Abstract
Low doses of methylphenidate reduce hyperactivity and improve attention in individuals with attention deficit hyperactivity disorder (ADHD) as well as in healthy humans and animals. Despite its extensive use, relatively little is known about its mechanisms of action. This study investigated the effects of methylphenidate on working memory performance, impulsivity, response accuracy and precision, and the ability to stay on task in rhesus monkeys using an oculomotor delayed response task. Methylphenidate affected task performance in an inverted-U manner in all three subjects tested. The improvements resulted from a reduction in premature responses and, importantly, not from improvement in the memory of target location. The length of time subjects participated in each session was also affected dose dependently. However, the dose at which the length of participation was maximally increased significantly impaired performance on the working memory task. This dissociation of effects has implications for the treatment of ADHD, for the non-prescription use of methylphenidate for cognitive enhancement, and for furthering the basic understanding of the neural substrate underlying these processes.
INTRODUCTION
The administration of psychostimulants such as methylphenidate can significantly alter the function of the nervous system. Whereas high doses cause locomotor activation and cognitive impairment (Sproson, Chantrey, Hollis, Marsden, & Fone, 2001; Gaytan, Ghelani, Martin, Swann, & Dafny, 1997), lower doses may improve cognitive and behavioral functions and are thus used extensively for the treatment of attention deficit hyperactivity disorder (ADHD; Solanto, 1998, 2002; Greenhill, 2001). Methylphenidate, which inhibits the reuptake of dopamine (DA) and norepinepherine (NE), thereby increasing their extracellular levels (e.g., Volkow et al., 2001), is also used without prescription to improve cognitive function (Greely et al., 2008) because it is also effective in healthy humans and animals (Gamo, Wang, & Arnsten, 2010; Berridge et al., 2006; Arnsten & Dudley, 2005; Mehta, Sahakian, & Robbins, 2001; Solanto, 1998; Rapoport et al., 1980).
Interestingly, despite the extensive use of methylphenidate, its effects on cognition, behavior, and the underlying neural mechanisms are not well understood (Solanto, 1998, 2002). Studies of the effects of methylphenidate in research animals have been primarily done in the context of delayed alternation tasks in rodents (Berridge et al., 2006; Arnsten & Dudley, 2005) or the classic delayed response task in primates (Gamo et al., 2010), both of which are thought to be measures of working memory, where it has been shown to produce an inverted-U dose–response curve.
However, methylphenidate is also known to affect other aspects of cognition and behavior (Solanto, 2002; Tannock, Schachar, & Logan, 1995; Tannock, Schachar, Carr, & Logan, 1989; Sprague & Sleator, 1977; Reynolds, Salzberg, & Barker, 1968). Sprague and Sleator (1977), for instance, showed in children diagnosed with ADHD a dose-dependent dissociation between the effects of methylphenidate on cognitive performance, measured with a nonspatial working memory task, and social behavior in the classroom, evaluated by a teacher using an abbreviated Conners’ rating scale (Conners, 1969) that measures behavioral problems in the classroom. Specifically, cognitive performance was improved with a low dose of methylphenidate and was hindered by a higher dose. Most importantly, social behavior was most improved at the higher of the two doses. Differential effects on two different cognitive tasks by a single high dose of methylphenidate have also been shown by Clatworthy et al. (2009) and Dyme, Sahakian, Golinko, and Rabe (1982). Thus, although it has been shown that cognitive/behavioral functions can be differentially affected by the same dose of methylphenidate, the specific dose–response profiles documented by Sprague and Sleator (1977) have not been replicated (Tannock et al., 1989, 1995).
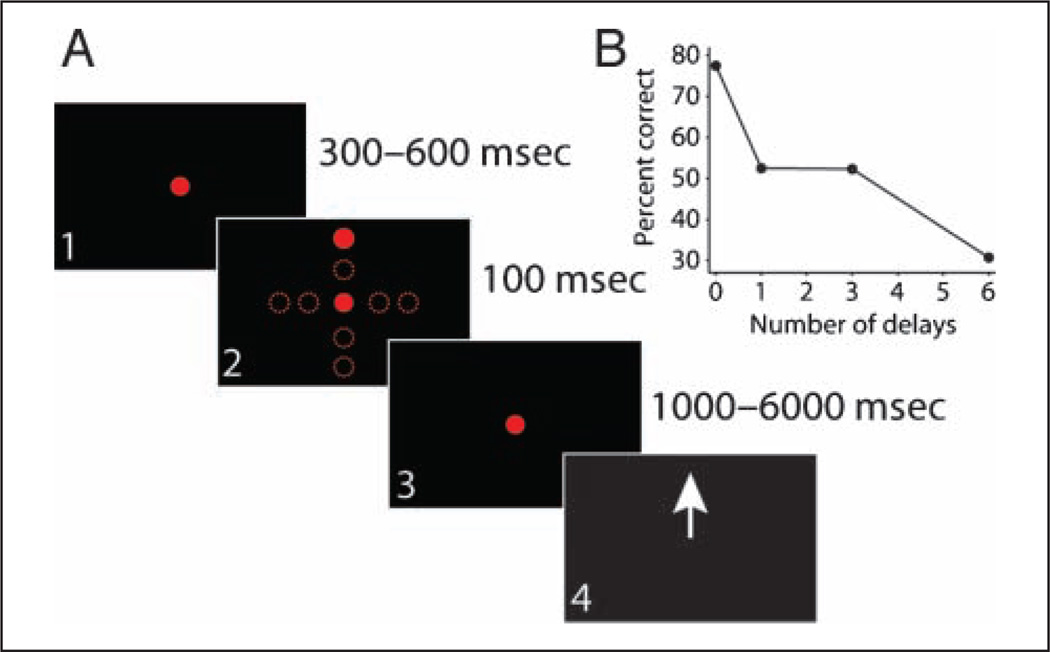
This study tested the hypothesis that different cognitive functions and behaviors are specifically improved/hindered by different doses of methylphenidate in rhesus monkeys, the animal model closest to humans available for studying nervous system function, using an oculomotor delayed response task (Figure 1A) tailored to measure working memory performance, impulsivity, response accuracy and precision, and ability to stay on task.
Figure 1.
Experimental task. (A) Diagram of the experimental task. (1) Upon the start of a trial, a fixation light located straight ahead was turned on, and the subjects were expected to look at it and maintain fixation until it was extinguished. (2) During fixation, a 100-msec visual target was presented. (3) The time between the onset of the target and the offset of the fixation light, the delay period, was varied randomly from trial to trial (1–6 sec). (4) The subjects were required to withhold response to the target until the fixation light was turned off, which was their instruction to respond. (B) Percent success as a function of the number of delays presented in a session. The average duration of the delays was kept constant (e.g., one delay of 3 sec; three delays of 2, 3, and 4 secs). As the number of delays randomly intermixed within a session increased, performance decreased as a result of the increased level of difficulty and cognitive/attentional demands. This study used six delays, 1–6 sec in steps of 1 sec, thereby preventing subjects from anticipating the timing of the response and requiring them to attend for the signal to respond.
METHODS
Subjects and Surgery
Three adult male rhesus monkeys (Macaca mulatta) ranging from 8 to 13 kg participated in this study. These animals were purchased from the Wisconsin Regional Primate Research Center. The three animals were prepared for eye movement recordings by implanting scleral search coils (Judge, Richmond, & Chu, 1980), constructed from teflon-coated stainless steel wire (SA632; Cooner Wire, Chatsworth, CA) and a lightweight titanium head post, which was used to restrain the head for experimental sessions and for cleaning the implant area. All surgical procedures were approved by the University of Wisconsin Animal Care and Use Committee and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Experimental Setup
The experiments were carried out in a 3 × 2.8 × 2 m double-walled soundproof chamber (Acoustic Systems, Austin, TX) under dim illumination. Eye movements were measured with the scleral search coil technique (Robinson, 1963) using a phase angle system (CNC Engineering, Seattle, WA). The signals representing horizontal and vertical eye position were low-pass filtered at 250 Hz (Krohn-Hite, Co., Avon, MA) and digitally sampled at 500 Hz with an analog to digital converter (Tucker Davis Technologies, Alachua, FL). Linear equations were fit to the horizontal and vertical eye movement data, and the coefficients were used to trans form the voltage output of the coil system into degrees of visual angle. The coils were calibrated with a behavioral procedure that relied on the animals’ tendency to look at spots of light presented in a darkened environment (Populin & Yin, 1998). Data acquisition was performed using custom software. The digitized eye position signals were stored in a relational database for off-line analysis.
Behavioral Training and Experimental Task
The subjects were trained to accept being handled with a pole and to enter a primate chair (Crist Instrument Co., Inc, Hagerstown, MD) using positive reinforcement. Animals were trained to make eye movements to light-emitting diodes (LEDs) presented within the frontal hemifield using operant conditioning. Water was delivered after the completion of successful trials, which required meeting both temporal and spatial criteria. Temporal criteria included maintaining fixation on the LED straight ahead until turned off and executing an eye movement within 700 msec. Spatial criteria involved making a saccade with end position falling within a (4°, 4°) acceptance window set around each target; the size of the acceptance window defined the margin of error allowed in the saccades to targets. Failure to meet these criteria resulted in termination of the trial at the time the error was made. The volume of water received in each successful trial was 0.5 ml.
Memory Saccade Task
This task (Figure 1A) was used to study working memory performance and was adapted from Funahashi, Bruce, and Goldman-Rakic (1989). It required subjects to withhold responses to a visual target and make a memory-guided saccade to the remembered location of the target following a delay period. The subjects were first required to fixate a red LED at the straight ahead position for a variable period (300–600 msec) at which point a 100-msec target was presented from one of eight target locations along the main axes (±10° and 20°), to which the subject was required to withhold response. After a variable delay period (1–6 sec), the fixation LED was extinguished signaling the subjects to make an eye movement to the remembered location of the target.
Experimental Sessions
Memory saccade trials of six delays and eight target positions were presented in random order and randomly intermixed with visually guided saccades to maintain the subjects’ interest in participating. Seven hundred milliseconds were allowed for the subject to respond after the offset of the fixation LED. The subjects participated in each experimental session until sated.
Importantly, unlike the task used by Funahashi et al. (1989), in which all targets were presented at 13° eccentricity, targets in the present task were presented at two different eccentricities (± 10° and 20°) in each direction, therefore requiring subjects to remember not only the direction but the actual spatial locations. Additionally, the trials were not blocked by delay. We chose to randomly vary six delay periods in the experimental sessions based on the results of a control study in which we varied the number of delays (0, 1, 3, 6) within a session maintaining an average length of 3 sec (Figure 1B). Accordingly, this resulted in overall success rates much lower than that of blocked experiments, which enabled room for performance to improve or deteriorate with the administration of methylphenidate. Because subjects’ performance varied from week to week, experimental sessions in which the subjects received methylphenidate were compared with the session performed the previous day.
Drug Delivery and Dosing
Methylphenidate (Sigma-Aldrich, St. Louis, MO) was administered orally, dissolved in 0.5–1 ml grape–cranberry juice 40–45 min before the start of an experimental session. The doses used in this study were chosen based on the work of Doerge, Fogle, Paule, McCullagh, and Bajic (2000), who determined that an acute oral dose of 3 mg/kg methylphenidate, prepared in a similar manner as in the current study, in the monkey resulted in plasma levels similar to those used therapeutically in humans treated for ADHD, with a half-life of 1.7 hr. The doses studied were 1.5, 3, 6, and 9 mg/kg; two sessions of each dose were collected from each of the subjects with the exception of the 9 mg/kg dose, which caused the monkeys to become agitated, accordingly only one session was recorded from each subject. Drug dosing was calculated in mg/kg and based on the animals’ weight the day of the experimental session. Grape and cranberry juices were mixed in an effort to mask the taste of the drug. The drug was administered while the subjects were in the primate chair to ensure that they received the full dose. Vehicle (juice only) was administered in a similar manner on control and nontest days.
Water Control Experiment
To control for the potential effects of methylphenidate on water consumption, two subjects were taken off study and provided with unlimited access to water for 8 hr a day, the length of time spent in the chair during the longest experimental session under 6 mg/kg methylphenidate. The volume of water each animal consumed was recorded every day, and testing began after water consumption stabilized, approximately 2 weeks. The subjects were placed in the primate chair and administered either vehicle or 6 mg/kg methylphenidate, as described above for experimental sessions. They were then returned to their home cage and provided water, and the volume consumed was recorded following 8 hr of ad lib drinking. Four controls and two dosing experiments were performed with each subject.
Dependent Variables and Measurements of Error
A within-subject experimental design was used. The dependent variables were (1) percent correct of the entire session and of memory saccade trials only, used to measure overall performance in the working memory task; (2) length of experimental session, used to measure the subjects’ ability to stay on task; (3) percent of specific error types (premature responses, inaccurate responses, and failure to fixate); and (4) final saccade end position, used to measure the error in remembering the location of the target, thus a measure of actual working memory.
The end of a session was defined as the point after which the subject was closing his eyes for 10–100 trials (varied depending on subject) in a row. Success was determined based on spatial and temporal criteria, as described above. Accordingly, failure could result from three distinct types of errors.
Premature Response
Failure to meet temporal criteria, by responding before the offset of the fixation LED, resulted in a premature response.
Inaccurate Response
Failure to meet the spatial criteria resulted in an inaccurate response. That is, the final gaze position of the eye movement(s) made to the remembered location of the target did not fall into the defined (4°, 4°) window for acceptance around the actual target location.
Failure to Fixate
Failure to fixate or remain fixated on the first LED before target presentation resulted in termination of the trial.
Data Analysis and Statistics
Measures of percent success, session length, percent of error types, and water consumption from experimental sessions in which methylphenidate was administered were averaged across sessions of the same dose and normalized to the average of the controls for those sessions performed the day before each treatment with methylphenidate. The standard error of the sample mean (Table 1) was computed from the normalized average of the two sessions that each subject performed at each dose. Normalization was necessary because of the inherent variability in the subjects’ performance from week to week and to compare the effects of methylphenidate across subjects. Thus, improvements in behavioral performance relative to control resulted in values greater than one, whereas decrements resulted in values less than one. Significance for success, session length, and water consumption for the normalized data of the three subjects at each dose was evaluated using 95% confidence intervals; a value was significant if its confidence interval did not include the control value of one.
Table 1.
Individual Normalized Performance
| Normalized Mean Percent Correct |
Standard Error of Mean |
||||
|---|---|---|---|---|---|
| Subject | Dose MPH (mg/kg) | Entire Session | Working Memory | Entire Session | Working Memory |
| Shepard | 1.5 | 1.1997 | 1.2726 | – | – |
| 3 | 1.3182 | 1.5183 | 0.049 | 0.060 | |
| 6 | 0.9983 | 0.9787 | 0.095 | 0.104 | |
| 9 | 1.0824 | 1.2121 | – | – | |
| Swigert | 1.5 | 0.9756 | 0.9758 | 0.056 | 0.086 |
| 3 | 1.1809 | 1.1890 | 0.012 | 0.006 | |
| 6 | 0.6254 | 0.6313 | 0.165 | 0.150 | |
| 9 | 0.8246 | 0.8347 | – | – | |
| Mitchell | 1.5 | 1.8577 | 1.8256 | 0.287 | 0.201 |
| 3 | 1.2681 | 1.2658 | 0.154 | 0.156 | |
| 6 | 0.5791 | 0.6091 | 0.354 | 0.354 | |
| 9 | 0.3756 | 0.3706 | – | – | |
The effect of methylphenidate on percent correct observed for the entire experimental session, consisting of memory- and visually guided saccades, mirrored the effect of methylphenidate on the memory-guided saccade trials only. The standard errors for the entire session and working memory trials illustrate within-subject variability between the two sessions for each dose of methylphenidate tested. (1) Only one drug treatment session was carried out at the 9 mg/kg dose in all three subjects, and (2) subject Shepard was tested only once with 1.5 mg/kg methylphenidate.
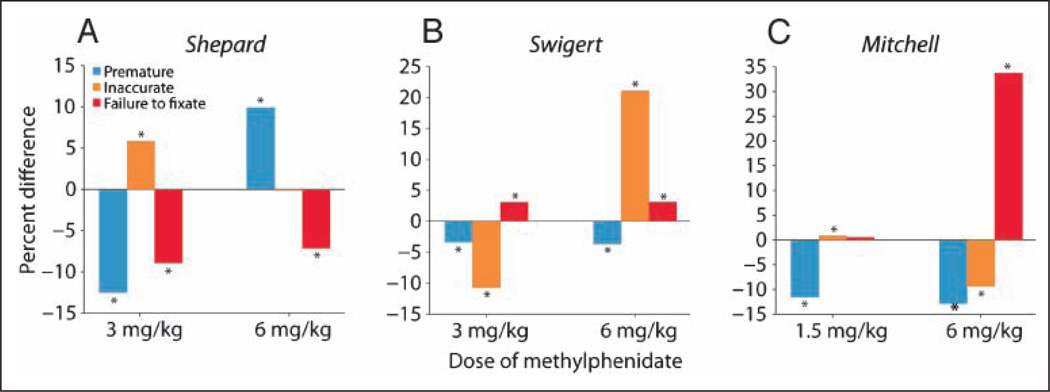
Analysis of error types, as defined above, was performed for the doses that maximally improved and impaired performance for each subject. Each subject’s data were analyzed separately because of differences in the doses of methylphenidate eliciting peak performance, for example, 1.5 and 6 mg/kg methylphenidate for subject Mitchell and 3 and 6 mg/kg for subjects Shepard and Swigert, and to evaluate differences in the effect of methylphenidate between subjects. Because of the small sample size, assumptions about the shape of the distribution could not be made. Thus, a binomial test was used to evaluate significance at p = .05 between the normalized values of each treatment group and control for each error type of each subject. For clarity, the data are presented as percent difference from control (Figure 5).
Figure 5.
Breakdown of error types by subject. Percent difference from control of the three error types for each subject (A–C, see Methods and Results for details). Premature response, blue; inaccurate response, yellow; failure to fixate, red. Significance at p = .05 evaluated using a binomial test, *p < .0005; Shepard, 6 mg/kg inaccurate, p = .15; Mitchell, 1.5 mg/kg failure to fixate, p = .986 (two-tailed).
Changes in accuracy and precision of saccades, the behavioral responses used to measure working memory performance, were analyzed using spherical statistics (Fisher, Lewis, & Embleton, 1987). Accuracy was defined as the closeness of final gaze position to actual target location, and precision as the consistency of final gaze position from trial to trial (Heffner et al., 2005). Angular error, the mean of the unsigned angles between the final gaze position and actual target location, was used to measure accuracy. Precision was measured using 1/kappa, the length of the summed vector of all the individual saccade end positions. Sessions were combined by treatment; angular error and 1/kappa were calculated for each target and averaged. Targets to which the subjects did not orient under control conditions were excluded from analysis.
RESULTS
Dose-dependent Dissociation of Performance and Session Length
The administration of methylphenidate produced significant changes in the behavior of all three subjects (Figure 2). The average percent correct of the entire experimental session, normalized to control, is plotted as a function of methylphenidate dose (Figure 2, filled symbols, dashed line); the controls were computed separately for each methylphenidate dose from the average percent correct of sessions performed the day before each methylphenidate treatment. Tests with methylphenidate were conducted in the middle of the workweek, after water consumption had stabilized following ad lib consumption during weekends. A total of 68,001 trials were collected from the three subjects in control and treatment sessions; 30,717 of those trials were memory-guided saccades.
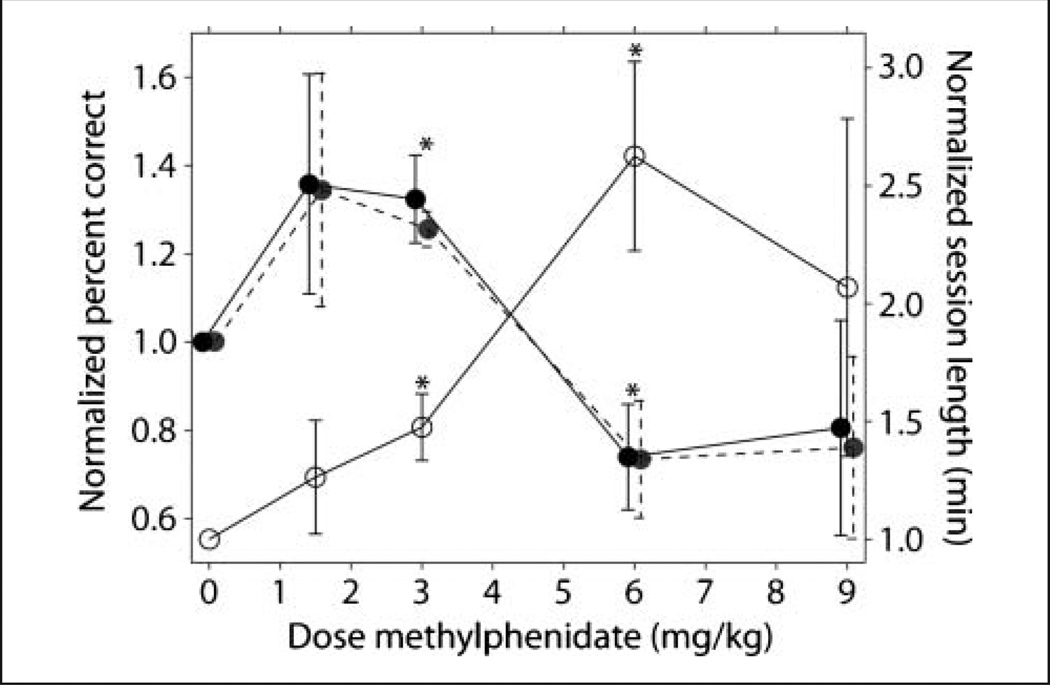
Figure 2.
Dissociated dose–response curves for performance and session length. Normalized percent correct and normalized session length for an experimental session plotted as a function of dose of methylphenidate. Methylphenidate significantly improved percent correct at 3 mg/kg and significantly impaired performance at 6 mg/kg across subjects. Concurrently, methylphenidate significantly increased the length of the experimental session at 6 mg/kg. Filled symbols with solid lines represent normalized percent correct in the working memory task only; filled symbols with dashed lines represent normalized percent correct of the entire experimental session. Open symbols with solid lines represent normalized session length (min). Data from three subjects are averaged, and error bars represent SEM. Significance evaluated using 95% confidence intervals, *p < .05 (two-tailed).
Consistent with the findings of Sprague and Sleator (1977), the effects of methylphenidate were observed in the most cognitively demanding task—thememory-guided saccade (Figure 2, filled symbols, solid line). Experimental sessions consisted of visually guided and memory-guided saccades. A significant improvement in working memory performance was measured at 3 mg/kg methylphenidate. The 1.5 mg/kg dose also improved performance, although the effect did not reach significance because large variability resulted from improvement in only one subject (Table 1).
In contrast, the 6 mg/kg dose of methylphenidate significantly impaired working memory performance. The highest dose of methylphenidate tested (9 mg/kg) resulted in more variable results, although the mean was similar to 6 mg/kg. This dose of methylphenidate caused the monkeys to become agitated, accordingly only one session was recorded from each subject. Therefore, consistent with previous observations (Gamo et al., 2010; Berridge et al., 2006; Arnsten & Dudley, 2005; Sprague & Sleator, 1977), methylphenidate affected overall percent success in an inverted-U manner across all three subjects.
In addition to changes in the percent correct in the working memory task, we also observed changes in the duration of the experimental session, an aspect of behavior that suggests changes in the subjects’ ability to remain on task (Figure 2, open symbols). Typically, the length of the session is determined by factors such as the size of the rewards, task difficulty, and the subject’s thirst. When the subject has reached a certain level of satiation, he may shake the chair or close his eyes signaling the end of the experiment. The 6 mg/kg dose of methylphenidate resulted in a significant 2.5-fold increase in session length (Figure 2, open symbols) compared with control sessions performed with the same reward configuration despite significantly impairing performance of the working memory task (Figure 2, filled symbols). This change in behavior under 6 mg/kg methylphenidate was out of the ordinary given that the subjects worked at a significantly lower rate. It must be noted that subjects did not simply work longer to compensate for the lower rate of reward, because at this dose they earned on average twice as much water as in the control (Figure 7A). The session length was also increased at 3 mg/kg, but to a smaller extent. These effects are illustrated in Figure 3, which shows cumulative success functions from the control, 3 mg/kg, and 6 mg/kg sessions from subject Shepard; similar functions were obtained from the other two subjects. The effect on task performance, as indicated by the slope of the functions, was consistent throughout the length of the sessions. Thus, the time subjects participated in an experiment was also affected by methylphenidate in a dose-dependent manner.
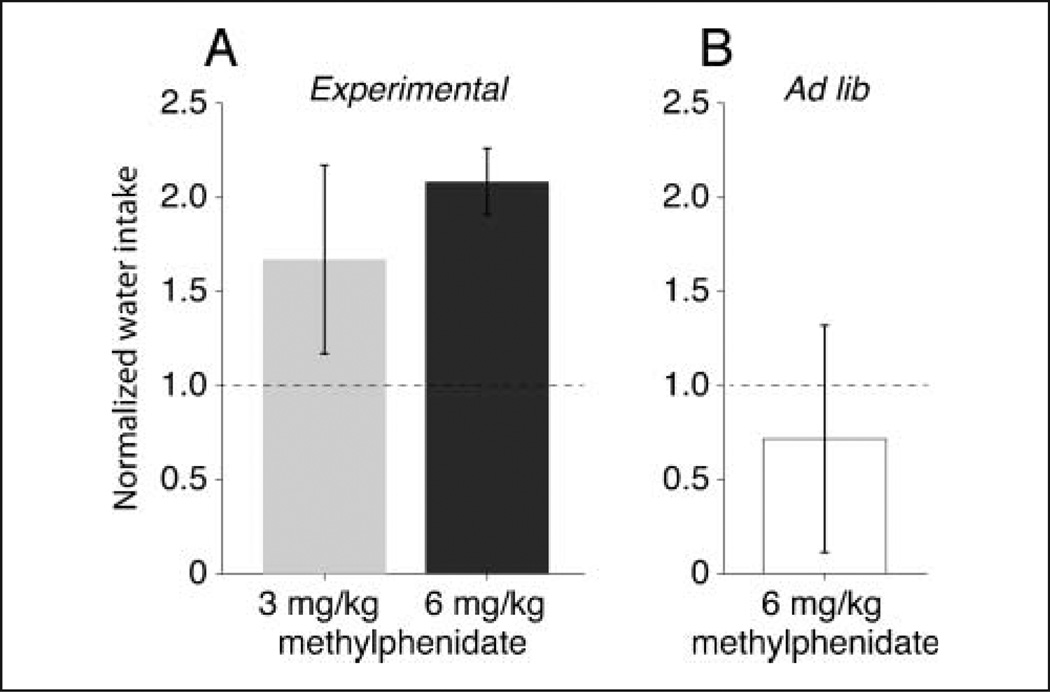
Figure 7.
Water control experiment. (A) Normalized volume of water consumed under 3 and 6 mg/kg methylphenidate during experimental sessions. (B) Normalized volume of water consumed while in home cage with ad lib access to water for 8 hr. Subjects given 6 mg/kg methylphenidate and provided with water in their home cages did not drink significantly more than the home cage control. The broken horizontal lines represent the control from sessions in which only vehicle was administered orally before experimental sessions (A) and before control sessions (B). Error bars represent 95% confidence intervals, *p < .05 (two-tailed).
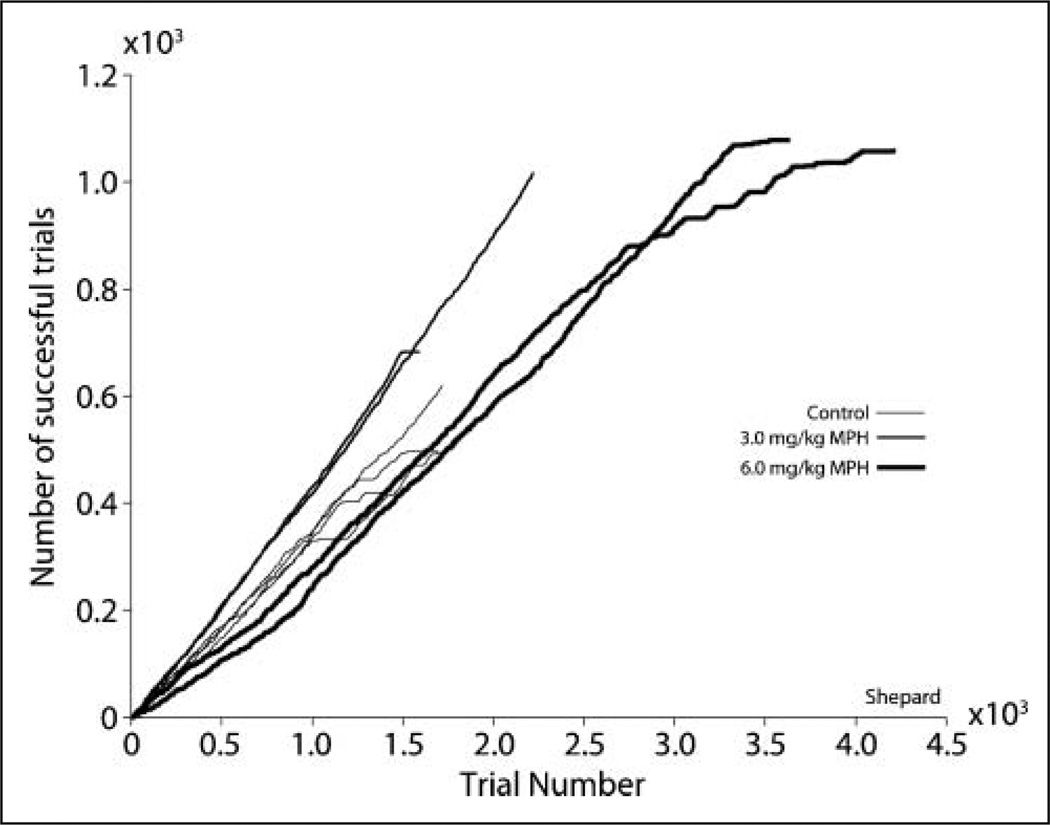
Figure 3.
Cumulative success functions illustrating the effect of methylphenidate on performance over the time course of the session for subject Shepard. The two 3 mg/kg and the 6 mg/kg methylphenidate sessions and their corresponding controls are plotted from one representative subject. Task performance improved at 3 mg/kg methylphenidate and deteriorated at 6 mg/kg methylphenidate, whereas the length of the experimental session was significantly increased.
Consistent with our hypothesis and the results of Sprague and Sleator (1977), the data show that distinct behavioral/cognitive functions are dissociated by different doses of methylphenidate. Although the ability to remain on task, measured by session duration, improved, cognitive function, measured by performance in the working memory task, was impaired (Figure 2; note the different scales).
Specific Effects of Methylphenidate on the Working Memory Task
Completion of the working memory task required (1) acquiring the fixation LED, which led to the presentation of the target; (2) maintaining fixation on the LED while it remained illuminated; and (3) upon offset of the fixation LED, making a saccadic eye movement to the remembered location of the target within 700 msec and with a final gaze position falling within a (4°, 4°) acceptance window. The specific effects of methylphenidate on task performance were analyzed in the context of these three requirements, and the data from each subject are presented separately because each responded differently to methylphenidate (Table 1); only data from the doses in which performance was significantly improved (1.5 mg/kg for Mitchell and 3 mg/kg for Shepard and Swigert; Table 1) and impaired (6 mg/kg for all monkeys) were included.
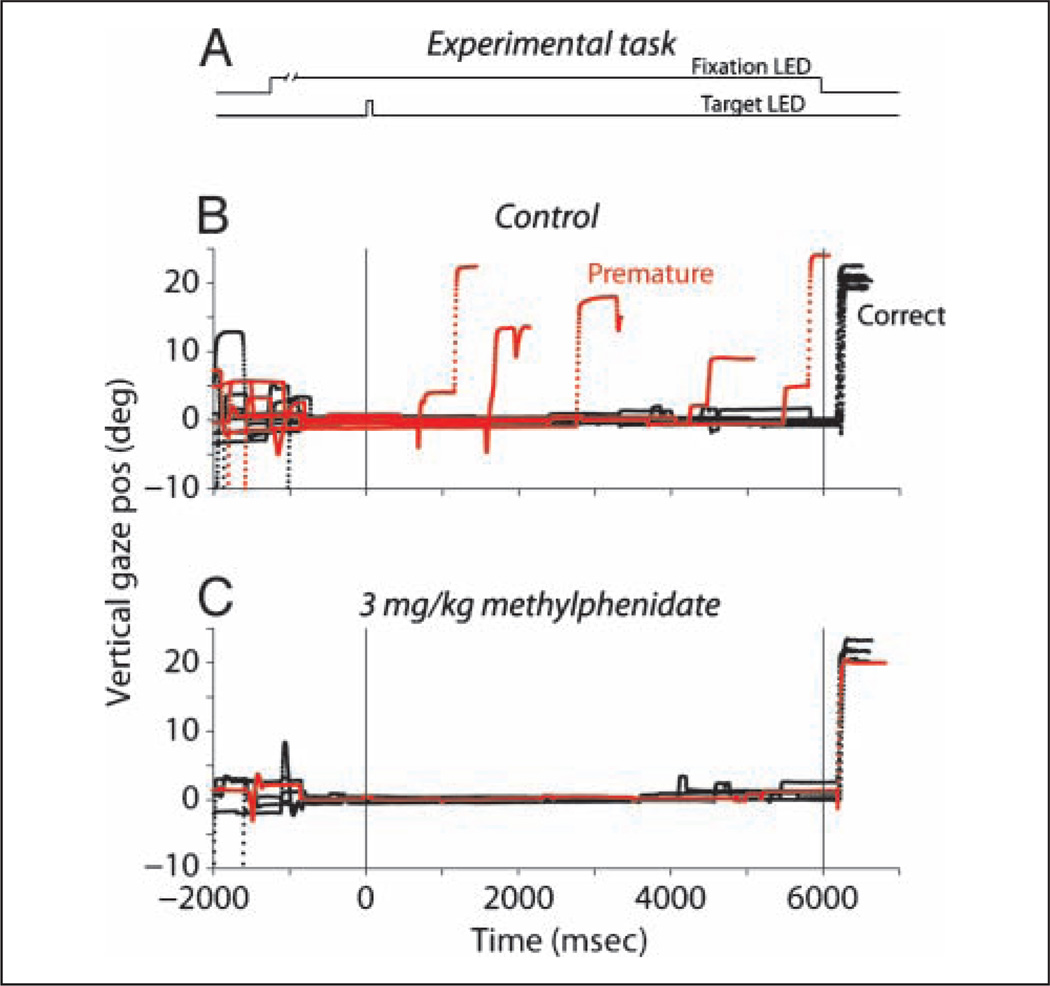
The configuration of the experimental sessions, which included a random mixture of targets at different eccentricities and delays, induced the monkeys to generate large numbers of premature responses. An example of this type of response under controlled conditions is illustrated in Figure 4B. Note the large number of premature responses plotted in red. In stark contrast to the control, the administration of 3 mg/kg methylphenidate significantly reduced premature responses and, for some targets, completely eliminated them (Figure 4C).
Figure 4.
Eye movement traces illustrating the effect of methylphenidate on premature responses in the memory saccade task with 6-sec delay. Vertical components of eye movements to a target presented at (0°, 20°) 300–600 msec after the subject acquired the fixation LED from a control and a 3 mg/kg methylphenidate session. (A) Schematic diagram of the memory saccade task. (B) Eye movements from the control session. Successful trials are plotted in black, and trials in which the subject responded prematurely, thus violating the temporal criterion, are plotted in red. (C) Eye movements from the 3 mg/kg methylphenidate session. Premature responses were eliminated. The single red trace represents an error due to an inaccurate response as the final gaze position landed outside the acceptance window for success.
The data in Figure 5A show, as the average percent difference from control, that the performance of subject Shepard in the working memory task improved at 3 mg/kg methylphenidate primarily as a result of a decrease in premature responses and that it also improved because of a reduction in the number of trials in which he failed to acquire the fixation LED at the start of the trial, defined as failure to fixate errors. There was, however, an increase in the number of inaccurate responses, trials that did not fall into the acceptance window set about the target. Conversely, at the 6 mg/kg dose of methylphenidate, subject Shepard made significantly more premature responses and, at 3 mg/kg, significantly fewer failure to fixate errors (Figure 5A). Note also that there was no change in the number of inaccurate responses at this dose. These data indicate, therefore, that methylphenidate affected primarily this subject’s ability to inhibit inappropriate responses by making him either more or less likely to respond prematurely to the target during the delay period.
Subject Swigert was affected by 3mg/kg methylphenidate differently than subject Shepard in two of the three measures (Figure 5B). Like subject Shepard, he showed a significant decrease in premature responses at 3 mg/kg methylphenidate, but unlike Shepard, his percent of inaccurate responses was significantly decreased at this performance-improving dose. The combination of these effects resulted in an overall increase in performance of the task. At 6 mg/kg methylphenidate, however, his performance was significantly impaired (Table 1) primarily because of an increase in inaccurate responses. Interestingly, the reduction in premature responses and the increase in failure to fixate errors was very similar at the 6 and 3 mg/kg methylphenidate doses.
A reduction in premature responses was also documented in subject Mitchell’s performance at the lower, performance-improving 1.5 mg/kg dose of methylphenidate (Figure 5C). This change accounted for the increase in success overall. At 6 mg/kg methylphenidate, subject Mitchell’s performance impairment was the result of a significant increase in failure to fixate errors (Figure 5C). It should be noted that he did not simply close his eyes during these trials, as that would have resulted in the termination of the experimental session. He continuously made eye movements but was unable to maintain fixation for a long enough period for the target to be presented and, consistent with the other subjects, Mitchell remained engaged in the experiment for a significantly longer period without closing his eyes or shaking the chair (Figure 2, open symbols).
Thus, the data show that although methylphenidate affected performance in the working memory task consistently across subjects, it did so by affecting error types in a subject-specific manner. It must be noted, however, that common to all subjects was a reduction in premature responses at the performance-improving lower dose.
Does Methylphenidate Improve Working Memory?
As indicated at the outset, working memory tasks have been used extensively to study behavioral/cognitive effects of methylphenidate. Interestingly, a lingering question concerns the extent to which the drug improves the actual memory as opposed to other aspects of cognitive function that could result in improved performance in working memory tasks. This question can be answered neither in the context of the traditional delay tasks used with nonhuman primates, nor in the context of t-maze tasks used with rodents because they only require a left versus right response. The accuracy and precision of final eye movement position, on the other hand, constitutes a more suitable measure of the effects of methylphenidate on the subjects’ ability to remember the actual spatial location of the target. Accordingly, we computed spherical measures of accuracy (angular error) and precision (1/kappa) for each subject (Fisher et al., 1987). Only trials in which the subjects met temporal criteria and made a response to the target, regardless of whether or not the saccadic eye movement landed within the acceptance window, were included in the analysis. Targets to which subjects did not routinely respond under control conditions were also excluded from the analysis.
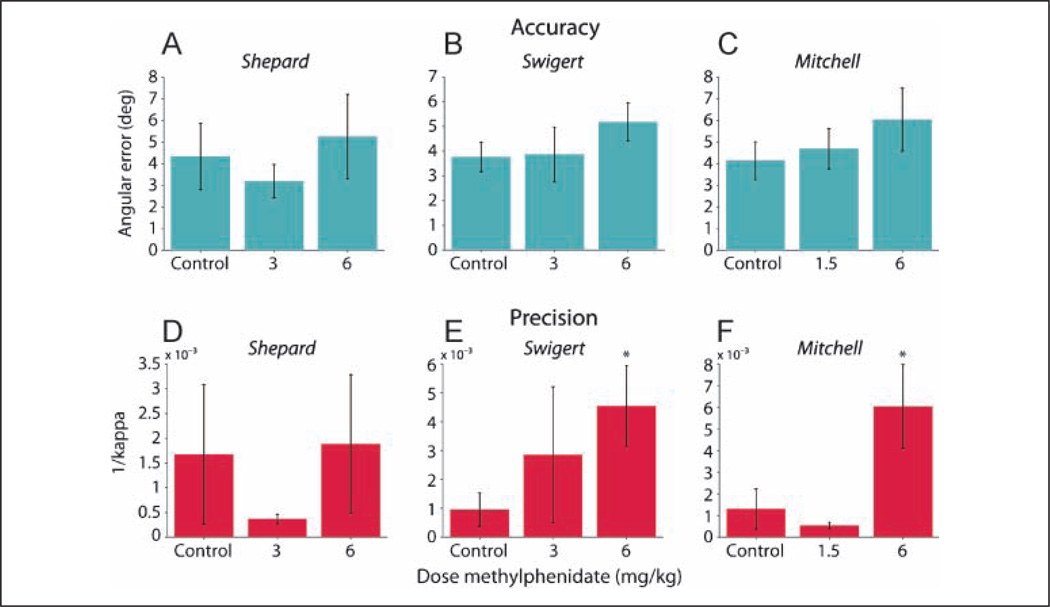
For all three subjects, there was no significant change in the accuracy of saccadic eye movements to remembered targets under either drug condition (Figure 6A–C), although there was a trend of increased angular error for all three subjects at the 6 mg/kg dose of methylphenidate. For subject Shepard, there was also a trend toward a decrease in angular error at 3 mg/kg methylphenidate (Figure 6A), but it was not significant.
Figure 6.
Accuracy and precision of final gaze position for each subject. Accuracy was measured using angular error (A–C) and precision using 1/kappa (D–F; see Methods and Results for details). Note that no improvement in working memory was recorded. Error bars represent 95% confidence intervals, *p < .05 (two-tailed).
Although there were no changes in the accuracy of the eyemovements to the remembered targets, their precision was affected by methylphenidate (Figure 6D–F). Again, each individual was affected differently. Subjects Shepard and Mitchell showed a trend, although not significant, of improvement in precision at the performance-improving dose of methylphenidate (3 and 1.5 mg/kg, respectively; Figure 6D and F). Subject Swigert, on the other hand, had no significant change in precision at 3 mg/kg methylphenidate (Figure 6E). Conversely, the precision of eye movements by subjects Swigert and Mitchell at 6 mg/kg methylphenidate was significantly decreased (Figure 6E and F), whereas subject Shepard showed no change (Figure 6D).
Thus, the data show that, at the lower, performance-improving doses of methylphenidate there was no significant improvement in accuracy or precision of remembered target location. At 6 mg/kg methylphenidate, there was no change in accuracy, but precision was impaired in two of the three subjects (Figure 6E and F). Therefore, methylphenidate did not improve working memory.
Water Control Experiment: Did Methylphenidate Increase Thirst?
The significant increase in the duration of the experimental sessions documented under 6 mg/kg methylphenidate (Figure 2, open symbols) resulted from an increase in the number of trials performed relative to control. Despite the lower rate of success (Figure 2, filled symbols), there was a significant twofold increase in the amount of water consumed relative to control (Figure 7A), which could have resulted from increased thirst caused by the administration of methylphenidate. Note that under 3 mg/kg methylphenidate water consumption also increased significantly (Figure 7A), but this increase resulted primarily from a higher rate of success (Figure 2, filled symbols).
To resolve this issue, two animals were taken off study and provided with ad lib water for 8 hr/day, the total time spent in the laboratory during the longest experimental session recorded under 6 mg/kg methylphenidate, until consumption was stabilized (approximately 2 weeks). This was necessary to obtain an uncontaminated measure of the effect on methylphenidate on water consumption because, in our experience, animals whose access to water is limited to daily experimental sessions during the workweek and are operantly conditioned to perform for water rewards drink excessively if water is made available without behavioral requirements. It must be noted that in the paradigm used in this study no limits were placed on the number of trials an animal could perform in an experimental session. After water consumption stabilized, four control and two 6 mg/kg methylphenidate testing sessions were carried out with each animal.
In contrast to the amount of water consumed during experimental sessions, the administration of 6 mg/kg methylphenidate in the animal quarters while drinking water at lib did not affect water consumption relative to the ad lib control. Accordingly, the significantly longer sessions recorded under 6 mg/kg methylphenidate did not result from increased thirst but, rather, could have resulted from a change in the animals’ ability to remain on task or from changes in the processing of rewards/failures.
DISCUSSION
This study shows that methylphenidate affects different cognitive and behavioral functions differently in a dose-dependent inverted-U manner, a pattern that closely resembles the dose–response profiles published by Sprague and Sleator (1977). Low doses of methylphenidate improved performance in the working memory task, whereas a higher dose significantly hindered it and, at the same time, significantly increased the time subjects remained on task despite their impaired performance. Interestingly, the improvement in working memory performance resulted from a significant reduction in the number of premature responses, not from improvement of working memory itself.
The data in Figure 2 resemble those of Sprague and Sleator (1977) from children with ADHD who found that cognitive performance peaked at a lower dose of methylphenidate than social behavior in the classroom. Most notably, in both studies, performance in social behavior (Sprague & Sleator, 1977) and the ability to remain on task (present study) peaked at the dose of methylphenidate that significantly hindered performance in the working memory task. Dissociated effects produced by methylphenidate were also obtained from a chimpanzee in a visual illusion task in which psychophysical judgments and response latency were affected differentially (Sprague & Sleator, 1975; Reynolds et al., 1968) and, importantly, with similar response profiles to those obtained in this study.
Direct comparison of what effects were dissociated in Sprague and Sleator’s (1977) and this study is difficult because, in addition to species differences, the experimental conditions and tasks were quite distinct and, therefore, must have imposed different cognitive/behavioral demands. For instance, in this study, subjects were conditioned, whereas in Sprague and Sleator (1977), subjects participated voluntarily. Despite these differences, there are similarities in the results indicating that different behavioral/cognitive functions are affected differentially by methylphenidate. These results may have implications for the dosing of patients with ADHD considering the distinct attentional/cognitive and hyperactive/behavioral symptoms of different subtypes of the disease (Solanto, 2002) and for healthy individuals taking methylphenidate without prescription to improve cognitive performance (Greely et al., 2008).
Participation in experimental sessions and the execution of complex tasks, such as the memory-guided saccade, require the contribution of various cognitive/behavioral functions, which can be difficult to differentiate (Wise, 2008). These functions are thought to be modulated by different neurotransmitter systems, for example, DA and NE, and their receptor subtypes. Accordingly, this dissociation of effects by methylphenidate could be explained by the different affinities of DA and NE receptors, which could be activated distinctly by increased levels of catecholamines that result from methylphenidate blocking the DA transporter or by methylphenidate acting in different brain regions (Solanto, 2002). Improvement in working memory performance has been hypothesized to result from optimal activation of D1 DA and α2 NE receptors (Arnsten, 2009; Arnsten & Dudley, 2005). Behavioral improvements at higher doses of methylphenidate likely result from either actions on lower affinity receptor subtypes such as α1 NE or actions in other brain regions given that, as in clinical use, the drug was administered systemically.
The significant improvement in working memory task performance came from the inhibition of premature responses, not from improved memory. It must be noted that premature responses under control conditions did not result from lack of behavioral training, as one might conclude from the performance of monkeys in other studies that used a similar task. Funahashi et al.’s (1989) subjects, for instance, performed at 90% correct or better, but the task included one target eccentricity (13°) and importantly the delays were blocked. The present experimental task imposed more stringent spatial memory requirements with targets presented at two eccentricities (Figure 1A). It also imposed a greater demand on attention/response inhibition because subjects had to attend to the fixation LED to detect its offset, the signal to respond, which was varied randomly (1–6 sec). Thus, the subjects of this study could not simply execute highly practiced saccadic eye movements of fixed amplitude in the remembered angular direction of the target, nor could they anticipate the time of fixation offset.
This effect of low doses of methylphenidate on premature responses is novel in the context of a delayed response task designed to measure working memory and could not have been observed in previous studies because the subjects are physically prevented from responding during the delay period; in the delayed alternation task the animal is held during the delay period (e.g., Berridge et al., 2006; Arnsten & Dudley, 2005), and in the delayed response task a barrier is placed between the animal and the wells (e.g., Gamo et al., 2010). The present results are in accordance with those obtained using tasks specifically designed to test for impulsivity such as the go/no-go task (Broyd et al., 2005; Trommer, Hoeppner, & Zecker, 1991).
The reduction in the number of premature responses resembles the reduction in impulsivity documented in individuals with ADHD who are treated with methylphenidate (Solanto, 1998, 2002; Greenhill, 2001). Relatively little is known about the physiological and pharmacological basis of this effect. It is possible that it could have resulted from the strengthening of inhibitory networks that prevent the untimely execution of programmed responses or from the strengthening of networks underlying the maintenance of attention necessary for the execution of the working memory task (Postle, 2006). Alternatively, and assuming that premature responses could have been prompted by a deterioration of the representation of the target, methylphenidate could have strengthened the memory of the target location (Arnsten, 2009). If this were the case, however, an improvement in the remembered location of the target should have been observed.
We addressed this issue by comparing the accuracy and precision of the final position of saccadic eye movements directed to the remembered location of the targets in trials in which the temporal criteria for success were fulfilled. Detailed measurements of remembered spatial location are neither available from studies that used the delayed alternation task nor from studies that used the traditional delayed response task because, in both instances, all response options are visible to the animal who simply must make a choice among them. In the memory-guided saccade task, on the other hand, the target is presented briefly and is not visible at the time of the response, and most importantly, the subject can respond with saccadic eye movements of infinite amplitudes and directions. The accuracy and precision of this type of memory-guided response, accordingly, allows for a measure of the effects of methylphenidate on the actual memory, that is, exactly where does the subject remember the location of the target in relation to where it was presented.
As illustrated in Figure 6, the effect of methylphenidate on working memory did not account for the improvement observed in the performance of the memory-guided saccade task. These results are consistent, therefore, with the notion that improvements in working memory task performance result from effects on other cognitive functions such as inhibition of inappropriate responses or attention. In addition, comparison of the error type profiles obtained from the three subjects reveals that, although the overall effect of methylphenidate on performance was consistent, each subject was affected differently.
Albeit consistent with the hypothesis that methylphenidate affects various cognitive/behavioral functions differently and dose-dependently, the magnitude of the increase in session length brought about by the 6 mg/kg dose of methylphenidate was unexpected and clearly shown not to be the result of an increase in thirst. Furthermore, the effect over this period can be attributed to the pharmacological action of methylphenidate given that it takes four to five half-lives for a drug to be completely eliminated from the system (Rowland & Tozer, 2010). Therefore, the increase in the length of time the subjects participated in the experimental session reflected a change in their ability to stay on task, which could result from improvement in sustained attention or changes in the processing of reward value (Rajala & Populin, 2011). Despite the differences in species and methodology, we consider these effects analogous to those reported by Sprague and Sleator (1977) for the larger dose of methylphenidate. The subjects in this study extended their participation by about 2.5 times in response to the larger dose of methylphenidate despite performing at a lower level of success and thus receiving rewards at a reduced rate. Similar to the effect of methylphenidate on premature responses, the effect on the ability to remain on task could not have been observed in previous studies because experimental sessions consisted of a preset number of trials (e.g., Gamo et al., 2010; Berridge et al., 2006; Arnsten & Dudley, 2005), whereas in this study the length of the experimental sessions was determined by the subjects’ participation.
In summary, the most relevant findings are the lack of improvement in the actual memory of target location and the dose-dependent dissociation of different effects that result from the administration of methylphenidate, as first reported by Sprague and Sleator (1977); their potential clinical implications cannot be overstated. Dosing based only on evaluation of social behavior could have detrimental consequences on cognitive function and instead should be based on an individual’s specific symptoms and sensitivity to the drug. Furthermore, the present results show that methylphenidate is an effective tool for the basic study of cognitive function because it can distinctly affect neural processes that are inherently difficult to uncouple.
Acknowledgments
This work was supported by grants from the Wisconsin Institutes for Discovery, the National Science Foundation (IOB- 0517458), and the National Institutes of Health (DC003693). We thank Katharine Reininger and Kimberly Lancaster for help with animal care and data collection, Yonghe Yan and Jane Sekulski for computer programming; Brad Postle, John Harting, and Craig Berridge for comments on an earlier version of this manuscript; and Craig Berridge for suggestions on dosing at the start of the project.
REFERENCES
- Arnsten AF. Stress signaling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Dudley AG. Methylphenidate improves prefrontal cortical cognitive function through alpha2 adrenoceptor and dopamine D1 receptor actions: Relevance to therapeutic effects in attention deficit hyperactivity disorder. Behavioral and Brain Functions. 2005;1:2. doi: 10.1186/1744-9081-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AFT, Kelley AE, Schmeichel B, et al. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biological Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Broyd SJ, Johnstone SJ, Barry RJ, Clarke AR, McCarthy R, Selikowitz M, et al. The effect of methylphenidate on response inhibition and the event-related potential of children with attention deficit/hyperactivity disorder. International Journal of Psychophysiology. 2005;58:47–58. doi: 10.1016/j.ijpsycho.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Clatworthy PL, Lewis SJG, Brichard L, Hong YT, Izquierdo D, Clark L, et al. Dopamine release in dissociable striatal subregions predicts the different effects of oral methylphenidate on reversal learning and spatial working memory. Journal of Neuroscience. 2009;29:4690–4696. doi: 10.1523/JNEUROSCI.3266-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK. A teacher rating scale for use in drug studies with children. American Journal of Psychiatry. 1969;126:884–888. doi: 10.1176/ajp.126.6.884. [DOI] [PubMed] [Google Scholar]
- Doerge DR, Fogle CM, Paule MG, McCullagh M, Bajic S. Analysis of methylphenidate and its metabolite ritalinic acid in monkey plasma by liquid chromatography/ electrospray ionization mass spectrometry. Rapid Communications in Mass Spectrometry. 2000;14:619–623. doi: 10.1002/(SICI)1097-0231(20000430)14:8<619::AID-RCM916>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Dyme IZ, Sahakian BJ, Golinko BE, Rabe EF. Perseveration induced by methylphenidate in children: Preliminary findings. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1982;6:269–273. doi: 10.1016/s0278-5846(82)80177-2. [DOI] [PubMed] [Google Scholar]
- Fisher NI, Lewis T, Embleton EJ. Statistical analysis of spherical data. Cambridge: Cambridge University Press; 1987. [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. Journal of Neurophysiology. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Gamo NJ, Wang M, Arnsten AF. Methylphenidate and atomoxetine enhance prefrontal function through α2-adrenergic and dopamine D1 receptors. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49:1011–1023. doi: 10.1016/j.jaac.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaytan O, Ghelani D, Martin S, Swann A, Dafny N. Methylphenidate: Diurnal effects on locomotor and stereotypic behavior in the rat. Brain Research. 1997;777:1–12. doi: 10.1016/s0006-8993(97)00880-9. [DOI] [PubMed] [Google Scholar]
- Greely H, Sahakian B, Harris J, Kessler RC, Gazzaniga M, Campbell P, et al. Towards responsible use of cognitive-enhancing drugs by the healthy. Nature. 2008;456:702–705. doi: 10.1038/456702a. [DOI] [PubMed] [Google Scholar]
- Greenhill LL. Stimulant drugs and ADHD: Basic and clinical neuroscience. Oxford, UK: Oxford University Press; 2001. Clinical effects of stimulant medication in ADHD; pp. 31–71. [Google Scholar]
- Heffner HE, Heffner RS, Tollin DJ, Populin LC, Moore JM, Ruhland JL, et al. The sound-localization ability of cats. Journal of Neurophysiology. 2005;94:3653–3655. doi: 10.1152/jn.00720.2005. [DOI] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: An improved method. Vision Research. 1980;20:535–537. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Sahakian BJ, Robbins TW. Stimulant drugs and ADHD: Basic and clinical neuroscience. Oxford: Oxford University Press; 2001. Comparative psychopharmacology of methylphenidate and related drugs in human volunteers, patients with ADHD, and experimental animals; pp. 303–331. [Google Scholar]
- Populin LC, Yin TCT. Behavioral studies of sound localization in the cat. Journal of Neuroscience. 1998;18:2147–2160. doi: 10.1523/JNEUROSCI.18-06-02147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR. Working memory as an emergent property of the mind and brain. Neuroscience. 2006;139:23–38. doi: 10.1016/j.neuroscience.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala AZ, Populin LC. Program No. 272.02. Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2011. Methylphenidate-induced changes in PFC activity are correlated with altered tasks-witching performance. On-line. [Google Scholar]
- Rapoport JL, Buchsbaum MS, Weingartner H, Zahn TP, Ludlow C, Mikkelsen EJ. Dextroamphetamine: Cognitive and behavioral effects in normal and hyperactive boys and normal men. Archives of General Psychiatry. 1980;37:933–943. doi: 10.1001/archpsyc.1980.01780210091010. [DOI] [PubMed] [Google Scholar]
- Reynolds HH, Salzberg CL, Barker LM. Effect of methylphenidate hydrochloride (ritalin) on psychophysical judgments by chimpanzees. Perceptual & Motor Skills. 1968;27:927–933. doi: 10.2466/pms.1968.27.3.927. [DOI] [PubMed] [Google Scholar]
- Robinson DA. A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Transactions on Biomedical Engineering. 1963;10:137–145. doi: 10.1109/tbmel.1963.4322822. [DOI] [PubMed] [Google Scholar]
- Rowland M, Tozer TN. Clinical pharmacokinetics and pharmacodynamics: Concepts and applications. 4th ed. Baltimore, MD: Williams and Wilkins; 2010. [Google Scholar]
- Solanto M. Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: A review and integration. Behavioural Brain Research. 1998;94:127–152. doi: 10.1016/s0166-4328(97)00175-7. [DOI] [PubMed] [Google Scholar]
- Solanto M. Dopamine dsyfunction in AD/HD: Integrating clinical and basic neuroscience research. Behavioural Brain Research. 2002;130:65–71. doi: 10.1016/s0166-4328(01)00431-4. [DOI] [PubMed] [Google Scholar]
- Sprague RL, Sleator EK. What is the proper dose of stimulant drugs in children? International Journal of Mental Health. 1975;4:75–104. [Google Scholar]
- Sprague RL, Sleator EK. Methylphenidate in hyperkinetic children: Differences in dose effects on learning and social behavior. Science. 1977;198:1274–1276. doi: 10.1126/science.337493. [DOI] [PubMed] [Google Scholar]
- Sproson EJ, Chantrey J, Hollis C, Marsden MA, Fone KCF. Effect of repeated methylphenidate administration on presynaptic dopamine and behaviour in young adult rats. Journal of Psychopharmacology. 2001;15:67–75. doi: 10.1177/026988110101500202. [DOI] [PubMed] [Google Scholar]
- Tannock R, Schachar RJ, Carr RP, Logan GD. Dose-response effects of methylphenidate on academic performance and overt behavior in hyperactive children. Pediatrics. 1989;84:648–657. [PubMed] [Google Scholar]
- Tannock R, Schachar RJ, Logan GD. Methylphenidate and cognitive flexibility: Dissociated dose effects in hyperactive children. Journal of Abnormal Child Psychology. 1995;23:235–266. doi: 10.1007/BF01447091. [DOI] [PubMed] [Google Scholar]
- Trommer BL, Hoeppner JA, Zecker SG. The go-no go test in attention deficit disorder is sensitive to methylphenidate. Journal of Child Neurology. 1991;6(Suppl.):S128–S131. doi: 10.1177/0883073891006001s13. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G, Fowler JS, Logan J, Gerasimov M, Maynard L, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. Journal of Neuroscience. 2001;21:1–5. doi: 10.1523/JNEUROSCI.21-02-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise S. Forward frontal fields: Phylogeny and fundamental function. Trends in Neuroscience. 2008;31:599–608. doi: 10.1016/j.tins.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]