Abstract
Specification of the dorsoventral (DV) axis is critical for the subsequent differentiation of regional fate in the primary germ layers of the vertebrate embryo. We have identified a novel factor that is essential for dorsal development in embryos of the frog Xenopus laevis. Misexpression of Xenopus mab21-like 3 (Xmab21l3) dorsalizes gastrula-stage mesoderm and neurula-stage ectoderm, while morpholino-mediated knockdown of Xmab21l3 inhibits dorsal differentiation of these embryonic germ layers. Xmab21l3 is a member of a chordate-specific subclass of a recently characterized gene family, all members of which contain a conserved, but as yet ill-defined, Mab21 domain. Our studies suggest that Xmab21l3 functions to repress ventralizing activity in the early vertebrate embryo, via BMP/Smad and Ras/ERK signaling.
Keywords: Xmab21l3, Xema, neural induction, mesoderm, Xenopus
1. Introduction
The establishment of the vertebrate body plan is dependent upon the accurate dorsoventral regionalization of the embryonic germ layers. Formation of the dorsal axis is mediated by nuclear accumulation of β-catenin triggered by, and located opposite to, the site of sperm entry (Heasman, 2006). Germ layer specification, in turn, is regulated by Nodal-class Transforming Growth Factor-β (TGFβ) signaling—high levels stimulate endoderm development in the vegetal pole, moderate levels induce mesoderm in the equatorial “marginal zone,” and the absence of Nodal signaling allows for ectodermal differentiation in the animal pole (Heasman, 2006). The colocalization of dorsal and mesendodermal signals lead to the formation of the Spemann Organizer, a source of secreted antagonists that function to inhibit receptor-mediated induction of ventral fates. Bone Morphogenetic Protein (BMP)-2, 4, and 7, also members of the TGFβ ligand superfamily and widely expressed in the gastrula ectoderm and mesoderm, are the primary ventralizing signals in the vertebrate embryo; several structurally unrelated factors expressed in Spemann’s organizer, including Chordin, Follistatin, and Noggin, function as BMP pathway inhibitors (Weinstein and Hemmati-Brivanlou, 1999). Establishment of a BMP-depleted zone promotes the formation of dorsal fates, including notochord and the differentiated neurons of the primary nervous system in the mesoderm and ectoderm, respectively; ventrally, BMP receptor activation promotes Smad1/5 activation, heterodimerization with Smad4, nuclear localization, and subsequent transcription of target genes mediating ventral fates, including blood (mesoderm) and epidermis (ectoderm)(Heasman, 2006).
We report here the identification of a novel factor, Xenopus mab21-like 3 (Xmab21l3), which is both sufficient and necessary for the development of dorsal ectodermal and mesodermal fates in Xenopus laevis. Misexpression of Xmab21l3 induces dorsal axis duplication, promotes dorsal mesoderm formation in response to the TGFβ ligand Activin, and neuralizes isolated ectodermal (animal cap) explants; Xmab21l3 knockdown inhibits dorsal mesoderm formation. Our studies suggest that Xmab21l3 regulates dorsal fate via the Ras/ERK and BMP/Smad pathways, and provide important insights into the regulation of the signaling cascades that mediate dorsoventral patterning in the early vertebrate embryo.
2. Results
2.1. Xmab21l3, a novel Mab-21 protein
Previous studies in our lab and by others characterized Xema/Foxi1e, a Foxi class transcription factor involved in ectopic mesendoderm suppression and ectodermal patterning in the early Xenopus embryos (Mir et al., 2007; Suri et al., 2005). To identify potential Xema targets, we used gene chip studies to isolate a transcript (Tx1, for Target of Xema1), that was both upregulated in Xema RNA- injected and downregulated in Xema morpholino-injected samples; this regulation was confirmed by RT-PCR (Figs. 1A, B, and data not shown).
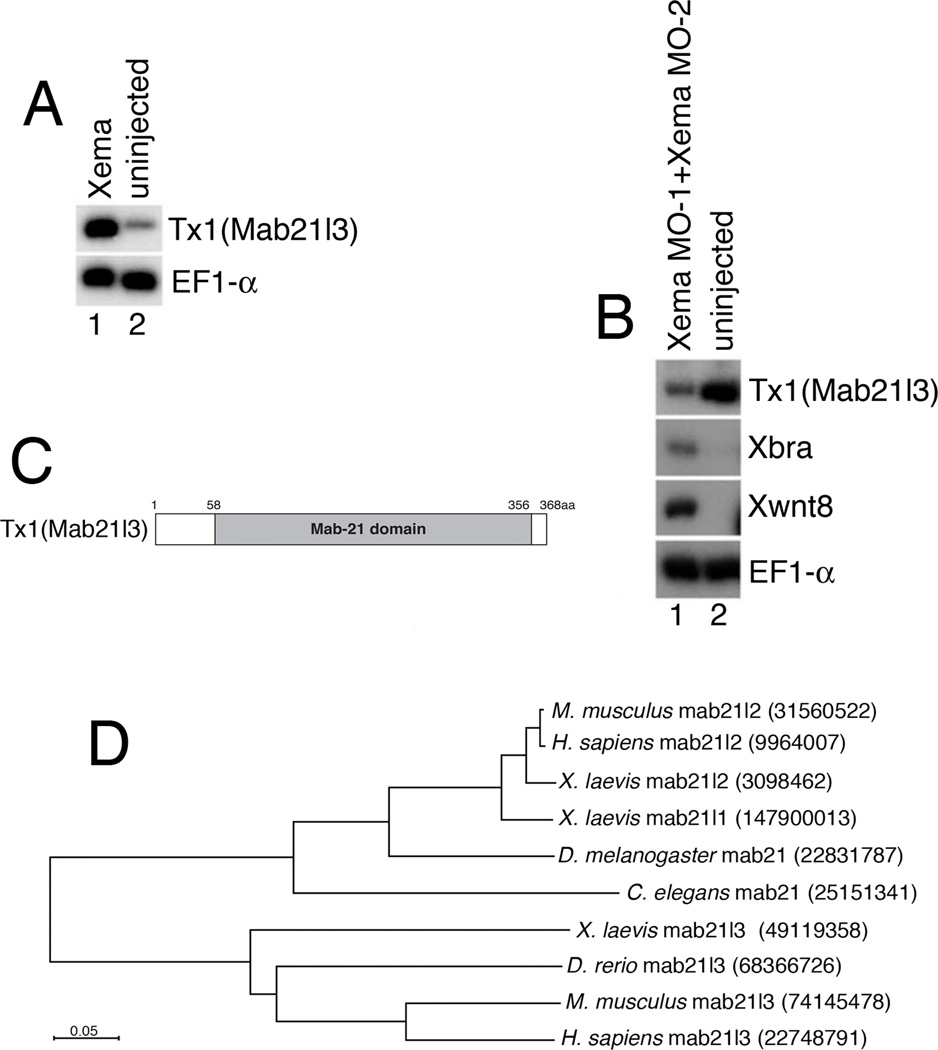
Fig.1. Xmab21l3, a novel Mab-21 family protein, is a target of Xema.
(A) Xema misexpression upregulates Tx1 in animal caps. RT-PCR analysis of Tx1 expression in animal caps derived from Xema RNA-injected embryos. 25 cycles of PCR were used for detection of Tx1. (B) Xema knockdown induces mesoderm and inhibits expression of Tx1. 33 cycles of PCR were used for detection of Tx1. (C) Tx1/Xmab21l3 encodes a Mab-21 family protein domain (Xmab21l3 aa 58–356). (D) The Mab21l3 proteins are more similar to each other than to other Mab-21 family proteins. Dendrogram highlighting the percentage similarity between Xmab21l3 and other Mab-21 family proteins in H. sapiens, M. musculus, X. laevis, D. melanogaster, C. elegans. The dendrogram was generated using ClustalW (http://www.genome.jp/tools/clustalw/) and visualized by NJpolt software (http://pbil.univ-lyon1.fr/software/njplot.html). Branch lengths are proportional to distance. Numbers indicate GI.
Sequence analysis of Tx1 revealed a region of similarity with members of the Mab-21 gene family that spans most of the length of the transcript (Fig. 1C). Male abnormal 21 (Mab-21), the founding member of this family, was identified in C. elegans as a factor whose loss of function causes sensory ray posterior-to-anterior homeotic transformations (Baird et al., 1991; Chow et al., 1995; Lau et al., 2001). Two genes, closely related to each other and to C. elegans Mab-21 have been reported in human, mouse, frogs and zebrafish; a single, putative Drosophila mab-21 gene has also been identified (Mariani et al., 1999). Xmab21l1 and Xmab21l2, the two putative Mab-21 homologs in Xenopus, are highly similar (97%) to each other and share 40% and 44% sequence similarity with Tx1, respectively. Based on this analysis, and in accordance with unpublished sequences obtained through database searches, we have assigned Tx1 the name Xenopus Mab-21-like 3 (Xmab21l3). BLAST sequence analysis identified putative Xmab21l3 homologs in zebrafish, chicken, mouse and human, but not in any invertebrate species, including C. elegans or D. melanogaster (Fig. 1D); Xmab21l3 thus appears to represent a vertebrate- or chordate-specific subclass of the Mab-21 family.
2.2. Spatio-temporal expression profile of Xmab21l3
Xmab21l3 transcripts are initially detected at blastula stage 9, concurrent with the onset of the organizer gene chordin, and slightly preceded by the onset of Xema expression (Fig. 2A)(Sasai et al., 1994) (Sasai et al., 1994); this initiation sequence further suggests that Xema regulates the onset of Xmab21l3 expression during early development. Xmab21l3 expression persists through late tadpole stages (stage 43) (Fig. 2B).
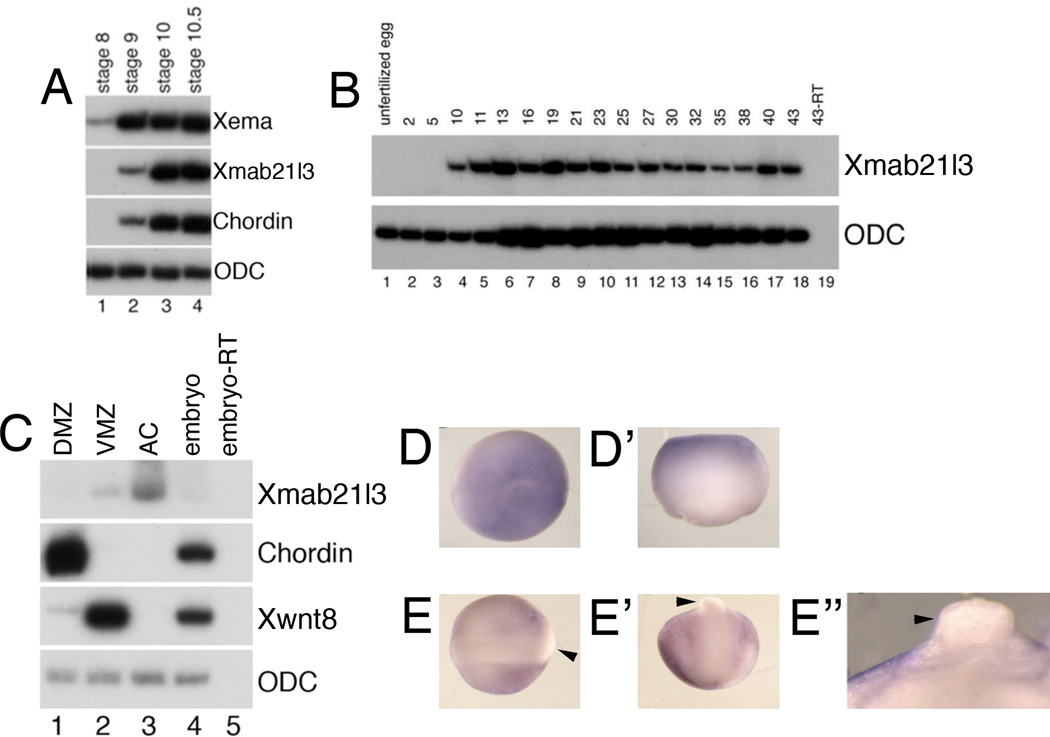
Fig.2. Spatiotemporal expression of Xmab21l3.
(A) Xmab21l3 expression is preceded by the onset of Xema expression. RT-PCR analysis of embryos collected at the indicated stages. The Spemann organizer marker chordin is first detected at stage 9 and increases dramatically at the initiation of gastrulation at stage 10. (B) RT-PCR analysis of embryos collected at the indicated stages. (C) RT-PCR of Xmab21l3 in explants of gastrula stage embryos. Xmab21l3 is expressed predominantly in the animal cap (AC) explants and to a lesser extent in ventral marginal zone (VMZ) explants of stage 10 embryos. DMZ, dorsal marginal zone. (D, D’) Xmab21l3 is expressed in the animal pole ectoderm of gastrula stage embryos. Animal and lateral views of whole mount in situ hybridization analysis of gastrula stage embryos. (E–E’’) Xmab21l3 is excluded from the dorsal neural tube (arrowheads). (E) Dorsal view of stage 20 embryos with anterior to the left. (E’) Posterior view of stage 20 embryos with anterior to the top. (E’’) Transverse cross-section of the dorsal neural tube. Dorsal is to the top.
Xmab21l3 is largely restricted to the presumptive ectoderm of the animal pole at mid-gastrula stage; additional weak expression is also detected in the ventral marginal zone (Fig. 2C). In situ analysis of Xmab21l3 expression in gastrula stage embryos confirmed animal pole localization (Fig. 2D–D’). At neurula stages, expression is restricted to non-neural ectoderm and is clearly excluded from the neural tube at stage 20 (Fig. 2E–E”). This expression profile is reminiscent of Xema expression in Xenopus at similar stages (Suri et al., 2005).
2.3. Ectopic Xmab21l3 dorsalizes mesoderm and neuralizes ectoderm
We have previously demonstrated that Xema regulates germ layer formation and patterning (Suri et al., 2005). In order to investigate the potential function of Xmab21l3 in these processes, we misexpressed Xmab21l3 in embryos. Injection of RNA encoding Xmab21l3 resulted in formation of ectopic protrusions in tail-bud stage embryos and defects in head formation (Fig.3A). Immunohistochemical staining revealed that some protrusions are positive for the somite-specific epitope 12/101, indicating that these represent partial axial duplications, and suggesting that Xmab21l3 plays a role in patterning or specification of the mesoderm (Fig. 3B).
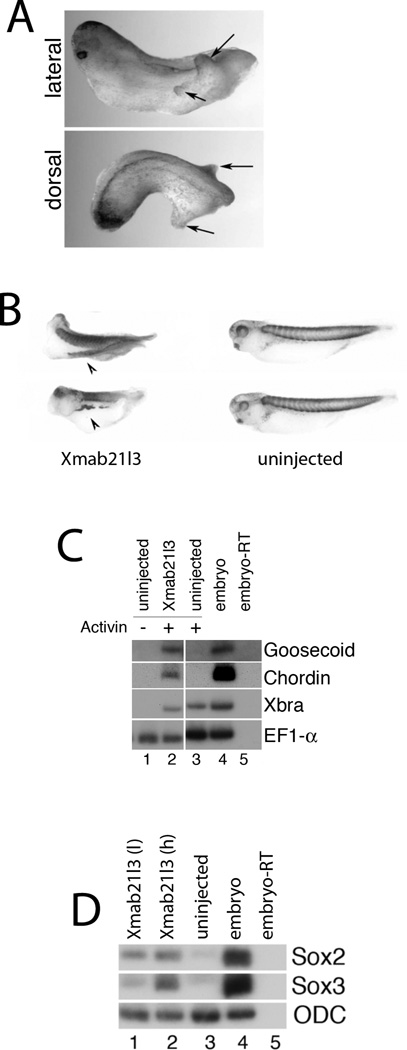
Fig.3. Xmab21l3 misexpression promotes dorsalization.
(A) Overexpression of Xmab21l3 in embryos causes head defects and ectopic tail-like lateral protrusions. Lateral (top) and dorsal (bottom) views of stage 35 embryos injected with 1ng of Xmab21l3 RNA in the animal pole of both blastomeres at the 2-cell stage. (B) Xmab21l3-induced lateral protrusions are positive for a somite-specific antigen. Whole mount immunohistochemistry of Xmab21l3 RNA-injected tadpoles with the 12/101 antibody (Kintner and Brockes, 1984). Arrows in (A) indicate protrusions; arrowheads in (B) indicate secondary axes. (C) Xmab21l3 overexpression promotes induction of dorsal markers chordin and goosecoid by Activin. RT-PCR analysis of uninjected and Xmab21l3 RNA-injected animal cap explants cultured until stage 10.5 in the absence or presence of a low dose of Activin (5ng/ml). (D) Xmab21l3 overexpression induces neural markers sox2 and sox3 in competent ectoderm. RTPCR analysis of animal cap explants from Xmab21l3 RNA-injected embryos cultured until stage 18. Xmab21l3 (l) and Xmab21l3 (h) indicate 0.5ng/embryo and 1ng/embryo doses, respectively, of injected Xmab21l3 RNA.
To better define Xmab21l3 function, we assayed its activity in ectodermal “animal cap” explants, in the presence and absence of the TGFβ ligand Activin. Treatment with low doses of Activin induces ventrolateral mesoderm in animal caps, as demonstrated by the induction of Xbrachyury and Xwnt8 expression (Fig. 3C and data not shown)(Smith et al., 1990); Activin treatment in explants derived from embryos injected with Xmab21l3 RNA additionally enhances expression of the dorsal mesodermal marker genes chordin and goosecoid (Fig. 3C) (Cho et al., 1991; Sasai et al., 1994). Xmab21l3 misexpression does not, however, enhance the expression of Xbrachyury or Xwnt8 in these cultures (Fig. 3C and data not shown). Xmab21l3 RNA overexpression does not induce mesoderm in the absence of Activin (data not shown); however, injection of Xmab21l3 RNA upregulates the early neural markers sox2 and sox3, in a dose dependent manner, in stage 18 animal caps (Fig. 3D)(Collignon et al., 1996; Kamachi et al., 1995; Uwanogho et al., 1995). Xmab21l3 therefore has the ability to dorsalize ectoderm as well as mesoderm; taken together, these data suggest a role for Xmab21l3 in dorsoventral patterning of the early Xenopus embryo.
2.4. Xmab21l3 does not markedly repress transcription
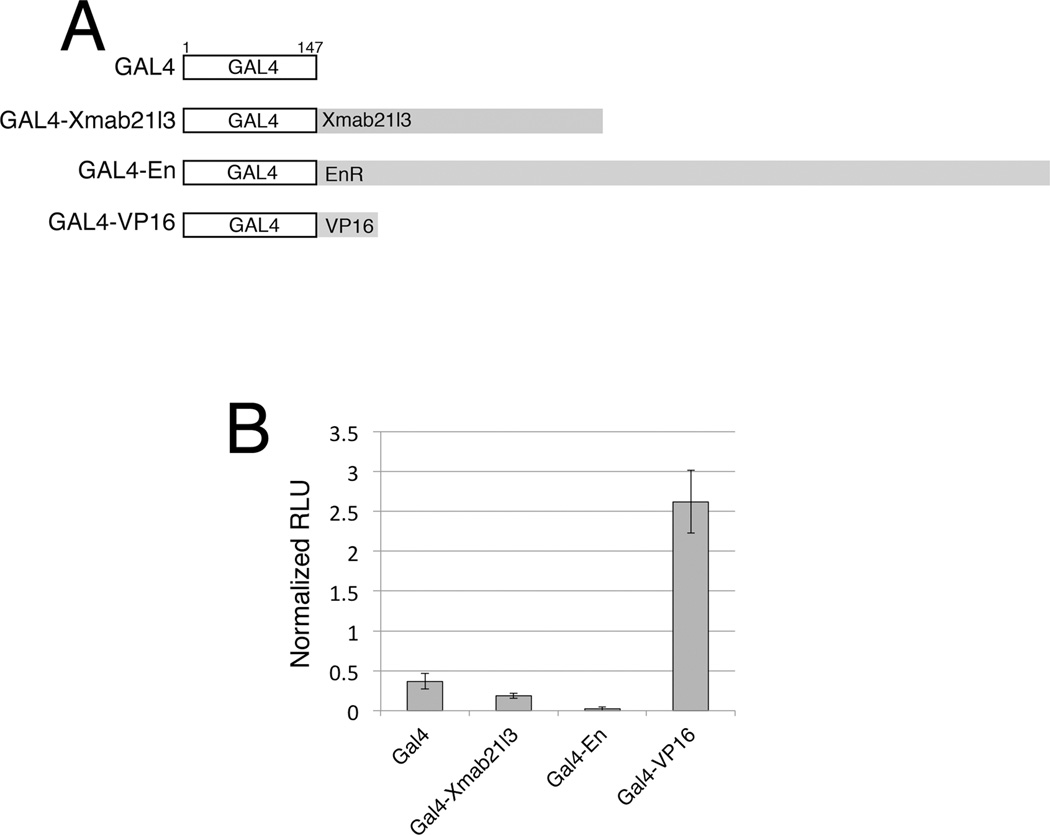
The Mab21 domain spans approximately 85% of the Xmab21l3 protein. Xmab21l2, a related Mab-21 protein, was reported to repress transcription from a heterologous promoter in mammalian cultured cells (Baldessari et al., 2004). To determine if Xmab21l3 itself possesses repressor activity, we analyzed the effects of Xmab21l3 on gene activation of a heterologous reporter. We designed a construct of full-length Xmab21l3 fused to the yeast Gal4-DBD (DNA binding domain) (Gal4-Xmab21l3)(Brand and Perrimon, 1993; Kim et al., 2002). To confirm the efficiency of the assay system, and for comparison, we also constructed fusions of Gal4-DBD to either the Drosophila Engrailed repressor domain (Gal4-En), or to the Herpes virus VP16 transcriptional activator domain (Gal4-VP16)(Fig.4A)(Kessler, 1997). We examined the effects of these chimeric constructs on transactivation of a Gal4-responsive Luciferase reporter gene (Kim et al., 2002). Gal4-En completely blocks expression of the reporter, while Gal4-VP16 strongly induces reporter activity; Gal4-Xmab21l3, however, showed minimal change in the levels of reporter expression compared to Gal4 alone in this assay (Fig.4B). These data suggest that Xmab21l3 does not directly repress transcription of target genes in the early embryo, or possesses only minimal repressor activity.
Fig.4. Xmab21l3 does not directly repress transcription of a heterologous promoter.
(A) Gal4 constructs used in heterologous promoter repression assays. The constructs were designed for expression of the Gal4 DNA binding domain (1–147aa) alone or fused to full length Xmab21l3 (1–368) (Gal4-Xmab21l3), the Engrailed repressor domain (169–1057aa) (Gal4-En) or the VP16 activation domain (410–490aa) (Gal4-VP16). (B) Expression of a Gal4-Xmab21l3 fusion protein has little or no effect on Luciferase reporter gene expression. Gal4-En blocks reporter expression completely and Gal4-VP16 strongly upregulates reporter expression. Graph measures expression in RLU (Relative luciferase units).
2.5. Xmab21l3 loss-of-function affects early development
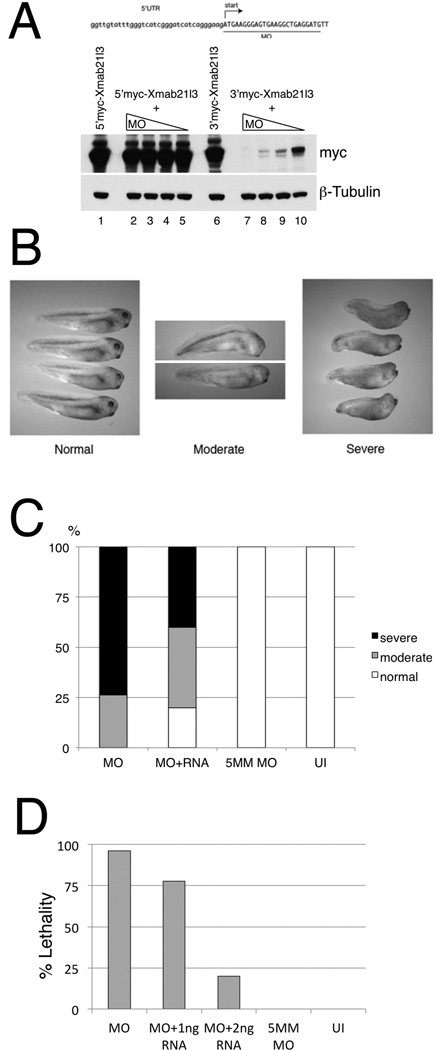
To address the potential requirement for Xmab21l3 in early development, we designed morpholino antisense oligonucleotides against Xmab21l3. A Xmab21l3 morpholino (Xmab21l3MO), designed to target the 5’ coding region of Xmab21l3, effectively blocks translation in vivo of a 3’myc-tagged fusion of Xmab21l3 in a dose-dependent manner; in contrast, expression of a 5’Myc-tagged fusion of Xmab21l3 was unchanged, confirming that the morpholino could not block translation when the morpholino target sequence was separated from the translational start site by the epitope tag (Fig. 5A). Having confirmed the effectiveness of the morpholino, we injected Xmab21l3MO into embryos and examined the effects of Xmab21l3 loss-of-function. Xmab21l3MO injection causes a range of defects that become visible at the tadpole stage. At low doses, defects are confined to a loss of eyes (Fig. 5B, middle panel); Mab-21 homologs in other species have also been reported to be important for eye development (Mariani et al., 1998; Yamada et al., 2004). At higher doses, morpholino injection leads to more pronounced defects including a shortened and curved body axis; eye loss and additional head defects are also more severe at higher doses (Figs. 5B, right panel). These defects are partially rescued by co-injection of a morpholino-insensitive silent mutant form of Xmab21l3 RNA (Fig. 5C). At high doses of morpholino, Xmab21l3 knockdown is lethal at early neurula stages; this effect can be rescued in a dose dependent manner by co-injection of morpholino-insensitive Xmab21l3 RNA (Fig. 5D). Injection of a morpholino that differs from Xmab21l3MO at five base pairs (5MM MO) does not affect normal development, indicating that the morphant phenotypes we observe are caused by a specific decrease in Xmab21l3 during development (Figs. 5C, D).
Fig.5. Loss of function of Xmab21l3 causes anterior defects and lethality.
Xmab21l3MO blocks translation of a 3’Myc-tagged Xmab21l3 protein in a dose-dependent manner. Western blot analysis was used to detect Myc-tagged Xmab21l3 protein. (B) Loss of Xmab21l3 affects eye development and axis formation. Low doses of Xmab21l3 MO (2.5ng) leads to a loss of eyes and/or mild axis curvature in a subset of embryos (middle). Higher doses of Xmab21l3 MO (6.4ng) give rise to pronounced head defects and shortened body axes (right). Embryos in left panel were injected with 20ng of scrambled (control) MO. (C) Coinjection of 1ng RNA encoding a Xmab21l3MO-insensitive silent mutant (Xmab21l3SMT) decreases the percentage of embryos with severe phenotypes. (D) High levels of Xmab21l3MO (250ng) cause lethality in 95% of gastrula stage embryos. Survival can be rescued in a dose-dependent manner by injection of Xmab21l3SMT. (C, D) Injection of a 5 base-pair mismatch (5MM) control morpholino does not give rise to developmental defects or lethality. Representative experiments shown in C, D; n≥15 for each condition.
2.6. Xmab21l3 knockdown inhibits dorsalization
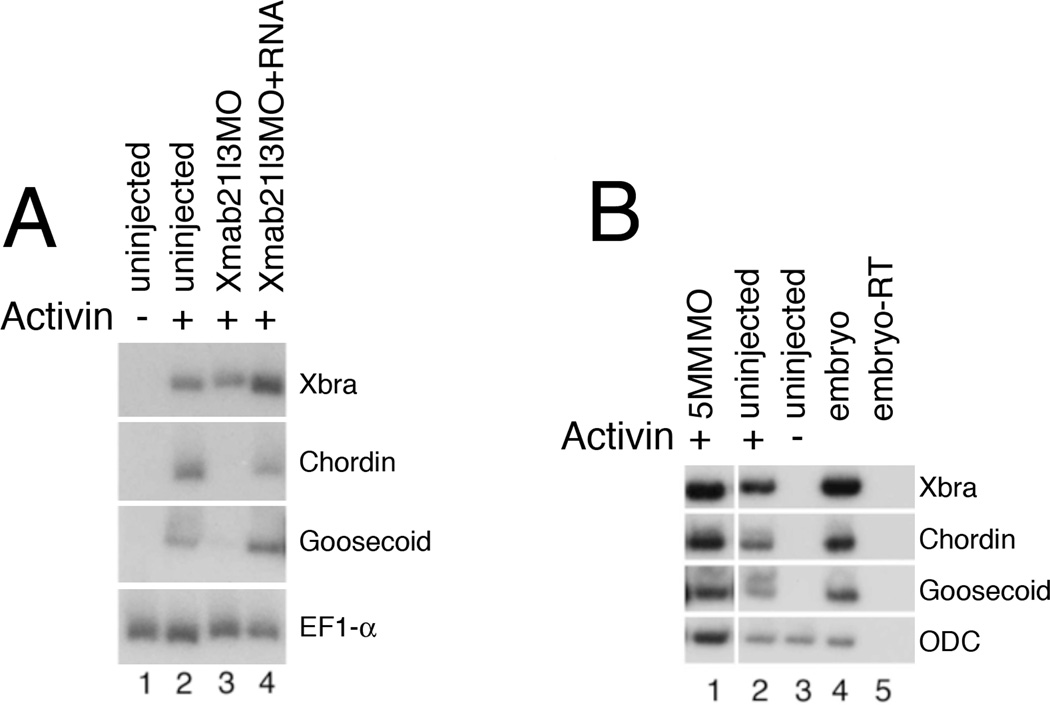
Since Xmab21l3 gain-of-function enhances dorsal mesoderm formation, we hypothesized that loss of Xmab21l3 would lead to a reduction in dorsal differentiation. Consistently, Xmab21l3 knockdown blocks Activin-induced expression of the dorsal markers chordin and goosecoid; effects of Xmab21l3MO were rescued by co-injection of the morpholino-insensitive Xmab21l3 RNA (Fig. 6A); moreover, the 5 base pair mismatched morpholino Xmab21l3 5MM MO has little or no inhibitory activity on the expression of Activin-induced dorsal mesodermal marker genes (Fig. 6B).
Fig.6. Xmab21l3 is required for dorsal fates.
(A) Xmab21l3MO injection inhibits induction of chordin and goosecoid, but not Xbrachyury, by Activin. Injection of RNA encoding the Xmab21l3MO-insensitive silent mutant Xmab21l3SMT rescues this effect. (B) A 5 base pair-mismatch Xmab21l3MO (5MM) morpholino does not inhibit mesodermal marker expression by Activin.
2.7. Antagonistic activity of Xmab21l3 and BMP-2 on the ectoderm
BMP signaling levels regulate dorsoventral patterning in the early vertebrate embryo: BMP-Smad1/5 activity promotes ventral fates, while antagonism of this pathway allows for dorsal development of the germ layers, including neutralization of the ectoderm (Heasman, 2006; Weinstein and Hemmati-Brivanlou, 1999). We therefore examined whether Xmab21l3-mediated dorsalization involved regulation of BMP signaling.
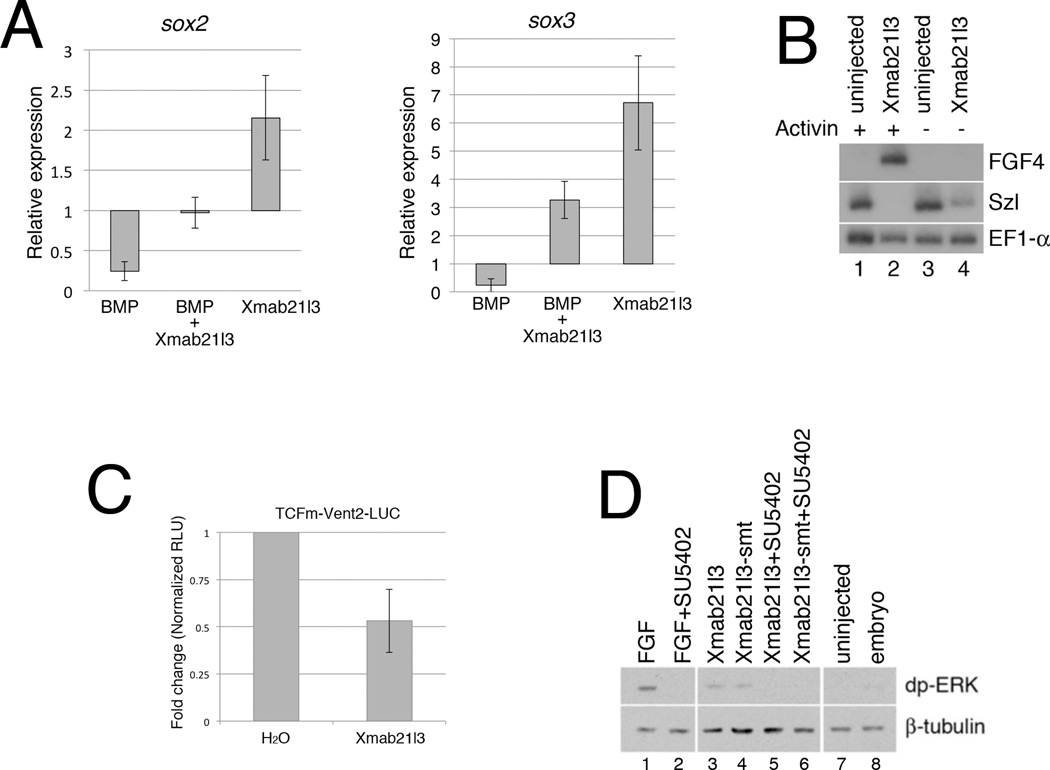
As shown earlier, animal cap explants derived from embryos injected with Xmab21l3 RNA express the early neural markers sox2 and sox3 (Fig. 3D). We find that Xmab21l3 and BMP2 RNA have antagonistic activity with respect to expression of sox2 and sox3 (Fig. 7A). Consistently, Xmab21l3 overexpression inhibits expression of the BMP-responsive gene sizzled (szl) in RT-PCR assays (Fig. 7B)(Collavin and Kirschner, 2003). We next tested the ability of Xmab21l3 to modulate expression of a BMP-responsive reporter gene, in which a modified Vent-2 promoter, lacking Wnt-responsive TCF binding sites, drives expression of firefly Luciferase (Hikasa et al., 2010). Ectopic Xmab21l3 inhibits Luciferase expression from this reporter in animal cap explants (Fig. 7C). These data suggest that Xmab21l3 dorsalizes ectoderm and mesoderm via regulation of BMP activity at or above the level of BMP-responsive target genes.
Fig.7. Analysis of effects of Xmab21l3 on BMP and FGF/ERK signaling.
(A) Injection of BMP2 RNA inhibits neuralization by Xmab21l3. Graphs show relative expression, assayed by RT-PCR, of the neural markers sox2 and sox3 in animal caps normalized to expression in uninjected explants. (B) Xmab21l3 misexpression inhibits expression of the BMP-responsive gene sizzled (szl), and induces expression of the dorsal marker fgf4. (C) Xmab21l3 misexpression inhibits expression of a Vent2-Luciferase reporter fusion protein; this construct includes a mutation in a TCF binding site that renders it insensitive to Wnt activation (Hikasa et al., 2010). (D) Xmab21l3 activates ERK1/2 signaling in animal cap explants. Western blot analysis using an antibody against di-phosphorylated ERK1/2 demonstrates that ERK1/2 is activated in response to treatment with FGF or following injection of Xmab21l3 RNA. The FGFR1 inhibitor SU5402 blocks ERK activation by FGF, Xmab21l3 and Xmab21l3SMT.
2.8. Xmab21l3 activates the ERK signaling pathway
Signaling through the ERK MAP kinase can neuralize ectoderm via inhibition of BMP signaling (Pera et al., 2003). Interestingly, the ectopic lateral tail-like protrusions and anterior defects caused by overexpression of Xmab21l3 in embryos closely resemble the overexpression phenotype of FGF/ERK-pathway components (Fig.3A)(Pownall et al., 1996; Weinstein et al., 1998). To address whether Xmab21l3 might regulate ERK signaling, we used an antibody that recognizes the double tyrosine phosphorylated (and thus activated) form of ERK1/2 (dpERK)(Umbhauer et al., 1995). Levels of dpERK are upregulated in animal cap explants treated with bFGF (FGF2) at the blastula stage, relative to untreated controls (Fig. 7D); Xmab21l3 RNA-injected samples also show elevated levels of di-phosphorylated ERK1/2, indicating that Xmab21l3 may regulate ERK/MAP kinase signaling during normal development (Fig. 7D).
In the presence of the FGF Receptor 1 (FGFR1) inhibitor SU5402, FGF-induced ERK phosphorylation is blocked; interestingly, SU5402 also blocks activation of ERK1/2 by Xmab21l3 (Fig. 7D)(Mohammadi et al., 1997). These data indicate that Xmab21l3 requires signaling through FGFR1 to induce or maintain ERK activation, and suggest that Xmab21l3 stimulates production of an extracellular FGF/ERK pathway component. Consistently, in the presence of Activin, Xmab21l3 misexpression induces expression of FGF-4/eFGF, which is expressed in the dorsal marginal zone at blastula stages (Fig. 7B) (Isaacs et al., 1995). These results demonstrate that Xmab21l3 can promote ERK activation, and suggest a mechanism by which Xmab21l3 could antagonize BMP-mediated ventralization.
3. Discussion
Xmab21l3 was isolated as a target of Xema, a transcription factor involved in the suppression of ectopic germ layer formation in the presumptive ectoderm. Xmab21l3 is a Mab-21 family protein expressed predominantly in the animal hemisphere and, to a lesser extent, in the ventral marginal zone of gastrula stage Xenopus embryos. Misexpression of Xmab21l3 leads to the induction of lateral protrusions, including secondary axes, neural induction in ectodermal explants, mesoderm dorsalization, and inhibition of BMP signaling. Finally, Xmab21l3 is required for dorsal differentiation in mesodermal and ectodermal explants, and for anterior differentiation and survival of Xenopus embryos.
The gastrula stage expression of Xmab21l3 in the animal pole and ventral marginal zone is ostensibly surprising, given the dorsalizing activity of this factor in gain-of-function assays. The demonstration, however, that Xmab21l3 knockdown inhibits dorsal mesoderm differentiation by Activin suggests that Xmab21l3 functions in vivo to confer responsiveness to endogenous dorsalizing signals. Axial duplications seen following Xmab21l3 overexpression, then, may not directly reflect the activity of lower, endogenous levels of native Xmab21l3.
The Xmab21l2 protein has been reported to possess transcriptional repressor activity (Baldessari et al., 2004). We find that a Gal4 DNA-binding domain-Xmab21l3 fusion protein does not significantly repress transcription when targeted to a heterologous promoter; thus, if Xmab21l3 is involved in mediation of transcriptional repression, it is likely to do so indirectly, via recruitment of additional proteins. Consistently, C.elegans mab-21 interacts with Sin3, a histone deacetylase (HDAC) repressor complex-associated scaffolding protein (Choy et al., 2007); the Sin3-HDAC repressor complex lacks DNA-binding activity and must be targeted to promoters by DNA binding proteins (Grzenda et al., 2009). Xmab21l3 could function similarly to bring repressor proteins to target sites on DNA. While it remains to be determined whether Xmab21l3 binds DNA directly, recent sequence analyses suggest that Mab-21 belongs to the nucleotidyl transferase family of proteins and may thus possess DNA/RNA binding activity (Kuchta et al., 2009).
Inhibition of BMP signaling is a central mechanism driving dorsalization in early Xenopus embryos, and our studies suggest that Xmab21l3 activity is mediated through antagonism of BMP signaling. Several receptor tyrosine kinase (RTK) cascades antagonize BMP signaling via phosphorylation of the linker region of the BMP effector Smad1 (Kretzschmar et al., 1997; Kuroda et al., 2005; Pera et al., 2003). ERK activation may thus play a role in Xmab21l3-mediated dorsalization; given the inhibition of Xmab21l3-mediated ERK phosphorylation by SU5402, this regulation may involve induction of FGF4 (eFGF) and/or other extracellular or cell-surface RTK pathway components (Lea et al., 2009; Mohammadi et al., 1997). We note that Xmab21l3, in the absence of Activin, induces ERK phosphorylation but not FGF4 expression, raising the possibility that Xmab21l3 activates ERK via both FGF4-dependent and FGF4-independent mechanisms.
Other Mab-21 proteins have also been reported to antagonize BMP signaling. One study places the C.elegans mab-21 gene downstream of the TGFβ ligand dbl-1/cet-1 and its sma (small) effectors in regulation of sensory ray specification (Morita et al., 1999). In Xenopus, Mab21l2 was shown to be antagonistic to BMP4: Xmab21l2 rescues ventralization of embryos by BMP4 and can also interact with the cytoplasmic BMP effector Smad1 (Baldessari et al., 2004). Xmab21l2 is first expressed in gastrula stage dorsal tissue in Xenopus, with strong expression detectable from stage 12; moreover, Xmab21l2 knockdown leads to defects in gastrulation and neural tube closure (data not shown)(Lau et al., 2001). Our data indicate that Xmab21l3 is specifically required for the development of dorsal fate; it remains to be determined whether Xmab21l2 is similarly essential.
We identified Xmab21l3 as a novel target of Xema, the latter a Foxi-class transcription factor that is both necessary and sufficient for the suppression of ectopic mesendoderm (Suri et al., 2005). Xema also promotes, and is required for, ectodermal fates including neurectoderm, and downregulates expression of the BMP target genes sizzled and vent2 (Mir et al., 2007)(data not shown). Our data suggest that Xmab21l3 mediates germ layer patterning, but not germ layer suppression, downstream of Xema. Strikingly, Xmab21l3 is required for the differentiation of dorsal mesoderm in response to Activin, while Xema is required for the suppression of ectopic mesoderm. This suggests that, in vivo, Xema both suppresses inappropriate germ layer formation and, via Xmab21l3, facilitates the competence of the presumptive ectoderm to mesoderm-inducing and dorsoventral patterning cues.
4. Experimental procedures
4.1. Gene chip analysis
RNA from 80 animal cap explants, cultured to stage 11, were used to generate hybridization probes for use on Affymetrix GeneChip Xenopus laevis Genome Arrays; hybridization was performed with the help of the Mount Sinai Microarray Shared Research Facility (http://www.mssm.edu/research/resources/microarray/). 1ng Xema or β-galactosidase RNA, 63ng 1:2 Xema MO1:Xema MO2, or 62.5ng scrambled morpholino (CMO) were injected, as described (Suri et al., 2005). Microarray data were normalized by RMA (Irizarry et al., 2003) and analyzed using the affylmGUI Bioconductor package (Wettenhall et al., 2006).
4.2. Isolation and Cloning of Xmab21l3
Xenopus Xmab21l3 was isolated in a microarray screen to identify transcriptional targets of Xema (Xenopus ectodermally expressed mesoderm antagonist) (Suri et al., 2005). The probe set (Xl.12881.2.A1_at) corresponding to Xmab21l3 was up-regulated in stage 11 animal pole ectodermal explants overexpressing Xema RNA, and down-regulated in Xema morpholino-injected explants when compared to uninjected or control scrambled morpholino-injected samples, respectively. A full-length Xmab21l3 cDNA was subsequently obtained from OpenBiosystems (IMAGE: 5569830).
4.3. Preparation of Xmab21l3 constructs
Full length Xmab21l3 was subcloned downstream of the Gal4 DNA binding domain (aa1-147) in the pGBT9 vector (Clontech)(Kim et al., 2002). For the Gal4-VP16 and Gal4-EnR constructs, residues 410–490 of the VP16 activator (Kessler, 1997) and residues 1–298 of the Drosophila Engrailed repressor, respectively, were cloned downstream of the Gal4 DNA-binding (aa1-147) domain. Fusion constructs were subcloned into the pCS2++ vector for in vitro RNA synthesis. A morpholino-insensitive Xmab21l3 silent mutation, Xmab21l3SMT, was generated by PCR using KOD Hot Start Polymerase (EMD, Rockland, MA), with the following primers:
Xtox1silent-U TTTAACACCTTTCATGCTGAATTC
Xtox1silent-D (P)-gCcGAaGAcGTTCATCATTTCCTAC
4.4. RNA preparation, explant dissection, and cell culture
RNA was synthesized in vitro in the presence of cap analog using the mMessage mMachine kit (Ambion). Microinjection, explant dissection, and cell culture were performed as described (Hemmati-Brivanlou and Melton, 1994; Wilson and Hemmati-Brivanlou, 1995).
4.5. Luciferase assays
Embryos were injected at the 2 cell stage with 1ng RNA encoding the appropriate Gal4 fusion constructs, and 125pg of luciferase reporter plasmid p17X4TKlucSV40pA (a gift of PD McCrea) containing a minimal thymidine kinase promoter under the control of four Gal4 binding sites. 600pg of Renilla luciferase reporter was coinjected with all samples as an internal control. Samples of five embryos each were collected in triplicate at stage 11 for analysis.
4.6. Whole mount in situ hybridization and immunocytochemistry
Whole mount in situ hybridization was carried out using standard protocols (Harland, 1991). BM Purple (Roche) was used for chromogenic reactions. Whole-mount antibody staining was performed as described (Hemmati-Brivanlou and Melton, 1994). The 12/101 antibody (ascites, Developmental Studies Hybridoma Bank) was used at a 1:1 dilution. The Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204)antibody (Sigma M8159) was used at a 1:100 dilution. The secondary antibody, a donkey anti-mouse IgG coupled to horseradish peroxidase (Jackson Laboratories), was used at a 1:1000 dilution. Color reaction for immunostaining was performed using the Vector SG kit (Vector Laboratories).
4.7. Morpholinos
Morpholino antisense oligonucleotides (Genetools LLC) were designed to hybridize to the 5’ region of target mRNAs to block translation. Morpholinos were heated at 65°C for 5 minutes, and then cooled on ice and centrifuged prior to microinjection. Morpholinos designed for this study are as follows:
Xmab21l3 MO: 5’-CATCCTCAGCCTTCACTCCCTTCAT-3’
Xmab21l3 5MM (mismatch) MO: 5’-CAACGTCAGGCTTCAGTGCCTTCAT-3’
4.8. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Xenopus laevis embryos were staged according to (Nieuwkoop and Faber, 1967) and harvested at appropriate stages according to morphological criteria. RNA was prepared using RNA Bee RNA isolation reagent (Tel-Test Inc.). RT-PCR was performed as described (Wilson and Hemmati-Brivanlou, 1995). Primers used in this study are as follows:
Xmab21l3-F: 5’-CAAAAGAGCACTCCCCATTG-3’
Xmab21l3-R: 5’-AAAGAAACGAGGCCACTGAG-3’
goosecoid-F: 5’-TCTTATTCCAGAGGAACC-3’
goosecoid-R: 5’-AGAGTTCATCTAGAGAG-3’
Xwnt8–F: 5’-GTTCAAGCATTACCCCGGAT-3’
Xwnt8-R: 5’-CTCCTCAATTCCATTCTGCG-3’
sox2-F: 5’-GAGGATGGACACTTATGCCCAC-3’
sox2-R: 5’-GGACATGCTGTAGGTAGGCGA-3’
sox3-F: 5’-ATCCCATTGACAAGGACCTG-3’
sox3-R: 5’-ATACGAACCAAAGGGGGAAA-3’
odc-F: 5’-AATGGATTTCAGAGACCA-3’
odc-R: 5’-CCAAGGCTAAAGTTGCAG-3’
chordin-F: 5’-CAGTCAGATGGAGCAGGATC-3’
chordin-R: 5’-AGTCCCATTGCCCGAGTTGC-3’
fgf4-F: 5’-CGGAAGGATAAATGGCATGC-3’
fgf4-R: 5’-TTTTGCCCAGAGCGATGTAC-3’
Szl-F: 5'-CATGTCCGGAGTCTTCCTGC-3'
Szl-R: 5'-GGATGAACGTGTCCAGGCAG-3'
Highlights.
We report the identification of a novel factor, Xenopus mab21-like 3 (Xmab21l3).
Misexpression of Xmab21l3 promotes dorsal mesoderm formation.
Misexpression of Xmab21l3 neuralizes ectodermal explants.
Xab21l3 knockdown inhibits dorsal mesoderm formation.
Xmab21l3 regulates dorsal fate via BMP/Smad signaling.
Acknowledgments
We thank S Sokol, D Kessler, and PD McCrea for gifts of reagents. This work is supported by PHS grant R01-GM61671 (DCW) and funds from Queens College of the City University of New York.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Baird SE, Fitch DH, Kassem IA, Emmons SW. Pattern formation in the nematode epidermis: determination of the arrangement of peripheral sense organs in the C. elegans male tail. Development. 1991;113:515–526. doi: 10.1242/dev.113.2.515. [DOI] [PubMed] [Google Scholar]
- Baldessari D, Badaloni A, Longhi R, Zappavigna V, Consalez GG. MAB21L2, a vertebrate member of the Male-abnormal 21 family, modulates BMP signaling and interacts with SMAD1. BMC Cell Biol. 2004;5:48. doi: 10.1186/1471-2121-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Cho KW, Blumberg B, Steinbeisser H, De Robertis EM. Molecular nature of Spemann's organizer: the role of the Xenopus homeobox gene goosecoid. Cell. 1991;67:1111–1120. doi: 10.1016/0092-8674(91)90288-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow KL, Hall DH, Emmons SW. The mab-21 gene of Caenorhabditis elegans encodes a novel protein required for choice of alternate cell fates. Development. 1995;121:3615–3626. doi: 10.1242/dev.121.11.3615. [DOI] [PubMed] [Google Scholar]
- Choy SW, Wong YM, Ho SH, Chow KL. C. elegans SIN-3 and its associated HDAC corepressor complex act as mediators of male sensory ray development. Biochem Biophys Res Commun. 2007;358:802–807. doi: 10.1016/j.bbrc.2007.04.194. [DOI] [PubMed] [Google Scholar]
- Collavin L, Kirschner MW. The secreted Frizzled-related protein Sizzled functions as a negative feedback regulator of extreme ventral mesoderm. Development. 2003;130:805–816. doi: 10.1242/dev.00306. [DOI] [PubMed] [Google Scholar]
- Collignon J, Sockanathan S, Hacker A, Cohen-Tannoudji M, Norris D, Rastan S, Stevanovic M, Goodfellow PN, Lovell-Badge R. A comparison of the properties of Sox-3 with Sry and two related genes, Sox-1 and Sox-2. Development. 1996;122:509–520. doi: 10.1242/dev.122.2.509. [DOI] [PubMed] [Google Scholar]
- Grzenda A, Lomberk G, Zhang JS, Urrutia R. Sin3: master scaffold and transcriptional corepressor. Biochim Biophys Acta. 2009;1789:443–450. doi: 10.1016/j.bbagrm.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland RM. In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- Heasman J. Patterning the early Xenopus embryo. Development. 2006;133:1205–1217. doi: 10.1242/dev.02304. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Melton DA. Inhibition of activin receptor signaling promotes neuralization in Xenopus. Cell. 1994;77:273–281. doi: 10.1016/0092-8674(94)90319-0. [DOI] [PubMed] [Google Scholar]
- Hikasa H, Ezan J, Itoh K, Li X, Klymkowsky MW, Sokol SY. Regulation of TCF3 by Wnt-dependent phosphorylation during vertebrate axis specification. Dev Cell. 2010;19:521–532. doi: 10.1016/j.devcel.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- Isaacs HV, Pownall ME, Slack JM. eFGF is expressed in the dorsal midline of Xenopus laevis. Int J Dev Biol. 1995;39:575–579. [PubMed] [Google Scholar]
- Kamachi Y, Sockanathan S, Liu Q, Breitman M, Lovell-Badge R, Kondoh H. Involvement of SOX proteins in lens-specific activation of crystallin genes. Embo J. 1995;14:3510–3519. doi: 10.1002/j.1460-2075.1995.tb07357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler DS. Siamois is required for formation of Spemann's organizer. Proc Natl Acad Sci U S A. 1997;94:13017–13022. doi: 10.1073/pnas.94.24.13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SW, Fang X, Ji H, Paulson AF, Daniel JM, Ciesiolka M, van Roy F, McCrea PD. Isolation and characterization of XKaiso, a transcriptional repressor that associates with the catenin Xp120(ctn) in Xenopus laevis. J Biol Chem. 2002;277:8202–8208. doi: 10.1074/jbc.M109508200. [DOI] [PubMed] [Google Scholar]
- Kintner CR, Brockes JP. Monoclonal antibodies identify blastemal cells derived from dedifferentiating limb regeneration. Nature. 1984;308:67–69. doi: 10.1038/308067a0. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M, Doody J, Massague J. Opposing BMP and EGF signalling pathways converge on the TGF-beta family mediator Smad1. Nature. 1997;389:618–622. doi: 10.1038/39348. [DOI] [PubMed] [Google Scholar]
- Kuchta K, Knizewski L, Wyrwicz LS, Rychlewski L, Ginalski K. Comprehensive classification of nucleotidyltransferase fold proteins: identification of novel families and their representatives in human. Nucleic Acids Res. 2009;37:7701–7714. doi: 10.1093/nar/gkp854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda H, Fuentealba L, Ikeda A, Reversade B, De Robertis EM. Default neural induction: neuralization of dissociated Xenopus cells is mediated by Ras/MAPK activation. Genes Dev. 2005;19:1022–1027. doi: 10.1101/gad.1306605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau GT, Wong OG, Chan PM, Kok KH, Wong RL, Chin KT, Lin MC, Kung HF, Chow KL. Embryonic XMab21l2 expression is required for gastrulation and subsequent neural development. Biochem Biophys Res Commun. 2001;280:1378–1384. doi: 10.1006/bbrc.2001.4290. [DOI] [PubMed] [Google Scholar]
- Lea R, Papalopulu N, Amaya E, Dorey K. Temporal and spatial expression of FGF ligands and receptors during Xenopus development. Dev Dyn. 2009;238:1467–1479. doi: 10.1002/dvdy.21913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani M, Baldessari D, Francisconi S, Viggiano L, Rocchi M, Zappavigna V, Malgaretti N, Consalez GG. Two murine and human homologs of mab-21, a cell fate determination gene involved in Caenorhabditis elegans neural development. Hum Mol Genet. 1999;8:2397–2406. doi: 10.1093/hmg/8.13.2397. [DOI] [PubMed] [Google Scholar]
- Mariani M, Corradi A, Baldessari D, Malgaretti N, Pozzoli O, Fesce R, Martinez S, Boncinelli E, Consalez GG. Mab21, the mouse homolog of a C. elegans cell-fate specification gene, participates in cerebellar, midbrain and eye development. Mech Dev. 1998;79:131–135. doi: 10.1016/s0925-4773(98)00180-4. [DOI] [PubMed] [Google Scholar]
- Mir A, Kofron M, Zorn AM, Bajzer M, Haque M, Heasman J, Wylie CC. FoxI1e activates ectoderm formation and controls cell position in the Xenopus blastula. Development. 2007;134:779–788. doi: 10.1242/dev.02768. [DOI] [PubMed] [Google Scholar]
- Mohammadi M, McMahon G, Sun L, Tang C, Hirth P, Yeh BK, Hubbard SR, Schlessinger J. Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science. 1997;276:955–960. doi: 10.1126/science.276.5314.955. [DOI] [PubMed] [Google Scholar]
- Morita K, Chow KL, Ueno N. Regulation of body length and male tail ray pattern formation of Caenorhabditis elegans by a member of TGF-beta family. Development. 1999;126:1337–1347. doi: 10.1242/dev.126.6.1337. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P, Faber J. Normal table of Xenopus Laevis. Amsterdam, The Netherlands: North Holland Publishing Co.; 1967. [Google Scholar]
- Pera EM, Ikeda A, Eivers E, De Robertis EM. Integration of IGF FGF, and anti-BMP signals via Smad1 phosphorylation in neural induction. Genes Dev. 2003;17:3023–3028. doi: 10.1101/gad.1153603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pownall ME, Tucker AS, Slack JM, Isaacs HV. eFGF, Xcad3 and Hox genes form a molecular pathway that establishes the anteroposterior axis in Xenopus. Development. 1996;122:3881–3892. doi: 10.1242/dev.122.12.3881. [DOI] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK, De Robertis EM. Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994;79:779–790. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Symes K, Hynes RO, DeSimone D. Mesoderm induction and the control of gastrulation in Xenopus laevis: the roles of fibronectin and integrins. Development. 1990;108:229–238. doi: 10.1242/dev.108.2.229. [DOI] [PubMed] [Google Scholar]
- Suri C, Haremaki T, Weinstein DC. Xema, a foxi-class gene expressed in the gastrula stage Xenopus ectoderm, is required for the suppression of mesendoderm. Development. 2005;132:2733–2742. doi: 10.1242/dev.01865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbhauer M, Marshall CJ, Mason CS, Old RW, Smith JC. Mesoderm induction in Xenopus caused by activation of MAP kinase. Nature. 1995;376:58–62. doi: 10.1038/376058a0. [DOI] [PubMed] [Google Scholar]
- Uwanogho D, Rex M, Cartwright EJ, Pearl G, Healy C, Scotting PJ, Sharpe PT. Embryonic expression of the chicken Sox2, Sox3 and Sox11 genes suggests an interactive role in neuronal development. Mech Dev. 1995;49:23–36. doi: 10.1016/0925-4773(94)00299-3. [DOI] [PubMed] [Google Scholar]
- Weinstein DC, Hemmati-Brivanlou A. Neural induction. Annu Rev Cell Dev Biol. 1999;15:411–433. doi: 10.1146/annurev.cellbio.15.1.411. [DOI] [PubMed] [Google Scholar]
- Weinstein DC, Marden J, Carnevali F, Hemmati-Brivanlou A. FGF-mediated mesoderm induction involves the Src-family kinase Laloo. Nature. 1998;394:904–908. doi: 10.1038/29808. [DOI] [PubMed] [Google Scholar]
- Wettenhall JM, Simpson KM, Satterley K, Smyth GK. affylmGUI: a graphical user interface for linear modeling of single channel microarray data. Bioinformatics. 2006;22:897–899. doi: 10.1093/bioinformatics/btl025. [DOI] [PubMed] [Google Scholar]
- Wilson PA, Hemmati-Brivanlou A. Induction of epidermis and inhibition of neural fate by Bmp-4. Nature. 1995;376:331–333. doi: 10.1038/376331a0. [DOI] [PubMed] [Google Scholar]
- Yamada R, Mizutani-Koseki Y, Koseki H, Takahashi N. Requirement for Mab21l2 during development of murine retina and ventral body wall. Dev Biol. 2004;274:295–307. doi: 10.1016/j.ydbio.2004.07.016. [DOI] [PubMed] [Google Scholar]