Abstract
The Minority Aging Research Study (MARS) is a longitudinal, epidemiologic cohort study of decline in cognitive function and risk of Alzheimer’s disease (AD) in older African Americans, with brain donation after death added as an optional component for those willing to consider organ donation. In this manuscript, we first summarize the study design and methods of MARS. We then provide details of ongoing efforts to achieve neuropathologic data on over 100 African Americans participating in MARS and in three other clinical-pathologic cohort studies at Rush University Medical Center. The results examine strategies for recruiting and consenting African Americans without dementia; 2) efforts to maintain high rates of follow-up participation; 3) strategies for achieving high rates of agreement to brain donation; and 4) the methodology of obtaining rapid brain autopsy at death. The implications of these efforts are discussed.
Keywords: Neuropathology, African American, aging, epidemiologic studies, longitudinal
INTRODUCTION
Cognitive decline frequently occurs in older persons [1] and is associated with adverse health consequences, including increased disability and death [2–4]. Due to the rapidly expanding older population, there will likely be a greater number of older adults who have cognitive impairment. The African American population is growing at a faster rate than other racial/ethnic groups [5] and some studies suggest they may be at higher risk for dementia compared to other groups [6–9]. The African American older population was 3.2 million in 2008 (8.3% of overall United States older population) and is projected to grow to over 9.9 million by 2050 (to 11%) [10]. Development of successful interventions for delaying cognitive impairment will benefit from the translation of knowledge about the neuropathologic basis of cognitive impairment into improved diagnosis and treatment. To date, most of the understanding of the neuropathologic basis of cognitive impairment in older persons has been based almost exclusively on studies of older whites. Clinical-pathologic studies in whites have demonstrated that cognitive decline is related to the quantity and distribution of amyloid deposition and neurofibrillary tangles associated with Alzheimer’s disease (AD) pathology, along with the presence of other common neuropathologies including infarcts and Lewy Bodies [11–13]. There are limited data on the relationship of these pathologic indices and cognitive impairment in African Americans, particularly in African Americans without clinically diagnosed dementia.
Of the few studies that have examined neuropathology among African Americans, most have been descriptive retrospective designs that included autopsies from medical examiners’ cases with little to no information on premortem clinical or cognitive status [14–16]; see (Table 1). One study reported that the frequency of histopathologic lesions of Alzheimer’s disease in a group of 100 neurologically normal patients was significantly higher among the white patients compared with black patients [14]. Others reported no racial differences in the frequency of AD pathology [15–16]. To our knowledge, only three clinical-pathologic studies have correlated clinical and neuropathologic diagnoses in older African Americans, and all three included persons diagnosed with clinical dementia at death, and had small sample sizes (n<15), limiting any conclusions regarding the association of neuropathology to cognitive impairment [17–19].
Table 1.
Studies with Brain Tissue from African Americans
| Study | No. of Subjects with Autopsy | Study Type | Pre-Mortem Cognitive Status of Subjects |
|---|---|---|---|
| De la Monte et al., [14] | 50 blacks, 50 whites | Review of pathology data from autopsy series at Johns Hopkins Medical Institutions | Neurologically normal by medical record review |
| Sandberg et al., [15] | 54 blacks, 84 whites | Autopsy series from Chief Examiner’s office, State of Maryland | Unknown |
| Miller et al., [16] | 25 blacks, 174 whites | Autospy series from patients at University of Michigan Medical Center | Unknown |
| Wilkins et al., [17] | 10 blacks, 10 whites | Participants from the Alzheimer’s Disease Research Center, Washington University | CDR scores ranging 0.5–3 by ante-mortem clinical evalutation |
| Bonner et al., [18] | 10 blacks | Autopsy recruitment program of caregivers and patients | Dementia by ante-mortem clinical evaluation |
| Pytel et al., [19] | 13 blacks | Autopsy study of larger hospital-based cohort of 270 African Americans | Dementia by medical record review |
Clinical-pathologic studies in minorities without dementia will fill an important gap in knowledge on the transition of healthy aging to dementia in a population at high risk for disease. Progress in understanding this transition will require substantial numbers of older, racially and ethnically diverse persons without dementia agreeing to clinical evaluations at regular intervals and eventually coming to autopsy so that the neuropathologic substrates underlying the clinical spectrum of cognition from normal to mild cognitive impairment to dementia can be documented and quantified. According to data from the National Alzheimer’s Coordination Center [20], of 35 centers across the U.S. who have contributed neuropathologic data, approximately 628 of 12,230 autopsies have come from minorities (5.1%), and even fewer are from minorities without dementia (n=223). It is clear that the neuropathology of cognitive impairment in African Americans has been understudied.
In the present paper, we describe the study design of the Minority Aging Research Study (MARS) [21], a longitudinal, clinical-pathologic cohort study of older African Americans without known dementia. The overall goal of the study is to identify risk factors for and determine the neuropathologic basis of cognitive impairment in older African Americans. We describe the strategies implemented to achieve this scientific goal including the critical steps of: 1) recruiting and consenting African Americans without dementia; 2) maintaining high rates of follow-up participation; 3) achieving high rates of agreement to brain donation; and 4) obtaining rapid brain autopsy at death.
OVERVIEW OF STUDY DESIGN
The Minority Aging Research Study, a longitudinal study of risk factors for cognitive decline, began data collection in August 2004 and was re-funded in July 2010 to include an optional arm in which existing and newly recruited participants may participate in brain donation. Unlike studies of the majority population, which have successfully required organ donation as a condition of entry, the renewal for the Minority Aging Research Study proposes to continue to follow existing participants in order to further build relationships and increase the likelihood that the participants and their family members will consider brain donation. Requiring organ donation for a population that has a legacy of experiencing discrimination and exploitation in the health care system and medical research would require considerable resources and time to obtain sufficient numbers [22]. It is our experience that the decision to agree to autopsy is often made after establishing a relationship based on mutual trust and respect. This is particularly true for historically disenfranchised racial and ethnic minorities. Further, because brain donation is a sensitive topic for African Americans, it is important that potential participants discuss the study and their wishes for brain donation with their family members before agreeing to donate their brains. Thus, our strategy in MARS is to expend resources to build relationships, making brain donation an optional component that can be endorsed at a later date, and thereby increasing the likelihood that participants and their family members will consider donation at some point during the study and actually follow through with contacting our team at the time of death. Due to the unique challenges associated with organ donation studies in general [23–25], and in minority populations in particular, the Minority Aging Research Study will take advantage of existing clinical data and brain tissue donated by three other NIA funded studies at Rush with similar study designs, including the Rush Memory and Aging Project [26], the Religious Orders Study [27], and the Clinical Core of the Rush Alzheimer’s Disease Core Center. There are at least two advantages of sharing data with other large longitudinal studies. First, like other successful initiatives that combine data from several centers and projects to examine important research questions in the majority white population, similar strategies will need to be implemented for studies of non-demented African Americans where the data are much more limited and the work is more challenging. Combining the different cohort studies at Rush will increase the potential sample size of African Americans with neuropathologic data since all of our studies employ a large common core of uniform, structured clinical and post-mortem data collection procedures, and all share substantial overlap in risk factors and cognitive performance measures. In fact, we have already begun to publish clinical papers that merge data across the Minority Aging Research Study and the Rush Memory and Aging Project [28–32]. Further, both the Rush Memory and Aging Project and the Religious Orders Study require organ donation and have more than 10% minorities enrolled in each study of whom the majority are African American. Second, both the Rush Memory and Aging Project and the Religious Orders Study have clinical data and brain tissue on a large number of whites, which will allow us to conduct analyses across race to test hypotheses of racial differences in neuropathology and its relationship with risk factors and cognition. Thus, the enhanced sample size that will result from combining the four cohort studies will allow not only for detailed examinations of individual differences within race, but will also make it possible to examine neuropathologic correlates of cognition and risk factors for cognitive impairment and neuropathology across race so that we may better understand the factors that may contribute to any observed racial differences in the neurobiology of cognitive impairment. Together, these studies, along with the Rush Clinical Core, include more than 3,900 older persons, and will eventually include more than 1000 African Americans without known dementia at baseline who agreed to annual detailed clinical evaluations, and a sizable proportion who has already agreed to brain donation at death.
In the sections that follow, we will describe the Minority Aging Research Study as well as the three cohort studies providing additional clinical and pathological data for the Minority Aging Research Study. Then we will discuss the tremendous effort it takes to obtain clinical and neuropathologic data in a cohort study of older African Americans without dementia including, the procedures we follow for recruiting and consenting, maintaining high rates of follow-up participation, obtaining agreement for brain donation at time of death, and achieving rapid autopsy.
METHODS
Cohort Studies at Rush
Minority Aging Research Study
The Minority Aging Research Study (MARS), a longitudinal clinical-pathologic study of aging and risk factors for cognitive decline, enrolls older African Americans free of dementia, and performs annual uniform, structured, clinical evaluations that include a detailed assessment of risk factors, neurological examination, donation of a blood sample for genetic testing, and comprehensive neuropsychological testing. To be eligible, potential participants have to be 65 years or older, no prior diagnosis of dementia, not taking medications typically prescribed for Alzheimer’s disease, and self-identify as African Americans using questions from the 1990 U.S. Census. The specific question is: With which group do you most closely identify yourself? White; Black, Negro, African-American; Native American, Indian; Eskimo; Aleut; Asian or Pacific Island. They are then asked whether they are of Spanish/Hispanic/Latino origin (yes/no). The study is funded by the National Institute on Aging and was approved by the Institutional Review Board of Rush University Medical Center.
Recruitment and Consent
Study participants were recruited from a variety of settings including churches, subsidized senior housing facilities, retirement communities, African American clubs, organizations, fraternities and sororities, and social service centers that cater to seniors in the metropolitan Chicago area and outlying suburbs. By utilizing these community recruitment centers, potential participants reflect a wide range of educational attainment and lifestyle experiences. Recruitment efforts began with relationship building in various African American communities in the city by culturally diverse staff connected with these communities. Several existing community-based advisory groups consisting of various African American community leaders and service providers were consulted to coordinate our outreach efforts. Based on input from leaders regarding the needs of older African Americans in their communities which could be addressed by the Rush Alzheimer’s Disease Center, culturally relevant outreach was provided. As community leaders appreciated that the Rush Alzheimer’s Disease Center could be a trusted partner in meeting community needs, the administrators of various organizations were contacted and arrangements were made for potential participants to be invited to attend a presentation about the study. The presentations were conducted at community facilities (e.g., senior housing building, church, or hall where the organization held their monthly meetings), and lasted approximately 1 hour, including questions. An update on aging, cognition, and AD, and the importance of minority participation in research was discussed. All details of the study were described, including the annual cognitive and neurological evaluations and blood draw, and written information was provided so that potential participants could discuss the project with family members. Potential participants were asked to complete a Rush Institutional Review Board approved form describing their level of interest in the study. The forms are used to both determine the number of people in attendance at each presentation and to gauge willingness to participate in the study. At a later date, those people who expressed interest were contacted by the same individuals who had been at presentations to arrange a time to discuss the study in detail and review and sign the written informed consent.
From September 2004 through November 2010, details of the study were presented to over 1,500 persons. Of those, 1113 expressed some interest in the study and the first 772 were approached for recruitment. After discussing the study in detail with each potential participant, 223 were found to be inelligible because they were either too young, had a prior dementia diagnosis, or were taking AD medications, 112 refused, 71 were reluctant, and 366 persons enrolled in the study. The study is 99.4% non-Hispanic African American (2 are Hispanic African American) and 28.1% male, with a wide spectrum of education from 3 years to 30 years.
Baseline and Follow-Up Examinations
After consent is obtained, a baseline evaluation is scheduled and performed in the participant’s home. At the baseline visit, a uniform, structured clinical evaluation is completed consisting of an interview to ascertain a variety of lifestyle and experiential risk factors, a neurological examination, a blood draw, and a comprehensive neuropsychological battery of 23 cognitive tests (see Tables 2–5). The clinical evaluation is repeated on an annual basis. Follow-up clinical evaluations, identical in all essential details to the baseline, are evenly spaced at one year intervals and performed by examiners blind to previously collected data. Overall follow-up participation among survivors is 90.5%, with 37 (10.1%) persons withdrawing from the study during follow-up.
Table 2.
Cognitive Tests in the Minority Aging Research Study and available in the other Cohort Studies at Rush
| MARS | MAP | ROS | CORE | |
|---|---|---|---|---|
| MMSE | X | X | X | X |
| Complex Ideational Material | X | X | X | X |
| Episodic Memory | ||||
| Logical Memory Ia | X | X | X | X |
| Logical Memroy Iia | X | X | X | X |
| East Boston Story Immediate recall | X | X | X | |
| East Boston Story Delayed recall | X | X | X | |
| Word List Memory | X | X | X | X |
| Word List Recall | X | X | X | X |
| Word List Recognition | X | X | X | X |
| Semantic Memory | ||||
| Boston Naming Test | X | X | X | X |
| Verbal Fluency | X | X | X | X |
| Wide Range Achievement Test | X | |||
| Working Memory | ||||
| Digit Span Forward | X | X | X | X |
| Digit Span Backward | X | X | X | X |
| Digit Span Ordering | X | X | X | |
| Perceptual Speed | ||||
| Symbol Digit | X | X | X | X |
| Number Comparison | X | X | X | |
| Stroop Word Reading | X | X | ||
| Stroop Word Color Naming | X | X | ||
| Visuospatial Ability | ||||
| Line Orientation | X | X | X | X |
| Progressive Matrices | X | X | X | X |
| Executive Function | ||||
| Trails A | X | X | ||
| Trails B | X | X |
Table 5.
Clinical Diagnoses in the Minority Aging Research Study and available in other cohort studies at Rush
| MARS | MAP | ROS | CORE | |
|---|---|---|---|---|
| Dementia | X | X | X | X |
| Alzheimer’s Disease | X | X | X | X |
| MCI | X | X | X | X |
| Stroke | X | X | X | X |
| CI due to stroke | X | X | X | X |
| Parkinsonism | X | X | X | X |
| Parkinson’s Disease | X | X | X | X |
| Depression | X | X | X | X |
As the study was recently refunded in June 2010, there have been no autopsies to date. Newly recruited participants are introduced to the optional brain donation component of the study at the time of enrollment and participants from the first funding cycle are asked to consider brain donation at the time of death at each evaluation. To date, 26 persons have enrolled in the brain donation component of the study by signing an Anatomical Gift Act, a legal document allowing us to harvest the brain as a gift.
Rush Memory and Aging Project [26]
The Rush Memory and Aging Project (MAP) is an ongoing longitudinal clinical-pathological study of common chronic conditions of old age. Participants are community-dwelling older adults recruited from about 40 continuous care retirement communities and subsidized housing facilities in and around the Chicago metropolitan area. Eligibility criteria for the study include agreeing to annual clinical evaluation and brain donation at the time of death. The study was approved by the Institutional Review Board of Rush University Medical Center. From September 1997 to November 2010, details of the study have been presented to over 4400 persons, with 2854 persons expressing some interest. Of those, 1407 enrolled in the study and have had a baseline clinical evaluation. Recruitment procedures for the African Americans in MAP are the same as those in the Minority Aging Research Study, including the educational outreach staff and the places from which participants are recruited, with one exception; all participants in MAP have signed a Uniform Anatomical Gift Act donating their brains to the Rush Investigators at the time of death as a condition of entry. The study is 86.6% non-Hispanic white, and includes 109 African Americans. It is 27.1% male. The overall follow-up of survivors approaches 95%. The autopsy rate exceeds 80% with 366 autopsies of 449 deaths (81.5%). There have been 9 autopsies from African Americans.
Religious Orders Study [27]
The Religious Orders Study (ROS) is an ongoing clinical-pathological study of aging and AD among older Catholic nuns, priest, and brothers. The study involves annual clinical evaluations and brain donation at death. It was approved by the Institutional Review Board of Rush University Medical Center. From January 1994 to October 2010, details of the study have been presented to over 1400 persons, with 1365 persons expressing some interest. Of those, 1157 enrolled in the study and the baseline clinical evaluation has been completed on 1,153. There are only three African American Orders, and we have recruited 91 African Americans over the age of 65 from these orders. All participants have signed a Uniform Anatomical Gift Act. The study is 87.9% non-Hispanic white and 30.9% male. The overall follow-up of survivors approaches 95%. The autopsy rate approaches 95% with 504 autopsies of 536 deaths (94.2%). There have been 14 autopsies from African Americans.
Clinical Core of the Rush Alzheimer’s Disease Core Center
The Rush Clinical Core is one of five cores within the Alzheimer’s Disease Core Center. The overall goal of the Clinical Core is to generate data and biospecimens required to support high quality, cutting edge, externally-funded clinical and clinical-pathologic studies that focus on the full spectrum of cognition from normal aging to MCI to the earliest stages of dementia among older African Americans. In 2008, based on a number of strategic decisions, the Clinical Core transitioned from a clinic-based study in which most participants were clinic patients with dementia and began enrolling older African Americans without dementia. Data collection was expanded to be compatible with the Religious Orders Study, the Minority Aging Research Study and the Rush Memory and Aging Project. Similar to MARS, the Clinical Core now performs annual clinical evaluations in participants’ homes, and works to obtain brain tissue with a short postmortem interval in African Americans without dementia. The major difference between the Clinical Core and the Minority Aging Research Study is the Clinical Core also requires a knowledgeable informant to be enrolled in the study with the participant and administration of the Uniform Data Set [20]. From January 2008 (when we made the transition to enroll African Americans without dementia) to November 2010, details of the study have been presented to over 2100 persons, with 493 persons expressing some interest. Of those, 218 African Americans without dementia have enrolled in the study. The overall follow-up of survivors approaches 95%. Participants enrolled since 2008 are asked at each evaluation to consider organ donation at the time of death. There have been no autopsies in the newly recruited African Americans, but 125 persons have signed an Anatomical Gift Act to date.
RESULTS
Efforts to Ensure High Rates of Follow-up and Participation in Brain Autopsy
Follow-Up Participation
Achieving high rates of follow-up is critical to studies of cognitive aging and the transition from normal aging to dementia. Once a person consents to enrolling into one of our cohorts, targeted efforts are initiated at the level of the Principal Investigator, and instituted by the study coordinator and staff to collect high quality data and retain participants throughout the follow-up period. The Principal Investigators, together with the education and outreach staff of the Rush Alzheimer’s Disease Center, have substantial experience with the techniques necessary to facilitate enrollment, follow-up and recrutiment for organ donation among the majority white population. This expertise has successfully been used to facilitate the entry of minorities into our studies and to provide culturally sensitive educational outreach to increase awareness on the importance of brain donation in underserved communities of color. Using a three-prong approach that serves to build a foundation of trust and reciprocity, several strategies are implemented to ensure that African Americans are retained in our studies and that they participate in brain donation at the time of death including: 1) overcoming barriers to participation by conducting all evaluations in the participants’ home; 2) frequent contact with participants including quarterly phone calls, newsletters, and acknowledgement cards for special occasions such as birthdays and holidays; 3) frequent dissemination of research findings and educational presentations on AD and healthy aging.
Achieving High Rates of Participation in Brain Donation
Efforts to ensure high rates of participation in brain donation in our studies consist of culturally-tailored educational programs to increase awareness of the importance of brain donation in minority populations. It is our experience that participants will often agree to autopsy after they have established a relationship with our staff that is based on mutual trust and respect. Thus, as opposed to studies of the majority population which can require organ donation as a condition of entry, for studies that focus exclusively on minority populations, we have learned that it is important to build relationships by making brain donation an optional component that can be endorsed at a later date, which is consistent with information obtained from qualitative studies with minorities regarding brain donation [33–35]. Then, we have to work to increase the likelihood that the participants and their family members will agree to organ donation at some point during the study by nurturing the relationships, being transparent in our goals, and maintaining communication with family members regarding study progress and the participant’s wishes for organ donation at the time of death. Participants are asked at each evaluation to consider brain donation at the time of death by study clinicians. If interested, they sign the Anatomical Gift Act and they are given a packet of information for their family members regarding procedures to follow at the time of death. If the participant is reluctant to sign the Anatomical Gift Act, but still considering, they are given a packet of information that addresses frequently asked questions and asked to share with their family. Our staff also meets with family members to discuss autopsy if the participant is interested but the family is reluctant. The approach is highly labor intensive and involves redundant procedures to ensure that we are notified at the time of death. However, in our experience, these procedures are essential to achieving the high follow-up participation and autopsy rates necessary for the validity of longitudinal cohort studies that include brain donation.
Achieving Rapid Autopsy at Time of Death
Rapid autopsy requires careful planning and the coordinated efforts of many individuals, before and after death. To honor the wishes of the participant to participate in organ donation, all participants who want to participate in organ donation sign a Uniform Anatomical Gift Act at the time of enrollment, donating their brain to Rush investigators for studies related to aging and AD. Unlike routine autopsy consent procedures that require the witnessed consent of the next of kin, the Uniform Anatomical Gift Act does not require the consent of a family member after death. In addition to honoring the participant’s wishes, use of the Uniform Anatomical Gift Act also expedites the autopsy process since we do not have to rely on notification from the next of kin at the time of death. However, this does not ensure that we will be notified at the time of death. Therefore, we have instituted a variety of strategies to ensure high autopsy rates. First, family members are provided with documentation of the study, instructions regarding whom to call at the time of death, and information about the autopsy procedures. Participants and family members are encouraged to call the Principal Investigator or the study coordinator if they are unclear on the instructions. Second, participants are encouraged to identify the most likely funeral home that they will employ so that we can contact the funeral home. This step accomplishes three objectives. First, it allows us to provide detailed instructions to the funeral home directors about how to go about getting the autopsy performed in a timely manner in order not to delay the funeral. Second, it also allows us to inform the funeral home as to what to expect at the time of autopsy so that they will be in a better position to work with the body so that it is presentable to the family and there is no evidence of brain removal. Third, it sets up another opportunity for us to be informed of a death in the event that the family member forgets to call. We provide the funeral directors with a copy of the Anatomical Gift Act so that they will also notify our staff at the time of death. If the participant is in the hospital or hospice, or other long-term care facilities including skilled and unskilled nursing units, adhesive labels that can be attached to the chart, with information that the participant is involved in a brain organ donation study and what number to call in the event of death, are provided. The study coordinators are contacted at the time of autopsy so that they can follow up with the family after the procedure is over and offer assistance if needed. Our study personnel are on-call 24 hours/day, 7 days/week to receive a call in the event of death and work with the funeral home and the Rush Alzheimer’s Disease Center autopsy team (also available 24 hours/day, 7 days/week) to ensure that the brain is removed with a short post-mortem interval. We also work directly with the funeral homes to ensure that we get the bill for all incidentals including but not limited to payment for transporting the body, to avoid having a family member receive the bill for these services and to prevent financial constraints from delaying or preventing the autopsy.
Summary of African American Cohort from Four Studies
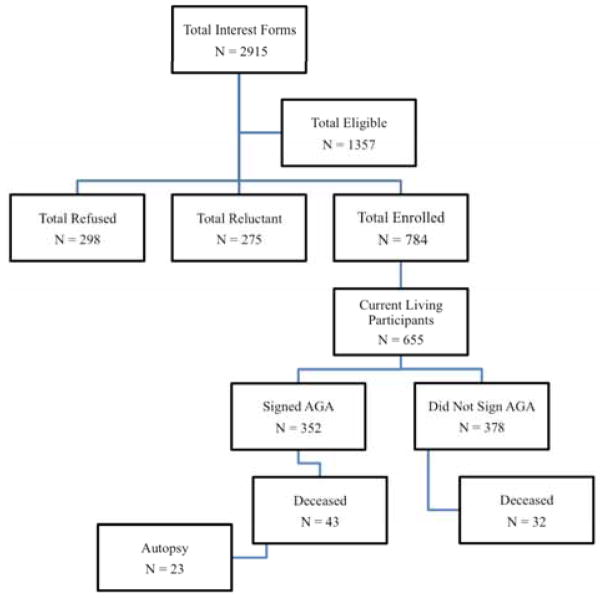
A flow chart summarizing the process of African Americans enrolling in one of our studies and eventually coming to autopsy is shown in Fig. (1). Of 2915 African Americans who fill out a study specific interest form, 1357 are eligible to enroll in one of our studies and 784 have been consented. Over 350 participants have signed an Anatomical Gift Act agreeing to organ donation. Others who have not signed to date are approached annually to see if they will consider organ donation, unless they firmly refuse to consider. To date, fewer than 30 people have refused to consider brain donation.
Fig. 1.
Flow chart of African American participants from recruitment to organ donation for the Minority Aging Research Study.
Table 6 shows the baseline demographic characteristics of the 784 African Americans enrolled in one of the four cohort studies. Fifteen persons had not yet completed their baseline clinical evaluation by the time of these analyses to render a clinical diagnosis. Follow-up participation among survivors approaches 95% and there have been 23 autopsies of 43 deaths from those who signed an Anatomical Gift Act (53.4% autopsy rate).
Table 6.
Demographic and Clinical Characteristics of 784 African Americans Enrolled in the Rush Cohort Studies
| Baseline Characteristics | N = 784 |
|---|---|
| Age, mean (sd) | 72.8 (6.6) |
| Education, y, mean (sd) | 14.8 (3.7) |
| Male, N (%) | 167 (21.3%) |
| Hispanic ethnicity, N (%) | 13 (1.6%) |
| Mini-Mental State Examination Score, mean (sd) | 27.6 (3.1) |
| Dementia at baseline, N (%) | 35 (4.4%) |
Table 7 provides descriptive characteristics of the 23 African Americans who signed an Anatomical Gift Act and came to autopsy to the 32 deceased African Americans who did not sign an Anatomical Gift Act and thus did not receive autopsy. There were no differences in age, education, or gender between the two groups. Those persons who came to autopsy tended to have a lower MMSE score at death than those who did not receive autopsy.
Table 7.
Descriptive Characteristics of African Americans who Received Autopsy (n = 23) to those who did not (n = 32)
| Autopsy (n = 23) | No Autopsy (n = 32) | |
|---|---|---|
| Age at death, years (sd), range | 80.9 (8.5) range = 65.9–97.7 | 78.7 (7.3) range = 59.3–92.4 |
| Education, years (sd), range | 14. 2 (4.2) range = 5.0–22.0 | 14.5 (3.5) range = 9.0 – 29.0 |
| % male (Number) | 27 (6) | 31 (10) |
| Last MMSE score proximate to death | 21.6 (8.8) range = 0–30 | 26.9 (2.9) range = 15–30 |
DISCUSSION
It is clear that with the rapid growth of the aging African American population and the suspected increased number of older African Americans who will experience cognitive decline, efforts will need to be extended to increase participation in studies that include brain donation. Similar to the considerable effort that is required to have high numbers of minorities participating in longitudinal cohort studies that emphasize high follow-up participation, the effort to obtain minority autopsies will need to be substantial as well. Although the flowchart likely underestimates the number of people we reach through our outreach efforts (because approximately 25% of attendees do not fill out an interest form or provide enough information to locate them after the presentation), it is clear that the entire process from recruitment to autopsy requires considerable staff time and effort. The results to date demonstrate that having a visible positive presence in the minority community and strengthening community partnerships are effective strategies for relieving the barriers to participation in brain donation studies.
Having pathologic data on relatively large numbers of well-characterized African Americans with comprehensive cognitive testing will not only contribute to our understanding of the neurobiologic basis of cognitive impairment, but will facilitate our ability to understand the impact of race and racial differences on neuropathology and on the relationship between neuropathology and cognition. It is important to note that there has been considerable debate in epidemiological research on the conceptualization of race in studies of racial differences [36] with some proposing that race is a biologic concept that should serve as a proxy for genetic variation and others proposing that race is solely a social concept driven by social and economic forces [e.g., 37–38]. Studies of racial differences in neuropathology and cognition will be particularly susceptible to a biologic conceptualization of race. However, there are no known biologic criteria on a phenotypic level to determine ones race [39–40], and at the level of the genotype, there is greater within-group heterogeneity than between-group heterogenity [e.g., 41–42]. Although there is evidence that some genetic factors may vary along racial/ethnic categorizations (e.g., sickle cell anemia, lupus, Tay-Sachs), most racial differences stem from multiple cultural and social attributes often associated with race including, but not limited to, socioeconomic status, low literacy and education, racial discrimination, residential segregation, and lack of access to quality healthcare [e.g., 43–44]. Rather than attempting to define race in strict biological or social terms, our goal is to develop a better understanding of the ways in which racial differences lead to disease biology and its functional consequences [45–46]. To the extent that health disparities arise from insults to a complex system that is represented by the interaction between genes and environments, identifying the conditions under which environmental triggers modify genetic risk or vice versa will place us in a better position to compare different population groups and understand disparities in health when we find them [47].
This study has an important limitation. The study uses a volunteer cohort of people drawn from the community that may not be representative of older community-dwelling African Americans. However, participants in MARS have a wide range of education and lifestyle experiences, and published data suggests that they are comparable to other cohorts of older African Americans in terms of cognitive test performance [48–50]. Moreover, our goal of determining the neuropathologic basis of cognitive impairment in older African Americans necessitates labor-intensive, in-depth characterization of clinical features proximate to death, and high rates of follow-up participation and autopsy, which would be difficult to achieve in a population-based setting. We are not aware of any large longitudinal cohort studies of African Americans that include organ donation on sufficient numbers of persons for meaningful analyses on the relation of neuropathology to cognition, or on the risk factors to neuropathology. Our recruitment results to date are consistent with other investigators who do research with underserved populations [51–55], and show that making authentic connections with potential participants and providing needed services to those who may not have access to up-to-date health information, helps to solidify relationships and creates a sense of “giving first” before asking for anything from the community. This reciprocity is not only important for establishing a culture of trust, but builds an infrastructure that allows the research to continue beyond individual projects.
The novel strategies, resources and infrastructure that have been put in place for the cohort studies at Rush will be fully utilized to insure that we continue to successfully educate, enroll, and obtain autopsy on more than 100 African Americans. The clinical and neuropathologic data generated from the Minority Aging Research Study, together with the other aging cohort studies at the Rush Alzheimer’s Disease Center is likely to greatly expand our understanding of the neuropathologic basis of cognitive impairment in older African Americans.
Table 3.
Data Collection in the Minority Aging Research Study and available in the other Cohort Studies at Rush
| Demographic Characteristics | MARS | MAP | ROS | CORE |
|---|---|---|---|---|
| Age, years | X | X | X | X |
| Gender | X | X | X | X |
| Education, years | X | X | X | X |
| Race and Ethnicity | X | X | X | X |
| Address at birth and age 12 (Country, State) | X | X | X | |
| Personal Income and/or household income | X | X | X | X |
| Occupational History | X | X | X | X |
| Marital Status | X | X | ||
| Objective Clinical Measures | ||||
| Dementia Diagnosis | X | X | X | X |
| Mild Cognitive Impairment Diagnosis | X | X | X | X |
| Stroke Diagnois and History | X | X | X | X |
| Parkinson’s Disease | X | X | X | X |
| Blood Pressure | X | X | X | X |
| Body mass Index | X | X | X | X |
| UPDRS Score | X | X | X | X |
| Visual Acuity | X | X | X | |
| Motor Performance (e.g, Timed walk, balance) | X | X | X | |
| Purdue Pegboard | X | X | X | |
| Medications (visually inspected) | X | X | X | X |
| Self-Report Clinical Measures | ||||
| Health History (e.g. head injury, hypertension, myocardial infarction, congestive heart failure, diabetes, etc) | X | X | X | X |
| Hormone Replacement Use and Menses History | X | X | X | |
| Incontinence (Urinary & Fecal) | X | X | X | |
| Tobacco Use | X | X | X | X |
| Alcohol Use | X | X | X | |
| Depressive Symptoms | X | X | X | X |
| Memory Complaints | X | X | X | X |
| Functional Status (ADLs, IADLs) | X | X | X | X |
| Family history (AD related disorders, education) | X | X | X | X |
| Driving status | X | X | ||
| Psychosocial Risk Factors | ||||
| Lifetime and Current Cognitive Activity | X | X | X | X |
| Physical Activity | X | X | X | |
| Social Networks | X | X | X | X |
| Social Activity | X | X | X | X |
| Social Isolation | X | X | ||
| Neuroticism | X | X | X | X |
| Purpose in Life | X | X | X | |
| Perceived Discrimination | X | X | X | |
| Racial Identity | X | |||
| Neighborhood Factors | X | |||
| Finanical Burden Scale | X | |||
| Caregiver Stress | X | |||
| History of Attending Segregated Schools | X | |||
| Perceived Control | X | X | ||
| Spirituality or Religious Activity | X | X | ||
| Coping and Resilience | X | |||
| Life Space | X | X | X |
Table 4.
Blood Measures in the Minority Aging Research Study and available in the other Cohort Studies at Rush
| MARS | MAP | ROS | CORE | |
|---|---|---|---|---|
| Lipid Panel | X | X | X | X |
| Hemogram with platelet count | X | X | X | X |
| Metabolic Panel | X | X | X | X |
| Hemoglobin A1c | X | X | X | X |
| Thyroid Stimulating hormone | X | X | X | X |
| Stored DNA | X | X | X | X |
| Apoe E4 Allele Status | X | X | X | X |
Acknowledgments
The authors thank the participants of the Minority Aging Research Study, the Rush Memory and Aging Project, the Religious Order Study, and the Rush Clinical Core for their invaluable contributions.
We thank Charlene Gamboa, MPH; Tracy Colvin, MPH; Tracey Nowakowski, MS; Rebecca Myers, Theresa Jenkins, Barbara Eubeler, Karen Lowe-Graham, MS, and Karen Skish, MS, PA(ASCP) MT, for study recruitment and coordination, and John Gibbons, MS and Greg Klein for data management, and the staff of the Rush Alzheimer’s Disease Center.
This research was supported by National Institute on Aging Grants R01AG22018, R01AG17917, P30G10161, and the Illinois Department of Public Health.
Footnotes
CONFLICT OF INTEREST
None declared.
References
- 1.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Public health and aging: Trends in aging – United States and Worldwide. JAMA. 2003;289:1371–1373. [PubMed] [Google Scholar]
- 3.Barnes DE, Cauley JA, Lui LY, et al. Women who maintain optimal cognitive function into old age. J Am Geriatr Soc. 2007;55:259–264. doi: 10.1111/j.1532-5415.2007.01040.x. [DOI] [PubMed] [Google Scholar]
- 4.Yaffe K, Lindquist K, Vittinghoff E, et al. The effect of maintaining cognition on risk of disability and death. J Am Geriatr Soc. 2010;58:889–894. doi: 10.1111/j.1532-5415.2010.02818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Current population reports, Series P25-1104, Population projections of the United States by age, sex, race, and Hispanic origin: 1993 to 2050.
- 6.Froehlich TE, Bogardus ST, Jr, Inouye SK. Dementia and race: are there differences between African Americans and Caucasians? J Am Geriatr Soc. 2001;49:477–484. doi: 10.1046/j.1532-5415.2001.49096.x. [DOI] [PubMed] [Google Scholar]
- 7.Tang MX, Cross P, Andrews H, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56:49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 8.Tang MX, Stern Y, Marder K, et al. The APOE-episilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA. 1998;279:751–755. doi: 10.1001/jama.279.10.751. [DOI] [PubMed] [Google Scholar]
- 9.Green RC, Cupples LA, Go R, et al. Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA. 2002;287:329–336. doi: 10.1001/jama.287.3.329. [DOI] [PubMed] [Google Scholar]
- 10.http://www.aoa.gov/aoaroot/aging_statistics/minority_aging/Facts-on-Black-Elderly-plain_format.aspx
- 11.Morris JC, Storandt M, McKeel DW, Jr, et al. Cerebral amyloid deposition and diffuse plaques in “normal” aging: evidence for pre-symptomatic and very mild Alzheimer’s disease. Neurology. 1996;46:707–719. doi: 10.1212/wnl.46.3.707. [DOI] [PubMed] [Google Scholar]
- 12.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol; 2004;61:378–384. doi: 10.1001/archneur.61.3.378. [DOI] [PubMed] [Google Scholar]
- 13.Wilson RS, Leurgans SE, Boyle PA, Schneider JA, Bennett DA. Neurodegenerative basis of age-related cognitive decline. Neurology. 2010;75:1070–1078. doi: 10.1212/WNL.0b013e3181f39adc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de la Monte SM, Hutchins GM, Moore GW. Racial differences in the etiology of dementia and frequency of Alzheimer lesions in the brain. J Natl Med Assoc. 1989;81:644–652. [PMC free article] [PubMed] [Google Scholar]
- 15.Sandberg G, Stewart W, Smialek J, Troncoso JC. The prevalence of the neuropathological lesions of Alzheimer’s disease is independent of race and gender. Neurobiol of Aging. 2001;22:169–175. doi: 10.1016/s0197-4580(00)00236-0. [DOI] [PubMed] [Google Scholar]
- 16.Miller FD, Hicks SP, D’Amato CJ, Landis JR. A descriptive study of neuritic plaques and neurofibrillary tangles in an autopsy population. Am J Epidemiol. 1984;120:331–341. doi: 10.1093/oxfordjournals.aje.a113897. [DOI] [PubMed] [Google Scholar]
- 17.Wilkins CH, Grant EA, Schmitt SE, McKeel DW, Morris JC. The neuropathology of Alzheimer disease in African American and White Individuals. Arch Neurol. 2006;63:87–90. doi: 10.1001/archneur.63.1.87. [DOI] [PubMed] [Google Scholar]
- 18.Bonner GJ, Darkwa OK, Gorelick PB. Autopsy recruitment program for African Americans. Alzheimer Dis Dis Assoc Disorder. 2000;14:202–208. doi: 10.1097/00002093-200010000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Pytel P, Cochran EJ, Bonner G, Nyenhuis DL, Thomas C, Gorelick PB. Vascular and Alzheimer-type pathology in an autopsy study of African Americans. Neurology. 2006;66:433–435. doi: 10.1212/01.wnl.0000196472.93744.57. [DOI] [PubMed] [Google Scholar]
- 20.Beekly DL, Ramos EM, van Belle G, et al. The National Alzheimer’s Coordinating Center (NACC) Database. Alzheimer Dis Assoc Disord. 2004;18:270–277. [PubMed] [Google Scholar]
- 21.Lewis TT, Aeillo AE, Leurgans SE, Kelly J, Barnes LL. Self-reported experiences of everyday discrimination are associated with elevated C-Reactive Protein levels in older African-American adults. Brain, Behavior, and Immunity. 2010;24:438–443. doi: 10.1016/j.bbi.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boulware E, Ratner LE, Cooper LA, Sosa JA, Laveist TA, Powe NR. Race and Gender Differences in Willingness to Donate Blood and Cadaveric Organs: Understanding Disparities in Donor Behavior. Medical Care. 2002;40:85–95. doi: 10.1097/00005650-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Siminoff LA, Gordon N, Hewlett J, Arnold RM. Factors influencing families’ consent for donation of solid organs for transplantation. JAMA. 2001;286:71–77. doi: 10.1001/jama.286.1.71. [DOI] [PubMed] [Google Scholar]
- 24.Stevens M. Factors influencing decisions about donation of the brain for research purposes. Age Ageing. 1998;27:623–629. doi: 10.1093/ageing/27.5.623. [DOI] [PubMed] [Google Scholar]
- 25.Schmitt FA, Wetherby MM, Wekstein DR, Dearth CM, Markesbery WR. Brain donation in normal aging: procedures, motivations, and donor characteristics from the Biologically Resilient Adults in Neurological Studies (BRAiNS) Project. Gerontologist. 2001;41:716–722. doi: 10.1093/geront/41.6.716. [DOI] [PubMed] [Google Scholar]
- 26.Bennett DA, Schneider JA, Buchman AS, Barnes LL, Wilson RS. Overview and findings from the Rush Memory and Aging Project. Current Alzheimer’s Research. 2011 doi: 10.2174/156720512801322663. (Current Issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett DA, Schneider JA, Wilson RS. Overview and findings from the Religious Orders Study. Current Alzheimer’s Research. 2011 doi: 10.2174/156720512801322573. (Current Issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arvanitakis Z, Bennett DA, Wilson RS, Barnes LL. Diabetes and cognitive systems in older black and white persons. Alzheimer’s Disease and Associated Disorders. 2010;24:37–42. doi: 10.1097/WAD.0b013e3181a6bed5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boyle P, Barnes LL, Buchman AS, Bennett DA. Purpose in life is associated with mortality among community-dwelling older persons. Psychosomatic Medicine. 2009;71:574–79. doi: 10.1097/PSY.0b013e3181a5a7c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buchman AS, Boyle PA, Leurgans SE, Barnes LL, Bennett DA. Cognitive Function is Associated with the Development of Mobility Impairments in Community-Dwelling Elders. Am J Geriatr Psychiatry. 2011;19:571–580. doi: 10.1097/JGP.0b013e3181ef7a2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fyffe DC, Mukherjee S, Barnes LL, Manly JJ, Bennett DA, Crane PK. Relationship between cognitive reserve and measurement invariance on memory test performance among Black and White older adults. JINS. doi: 10.1017/S1355617711000476. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.James BD, Boyle PA, Buchman AS, Barnes LL, Bennett DA. Life space and risk of Alzheimer’s disease, mild cognitive impairment, and cognitive decline in old age. Am J Geriatr Psychiatry. 2011 Mar 22; doi: 10.1097/JGP.0b013e318211c219. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lambe S, Cantwell N, Islam F, Horvath K, Jefferson AL. Perception, knowledge, incentives, and barriers of brain donation among African American elders enrolled in an Alzheimer’s research program. Gerontologist. 2011;51:28–38. doi: 10.1093/geront/gnq063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arriola KRJ, Perryman JP, Doldren M. Moving beyond attitudinal barriers: understanding African Americans’ support for organ and tissue donation. J Natl Med Association. 2005;97:339–350. [PMC free article] [PubMed] [Google Scholar]
- 35.Zaramo CE, Morton T, Yoo JW, Bowen GR, Modlin CS. Culturally competent methods to promote organ donation rates among African Americans using venues of the Bureau of Motor Vehicles. Transplant Proc. 2008;40:1001–1004. doi: 10.1016/j.transproceed.2008.03.060. [DOI] [PubMed] [Google Scholar]
- 36.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Painting a truer picture of US socioeconomic and racial/ethnic health inequalities: the Public Health Disparities Geocoding Project. Am J Public Health. 2005;95:312–323. doi: 10.2105/AJPH.2003.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wyatt SB, Williams DR, Calvin R, Henderson FC, Walker ER, Winters K. Racism and cardiovascular disease in African Americans. Am J Med Sci. 2003;325:315–331. doi: 10.1097/00000441-200306000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Dressler WW, Oths KS, Gravlee CC. Race and ethnicity in public health research: models to explain health disparities. Annu Rev Anthropol. 2005;34:231–252. [Google Scholar]
- 39.Garte S. The racial genetics paradox in biomedical research and public health. Public Health Rep. 2002;117:421–425. doi: 10.1016/S0033-3549(04)50181-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenthal L, Lobel M. Explaining racial disparities in adverse birth outcomes: unique sources of stress for Black American women. Social Science and Medicine. 2011;72:977–983. doi: 10.1016/j.socscimed.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 41.Rosenberg NA, Pritchard JK, Weber JL, et al. Genetic structure of human populations. Science. 2002;298:2381–5. doi: 10.1126/science.1078311. [DOI] [PubMed] [Google Scholar]
- 42.Jorde LB, Wooding SP. Genetic variation, classification and ‘race.’. Nat Genet. 2004;36:S28–S33. doi: 10.1038/ng1435. [DOI] [PubMed] [Google Scholar]
- 43.LaVeist T. Disentangling race and socioeconomic status: a key to understanding health inequalities. J Urban Health. 2005;82:iii26–iii34. doi: 10.1093/jurban/jti061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams DR. Race, socioeconomic status, and health. The added effects of racism and discrimination. Ann N YAcad Sci. 1999;896:173–188. doi: 10.1111/j.1749-6632.1999.tb08114.x. [DOI] [PubMed] [Google Scholar]
- 45.Gravlee CC. How race becomes biology: Embodiment of social inequality. American Journal of Physical Anthropology. 2009;139:47–57. doi: 10.1002/ajpa.20983. [DOI] [PubMed] [Google Scholar]
- 46.Cooper RS, Kaufman JS, Ward R. Race and Genomics. NEJM. 2003;348:1166–1170. doi: 10.1056/NEJMsb022863. [DOI] [PubMed] [Google Scholar]
- 47.Kuzawa CW, Sweet E. Epigenetics and the Embodiment of Race: Developmental Origins of US Racial Disparities in Cardiovascular Health. American Journal of Human Biology. 2009;21:2–15. doi: 10.1002/ajhb.20822. [DOI] [PubMed] [Google Scholar]
- 48.Barnes LL, Wilson RS, Hebert LE, Scherr PA, Evans DA, Mendes de Leon CF. Racial differences in the association of education with physical and cognitive function in older Blacks and Whites. J Gerontol B Psychol Sci Soc Sci. 2011;66:354–363. doi: 10.1093/geronb/gbr016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Masel MC, Peek MK. Ethnic differences in cognitive function over time. Ann Epidemiol. 2009;19:778–783. doi: 10.1016/j.annepidem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwartz BS, Glass TA, Bolla KL, et al. Disparities in cognitive functioning by race/ethnicity in the Baltimore Memory Study. Environ Health Perspect. 2004;112:314–320. doi: 10.1289/ehp.6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levkoff S, Sanchez H. Lessons learned about minority recruitment and retention from the Centers on Minority Aging and Health Promotion. Gerontologist. 2003;43:18–26. doi: 10.1093/geront/43.1.18. [DOI] [PubMed] [Google Scholar]
- 52.Keyzer JF, Melnikow J, Kuppermann M, et al. Recruitment strategies for minority participation: challenges and cost lessons from the POWER interview. Ethn Dis. 2005;15:395–406. [PubMed] [Google Scholar]
- 53.McCaskill-Stevens W, McKinney MM, Whitman CG, Minasian LM. Increasing minority participation in cancer clinical trials: the minority-based community clinical oncology program experience. J Clin Oncol. 2005;23:5247–5254. doi: 10.1200/JCO.2005.22.236. [DOI] [PubMed] [Google Scholar]
- 54.Grann VR, Jacobson JS, Troxel AB, et al. Barriers to minority participation in breast carcinoma prevention trials. Cancer. 2005;104:374–379. doi: 10.1002/cncr.21164. [DOI] [PubMed] [Google Scholar]
- 55.Herring P, Montgomery S, Yancey AK, Williams D, Fraser G. Understanding the challenges in recruiting blacks to a longitudinal cohort study: the Adventist health study. Ethn Dis. 2004;14:423–430. [PubMed] [Google Scholar]