Abstract
Substance abuse and addiction are associated with an apparent devaluation of, and inattention to, natural rewards. This consequence of addiction can be modeled using a reward comparison paradigm where rats avoid intake of a palatable taste cue that comes to predict access to a drug of abuse. Evidence suggests rats avoid intake following such pairings, at least in part, because the taste cue pales in comparison to the highly rewarding drug expected in the near future. In accordance, lesions of the gustatory thalamus or cortex eliminate avoidance of a taste cue when paired with either a drug of abuse or a rewarding sucrose solution, but not when paired with the aversive agent, LiCl. The present study used bilateral ibotenic acid lesions to evaluate the role of a neighboring thalamic structure, the trigeminal orosensory area (TOA), in avoidance of a gustatory cue when paired with sucrose (Experiment 1), morphine (Experiment 2), cocaine (Experiment 3), or LiCl (Experiment 4). The results show that the TOA lesion disrupts, but does not eliminate avoidance of a taste cue that predicts access to a preferred sucrose solution and leaves intact the development of a LiCl-induced conditioned taste aversion. The lesion does, however, eliminate the suppression of intake of a taste cue when paired with experimenter administered morphine or cocaine using our standard parameters. As such, this is the first manipulation found to dissociate avoidance of a taste cue when mediated by a sweet or by a drug of abuse.
Keywords: Trigeminal, Thalamus, Ibotenic Acid Lesion, Contrast, Reward Comparison
Addiction is a disease of chronic relapse (Leshner, 1999; Volkow & Li, 2005) that costs society an estimated $500 billion per year (ONDCP) as the addict repeatedly cycles from addiction to abstinence, withdrawal, drug-seeking, and relapse. Addiction also comes at a great cost to the addict and his or her family as substance abuse, dependence, and addiction are associated with an apparent devaluation of, and inattention to, natural rewards. Indeed, according to the DSM-IV, substance abuse and dependence involve a failure to fulfill major obligations at work, school, or home, the giving up of important social, occupational, or recreational activities, and continued drug use in spite of recurrent physical, legal, social, or psychological problems (Goldstein, et al., 2007; Jones, Casswell, & Zhang, 1995; Nair, et al., 1997; Santolaria-Fernandez, et al., 1995).
For decades, it has been known that rats will avoid intake of a taste cue when paired with a drug of abuse (Cappell & LeBlanc, 1971; Cappell, LeBlanc, & Endrenyi, 1973; Glowa, Shaw, & Riley, 1994; Grigson, Twining, & Carelli, 2000; LeMagnen, 1969; Miller, Kelly, Neisewander, McCoy, & Bardo, 1990; Sherman, Pickman, Rice, Liebeskind, & Holman, 1980). Because avoidance of the taste cue is also seen in the conditioned taste aversion model (CTA), findings of this nature have been interpreted as resulting from the development of a conditioned taste aversion, despite the well-known rewarding properties of these drugs (Berger, 1972; Cappell & LeBlanc, 1971; Cappell, et al., 1973; Gamzu, 1977; Hunt & Amit, 1987; Lester, Nachman, & LeMagnen, 1970; Vogel & Nathan, 1975). However, rats also suppress intake of a taste cue (e.g., saccharin) when paired with a highly rewarding sucrose solution (Flaherty & Checke, 1982; Flaherty & Grigson, 1988; Flaherty & Rowan, 1985). This observation led us to hypothesize that rats may avoid intake of a saccharin cue following pairings with a drug of abuse because they are anticipating the availability of the highly rewarding properties of the drug (Grigson, 1997). In accordance, manipulations such as deprivation state (Bell, Thiele, Seeley, Bernstein, & Woods, 1998; Gomez & Grigson, 1999; Grigson, Lyuboslavsky, Tanase, & Wheeler, 1999), drug history (Grigson, Wheeler, Wheeler, & Ballard, 2001), strain (Glowa, et al., 1994; Grigson & Freet, 2000), and even bilateral lesions of the gustatory thalamus (Flynn, Grill, Schulkin, & Norgren, 1991; Grigson, Lyuboslavsky, & Tanase, 2000; Reilly, Bornavolova, & Trifunovic, 2004; Reilly & Pritchard, 1996a; Reilly & Trifunovic, 1999; Schroy, et al., 2005) and cortex (Geddes, Han, Baldwin, Norgren, & Grigson, 2008) can similarly affect drug and sweet-induced suppression of conditioned stimulus (CS) intake, while having relatively little impact on a LiCl-induced conditioned taste aversion (for reviews, see Grigson, 2008; Grigson, Twinning, Freet, Wheeler, & Geddes, 2009).
In addition to drug-induced devaluation, we also have evidence that, with experience, rats avoid the CS because it comes to elicit the onset of a conditioned aversive state, possibly involving craving and withdrawal. In support, drug-induced avoidance of the CS is accompanied by a conditioned elevation of circulating corticosterone (Gomez, Leo, & Grigson, 2000), blunted accumbens dopamine (Grigson & Hajnal, 2007), and the onset of aversive taste reactivity (i.e., gapes) (Wheeler, et al., 2008). Further, as might be expected in a conditioned state of withdrawal, greater aversive taste reactivity is associated with a shorter latency to take drug (i.e., cocaine) and with greater load-up behavior (Wheeler, et al., 2008). Interestingly, withdrawal induced by an opiate antagonist also is associated with elevated corticosterone (Nunez, Foldes, Laorden, Milanes, & Kovacs, 2007), blunted dopamine in the nucleus accumbens (Shaham & Stewart, 1995), and the onset of aversive taste reactivity following intraoral delivery of a naloxone-paired cue (McDonald, Parker, & Siegel, 1997). Thus, while rats may initially avoid intake of the CS because it pales in comparison to the drug’s value, we hypothesize that, ultimately, avoidance is mediated by the onset of a conditioned aversive state involving craving and withdrawal in anticipation of drug availability (Grigson, 2008; Grigson, et al., 2009).
Progress has been made in identifying the underlying circuitry involved in drug-induced avoidance of a taste cue. For instance, lesions of the nucleus accumbens do not disrupt anticipatory contrast (Leszczuk & Flaherty, 2000) and 6-OHDA lesions of the ventral tegmental area have no effect on the suppression of intake of a taste cue that predicts morphine or cocaine administration (Twining, et al., 2005). An intact gustatory thalamus and cortex, on the other hand, is critical for drug-induced suppression of CS intake, but these structures need not be intact for the development of a LiCl-induced CTA (Geddes, et al., 2008; Grigson, et al., 2000; Scalera, Grigson, & Norgren, 1997). As with drug-induced suppression of CS intake, both the thalamic and cortical lesions also prevent the development of an anticipatory contrast effect (ACE) where, as mentioned, rats avoid a lesser valued saccharin cue in anticipation of the availability of a preferred sucrose reward (Reilly, et al., 2004; Schroy, et al., 2005). Again, these data are consistent with other data suggesting that drug-induced suppression of CS intake is similar to the suppressive effects mediated by a rewarding sucrose solution, and different from a LiCl-induced CTA (Gomez & Grigson, 1999; Grigson, Cornelius, & Wheeler, 2001; Grigson & Freet, 2000; Grigson, et al., 1999; Grigson, et al., 2000; Grigson, et al., 2001).
As outlined, our initial evaluations of the underlying circuitry have focused upon the gustatory pathway, in particular the region of the thalamus that is responsive to stimulation of the tongue via gustatory stimuli, hereafter referred to as the thalamic taste area (TTA). The focus here is shifted to a region of the thalamus located 500 μm lateral to the gustatory area which receives trigeminal orosensory input. This area was referred to as the trigeminal orosensory area (TOA) of the thalamus by Liang and colleagues (2012a–c). In this set of studies (Liang, Freet, Grigson, & Norgren, 2012a; Liang, Grigson, & Norgren, 2012b; Liang, Norgren, & Grigson, 2012c), rats with lesions centered on the TOA, but also encompassing the majority of the TTA, exhibited increased operant responding for sucrose and corn oil under sham feeding (i.e., open gastric fistula) conditions (Liang, et al., 2012a). This finding is surprising, considering that lesions encompassing only the TTA do not affect operant responding for natural rewards (Reilly & Trifunovic, 1999). Rats with TOA lesions also yielded higher break points for sucrose in a progressive ratio task compared with Sham-lesioned subjects, indicating increased motivation for sweet reward (Liang, et al., 2012a). This disinhibition in the lesioned subjects suggests that the TOA may exert tonic inhibition on responding for sweet reward. While this appears to be the case, lesions of the TOA have no effect on the development of CTA when sham-fed corn oil or sucrose is paired with LiCl (Liang, et al., 2012b). This structure, then, is not essential for associating such natural rewards with LiCl-induced illness. Finally, while lesions of the TTA completely prevent ACE for sucrose (Reilly, et al., 2004; Schroy, et al., 2005), rats with TOA lesions, that often encompass both taste and trigeminal thalamic relays, are capable of developing an ACE for disparate concentrations of sham fed sucrose or corn oil (Liang, et al., 2012c). Together, these data suggest that the TOA is involved in the motivation to respond for a sweet reward. This increased motivation for a natural reward could rival the perceived rewarding value of a drug of abuse, and therefore the lesion may protect against the devaluation of such natural rewards.
Experiment 1
In light of the results of Liang et al. (2012c), it was imperative to investigate the influence of the TOA on sucrose reward under natural feeding conditions. While sham feeding is essential for studying the influence of oral sensation in the absence of post-ingestive feedback, it does have potential drawbacks. For example, the gastric fistula itself as well as daily flushing of the stomach could be stressful and present discomfort, which ultimately could alter learning behaviors. Additionally, leakage into the duodenum still may occur (Sclafani & Nissenbaum, 1985). Most importantly, the procedure is by no means natural feeding, even with the fistula closed. Given the unexpected role of the TOA in sucrose reward, Experiment 1 was designed to verify that this structure is not involved in the development of a sucrose ACE under natural feeding conditions.
Method
Subjects
The subjects were run in two batches that totaled 64 naïve male, Sprague-Dawley rats delivered from Charles River at approximately 50 days of age. Following one week of quarantine, the rats were housed individually in stainless steel hanging cages in a temperature-controlled (21 °C) animal care facility with a 12-h light-dark cycle (lights on at 7 a.m.). They were maintained on dry Harlan Teklad rodent diet (Madison, WI) and water ad libitum, except where noted otherwise.
Surgery
Following acclimation to the colony room, the subjects (at least 300 g) underwent either bilateral ibotenic acid lesions of the thalamic trigeminal orosensory area (TOAx) or vehicle infusions (Sham). Twenty min prior to anesthesia, all rats were injected with atropine sulfate (0.1 mg/rat, ip) and Bicillin (200,000 U/0.3ml, im). They were anesthetized with sodium pentobarbital (50 mg/kg, ip) and supplemented as necessary throughout surgery. Body temperature was maintained at 37+/−1 °C. The rat’s head was mounted in a stereotaxic instrument using non-traumatic earbars. The skin over the skull was cleaned with Betadine and opened with a mid-line incision. Using a 4 mm diameter trephine, two holes were drilled in the skull centered 3.0 mm posterior to β and 1.2 mm lateral of the midline on either side. The dura mater remained intact and moist throughout surgery with physiological saline. The skull was leveled between β and λ and a search electrode (glass-insulated tungsten microelectrode; Z = 0.5 – 1.2 Mohms at 1kHz) was lowered into the left or right gustatory thalamus. The taste area was located by recording neural activity while stimulating the anterior tongue with 0.3 M NaCl (approximate coordinates: − 3.8 mm posterior to β, +/− 1.2 mm lateral to the midsagittal suture, and 6.0 mm below dura). Once having located the gustatory thalamus, the search electrode was removed and the lesion micropipette lowered. The lesion was made using an ibotenic acid filled glass micropipette (diameter 20 – 24 μm at the tip) that was glued directly onto the needle of a 1.0 μl Hamilton microsyringe. The presence of taste neurons was confirmed by recording background taste responses with the glass micropipette. The micropipette was then raised and lowered again 500 microns lateral to the recorded area and 0.2 μl (20 μg/μl) of ibotenic acid was infused over 10 min. The pipette remained in situ for another 10 min. This same procedure was repeated on the contralateral side. After removal of the pipette, the holes in the skull were filled with Gelfoam and the wound closed with wound clips. The animals recovered over 2–3 days and body weight returned to presurgical levels within less than a week.
Apparatus
Rats were trained in one of six identical modular operant chambers (MED Associates, Inc., St. Albans, VT), measuring 30.5 × 24.0 × 29.0 cm (length × width × height). All chambers have a clear Plexiglas top, front, and back wall. Sidewalls are made of aluminum. The grid floors consist of nineteen 4.8-mm stainless steel rods spaced 1.6-cm apart (center to center). Each chamber is equipped with two retractable sipper tubes that can advance into the chamber through 1.3-cm diameter holes spaced 16.4-cm apart (center to center). A lickometer circuit is used to monitor licking. A shaded bulb, which reflects light off the ceiling, is located to the right of the cage and a white noise speaker is on the left-end wall, opposite to the sipper tubes. Each chamber is housed in a light and sound attenuated cubicle that is fitted with a ventilation fan and a white noise source that provides a background noise level of 75 dB. Control of events in the chamber and collection of the data are carried out on-line using a Pentium computer and programs are written in the Medstate notation language (MED Associates, Inc., St. Albans, VT).
Solutions
Sodium saccharin and sucrose (Sigma Chemical, St. Louis, MO) were dissolved in distilled water (dH2O) and presented at room temperature.
Procedure
Once having recovered from surgery (no less than 1 week), all rats were food-deprived to 90% of their free-feeding body weight, maintained by a once per day feeding. Subjects were divided into Saccharin-Saccharin (Sham n=12, TOAx n=18) and Saccharin-Sucrose groups (Sham n=14, TOAx n=20). They were then habituated to the chambers for 5 min a day for 3 days with the house light and white noise on. During testing, each rat was placed in the apparatus with the house light and white noise on. The first bottle (located on the left) advanced and the rat had 3 min access to 0.15% saccharin. Thereafter, the first bottle retracted and a second bottle advanced on the right for a 3 min access period. The second bottle contained either more 0.15% saccharin (Saccharin-Saccharin group) or 1.0 M sucrose (Saccharin-Sucrose group). The bottle was then retracted and the subject was immediately removed from the chamber. Daily feeding occurred one hour after removal from the chambers. There was one such taste-taste pairing a day for 16 days and the latency to first lick and number of licks made on each bottle was recorded.
Histology
At the end of all behavioral tests, the rats were given an overdose of Pentobarbital Sodium (100 mg/kg, ip) and, once deeply anesthetized, perfused transcardially for 5 min with cold physiological saline plus heparin (1.5 U/ml), immediately followed by cold 4% paraformaldehyde for 20 min. The brains were removed and stored in paraformaldehyde for 2 h, then in a mixture of 20% sucrose + 0.1 M phosphate buffer overnight. They were frozen and sliced coronally in 50 μm sections. One series of alternate sections was stained for cell bodies with cresyl Lecht violet and the other for Neuronal Nuclei protein (NeuN). The adequacy of the lesions was judged by comparing the acellular areas in the lesioned subjects to that of the Sham-lesioned controls. The boundaries of the gustatory and oral tactile nuclei have been defined from neuroanatomical and electrophysiological data (Kosar, Grill, & Norgren, 1986a, 1986b; Norgren, 1976; for reviews, see Norgren, 1984; 1995). Here and elsewhere, data from subjects found to have misplaced or incomplete lesions were excluded from further analysis.
Results and Discussion
Histology
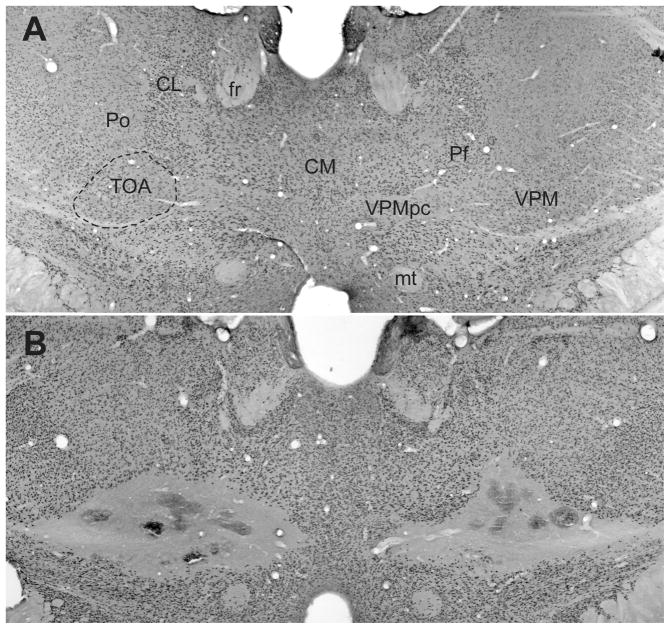
Sixty-four rats were tested in Experiment 1; data from five TOAx rats were excluded from statistical analysis due to incomplete lesions, and from one control subject that failed to establish consistent licking behavior. Typical lesions damaged the middle third of the VPM as well as the majority of the taste area in the parvicellular area of the VPM (VPMpc). A few subjects had extensive damage to the VPMpc with lesions crossing the midline. Additionally, the lesion encompassed small parts of adjacent nuclei such as the paracentral nucleus (PC), centrolateral nucleus (CL), central medial nucleus (CM), parafascicular nucleus (Pf), and the posterior nucleus (Po) (see Figure 1).
Figure 1.
Digital photomicrographs of coronal sections of Sham (A) and TOAx (B) stained with NeuN. Abbreviations: CL, centrolateral nucleus; CM, central medial nucleus; Pf, parafascicular nucleus; Po, posterior nucleus; VPM, ventral posteromedial nucleus; VPMpc, parvicellular subdivision of the VPM (thalamic taste area); mt, mammillary tract; fr, fasciculus retroflexus.
CS Intake (Licks/3 min)
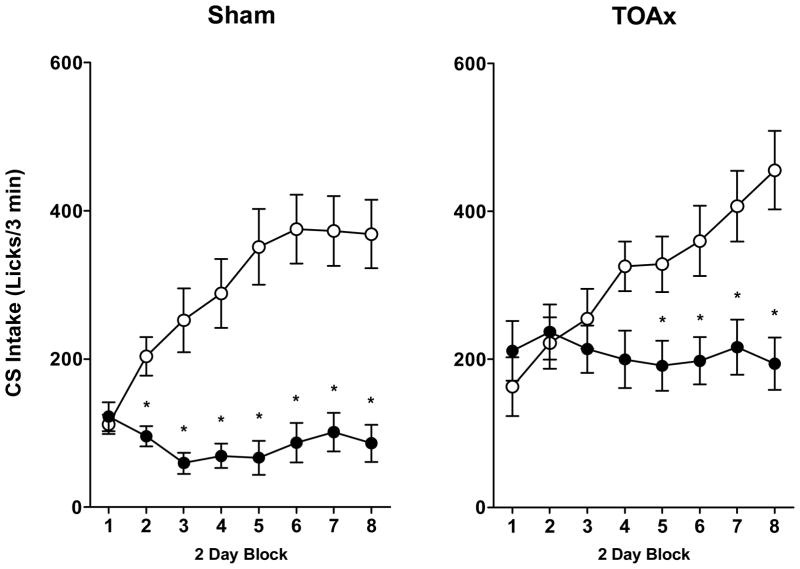
The number of licks on the CS spout was analyzed using a mixed factorial analysis of variance (ANOVA) with Lesion (Sham or TOAx) and unconditioned stimulus (US; Saccharin or Sucrose) as the between-subjects factors and 2-Day Blocks (1–8) as the within-subjects factor. Post hoc Newman-Keuls tests were conducted where appropriate. Data were considered statistically significant when p < 0.05. The results showed in real feeding rats, like sham feeding rats in the Liang et al. (2012c) study, that TOA lesions do not prevent the development of an ACE, i.e., sucrose-induced suppression of CS intake (see Figure 2).
Figure 2.
Mean 3-min intake (± SEM) of 0.15% saccharin in Sham (left panel) or TOAx (right panel) rats paired with 0.15% saccharin (open circles) or 1 M sucrose US for a total of 16 CS-US pairings. Data are represented as 2-day blocks. Sham: Sac-Sac N=11, Sac-Sucrose N=14; TOAx: Sac-Sac N=15, Sac-Sucrose N=18.
This conclusion was confirmed by a significant main effect of US, F(1,54) = 24.59, p < 0.001, indicating that all rats in the Saccharin-Sucrose condition made fewer licks for the saccharin cue than did the Saccharin-Saccharin controls overall. The US × Block interaction also attained statistical significance, F (7,378) = 19.64, p < 0.001, and post hoc tests revealed that anticipation of the availability of sucrose suppressed intake of the saccharin cue for all rats (Sham and TOAx), beginning with the 4th block (p < 0.05). There was no significant Lesion × US interaction, F(1,54) = 2.08, p = 0.15, and while contrast was significant for both the Sham and the TOAx rats, post hoc analysis indicates higher CS intake in the TOAx Saccharin-Sucrose condition compared with the Sham rats in the Saccharin-Sucrose condition (p < 0.05), suggesting a reduced anticipatory contrast effect. Finally, while the main effect of Lesion was significant, F(1,54) = 4.08, p < 0.05 (TOAx rats consumed more of the first bottle solution overall), the Lesion × US × Block interaction did not attain statistical significance, F(7,378) = 1.45, p = 0.19.
In the Liang et al. (2012c) paper, the ACE for sucrose was slower to develop in the sham feeding TOAx rats. Thus, we conducted separate follow up analyses on the Sham and the TOAx rats. Analysis of the Sham rats revealed a significant US × Block interaction, F(7,161) = 17.29, p < 0.001, and post hoc tests confirmed lower CS intake in the Saccharin-Sucrose group relative to the Saccharin-Saccharin controls, beginning with the 2nd block, p < 0.05. A similar analysis for the TOAx rats also revealed a significant US × Block interaction, F(7,217) = 8.08, p < 0.001. Post hoc tests, however, evidenced a significant ACE for rats in the Saccharin-Sucrose group only for blocks 5 through 8, p < 0.05. Rats with the TOA lesion, then, clearly can acquire the sucrose ACE in the lick frequency measure, but with some delay. This pattern of data is unlike that obtained in rats with lesions centered on the TTA who are fully incapable of acquiring an ACE using identical testing parameters (Schroy et al., 2005).
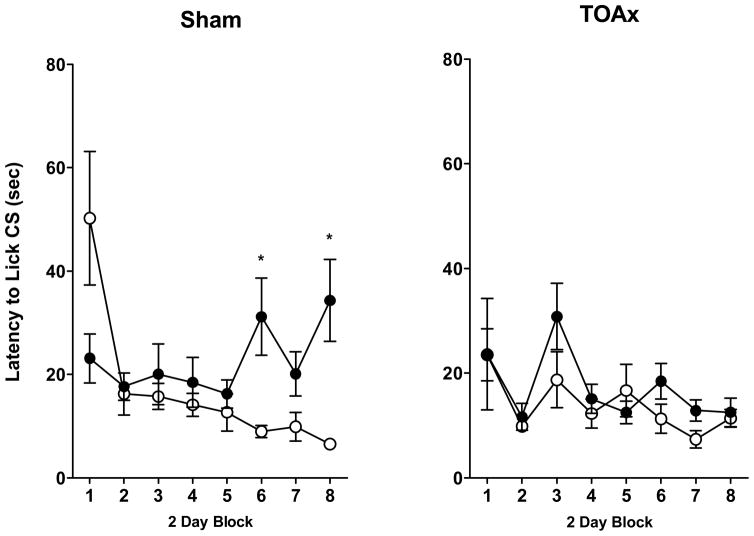
CS Latency
The Sham, but not the TOAx rats, exhibited a significant contrast effect in latency by taking longer to initiate licking for the saccharin cue when it predicted access to the preferred sucrose US than when it predicted access to more saccharin (see Figure 3).
Figure 3.
Latency to initiate licking of 0.15% saccharin CS (± SEM) in Sham (left panel) or TOAx (right panel) rats when the CS was paired with 0.15% saccharin (open circles) or 1 M sucrose US for a total of 16 CS-US pairings. Data are represented as 2-day blocks. Sham: Sac-Sac N=11, Sac-Sucrose N=14; TOAx: Sac-Sac N=15, Sac-Sucrose N=18.
This was evidenced by a significant Lesion × US × Block interaction, F(7,378) = 2.32, p < 0.05. Post hoc analyses conducted on this 3-way interaction observed reliable contrast in the latency to initiate licking of the sucrose-paired saccharin CS by the 6th and 8th blocks in the Sham subjects (see Figure 3, left panel) relative to their Saccharin-Saccharin controls, p < 0.05. No significant differences were evident in the TOAx rats.
US Intake (Licks/3 min)
Both the Sham and the TOAx rats exhibited a magnitude of reinforcement effect by making more licks for second bottle sucrose than for second bottle saccharin (see Figure 4).
Figure 4.
Mean 3-min intake (± SEM) of US in Sham (left panel) or TOAx (right panel) rats for 0.15% saccharin (open circles) or 1 M sucrose US for a total of 16 CS-US pairings. Data are represented as 2-day blocks. Sham: Sac-Sac N=11, Sac-Sucrose N=14; TOAx: Sac-Sac N=15, Sac-Sucrose N=18.
This observation was supported by the absence of a significant 3-way Lesion × US × Block interaction, F < 1, and a significant main effect of US, F(1,54) = 190.04, p < 0.001. The Lesion × US interaction also was significant, F(1,54) = 8.73, p < 0.01. Post hoc analyses indicated lower overall sucrose intake in the TOAx rats compared with the Sham group in the Saccharin-Sucrose condition, p < 0.001.
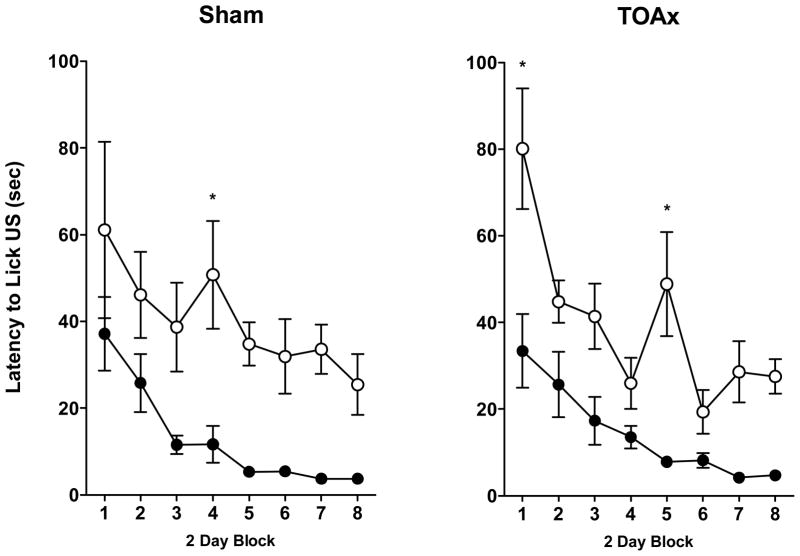
US Latency
Likewise, all rats, regardless of lesion condition, initiated licking faster for second bottle sucrose than for second bottle saccharin (see Figure 5).
Figure 5.
Latency to initiate licking of US (± SEM) in Sham (left panel) or TOAx (right panel) rats for 0.15% saccharin (open circles) or 1 M sucrose US for a total of 16 CS-US pairings. Data are represented as 2-day blocks. Sham: Sac-Sac N=11, Sac-Sucrose N=14; TOAx: Sac-Sac N=15, Sac-Sucrose N=18.
This conclusion is supported by the absence of a significant Lesion × US × Block interaction, F < 1, and a significant main effect of US, F(1,54) = 43.12, p < 0.001. The main effect of Block, F(7,378) = 15.96, p < 0.001, also was significant, showing that the latency to initiate licking decreased over successive trials, overall.
The results indicate that all rats, Sham and TOAx, made fewer licks for the first bottle saccharin solution when it predicted subsequent access to the preferred sucrose solution than when it predicted more access to the same saccharin solution. In addition, all subjects exhibited a normal magnitude of reward effect by initiating licking for, and making more licks of, second bottle sucrose than second bottle saccharin. The failure of the TOA lesion to completely eliminate ACE in the Liang et al. (2012c) study then was not due to the use of the sham-feeding regimen. As such, the TOA is not essential for the development of an ACE (i.e., sucrose-induced suppression of CS intake). That said, the lesion was not without effect. That is, while the ACE developed in first bottle licks for the TOAx rats, it was delayed relative to the Sham-lesioned subjects. Moreover, contrast also was evidenced in the Sham rats in the latency to lick the saccharin cue. Rats with lesions of the TOA, on the other hand, failed to exhibit contrast on this measure at all. TOAx rats initiated licking for first bottle saccharin very quickly, regardless of whether it predicted subsequent access to more saccharin or the preferred sucrose solution.
The delayed acquisition of ACE in the TOAx rats mirrored that seen in the Liang et al. (2012c) study. A number of hypotheses might be put forth to account for this effect. First, a reduced neophobic response by the TOAx rats may have contributed to delayed acquisition of the ACE. Neophobia is a common behavior in rats, where the animal will consume only small amounts of a novel food upon first exposure. In support, intake of the saccharin cue in the first 2-Day Block is higher in the lesioned group (see Figure 2), and while not a true test of neophobia, may suggest a reduced neophobic response in the lesioned animals. Counter to this argument, however, the same TOAx rats drank less, not more, of the 1.0 M sucrose solution upon the first exposure when presented in Bottle 2. A second hypothesis is that the delay in acquisition of the ACE is the result of an increase in motivation. The TOA lesioned rat may simply be more motivated, and therefore unwilling to wait for the preferred reward. In support, the TOAx rats did not only fail to suppress intake of the taste cue, they also were far quicker to initiate licking the taste cue than were the Sham-lesioned rats. Also, in the Liang et al. (2012a) study, TOAx rats showed increased operant responding for sucrose when compared with Sham subjects, indicating higher motivation amongst the TOAx group. While possible, this account also seems unlikely because the TOAx rats were no faster (or slower) to initiate licking 2nd bottle saccharin or sucrose relative to the Sham-lesioned controls. Finally, it is possible that these lesioned subjects are slower to associate a CS with a US. In the Liang et al. (2012c) study, however, similarly lesioned rats readily learned to associate a gustatory CS with LiCl-induced malaise in a CTA paradigm. It is possible, then, that the lesion may have a small, but uniform effect on the comparison of disparate rewards, particularly when they are of a sweet nature.
Experiment 2
As mentioned, rats will readily learn to suppress intake of a taste cue that is paired with a drug of abuse (Cappell & LeBlanc, 1971; Cappell, et al., 1973; Glowa, et al., 1994; Grigson, et al., 2000; Miller, et al., 1990; Sherman, et al., 1980). In previous studies, drug-induced suppression of CS intake was found to be eliminated in rats with bilateral lesions focused on the gustatory thalamus (Grigson, et al., 2000). Drug- and sucrose-induced suppression of CS intake are similarly affected by lesions of the gustatory thalamus (Grigson, et al., 2000; Reilly, et al., 2004; Schroy, et al., 2005) or cortex (Geddes, et al., 2008; Mackey, Keller, & Van der Kooy, 1986; Zito, Bechara, Greenwood, & Van der Kooy, 1988). It was expected, then, that the TOA lesion would have little or no effect on the development of drug-induced suppression of intake of a gustatory cue. Experiment 2 used morphine to test this hypothesis.
Method
Subjects
The subjects that served in Experiment 1 were used in Experiment 2 in a partial cross over design where half of each group (Saccharin-Saccharin or Saccharin-Sucrose) was assigned to one of two conditions (Saline or Morphine). They were returned to their free-feeding body weight and housed and maintained as described in Experiment 1. Five subjects from the TOAx condition were excluded due to inability to regain original body weight or as a result of dental occlusions. Food and water were freely available, except where noted below.
Apparatus
The experiment was conducted in the home cages. Fluid was presented in inverted graduated Nalgene cylinders with silicone stoppers and stainless steel spouts affixed to the front of each home cage with springs. Intake was measured to the nearest 0.5 ml.
Drugs and Solutions
Morphine sulfate was provided by the National Institute on Drug Abuse (Bethesda, MD) and was prepared in sterile saline immediately before testing. Polycose (Sigma Chemical, St. Louis, MO) was dissolved in dH2O and presented at room temperature.
Procedure
Once having recovered to their free-feeding body weight, the rats were placed on a water deprivation schedule that allowed for 5 min of access to dH2O in the morning and 1 h each afternoon. Following stabilization of morning water intake (approximately 9 days), rats were matched for 5 min water intake and history. Thus, half of the rats from the Saccharin-Saccharin condition, and half from the Saccharin-Sucrose condition in Experiment 1 served in each drug treatment group in Experiment 2: Saline (Sham n=12, TOAx n=18) and Morphine (Sham n=14, TOAx n=20) groups. All rats were given 5 min access to a 0.03 M Polycose CS, and after a 5-min interval, were injected intraperitoneally with either saline or a 15 mg/kg dose of morphine. There were six such taste-drug pairings occurring at 48-hour intervals. To maintain proper hydration, dH2O continued to be provided for 5 min on the days between injections and for 1 h every afternoon.
Results and Discussion
Sixty-four rats were tested in Experiment 2; as mentioned in Experiment 1, data from five TOAx rats were excluded from statistical analysis due to incomplete lesions. Additionally, data were excluded from five other TOAx subjects that failed to either regain free-fed body weight or had dental occlusion and could not complete the study.
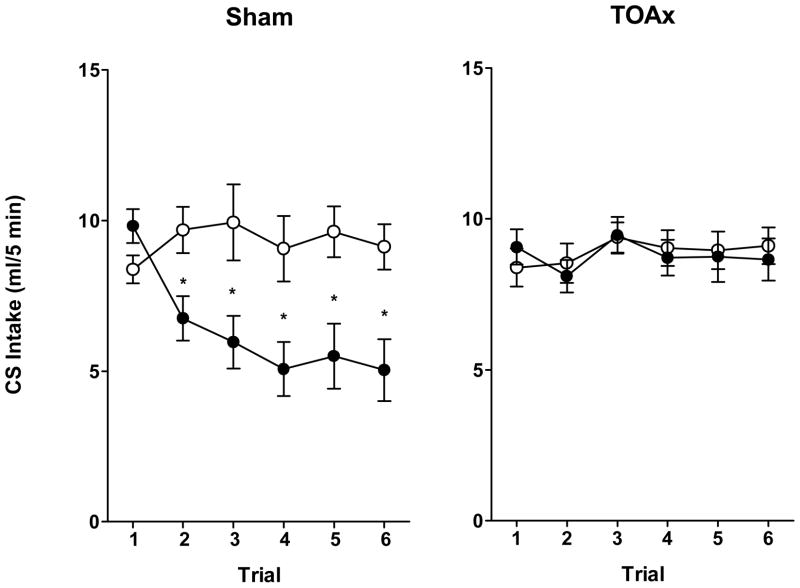
Relative to the saline treated controls, rats with Sham lesions suppressed intake of the Polycose CS following Polycose-morphine pairings. This same pattern was not evident in the lesioned group (see Figure 6).
Figure 6.
Mean 5-min intake (± SEM) of 0.03 M Polycose in Sham (left panel) or TOAx (right panel) rats injected intraperitonealy with saline (open circles) or morphine sulfate (15 mg/kg; solid circles) for a total of five taste-drug pairings and one CS-only test. Sham: Saline N=12, Morphine N=14; TOAx: Saline N=13, Morphine N=15.
Polycose intake was analyzed using a mixed factorial ANOVA with Lesion (Sham or TOAx) and US (Saline or Morphine) as the between-subjects factors and Trial (1–6) as the within-subjects factor, followed by Newman-Keuls post hoc tests. There was a significant Lesion × US × Trial interaction, F(5,250) = 3.89, p < 0.01, and a significant Lesion × US interaction, F(1,50) = 8.24, p < 0.01. Post hoc analysis indicated significant differences between the Sham saline and Sham morphine groups, beginning with trial 2, p < 0.05, as well between the Sham morphine group and both the TOAx saline and TOAx morphine groups, also beginning with trial 2, p < 0.05. No significant differences were found between the TOAx saline and TOAx morphine groups. This is the first intervention found to differentially influence suppression of intake of a taste cue when paired with a drug of abuse versus that which occurs when a similar taste cue is paired with a naturally rewarding sucrose solution. As mentioned, using similar parameters, these two paradigms have been strikingly parallel, as they have been found to be similarly affected by strain (Glowa, et al., 1994; Grigson & Freet, 2000; for a discussion, see Grigson, et al., 2001; but also see Riley, 2011), deprivation state (Bell, et al., 1998; Flaherty, Grigson, Checke, & Hnat, 1991; Gomez & Grigson, 1999; Grigson, et al., 1999), as well as lesions of the gustatory thalamus (Flynn, et al., 1991; Grigson, et al., 2000; Reilly, et al., 2004; Reilly & Pritchard, 1996b; Reilly & Trifunovic, 1999; Schroy, et al., 2005) or cortex (Geddes, et al., 2008). It is possible that, as in the ACE study, TOAx rats are just slower to suppress, and that an effect would have developed with more trials. While this is possible, it is unlikely as there is absolutely no evidence of suppression of CS intake by the TOAx rats following 5 pairings and one test. Moreover, in an unpublished study with the rats from the Liang et al. studies, TOAx rats failed to suppress intake of a Polycose CS following 9 pairings with morphine. It is important to note that the number of trials typically required to attain significant CS suppression in the anticipatory contrast paradigm is around eight pairings (or four 2-day blocks)(Schroy, et al., 2005), whereas suppression can be observed in the reward comparison paradigm following a single taste-drug pairing (Grigson, et al., 2000). The lack of suppression, therefore, is not likely to reflect slow learning but, rather, a complete attenuation of morphine-induced suppression of CS intake using the present parameters.
Experiment 3
Mu opioid receptors are densely concentrated throughout the area of the thalamus targeted by the ibotenic acid lesion (Mansour, Khachaturian, Lewis, Akil, & Wartson, 1987). For this reason, it is possible that the results obtained in Experiment 2 were due to the lesion interfering with the brain’s ability to detect and process the opiate. To test the merits of this hypothesis, we employed a different class of addictive drug, namely the stimulant, cocaine. Since cocaine’s mechanism of action is modulated by, but not dependent upon, mu opioid receptor activation (Becker, et al., 2002; Lesscher, et al., 2005), it is possible to determine whether the effect of the TOA lesion is opiate specific or whether the lesion can interfere with a more general drug reward mechanism.
Method
Subjects and Apparatus
The subjects were 36 naïve, male, Sprague-Dawley rats delivered from Charles River at approximately 50 days of age. The rats were housed and underwent lesion surgery as described in Experiment 1. The apparatus was the same as in Experiment 2. Histology was completed as described in Experiment 1.
Drugs and Solutions
Cocaine hydrochloride was provided by the National Institute on Drug Abuse (Bethesda, MD). The 10 mg/kg dose of cocaine was prepared in sterile saline and administered subcutaneously as a 1.5 mg/ml stock solution, adjusted by body weight to avoid necrosis (Durazzo, Gauvin, Goulden, Briscoe, & Holloway, 1994). Sodium saccharin (Sigma Chemical, St. Louis, MO) was dissolved in dH2O and presented at room temperature.
Procedure
The rats were placed on a water deprivation schedule that allowed for 5 min of access to dH2O in the morning and 1 h each afternoon. Following stabilization of morning water intake (approximately 9 days), all rats were given 5 min access to a 0.15% saccharin CS, and after a 5-min interval, were injected subcutaneously with either saline or a 10 mg/kg dose of cocaine as described. There were five such taste-drug pairings occurring at 48-h intervals. To maintain proper hydration, dH2O was provided for 5 min each morning between conditioning trials and for 1 h every afternoon.
Results and Discussion
Histology
Of the 36 rats in Experiment 3, three died during surgery and data from an additional three subjects were removed due to incomplete lesions. The following data include 16 subjects with bilateral ibotenic acid lesions of the trigeminal thalamic orosensory area (TOAx), and 14 with vehicle infusions (Sham). As mentioned, lesions damaged the middle third of the VPM as well as the majority of the taste area in the VPMpc. Subjects that failed to meet these criteria were not included in the analyses (see Figure 1).
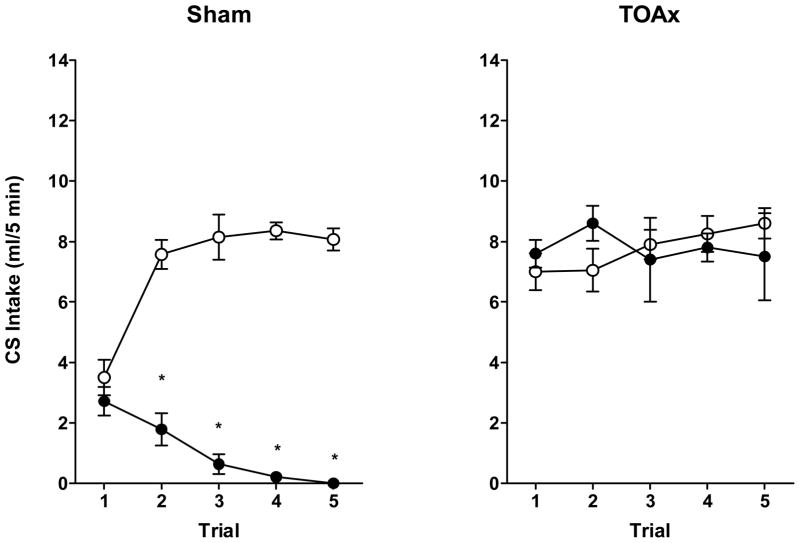
Saccharin intake was analyzed using a mixed factorial ANOVA with Lesion (Sham or TOAx) and US (Saline or Cocaine) as the between-subject factors and Trial (1–5) as the within-subject factor, followed by Newman-Keuls post hoc tests. As was the case when the taste cue predicted an injection of morphine, the TOA lesions interfered with cocaine-induced suppression of intake of the saccharin taste cue (see Figure 7).
Figure 7.
Mean 5-min intake (± SEM) of 0.15% saccharin in Sham (left panel) or TOAx (right panel) rats injected subcutaneously with saline (open circles) or cocaine hydrochloride (10 mg/kg; solid circles) for a total of four taste-drug pairings and one CS-only test. Sham: Saline N=7, Cocaine N=7; TOAx: Saline N=10, Cocaine N=6.
This conclusion was supported by a significant Lesion × US × Trial interaction, F(4,108) = 9.00, p < 0.001, and a significant Lesion × US interaction, F(1,27) = 33.78, p < 0.001. Post hoc tests of this significant 3-way ANOVA found that this effect was significant for the Sham group on the second through fifth trials (p < 0.001). In the TOAx group, however, CS intake in the Saline condition did not significantly differ from that in the cocaine condition on any trial, supporting the conclusion that the lesion interfered with suppression of intake of the taste cue. Furthermore, post hoc analyses indicated a significant difference in CS intake between the TOAx and Sham groups on trial 1. The trial 1 intake is higher in the TOAx subjects, indicating an apparent reduced neophobic response to the CS.
The results show that bilateral TOA lesions interfere with not only morphine-induced suppression of CS intake, but with cocaine-induced suppression of CS intake as well. As with the morphine reward comparison experiment, the TOAx rats appear to have an increased affinity for sweet reward, and may be unable to compare a natural gustatory reward with a drug reward. As in Experiment 1 (where saccharin served as the CS), the TOAx group appeared to lack a neophobic response to the novel saccharin taste cue and a failure to evidence neophobia has been posited as the cause for the disruptive effect of the thalamic taste area lesion (Lin, Arthurs, & Reilly, 2011). That said, reduced neophobia does not account for these results. The TOAx group showed reduced neophobia to a saccharin CS in Experiments 1 and 3, when paired with either sucrose or cocaine, respectively. However, after several pairings, the TOAx rats suppressed intake of the saccharin CS when it predicted sucrose, but failed to avoid the CS when it predicted cocaine. Furthermore, neither the Sham nor TOAx rats exhibited neophobia in Experiment 2 when a Polycose CS predicted morphine, yet the TOAx rats failed to avoid the CS after several pairings. Therefore, while a reduced neophobic response to the CS is a notable effect of the TOA lesion, as has been found with lesions of the gustatory pathway (Lin, et al., 2011); the determining factor here is not the CS, or a neophobic response to the CS, but rather the US.
Experiment 4
As described above, CTA is a paradigm that also results in suppression of intake of a taste cue. Traditionally, a CTA occurs by pairing a taste cue with an illness-inducing agent such as LiCl or x-radiation (Garcia, Kimmeldorf, & Koelling, 1955; Nachman, 1963; Smith, 1971). Unlike sweets and drugs of abuse, evidence suggests that LiCl is highly and uniformly aversive. For example, rats avoid a place associated with LiCl injections yet show preference for a place associated with a palatable sucrose solution or drugs of abuse (Bardo, Miller, & Neisewander, 1984; Blander, Hunt, Blair, & Amit, 1984; Katz & Gormezano, 1979; Reilly, Grigson, & Norgren, 1993; White & Carr, 1985). Additionally, rats have increased operant responding for sucrose or drug, but not for LiCl (Guttman, 1953; Hajnal, Acharya, Grigson, Covasa, & Twining, 2007; Liang, et al., 2012a; White, Sklar, & Amit, 1977; Wise, Yokel, & DeWit, 1976). Even so, like ACE learning, CTA learning also depends upon identifying a CS and associating this CS with the consequences of the US. As previously mentioned, the Liang et al. (2012b) study found CTA development to be intact in sham fed TOAx rats following three pairings of either 0.3 M sucrose or 100% corn oil with LiCl. To ensure there were no differences in real fed rats, a subset of the subjects from Experiments 1 and 2 underwent pairings of a novel CS and the illness-inducing agent, LiCl. Because of the robust nature of the response to LiCl-induced visceral malaise, prior experience should not affect suppression in this paradigm; however, subjects were assigned to each condition in a counterbalanced fashion to avoid generalization from previous studies. Furthermore, a neutral stimulus was selected as the CS to further avoid generalization between studies.
Method
Subjects and Apparatus
Following Experiments 1 and 2, a subset of 32 subjects was allowed several weeks’ recovery before water training began. Subjects were again divided into control and experimental conditions in a counterbalanced fashion based on lesion and the previous assignments in Experiments 1 and 2. The rats were housed as described in Experiment 1 and the apparatus (home cage testing) was the same as that described in Experiment 2.
Drugs and Solutions
Lithium chloride (Sigma Chemical, St. Louis, MO) was dissolved in dH2O immediately before testing. Sodium chloride (NaCl) (Sigma Chemical, St. Louis, MO) served as the gustatory CS and was prepared in dH2O and presented at room temperature.
Procedure
The rats were placed on a water deprivation schedule that allowed for 5 min of access to dH2O in the morning and 1 h each afternoon. Once 5 min intake stabilized, subjects were divided into Saline (Sham n=8, TOAx n=8) and LiCl (Sham n=8, TOAx n=8) groups. All rats were given 5 min access to 0.1 M NaCl (CS), followed 5 min later by an intraperitoneal injection of either saline or increasing concentrations of LiCl over trials. We intended to use our standard “low” concentration of 0.009 M LiCl, injected at a volume of 1.33 ml/100g body weight (5.07 mg/kg body weight) because the suppressive effects of this dose have been matched to those of a 15 mg/kg dose of morphine and a 10 mg/kg dose of cocaine (Grigson, 1997). Instead, the volume injected was 1 ml/kg body weight, which resulted in a much lower dose than anticipated. The concentration was gradually increased until an effective dose was established as follows: 0.009 M (0.38 mg/kg body weight) for the first 4 pairings, 0.018 M (0.76 mg/kg body weight) for pairings 5 and 6, 0.0375 M (1.59 mg/kg body weight) for pairings 7 and 8, and the final 4 pairings were 0.15 M (6.36 mg/kg body weight). Due to the volume of the injection, this final dose was only slightly higher than our standard low 0.009 M dose injected at a volume of 1.33 ml/100 g body weight. There was one such taste-drug pairing a day with 48 h between pairings for 11 trails, followed by one CS only test day. NaCl intake was recorded. To maintain proper hydration, dH2O was provided for 5 min each morning between conditioning trials and for 1 h each afternoon.
Results and Discussion
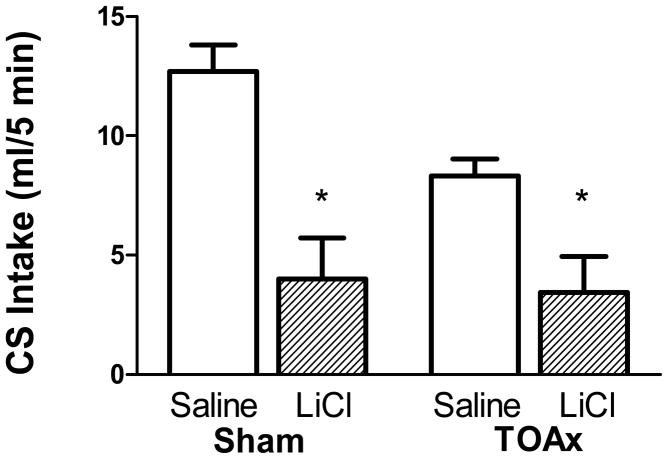
Sodium chloride intake on the final test day was analyzed using a 2 way ANOVA with Lesion (Sham or TOAx) and US (Saline or LiCl) as between-subject factors. The results verify those found in sham fed TOAx rats in the Liang et al. (2012b) study. Thus, on the final day of testing (see Figure 8), both the Sham and the TOAx rats suppressed intake of the LiCl-paired taste cue, indicating that the TOA lesion did not prevent a LiCl-induced conditioned taste aversion.
Figure 8.
Mean 5-min intake (± SEM) on final test day of 0.1 M NaCl in Sham (left panel) or TOAx (right panel) rats injected intraperitonealy with saline (open circles) or LiCl (solid circles) for a total of twelve taste-drug pairings. Sham: Saline N=8, LiCl N=8; TOAx: Saline N=8, LiCl N=8.
Support for this conclusion was provided by a non-significant Lesion × US interaction, F(1,28) = 1.31, p = 0.26, but a significant main effect of US, F(1,28) = 31.75, p < 0.001. The main effect of lesion was significant on the test day, F(1,28) = 5.15, p < 0.05, indicating lower overall intake in the TOAx saline group compared with the Sham saline group. The results indicate that the TOA lesions do not interfere with LiCl-induced conditioned taste aversion. As described, this also was the case in the TOAx rats in the Liang et al. (2012b) study following 3 pairings of 0.3 M sucrose or 100% corn oil with a potent 0.15 M (1.33ml/100g body weight, ip) LiCl. Therefore, we can assume that the TOAx rats also have a normal sensitivity to LiCl across a range of doses.
General Discussion
The results from Experiment 1 replicate and extend those obtained by Liang et al. (2012c) by showing that the TOA lesion does not block avoidance of a lesser valued taste cue that predicts access to a highly palatable sucrose solution under real feeding conditions. As such, the lesioned subjects were able to detect the taste cue, associate the taste cue with the US, compare the available reward with the anticipated reward, and respond accordingly by suppressing intake of the lesser valued taste cue. That said, as a group, the TOA lesioned rats did not develop an anticipatory contrast effect as fast as the Sham operated controls. In addition to contrast in lick frequency, the Sham group also exhibited contrast in latency as evidenced by an increase in the latency to lick the sucrose-paired saccharin cue. The TOAx group, however, did not develop contrast in the latency to lick the sucrose-paired cue. The lesion, then, disrupts, but does not eliminate, the development of an anticipatory contrast effect. Also consistent with the findings of Liang et al. (2012b) in sham feeding rats, the TOA lesion had no effect on the LiCl-induced CTA when assessed in real feeding rats in Experiment 4. Finally, in Experiments 2 and 3, the same TOA lesion fully prevented suppression of intake of a taste cue that was paired with the drugs of abuse morphine or cocaine in the reward comparison paradigm. The TOA lesion is, then, the first manipulation in which performance in one paradigm is intact, albeit slightly delayed (anticipatory contrast), while performance in the other is eliminated (drug-mediated reward comparison), and this is the case with both morphine and cocaine.
In previous studies, gustatory TTAx lesions (focused on the VPMpc) eliminated avoidance of a palatable taste cue when paired with a naturally rewarding sucrose solution or a drug of abuse, but had no effect on the development of a LiCl-induced conditioned taste aversion (Flynn, et al., 1991; Grigson, et al., 2000; Reilly, et al., 2004; Reilly & Pritchard, 1996b; Reilly & Trifunovic, 1999; Scalera, et al., 1997; Schroy, et al., 2005). Bilateral lesions of the insular cortex also disrupted the suppressive effect of a drug of abuse, but not those induced by LiCl (Geddes, et al., 2008; Mackey, et al., 1986). As described, lesions centered on the slightly lateral TOA, on the other hand, spare contrast effects involving the comparison of two disparate gustatory (i.e., saccharin vs. sucrose) or trigeminal (i.e., corn oil) rewards (current data and (Liang, et al., 2012c)), but block avoidance of a taste cue when paired with a drug of abuse. This would seem straight forward enough. Yet, squaring this circle is a challenge, as the TOA lesion encroaches on, and almost always includes, portions of the VPMpc. Indeed, our histological analysis suggests that subjects with greater damage to the gustatory thalamus may, in fact, be slower to learn the ACE and have lower overall intake compared with those with more sparing of the area. We can conclude that sparing of ACE relates, at least in part, to the degree of sparing of the VPMpc. We also can infer that the slightly different structures in the thalamus, then, serve very discrete functions as related to the comparison of, and responding to, disparate rewards over time. An intact VPMpc is required for avoidance of a lesser valued saccharin cue when paired with sucrose or when paired with a drug of abuse such as morphine or cocaine. The TOA, on the other hand, is involved predominantly when avoiding a taste cue that predicts the availability of drug.
The most parsimonious explanation is that ACE is accomplished in TOAx rats by some sparing of cells in the VPMpc. Thus, it is possible that, in moving the lesion 500 microns laterally, enough cells may have been spared to enable the comparison of two disparate natural rewards (anticipatory contrast), yet still attenuate the comparison of a natural reward with a drug of abuse. In support, there were a few subjects with TOA lesions that had extensive damage crossing the midline and these subjects seemed to require more pairings before suppressing CS intake in the anticipatory contrast paradigm compared with other lesioned subjects. However, in the reward comparison paradigms, where the CS predicted morphine or cocaine, all subjects failed to suppress intake of the drug-paired taste cue, suggesting that, unlike the ACE paradigm, the magnitude of effect is not influenced by the extent to which the lesion encroaches on the VPMpc. Of course, one might wonder how these rats with damage crossing the midline could acquire an ACE at all. Is the gustatory thalamic taste area (VPMpc) really the key thalamic nucleus for ACE? This determination will require further testing. For now, however, it is at least clear that ACE was spared when lesion damage was limited more to the TOA.
The disruptive effect of the TOA lesion also may be due to interference with descending corticothalamic input. For example, projections from the dysgranular anterior insular cortex terminate in the areas specifically damaged in the TOA lesion, including the VPM, VPMpc, CM, Po, PC, and Pf (Shi & Cassell, 1998). It is possible that the effects of the TOA lesion are a result of disconnection of this cortical circuit. Because the insular cortex has a direct contribution to morphine antinociception (Burkey, Carstens, Wenniger, Tang, & Jasmin, 1996), and is involved in cocaine-seeking behaviors (Kufahl, et al., 2009), it is possible that disconnection of the corticothalamic fibers may have contributed to the failure to avoid a taste cue following pairings with a drug of abuse. Indeed, asymmetric lesions of the gustatory thalamus and insular cortex also disrupt avoidance of a taste cue when paired with either morphine or cocaine (Geddes, et. al, in preparation). Lesions of the gustatory cortex also disrupt the establishment of an anticipatory contrast effect following saccharin-sucrose pairings as well (Geddes et al., submitted).
Furthermore, as explored here, avoidance of the drug-paired taste cue appears to be unlike avoidance of a LiCl-paired taste cue. As with other manipulations (Bell, et al., 1998; Geddes, et al., 2008; Gomez & Grigson, 1999; Grigson & Freet, 2000; Grigson, et al., 2000; Grigson, et al., 1999; Grigson, et al., 2001), lesions of the TOA prevented drug- but not LiCl-induced suppression of CS intake. The suppressive effects of a drug of abuse, then, appear to have little to do with aversive properties of the drug itself (as is the case with LiCl). In this case, and as mentioned in the Introduction, avoidance of the cue is due, in part, to devaluation of the taste cue in anticipation of drug availability. Unlike sucrose, however, the drug of abuse has a potent impact on the CNS. Consequently, with experience, the cue comes to elicit the onset of a conditioned opponent process (Solomon & Corbit, 1974) and this opponent process appears highly aversive, possibly involving the onset of craving and withdrawal. In accordance, and as mentioned above, ingestion of a drug paired taste cue, like naloxone supported conditioned withdrawal (McDonald, et al., 1997; Nunez, et al., 2007; Shaham & Stewart, 1995), involves a conditioned elevation of circulating corticosterone (Gomez, et al., 2000), reduced accumbens dopamine (Grigson & Hajnal, 2007; Wheeler et al., 2011), and, when intraorally infused, the onset of aversive taste reactivity behavior (Wheeler et al., 2008). Such an aversive conditioned state is also seen in addicted humans who are reported to show negative affect when having to wait for access to nicotine in the presence of drug-related cues (Sayette, et al., 2003). Negative affect is a potent precipitator of relapse (Sinha et al., 2009). Likewise, in the rodent model, both avoidance of the taste cue and the onset of aversive taste reactivity behavior predict the latency to take drug, the length of the load up period, and the speed with which rats will acquire cocaine self-administration behavior (Wheeler et al., 2008). Therefore, unlike LiCl, cues that predict drugs of abuse can lead to a conditioned aversive state involving craving and withdrawal (Becker, Gerak, Li, Koek, & France, 2010; McDonald, et al., 1997, see Grigson, 2008, for a review). The TOA, then, may be involved in the development and/or expression of this more complex process.
As this is the first manipulation to yield differing results in anticipatory contrast effect and suppression of CS intake when paired with a drug of abuse, it is possible that parametric differences between these two paradigms may contribute to the selective effect of the TOA lesion on drug-induced suppression of CS intake. Despite the number of parallels between avoidance of a CS when paired with sucrose and when paired with a drug of abuse (Gomez & Grigson, 1999; Grigson & Freet, 2000; Grigson, et al., 2001), there are, in fact, a number of differences between these two paradigms such as the length of access to the CS, length of the interval between the CS and US, and route of administration of the US. Understanding the influence of each of these factors in relation to the lesion is paramount in determining the role of the TOA and its involvement in the processing of drug-related cues.
One difference between the two paradigms is the length of CS access. Rats are allowed 3 min access to the CS in the ACE paradigm and 5 min access in the drug pairings. In rats with lesions of the insular cortex, Lin et al. (2011) found moderate drug-induced suppression of CS intake when given a longer 15 min access period compared to a 5 min access period, suggesting that there is a possible ceiling effect as a result of the 5 min access period. Here, the TOA lesioned rats evidenced clear and reliable suppression of CS intake in the ACE paradigm when given only 3 min access to the saccharin CS (or, in the Liang et al. (2012) study, the corn oil CS). Accordingly, if the length of CS access is truly the mediating factor, one would expect more suppression in a 5 min access period compared to the 3 min access, not less. Furthermore, a 5 min access period was also used in the CTA study and the lesion did not prevent avoidance of the LiCl-paired CS. Therefore, the length of the access period is unlikely to account for the dissociation. Further studies will need to be conducted to determine whether the use of a longer 15 min CS access period will reduce the disruptive effect of the TOA lesion as the information provided by the cue, and the development of the opponent process, changes with the length of the CS access period (Wheeler et al., 2008; Wheeler et al., 2011).
In addition to the different access times, the interval between the CS and the US differs between the two paradigms. There is no CS-US interstimulus interval (ISI) for the ACE paradigm, while subjects must wait 5 min before receiving drug in the reward comparison paradigm. This may potentially lead to behavioral changes as the 5 min wait may interfere with associating the CS and the US in the lesioned rats. This is unlikely, however, because there is also a 5 min ISI in the LiCl-induced CTA paradigm. However, unlike LiCl, cues that predict drugs of abuse can lead to a conditioned aversive state involving craving and withdrawal (Becker, et al., 2010; McDonald, et al., 1997). According to Wheeler et al. (2011), when an intraorally infused taste cue predicts delayed access to drug, the CS elicits aversive responses such as decreased accumbens dopamine and aversive orofacial responses. Yet when the CS is delivered at the same time as drug (Wheeler et al., 2011), there is an increase in accumbens dopamine in response to the CS. Therefore, the avoidance of the CS when paired with drugs of abuse may very likely be the result of the onset of conditioned withdrawal in anticipation of impending drug availability. The TOA, then, may be involved in the development of this more complex process.
A final major disparity between the ACE and the reward comparison experiments is the fact that, for the ACE studies, the rats actively consume the sucrose US, while in the drug suppression studies described here, the drug is passively delivered by the experimenter. We plan to further evaluate this disparity using a self-administration paradigm, where the rats can actively self-administer the drug. While the drug is itself known to be rewarding, there is a body of evidence indicating that experimenter delivered drug is aversive (Chen, et al., 2008; Lecca, Cacciapaglia, Valentini, Acquas, & Di Chiara, 2007; Palamarchouk, Smagin, & Goeders, 2009; Stefanski, et al., 2007). We have previously demonstrated that yoked delivery of drug also is aversive, rendering such treated rats unwilling to work for cocaine and averse to a location associated with yoked delivery of drug (Twining, Bolan, & Grigson, 2009). However, when an intraorally infused CS is paired with either experimenter-delivered drug (Wheeler, et al., 2011), or self-administered drug (Wheeler, et al., 2008) rats emit aversive orofacial responses (i.e., gapes), but in the case of self-administered drug, these conditioned aversive taste reactivity behaviors develop when the signal indicates that the rat must wait 30 min for drug (Wheeler et al., 2008; Wheeler et al., 2011), not when presentation of the taste cue has been commensurate with drug delivery (Wheeler et al., 2011). The role of the TOA, then, may not have to do with the mode of administration of the US, per se, but whether the cue signals an immediate or a delayed reward. Confirmation of such a hypothesis awaits further testing.
In summary, the TOA appears to play a role in modulating responsiveness to a gustatory cue that predicts the availability of drug. It is not essential for avoidance of a similar taste cue when paired with a sweet in the anticipatory contrast paradigm or when paired with LiCl in the conditioned taste aversion paradigm. As such, the lesion of the TOA appears to prevent devaluation of the gustatory cue, whether mediated by comparison with the more intense drug of abuse, and/or by the onset of a conditioned aversive state, possibly involving craving and withdrawal. The selective role of the TOA in avoidance of a drug-paired taste cue appears not to depend upon sensitivity to neophobia or the length of CS access, but possibly depends upon the information provided by the taste cue – i.e., the information that drug availability, while immanent, is delayed. Future studies will test the merits of this hypothesis involving sweets and/or drugs. If confirmed, the TOA will be implicated in the development of the critical opponent process that is part and parcel to the development of addiction and, once learned, to the precipitation of cue-induced relapse.
Acknowledgments
This research article was supported in part by DA012473, F31-DA029369, and under a grant with the Pennsylvania Department of Health using Tobacco Settlement Funds.
Contributor Information
Jennifer E. Nyland, Department of Neural and Behavioral Sciences, The Pennsylvania State University College of Medicine; Hershey, Pennsylvania
Danielle N. Alexander, Department of Neural and Behavioral Sciences, The Pennsylvania State University College of Medicine; Hershey, Pennsylvania
Nu-Chu Liang, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine; Baltimore, Maryland.
Patricia S. Grigson, Department of Neural and Behavioral Sciences, The Pennsylvania State University College of Medicine. Hershey, Pennsylvania
References
- Bardo MT, Miller JS, Neisewander JL. Conditioned place preference with morphine: The effect of extinction training on the reinforcing conditioned response. Pharmacology Biochemistry and Behavior. 1984;21(4):545–549. doi: 10.1016/S0091-3057(84)80037-4. [DOI] [PubMed] [Google Scholar]
- Becker A, Grecksch G, Kraus J, Loh HH, Schroeder H, Hollt V. Rewarding effects of ethanol and cocaine in μ opioid receptor-deficient mice. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2002;365(4):296–302. doi: 10.1007/s00210-002-0533-2. [DOI] [PubMed] [Google Scholar]
- Becker GL, Gerak LR, Li JX, Koek W, France CP. Precipitated and conditioned withdrawal in morphine-treated rats. Psychopharmacology. 2010;209:85–94. doi: 10.1007/s00213-009-1773-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SM, Thiele TE, Seeley RJ, Bernstein IL, Woods SC. Effects of food deprivation on conditioned taste aversions in rats. Pharmacology Biochemistry and Behavior. 1998;60(2):459–466. doi: 10.1016/S0091-3057(98)00024-0. [DOI] [PubMed] [Google Scholar]
- Berger BD. Conditioning of food aversions by injections of psychoactive drugs. Journal of Comparative and Physiological Psychology. 1972;81:21–26. doi: 10.1037/h0033316. [DOI] [PubMed] [Google Scholar]
- Blander A, Hunt T, Blair R, Amit Z. Conditioned place preference: An evaluation of morphine’s positive reinforcing properties. Psychopharmacology. 1984;84:124–127. doi: 10.1007/BF00432040. [DOI] [PubMed] [Google Scholar]
- Burkey AR, Carstens E, Wenniger JJ, Tang J, Jasmin L. An opioidergic cortical antinociception triggering site in the agranular insular cortex of the rat that contributes to morphine antinociception. The Journal of Neuroscience. 1996;16(20):6612–6623. doi: 10.1523/JNEUROSCI.16-20-06612.1996. 0270-6474/96/166612-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappell H, LeBlanc AE. Conditioned aversion to saccharin by single administrations of mescaline and d-amphetamine. Psychopharmacologia. 1971;22:22–356. doi: 10.1007/BF00406873. [DOI] [PubMed] [Google Scholar]
- Cappell H, LeBlanc AE, Endrenyi L. Aversive conditioning by psychoactive drugs: Effects of morphine, alcohol, and chlordiazepoxide. Psychopharmacology. 1973;29:239–232. doi: 10.1007/BF00414038. [DOI] [PubMed] [Google Scholar]
- Chen BT, Bowers MS, Martin M, Hopf W, Guillory AM, Carelli RM, et al. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron. 2008;59:288–297. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Gauvin DV, Goulden KL, Briscoe RJ, Holloway FA. The subcutaneous administration of cocaine in the rat. Pharmacology, Biochemistry and Behavior. 1994;49:1007–1010. doi: 10.1016/0091-3057(94)90256-9. [DOI] [PubMed] [Google Scholar]
- Flaherty CF, Checke S. Anticipation of incentive gain. Animal Learning & Behavior. 1982;10:177–182. doi: 10.3758/BF03212267. [DOI] [Google Scholar]
- Flaherty CF, Grigson PS. From contrast to reinforcement: Role of response contingency in anticipatory contrast. Journal of Experimental Psychology & Animal Behavioral Processes. 1988;14:165–176. doi: 10.1037/0097-7403.14.2.165. [DOI] [PubMed] [Google Scholar]
- Flaherty CF, Grigson PS, Checke S, Hnat K. Deprivation state and temporal horizons in anticipatory contrast. Journal of Experimental Psychology: Animal Behavior Processes. 1991;17:503–518. doi: 10.1037/0097-7403.17.4.503. [DOI] [Google Scholar]
- Flaherty CF, Rowan GA. Anticipatory contrast: Within-subjects analysis. Animal Learning & Behavior. 1985;13:2–5. doi: 10.3758/BF03213357. [DOI] [Google Scholar]
- Flynn FW, Grill HJ, Schulkin J, Norgren R. Central gustatory lesions: II. Effects on sodium appetite, taste aversion learning, and feeding behaviors. Behavioral Neuroscience. 1991;105(6):944–954. doi: 10.1037/0735-7044.105.6.944. [DOI] [PubMed] [Google Scholar]
- Gamzu E. The multifaceted nature of taste-aversion inducing agents: Is there a single common factor? In: Barker L, Best M, Domjan M, editors. Learning Mechanisms in Food Selection. Waco, TX: Baylor University Press; 1977. [Google Scholar]
- Garcia J, Kimmeldorf D, Koelling R. Conditioned aversions to saccharin resulting from exposure to gamma radiation. Science. 1955;122:157–158. doi: 10.1126/science.122.3179.1089. [DOI] [PubMed] [Google Scholar]
- Geddes RI, Han L, Baldwin AE, Norgren R, Grigson PS. Gustatory insular cortex lesions disrupt drug-induced, but not lithium chloride-induced, suppression of conditioned stimulus intake. Behavioral Neuroscience. 2008;122(5):1038–1050. doi: 10.1037/a0012748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowa JR, Shaw AE, Riley AL. Cocaine-induced conditioned taste aversions: Comparison between effects in LEW/N and F344/N rat strains. Psychopharmacology. 1994;114:229–232. doi: 10.1007/BF02244841. [DOI] [PubMed] [Google Scholar]
- Goldstein R, Tomasi D, Alia-Klein N, Cottone L, Zhang L, Telang F. Subjective sensitivity to monetary gradients is associated with frontolimbic activation to reward in cocaine abusers. Drug and Alcohol Dependence. 2007;87(2–3):233–240. doi: 10.1016/j.drugalcdep.2006.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez F, Grigson PS. The suppressive effects of LiCl, sucrose, and drugs of abuse are modulated by sucrose concentration in food-deprived rats. Physiology & Behavior. 1999;67(3):351–357. doi: 10.1016/S0031-9384(99)00079-7. [DOI] [PubMed] [Google Scholar]
- Gomez F, Leo NA, Grigson PS. Morphine-induced suppression of saccharin intake is correlated with elevated corticosterone levels. Brain Research. 2000;863(1–2):52–58. doi: 10.1016/s0006-8993(00)02093-x. doi:1016/S0006-8993(00)02093-X. [DOI] [PubMed] [Google Scholar]
- Grigson PS. Conditioned taste aversions and drugs of abuse: A reinterpretation. Behavioral Neuroscience. 1997;111(1):129–136. doi: 10.1037/0735-7044.111.1.129. [DOI] [PubMed] [Google Scholar]
- Grigson PS. Reward comparison: The Achilles’ heel and hope for addiction. Drug Discovery Today: Disease Models. 2008;5(4):227–233. doi: 10.1016/j.ddmod.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigson PS, Cornelius K, Wheeler DS. The suppressive effects of intraperitoneal cocaine are augmented when evaluated in nondeprived rats. Pharmacology, Biochemistry and Behavior. 2001;69(1–2):117–123. doi: 10.1016/S0091-3057(01)00501-9. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Freet CS. The suppressive effects of sucrose and cocaine, but not lithium chloride, are greater in Lewis than in Fischer rats: Evidence for the reward comparison hypothesis. Behavioral Neuroscience. 2000;114(2):353–363. doi: 10.1037/0735-7044.114.2.353. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Hajnal A. Once is too much: Conditioned changes in accumbens dopamine following a single saccharin–morphine pairing. Behavioral Neuroscience. 2007;121(6):1234–1242. doi: 10.1037/0735-7044.121.6.1234. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Lyuboslavsky P, Tanase D. Bilateral lesions of the gustatory thalamus disrupt morphine- but not LiCl-induced intake suppression in rats: Evidence against the conditioned taste aversion hypothesis. Brain Research. 2000;858(2):327–337. doi: 10.1016/S0006-8993(00)01939-9. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Lyuboslavsky P, Tanase D, Wheeler RA. Water-deprivation prevents morphine-, but not LiCl-induced, suppression of sucrose intake. Physiology & Behavior. 1999;67(2):277–286. doi: 10.1016/S0031-9384(99)00080-3. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Twining RC, Carelli RM. Heroin-induced suppression of saccharin intake in water-deprived and water-replete rats. Pharmacology, Biochemistry and Behavior. 2000;66(3):603–608. doi: 10.1016/S0091-3057(00)00253-7. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Twinning RC, Freet CS, Wheeler RA, Geddes RI. Drug-induced suppression of CS intake: Reward, aversion, and addiction. In: Reilly S, Schachtman TR, editors. Conditioned Taste Aversion: Behavioral and Neural Processes. New York: Oxford University Press; 2009. pp. 74–91. [Google Scholar]
- Grigson PS, Wheeler RA, Wheeler DS, Ballard SM. Chronic morphine treatment exaggerates the suppressive effects of sucrose and cocaine, but not lithium chloride, on saccharin intake in Sprague-Dawley rats. Behavioral Neuroscience. 2001;115(2):403–416. doi: 10.1037/0735-7044.115.2.403. [DOI] [PubMed] [Google Scholar]
- Guttman N. Operant conditioning, extinction, and periodic reinforcement in relation to concentration of sucrose used as reinforcing agent. Journal of Experimental Psychology. 1953;46(4):213–224. doi: 10.1037/h0061893. [DOI] [PubMed] [Google Scholar]
- Hajnal A, Acharya NK, Grigson PS, Covasa M, Twining RC. Obese OLETF rats exhibit increased operant performance for palatable sucrose solutions and differential sensitivity to D2 receptor antagonism. American Journal of Physiology - Regulatory, Integrative, and Comparative Physiology. 2007;293:R1846–R1854. doi: 10.1152/ajpregu.00461.2007. [DOI] [PubMed] [Google Scholar]
- Hunt T, Amit Z. Conditioned taste aversion induced by self-administered drugs: Paradox revisited. Neuroscience and Biobehavioral Reviews. 1987;1:107–130. doi: 10.1016/S0149-7634(87)80005-2. [DOI] [PubMed] [Google Scholar]
- Jones S, Casswell S, Zhang JF. The economic costs of alcohol-related absenteeism and reduced productivity among the working population of New Zealand. Addiction. 1995;90:1455–1461. doi: 10.1046/j.1360-0443.1995.901114553.x. [DOI] [PubMed] [Google Scholar]
- Katz R, Gormezano G. A rapid and inexpensive technique for assessing the reinforcing effect of opiate drugs. Pharmacology, Biochemistry and Behavior. 1979;11(2):231–233. doi: 10.1016/0091-3057(79)90019-4. [DOI] [PubMed] [Google Scholar]
- Kosar E, Grill HJ, Norgren R. Gustatory cortex in the rat. I. Physiological properties and cytoarchetecture. Brain Research. 1986a;379:329–341. doi: 10.1016/0006-8993(86)90787-0. [DOI] [PubMed] [Google Scholar]
- Kosar E, Grill HJ, Norgren R. Gustatory cortex in the rat. II. Thalamocortical projections. Brain Research. 1986b;379:342–352. doi: 10.1016/0006-8993(86)90788-2. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Zavala AR, Singh A, Thiel KJ, Dickey ED, Joyce JN, et al. c-Fos expression associated with reinstatement of cocaine-seeking behavior by response-contingent conditioned cues. Synapse. 2009;63:823–835. doi: 10.1002/syn.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca D, Cacciapaglia F, Valentini V, Acquas E, Di Chiara G. Differential neurochemical and behavioral adaptation to cocaine after response contingent and noncontingent exposure in the rat. Psychopharmacology. 2007;191:653–667. doi: 10.1007/s00213-006-0496-y. [DOI] [PubMed] [Google Scholar]
- LeMagnen J. Peripheral and systemic actions of food in caloric regulation of intake. Annals of the New York Academy of Sciences. 1969;157:1126–1157. doi: 10.1111/j.1749-6632.1969.tb12940.x. [DOI] [PubMed] [Google Scholar]
- Leshner A. Science-based views of drug addiction and its treatment. Journal of the American Medical Association. 1999;282:1314–1316. doi: 10.1001/jama.282.14.1314. [DOI] [PubMed] [Google Scholar]
- Lesscher H, Hordijk M, Bondar N, Alekseyenko O, Burbach J, Van Ree J, et al. Mu-opioid receptors are not involved in acute cocaine-induced locomotor activity nor in development of cocaine-induced behavioral sensitization in mice. Neuropsychopharmacology. 2005;30(2):278–285. doi: 10.1038/sj.npp.1300529. [DOI] [PubMed] [Google Scholar]
- Lester D, Nachman M, LeMagnen J. Aversive conditioning by ethanol in the rat. Quarterly Journal of the Study of Alcohol. 1970;31:578–586. [PubMed] [Google Scholar]
- Leszczuk MH, Flaherty CF. Lesions of nucleus accumbens reduce instrumental but not consummatory negative contrast in rats. Behavioural Brain Research. 2000;116(1):61–79. doi: 10.1016/S0166-4328(00)00265-5. [DOI] [PubMed] [Google Scholar]
- Liang N-C, Freet CS, Grigson PS, Norgren R. Pontine and thalamic influences on fluid rewards: I. Operant responding for sucrose and corn oil. [Epub ahead of print] Physiology & Behavior. 2012a doi: 10.1016/j.physbeh.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang N-C, Grigson PS, Norgren R. Pontine and thalamic influences on fluid rewards: II. Sucrose and corn oil conditioned aversions. [Epub ahead of print] Physiology & Behavior. 2012b doi: 10.1016/j.physbeh.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang N-C, Norgren R, Grigson PS. Pontine and thalamic influences on fluid rewards: III. Anticipatory contrast for sucrose and corn oil. [Epub ahead of print] Physiology & Behavior. 2012c doi: 10.1016/j.physbeh.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Arthurs J, Reilly S. Role of the insular cortex in morphine-induced conditioned taste avoidance. Brain Research. 2011;1384:80–88. doi: 10.1016/j.brainres.2011.01.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey W, Keller J, Van der Kooy D. Visceral cortex lesions block conditioned taste aversions induced by morphine. Pharmacology, Biochemistry and Behavior. 1986;24:71–78. doi: 10.1016/0091-3057(86)90047-X. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis M, Akil H, Wartson S. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. Journal of Neuroscience. 1987;7(8):2445–2464. 0270-6474/87/082445-20. [PMC free article] [PubMed] [Google Scholar]
- McDonald R, Parker L, Siegel S. Conditioned sucrose aversions produced by naloxone-precipitated withdrawal from acutely administered morphine. Pharmacology, Biochemistry and Behavior. 1997;58(4):1003–1008. doi: 10.1016/S0091-3057(97)00313-4. [DOI] [PubMed] [Google Scholar]
- Miller JS, Kelly KS, Neisewander JL, McCoy DF, Bardo MT. Conditioning of morphine-induced taste aversion and analgesia. Psychopharmacology. 1990;101:472–480. doi: 10.1007/BF02244224. [DOI] [PubMed] [Google Scholar]
- Nachman M. Learned aversion to the taste of lithium chloride and generalization to other salts. Journal of Comparative and Physiological Psychology. 1963;56(2):343–349. doi: 10.1037/h0046484. [DOI] [PubMed] [Google Scholar]
- Nair P, Black M, Schuler M, Keane V, Snow L, Rigney B. Risk factors for disruption in primary care giving among infants of substance abusing woman. Child Abuse & Neglect. 1997;21:1039–1051. doi: 10.1016/S0145-2134(97)00064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgren R. Taste pathways to hypothalamus and amygdala. The Journal of Comparative Neurology. 1976;166(1):17–30. doi: 10.1002/cne.901660103. [DOI] [PubMed] [Google Scholar]
- Norgren R. Central neural mechanisms of taste. In: Brookhart M, Mountcastle V, editors. Handbook of Physiology: Sect. 1. The nervous system: Vol 3: Sensory Process. Bethesda, MD: American Physiological Society; 1984. pp. 1087–1128. [Google Scholar]
- Norgren R. Gustatory system. In: Paxinos G, editor. The rat nervous system. 2. San Diego: Academic Press; 1995. pp. 751–771. [Google Scholar]
- Nunez C, Foldes A, Laorden M, Milanes M, Kovacs K. Activation of stress-related hypothalamic neuropeptide gene expression during morphine withdrawal. Journal of Neurochemistry. 2007;101(4):1060–1071. doi: 10.1111/j.1471-4159.2006.04421.x. [DOI] [PubMed] [Google Scholar]
- ONDCP. The economic costs of drug abuse in the United States, 1992–2002. Washington, DC: Executive Office of the President; 2004. [Google Scholar]
- Palamarchouk V, Smagin G, Goeders NE. Self-administered and passive cocaine infusions produce different effects on corticosterone concentrations in the medial prefrontal cortex (MPC) of rats. Pharmacology Biochemistry and Behavior. 2009;94:163–168. doi: 10.1016/j.pbb.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly S, Bornavolova M, Trifunovic R. Excitotoxic lesions of the gustatory thalamus spare simultaneous contrast effects but eliminate anticipatory negative contrast: Evidence against a memory deficit. Behavioral Neuroscience. 2004;118(2):365–376. doi: 10.1037/0735-7044.118.2.365. [DOI] [PubMed] [Google Scholar]
- Reilly S, Grigson PS, Norgren R. Parabrachial nucleus lesions and conditioned taste aversion: Evidence supporting an associative deficit. Behavioral Neuroscience. 1993;107:1005–1017. doi: 10.1037/0735-7044.107.6.1005. [DOI] [PubMed] [Google Scholar]
- Reilly S, Pritchard T. Gustatory thalamus lesions in the rat: I. Innate taste preferences and aversion. Behavioral Neuroscience. 1996a;110:737–745. doi: 10.1037/0735-7044.110.4.737. [DOI] [PubMed] [Google Scholar]
- Reilly S, Pritchard T. Gustatory thalamus lesions in the rat: II. Aversive and appetitive taste conditioning. Behavioral Neuroscience. 1996b;110:746–759. doi: 10.1037/0735-7044.110.4.746. [DOI] [PubMed] [Google Scholar]
- Reilly S, Trifunovic R. Progressive ratio performance in rats with gustatory thalamic lesions. Behavioral Neuroscience. 1999;113:1008–1019. doi: 10.1037/0735-7044.113.5.1008. [DOI] [PubMed] [Google Scholar]
- Riley AL. The paradox of drug taking: The role of the aversive effects of drugs. Physiology & Behavior. 2011;103:69–78. doi: 10.1016/j.physbeh.2010.11.021. [DOI] [PubMed] [Google Scholar]
- Santolaria-Fernandez F, Gomez-Sirvent J, Gonzalez-Reimers C, Batista-Lopez J, Jorge-Hernandez J, Rodriguez-Moreno F, et al. Nutritional assessment of drug addicts. Drug and Alcohol Dependence. 1995;38:11–18. doi: 10.1016/0376-8716(94)01088-3. [DOI] [PubMed] [Google Scholar]
- Sayette M, Wertz J, Martin C, Cohen J, Perrott M, Hobel J. Efffects of smoking opportunity on cue-elicited urge: A facial coding analysis. Experimental and Clinical Psychopharmacology. 2003;11:218–227. doi: 10.1037/1064-1297.11.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalera G, Grigson PS, Norgren R. Gustatory functions, sodium appetite, and conditioned taste aversions survive excitotoxic lesions of the thalamic taste area. Behavioral Neuroscience. 1997;111:633–645. doi: 10.1037/0735-7044.111.3.633. [DOI] [PubMed] [Google Scholar]
- Schroy PL, Wheeler RA, Davidson C, Scalera G, Twining RC, Grigson PS. Role of gustatory thalamus in anticipation and comparison of rewards over time in rats. American Journal of Physiology -Regulatory, Integrative, and Comparative Physiology. 2005;288(4):R966–R980. doi: 10.1152/ajpregu.00292.2004. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Nissenbaum JW. Is gastric sham feeding really sham feeding? American Journal of Physiology. 1985;248(3 Pt 2):R387–390. doi: 10.1152/ajpregu.1985.248.3.R387. 0363-6119/85. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Stress reinstates heroin-seeking in drug free animals: An effect mimicking heroin, not withdrawal. Psychopharmacology. 1995;199:334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- Sherman JE, Pickman C, Rice A, Liebeskind JC, Holman EW. Rewarding and aversive effects of morphine: Temporal and pharmacological properties. Pharmacology, Biochemistry and Behavior. 1980;13:501–505. doi: 10.1016/0091-3057(80)90271-3. [DOI] [PubMed] [Google Scholar]
- Shi CJ, Cassell MD. Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. The Journal of Comparative Neurology. 1998;399(4):440–468. doi: 10.1002/(SICI)1096-9861(19981005). [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox H, Hong K, Bergquist K, Bhagwagar Z, Siedlarz K. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34:1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. Radiation: Its detection and its effect on taste preference. Progress in Physiological Psychology. 1971;4:53–118. [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation: I. Temporal dynamics of affect. Psychological Review. 1974;81(2):119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Stefanski R, Ziolkowska B, Kusmider M, Mierzejewski P, Wyszogrodzka E, Dziedzickz-Wasylewska M, et al. Active versus passive cocaine administration: Differences in the neuroadaptive changes in the brain dopaminergic system. Brain Research. 2007;1157:1–10. doi: 10.1016/j.brainres.2007.04.074. [DOI] [PubMed] [Google Scholar]
- Twining RC, Bolan M, Grigson PS. Yoked delivery of cocaine is aversive and protects against the motivation for drug in rats. Behavioral Neuroscience. 2009;123:913–925. doi: 10.1037/a0016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twining RC, Hajnal A, Han L, Bruno K, Hess EJ, Grigson PS. Lesions of the ventral tegmental area disrupt drug-induced appetite stimulating effects but spare reward comparison. The International Journal of Comparative Psychology. 2005;18(4):372–396. [Google Scholar]
- Vogel J, Nathan B. Learned taste aversions induced by hypnotic drugs. Pharmacology, Biochemistry and Behavior. 1975;3:189–194. doi: 10.1016/0091-3057(75)90147-1. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Li TK. Drugs and alcohol: Treating and preventing abuse, addiction and their medical consequences. Pharmocology & Therapeutics. 2005;108:3–17. doi: 10.1016/j.pharmthera.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Wheeler RA, Aragona BJ, Fuhrmann KA, Jones JL, Day JJ, Cacciapaglia F, et al. Cocaine cues drive opposing context-dependent shifts in reward processing and emotional state. Biological Psychiatry. 2011;69:1067–1074. doi: 10.1016/j.biopsych.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RA, Twining RC, Jones JL, Slater JM, Grigson PS, Carelli RM. Behavioral and electrophysiological indices of negative affect predict cocaine self-administration. Neuron. 2008;57(5):774–785. doi: 10.1016/j.neuron.2008.01.024. [DOI] [PubMed] [Google Scholar]
- White NM, Carr GD. The conditioned place preference is affected by two independent reinforcement processes. Pharmacology Biochemistry and Behavior. 1985;23:37–42. doi: 10.1016/0091-3057(85)90127-3. [DOI] [PubMed] [Google Scholar]
- White NM, Sklar L, Amit Z. The reinforcing action of morphine and its paradoxical side effect. Psychopharmacology. 1977;52:63–66. doi: 10.1007/BF00426601. [DOI] [PubMed] [Google Scholar]
- Wise RA, Yokel R, DeWit H. Both positive reinforcement and conditioned aversion from amphetamine and from apomorphine in rats. Science. 1976;191:1273–1275. doi: 10.1126/science.1257748. [DOI] [PubMed] [Google Scholar]
- Zito KA, Bechara A, Greenwood C, Van der Kooy D. The dopamine innervation of the visceral cortex mediates the aversive effects of opiate. Pharmacology Biochemistry and Behavior. 1988;30:693–699. doi: 10.1016/0091-3057(88)90086-X. [DOI] [PubMed] [Google Scholar]