/awq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wilson RS, Leurgans SE, Foroud TM, et al. Assessment of cognitive function in the late onset Alzheimer’s disease family study. Archives of Neurology. 2010;67:855–861. doi: 10.1001/archneurol.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wilson RS, Barral S, Lee JH, et al. Heritability of different forms of memory in the late onset Alzheimer’s disease family study. Journal of Alzheimer’s Disease. 2011;23:249–55. doi: 10.3233/JAD-2010-101515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Naj AC, Jun G, Beecham GW, et al. Genome-wide association study of late-onset Alzheimer disease identifies disease associated variants in MS4A4/MS4A6E, CD2AP, CD33, and EPHA1. Nature Genetics. 2011;43:436–41. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shulman JM, Chipendo P, Aubin C, et al. Functional validation of Alzheimer’s pathology genome-wide association signals in Drosophila. American Journal of Human Genetics. 2011;88:232–238. doi: 10.1016/j.ajhg.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wijsman EM, Pankratz N, Choi Y, et al. Genome wide association of familial late onset Alzheimer’s disease replicates BIN1 and CLU, and nominates CUGBP2 in interaction with APOE. PLoS Genetics. 2011;7:e1001308. doi: 10.1371/journal.pgen.1001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kramer PL, Xu H, Westaway SK, et al. Alzheimer disease pathology in cognitively healthy elderly: a genome-wide study. Neurobiology of Aging. 2011;32:2113–2122. doi: 10.1016/j.neurobiolaging.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Treusch S, Hamamichi S, Goodman JL, et al. Functional links in yeast between Aβ toxicity, endocytic trafficking and Alzheimer’s disease risk factors. Science. 2011 doi: 10.1126/science.1213210. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bennett DA, De Jager PL, Leurgans SE, Schneider JA. Neuropathologic intermediate phenotypes enhance association to Alzheimer susceptibility alleles. Neurology. 2009;72:1495–1503. doi: 10.1212/WNL.0b013e3181a2e87d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Berry-Kravis E, Arnold SE. Amyloid mediates the association of apolipoprotein E ε4 to level of cognitive function in older persons. J Neurol Neurosurg Psychiatry. 2005;76:1194–1199. doi: 10.1136/jnnp.2004.054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schneider JA, Bienias JL, Wilson RS, Berry-Kravis E, Evans DA, Bennett DA. Relation of the apolipoprotein E ε4 allele to cerebral infarction in older persons. Stroke. 2005;36:954–959. doi: 10.1161/01.STR.0000160747.27470.2a. [DOI] [PubMed] [Google Scholar]
- 145.Biffi A, Shulman JM, Jagiella JM, et al. Genetic Variation at CR1 Increases Risk and Severity of Cerebral Amyloid Angiopathy. Neurology. doi: 10.1212/WNL.0b013e3182452b40. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shulman JM, Chibnik L, Aubin C, Schneider JA, Bennett DA, De Jager PL. Intermediate phenotypes identify divergent pathways Alzheimer’s disease. PLoSONE. 2010 Jun 21;5:e11244. doi: 10.1371/journal.pone.0011244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Arvanitakis Z, Schneider JA, Wilson RS, et al. Diabetes mellitus is related to cerebral infarction but not Alzheimer’s disease pathology in older persons. Neurology. 2006;67:1960–1965. doi: 10.1212/01.wnl.0000247053.45483.4e. [DOI] [PubMed] [Google Scholar]
- 148.Bennett DA, Wilson RS, Schneider JA, et al. Education modifies the relation of AD pathology to cognitive function in older persons. Neurology. 2003;60:1909–1915. doi: 10.1212/01.wnl.0000069923.64550.9f. [DOI] [PubMed] [Google Scholar]
- 149.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Education modifies the association of amyloid, but not tangles, with cognitive function. Neurology. 2005;65:953–956. doi: 10.1212/01.wnl.0000176286.17192.69. [DOI] [PubMed] [Google Scholar]
- 150.Barnes LL, Wilson RS, Schneider JA, Bienias, Evans DA, Bennett DA. Sex differences in the clinical manifestations of Alzheimer’s disease pathology. Arch Gen Psychiatry. 2005;62:685–691. doi: 10.1001/archpsyc.62.6.685. [DOI] [PubMed] [Google Scholar]
- 151.Wilson RS, Schneider JA, Bienias JL, Arnold S, Evans DA, Bennett DA. Depressive symptoms, clinical AD, and cortical plaques and tangles in older persons. Neurology. 2003;61:1102–1108. doi: 10.1212/01.wnl.0000092914.04345.97. [DOI] [PubMed] [Google Scholar]
- 152.Bennett DA, Wilson RS, Schneider JA, Bienias JL, Arnold SE. Cerebral infarctions and the relation of depressive symptoms to level of cognitive function in older persons. American Journal of Geriatric Psychiatry. 2004;12:211–219. [PubMed] [Google Scholar]
- 153.Wilson RS, Arnold SE, Schneider JA, Li Y, Bennett DA. Chronic distress, age-related neuropathology, and cognitive impairment in old age. Psychosomatics Medicine. 2007;69:47–53. doi: 10.1097/01.psy.0000250264.25017.21. [DOI] [PubMed] [Google Scholar]
- 154.Soetanto A, Wilson RS, Un A, et al. Anxiety and depression are associated with hippocampal CA3 dendrite and spine density in older humans. Archives of General Psychiatry. 2010;67:448–457. doi: 10.1001/archgenpsychiatry.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.MacQueen GM, Campbell S, McEwen BS, et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci U S A. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stoub TR, deToledo-Morrell L, Stebbins GT, et al. Hippocampal disconnection contributes to the memory dysfunction in individuals at risk of Alzheimer’s disease. Proceedings of the National Academy of Sciences. 2006;103:10041–10045. doi: 10.1073/pnas.0603414103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Medina D, deToldeo-Morrell L, Urresta F, et al. White matter changes in mild cognitive impairment and AD: a diffusion tensor imaging study. Neurobiology of Aging. 2006;27:663–672. doi: 10.1016/j.neurobiolaging.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 158.Wang DS, Bennet DA, Mufson EJ, Cochran EJ, Dickson DW. Contribution of changes in ubiquitin and myelin basic protein to age-related cognitive decline. Neuroscience Research. 2004;48:93–100. doi: 10.1016/j.neures.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 159.Stoub TR, Bulgakova M, Leurgans S, et al. MRI-predictors of risk of Alzheimer’s disease: A longitudinal study. Neurology. 2005;64:1520–1524. doi: 10.1212/01.WNL.0000160089.43264.1A. [DOI] [PubMed] [Google Scholar]
- 160.Stoub TR, Rogalski EJ, Leurgans SE, Bennett DA, deToledo-Morrell L. Rate of entorhinal and hippocampal atrophy in incipient and mild AD: Relation to memory function. Neurobiology of Aging. 2010;31:1089–1098. doi: 10.1016/j.neurobiolaging.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kordower JH, Chu Y, Stebbins GT, et al. Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment. Ann Neurol. 2001;49:202–213. [PubMed] [Google Scholar]
- 162.Mufson EJ, Ma SY, Cochran EJ, et al. Loss of nucleus basalis neurons containing trka immunoreactivity in individuals with mild cognitive impairment and early Alzheimer’s disease. Journal of Comparative Neurology. 2000;427:19–30. doi: 10.1002/1096-9861(20001106)427:1<19::aid-cne2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 163.Chu YP, Cochran EJ, Bennett DA, Mufson EJ, Kordower JH. Down regulation of trkA mRNA within nucleus basalis neurons in individuals with mild cognitive impairment and Alzheimer’s disease. Journal of Comparative Neurology. 2001;437:296–307. doi: 10.1002/cne.1284. [DOI] [PubMed] [Google Scholar]
- 164.DeKosky ST, Ikonomovic MD, Styren SD, et al. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann Neurol. 2002;51:145–55. doi: 10.1002/ana.10069. [DOI] [PubMed] [Google Scholar]
- 165.Ikonomovic MD, Mufson EJ, Wuu J, Cochran EJ, Bennett DA, DeKosky ST. Cholinergic plasticity in hippocampus of individuals with mild cognitive impairment: correlation with Alzheimer’s neuropathology. JAD. 2003;5:39–48. doi: 10.3233/jad-2003-5106. [DOI] [PubMed] [Google Scholar]
- 166.Ikonomovic MD, Mufson EJ, Wuu J, Bennett DA, DeKosky ST. Reduction of choline acetyltransferase in primary visual cortex in mild Alzheimer’s disease. Arch Neurol. 2005;62:425–430. doi: 10.1001/archneur.62.3.425. [DOI] [PubMed] [Google Scholar]
- 167.Mufson EJ, Ikonomovic MD, Styren SD, et al. Preservation of Brain Nerve Growth Factor in the Elderly with Mild Cognitive Impairment. Archives of Neurology. 2003;60:1143–1148. doi: 10.1001/archneur.60.8.1143. [DOI] [PubMed] [Google Scholar]
- 168.Bell KFS, Ducatenzeiler A, Ribeiro-da-Silva A, et al. The Alzheimer’s disease-like amyloid pathology progresses in a neurotransmitter-specific manner. Neurobiology of Aging. 2006;27:1644–1657. doi: 10.1016/j.neurobiolaging.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 169.Bell KFS, Bennett DA, Cuello AC. Paradoxical cortical upregulation of glutamatergic synapses in mild cognitive impairment. Journal of Neuroscience. 2007;27:10810–7. doi: 10.1523/JNEUROSCI.3269-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rissman RA, Bennett DA, Armstrong DM. Subregional analysis of GABAa receptor subunit mRNAs in the hippocampus of older persons with and without cognitive impairment. J Chem Neuroanat. 2004;28:17–25. doi: 10.1016/j.jchemneu.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 171.Arvanitakis Z, Wilson RS, Schneider JA, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and progression of rigidity and gait disturbance in older persons. Neurology. 2004;63:966–1002. doi: 10.1212/01.wnl.0000138432.16676.4b. [DOI] [PubMed] [Google Scholar]
- 172.Buchman AS, Leurgans SE, Nag S, Bennett DA, Schneider JA. Cerebrovascular disease pathology and Parkinsonian signs in old age. Stroke. 2011;42:3183–3189. doi: 10.1161/STROKEAHA.111.623462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Schneider JA, Li JL, Li Y, Wilson RS, Kordower JH, Bennett DA. Neurofibrillary tangles in the substantia nigra are related to gait impairment in older persons. Annals of Neurology. 2006;59:166–173. doi: 10.1002/ana.20723. [DOI] [PubMed] [Google Scholar]
- 174.Buchman AS, Schneider JA, Wilson RS, Bienias JL, Bennett DA. Body mass index in older persons is associated with Alzheimer’s disease pathology. Neurology. 2006;67:1949–1954. doi: 10.1212/01.wnl.0000247046.90574.0f. [DOI] [PubMed] [Google Scholar]
- 175.Krueger KR, Wilson RS, Shah RC, Tang Y, Bennett DA. Personality and incident disability in older persons. Age and Ageing. 2006;35:428–433. doi: 10.1093/ageing/afl028. [DOI] [PubMed] [Google Scholar]
- 176.Wilson RS, Bienias JL, Mendes de Leon CF, Evans DA, Bennett DA. Negative affect and mortality in older persons. Am J Epidemiol. 2003;158:827–835. doi: 10.1093/aje/kwg224. [DOI] [PubMed] [Google Scholar]
- 177.Wilson RS, Mendes de Leon CF, Bienias JL, Evans DA, Bennett DA. Personality and mortality in old age. Journals of Gerontology: Psychological Sciences. 2004;59:P110–P116. doi: 10.1093/geronb/59.3.p110. [DOI] [PubMed] [Google Scholar]
- 178.Fleischman DA, Gabrieli JDE, Wilson RS, Moro TT, Bennett DA. Repetition priming and recognition memory in young and old persons: Performance and Temporal Stability. Neuropsychology. 2005;19:750–759. doi: 10.1037/0894-4105.19.6.750. [DOI] [PubMed] [Google Scholar]
- 179.Fleischman DA, Wilson RS, Gabrieli JDE, Bienias JL, Bennett DA. A Longitudinal study of implicit and explicit memory in old persons. Psychology & Aging. 2004;19:617–625. doi: 10.1037/0882-7974.19.4.617. [DOI] [PubMed] [Google Scholar]
- 180.Fleischman DA, Bienias JL, Bennett DA. Repetition priming and change in functional ability in older persons without dementia. Neuropsychology. 2009;23:98–104. doi: 10.1037/a0013994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fleischman DA, Buchman AS, Bienias JL, Bennett DA. Visuoperceptual repetition priming and progression of parkinsonian signs in aging. Neurobiology of Aging. 2009;30:441–449. doi: 10.1016/j.neurobiolaging.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Fleischman DA, Wilson RS, Gabrieli JDE, Schneider JA, Bienias JL, Bennett DA. Implicit memory and Alzheimer’s disease neuropathology. Brain. 2005;128:2006–2015. doi: 10.1093/brain/awh559. [DOI] [PubMed] [Google Scholar]
- 183.Koliatsos VE, Kecojevic A, Troncoso JC, Gastard MC, Bennett DA, Schneider JA. Early involvement of small inhibitory cortical interneurons in Alzheimer’s disease. Acta Neuropathologica. 2006;112:147–162. doi: 10.1007/s00401-006-0068-6. [DOI] [PubMed] [Google Scholar]
- 184.Albrecht S, Bourdeau M, Bennett DA, Mufson EJ, Bhattachargee M, LeBlanc AC. Activation of caspase-6 in aging and mild cognitive impairment. American Journal of Pathology. 2007;170:1200–9. doi: 10.2353/ajpath.2007.060974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Horowitz PM, Patterson K, Guillozet-Bongaarts AL, et al. Early N-terminal changes and aspase-6 cleavage of tau in Alzheimer’s disease. J Neurosci. 2004;24:7895–7902. doi: 10.1523/JNEUROSCI.1988-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Newman J, Rissman RA, Sarsoza F, et al. Caspase-cleaved tau accumulation in neurodegenerative diseases associated with tau and α-synuclein pathology. Acta Neuropathologica. 2005;110:135–144. doi: 10.1007/s00401-005-1027-3. [DOI] [PubMed] [Google Scholar]
- 187.Halawani D, Tessier S, Anzellotti D, Bennett DA, Latterich M, LeBlanc AC. Identification of caspase-6 mediated processing of the valosin containing protein (p97) in Alzheimer’s disease: a novel link to dysfunction in ubiuquitin proteasome system-mediated protein degradation. Journal of Neuroscience. 2010;28:6132–6142. doi: 10.1523/JNEUROSCI.5874-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Louneva N, Cohen JW, Han L-Y, et al. Caspase-3 is enriched in postsynaptic densities and increased in Alzheimer’s disease. American Journal of Pathology. 2008;173:1488–95. doi: 10.2353/ajpath.2008.080434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang DS, Simon BP, Bennett DA, Schneider JA, Malter JS, Wang DS. The significance of Pin1 in the development of Alzheimer’s disease. Journal of Alzheimer’s Disease. 2007;11:13–23. doi: 10.3233/jad-2007-11105. [DOI] [PubMed] [Google Scholar]
- 190.Julien C, Tremblay C, Emond V, et al. Sirtuin 1 reducation parallels the accumulation of tau in Alzheimer’s disease. Journal of Neuropathology and Experimental Neurology. 2009;68:48–58. doi: 10.1097/NEN.0b013e3181922348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang DS, Ishikado H, Bennett DA, et al. Cognitive performance correlates with cortical isopeptide immunoreactivity as well as Alzheimer type pathology. Journal of Alzheimer’s Disease. 2008;13:53–66. doi: 10.3233/jad-2008-13106. [DOI] [PubMed] [Google Scholar]
- 192.Wang S, Wang R, Chen L, Bennett DA, Dickson DW, Wang DS. Expression and functional profiling of neprilysin, insulin degrading enzyme and endothelin converting enzyme in prospectively studied elderly and Alzheimer’s brain. Journal of Neurochemistry. 2010;115:47–57. doi: 10.1111/j.1471-4159.2010.06899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bruno A, Huang J, Bennett DA, Marr R, Hastings ML, Stutzmann GE. Altered ryanodine receptor expression in mild cognitive impairment and Alzheimer’s disease. Neurobiology of Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.03.011. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Huang JY, Hafez DM, James BD, Bennett DA, Marr RA. Altered NEP2 expression and activity in mild cognitive impairment and Alzheimer’s disease. Journal of Alzheimer’s Disease. 2011 doi: 10.3233/JAD-2011-111307. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wang HY, Stucky A, Hahn CG, Wilson RS, Bennett DA, Arnold SE. BDNF-TrkB signaling and late life cognitive decline and Alzheimer’s disease. Translational Neuroscience. 2011;2:91–100. [Google Scholar]
- 196.Schipper HM, Bennett DA, Lieberman A, et al. Heme oxygenase-1 expression in MCI and early AD. Neurobiology of Aging. 2006;27:252–261. doi: 10.1016/j.neurobiolaging.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 197.Assaraf MI, Diaz Z, Liberman A, et al. Brain erythropoietin receptor expression in Alzheimer’s disease and mild cognitive impairment. Journal of Neuropathology and Experimental Neurology. 2007;66:389–398. doi: 10.1097/nen.0b013e3180517b28. [DOI] [PubMed] [Google Scholar]
- 198.Hascalovici JR, Vaya J, Khatib S, et al. Brain sterol dys-regulation in sporadic AD and MCI: Relationship to heme oxygenase-1. Journal of Neurochemistry. 2009;110:1241–1243. doi: 10.1111/j.1471-4159.2009.06213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Loeffler DA, Camp DM, Bennett DA. Plaque complement activation and cognitive loss in Alzheimer’s disease. Journal of Neuroinflammation. 2008;5:9. doi: 10.1186/1742-2094-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lopez MF, Mikulskis A, Golenko E, et al. Microscale fractionation facilitates detection of differentially expressed proteins in Alzheimer’s disease brain samples. Electrophoresis. 2004;25:2557–2563. doi: 10.1002/elps.200406011. [DOI] [PubMed] [Google Scholar]
- 201.Lopez MF, Mikulskis A, Kuzdzal S, et al. High-Resolution serum proteomic profiling of Alzheimer’s Disease samples reveals disease-specific, carrier-protein bound mass signatures. Clinical Chemistry. 2005;51:1946–1954. doi: 10.1373/clinchem.2005.053090. [DOI] [PubMed] [Google Scholar]
- 202.Choi SR, Schneider JA, Bennett DA, et al. Correlation of amyloid PET ligand florbetapir F 18 (18F-AV-45) binding with β-amyloid aggregation and neuritic plaque deposition in postmortem brain tissue. Alzheimer’s Disease and Associated Disorders. 2011 doi: 10.1097/WAD.0b013e31821300bc. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Walter S, Atzmon G, Demerath EW, et al. A genome-wide association study of aging. Neurobiology of Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.05.026. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wilson RS, Leurgans SE, Boyle PA, Bennett DA. Cognitive decline in prodromal Alzheimer’s disease and mild cognitive impairment. Archives of Neurology. 2011;68:3551–358. doi: 10.1001/archneurol.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hall CB, Lipton RB, Sliwinski M, Stewart WF. A change point model for estimating the onset of cognitive decline in preclinical Alzheimer’s disease. Stat Med. 2000;19:1555–1566. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1555::aid-sim445>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 206.Johnson DK, Storandt M, Morris JC, Galvin JE. Longitudinal study of the transition from healthy aging to Alzheimer disease. Arch Neurol. 2009;66:1254–1259. doi: 10.1001/archneurol.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Small BJ, Backman L. Longitudinal trajectories of cognitive change in preclinical Alzheimer’s disease: a growth mixture modeling analysis. Cortex. 2007;43:826–834. doi: 10.1016/s0010-9452(08)70682-8. [DOI] [PubMed] [Google Scholar]
- 208.Grober E, Hall CB, Lipton RB, Zonderman AB, Resnick SM, Kawas C. Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer’s disease. J Int Neuropsychol Soc. 2008;14:266–278. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Howieson DB, Carlson NE, Moore MM, et al. Trajectory of mild cognitive impairment onset. J Int Neuropsychol Soc. 2008;14:192–198. doi: 10.1017/S1355617708080375. [DOI] [PubMed] [Google Scholar]
- 210.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Troncoso JC, Zonderman AB, Resnick SM, Crain B, Pletnikova O, O’Brien RJ. Effect of infarcts on dementia in the Baltimore longitudinal study of aging. Ann Neurol. 2008;64:168–76. doi: 10.1002/ana.21413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Petrovitch H, Ross GW, Steinhorn SC, et al. AD lesions and infarcts in demented and non-demented Japanese-American men. Ann Neurol. 2005;57:98–103. doi: 10.1002/ana.20318. [DOI] [PubMed] [Google Scholar]
- 215.Neuropathology Group of the Medical Research Council Cognitive Function and Aging Study (MRC CFAS) Pathologic correlates of late onset dementia in a multicentre, community based population in England and Wales. Lancet. 2001;357:169–175. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- 216.Sonnen JA, Larson EB, Crane PK, et al. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol. 2007;62:406–413. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- 217.Nelson PT, Jicha GA, Schmitt FA, et al. Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J Neuropathol Exp Neurol. 2007;66:1136–1146. doi: 10.1097/nen.0b013e31815c5efb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Snowdon DA, Greiner LH, Mortimer JA, et al. Brain infarction and the clinical expression of AD. The nun study JAMA. 1997;277:813–817. [PubMed] [Google Scholar]
- 219.Ahtiluoto S, Polvikoski T, Peltonen M, et al. Diabetes, Alzheimer disease, and vascular dementia: a population-based neuropathologic study. Neurology. 2010;75:1195–202. doi: 10.1212/WNL.0b013e3181f4d7f8. [DOI] [PubMed] [Google Scholar]
- 220.Peila R, Rodriguez BL, Launer LJ Honolulu-Asia Aging Study. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–62. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 221.Sonnen JA, Larson EB, Brickell K, et al. Different patterns of cerebral injury in dementia with or without diabetes. Arch Neurol. 2009;66:315–22. doi: 10.1001/archneurol.2008.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Beeri MS, Silverman JM, Davis KL, et al. Type 2 diabetes is negatively associated with Alzheimer’s disease neuropathology. J Gerontol A Biol Sci Med Sci. 2005;60:471–5. doi: 10.1093/gerona/60.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ganguli M, Kukull WA. Lost in translation: epidemiology, risk, and Alzheimer disease. Arch Neurol. 2010;67:107–11. doi: 10.1001/archneurol.2009.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Roe CM, Xiong C, Miller JP, Morris JC. Education and Alzheimer disease without dementia: support for the cognitive reserve hypothesis. Neurology. 2007;68:223–8. doi: 10.1212/01.wnl.0000251303.50459.8a. [DOI] [PubMed] [Google Scholar]
- 226.Koepsell TD, Kurland BF, Harel O, Johnson EA, Zhou XH, Kukull WA. Education, cognitive function, and severity of neuropathology in Alzheimer disease. Neurology. 2008;70:1732–9. doi: 10.1212/01.wnl.0000284603.85621.aa. [DOI] [PubMed] [Google Scholar]
- 227.Roe CM, Mintun MA, D’Angelo G, Xiong C, Grant EA, Morris JC. Alzheimer disease and cognitive reserve: variation of education effect with carbon 11-labeled Pittsburgh Compound B uptake. Arch Neurol. 2008;65:1467–71. doi: 10.1001/archneur.65.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Rentz DM, Locascio JJ, Becker JA, et al. Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol. 2010;67:353–64. doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Rapp MA, Schnaider-Beeri M, Grossman HT, et al. Increased hippocampal plaques and tangles in patients with Alzheimer disease with a lifetime history of major depression. Arch Gen Psychiatry. 2006;63:161–7. doi: 10.1001/archpsyc.63.2.161. [DOI] [PubMed] [Google Scholar]
- 230.Negash S, Bennett DA, Wilson RS, Schneider JA, Arnold SE. Cognition and Neuropathology in Aging: Multidimensional Perspectives from the Rush Religious Orders Study and Rush Memory and Aging Project. Current Alzheimer’s Research. 2011;8:336–340. doi: 10.2174/156720511795745302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Richards M, Stern Y, Mayeux R. Subtle extrapyramidal signs can predict the development of dementia in elderly individuals. Neurology. 1993;43:2184–8. doi: 10.1212/wnl.43.11.2184. [DOI] [PubMed] [Google Scholar]
- 232.Louis ED, Tang MX, Mayeux R. Parkinsonian signs in older people in a community-based study: risk of incident dementia. Arch Neurol. 2004;61:1273–6. doi: 10.1001/archneur.61.8.1273. [DOI] [PubMed] [Google Scholar]
- 233.Liu Y, Stern Y, Chun MR, Jacobs DM, Yau P, Goldman JE. Pathological correlates of extrapyramidal signs in Alzheimer’s disease. Ann Neurol. 1997;41:368–74. doi: 10.1002/ana.410410312. [DOI] [PubMed] [Google Scholar]
- 234.Burns JM, Galvin JE, Roe CM, Morris JC, McKeel DW. The pathology of the substantia nigra in Alzheimer disease with extrapyramidal signs. Neurology. 2005;64:1397–403. doi: 10.1212/01.WNL.0000158423.05224.7F. [DOI] [PubMed] [Google Scholar]
- 235.Attems J, Quass M, Jellinger KA. Tau and alpha-synuclein brainstem pathology in Alzheimer disease: relation with extrapyramidal signs. Acta Neuropathol. 2007;113:53–62. doi: 10.1007/s00401-006-0146-9. [DOI] [PubMed] [Google Scholar]
- 236.Hughes TF, Borenstein AR, Schofield E, Wu Y, Larson EB. Association between late-life body mass index and dementia: The Kame Project. Neurology. 2009;72:1741–6. doi: 10.1212/WNL.0b013e3181a60a58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Fitzpatrick AL, Kuller LH, Lopez OL, et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66:336–42. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Johnson DK, Wilkins CH, Morris JC. Accelerated weight loss may precede diagnosis in Alzheimer disease. Arch Neurol. 2006;63:1312–7. doi: 10.1001/archneur.63.9.1312. [DOI] [PubMed] [Google Scholar]
- 239.Stewart R, Masaki K, Xue QL, et al. A 32-year prospective study of change in body weight and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol. 2005;62:55–60. doi: 10.1001/archneur.62.1.55. [DOI] [PubMed] [Google Scholar]
- 240.Dawe RJ, Bennett DA, Schneider JA, Vasireddi SK, Arfanakis K. Post-mortem MRI of Human brain hemispheres: T2 relaxation times during formaldehyde fixation. Magnetic Resonance in Medicine. 2009;61:810–818. doi: 10.1002/mrm.21909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Dawe RJ, Bennett DA, Schneider JA, Arfanakis K. Neuropathologic correlates of hippocampal atrophy in the elderly: a clinical, pathologic, postmortem MRI study. PLoS One. doi: 10.1371/journal.pone.0026286. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Bienias JL, Beckett LA, Bennett DA, Wilson RS, Evans DA. Design of the Chicago Health and Aging Project (CHAP) The Journal of Alzheimer’s Disease. 2003;5:349–355. doi: 10.3233/jad-2003-5501. [DOI] [PubMed] [Google Scholar]
- 243.Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and cognitive decline in a biracial community population. Neurology. 2003;61:812–816. doi: 10.1212/01.wnl.0000083989.44027.05. [DOI] [PubMed] [Google Scholar]
- 244.Wilson RS, Mendes de Leon CF, Bennett DA, Bienias JL, Evans DA. Depressive symptoms and cognitive decline in a community population of older persons. Journal of Neurology, Neurosurgery, & Psychiatry. 2004;75:126–129. [PMC free article] [PubMed] [Google Scholar]
- 245.Wilson RS, Bennett DA, Bienias JL, Mendes de Leon CF, Morris MC, Evans DA. Distress proneness and cognitive decline in a biracial population of older persons. Psychoneuroendocrinology. 2005;30:11–17. doi: 10.1016/j.psyneuen.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 246.Wilson RS, Barnes LL, Bennett DA, et al. Proneness to psychological distress and risk of Alzheimer’s disease in a biracial community. Neurology. 2005;64:380–382. doi: 10.1212/01.WNL.0000149525.53525.E7. [DOI] [PubMed] [Google Scholar]
- 247.Wilson RS, Scherr PA, Bienias JL, et al. Socioeconomic characteristics of the community in childhood and cognition in old age. Experimental Aging Research. 2005;31:393–407. doi: 10.1080/03610730500206683. [DOI] [PubMed] [Google Scholar]
- 248.Grodstein F, Skarupski KA, Bienias JL, Wilson RS, Bennett DA, Evans DA. Anti-Inflammatory agents and cognitive decline in a bi-racial population. Neuroepidemiology. 2008;30:45–50. doi: 10.1159/000115749. [DOI] [PMC free article] [PubMed] [Google Scholar]