Abstract
Population-based longitudinal clinicopathological studies provide an ideal opportunity to study a variety of risk and protective factors in relation to pathology associated with dementia in individuals who are representative of the general population. The 90+ Study is a population-based study designed specifically to study aging and dementia as well as its neuropathological correlates in participants 90 years of age and older. We present demographic and pathological data on the first 104 participants to come to autopsy from the brain donation component of the study, The 90+ Autopsy Study. Cognitive diagnosis was assigned according to Diagnostic and Statistical Manual 4th edition criteria for dementia and neuropathological diagnoses were made according to the Consortium to Establish a Registry for Alzheimer’s Disease protocol. Dementia was present in 61% of autopsied participants, the majority of whom were diagnosed with Alzheimer’s disease (85%). Many different types of pathology typically associated with dementia were common in the oldest-old, and included neurofibrillary tangles, neuritic plaques, diffuse plaques, Lewy bodies, hippocampal sclerosis, and cerebral infarctions. Most types of pathology were more frequently found in participants suffering from dementia but there was extensive overlap in pathology among those with and without dementia. In addition, 22% of demented participants did not have sufficient pathology to account for their cognitive loss. Our results highlight the poor associations between these common pathological lesions and dementia in the oldest-old and the importance of considering many different types of pathology, possibly including some yet to be identified, in order to account for all dementias in the oldest-old.
Keywords: Dementia, Alzheimer’s disease, cohort study, longitudinal studies, neuropathology, oldest-old
INTRODUCTION
The oldest-old are the fastest growing segment of our population. Currently numbering around 2 million people [1], by 2050 about 10 million Americans will be 90 years of age or older [2]. The striking increase in the number of the oldest-old presents a public health challenge to promote the quality as well as the quantity of life. Up to 40% of people in this age group will have dementia [3] and a greater percentage will endure functional disabilities [4]. Prospective longitudinal investigations are essential to identify risk and protective factors that influence cognitive and functional status in this age group. Furthermore, being able to relate the cognitive and functional abilities of well-characterized individuals to neuropathological findings is crucial to understanding disorders in these individuals.
Population-based prospective clinical pathological studies are the ideal setting to study a variety of risk and protective factors in relation to dementing pathologies in participants with a wide variety of cognitive and functional abilities. Unfortunately, these types of studies are uncommon [5]. Most clinical-pathological studies in the elderly are composed of volunteer cohorts, hospital patients, or clinic-based cases of Alzheimer’s disease. Therefore, participants in those studies are not likely to be representative of the general population. Although much has been learned from these pathological studies of the elderly, the relative importance of certain pathologies or the relationships between pathologies and risk factors may not be generalizable to the elderly population as a whole [5].
The handful of existing prospective population-based pathological studies of aging and dementia include few oldest-old participants. In addition, these studies are typically designed to study a wide age range of elderly. Thus, some aspects of the study design may not be adequate for the very elderly participants in these studies. For example, because of the large size of these prospective studies, the interval between evaluations is usually of several years, but this interval may be too long to capture the rapid rates of cognitive and functional deterioration in the oldest-old.
The 90+ Study was specifically created and designed to fill the void in current studies and to try to answer some important questions about our oldest-old citizens. The 90+ Study is a population-based epidemiological longitudinal study of aging and dementia in people aged 90 years and older with a subset of participants who have agreed to post-mortem examination. Evaluations are done every 6 months, a shorter interval than most studies. By design, participants in The 90+ Study are expected to be free of some of the selection biases in other studies. Thus, they are more representative of the oldest-old population as a whole and have a full range of cognitive and functional abilities, comorbidities, medication use, and risk and protective factors.
Here, we summarize the methods, assessment, and enrollment of The 90+ Study and its brain donation component, The 90+ Autopsy Study. We also describe basic demographic characteristics of the participants in the two study components. Finally, we present pathological data and how the pathology relates to cognitive status in 104 oldest-old participants from The 90+ Autopsy Study.
METHODS
Participants
Participants in The 90+ Study are survivors from the Leisure World Cohort Study (LWCS), an epidemiologic investigation of a retirement community in Orange County, CA (Leisure World, Laguna Woods). The LWCS was initiated when researchers mailed a health survey to all residents in the Leisure World retirement community [6]. A total of 13,978 residents (61%) with a median age of 73 years returned the questionnaire. In addition to the original 1981 survey, cohort members were sent follow-up questionnaires in subsequent years. The Leisure World cohort is an ideal sample for studies of the oldest-old since it is an intact, highly cooperative cohort, with nearly all surviving members of the cohort aged 90+ years.
The 90+ Study was initiated in 2003 when the 1,146 surviving participants from the original LWCS who were aged 90 years and older on January 1, 2003 were invited to join. From the original 13,978 LWCS participants, the majority did not meet criteria for enrollment, either because they had died before January 1, 2003 (79%) or where younger than 90 years of age (13%). Of the 1,146 people who did meet criteria for participation, we were able to enroll 961 (84%) with the remaining either refusing (11%) or untraceable (5%). We also extended an invitation to join The 90+ Study to those participants from the original cohort who were 90 years and older on January 1, 2008 and to those who turn 90 thereafter. As of January 2010, about 1,400 participants had joined The 90+ Study.
Recruitment
Although it is not standardized to the whole U.S. Population, The 90+ Study is a population-based study because subjects comprise survivors of a study established in 1981 in a geographically defined area (The Leisure World Retirement Community). In addition, as described in the following sections, participants lived at home as well as in institutions, resided across the country, represent the full spectrum of health and cognitive abilities, and are representative of the oldest-old population in Orange County.
To maintain the population-based aspect of the cohort it was crucial to recruit as many people as possible from the original cohort. Given the advanced age of the participants and the full spectrum of cognitive and functional abilities, health status, and geographic locations, we provided much flexibility in terms of how and where evaluations took place.
All cohort members are initially asked to undergo a comprehensive in-person evaluation. In many cases, the evaluation is done at the clinic but very often participants cannot travel to the clinic and the evaluation is instead done at their place of residence, including assisted living facilities, and nursing homes. Sometimes participants, or their relatives on their behalf, do not agree to an in-person evaluation, usually due to frailty, cognitive impairment, or poor health. In such situations, we obtain information via telephone or mail either from the participants themselves or from their informants. Due to the advanced age of the cohort, we obtained information via telephone or mail from suitable informants for participants who had passed away before we were able to contact them.
Although most participants still resided in or near the Leisure World Community, about a third had moved out of the community often to be closer to relatives who could help care for them. In those situations, we visit participants at their homes and have traveled to 31 states to perform evaluations.
We have used several different strategies to trace and locate participants in the LWCS and 90+ Study. Participant’s addresses are kept up-to-date by mailing periodic newsletters to cohort members updating them of study findings. Addresses of those returned as undeliverable are updated with addresses provided by the post office. When participants cannot be located, we contact their next-of-kin. We also use internet and commercial databases to locate participants and their relatives.
Enrollment
The 90+ Study is composed of two main cohorts recruited from surviving participants of the original LWCS: the 1,146 who were aged 90 years and older on January 1, 2003 (2003 Cohort) and those who were aged 90 years and older on or after January 1, 2008 (2008 Cohort). As of January 2010, a total of 961 participants (84%) had been recruited to the 2003 Cohort and 402 participants (74%) had joined the 2008 Cohort.
Over 70% (N=814) of enrolled participants who were alive at first contact agreed to in-person longitudinal examinations. During every subsequent 6-month follow-up examination of the in-person subjects, we completed data collection on more than 94% of survivors, resulting in an excellent follow-up rate. We have completed two or more visits on over 700 participants, three or more visits on over 500 participants, and some participants have been seen as many as 14 times.
Brain donation is an important component of The 90+ Study and thus all participants evaluated in-person are invited to take part of The 90+ Autopsy Study. A total of 202 participants, about 35% of all participants with in-person clinical evaluations, had enrolled in The 90+ Autopsy Study as of January 2010. Given the rarity of well-characterized oldest-old subjects participating in neuropathological studies, we also enrolled a small group of 29 volunteers who approached us for participation in The 90+ Autopsy Study but who were not part of the original Leisure World Cohort. These volunteers were mostly friends and family of study participants. Although many participants sign up for the autopsy study after their initial visit, most have agreed after several visits, including participants who have consented after being seen more than 10 times. Thus, we continue to discus brain donation with participants during every visit. All participants evaluated in-person are invited to participate in The 90+ Autopsy Study, regardless of where they reside. For participants who live more than two hours away from UCI, we arrange for brain collection with local facilities, such as ADRCs, research universities, or hospitals. These facilities perform the brain removal according to a standard procedure and then ship the brain whole to UCI for autopsy.
As of January 2010, of the 202 participants enrolled in the Autopsy study, 120 autopsies had been completed on the 134 people who died, giving us an autopsy rate of 90%. As we continue to follow participants who have consented to brain donation and continue to enroll participants, we expect to have one of the largest collections of brains of oldest-old participants available for clinical-pathological studies.
Clinical Assessments
The full clinical evaluation includes a neurological exam (with mental status testing and assessment of functional abilities) by a trained physician or nurse practitioner and a comprehensive neuropsychological test battery [7] (including the Mini-Mental State Exam (MMSE; score range: 0–30) [8]). Participants evaluated by phone complete the short version of the Cognitive Abilities Screening Instrument (CASI-short; score range: 0–34) [9]. In addition, all participants (or their informants) are asked to provide information about demographics, past medical history, and medication use. Informants of all participants complete a mailed questionnaire about the participant’s cognitive status [10] and functional abilities [11–12]. The Dementia Questionnaire (DQ) [13–14] is done over the phone with informants of participants who show signs of cognitive decline and for all participants shortly after their death, in order to obtain details about the onset of cognitive problems.
The assessments for The 90+ Study were designed keeping in mind the advanced age of the participants and the high prevalence of medical comorbidities, motor limitations, and sensory losses in this population, factors that can affect the validity and reliability of the data obtained [15]. To accommodate the wide variety of cognitive and physical abilities of the participants and to minimize missing data, we made modifications and allowances to the assessments and visits. For example, we provide printed instructions and sound amplifiers for participants with sensory impairments. We also use short forms of tests, keep visits as short as possible, provide frequent breaks, or divide visits into more than one session to minimize fatigue. The presence of comorbidities, sensory losses, and other limitations also make documenting functional loss due to cognitive difficulties in these oldest-old participants very challenging. To overcome these challenges, we modified our functional activities questions to probe in more detail about their functional abilities in order to help us differentiate between functional losses due to cognitive vs. other physical reasons. In addition, we rely heavily on relatives and caregivers of the participants for insight and information regarding functional abilities.
Another aspect of The 90+ Study different from most studies is the short interval between evaluations. As these participants have a high incidence of dementia [16] and disability [17] and a fast rate of cognitive [15] and functional decline, we repeat in-person evaluations every 6 months allowing us to capture fast and frequent changes in cognition and function in this very elderly group.
Cognitive Diagnosis Determination
A determination of cognitive status is made for all participants at every visit. For participants that are seen in-person, cognitive status is determined by a neurological examiner using only information from their current examination and the MMSE test results applying Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) criteria for dementia [18]. Although examiners are not provided with data collected on previous visits to make their diagnoses, they are not completely blinded to previously collected information as they may have evaluated the same participants in multiple occasions. For participants not seen in-person, cognitive status is determined using other available information including the CASI-short or informant questionnaires (for details see [3, 16]).
After a participant dies, another determination of cognitive status is done during a multidisciplinary diagnostic conference, this time using all available information, including the participants’ full evaluations, information collected from informants, laboratory tests, medical records, including CT or MRI scans of the head, and any other relevant studies. All the information is presented and discussed during the diagnostic conference led by the study principal investigator with conferees blinded to pathological diagnosis. Participants are classified as normal, cognitively impaired not demented (CIND), or demented (DSM-IV criteria). CIND is assigned when a participant has evidence of cognitive or functional loss but not of sufficient severity to meet criteria for dementia. If a participant is diagnosed with dementia, the primary and secondary etiologies of the dementia are specified as well as the date when dementia criteria were met.
Brain Tissue Collection and Pathological Evaluation
All procedures for procuring and preparing brain tissues are done according to the uniform datasets and forms of the National Alzheimer Coordinating Center (NACC) and the Alzheimer Disease Research Centers (ADRC). The NIA-Reagan Working Group [19] modification of the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) format [20–21] is used as a standardized diagnostic protocol. The leader of the UCI ADRC Pathology Core performs all diagnostic pathologic evaluations blinded to cognitive diagnosis.
RESULTS
In the next few sections, we present baseline demographic characteristics and pathological findings of participants in The 90+ Study and The 90+ Autopsy Study. We only present characteristics of those participants recruited to the 2003 Cohort, as recruitment is still ongoing for the 2008 Cohort.
Baseline Demographic and Clinical Characteristics
The 90+ Study
The 961 participants enrolled in the 2003 Cohort had a baseline average age of 94 years (range: 90–107), 78% were women, were predominantly Caucasian (99%) and well educated, with 63% having some college education or higher (Table 1). These demographics are similar to that of the elderly population in Orange County in the early 2000s, where according to the US Census, 90+ year olds were mostly women (76%) and Caucasian (91%) [22] and more than half of 65+ year olds had some college education or higher (54%) [23].
Table 1.
Baseline characteristics of non-autopsy and autopsy participants in The 90+ Study 2003 cohort
| Characteristic | All Participants (N = 961)1 | Non-Autopsy (N = 811)1 | Autopsy (N = 150)1 | p-value2 |
|---|---|---|---|---|
|
| ||||
|
Median (range)
| ||||
| Age at entry (y) | 94.9 (90–107) | 95.0 (90–107) | 94.5 (90–104) | 0.10 |
|
| ||||
| Age at death (y) | 96.8 (90–109) | 96.6 (90–109) | 97.9 (92–106) | <0.001 |
|
| ||||
|
Number (%)
| ||||
| Women | 751 (78) | 639 (79) | 112 (75) | 0.26 |
|
| ||||
| Education | ||||
| ≤High School | 351 (37) | 304 (38) | 47 (32) | 0.25 |
| Any College | 414 (44) | 346 (43) | 68 (46) | |
| Any Graduate School | 182 (19) | 148 (19) | 34 (23) | |
|
| ||||
| Living situation | ||||
| In own home alone | 277 (29) | 227 (29) | 50 (34) | 0.04 |
| In own home with relative or caregiver | 267 (28) | 220 (28) | 47 (32) | |
| Assisted living or board and care | 245 (26) | 207 (26) | 13 (26) | |
| Nursing home | 155 (16) | 142 (18) | 30 (9) | |
|
| ||||
| Medical histories at baseline | ||||
| Falls in previous year | 483 (53) | 409 (54) | 74 (52) | 0.76 |
| Heart disease | 404 (45) | 341 (45) | 80 (44) | 0.85 |
| Hypertension | 407 (47) | 336 (46) | 71 (51) | 0.21 |
| TIA | 200 (24) | 175 (25) | 25 (19) | 0.14 |
| Stroke | 133 (15) | 117 (16) | 16 (12) | 0.22 |
|
| ||||
| Neurological exam cognitive diagnosis | ||||
| Normal | 117 (31) | 78 (30) | 39 (35) | 0.58 |
| CIND | 142 (38) | 103 (39) | 39 (35) | |
| Demented | 113 (30) | 80 (31) | 33 (30) | |
Numbers do not always add to the total because of missing data.
p-values are for Wilcoxon rank sum test for continuous variables, Fisher’s exact test for binary variables, chi-square tests for categorical variables, and compare non-autopsy vs. autopsy participants.
Abbreviations: TIA=Transient ischemic attack; CIND=cognitively impaired not demented
At the baseline evaluation, most of the participants lived at home (57%), although many lived in assisted living facilities or nursing homes (42%). About half of participants reported a history of heart disease (45%), hypertension (47%), and falls in the previous year (53%). Less common were a history of stroke (15%) or TIA (24%). Of those evaluated in-person, the cognitive diagnoses were evenly distributed with about a third of participants falling in each of the cognition groups: normal cognition (31%), CIND (38%), and dementia (30%).
Estimates of prevalence and incidence of all-cause dementia and functional disability in The 90+ Study have been found to be very high. The prevalence of dementia was estimated as 41% and was found to increase with age in women but not men [3]. The incidence of dementia was found to increase exponentially with age for both men and women, from 13% per year at ages 90–94, to as high as 41% per year in centenarians [16]. Functional disability was also found to be very prevalent in the oldest-old, with more than half of participants (56%) being dependent on at least one activity of daily living [4].
The 90+ Autopsy Study
The 150 participants enrolled in The 90+ Autopsy Study 2003 Cohort do not differ from non-autopsy participants in most regards including age at entry, gender, education, cognitive diagnosis, or history of stroke or TIA (Table 1). The few differences found are that members of the autopsy cohort are slightly older at time of death and less likely to live in nursing homes than non-autopsy participants. An interesting finding is that the 29 autopsy volunteers who were not part of the initial LWC do differ from the other participants in some basic demographics; they are younger, more frequently male, and more educated (data not shown). This disparity illustrates the potential marked differences in participants who volunteer vs. those who are part of a population-based sample and the potential biases that self-selected participants may bring.
Table 2 shows characteristics of the 104 participants from The 90+ Autopsy Study 2003 Cohort on whom a pathological report was available as of January 2010. The median post-mortem interval was 4.9 hours and the mean interval between last evaluation and death was 3.8 months. The median MMSE score was 18 and covered the full spectrum of cognitive abilities (range: 0–30). Of all participants, 39% were not found to meet criteria for dementia and about half of these were classified as cognitively normal (49%) and half as CIND (51%). On the other hand, the majority of autopsied participants (61%) did meet criteria for dementia. By far, the most common clinical dementia diagnosis was AD (85%) either alone or in combination with other etiologies. Other clinical diagnoses included vascular dementia (8%), fronto-temporal dementia (1.5%), Lewy body disease (1.5%) and two cases on whom the etiology could not be specified (3%).
Table 2.
Characteristics of demented and non-demented autopsy participants in The 90+ Study 2003 cohort
| Characteristic | All Participants (N=104) | Not Demented (N=41) | Demented (N=63) | p-value1 |
|---|---|---|---|---|
|
| ||||
|
Median (Range)
| ||||
| Age at death (y) | 97.3 (92 – 106) | 97.9 (92 – 104) | 96.7 (92 – 106) | 0.19 |
|
| ||||
| Brain weight (g) | 1128 (871 – 1403) | 1157 (872 – 1390) | 1100 (871 – 1403) | 0.005 |
|
| ||||
| MMSE score at last visit | 18.0 (0 – 30) | 27 (16 – 30) | 11 (0 – 28) | <0.001 |
|
| ||||
| Last visit to death interval (months) | 3.8 (0.2 – 43.4) | 3.9 (0.3 – 25.9) | 3.8 (0.2 – 43.4) | 0.84 |
|
| ||||
| Postmortem - interval (hrs) | 4.9 (1.0 – 53.7) | 4.8 (2.2 – 20.8) | 5.0 (1.0 – 53.7) | 0.65 |
|
| ||||
|
Number (%)
| ||||
| Women | 82 (79) | 31 (75) | 51 (81) | 0.62 |
|
| ||||
| APOE e4 present | 24 (23) | 6 (15) | 18 (29) | 0.10 |
|
| ||||
| APOE e2 present | 17 (17) | 9 (22) | 8 (13) | 0.28 |
|
| ||||
| Braak tangle stage | ||||
| I – II | 23 (22) | 12 (29) | 11 (17) | 0.01 |
| III – IV | 40 (38) | 20 (49) | 20 (32) | |
| V – VI | 41 (39) | 9 (22) | 32 (51) | |
|
| ||||
| Neuritic plaques | ||||
| None | 18 (17) | 10 (24) | 8 (13) | 0.02 |
| Sparse | 24 (23) | 8 (20) | 16 (25) | |
| Moderate | 35 (34) | 18 (44) | 17 (27) | |
| Frequent | 27 (26) | 5 (12) | 22 (35) | |
|
| ||||
| Diffuse plaques | ||||
| None | 16 (15) | 8 (19) | 8 (13) | 0.15 |
| Sparse | 18 (17) | 7 (17) | 11 (17) | |
| Moderate | 24 (23) | 13 (32) | 11 (17) | |
| Frequent | 46 (44) | 13 (32) | 33 (52) | |
|
| ||||
| NIA-Reagan criteria | ||||
| None | 18 (17) | 10 (24) | 8 (13) | 0.03 |
| Low | 30 (29) | 11 (27) | 19 (30) | |
| Intermediate | 32 (31) | 16 (39) | 16 (25) | |
| High | 24 (23) | 4 (10) | 20 (32) | |
|
| ||||
| Hippocampal sclerosis | 11 (11) | 0 (0) | 11 (17) | 0.003 |
|
| ||||
| Diffuse Lewy body disease | 8 (8) | 0 (0) | 8 (13) | 0.02 |
|
| ||||
| Cerebral infarcts2 | 31 (30) | 10 (24) | 21 (33) | 0.38 |
p-values are for Wilcoxon rank sum test for continuous variables, Fisher’s exact test for binary variables, chi-square tests for categorical variables, and compare demented vs. not demented participants.
Includes large infarcts, lacunes, or micro-infarcts.
Abbreviations: MMSE=Mini-Mental State Exam; APOE=apolipoprotein; NIA=National Institute on Aging
Pathological Findings
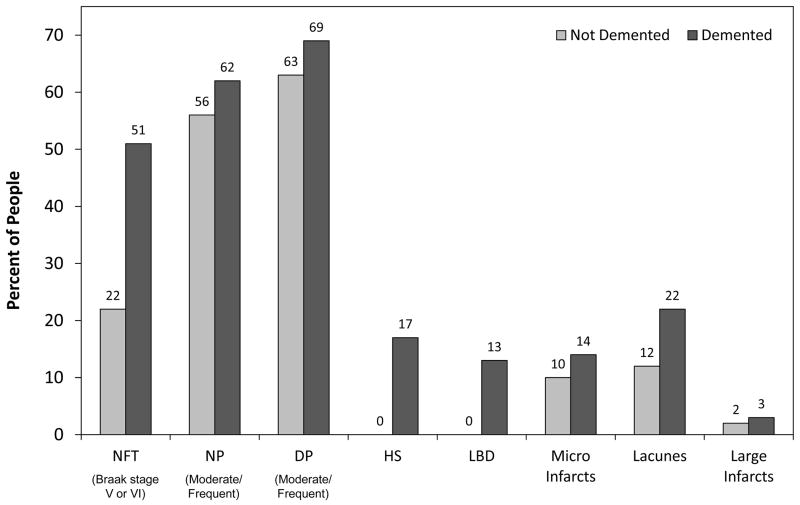
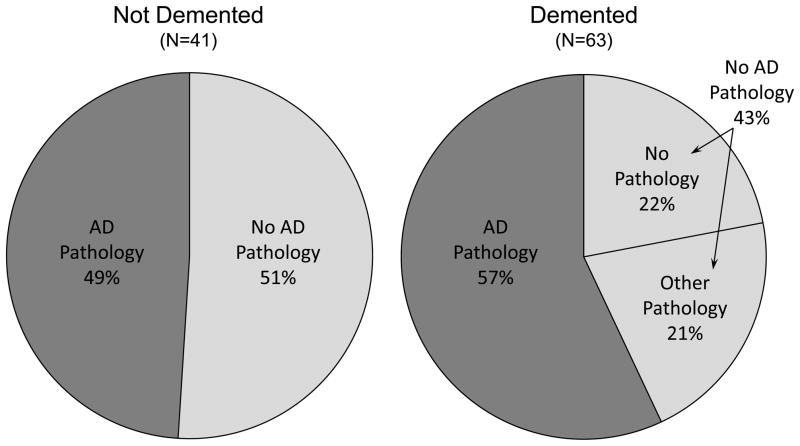
Several different pathologies usually associated to dementia were common in the oldest-old, and included neurofibrillary tangles (NFT), neuritic plaques (NP), diffuse plaques (DP), Lewy bodies, hippocampal sclerosis, and cerebral infarctions (Table 2). Most types of pathology were more frequently found in participants suffering from dementia and several of these pathologies were significantly related to the likelihood of dementia (Table 2 and Figure 1). However, there was extensive overlap in pathology among those with and without dementia, particularly for tangles, plaques, and infarcts (Figure 1). For example, in non-demented participants, 22% had a tangle stage of V or VI, 56% had moderate or frequent neuritic plaques, 64% had moderate or frequent diffuse plaques, and 34% had infarcts. As seen in Figure 2, half of all non-demented participants (49%) and just over half of demented participants (57%) met pathological criteria for AD, defined as intermediate or high likelihood according to NIA-Reagan criteria [19]. There were no other pathological diagnoses given to nondemented participants other than Alzheimer’s disease. In contrast, several other pathological diagnoses were seen among demented participants alone or in combination, including hippocampal sclerosis, diffuse Lewy body disease, corticobasal degeneration, and vascular dementia. The pathologies examined to date failed to explain all dementia in this cohort, as almost one quarter (22%) of all demented participants did not have significant AD or any other pathology to explain their cognitive loss.
Figure 1.
Types of Pathology in Demented and Non-Demented Participants in The 90+ Autopsy Study 2003 Cohort (N=104)
NFT=neurofibrillary tangles; NP=neuritic plaques; DP=diffuse plaques; HS=hippocampal sclerosis; LBD=Lewy body disease; infarcts do not include terminal events
Figure 2.
NIA-Reagan Criteria for Neuropathological Alzheimer’s Disease in Demented and Non-Demented Autopsy Participants in The 90+ Study 2003 Cohort (N=104)
AD Pathology defined as intermediate or high likelihood of AD based on NIA-Reagan Criteria
Other types of pathology include hippocampal sclerosis, diffuse Lewy body disease, Corticobasal degeneration, Braak tangle stage≥5, and vascular dementia pathology
In recent publications and presentations, we have compared the relative importance of pathologies to the likelihood of dementia, have examined factors that may help explain the poor clinical-pathological correlations, and have started to explore other pathologies that may be related to cognitive impairment. Below we summarize some of these initial findings from The 90+ Autopsy Study.
We examined the sensitivity and specificity of neuropathological measures in relation to dementia in the oldest-old in the first 85 participants to come to brain autopsy from The 90+ Study [24]. The area under the curve [AUC] from receiving operating characteristic (ROC) analyses were used to compare Braak plaque stage, Braak tangle stage, and NIA-Reagan criteria in their association with dementia. NIA-Reagan criteria (AUC=.59) and Braak plaque stage (AUC=.59) were poor at predicting clinical dementia. Braak tangle stage had better overall accuracy identifying dementia (AUC=.71), although still not very high. The findings supported the notion that tangles may play a more critical role in dementia in the oldest-old than neuritic plaques, potentially lessening the utility in the oldest-old of biomarkers and therapies that target β-amyloid.
As seen in Figure 2, half of non-demented participants met pathological criteria for AD. We considered the possibility that those participants who met pathological criteria for AD may have had more cognitive impairment than those without AD pathology. This was not the case, as MMSE scores and the proportion of people who were CIND were about the same in the two pathological groups (median MMSE: 26 in the no pathology group vs. 28 in the AD pathology group; CIND: 52% in the no pathology group vs. 55% in the AD pathology group). Thus, other factors must be responsible for allowing some participants to sustain relatively high levels of AD pathology without affecting their cognitive ability. In a recent publication with the first 82 genotyped participants from The 90+ Autopsy Study, we found that this finding can be partially explained by Apolipoprotein E (APOE) genotype [25]. The study included 11 APOE2 carriers, 15 APOE4 carriers, and 56 individuals with APOE3/3 genotype. Compared to those with APOE3/3, APOE4 carriers were more likely to be diagnosed with dementia (odds ratio [OR] = 12.2, 95%CI = 1.5–102.0), whereas APOE2 carriers were not (OR = 0.3, 95% CI = 0.1–1.3). Surprisingly, both APOE4 (OR = 4.6, 95% CI = 1.3–16.5) and APOE2 (OR = 7.8, 95% CI = 1.5–40.2) carriers were more likely to meet neuropathologic criteria for AD (NIA-Reagan criteria) than those with APOE3/3 genotype. Therefore, in the oldest-old, it seems the presence of the APOE2 is associated with a somewhat reduced risk of dementia, but paradoxically is associated with increased AD neuropathology. Oldest- old APOE2 carriers may have some mechanism that contributes to the maintenance of cognition independently of the formation of AD pathology.
We have also begun to explore other pathological markers that may have better associations with cognition in the oldest-old. In a small preliminary study with participants from The 90+ Autopsy Study, we found an association between pre-synaptic protein levels and cognition in the oldest-old [26]. Synaptophysin was studied in the frontal cortex of 32 participants with a range of cognitive function. In these participants, synaptophysin levels were significantly reduced in individuals with dementia and correlated strongly with MMSE scores. These results point to an additional neuropathological marker that may be relevant to maintaining intact cognition in the oldest-old. To see if this association persists in a larger sample, we are in the process of extending investigations of synaptic proteins to all available brains from The 90+ Autopsy Study.
Established Collaborations
To expand on our initial observations of relatively poor associations between brain pathology and cognition and to explore other types of neuropathology that may be relevant for dementia in the oldest-old, we have established collaborations with investigators in several academic institutions to conduct additional pathological measurements in participants from The 90+ Autopsy Study. These collaborations include image-based quantitative analysis to detect pathological burden measured by neocortical total Aβ area and hippocampal tau area, stereology measures of neuronal volume, measurements of vessel density and atherosclerosis, immunohistochemical analyses of different types of amyloid aggregates, and additional investigations of synaptic proteins.
CONCLUSIONS
The 90+ Study is one of only a handful of population-based prospective studies of aging and dementia designed specifically to study oldest-old participants. Its brain donation component, The 90+ Autopsy Study, maintains the population-based characteristic of the overall study, as its participants are not different from the rest of The 90+ Study participants in most respects. The 90+ Autopsy Study has collected the brains of 120 nonagenarians and centenarians and continues to enroll participants to the brain donation program.
There were a variety of pathologies, including neurofibrillary tangles, neuritic and diffuse plaques, hippocampal sclerosis, diffuse Lewy body disease, and infarcts detected in this oldest-old sample. We found that although AD and cerebrovascular pathologies were common in the oldest-old, they do not relate to dementia very strongly, as we found significant overlap in pathology between demented and non-demented participants. We also found that some pathologies (such as hippocampal sclerosis, diffuse Lewy body disease, and corticobasal degeneration) were only present in demented participants. Finally, in spite of the high frequency of pathological lesions, we could not determine the dementia etiology for at least one quarter of demented participants in this cohort. Some of our findings are consistent with other pathological population-based studies that have investigated very old participants. Most studies have noted that the association between AD pathology and dementia is weaker in older elderly compared with younger elderly [27–28]. Furthermore, studies have found that pathologies other than AD play a more important role in dementia expression of the oldest-old compared to younger elderly [27, 29–30]. Our results highlight the poor associations between typical pathological lesions and cognition in the oldest-old and the importance of considering many different pathologies or combinations of pathologies, possibly including some that we have yet to identify, in order to account for all dementias in the oldest-old.
Acknowledgments
This research was funded in part by grants from the National Institutes of Health (R01AG21055, P50AG16573, and T32AG00096). We thank the staff of the UCI brain repository, especially Dr. Ronald Kim, for the collection and neuropathologic analysis of the brain tissue in this study. We also thank the participants and their relatives, testers, and examiners of The 90+ Study.
Footnotes
The authors have no potential conflicts of interest relevant to the subject matter discussed in this manuscript.
References
- 1.US Census Bureau. Annual Estimates of the Population by Sex and Five-Year Age Groups for the United States: April 1, 2000 to July 1, 2007 (NC-EST2007-01.xls) Population Division, U.S. Census Bureau; May 1, 2008. [Accessed: May 5, 2009]. Available from: http://www.census.gov/popest/national/asrh/NC-EST2007-sa.html. [Google Scholar]
- 2.US Census Bureau. Projected Population by Single Year of Age, Sex, Race, and Hispanic Origin for the United States: July 1, 2000 to July 1, 2050. (NP2008_D1.xls) Population Division, U.S. Census Bureau; Aug 14, 2008. [Accessed: May 5, 2009]. Available from: http://www.census.gov/population/www/projections/downloadablefiles.html. [Google Scholar]
- 3.Corrada MM, Brookmeyer R, Berlau D, Paganini-Hill A, Kawas CH. Prevalence of dementia after age 90: results from the 90+ study. Neurology. 2008;71:337–343. doi: 10.1212/01.wnl.0000310773.65918.cd. [DOI] [PubMed] [Google Scholar]
- 4.Berlau DJ, Corrada MM, Kawas CH. The prevalence of disability in the oldest-old is high and continues to increase with age: findings from The 90+ Study. Int J Geriatr Psychiatry. 2009;24:1217–1225. doi: 10.1002/gps.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaccai J, Ince P, Brayne C. Population-based neuropathological studies of dementia: design, methods and areas of investigation - a systematic review. BMC Neurol. 2006;6:2. doi: 10.1186/1471-2377-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paganini-Hill A, Ross RK, Henderson BE. Prevalence of chronic disease and health practices in a retirement community. J Chronic Dis. 1986;39:699–707. doi: 10.1016/0021-9681(86)90153-0. [DOI] [PubMed] [Google Scholar]
- 7.Whittle C, Corrada MM, Dick M, et al. Neuropsychological data in nondemented oldest-old: The 90+ Study. J Clin Exp Neuropsychol. 2007;29:290–299. doi: 10.1080/13803390600678038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 9.Teng EL, Hasegawa K, Homma A, et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 1994;6:45–58. doi: 10.1017/s1041610294001602. [DOI] [PubMed] [Google Scholar]
- 10.Clark CM, Ewbank DC. Performance of the dementia severity rating scale: a caregiver questionnaire for rating severity in Alzheimer disease. Alzheimer Dis Assoc Disord. 1996;10:31–39. [PubMed] [Google Scholar]
- 11.Pfeffer RI, Kurosaki TT, Harrah CH, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 12.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 13.Silverman JM, Breitner JC, Mohs RC, Davis KL. Reliability of the family history method in genetic studies of Alzheimer’s Disease and related dementias. Am J Psychiatry. 1986;143:1279–1282. doi: 10.1176/ajp.143.10.1279. [DOI] [PubMed] [Google Scholar]
- 14.Kawas CH, Segal J, Stewart WF, Corrada MM, Thal LJ. A validation study of the Dementia Questionnaire. Arch Neurol. 1994;51:901–906. doi: 10.1001/archneur.1994.00540210073015. [DOI] [PubMed] [Google Scholar]
- 15.Kahle-Wrobleski K, Corrada MM, Kawas CH. Dementia and cognition in the oldest-old. In: Miller BL, Boeve BF, editors. The Behavioral Neurology of Dementia. Cambridge: Cambridge University Press; 2009. pp. 254–263. [Google Scholar]
- 16.Corrada MM, Brookmeyer R, Paganini-Hill A, Berlau D, Kawas CH. Dementia incidence continues to increase with age in the oldest-old: The 90+ Study. Ann Neurol. 2010;67:114–121. doi: 10.1002/ana.21915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berlau DJ, Corrada MM, Peltz CB, Kawas CH. Disability in the oldest-old: Incidence and risk factors in The 90+ Study. Am J Geriatr Psychiatry. 2011 doi: 10.1097/JGP.0b013e31820d9295. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 19.The National Institute on Aging and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- 20.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 21.Mirra SS. The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer’s disease: A commentary. Neurobiol Aging. 1997;18:S91–S94. doi: 10.1016/s0197-4580(97)00058-4. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Census Bureau. Census 2000 Summary File 2 (SF 2) 100-Percent Data. [Accessed: February 7, 2011];PCT3 Sex by Age. 2001 Available from: http://factfinder.census.gov.
- 23.U.S. Census Bureau. American Community Survey Summary Tables. [Accessed: February 7, 2011];PCT033 Sex by Age by Educational Attainment for the Population 18 years and Over (2002) 2002 Available from: http://factfinder.census.gov/
- 24.Berlau D, Corrada MM, Kawas CH. Clinical dementia is more highly associated with Braak tangle stage than NIA-Reagan criteria in the oldest-old. Presented at the 61st Annual American Academy of Neurology Meeting; April 25–May 2; Seattle, WA. 2009. [Google Scholar]
- 25.Berlau DJ, Corrada MM, Head E, Kawas CH. APOE epsilon2 is associated with intact cognition but increased Alzheimer pathology in the oldest old. Neurology. 2009;72:829–834. doi: 10.1212/01.wnl.0000343853.00346.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Head E, Corrada MM, Kahle-Wrobleski K, et al. Synaptic proteins, neuropathology and cognitive status in the oldest-old. Neurobiol Aging. 2009;30:1125–1134. doi: 10.1016/j.neurobiolaging.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haroutunian V, Schnaider-Beeri M, Schmeidler J, et al. Role of the neuropathology of Alzheimer disease in dementia in the oldest-old. Arch Neurol. 2008;65:1211–1217. doi: 10.1001/archneur.65.9.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Age, neuropathology, and dementia. N Engl J Med. 2009;360:2302–2309. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- 29.Crystal HA, Dickson D, Davies P, Masur D, Grober E, Lipton RB. The relative frequency of “dementia of unknown etiology” increases with age and is nearly 50% in nonagenarians. Arch Neurol. 2000;57:713–719. doi: 10.1001/archneur.57.5.713. [DOI] [PubMed] [Google Scholar]
- 30.Brayne C, Richardson K, Matthews FE, et al. Neuropathological Correlates of Dementia in Over-80-Year-Old Brain Donors from the Population-Based Cambridge City over-75s Cohort (CC75C) Study. J Alzheimers Dis. 2009;18:645–658. doi: 10.3233/JAD-2009-1182. [DOI] [PubMed] [Google Scholar]