Abstract
Cognitively intact elderly research volunteers at the University of Kentucky have been recruited, followed longitudinally, and autopsied with extensive neuropathological evaluations since 1989. To date, the cohort has recruited 1,030 individuals with 552 participants being actively followed, 363 deceased, and 273 autopsied. An extensive database has been constructed with continuous updates that include textured clinical, neuropsychological, neuroimaging, and pathological information. The history, demographics, clinical observations, and pathological features of this research cohort are described. We also explain some of the evolving methodologies and the academic contributions that have been made due to this motivated group of older Kentuckians.
Keywords: Aging, Alzheimer’s, autopsy, brain, dementia, Lewy bodies, longitudinal, neuropathology, neurocognition, neuritic plaques, neurofibrillary tangles
INTRODUCTION
This project highlights a unique group of older Kentuckians who are motivated by their desire to contribute to our scientific understanding of brain aging and dementia [1]. Alzheimer’s disease (AD) is thought to involve mechanisms in the brain that evolve well before clinical symptoms are observed [2, 3]. Recruitment of non-demented older adults began in 1989 at the University of Kentucky Sanders-Brown Center on Aging as a core component of a program project grant investigating pathogenetic mechanisms in AD. This effort was led by Drs. William Markesbery and David Wekstein who recognized the importance of longitudinal assessments of cognition and health with prearranged postmortem brain examination to more thoroughly study normal and pathologic brain aging.
Recruitment efforts (beginning fall 1989) involved contacting potential volunteers from a registry of over 4,500 community residents over 60 years of age, who had earlier indicated a willingness to participate in research, following a mailing to all registered voters in Fayette County, Kentucky. Potential participants received an introductory letter summarizing the importance of autopsy, followed by a visit with a center staff member to provide information about the project. Other volunteers came to the program following articles in the local press and broadcast news media.
The first 42 participants were recruited during the last quarter of 1989. At this time, the recruitment, evaluation, and follow-up activities for this project, based on National Institute on Aging recommendations, were incorporated into the ongoing Alzheimer’s Disease Research Center’s (ADC) Clinical Core with its renewal in 1990. Very few prospectively studied normal elderly subjects with autopsy findings had been published up to this time [4–6]. The efforts of the ADC and related program project began to focus on autopsy comparisons between the ‘normal’ aging brain and brain diseases including AD. An additional 117 participants were recruited during 1990. The initial success of this effort also led to the transformation of the longitudinal study of the School Sisters of Notre Dame in 1991 from a focus on breast cancer to a study devoted to cognitive aging and dementia [7].
In 1992, study participants selected ‘Biologically Resilient Adults in Neurological Studies’ as their official acronym and the ‘BRAiNS’ cohort continued to grow to over 1,000 participants. With the addition of a memory clinic in the local African American community, the cohort’s name was recently changed to ‘Sanders-Brown Healthy Brain Aging Volunteers’. This presentation provides a summary of the cohort’s demographics, procedures employed during the past two decades, a summary of findings and affiliated research that has used clinical data, and biological samples provided
PARTICIPANTS
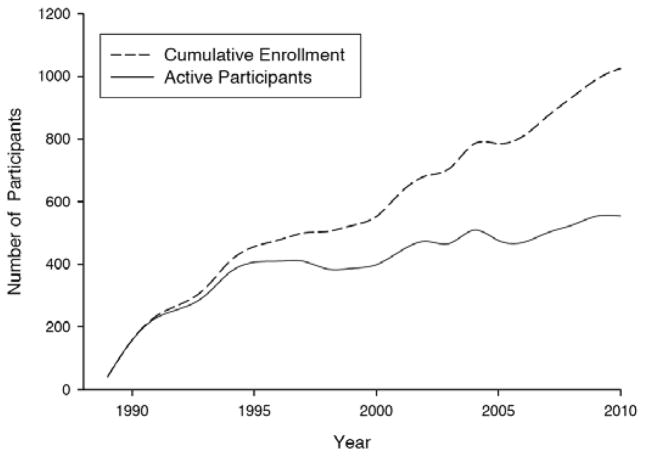
As of this writing, 1,030 individuals have joined this cohort and volunteered to be followed to autopsy; these individuals were recruited in ‘waves’. The current goal is to maintain a cohort of 500 active participants in annual followup to meet research needs regarding transitions from normal aging to conditions that result in impaired cognition. Forty-nine (4.8%) volunteers from this project presented with memory concerns and were diagnosed with mild cognitive impairment (MCI) and subsequently followed through the ADC clinical core as part of a separate cohort. The cohort’s demographics at enrollment are shown in (Table 1a) and ‘waves’ of recruitment are indicated in Fig. (1). Clearly, this sample does not reflect a true epidemiological cohort given the nature of participant recruitment. These volunteers are predominantly female (63.8%), generally well educated (15.9±2.6 years of education), have a relatively high prevalence of at least one apolipoprotein E4 allele (30.8% of those tested) and, on average, have had 7.6 (range: 1–21) annual visits. Since the program’s inception, autopsies have been performed on 273 of 353 deceased individuals with a 75% autopsy rate see (Table 1b). This autopsy rate reflects participants who initially enrolled in the project but later moved from the area or withdrew consent (during the study (N=115) or due to family decisions did not follow through with autopsy; N=90) contrasted with active participants who came to autopsy (N=273). Since 2005, the autopsy rate for all persons who had consented to join the program has been 85%.
Table 1a.
Participant Baseline Demographics (n = 1030)
| Baseline age, years (mean±SD) | 72.8±7.9 |
| Years of education (mean±SD) | 15.9±2.6 |
| Sex (M:F) | 371:659 |
| Family history of dementia | 352 (34.2%) |
| APOE E4 (positive/negative/not done) | 276/626/128 |
| Normal dx at baseline | 985 (95.6%) |
| Deceased | 363 (35.3%) |
| Currently active | 552 (53.6%) |
Fig. (1).
Cumulative enrollment of normal volunteers and number of active participants in the cohort by year.
Table 1b.
Demographic and Clinical Characteristics of Autopsied Participants.* (n = 273)
| Age at death, years (mean±SD) | 86.3±7.6 |
| Years of education (mean±SD) | 16.0±2.5 |
| Sex (M:F) | 114:159 |
| Family history of dementia (N) | 77 (28.2%) |
| APOE E4 (positive/negative/not done) | 74/190/9 |
| Post mortem interval, hours (median; mean±SD) | 2.9; 4.8±7.2 |
| Time since last visit before autopsy, years (median; mean±SD) | 0.7; 0.9±0.9 |
| Normal diagnosis at baseline | 273 (100%) |
Note: See Figure 2 for autopsy diagnoses of these initially normal older adults.
Autopsy diagnoses are summarized in Fig. (2). Note that over half of these autopsies (52%) had a neuropathological diagnosis consistent with some form of dementia with the following two interesting subsets: 3% of autopsies had ‘pure’ Lewy Body disease (DLB) and 5% had sufficient pathology for a ‘pure’ AD diagnosis (ADP-NRM) despite having clinically normal assessments prior to death. Approximately 40% of autopsies were consistent with normal brain aging without signs of dementia or other neuropathologies (NRM), thus providing a robust group of control brains for comparison. Although AD is present in fewer cases than would be suggested by earlier reviews [8], these data compare well with more recent reports from autopsy based cohorts [9, 10].
Fig. (2).
Neuropathological diagnoses from 273 autopsied control volunteers shown as percent of total sample.
METHODS AND PROCEDURES
Procedures for this longitudinal project have evolved over time. For the first 15 years of study, the inclusion criteria for this project (on enrollment) were: 1) age greater than 60 years; 2) absence of National Institute for Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association criteria for AD [11]; 3) absence of medical, neurological, and psychiatric conditions that affect cognition; 4) initial mental status examination scores above standard clinical cut points for dementia (e.g., Mini-Mental State Examination [12] MMSE> 24); 5) willingness to complete annual mental status examinations; and 6) brain donation at death (78% of donors also granted permission to remove other tissues). At enrollment, cerebrovascular disease (e.g., documented stroke or TIA) was exclusionary and even though treated hypertension was not exclusionary, overall vascular risks were low as reflected in their average (SD) modified Hachinski [13] score of 0.96 (±1.33; median=1.0). With the renewal of the ADC grant in 2000, annual neurological and medical examinations were initiated and the enrollment age was increased to age 70. Current participant enrollment inclusion and exclusion criteria are shown in (Table 2) and reflect the primary change in minimum age at enrollment.
Table 2.
Current Enrollment Criteria for Normal Control Volunteers
| INCLUSION CRITERIA | EXCLUSION CRITERIA |
|---|---|
| Minimum age 70 (or 65 for African Americans) | History of substance abuse (including alcohol) |
| Cognitively and neurologically normal | Major head injury |
| Family history of AD or dementia is preferred | Major psychiatric or neurological illness |
| Agree to brain donation at death | Medical illnesses that are non-stable, impairing, and/or have an effect on the CNS |
| Live within a 2 hour drive of Lexington, KY | Chronic infectious disease (e.g., HIV) |
| Must have a designated informant for structured interviews (e.g., CDR) | Strokes |
| Willing to undergo annual cognitive testing, physical and neurological exam | History of encephalitis, meningitis or epilepsy |
CLINICAL PROCEDURES
Participants consented to annual mental status testing, telephone health and medication interviews every 6 months, biennial physical and neurological examination, ApoE testing (starting in 1996), and donation of their brain at death. Cognitive assessments were completed at the participant’s residence and included the Iowa Dementia Screening Battery (Temporal Orientation, Benton Visual Retention Test, and Verbal Fluency [14]), the Washington University Battery [15] (Wechsler Memory, Logical Memory and Mental Control, Verbal Fluency, Trailmaking; MMSE, Memory Information Test, Boston Naming Test (15-item version), Alzheimer Disease Assessment Scale (word list learning), delayed recall, and recognition [16]), and copying of geometric figures [17]. A baseline level of mental ability was estimated with the Vocabulary subtest of the Wechsler Adult Intelligence Scale-Revised (WAIS-R) and Wide Range Achievement Test reading subtest while speed of mental processing was assessed with the Digit Symbol Substitution task from the WAIS-R (cf., [18] for descriptions of many of these procedures).
It was later recognized (in 1999 [19]) that moderate neuropathological changes along the AD spectrum may be seen in some individuals who evidenced little change on standard clinical assessments [19]. Therefore, new cognitive and clinical measures were piloted in a subgroup of 135 participants. These included the Clinical Dementia Rating [20] scale, structural magnetic resonance imaging [21], and the list memory task whose sensitivity was increased with the Rey Auditory Verbal Learning Test. Additional cognitive measures (cf. [18] for descriptions of these procedures) were explored such as the Stroop Test as a marker of executive functions, and more detailed activities of daily living were documented. As another estimate of intellectual function, irregular word reading was used with the addition of the National Adult Reading Test-Revised [22] at baseline.
Given the current focus on early AD detection, the transition from normal to MCI was seen as an important factor in determining the size of this cohort. Our biostatistics group modeled the transition from normal cognition to MCI in our participants and analyzed historical data as well as mortality [23]. Analyses of 15 years of data from the project’s inception supported a target cohort size of 500 active participants given a 2.6% annual conversion rate from normal aging to MCI. This rate would result in 13±3 transition events from normal cognition to MCI per year and a total of between 50–80 transitions over 5 years. Increasing the enrollment age to 70 years was deemed necessary based on mortality estimates within this cohort (Table 2) and the desire to study preclinical dementia [24] as well as ‘successful cerebral aging’.
More recently, as this normal aging cohort was incorporated into the ADC, in 2005 a major change in clinical procedures was initiated with the adoption of the National Alzheimer Coordinating Center Uniform Data Set (UDS) [25] procedures. Existing cognitive and clinical procedures were changed to match those required in the UDS and have been used for the past half-decade. However, with the initiation of these new standards we retained measures such as the MMSE, Trailmaking, verbal fluency, and paragraph memory as tests that have been part of annual procedures since the project’s inception [1, 19, 24]. The annual follow-up rate has remained high at 80% with only 115 volunteers withdrawing from the cohort (primarily due to relocation) and 90 deaths without autopsy (primarily due to late-life relocation or family members withdrawing consent for autopsy).
NEUROPATHOLOGICAL PROCEDURES
These procedures have two major goals. The first is to provide a thorough evaluation of the brain incorporating National Institute on Aging-Reagan Institute criteria [26] yielding an accurate and detailed postmortem diagnosis. The second is to provide a tissue bank consisting of valuable brain specimens, cerebrospinal fluid (CSF), and synaptosome preparations from longitudinally evaluated patients in the ADC. Given the emphasis on gaining a better understanding of the neuropathology of persons with preclinical AD, MCI, early AD, mixed dementia syndromes, and normal brain aging, every effort is made to obtain quality data and specimens for investigators at our institution and to provide these biospecimens to other research centers that study AD and other dementing disorders. Special emphasis is placed on clinical-pathological correlation in advanced age (> 85 years old) and those persons with “successful cerebral aging”.
A diagram of the rapid autopsy program is provided in Fig. (3). Rapid autopsies are performed on ‘normal’ control participants. On average, the postmortem interval for these cases is 2.8 hours (SD=1.2). In the event that the clinical status reflects MCI, early dementia, or a unique dementing condition, rapid autopsies are also performed. However, if the participant meets clinical criteria for an advanced dementia or certain other criteria such as an extended agonal hypoxic episode, ‘routine’ autopsy replaces our more rapid approach but brain specimens are derived and stored in a similar fashion as shown in Fig. (3).
Fig. (3).
Flow diagram of autopsy procedures.
The methodology used for the assessment of neuropathology on this cohort has remained relatively stable over time with a strong emphasis on quantitative (as opposed to only semiquantitative or ordinal) metrics of pathology, which offers excellent opportunities for clinical-pathological correlations because all pathological data are managed in the centralized database. The neuropathological methodologies applied to all cohorts at the UK ADC were previously described [27–29]. Briefly, at least 24 samples are taken from each brain, including middle frontal gyrus (Brodmann Area 9), superior and middle temporal gyri (Brodmann Areas 21 and 22), inferior parietal lobule (Brodmann Areas 39 and 40), and occipital lobe including primary visual area (Brodmann Areas 17 and 18). Amyloid plaques were counted separately as diffuse plaques (plaques without neurites) and neuritic plaques in each region, as previously described with the criteria based on assessment of Bielschowsky-stained sections. An arithmetic mean was calculated from counts of diffuse plaques (number/2.35 mm2), neuritic plaques (number/2.35 mm2), and neurofibrillary tangles (number/0.586 mm2) for each region in the 5 fields that were subjectively determined to have the greatest involvement. As previously described [28, 29] (alpha-synuclein, PHF-1, and TDP-43 immunolabeling are now routinely performed and more specific antibodies (e.g. Anti-FUS) are used as necessary.
RESULTS AND FINDINGS
With the maturation of this cohort, statistical models of transitions from normal cognitive aging became possible. In an investigation of risk factors using a novel Markov statistical model, we showed that transitions to MCI and early dementia (both AD and mixed cases of MCI [amnestic plus a decline in another cognitive domain]) could be predicted by age, ApoE genotype, and educational attainment [23]. This research (beginning in 1999) also stimulated several conferences on statistical issues associated with longitudinal aging [30], and a smaller series of conferences focusing on longitudinal findings that were held at the University of Kentucky.
In addition, rigorous clinical-pathological correlation studies from these longitudinally followed nondemented participants, who have been assessed for more than two decades with continuously gathered clinical and pathological data, have focused on still-controversial but fundamental issues such as the impact of diabetes on AD pathology [31], and cases with AD-related pathologies that fall outside of current NIA-RI recommendations [32, 33]. Also reported has been the impact of neuropathological subtypes in “pure” AD cases [27] as well as the earliest pathological changes that are seen in MCI or in individuals who were cognitively intact before death [34–37]. In a different experimental model, the statistical power of the University of Kentucky-ADC was harnessed to evaluate the associations between cerebral amyloid angiopathy, hippocampal sclerosis, and dozens of other pathological indices in multivariable statistical models [38].
When combined with other databases, clinical-pathological correlation studies in dementia with Lewy bodies (DLB) show that “pure” neocortical Lewy body pathology is far more frequently seen in males than females [39], and even extensive neocortical Lewy body pathology is not generally a substrate for “end-stage” dementia as indexed by the MMSE [40]. Neuropathological and clinical data from this and other cohorts have helped to define the expectations of neocortical LB pathology in non-demented persons[41, 42], demonstrated that DLB diagnostic sensitivity is imperfect [43], and compared the clinical course of “pure” AD with AD+DLB with or without acetylcholinesterase inhibitor therapies [44].
Clinical and pathological data from this cohort have also highlighted aspects of brain pathology in preclinical AD. For example, participants with early AD neuropathology as defined by National Institute on Aging-Reagan Institute criteria show reliable within subject declines in memory over time [24]. Further, participants with clinical MCI or very early AD symptoms at death show clear differences in neurofibrillary tangle loads when contrasted with clinically intact individuals in those brain regions affected by AD [34]; Fig. (4). Data from the BRAiNS cohort have been cross-correlated with other neuropathology data gathered at the University of Kentucky, such as the Nun Study, and combining the database has strengthened research projects on topics including AD-type pathology, hippocampal sclerosis, and other brain diseases [29, 33, 45].
Fig. (4).
Average (SEM) neurofibrillary (NFT) counts in five brain regions by last clinical diagnosis in 30 initially normal volunteers (N=10 per group).
A third area of investigation traces back to the original program project grant that established this cohort, namely the role of oxidative stress and neuroinflammation in AD. Tissue from the normal aging cohort and ADC participants has been supplied for research on oxidative stress to University of Kentucky researchers including Drs. Allan Butterfield, Stephen Scheff, Rodney Guttmann, Mark Lovell, and outside researchers such as Jeffrey Keller, Anna Bruce-Keller (Louisiana State University), Dr. Mark Mattson (National Institute on Aging), and the late Dr. Mark A. Smith (Case Western University). More than 100 papers have been published on oxidative stress in neurodegenerative diseases based on studies using UK-ADC tissues during the past 5 years.
Multiple studies of postmortem tissue specimens from normal control subjects who converted to MCI during follow up showed significantly increased lipid peroxidation [46], oxidative modification of multiple proteins (reviewed [47] and oxidative modification of DNA [48] and RNA [49] compared to control subjects who remained cognitively normal at death. In more recent studies analysis of brain specimens from subjects with preclinical AD showed significantly increased levels of aldehydic markers of lipid peroxidation but not protein oxidation in vulnerable brain regions compared to cognitively normal control subjects [50] suggesting lipid peroxidation occurs early in the progression of AD.
This program project grant also spawned multiple animal and cell studies on the use of antioxidants to prevent the disease, which in turn provided the scientific rationale for the ongoing prevention trial on the use of the anti-oxidants selenium and vitamin E used alone or in combination to prevent AD (Prevention of AD with Vitamin E and Selenium, PREADVISE). The translational project PREADViSE, centered at UK, enrolled 7,500 men aged 62 or older and has recently evolved into an exposure study [51].
A fourth area of study harnesses the resources of this longitudinal cohort in conjunction with the Religious Orders Study (see manuscript in this issue) to investigate synaptic changes in normal aging and synaptic pathology in MCI and AD. Under the leadership of Dr. Stephen Scheff, these studies probe the early stages of the disease process and use state-of-the-art stereology to quantify earliest disease-related changes in synapse numbers and morphology [52–54].
The fifth primary use of biological samples from the control cohort focuses on advancing neurodegenerative disease genomics research. Several examples follow. The control cohort reflects over 400 brain specimens supplied to the Alzheimer’s Disease Genetic Consortium led by Dr. Gerard Schellenberg. Dr. Steven Estus at the University of Kentucky works on AD genomics using tissue samples from this group [55, 56] while Drs. Eric Blalock and Philip Landfield have provided key insights into AD transcriptomics using this tissue [57, 58]. Further, Dr. Peter Nelson performs research on microRNAs in aging and AD using brain specimens from these cohorts [59–67]. Finally, this cohort supports National Institute on Aging initiatives (Table 4) and research by a number of investigators throughout the nation. Altogether, over the last five years, more than 14,000 specimens have been provided to investigators to support research projects.
Table 4.
Interaction of UK-ADC Control Cohort with National Institute on Aging Initiatives
| Entity | Category | N | Source |
|---|---|---|---|
| GWAS/ADGC Phase 1 (autopsy) | Cognitively intact cases | 78 | 1 |
| GWAS/ADGC Phase 2 (UDS) | Cognitively intact cases | 247 | 2 |
| ADNI Phase 1 | Overall (Cases with MRIs) | 32 | 3 |
| ADNI Phase 1 | Cases with CSF | 22 | 4 |
| NACC/UDS | Return visits | 1,665 | 5 |
| NACC/UDS | Total Packets | 2,596 | 5 |
| LOAD | Cognitively intact cases | 325 | 6 |
| NCRAD | Immortalized cell lines from the normal cohort | 597 | 7 |
Sources:
Genome-wide association studies (GWAS) Phase I Summary Report - Samples Received by 07SEP10
GWAS Phase II Summary Report - Samples Received by 07SEP10
Clinical data download from the Alzheimer’s Disease Neuroimaging Initiative (ADNI), September 14, 2010
Clinical data download from ADNI, September 14, 2010
National Alzheimer’s Coordinating Center (NACC)-custom report generated by request September 17, 2010
Genetics of Late Onset Alzheimer’s Disease study (LOAD)-via September 15, 2010 email from Kelley Faber, Clinical Research Manager, NCRAD
The National Cell Repository for Alzheimer’s Disease (NCRAD)-via September 20, 2010 email from Kelley Faber, Clinical Research Manager, NCRAD
Additional efforts have led to volunteers from this cohort enrolling in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) and they reflected 18% of the 32 local ADNI recruits. Further work in statistical models of dementia risk harnessed the longitudinal clinical data from this cohort as reflected in our group’s work on Markov models of transitions from normal aging see (Table 5). In turn, we have applied these approaches to data from the Nun study cohort and showed that age, education, and apolipoprotein E genotype are risk factors for transitions to MCI with progression to dementia being dependent on advancing age and the competing risk of mortality [68]. Additional efforts to define brain changes associated with aging and dementia incorporated the work of our team under the leadership of our neuropathologists, Drs. Markesbery, Davis, Nelson, and Wilson and linked data from the Nun, Religious Orders, and our longitudinal cohort studies are highlighted in (Table 5).
Table 5.
Selected Findings from the UK-ADC Aging Autopsy Cohort
| Cognitive changes and AD pathology* |
|
|
|
|
| Synaptic changes^ |
|
|
|
| Oxidative stress |
|
|
|
|
|
| Biostatistics, Imaging, and Genetics |
|
|
|
Note:
includes datasets that incorporate National Alzheimer’s Coordinating Center and Nun Study with UK-ADC longitudinal participants.
incorporates data from the Religious Orders Study.
CONCLUSIONS
We conclude that the study of healthy aging and preclinical disease states has only become more topical over the past several decades. The study of diseases of aging require well-characterized control subjects, stable populations that can be followed over time, and an insightful approach to understanding the sometimes blurry lines between “normal” and disease states. These requirements span the research fields of genetics, imaging, neuropsychology, neuropathology, biostatistics, biochemistry, and other areas. We have been humbled by the passion and commitment of our research volunteers who have made scientific progress possible and embody our hope for future progress.
Table 3.
Incidence Rates for MCI, Dementia, Death, and Drop out Computed Using Data from Cognitively intact individuals at Baseline
| Age | N | Person Years at Risk* | MCI Rate** | Dementia Rate | Death Rate | Dropout Rate | Death / Dropout in Person Years |
|---|---|---|---|---|---|---|---|
| <60+ | 37 | 424.6 – 431.2 | 0.24 | 0.00 | 0.46 | 2.55 | 431.2 |
| 60–64 | 112 | 1023.8 – 1098.3 | 1.56 | 0.83 | 1.27 | 1.64 | 1098 |
| 65–69 | 187 | 1289.3 – 1429.3 | 2.25 | 0.96 | 2.52 | 1.47 | 1429 |
| 70–74 | 260 | 1740.4 – 2037.8 | 2.82 | 1.30 | 3.04 | 1.03 | 2038 |
| 75–79 | 229 | 1403.7 – 1731.4 | 3.28 | 1.97 | 7.16 | 1.10 | 1731 |
| 80–84 | 137 | 678.9 – 942.3 | 4.71 | 2.91 | 7.96 | 0.74 | 942.3 |
| 85–89 | 51 | 188.5 – 262.8 | 3.18 | 3.00 | 13.70 | 1.14 | 262.8 |
| >89 | 17 | 53.8 – 61.4 | 1.86 | 0.00 | 21.17 | 0.00 | 61.4 |
| Total | 1030 | 6803.0 – 7994.5 | 2.65 | 1.47 | 4.53 | 1.25 | 7995 |
Reported years of risk adjusted for the events dementia and death; for example, a participant who converted to MCI at second visit continued to be at risk for conversion to dementia or death. The drop rate includes only those who dropped out without converting to MCI or dementia.
All rates expressed per year per hundred; e.g., 5.7 cases per year per 100 subjects at risk. There is a larger number of years at risk for death than for transition to MCI because once someone is diagnosed with MCI, they remain at risk for death but are no longer at risk for MCI.
Some participants were younger than 60 years of age during initial (1989 through 1992) recruitment stages given a strong family history of AD.
Acknowledgments
STUDY FUNDING
Supported by NIH (P01 AG05119, R01 NS061933, P30 AG028383, R01HD064993, R01AG019241, R01AG027219, U01 AG016976).
The authors wish to acknowledge the outstanding efforts of Drs. William Markesbery and David Wekstein which culminated in the establishment of the Sanders-Brown Center on Aging and set high standards for the research that is described herein. We also wish to thank the staff of the Sanders-Brown Center on Aging and Alzheimer’s Disease Center for their ongoing contributions to this research endeavor. Their efforts from “bench to bedside” have made this work so successful. Finally, the authors recognize the gifts of time and brain donation of our dedicated research volunteers who have invested themselves to the betterment of future generations through brain aging research.
References
- 1.Schmitt FA, Wetherby MM, Wekstein DR, Dearth CM, Markesbery WR. Brain donation in normal aging: procedures, motivations, and donor characteristics from the Biologically Resilient Adults in Neurological Studies (BRAiNS) Project. Gerontologist. 2001;41(6):716–22. doi: 10.1093/geront/41.6.716. [DOI] [PubMed] [Google Scholar]
- 2.Braak H, Del Tredici K. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol. 2011 Feb;121(2):171–81. doi: 10.1007/s00401-010-0789-4. [DOI] [PubMed] [Google Scholar]
- 3.Scarmeas N, Habeck CG, Hilton J, Anderson KE, Flynn J, Park A, et al. APOE related alterations in cerebral activation even at college age. J Neurol Neurosurg Psychiatry. 2005 Oct;76(10):1440–4. doi: 10.1136/jnnp.2004.053645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968 Jul;114(512):797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 5.Tomlinson BE, Blessed G, Roth M. Observations on the brains of non-demented old people. J Neurol Sci. 1968 Sep-Oct;7(2):331–56. doi: 10.1016/0022-510x(68)90154-8. [DOI] [PubMed] [Google Scholar]
- 6.Katzman R, Terry R, DeTeresa R, Brown T, Davies P, Fuld P, et al. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988 Feb;23(2):138–44. doi: 10.1002/ana.410230206. [Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 7.Greiner PA, Snowdon DA, Schmitt FA. The loss of independence in activities of daily living: the role of low normal cognitive function in elderly nuns. Am J Public Health. 1996 Jan;86(1):62–6. doi: 10.2105/ajph.86.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris JC. Differential diagnosis of Alzheimer’s disease. Clin Geriatr Med. 1994 May;10(2):257–76. [PubMed] [Google Scholar]
- 9.Crystal HA, Dickson D, Davies P, Masur D, Grober E, Lipton RB. The relative frequency of “dementia of unknown etiology” increases with age and is nearly 50% in nonagenarians. Arch Neurol. 2000 May;57(5):713–9. doi: 10.1001/archneur.57.5.713. [DOI] [PubMed] [Google Scholar]
- 10.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community- dwelling older persons. Neurology. 2007 Dec 11;69(24):2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 11.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984 Jul;34(7):939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 12.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 13.Rosen WG, Terry RD, Fuld PA, Katzman R, Peck A. Pathological verification of ischemic score in differentiation of dementias. Ann Neurol. 1980 May;7(5):486–8. doi: 10.1002/ana.410070516. [DOI] [PubMed] [Google Scholar]
- 14.Eslinger PJ, Damasio AR, Benton AL, Van Allen M. Neuropsychologic detection of abnormal mental decline in older persons. Jama. 1985 Feb 1;253(5):670–4. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] [PubMed] [Google Scholar]
- 15.Storandt M, Botwinick J, Danziger WL, Berg L, Hughes CP. Psychometric differentiation of mild senile dementia of the Alzheimer type. Arch Neurol. 1984 May;41(5):497–9. doi: 10.1001/archneur.1984.04050170043013. [Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 16.Mohs RC, Rosen WG, Davis KL. The Alzheimer’s disease assessment scale: an instrument for assessing treatment efficacy. Psychopharmacol Bull. 1983;19(3):448–50. [PubMed] [Google Scholar]
- 17.Welsh K, Butters N, Hughes J, Mohs R, Heyman A. Detection of abnormal memory decline in mild cases of Alzheimer’s disease using CERAD neuropsychological measures. Arch Neurol. 1991 Mar;48(3):278–81. doi: 10.1001/archneur.1991.00530150046016. [Research Support, U.S. Gov’t, Non-P.H.S. Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 18.Lezak MD. Neuropsychological assessment in behavioral toxicology-developing techniques and interpretative issues. Scand J Work Environ Health. 1984;10( Suppl 1):25–9. [PubMed] [Google Scholar]
- 19.Davis DG, Schmitt FA, Wekstein DR, Markesbery WR. Alzheimer neuropathologic alterations in aged cognitively normal subjects. J Neuropathol Exp Neurol. 1999 Apr;58(4):376–88. doi: 10.1097/00005072-199904000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Berg L. Clinical Dementia Rating. Br J Psychiatry. 1984 Sep;145:339. [PubMed] [Google Scholar]
- 21.Martin SB, Smith CD, Collins HR, Schmitt FA, Gold BT. Evidence that volume of anterior medial temporal lobe is reduced in seniors destined for mild cognitive impairment. Neurobiol Aging. 2010 Jul;31(7):1099–106. doi: 10.1016/j.neurobiolaging.2008.08.010. [Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berry DT, Carpenter GS, Campbell DA, Schmitt FA, Helton K, Lipke-Molby T. The New Adult Reading Test-Revised: accuracy in estimating WAIS-R IQ scores obtained 3.5 years earlier from normal older persons. Arch Clin Neuropsychol. 1994 May;9(3):239–50. [PubMed] [Google Scholar]
- 23.Kryscio RJ, Schmitt FA, Salazar JC, Mendiondo MS, Markesbery WR. Risk factors for transitions from normal to mild cognitive impairment and dementia. Neurology. 2006 Mar 28;66(6):828–32. doi: 10.1212/01.wnl.0000203264.71880.45. [DOI] [PubMed] [Google Scholar]
- 24.Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR. “Preclinical” AD revisited: neuropathology of cognitively normal older adults. Neurology. 2000 Aug 8;55(3):370–6. doi: 10.1212/wnl.55.3.370. [DOI] [PubMed] [Google Scholar]
- 25.Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006 Oct-Dec;20(4):210–6. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 26.Markesbery WR. Neuropathological criteria for the diagnosis of Alzheimer’s disease. Neurobiol Aging. 1997 Jul-Aug;18(4 Suppl):S13–9. doi: 10.1016/s0197-4580(97)00064-x. [DOI] [PubMed] [Google Scholar]
- 27.Nelson PT, Jicha GA, Schmitt FA, Liu H, Davis DG, Mendiondo MS, et al. Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J Neuropathol Exp Neurol. 2007 Dec;66(12):1136–46. doi: 10.1097/nen.0b013e31815c5efb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, Smith CD, et al. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol. 2010 Jan;20(1):66–79. doi: 10.1111/j.1750-3639.2008.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson PT, Schmitt FA, Lin Y, Abner EL, Jicha GA, Patel E, et al. Hippocampal sclerosis in advanced age: clinical and pathological features. Brain. 2011 May;134(Pt 5):1506–18. doi: 10.1093/brain/awr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendiondo MS, Ashford JW, Kryscio RJ, Schmitt FA. Modelling mini mental state examination changes in Alzheimer’s disease. Stat Med. 2000 Jun 15–30;19(11–12):1607–16. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1607::aid-sim449>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 31.Nelson PT, Smith CD, Abner EA, Schmitt FA, Scheff SW, Davis GJ, et al. Human cerebral neuropathology of Type 2 diabetes mellitus. Biochim Biophys Acta. 2008 Aug 22; doi: 10.1016/j.bbadis.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson PT, Kukull WA, Frosch MP. Thinking outside the box: Alzheimer-type neuropathology that does not map directly onto current consensus recommendations. J Neuropathol Exp Neurol. May;69(5):449–54. doi: 10.1097/NEN.0b013e3181d8db07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, Santacruz K, et al. Brains with medial temporal lobe neurofibrillary tangles but no neuritic amyloid plaques are a diagnostic dilemma but may have pathogenetic aspects distinct from Alzheimer disease. J Neuropathol Exp Neurol. 2009 Jul;68(7):774–84. doi: 10.1097/NEN.0b013e3181aacbe9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Markesbery WR, Schmitt FA, Kryscio RJ, Davis DG, Smith CD, Wekstein DR. Neuropathologic substrate of mild cognitive impairment. Arch Neurol. 2006 Jan;63(1):38–46. doi: 10.1001/archneur.63.1.38. [DOI] [PubMed] [Google Scholar]
- 35.Smith CD, Chebrolu H, Wekstein DR, Schmitt FA, Jicha GA, Cooper G, et al. Brain structural alterations before mild cognitive impairment. Neurology. 2007 Apr 17;68(16):1268–73. doi: 10.1212/01.wnl.0000259542.54830.34. [DOI] [PubMed] [Google Scholar]
- 36.Nelson PT, Abner EL, Scheff SW, Schmitt FA, Kryscio RJ, Jicha GA, et al. Alzheimer’s-type neuropathology in the precuneus is not increased relative to other areas of neocortex across a range of cognitive impairment. Neurosci Lett. 2009 Feb 6;450(3):336–9. doi: 10.1016/j.neulet.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nelson PT, Braak H, Markesbery WR. Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J Neuropathol Exp Neurol. 2009 Jan;68(1):1–14. doi: 10.1097/NEN.0b013e3181919a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, Smith CD, et al. Modeling the Association between 43 Different Clinical and Pathological Variables and the Severity of Cognitive Impairment in a Large Autopsy Cohort of Elderly Persons. Brain Pathol. 2008 Nov 19; doi: 10.1111/j.1750-3639.2008.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson PT, Schmitt FA, Jicha GA, Kryscio RJ, Abner EL, Smith CD, et al. Association between male gender and cortical Lewy body pathology in large autopsy series. J Neurol. Jun 20; doi: 10.1007/s00415-010-5630-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nelson PT, Kryscio RJ, Jicha GA, Abner EL, Schmitt FA, Xu LO, et al. Relative preservation of MMSE scores in autopsy-proven dementia with Lewy bodies. Neurology. 2009 Oct 6;73(14):1127–33. doi: 10.1212/WNL.0b013e3181bacf9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Markesbery WR, Jicha GA, Liu H, Schmitt FA. Lewy body pathology in normal elderly subjects. J Neuropathol Exp Neurol. 2009 Jul;68(7):816–22. doi: 10.1097/NEN.0b013e3181ac10a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jicha GA, Schmitt FA, Abner E, Nelson PT, Cooper GE, Smith CD, et al. Prodromal clinical manifestations of neuropathologically confirmed Lewy body disease. Neurobiol Aging. 2008 Nov 19; doi: 10.1016/j.neurobiolaging.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nelson PT, Jicha GA, Kryscio RJ, Abner EL, Schmitt FA, Cooper G, et al. Low sensitivity in clinical diagnoses of dementia with Lewy bodies. J Neurol. 2009 Oct 1; doi: 10.1007/s00415-009-5324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nelson PT, Kryscio RJ, Abner EA, Schmitt FA, Jicha GA, Mendiondo MS, et al. Acetylcholinesterase inhibitor treatment is associated with relatively slow cognitive decline in patients with Alzheimer’s disease and AD+DLB. J Alzheimers Dis. 2008 doi: 10.3233/JAD-2009-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abner EL, Kryscio RJ, Schmitt FA, Santacruz KS, Jicha GA, Lin Y, et al. “End-Stage” Neurofibrillary Tangle Pathology in Preclinical Alzheimer’s Disease: Fact or Fiction? J Alzheimers Dis. 2011 Apr 6; doi: 10.3233/JAD-2011-101980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams TI, Lynn BC, Markesbery WR, Lovell MA. Increased levels of 4-hydroxynonenal and acrolein, neurotoxic markers of lipid peroxidation, in the brain in Mild Cognitive Impairment and early Alzheimer’s disease. Neurobiol Aging. 2006 Aug;27(8):1094–9. doi: 10.1016/j.neurobiolaging.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Sultana R, Butterfield DA. Role of Oxidative Stress in the Progression of Alzheimer’s Disease. J Alzheimers Dis. 2009 Sep 11; doi: 10.3233/JAD-2010-1222. [DOI] [PubMed] [Google Scholar]
- 48.Wang J, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in mild cognitive impairment. J Neurochem. 2006 Feb;96(3):825–32. doi: 10.1111/j.1471-4159.2005.03615.x. [DOI] [PubMed] [Google Scholar]
- 49.Lovell MA, Markesbery WR. Oxidatively modified RNA in mild cognitive impairment. Neurobiol Dis. 2008 Feb;29(2):169–75. doi: 10.1016/j.nbd.2007.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bradley MA, Markesbery WR, Lovell MA. Increased levels of 4- hydroxynonenal and acrolein in the brain in preclinical Alzheimer disease. Free Radic Biol Med. 2010 Jun 15;48(12):1570–6. doi: 10.1016/j.freeradbiomed.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kryscio RJ, Mendiondo MS, Schmitt FA, Markesbery WR. Designing a large prevention trial: statistical issues. Stat Med. 2004 Jan 30;23(2):285–96. doi: 10.1002/sim.1716. [DOI] [PubMed] [Google Scholar]
- 52.Scheff SW, Price DA. Alzheimer’s disease-related alterations in synaptic density: neocortex and hippocampus. J Alzheimers Dis. 2006;9(3 Suppl):101–15. doi: 10.3233/jad-2006-9s312. [DOI] [PubMed] [Google Scholar]
- 53.Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2006 Oct;27(10):1372–84. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 54.Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology. 2007 May 1;68(18):1501–8. doi: 10.1212/01.wnl.0000260698.46517.8f. [DOI] [PubMed] [Google Scholar]
- 55.Gopalraj RK, Zhu H, Kelly JF, Mendiondo M, Pulliam JF, Bennett DA, et al. Genetic association of low density lipoprotein receptor and Alzheimer’s disease. Neurobiol Aging. 2005 Jan;26(1):1–7. doi: 10.1016/j.neurobiolaging.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 56.Zhu H, Taylor JW, Bennett DA, Younkin SG, Estus S. Lack of association of hepatic lipase polymorphisms with late-onset Alzheimer’s disease. Neurobiol Aging. 2008 May;29(5):793–4. doi: 10.1016/j.neurobiolaging.2006.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blalock EM, Chen KC, Stromberg AJ, Norris CM, Kadish I, Kraner SD, et al. Harnessing the power of gene microarrays for the study of brain aging and Alzheimer’s disease: statistical reliability and functional correlation. Ageing Res Rev. 2005 Nov;4(4):481–512. doi: 10.1016/j.arr.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 58.Kelly KM, Nadon NL, Morrison JH, Thibault O, Barnes CA, Blalock EM. The neurobiology of aging. Epilepsy Res. 2006 Jan;68( Suppl 1):S5–20. doi: 10.1016/j.eplepsyres.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 59.Nelson PT, Baldwin DA, Kloosterman WP, Kauppinen S, Plasterk RH, Mourelatos Z. RAKE and LNA-ISH reveal microRNAs expression and localization in archival human brain. RNA. 2006 Feb;12(2):187–91. doi: 10.1261/rna.2258506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nelson PT, Dimayuga J, Wilfred BR. MicroRNA in Situ Hybridization in the Human Entorhinal and Transentorhinal Cortex. Front Hum Neurosci. 2010 Feb 22;4:7. doi: 10.3389/neuro.09.007.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nelson PT, Kiriakidou M, Mourelatos Z, Tan GS, Jennings M, Xie K, et al. High-throughput experimental studies to identify miRNA targets directly, with special focus on the mammalian brain. Brain Res. Apr 6; doi: 10.1016/j.brainres.2010.03.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nelson PT, Wang WX. MiR-107 is Reduced in Alzheimer’s Disease Brain Neocortex: Validation Study. J Alzheimers Dis. Apr 22; doi: 10.3233/JAD-2010-091603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nelson PT, Wang WX, Rajeev BW. MicroRNAs (miRNAs) in neurodegenerative diseases. Brain Pathol. 2008;18(1):130–8. doi: 10.1111/j.1750-3639.2007.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nelson PT, Wang WX, Wilfred BR, Tang G. Technical variables in high-throughput miRNA expression profiling: much work remains to be done. Biochim Biophys Acta. 2008;1779(11):758–65. doi: 10.1016/j.bbagrm.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nelson PT, Wilfred BR. In situ hybridization is a necessary experimental complement to microRNA (miRNA) expression profiling in the human brain. Neurosci Lett. 2009 doi: 10.1016/j.neulet.2009.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang WX, Rajeev BW, Stromberg AJ, Ren N, Tang G, Huang Q, et al. The expression of microRNA miR-107 decreases early in Alzheimer’s disease and may accelerate disease progression through regulation of beta-site amyloid precursor protein-cleaving enzyme 1. J Neurosci. 2008;28(5):1213–23. doi: 10.1523/JNEUROSCI.5065-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang WX, Wilfred BR, Baldwin DA, Isett RB, Ren N, Stromberg A, et al. Focus on RNA isolation: obtaining RNA for microRNA (miRNA) expression profiling analyses of neural tissue. Biochim Biophys Acta. 2008;1779(11):749–57. doi: 10.1016/j.bbagrm.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tyas SL, Salazar JC, Snowdon DA, Desrosiers MF, Riley KP, Mendiondo MS, et al. Transitions to mild cognitive impairments, dementia, and death: findings from the Nun Study. Am J Epidemiol. 2007;165(11):1231–8. doi: 10.1093/aje/kwm085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schmitt FA, Miller JP, Kryscio RJ. Promoting interactions with basic scientists and clinicians: summary of the panel session. Stat Med. 2000;19(11–12):1463–8. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1463::aid-sim438>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 70.Riley KP, Jicha GA, Davis D, Abner EL, Cooper GE, Stiles N, et al. Prediction of Preclinical Alzheimer’s Disease: Longitudinal Rates of Change in Cognition. J Alzheimers Dis. 2011 doi: 10.3233/JAD-2011-102133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jicha GA, Abner EL, Schmitt FA, Kryscio RJ, Riley KP, Cooper GE, et al. Preclinical AD Workgroup staging: pathological correlates and potential challenges. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scheff SW, Price DA, Schmitt FA, Scheff MA, Mufson EJ. Synaptic loss in the inferior temporal gyrus in mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2011;24(3):547–57. doi: 10.3233/JAD-2011-101782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Keller JN, Schmitt FA, Scheff SW, Ding Q, Chen Q, Butterfield DA, et al. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64(7):1152–6. doi: 10.1212/01.WNL.0000156156.13641.BA. [DOI] [PubMed] [Google Scholar]
- 74.Ansari MA, Scheff SW. Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J Neuropathol Exp Neurol. 2010;69(2):155–67. doi: 10.1097/NEN.0b013e3181cb5af4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ansari MA, Scheff SW. NADPH-oxidase activation and cognition in Alzheimer disease progression. Free Radic Biol Med. 2011 doi: 10.1016/j.freeradbiomed.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang WX, Huang Q, Hu Y, Stromberg AJ, Nelson PT. Patterns of microRNA expression in normal and early Alzheimer’s disease human temporal cortex: white matter versus gray matter. Acta Neuropathol. 2011;121(2):193–205. doi: 10.1007/s00401-010-0756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang WX, Wilfred BR, Madathil SK, Tang G, Hu Y, Dimayuga J, et al. miR-107 regulates granulin/progranulin with implications for traumatic brain injury and neurodegenerative disease. Am J Pathol. 2010;177(1):334–45. doi: 10.2353/ajpath.2010.091202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nelson PT, Wang WX. MiR-107 is reduced in Alzheimer’s disease brain neocortex: validation study. J Alzheimers Dis. 2010;21(1):75–9. doi: 10.3233/JAD-2010-091603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Finnerty JR, Wang WX, Hebert SS, Wilfred BR, Mao G, Nelson PT. The miR-15/107 group of microRNA genes: evolutionary biology, cellular functions, and roles in human diseases. J Mol Biol. 2010;402(3):491–509. doi: 10.1016/j.jmb.2010.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang WX, Rajeev BW, Stromberg AJ, Ren N, Tang G, Huang Q, et al. The expression of microRNA miR-107 decreases early in Alzheimer’s disease and may accelerate disease progression through regulation of beta-site amyloid precursor protein-cleaving enzyme 1. J Neurosci. 2008;28(5):1213–23. doi: 10.1523/JNEUROSCI.5065-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]