Abstract
Bronchiolitis obliterans syndrome (BOS), the clinical correlate of chronic rejection after lung transplantation, is the leading obstacle to better long-term outcomes. We previously instituted a clinical protocol to screen for donor-specific human leukocyte antigen (HLA) antibodies (DSA) and a preemptive antibody-directed therapy protocol consisting of rituximab and/or intravenous immune globulin. In this study, we retrospectively analyzed serum samples from lung transplant recipients (n = 108) for antibodies to self-antigens (K-α 1 tubulin and collagen V) before and after antibody-directed therapy and correlated the results with the subsequent development of BOS. Seventy-two of the 108 recipients developed antibodies to self-antigens. There was a correlation between the development of antibodies to self-antigens and DSA. Sixteen of the 54 patients who had antibodies to self-antigens and were treated with antibody-directed therapy cleared the antibodies, and they were significantly less likely to develop BOS than those who had persistent antibodies. Furthermore, those who cleared DSA after treatment but had persistent antibodies to self-antigens were significantly more likely to develop BOS than those who cleared these antibodies. We conclude that antibodies to self-antigens are an important risk factor for the development of BOS.
Keywords: Autoimmunity, antibodies, BOS, lung transplantation
INTRODUCTION
Lung transplantation is the ultimate treatment for patients with a variety of end-stage lung diseases. However, despite improvements in donor management, early post-operative care, and immunosuppressive therapy, long-term outcomes remain disappointing. Indeed, according to the latest International Society for Heart and Lung Transplantation (ISHLT) Registry Report, the median survival in the most recent era (2000-2008) was 5.7 years (1, 2). Bronchiolitis obliterans syndrome (BOS), characterized by a progressive decline in lung function due to luminal obliteration of terminal and respiratory bronchioles is the leading cause of death beyond the first year after transplantation and the primary obstacle to better long-term outcomes (2). While the pathogenesis of BOS remains poorly understood, clinical risk factors provide important insights into the biological mechanisms that lead to airway obliteration. Among these, both alloimmune injury including acute rejection, lymphocytic bronchiolitis, and the development of human leukocyte antigen (HLA) antibodies and non-alloimmune injury including gastroesophageal reflux, primary graft dysfunction (PGD), and respiratory viral infections are implicated (3-8).
In addition, an important role for immune responses to self-antigens and the resultant autoimmunity has emerged in graft rejection in recent years. The development of antibodies to myosin, vimentin, and heat-shock proteins after heart transplantation has been linked to cardiac allograft vasculopathy, and antibodies to angiotensin type 1 receptor have been associated with kidney allograft rejection (9-12). Similarly, cellular and humoral immune responses to collagen V, a sequestered but immunogenic extracellular matrix protein, and K-α 1 tubulin, a gap junction protein have been associated with the development of BOS after lung transplantation (13-16). Importantly, the binding and ligation of K-α 1 tubulin on airway epithelial cells by its specific antibodies resulted in increased expression of fibrogenic growth factors, the activation of cell cycle signaling, and fibroproliferation suggesting that the antibodies to K-α 1 tubulin are directly pathogenic in the development of obliterative bronchiolitis (16). In addition, several studies suggest that the loss of self-tolerance characteristic of autoimmunity may result from alloimmune mediated graft injury and activation of Th 17 cells (13-18).
In 2006, we developed a clinical protocol to screen lung transplant recipients for the development of donor-specific HLA antibodies (DSA) after transplantation and preemptively treat those who developed DSA with rituximab and/or intravenous immune globulin (IVIG) (19). The goal of this study is to examine the effect of this antibody-directed therapy on antibodies to self-antigens (K-α 1 tubulin and collagen V) and to determine the role of antibodies to self-antigens in BOS development.
METHODS
Study design and patients
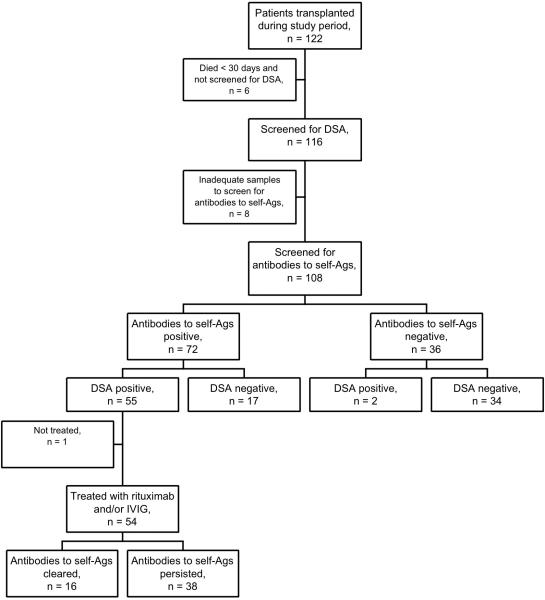
We conducted a retrospective cohort study of patients who underwent lung transplantation at our center between 7/1/2006 and 7/31/2008. During this time period, 122 adults underwent transplantation, but 6 patients died within 30 days and were not screened for DSA (Figure 1). The remaining 116 patients were prospectively screened for DSA after transplantation using the LABScreen Single Antigen assay (One Lambda Inc., Canoga Park, CA). Because of fluctuations in mean fluorescence intensity (MFI) between positive controls, the HLA lab at our center defines a positive DSA as a ratio of sample MFI to positive control MFI ≥ 0.2 (19). Among the 116 patients screened for DSA, 65 (56%) developed DSA with a mean MFI of 4358 ± 2233, and 61 of these were treated with rituximab and/or IVIG as previously described (19). We retrospectively analyzed serum samples collected for DSA screening for antibodies to collagen V and K-α 1 tubulin in 108 patients, and these patients constitute the cohort in this study (Figure 1). Eight of the original 116 patients did not have adequate samples for testing and were excluded from this analysis. The Institutional Review Board for human studies approved this retrospective data analysis.
Figure 1.
Flow chart of patients through the study including screening, treatments, and outcomes. DSA: donor-specific HLA antibodies. Antibodies to self-Ags: antibodies to self-antigens. IVIG: intravenous immune globulin.
Medical management and antibody-directed therapy
We previously described our clinical protocol in detail (19). Briefly, recipients are treated with either equine anti-thymocyte globulin or basiliximab for induction and tacrolimus, azathioprine, and prednisone for maintenance immunosuppression. Recipients undergo surveillance bronchoscopy at 1, 2, 3, 6, and 12 months, and are screened for DSA at these time points. BOS is diagnosed and staged according to the standard ISHLT criteria (20, 21). Recipients who developed DSA after transplantation were treated preemptively with rituximab and/or IVIG; those who were infected or colonized with multi-drug resistant organisms, or had a history of recurrent infections were treated with IVIG alone (500 mg/kg monthly for 6 months). Otherwise, patients were treated with a single dose of rituximab (375 mg/m2) and IVIG.
Enzyme-linked immunoassay (ELISA) for detection of antibodies to K-α 1tubulin and collagen V
To detect antibodies to collagen V and K-α1 tubulin, an ELISA plate (Nunc/Thermo Fisher Scientific, Rochester, NY) was coated with pure collagen V (1μg/ml; Chemicon) or recombinant K-α1 tubulin (1μg/ml) in phosphate-buffered saline (PBS) overnight at 4°C and blocked with 1% BSA for 2 hrs. The collagen V and K-α 1tubulin proteins were tested and found to be free of endotoxin contamination by chromogenic limulus amebocyte lysate (LAL) assay and isoelectric focusing. Patient and normal sera were tested at different dilutions up to 1:750 and 1:1250 respectively for antibodies to collagen V and K-α 1 tubulin as detailed in our previous publications (15, 22). Antibodies were detected using horseradish peroxidase conjugated anti-human IgG (1:10,000), developed using TMB substrate and read at 450 nm. A sample was considered positive if values were greater than the mean + 2 standard deviations from normal sera in 25 healthy controls (218 ng/ml for K-α 1 tubulin and 160 ng/ml for collagen V). Antibody concentration was calculated using a standard curve of known concentration of K-α 1 tubulin or collagen V antibodies (Santa Cruz Biotechnology). Serum concentrations of IL-1β, IFN-γ, IL-17, and IL-10 were measured using the Luminex assay according to the manufacturer's protocols (Invitrogen, Carlsbad, CA).
Statistical analysis
We compared continuous variables with normal distribution using the Student's t-test and skewed data using the Mann-Whitney test. Categorical variables were compared using the chi square test. We used the Kaplan-Meier method to evaluate freedom from BOS and compared groups using the log rank test. In addition, we constructed Cox proportional hazards models to conduct multivariable analyses and evaluated the development and clearance of antibodies to self-antigens as time-dependent variables. We conducted the statistical analysis using SPSS 17.0 and GraphPad Prism and considered p values < 0.05 statistically significant.
RESULTS
Follow-up was complete through 4/1/2011, and the study included 323 patient-years of follow-up. The median follow-up per patient was 3.1 years (mean ± standard deviation = 2.7 ± 1.4 years). Seventy-two of the 108 (67%) recipients were found to have antibodies to either K-α 1 tubulin or collagen V (Figure 2). Among these, 64 (89%) had antibodies to both K-α 1 tubulin and collagen V, 7 (10%) had antibodies only to K-α 1 tubulin, and 1 (1%) had antibodies only to collagen V. Recipient demographics are shown in Table 1. Men were more likely to have antibodies to self-antigens than women, but this was not statistically significant (p = 0.10, Table 1). There was no association between the underlying diagnosis and the development of antibodies to self-antigens, and the distribution of diagnoses spanned the spectrum of end-stage lung diseases. Most patients underwent bilateral transplantation, which is the routine practice at our center. Patients who had antibodies to self-antigens were more likely to be sensitized to HLA before transplantation, but this was not statistically significant (p = 0.16, Table 1). The two groups had similar ischemic times, frequency of needing cardiopulmonary bypass during transplantation, and PGD grades immediately after transplantation (Table 1). Finally, both groups had a similar number of HLA mismatches at the A, B, and DR loci. The acute rejection profile for patients who had antibodies to self-antigens and those who did not is shown in Table 2; there were no significant differences in the incidence or severity of acute rejection between the two groups (Table 2).
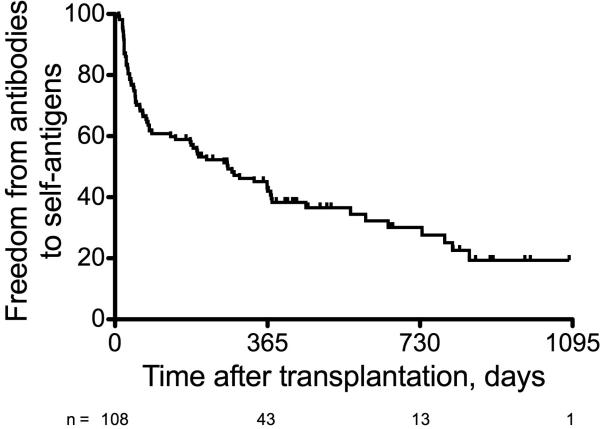
Figure 2.
Freedom from antibodies to self-antigens for the entire cohort.
Table 1.
Recipient demographics (n = 108).
| Variable | Auto-antibody positive (n = 72) | Auto-antibody negative (n = 36) | p |
|---|---|---|---|
| Age, years | 49.1 ± 15.1 | 48.4 ± 14.8 | 0.82 |
| Male gender, n (%) | 44 (61%) | 16 (44%) | 0.10 |
| Diagnosis, n (%) | 0.72 | ||
| COPD or α1-AE | 23 (32%) | 11 (31%) | |
| Cystic fibrosis | 15 (21%) | 9 (25%) | |
| Pulmonary fibrosis | 19 (26%) | 10 (28%) | |
| Pulmonary hypertension | 3 (4%) | 0 (0%) | |
| Re-transplant | 5 (7%) | 1 (3%) | |
| Other diagnosis | 7 (10%) | 5 (14%) | |
| Bilateral lung transplant, n (%) | 70 (97%) | 33 (92%) | 0.34 |
| Cardiopulmonary bypass, n (%) | 31 (43%) | 16 (44%) | 0.94 |
| Ischemic time, mean ± SD | 304 ± 52 | 295 ± 76 | 0.65 |
| CMV mismatch (R-/D+), n (%) | 17 (24%) | 13 (36%) | 0.17 |
| Allosensitized at transplant, n (%) | 30 (42%) | 10 (28%) | 0.16 |
| HLA mismatch, median | 5 | 5 | 0.45 |
| Primary graft dysfunction at T0 | 0.23 | ||
| PGD grade 0 | 16 (22%) | 12 (33%) | |
| PGD grade 1 | 19 (26%) | 13 (36%) | |
| PGD grade 2 | 21 (29%) | 6 (17%) | |
| PGD grade 3 | 16 (22%) | 5 (14%) | |
| Post-transplant ventilator days, median | 2 | 1 | .52 |
Table 2.
Acute rejection profile for recipients who developed antibodies to self-antigens and those who did not.
| Variable | Auto-antibody positive (n = 72) | Auto-antibody negative (n = 36) | p |
|---|---|---|---|
| Number of episodes of acute rejection | |||
| Grade ≥ A1, mean ± SD | 1.00 ± 1.13 | 1.33 ± 1.27 | 0.17 |
| Grade ≥ A2, mean ± SD | 0.56 ± 0.69 | 0.72 ± 0.94 | 0.30 |
| Grade ≥ A3, mean ± SD | 0.07 ± 0.26 | 0.06 ± 0.23 | 0.77 |
| Highest A grade, mean ± SD | 1.14 ± 1.05 | 1.19 ± 0.98 | 0.79 |
| Cumulative acute rejection score, mean ± SD | 1.64 ± 1.79 | 2.11 ± 2.23 | 0.24 |
There were no episodes of acute rejection grade A4 during the study period in either group.
During the study period, 57 of the 108 (53%) recipients developed DSA and 51 (47%) did not. Those who developed DSA were significantly more likely to have antibodies to self-antigens than those who did not; in fact, 55 of the 57 (96%) who developed DSA had antibodies to self-antigens, while 17 of the 51 (33%) who did not develop DSA had antibodies to self-antigens (p < 0.001). Among those who developed DSA and had auto-antibodies, 54 received preemptive antibody-directed therapy for DSA as part of our clinical protocol; one patient was not treated because of a concomitant severe critical illness. Among the 54 who were treated, 38 (70%) were treated with rituximab and IVIG and 16 (30%) were treated with IVIG alone. After treatment, 16 of the 54 (30%) patients cleared the antibodies to K-α 1 tubulin and collagen V. A greater proportion of those treated with rituximab and IVIG (14 of 38; 37%) cleared the antibodies to self-antigens than those treated with IVIG alone (2 of 16; 13%), but this was not statistically significant (p = 0.07).
The development of antibodies to self-antigens, analyzed as a time-dependent variable, was a significant risk factor for BOS in a Cox proportional hazards model (HR = 3.32; 95% CI: 1.86 – 5.92, p < 0.0005). In addition, there was a significant association between the serum concentration of antibodies to collagen V before treatment and the risk of BOS (HR = 1.001; 95% CI: 1.001 – 1.002, p = 0.002) and a significant association between the serum concentration of antibodies to K-α 1 tubulin before treatment and the risk of BOS (HR = 1.001; 95% CI: 1.001 – 1.002, p = 0.003). Furthermore, the development of antibodies to self-antigens, analyzed as a time-dependent variable, was a significant risk factor for death after transplantation (HR = 2.37; 95% CI: 1.03 – 5.46, p = 0.04).
We previously showed that patients who cleared the DSA after treatment were less likely to develop BOS than those who had persistent DSA (19). Similarly, 3 of the 15 (20%) recipients who cleared the antibodies to self-antigens in this cohort developed BOS compared to 27 of the 38 (71%) who had persistent antibodies to self-antigens (p < 0.0005). Furthermore, clearance of antibodies to self-antigens was associated with better freedom from BOS independent of DSA clearance. Among the 31 patients who cleared the DSA after treatment, 12 cleared the antibodies to self-antigens and 19 had persistent antibodies to self-antigens, and 2 of the 12 (17%) who cleared the antibodies to self-antigens developed BOS compared to 13 of the 19 (68%) who had persistent antibodies to self-antigens (p = 0.005). Likewise, among the 23 patients who had persistent DSA, 4 cleared the antibodies to self-antigens and 19 had persistent antibodies to self-antigens, and 1 of the 4 (25%) who cleared the antibodies to self-antigens developed BOS compared to 14 of the 19 (74%) who had persistent antibodies to self-antigens (p = 0.043).
In addition, to avoid assigning risk before event occurrence, we evaluated the impact of clearance of antibodies to self-antigens on the risk of BOS using a Cox proportional hazards model with clearance as a time-dependent variable. In this univariable analysis, clearance of antibodies to self-antigens was associated with a significantly lower risk of BOS (HR = 0.26; 95% CI: 0.09 – 0.75, p = 0.01). Furthermore, to evaluate the impact of clearance of antibodies to self-antigens on the risk of BOS in the context of other risk factors, we constructed a multivariable Cox proportional hazards model with clearance of antibodies to self-antigens as a time-dependent variable (Table 3). Clearance of antibodies to self-antigens was again associated with a significantly lower risk of BOS (HR = 0.24; 95%CI: 0.08 – 0.70, p = 0.009). Only episodes of acute rejection, lymphocytic bronchiolitis, and community-acquired respiratory viral infections that occurred before the diagnosis of BOS were included in this analysis. In addition, those who cleared the antibodies to self-antigens had a similar number of bronchoscopies and transbronchial lung biopsies (5.0 ± 1.3) as those who had persistent antibodies to self-antigens (5.14 ± 1.3; p = 0.73).
Table 3.
Multivariable Cox proportional hazards model of risk factors for bronchiolitis obliterans syndrome.
| Variable | HR | 95% CI | p value |
|---|---|---|---|
| Clearance of antibodies to self-antigens* | 0.24 | 0.08 – 0.70 | 0.009 |
| Primary graft dysfunction at T0 | 0.49 | ||
| PGD grade 1 | 0.38 | 0.09 – 1.66 | 0.20 |
| PGD grade 2 | 0.60 | 0.16 – 2.26 | 0.45 |
| PGD grade 3 | 0.81 | 0.21 – 3.11 | 0.76 |
| Acute rejection grade ≥ A2 | 1.25 | 0.89 – 2.71 | 0.12 |
| Lymphocytic bronchiolitis grade ≥ B2 | 0.49 | 0.15 – 1.59 | 0.36 |
| Community-acquired respiratory virus | 2.37 | 0.76 – 7.88 | 0.16 |
Clearance of antibodies to self-antigens was evaluated as a time-dependent variable.
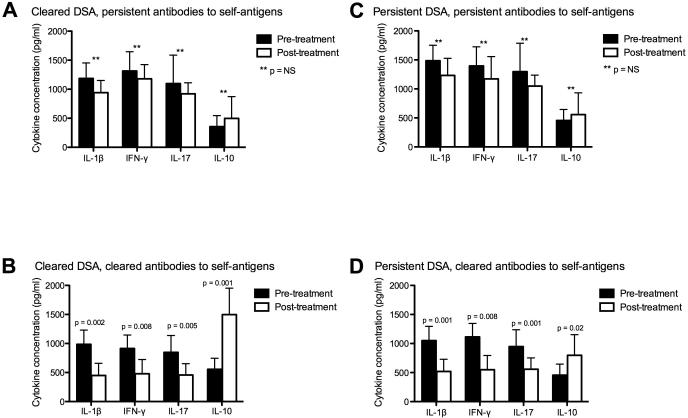
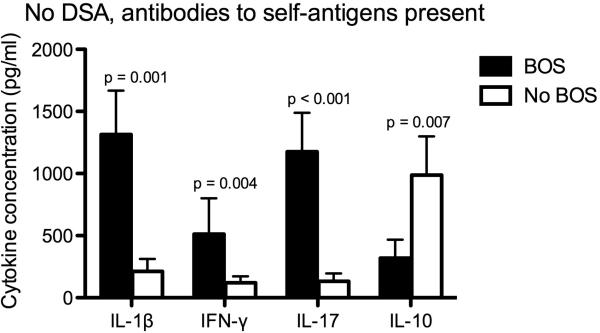
To explore the association between antibodies to self-antigens and other markers of inflammation, we measured serum pro-inflammatory and anti-inflammatory cytokine concentrations and correlated these with clinical outcomes. Among patients who cleared the DSA after treatment, those who had persistent antibodies to self-antigens and developed BOS had similar concentrations of IL-1β (1185 ± 267 vs. 941 ± 210 pg/ml; p = 0.23), IFN-γ (1314 ± 331 vs. 1179 ± 245 pg/ml; p = 0.31), IL-17 (1097 ± 491 vs. 921 ± 189 pg/ml; p = 0.12), and IL-10 (356 ± 190 vs. 497 ± 376 pg/ml; p = 0.67) before and after treatment (Figure 3A). In contrast, those who cleared the antibodies to self-antigens and did not develop BOS had a significant reduction in the concentrations of IL-1β (985 ± 245 vs. 449 ± 210 pg/ml; p = 0.002), IFN-γ (914 ± 231 vs. 479 ± 245 pg/ml; p = 0.008), and IL-17 (847 ± 291 vs. 457 ± 195 pg/ml; p = 0.005), and a significant increase in IL-10 (556 ± 190 vs. 1497 ± 456 pg/ml; p = 0.001) after treatment (Figure 3B). Similarly, among those with persistent DSA after treatment, those who had persistent antibodies to self-antigens and developed BOS had similar concentrations of IL-1β (1485 ± 296 vs. 1233 ± 280 pg/ml; p = 0.18), IFN-γ (1395 ± 339 vs. 1173 ± 385 pg/ml; p = 0.41), IL-17 (1297 ± 491 vs. 1049 ± 189; p = 0.39), and IL-10 (456 ± 190 vs. 557 ± 376 pg/ml; p = 0.57) before and after treatment (Figure 3C). On the other hand, those who cleared the antibodies to self-antigens and did not develop BOS had a significant reduction in the concentrations of IL-1β (1051 ± 241 vs. 519 ± 213 pg/ml; p = 0.001), IFN-γ (1114 ± 253 vs. 549 ± 205 pg/ml; p = 0.008), and IL-17 (947 ± 291 vs. 557 ± 195 pg/ml; p = 0.001), and a significant increase in IL-10 (456 ± 190 vs. 797 ± 356 pg/ml; p = 0.02) after treatment (Figure 3D). In addition, among patients who had antibodies to self-antigens but did not have DSA, those who developed BOS had significantly higher serum concentrations of IL-1β (1314 ± 354 vs. 212 ± 100 pg/ml; p = 0.001), IFN-γ (513 ± 287 vs. 121 ± 51 pg/ml; p = 0.004), and IL-17 (1176 ± 313 vs. 132 ± 64 pg/ml; p < 0.001), and significantly lower concentrations of IL-10 (319 ± 148 vs. 987 ± 312 pg/ml; p = 0.007) than those who did not develop BOS (Figure 4).
Figure 3.
A. Among patients who cleared DSA but had persistent antibodies to self-antigens and developed BOS (n = 13), there was no significant change in pro-inflammatory cytokine (IL-1β, IFN-γ, and IL-17) concentrations or in IL-10 concentrations before and after antibody-directed therapy.
B. Among patients who cleared DSA and antibodies to self-antigens and did not develop BOS (n = 10), there was a significant reduction in pro-inflammatory cytokine concentrations and a significant increase in IL-10 after antibody-directed therapy.
C. Among patients with persistent DSA and persistent antibodies to self-antigens who developed BOS (n = 14), there was no significant change in pro-inflammatory cytokine (IL-1β, IFN-γ, and IL-17) concentrations or in IL-10 concentrations before and after antibody-directed therapy.
D. Among patients with persistent DSA who cleared antibodies to self-antigens and did not develop BOS ( n = 3), there was a significant reduction in pro-inflammatory cytokine concentrations and a significant increase in IL-10 after antibody-directed therapy.
Figure 4.
Among patients who had antibodies to self-antigens but did not have DSA, those who developed BOS (n = 12) had significantly higher pro-inflammatory cytokine (IL-1β, IFN-γ, and IL-17) concentrations and lower IL-10 concentrations than those who did not develop BOS (n = 5).
DISCUSSION
In this study, we evaluated the incidence of antibodies to two self-antigens, collagen V and K-α 1 tubulin, and their role in BOS development. Our findings demonstrate that the development of antibodies to self-antigens is surprisingly common after lung transplantation and that they are linked to DSA development. In fact, 67% of recipients in this cohort had antibodies to self-antigens, and 96% of those who developed DSA also developed antibodies to self-antigens. However, persistence of antibodies to self-antigens after treatment was more common than DSA persistence. Indeed, the clearance rate of antibodies to self-antigens after treatment was only 30% compared to the clearance rate of DSA, which was over 60% in our previous study (19). This is consistent with our previous findings that antibodies to self-antigens can persist after DSA depletion (15). Nonetheless, clearance of antibodies to self-antigens was associated with a lower risk of BOS, and this was independent of DSA clearance. In addition, depletion of antibodies to self-antigens was associated with a significant reduction in pro-inflammatory cytokines (IL-1β, IFN-γ, and IL-17) and an increase in anti-inflammatory IL-10, and this correlated with freedom from BOS regardless of DSA depletion. We chose these cytokines since IL-1β is increased in systemic inflammatory responses (23), IFNγ has been shown to be important in Th1 cellular responses (24), IL-17 is a marker for Th17 cellular responses and induction of autoimmunity (25), and IL-10 is a Th2 and regulatory T-cell marker (26). In contrast, there was no significant change in pro-inflammatory cytokines or IL-10 concentrations after treatment among those who had persistent antibodies to self-antigens and developed BOS.
The association between DSA and the development of antibodies to self-antigens underscores the potential interaction between alloimmunity and autoimmunity. And although the specific biologic interactions have not been completely characterized in clinical transplantation, we have shown in a murine model that administration of antibodies to mismatched major histocompatibility complex (MHC) antigens intrabronchially can induce the development of antibodies to collagen V and K-α 1 tubulin and lead to small airway obliteration similar to human OB through an IL-17-dependent mechanism (17). These findings are consistent with human studies demonstrating that the alloimmune responses can induce cellular and humoral autoimmune responses to collagen V and K-α 1 tubulin through the IL-17 pathway (13, 15, 18). Importantly, the binding of K-α 1 tubulin by its antibodies to airway epithelial cells results in increased expression of transcription factors (TCF5 and c-Myc) and growth factors (HB-EGF, TGF-β, and VEGF) that activate the fibroproliferation cascade (16). In addition, the adoptive transfer of lymphocytes from collagen V-immunized rats induced small airway obliteration in a rat lung isograft model (13). Collectively, these findings suggest that the immune responses to self-antigens can directly lead to chronic rejection.
We propose that graft injury can expose cryptic self-antigens or their determinants leading to immune responses to these self-antigens, which promote graft rejection. While regulatory T-cells control autoreactive and alloreactive effector T-cells under normal conditions, loss of this peripheral tolerance can lead to chronic rejection (14). It is possible that standard maintenance immunosuppressive regimens using calcineurin inhibitors may promote the loss of peripheral tolerance by suppressing regulatory T-cell functions thereby facilitating the development of immune responses to self-antigens (27-29). Indeed, the persistence of antibodies to self-antigens after treatment was more common than the persistence of DSA.
There are several limitations inherent to this study's design. First, the clinical decision to initiate treatment was not based on the development of antibodies to self-antigens but rather on DSA development, and although antibodies to self-antigens and DSA were tightly linked, this may have introduced bias. In addition, this was not a randomized controlled trial, and the cohort did not include a control group. It is therefore difficult to make conclusions about the efficacy of treatment. Additionally, patients were not randomized to the treatment arms (rituximab and IVIG vs. IVIG alone), so no conclusions can be made regarding the efficacy of each regimen. Furthermore, although successful depletion of antibodies to self-antigens was associated with greater freedom from BOS, this was only accomplished in 30% of treated patients. Clearly, more effective regimens are necessary to improve clinical outcomes. Nevertheless, our findings emphasize an important role for immune responses to self-antigens in BOS development and suggest that the development of antibodies to self-antigens may be a useful biomarker of chronic graft rejection. Prevention of autoimmunity development and evolution of new strategies to treat autoimmunity such as IL-6 or IL-17 blockade may be necessary to prevent BOS development following human lung transplantation.
In conclusion, antibodies to collagen V and K-α 1 tubulin are common among lung transplant recipients, and they are associated with an increased risk of BOS development. Although successful antibody depletion mitigates this increased risk, the ideal clinical regimen is unknown. Clearly, additional studies to define the role of immune responses to self-antigens in the development of chronic graft rejection after lung transplantation and of clinical approaches to abrogate it are needed.
ACKNOWLEDGEMENTS
This work is supported by NIH RO1 HL056643-13A1, R34 HL105412, and the BJC foundation (RRH and TM).
Footnotes
DISCLOSURE
This work and manuscript were not prepared nor funded by any commercial organization. The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
REFERENCES
- 1.Thabut G, Christie JD, Kremers WK, Fournier M, Halpern SD. Survival differences following lung transplantation among US transplant centers. JAMA. 2010;304(1):53–60. doi: 10.1001/jama.2010.885. [DOI] [PubMed] [Google Scholar]
- 2.Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult lung and heart-lung transplant report – 2010. J Heart Lung Transplant. 2010;29(10):1083–1141. doi: 10.1016/j.healun.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Khalifah AP, Hachem RR, Chakinala MM, et al. Minimal acute rejection after lung transplantation: a risk for bronchiolitis obliterans syndrome. Am J Transplant. 2005;5(8):2022–30. doi: 10.1111/j.1600-6143.2005.00953.x. [DOI] [PubMed] [Google Scholar]
- 4.Glanville AR, Aboyoun CL, Havryck A, Plit M, Rainer S, Malouf MA. Severity of lymphocytic bronchiolitis predicts long-term outcome after lung transplantation. Am J Respir Crit Care Med. 2008;177(9):1033–40. doi: 10.1164/rccm.200706-951OC. [DOI] [PubMed] [Google Scholar]
- 5.Girnita AL, Duquesnoy R, Yousem SA, et al. HLA-specific antibodies are risk factors for lymphocytic bronchiolitis and chronic lung allograft dysfunction. Am J Transplant. 2005;5(1):131–8. doi: 10.1111/j.1600-6143.2004.00650.x. [DOI] [PubMed] [Google Scholar]
- 6.Davis RD, Lau CL, Eubanks S, et al. Improved lung allograft function after fundoplication in patients with gastroesophageal reflux disease undergoing lung transplantation. J Thorac Cardiovasc Surg. 2003;125(3):533–42. doi: 10.1067/mtc.2003.166. [DOI] [PubMed] [Google Scholar]
- 7.Daud SA, Yusen RD, Meyers BF, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007;175(5):507–13. doi: 10.1164/rccm.200608-1079OC. [DOI] [PubMed] [Google Scholar]
- 8.Khalifah AP, Hachem RR, Chakinala MM, et al. Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. Am J Respir Crit Care Med. 2004;170(2):181–7. doi: 10.1164/rccm.200310-1359OC. [DOI] [PubMed] [Google Scholar]
- 9.Jurcevic S, Ainsworth ME, Pomerance A, et al. Antivimentin antibodies are an independent predictor of transplant-associated coronary artery disease after cardiac trasnplantation. Transplantation. 2001;71(7):886–92. doi: 10.1097/00007890-200104150-00011. [DOI] [PubMed] [Google Scholar]
- 10.Morgun A, Shulzhenko N, Unterkircher CS, et al. Pre- and post-transplant anti-myosin and anti-heat shock protein antibodies and cardiac transplant outcome. J Heart Lung Transplant. 2004;23(2):204–9. doi: 10.1016/S1053-2498(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 11.Dragun D, Muller DN, Brasen JH, et al. Angiotensin II type1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005;352:558–69. doi: 10.1056/NEJMoa035717. [DOI] [PubMed] [Google Scholar]
- 12.Reinsmoen NL, Lai C, Heidecke H, et al. Anti-angiotensin type 1 receptor antibodies associated with antibody mediated rejection in donor HLA antibody negative patients. Transplantation. 2010;90:1473–1477. doi: 10.1097/TP.0b013e3181fd97f1. [DOI] [PubMed] [Google Scholar]
- 13.Burlingham WJ, Love RB, Jankowska-Gan E, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117:3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bharat A, Fields RC, Steward N, Trulock EP, Patterson GA, Mohanakumar T. CD4+25+ regulatory T cells limit Th1-autoimmunity by inducing IL-10 producing T cells following human lung transplantation. Am J Transplant. 2006;6:1799–1808. doi: 10.1111/j.1600-6143.2006.01383.x. [DOI] [PubMed] [Google Scholar]
- 15.Saini D, Weber J, Ramachandran S, et al. Alloimmunity-induced autoimmunity as a potential mechanism in the pathogenesis of chronic rejection of human lung allografts. J Heart Lung Transplant. 2011;30(6):624–31. doi: 10.1016/j.healun.2011.01.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goers TA, Ramachandra S, Aloush A, Trulock E, Patterson GA, Mohankumar T. De novo production of K-α 1 tubulin-specific antibodies: role in chronic lung allograft rejection. J Immunol. 2008;180:4487–4494. doi: 10.4049/jimmunol.180.7.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukami N, Ramachandran S, Saini D, et al. Antibodies to MHC class I induces autoimmunity: role in the pathogenesis of chronic rejection. J Immunol. 2009;182(1):309–318. doi: 10.4049/jimmunol.182.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan L, Benson HL, Vittal R, et al. Neutralizing IL-17 prevents obliterative bronchiolitis in murine orthotopic lung transplantation. Am J Transplant. 2011;11:911–922. doi: 10.1111/j.1600-6143.2011.03482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hachem RR, Yusen RD, Meyers BF, et al. Anti-human leukocyte antigen antibodies and preemptive antibody-directed therapy after lung transplantation. J Heart Lung Transplant. 2010;29:973–980. doi: 10.1016/j.healun.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–12242. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 21.Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 22.Bharat A, Saini D, Steward N, et al. Antibodies to self-antigens predispose to primary lung allograft dysfunction and chronic rejection. Ann Thorac Surg. 2010;90:1094–1101. doi: 10.1016/j.athoracsur.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dinarello CA. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur J Immunol. 2011;41:1203–17. doi: 10.1002/eji.201141550. [DOI] [PubMed] [Google Scholar]
- 24.Bradley LM, Dalton DK, Croft M. A direct role for IFN-gamma in regulation of Th1 cell development. J Immunol. 1996;157:1350–1358. [PubMed] [Google Scholar]
- 25.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annual Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 26.O'Garra A, Vieira PL, Vieira P, Goldfeld AE. IL-10-producing and naturally occurring CD4+ Tregs: limiting collateral damage. J Clin Invest. 2004;114:1372–1378. doi: 10.1172/JCI23215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeiser R, Nguyen VH, Beilhack A, et al. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood. 2006;108:390–399. doi: 10.1182/blood-2006-01-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakaguchi S, Sakaguchi N. Thymus and autoimmunity. Transplantation of the thymus from cyclosporine A-treated mice causes organ-specific autoimmune disease in athymic nude mice. J Exp Med. 1988;167:1479–1485. doi: 10.1084/jem.167.4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakaguchi S, Sakaguchi N. Organ-specific autoimmune disease induced in mice by elimination of T cell subsets. Neonatal administration of cyclosporine A causes autoimmune disease. J Immunol. 1989;142:471–480. [PubMed] [Google Scholar]