Abstract
Objective
To identify a cGMP-specific phosphodiesterase (PDE) in non-human primate germinal vesicle (GV) oocytes and establish a proposed effect on oocyte maturation through preliminary experiments in mouse GV oocytes.
Design
Controlled non-human primate and rodent experiments.
Setting
Academic research institution.
Animals
Rhesus macaques and B6/129F1 mice.
Interventions
Rhesus macaques were stimulated with FSH to collect GV oocytes and cumulus for gene expression analysis. Female mice were stimulated with PMSG to collect GV oocytes.
Main Outcome Measures
PDE transcript expression in primate GV oocytes and cumulus cells. Fluorescence polarization (FP) measurements of PDE3A activity. Spontaneous resumption of meiosis in mouse GV oocytes.
Results
Five PDE transcripts were detected in Rhesus GV oocytes, only PDE9A was cGMP-specific. FP assays indicated cGMP has an inhibitory effect on PDE3A while the PDE9 inhibitor, BAY73-6691, did not. Similarly, BAY73-6691, had little effect on preventing spontaneous maturation in oocytes, but did augment the inhibitory effects of cGMP. Inclusion of 0µM (control), 10µM, 100µM, and 1 mM BAY73-6691 significantly increased the proportion of mouse oocytes maintaining GV arrest in the presence of the cGMP analog 8-Br-cGMP at: 100µM (8.8%, 11.4%, 18.8%, and 28%), 500µM (21.1%, 38.1%, 74.5%,and 66.5%), and 1 mM (57.8%, 74.5%, 93.9%, and 94.0%) respectively, when P<0.05.
Conclusions
PDE9 is a cGMP-specific hydrolyzing enzyme present in primate oocytes, and PDE9 antagonists augment the inhibitory effect of cGMP during spontaneous in vitro maturation of GV mouse oocytes.
Keywords: oocyte, meiosis, phosphodiesterase 9A, cGMP, phosphodiesterase 3A
Introduction
Oocyte maturation is an integral part of the reproductive cycle and critical for normal fertility in women. Oocytes remain arrested at the germinal vesicle stage (GV) of prophase I by maintaining elevated levels of cyclic AMP (cAMP) (1, 2). The intra-oocyte cAMP levels drop dramatically following the LH surge, or upon the release of the oocyte from the follicular environment, due to decreased synthesis and increased hydrolysis of cAMP by selected cyclic nucleotide phosphodiesterases (PDEs). PDEs are a class of enzymes that hydrolyze the second messengers cAMP and cGMP (3). There are 11 different families of PDEs characterized by their relative affinities and binding capacities for each second messenger (4). Within the oocyte, PDE3A has been identified as the primary cAMP-hydrolyzing phosphodiesterase (5–9). Inhibition of PDE3 has been shown to prevent both the spontaneous and gonadotropin-induced maturation of oocytes in a number of species, including human and non-human primates (6, 10–17).
Recent studies have shown that cGMP plays an important role in maintaining the meiotic arrest state of the GV oocyte suppressing the activity of PDE3A (18). cGMP is produced by the granulosa cells and diffuses into the oocyte via gap junctions (18–21). When the LH surge occurs, gap junctions close, cutting off the supply of cGMP to the oocyte. The concentration of cGMP in the oocyte gradually declines which then permits PDE3A to hydrolyze cAMP into 5’AMP, leading to meiotic resumption and the production of a fertilizable egg (5, 22–25). It is likely that a PDE(s) is present in the oocyte which targets residual cGMP for degradation after gap junction closure, however the specific PDE has until now not been identified. Inhibitors for cGMP-specific PDEs in the oocyte could provide a targeted approach to prevent hydrolysis of cGMP that would in turn block down-stream degradation of cAMP and prolong oocyte meiotic arrest, even after ovulation.
Pharmacological inhibition of PDE3 has been shown to inhibit oocyte maturation and prevent pregnancy in rhesus macaques (26). However, unfavorable side effects and inconsistent bioavailability of currently available PDE3 inhibitors suggest the need for further investigation of alternate contraceptive strategies (26). We hypothesized that suppressing cGMP-targeting PDE(s) with specific inhibitors will maintain elevated intra-oocyte cGMP levels and block (or delay) PDE3A-induced cAMP degradation preventing timely resumption of meiosis and circumventing the need to target PDE3A directly. Herein we report the expression pattern of PDE genes in the primate follicle and the identification of a cGMP-targeting enzyme in the monkey GV oocyte. Additionally we evaluated the function of this PDE by assessing the effect of its inhibitor on recombinant PDE3A activity in a fluorescence polarization assay and on spontaneous mouse oocyte maturation, either alone or in combination with exogenous cGMP.
Materials and Methods
Animal use
All animal protocols and procedures were performed after approval from, and in strict accordance to the Oregon Health & Science University Institutional Animal Care and Use Committee and followed the National Institute of Health’s Guide for the Care and Use of Laboratory Animals.
Collection of rhesus monkey germinal vesicle oocytes and granulosa cells
Ovary aspiration and collection of GV oocytes in the Rhesus monkey (Macaca mulatta) has been previously described (27). Briefly, antral follicles were aspirated following a standard controlled ovary stimulation protocol modified to exclude administration of human chorionic gonadotropin (hCG) (28). GV oocytes were aspirated into warm TL Hepes medium (Lonza Walkersville Inc., Walkersville, Maryland, USA) supplemented with 5 IU/ml heparin (MP Biomedicals, Solon, Ohio, USA). Total volume of the aspirate was adjusted to 10 ml with fresh TL Hepes and 3 mg of hyaluronidase (Sigma-Aldrich, St. Louis, Missouri, USA) was then added. The aspirate was filtered through a 70-µm nylon cell strainer (BD Biosciences, Bedford, Massachusetts, USA), and remaining debris in the filter cup was collected with 10 ml fresh TL Hepes into a 60 mm × 15 mm petri dish. GV oocytes were collected and prepared for RNA isolation by removing cumulus cells with repeated pipetting through a fine bore glass capillary needle.
Granulosa cells from the filtered aspirate were collected into a 15-ml conical tube and pelleted by centrifugation at 3000 × g for 2 minutes. The cell pellet was resuspended in 500 µl warm DMEM cell culture medium (Invitrogen, Carlsbad, California, USA) and layered on top of a 3-ml 30% Percoll/DMEM mixture in a 15-ml conical tube. The suspension was centrifuged at 3000 × g for 30 minutes to remove the red blood cells from the granulosa cell mixture, and the top layer with the purified granulosa cells was washed in 10 ml of fresh DMEM and centrifuged for an additional 3 minutes at 3000 × g. After removal of the supernatant, the granulosa cell pellet was considered ready for RNA isolation.
Collection and preparation of mouse germinal vesicle oocytes
B6D2F1 (BDF-1) mice (Charles River Laboratories, Wilmington, Massachusetts, USA) at 3–6 weeks of age were stimulated with 5 IU of PMSG (Harbor UCLA, Torrance, California, USA), administered intraperitoneal to promote follicle growth. After 48 hours, ovaries were collected into warm M2 medium (Millipore, Billerica, Massachusetts, USA) containing 200 µM 3-Isobuty-methylxanthine (IBMX; Sigma-Aldrich), and follicles were repeatedly punctured with a 27G needle to release the oocytes. Cumulus granulosa cells were removed by repeated pipetting through a fine bore glass needle, and cumulus-free, GV-intact oocytes were washed in fresh KSOM medium (Millipore) without IBMX for additional culture experiments.
RNA isolation, RT-PCR, and quantitative PCR (qPCR)
RNA from rhesus monkey GV oocytes and granulosa cells was isolated following the manufacturer’s protocol for the Absolutely RNA™ Nanoprep Kit (Agilent Technologies, Inc., Wilmington, Delaware, USA). Isolated RNA samples were treated with RNase-free DNase (Promega, Madison, Wisconsin, USA) and eluted with 11 µl of nuclease-free water. The eluate was directly amplified with Superscript III reverse transcriptase and random hexamer primers (Invitrogen) to produce cDNA. The quality of cDNA for each sample was verified by PCR amplification of the housekeeping gene peptidylprolyl isomerase A (PPIA) using HotStar taq polymerase (QIAGEN, Valencia, California, USA); the product was then visualized on a 2% ethidium bromide-stained agarose gel. All RT-PCR primers were designed using Primer 3 software (Sigma-Aldrich). Although the zona pellucida was not removed from the oocytes prior to RNA isolation, all attached cumulus cells were removed to prevent transcript contamination in the samples. As cumulus-oocyte heteromeric gap junctions do not permit permeability of molecules greater than 0.1 kDa (PDE transcript average size ~600 kDa), it is expected trans-zonal projections would not be a source of RNA contamination (29).
Prior to qPCR amplification, the concentration for each cDNA sample was measured using a Nanodrop ND-1000, and nuclease-free water was used for all required dilutions. The standard curve method was implemented to detect 19 specific PDE genes in the pre-ovulatory rhesus monkey GV oocyte or the associated granulosa cells. Ribosomal protein S-10 (RPS-10) and 18s ribosomal RNA (18s-rRNA) were used as the endogenous control genes to establish expression levels of the PDE messages in granulosa cells and GV oocytes, respectively. qPCR primers were designed with an on-line program from Biosearch Technologies (Novato, California, USA) and ordered through Sigma-Aldrich (Supplemental Table 1). qPCR was performed in 1x Fast SYBR® Green PCR Master Mix (Applied Biosystems, Carlsbad, CA), 20 ng of cDNA and 80 nM of each primer in a 20-µl total volume reaction. Amplification and detection were performed with a 7500 Fast Real-Time PCR system (Applied Biosystems) over 50 cycles. For each gene, a standard curve was performed to assess the quality of the reaction and quantify relative expression levels of all PDE genes compared to the endogenous control.
PDE3A Inhibition Assay
Prior to mouse oocyte culture experiments, the direct inhibitory effects on PDE3A activity by 8-Br-cGMP and BAY 73-6691 were measured with a cell-free fluorescence polarization (FP) assay. FP assays corrects for rotation of the fluorophore, induced by absorbance of polarized light and interaction with a ligand, by measuring fluorescence intensity both parallel and perpendicular to the detectors. This method provides more accurate measurement of fluorescent biochemical activity than standard single-read assays. Using a PDE3A FP Assay Kit (Bioscience, San Diego, California, USA),the protocol was followed per manufacturer’s suggestions to determine the percent decrease of PDE3A activity achieved in the presence of increasing concentrations of either compound. Inhibitory activity of 8-Br-cGMP was measured at 1 µM, 10 µM, 100 µM, 1 mM, and 10 mM and BAY 73-6691 at 1 µM, 10 µM, 100 µM, and 1 mM. Five replicates were performed for each compound series over 3 different assay kits. Fluorescent cAMP was incubated with 400 pg of human PDE3A (92% homologous to mouse PDE3A) and different concentrations of either 8-Br-cGMP or BAY 73-6691 in a 96-well microtiter plate in triplicate. PDE3A activity was indicated by generation of fluorescent nucleotide monophosphates, a by-product of PDE3A induced cAMP hydrolysis; inhibition of PDE3A reduces the concentration of the monophosphates available for measurement. Fluorescence polarization measurements (mP) were obtained at 520 nm with a Molecular Devices SpectaMax M5 microplate reader. Data collected were then used to define a range of concentrations to evaluate during mouse oocyte culture and validate that dose response effects observed in these experiments were due to PDE3A inhibition.
Mouse GV oocyte co-culture
All culture experiments were carried out in KSOM medium at 37°C in a humidified 5% CO2 incubator for 20–22 hours. A membrane-permeable cGMP analog, 8-Br-cGMP (Sigma-Aldrich), was used as an exogenous cGMP source and dissolved in KSOM to make a 100 mM stock solution. The stock solution was diluted to 100 µM, 500 µM, 1 mM or 10 mM in KSOM medium and used for mouse GV oocyte culture. BAY 73-6691 (Sigma-Aldrich), a membrane permeable, specific PDE9 inhibitor, was dissolved in DMSO to make a 100 mM stock solution. The BAY 73-6691 stock solution was also diluted in KSOM medium at 10 µM, 100 µM, or 1 mM for oocyte incubation. Co-culture experiments with 8-Br-cGMP and BAY 73-6691 together at varying concentrations were also performed. Each experiment included a control group of oocytes incubated in KSOM without supplementation. The criterion for spontaneous oocyte maturation was the disappearance of a visible nucleus, i.e., germinal vesicle breakdown (GVBD).
Statistical analysis
All qPCR data sets represent a mean value based on three experimental replicates, over three different biological samples. Expression values for these data are graphically displayed as a relative fold change compared to an endogenous control gene (Figure 1). The first standard deviation is indicated; means were evaluated using One Way ANOVA and pair wise comparisons were performed with the Student-Newman-Keuls method with P < 0.001 considered significant to adjust for multiple comparisons.
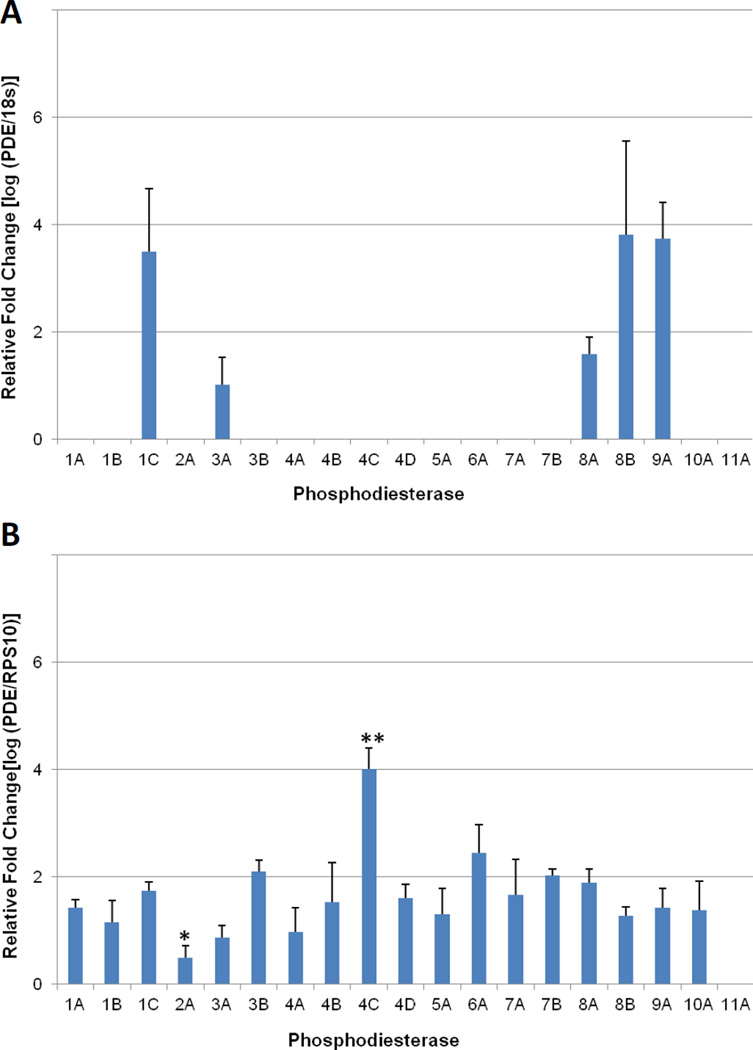
Figure 1.
Quantitative PCR (qPCR) profiling of PDE expression in rhesus monkey GV oocytes and granulosa cells collected from pre-ovulatory antral follicles. (A) Of the 19 PDE genes that were assayed, only PDE1C, PDE3A, PDE8A, PDE8B, and PDE9A were detectable in the GV oocyte. (B) PDE expression in granulosa cells, PDE11A was the only isoform not detectable in any of the samples evaluated. Expression ratios are based on a comparison to the endogenous control gene; 18s-rRNA for oocytes and RPS10 for granulosa cells. Error bars indicate standard deviation. One Way ANOVA was performed with a Student-Newman-Keuls post hoc test to determine significant changes in expression with P < 0.001. * Indicates expression is significantly less than all other isoforms. ** Expression is significantly greater than all other isoforms.
Data from the fluorescence polarization measurements were analyzed for the Z’-factor to assess data quality and variability (30). Z’-factor values between 0.5 and 1.0 are considered excellent (good quality, repeatable data) while data with Z’-factor values below 0.5 are considered less reliable. Significant changes in mP values were determined by One Way ANOVA followed by posthoc comparisons with the Student-Newman-Keuls test where P < 0.001. The first standard deviation is indicated for both compounds evaluated (Figure 2).
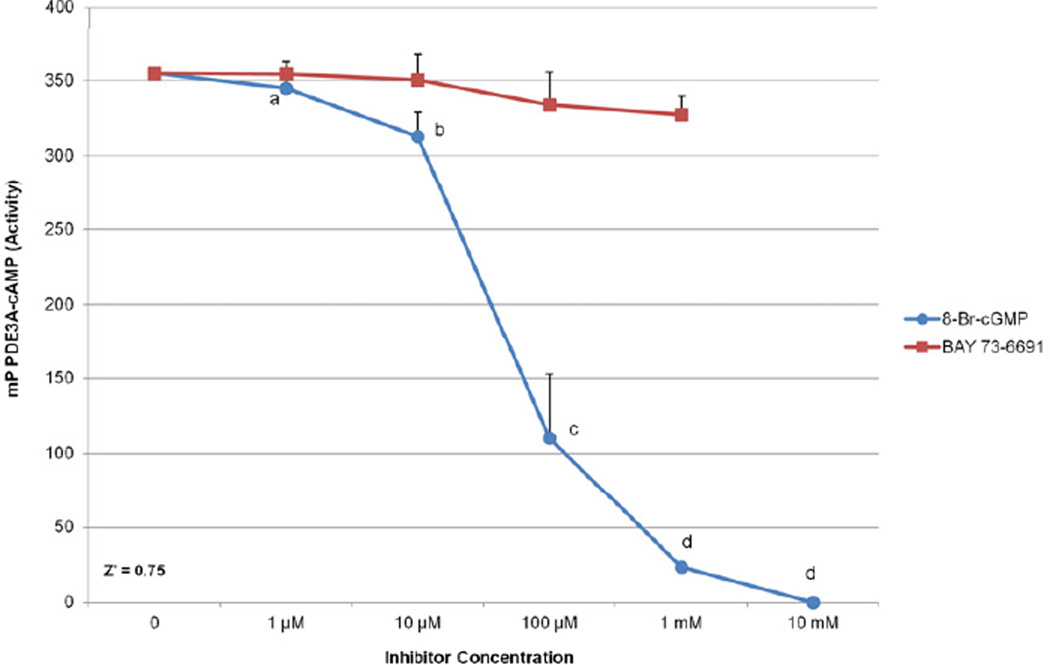
Figure 2.
Florescence polarization assay for PDE3A activity. Increasing concentrations of the PDE9 inhibitor, BAY 73-6691, were evaluated for inhibitory properties against PDE3A activity at 0 µM (no inhibitor), 1 µM, 10 µM, 100 µM, and 1 mM (Red). Similarly, the cGMP analog, 8-Br-cGMP, was assayed at 0 µM (no inhibitor), 1 µM, 10 µM, 100 µM, 1 mM and 10 mM (Blue). No significant changes were observed for the PDE9 inhibitor. Different letters indicate a significant decrease in PDE3A and Z’ = 0.75 for both assays. Significant changes in activity were analyzed by ANOVA followed by posthoc comparison with Student-Newman-Keuls test with P<0.001. Error bars indicate the first standard deviation.
A total of 32 mice were used to collect oocyte GV retention data over 5–6 experimental replicates. The SD is indicated for all the mean values of the proportions. The statistical significance was determined by Chi Square analysis with a criterion of P < 0.05.
Results
Cellular distribution of PDE transcripts in rhesus monkey antral follicles
Rhesus monkey GV oocyte and granulosa cells collected from preovulatory (no LH) antral follicles were analyzed by qPCR to identify the PDE genes actively expressed at this stage of development. Of the six genes in the PDE6 family (PDE6A, PDE6B, PDE6C, PDE6D, PDE6G, PDE6H), only PDE6A was selected for detection by qPCR. In preliminary experiments using macaque ovarian tissue, standard RT-PCR analysis failed to detect any of the remaining 5 genes in the PDE6 family (data not shown). Among the 19 PDE genes assayed in the GV oocyte, only five transcripts were detected: PDE1C, PDE3A, PDE8A, PDE8B, and PDE9A (Figure 1A). Of these only PDE9A is cGMP-specific. The remaining oocyte-localizing PDE transcripts predominantly target cAMP (PDE8A/B) or have an affinity for both cAMP and cGMP (PDE1C and PDE3A). There were no statistically significant differences in the expression levels of these five oocyte-localizing transcripts.
In contrast, all of the PDE genes assayed were detected in granulosa cells (Figure 1B) with the exception of PDE11A. PDE4C, which primarily targets cAMP for degradation, was the most abundantly expressed and displayed a significant 1.6–2.3 fold increase (P < 0.001) in signal compared to all other transcripts.
Effect of a PDE9 inhibitor and cGMP on PDE3A activity in vitro
To determine whether the PDE-9 specific inhibitor BAY 73-6691 could also inhibit PDE3A activity, we measured degradation of fluorescently labeled cAMP following co-incubation with PDE3A and increasing concentrations of the PDE9A inhibitor (Figure 2). There were no significant inhibitory effects of BAY 73-6691 on PDE3A activity [0% (1 µM); −1.1%, (10 µM), −5.9% (100 µM), and −7.6% (1 mM)]. These data demonstrate PDE9 does not directly hydrolyze cAMP or inhibit PDE3A activity to any significant extent and therefore should have little effect on meiosis and not contribute to GV retention during in vitro oocyte culture experiments. Higher concentrations of the inhibitor were not measured as precipitant forms at doses greater than 1 mM; the slight inhibition observed at the 1 mM concentration may be an artifact.
Increasing concentrations of 8-Br-cGMP were also assayed to determine the dose response for inhibition of PDE3A by cGMP. The lowest concentrations of 1 and 10 µM 8-Br-cGMP produced small, non-significant inhibition of PDE3A activity (−2.8% and −11.8%) (Figure 2) but higher concentrations produced significant (−68.9 (100 µM, −93.4 (1 mM), and −100% (10 mM) (P < 0.001). These data verify a dose-dependent inhibitory effect of 8-Br-cGMP on PDE3A hydrolysis of cAMP.
Effect of BAY 73-6691 on mouse oocyte maturation in vitro
Given the limited availability of monkey oocytes for culture experiments, preliminary functional data assessing PDE9 inhibitor activity was obtained using mouse oocytes. Prior to in vitro culture experiments with the PDE9 inhibitor, the transcript expression of cGMP-specific PDEs (Pde5A, Pde6A, and Pde9A) in mouse GV oocytes was verified by conventional RT-PCR (data not shown). Only Pde6A and Pde9A were detected and the expression level of Pde9A was twice that of Pde6A. Therefore, mouse GV oocytes were deemed an appropriate model for this preliminary investigation into the effects of the PDE9 inhibitor in mammalian oocytes.
Cumulus-free mouse GV oocytes were cultured in KSOM medium containing 10 µM, 100 µM, or 1 mM BAY 73-6691 for 20–22 hours, and GV retention rates were compared to the non-treated control (no inhibitor). Of the 4 treatment groups, only the oocytes cultured with 1 mM BAY 73-6691 exhibited a modest, but not statistically significant increase in meiotic inhibition (19.0%). No detectable difference was found among the proportion of oocytes cultured in 10 µM (7.4%) and 100 µM (11.8%) BAY 73-6691, compared to the control (7.8%) (Figure 3A).
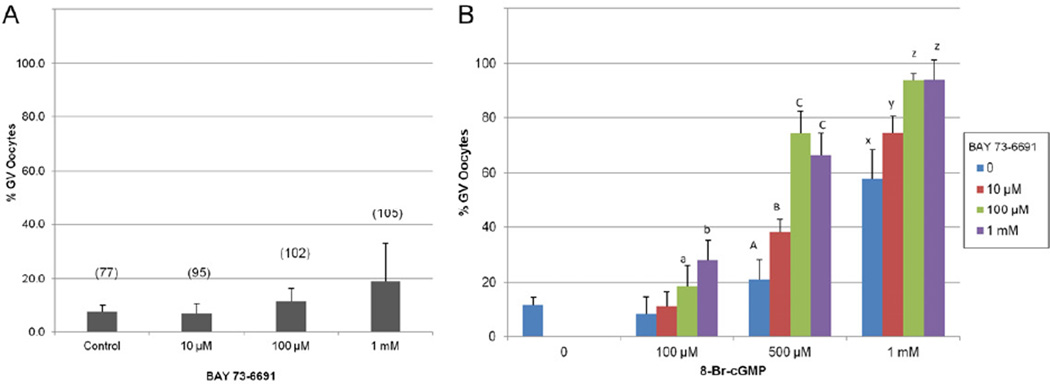
Figure 3.
Percentage of mouse oocytes that retained the GV after 20–22 hours of culture with (A) the selective PDE9 inhibitor BAY 73-6691, or (B) a combination of both 8-Br-cGMP and BAY 73-6691. Error bars represent the standard deviation; value in parenthesis represents the total number of oocytes in each treatment group for (A). Different letters at each level of 8-Br-cGMP in (B) represent a significant change from the control and determine by Chi Square analysis with P<0.001.
Effect of 8-Br-cGMP on mouse oocyte maturation in vitro
Cumulus-free GV oocytes were cultured with the membrane permeable cGMP analog, 8-Br-cGMP, to verify the minimum concentration of exogenous cGMP required to impede spontaneous meiosis in vitro (Figure 3B). We observed that meiosis was inhibited in a typical dose-response fashion as indicated by the retention of the germinal vesicle. Compared to the non-treated control (7.8% GV intact), 500 µM, 1 mM, and 10 mM 8-Br-cGMP maintained GV arrest in a significantly higher proportion of the total oocytes cultured [21.1%, 57.8%, and 97.8%, respectively]. There was no difference in the proportion of oocytes that maintained meiosis arrest in 100 µM 8-Br-cGMP (8.8%) when compared to the control treatment.
Inhibitory effects of 8-Br-cGMP and BAY 73-6691 co-treatment on spontaneous meiosis resumption in vitro
Cumulus-free mouse GV oocytes were co-cultured with 8-Br-cGMP and BAY 73-6691 in a multiple combinatorial approach with increasing concentrations of each compound. The proportion of oocytes that remained in the GV stage were graphically reported in Figure 3B. At every level of 8-Br-cGMP tested, the addition of BAY 73-6691 induced significant increases in the proportion of oocytes which retained the germinal vesicle throughout the culture period. As the level of 8-Br-cGMP increased, so did the impact of the PDE9 inhibitor, allowing fewer oocytes to exit prophase I arrest. The inclusion of 0 µM, 10 µM, 100 µM, and 1 mM BAY 73-6691 to the 8-Br-cGMP supplemented culture media, substantially increased the proportion of oocytes maintaining GV arrest (non-supplemented control = 11.8%); 8-Br-cGMP 100 µM [8.8%, 11.4%, 18.8%, and 28%], 500 µM [21.1%, 38.1%, 74.5%, and 66.5%], and 1 mM [57.8%, 74.5%, 93.9%, and 94.0%] respectively (Figure 3B).
Discussion
The regulatory roles of cyclic nucleotides and selected PDEs in the maturing follicle are well established (19). Elevated levels of cAMP within the GV oocyte maintain the meiotic arrest state by supporting PKA activity, thus preventing further downstream signaling in the meiosis activation pathway (31). PDEs target specific cyclic nucleotides during meiotic resumption and PDE3A has been identified as the dominant enzyme that actively hydrolyzes cAMP to promote oocyte maturation (6, 32). Signaling molecules in the oocyte, upstream of PDE3A, work in concert to prevent premature initiation of meiosis by inhibiting PDE3A activity. In particular, cGMP is produced in the cumulus granulosa cells and diffuses into the oocyte through gap junctions. High levels of cGMP in the oocyte inhibits PDE3A hydrolytic activity so that elevated levels of cAMP are sustained and the oocyte remains in meiotic arrest, allowing time for the accumulation of cytoplasmic competence (33). Following LH-surge induced closure of the gap junctions, the meiosis inhibiting cGMP is no longer supplied to the oocyte, but residual quantities must be reduced before PDE3A activity can begin degrading cAMP (34, 35).
Until now, it was unclear which PDE enzyme is present in the fully grown GV oocyte to participate in hydrolyzing the residual cGMP following gap-junction closure. We identified PDE9A as the only cGMP-specific degrading gene transcribed in the primate GV oocyte prior to LH-induced initiation of meiosis (Figure 1A), and a potential candidate responsible for removal of inhibitory cGMP from the oocyte. Microarray data collected for transcriptome analysis of human metaphase II oocytes confirms that of the three cGMP-specific PDEs, PDE9A is greatly expressed above background control values while PDE5A and PDE6A barely register (36). Additional studies on kinetics analysis calculate PDE9A as having a Km of 170 nM for cGMP making it the PDE with the greatest affinity for cGMP (37). Taken together, it is possible that PDE9A may be the dominant (if not only) cGMP-specific degrading enzyme in the oocyte; however future experiments are required to directly measure PDE9A activity in peri-ovulatory primate oocytes.
Our experiments also support a functional role of PDE9A during meiosis regulation. First, we demonstrated using a fluorescence polarization assay that the membrane permeable cGMP analog 8-Br-cGMP inhibits PDE3A activity in a dose dependent fashion, but the PDE 9 inhibitor BAY 73-669 does not. (Figure 2). While a slight decrease of PDE3 was observed at 1 mM with the PDE9 inhibitor, minor precipitation of the compound was observed and this could have interfered with the ability of the detector to precisely measure fluorescence affecting the reliability the assay at this level. Although the concentrations of 8-Br-cGMP in our experiments needed to inhibit PDE3 were very high, a dose response was observed and no precipitation of the compound was noted. Using [3H] cGMP, Vaccari, et al., demonstrated that levels of cGMP in the nanomolar range (IC50 = 96.9 nM) inhibited PDE3 derived from mouse oocytes extracts in a typical dose response fashion (18). The concentration of the cGMP isozyme, 8-Br-cGMP, needed in our experiments to completely inhibit PDE3A activity was much higher and non-physiologic, this is most likely due to the imidizole ring substitution, required to make the compound soluble and membrane permeable; this increases the IC50 65-fold compared to unmodified cGMP (38). Although a higher dose is required to exert a similar effect as native cGMP, 8-Br-cGMP is widely used in oocyte meiosis studies and is a suitable model agent for in vitro experiments (39, 40).
In our mouse oocyte culture, 8-Br-cGMP, prevented spontaneous in vitro maturation in up to nearly 100% of the oocytes at 10 mM, with a dose response similar to that reported in hamster, mouse, and pig oocytes (41–43). The PDE 9 inhibitor BAY 73-6691 alone had no inhibitory effect on spontaneous resumption of meiosis, suggesting that the cumulus-free oocyte is not a significant source of cGMP so that endogenous levels are low without the cumulus cell contribution. In addition, the rate of cGMP degradation in GV oocytes is unknown. Lower concentrations of the PDE9 inhibitor were not sufficient to stop the theoretically rapid hydrolysis of cGMP and may explain why the highest concentration (1 mM) had only a minor, and non-statistically significant inhibitory effect. It is possible that a higher concentration of BAY 73-6691 may be required to adequately slow the hydrolysis of cGMP in order to observe an impact on meiotic resumption. However, BAY 73-6691 and 8-Br-cGMP combined in culture showed a synergistic effect where PDE9 inhibition augmented the potency of cGMP to sustain meiotic arrest (Figure 3B). Addition of 100 µM or 1 mM BAY 73-6691 to 1 mM of 8-Br-cGMP was enough to prevent germinal vesicle breakdown in 94% of the oocytes which is similar to the percentage of inhibited oocytes cultured in 10 mM 8-Br-cGMP alone. In short, by inhibiting PDE9 activity in the oocyte, the amount of cGMP required to suppress PDE3A activity was decreased 10-fold. From these preliminary data we can conclude that PDE9A participates in the oocyte maturation pathway upstream of PDE3A and cGMP (Figure 4).
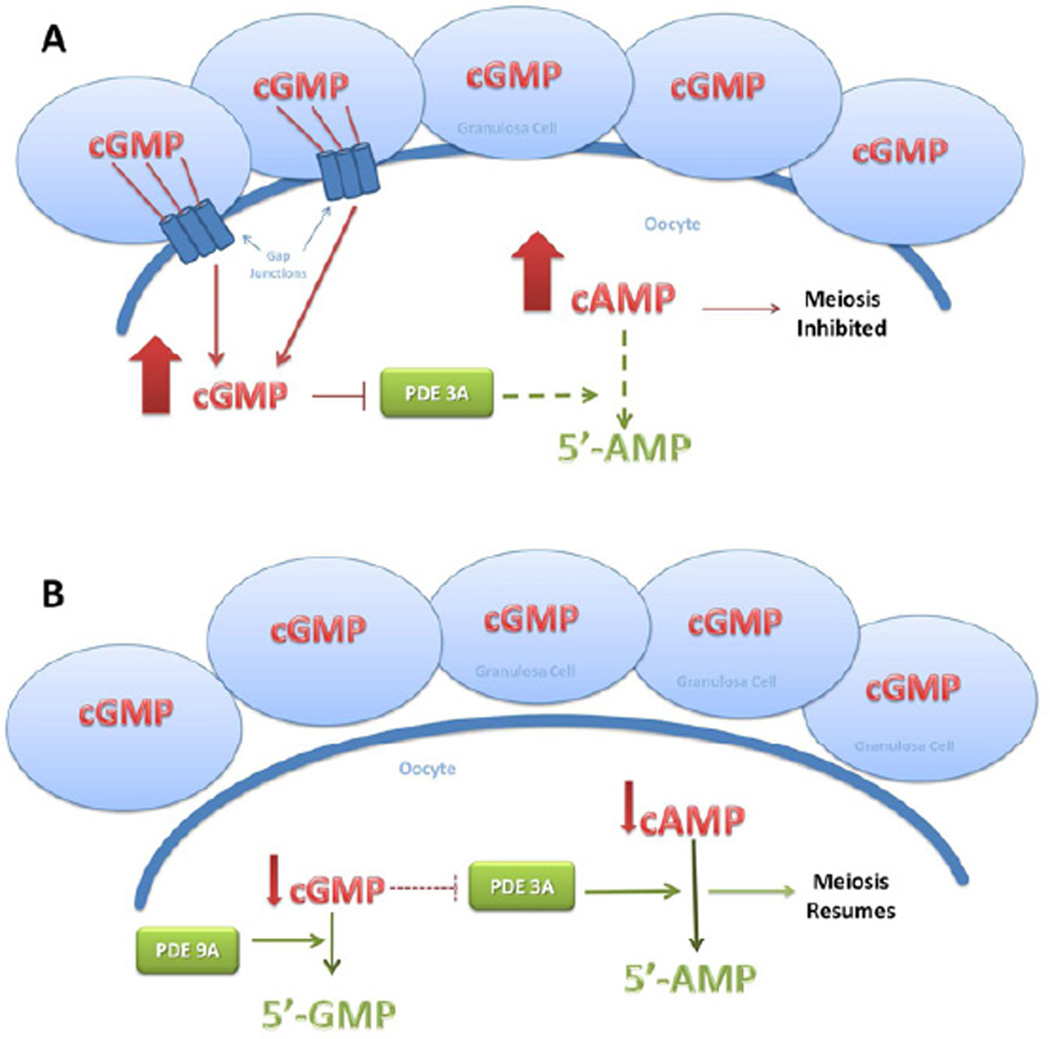
Figure 4.
Proposed PDE9 activity pathway in the oocyte. (A) cGMP from granulosa cells is actively transported through gap junctions into the GV oocyte. Elevated levels of cytoplasmic cGMP inhibit PDE3A hydrolysis of cAMP and maintain the oocyte in meiotic arrest. Based on our data, following the LH surge, gap junctions are closed, and residual cGMP is degraded by PDE9 (B). Decreased levels of cGMP permit PDE3A mediated hydrolysis of cAMP, lowering the cytoplasmic concentration and inducing meiotic resumption.
Previous work in our laboratory evaluated the pharmacological PDE3 inhibitor, ORG 9935, for its ability to prevent spontaneous and gonadotropin-induced oocyte maturation and as a contraceptive agent for socially-housed harems of rhesus monkeys (14–16, 26). ORG 9935 proved to be an effective inhibitor of meiosis both in vitro and in vivo and prevented pregnancy when present at serum levels above 300 nMol/L, but problems associated with drug bioavailability and tolerability prevented further investigation of this compound.
These experiments suggest that blocking PDE9 activity may be useful as part of a contraceptive strategy. Recent data demonstrated that when the catalytic domain of PDE9A was injected into mouse follicle enclosed oocytes, cAMP was drastically reduced after 1 hour and nuclear maturation resumed after 2 hours (19). The decrease in cAMP was not a direct result of PDE9A hydrolytic activity but rather as a result of decreased cGMP in the oocyte via PDE9A degradation. Although an enzymatic extract was used for these data, the ability of PDE9A to effectively reduce cGMP in oocyte cytoplasm appears to be robust (19). By extension, targeted inhibition of PDE9A would prevent cGMP breakdown and prolong the time to nuclear maturation, following the LH surge, through cGMP inhibition of PDE3A.
We theorize that a low-dose combination of PDE inhibitors targeting specific enzymes localized to the immature oocyte and granulosa cells may represent a novel, non-hormonal based contraceptive strategy. For example, tolerability to PDE3A blockade could be improved through dose reduction supported by the addition of a PDE9A inhibitor. In a theoretical model system, a PDE5 inhibitor (several currently approved drugs are available (44)) could also be included to inhibit cGMP degradation in the granulosa cells so that a higher concentration of cGMP would be available to diffuse through the gap junctions into the oocyte. Similarly, the cAMP hydrolyzing PDE4 is the only isoform demonstrated thus far to be active in human granulosa cells, and an inhibitor to PDE4 would also potentially supply the oocyte with greater levels of inhibitory cAMP (45, 46). Following the gap junction closure event, degradation of cGMP in the oocyte would be blocked by the PDE9 inhibitor which in turn would potentiate the action of the PDE3A inhibitor, preventing hydrolysis of cAMP. This synergistic approach to arrest of oocyte maturation with a low-dose selective “cocktail” approach to phosphodiesterase inhibition in the periovulatory follicle may reduce the risk of off-target side effects in a novel nonhormonal strategy.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the following core facilities at the Oregon National Primate Research Center: the Division of Animal Resources surgical team for assistance in monkey oocyte collection; the Assisted Reproductive Technologies Core for oocyte culture media; and the Endocrine Services Laboratory for monkey blood serum analysis.
J.T.J. is a consultant for Bayer Healthcare, HRA Pharma, Merck, and the Population Council. He is a speaker for Bayer Healthcare. He receives research support from Abbott Pharmaceuticals, Bayer Healthcare, M-360, the Population Council, and the NIH.
This work was supported by the NICHD grant HD042710 NICHD, the NIH Contraceptive Development Research Center grant U54 HD18185 SCCPIR and NCRR RR00163.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
C.B.H has nothing to disclose. S.Y. has nothing to disclose. X.W. has nothing to disclose.
References
- 1.Phillips BT, Gassei K, Orwig KE. Spermatogonial stem cell regulation and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1663–1678. doi: 10.1098/rstb.2010.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fair T. Mammalian oocyte development: checkpoints for competence. Reprod Fertil Dev. 2010;22:13–20. doi: 10.1071/RD09216. [DOI] [PubMed] [Google Scholar]
- 3.Nogueira D, Cortvrindt R, De Matos DG, Vanhoutte L, Smitz J. Effect of phosphodiesterase type 3 inhibitor on developmental competence of immature mouse oocytes in vitro. Biol Reprod. 2003;69:2045–2052. doi: 10.1095/biolreprod.103.021105. [DOI] [PubMed] [Google Scholar]
- 4.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: Molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- 5.Sun Q-Y, Miao Y-L, Schatten H. Towards a new understanding on the regulation of mammalian oocyte meiosis resumption. Cell Cycle. 2009;8:2741–2747. doi: 10.4161/cc.8.17.9471. [DOI] [PubMed] [Google Scholar]
- 6.Masciarelli A, Horner K, Liu C, Park SH, Hinckley M, Hockman S, et al. Cyclic nucleotide phosphodiesterase 3A-deficient mice as a model of female infertility. J Clin Invest. 2004;114:196–205. doi: 10.1172/JCI21804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sasseville M, Cote N, Guillemette C, Richard FJ. New insight into the role of phosphodiesterase 3A in porcine oocyte maturation. BMC Dev Biol. 2006;6:47. doi: 10.1186/1471-213X-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sasseville M, Albuz FK, Cote N, Guillemette C, Gilchrist RB, Richard FJ. Characterization of novel phosphodiesterases in the bovine ovarian follicle. Biol Reprod. 2009;81:415–425. doi: 10.1095/biolreprod.108.074450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsafriri A, Chun SY, Zhang R, Hsueh AJ, Conti M. Oocyte maturation involves compartmentalization and opposing changes of cAMP levels in follicular somatic and germ cells: studies using selective phosphodiesterase inhibitors. Dev Biol. 1996;178:393–402. doi: 10.1006/dbio.1996.0226. [DOI] [PubMed] [Google Scholar]
- 10.Vaccari S, Horner K, Mehlmann LM, Conti M. Generation of mouse oocytes defective in cAMP synthesis and degradation: endogenous cyclic AMP is essential for meiotic arrest. Dev Biol. 2008;316:124–134. doi: 10.1016/j.ydbio.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solc P, Schultz RM, Motlik J. Prophase I arrest and progression to metaphase I in mouse oocytes: comparison of resumption of meiosis and recovery from G2-arrest in somatic cells. Mol Hum Reprod. 2010;16:654–664. doi: 10.1093/molehr/gaq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nogueira D, Albano C, Adrianenssens T, Cortvrindt R, Bourgain C, Devroey P, et al. Human oocytes reversibly arrested in prophase I by phosphodiesterase type 3 inhibitor in vitro. Biol Reprod. 2003;69:1042–1052. doi: 10.1095/biolreprod.103.015982. [DOI] [PubMed] [Google Scholar]
- 13.Nogueira D, Ron-El R, Friedler S, Schachter M, Raziel A, Cortvrindt R, et al. Meiotic arrest in vitro by phosphodiesterase 3-inhibitor enhances maturation capacity of human oocytes and allows subsequent embryonic development. Biol Reprod. 2006;74:177–184. doi: 10.1095/biolreprod.105.040485. [DOI] [PubMed] [Google Scholar]
- 14.Jensen JT, Schwinof KM, Zelinski-Wooten MB, Conti M, DePaolo LV, Stouffer RL. Phosphodiesterase 3 inhibitors selectively block the spontaneous resumption of meiosis by macaque oocytes in vitro. Human Reprod. 2002;17 doi: 10.1093/humrep/17.8.2079. [DOI] [PubMed] [Google Scholar]
- 15.Jensen JT, Zelinski MB, Stanley JE, Fanton JW, Stouffer RL. The phosphodiesterase 3 inhibitor ORG 9935 inhibits oocyte maturation in the naturally selected dominant follicle in rhesus macaques. Contraception. 2008;77:303–307. doi: 10.1016/j.contraception.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen JT, Zelinski-Wooten MB, Schwinof KM, Vance JE, Stouffer RL. The phosphodiesterase 3 inhibitor ORG 9935 inhibits oocyte maturation during gonadotropin-stimulated ovarian cycles in rhesus macaques. Contraception. 2005;71:68–73. doi: 10.1016/j.contraception.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Vanhoutte L, De Sutter P, Nogueira D, Gerris J, Dhont M, Van der Elst J. Nuclear and cytoplasmic maturation of in vitro matured human oocytes after temporary nuclear arrest by phosphodiesterase 3-inhibitor. Human Reprod. 2007;22:1239–1246. doi: 10.1093/humrep/dem007. [DOI] [PubMed] [Google Scholar]
- 18.Vaccari S, Weeks JLn, Hsieh M, Menniti FS, Conti M. Cyclic GMP signaling is involved in the luteinizing hormone-dependent meiotic maturation of mouse oocytes. Biol Reprod. 2009;81:595–604. doi: 10.1095/biolreprod.109.077768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norris RP, Ratzan WJ, Freudzon M, Mehlmann LA, Krall J, Movsesian MA, et al. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development. 2009;136:1869–1878. doi: 10.1242/dev.035238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sasseville M, Cote N, Gagnon MC, Richard FJ. Up-regulation of 3'5'-cyclic guanosine monophosphate-specific phosphodiesterase in the porcine cumulus-oocyte complex affects steroidogenesis during in vitro maturation. Endocrinology. 2008;149:5568–5576. doi: 10.1210/en.2008-0547. [DOI] [PubMed] [Google Scholar]
- 21.Zhang M, Su Y, Sugiura K, Xia G, Eppig JJ. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science. 2010;330:366–369. doi: 10.1126/science.1193573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dekel N. Cellular, biochemical and molecular mechanisms regulating oocyte maturation. Mol Cell Endrocrinol. 2005;234:19–25. doi: 10.1016/j.mce.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Kovo M, Kandli-Cohen M, Ben-Haim M, Galiani D, Carr DW, Dekel N. An active protein kinase A (PKA) is involved in meiotic arrest of rat growing oocytes. Reproduction. 2006;132:33–43. doi: 10.1530/rep.1.00824. [DOI] [PubMed] [Google Scholar]
- 24.Mehlmann LA, Kalinowski RR, Ross LF, Parlow AF, Hewlett EL, Jaffe LA. Meiotic resumption in response to luteinizing hormone is independent of a Gi family G protein or calcium in the mouse oocyte. Dev Biol. 2006;299:345–355. doi: 10.1016/j.ydbio.2006.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehlmann LA, Teresa LA, Jaffe LA. Meiotic arrest in the mouse follicle maintained by a Gs protein in the oocyte. Science. 2002;297:1343–1345. doi: 10.1126/science.1073978. [DOI] [PubMed] [Google Scholar]
- 26.Jensen JT, Stouffer RL, Stanley JE, Zelinski MB. Evaluation of the phosphodiesterase 3 inhibitor ORG 9935 as a contraceptive in female macaques: initial trials. Contraception. 2010;81:165–171. doi: 10.1016/j.contraception.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.VandeVoort CA, Leibo SP, Tarantal AF. Improved collection and developmental competence of immature macaque oocytes. Theriogenology. 2003;59:699–707. doi: 10.1016/s0093-691x(02)01129-9. [DOI] [PubMed] [Google Scholar]
- 28.Young KA, Chaffin CL, Molskness TA, Stouffer RL. Controlled ovulation of the dominant follicle: a critical role for LH in the late follicular phase of the menstrual cycle. Human Reprod. 2003;18:2257–2263. doi: 10.1093/humrep/deg467. [DOI] [PubMed] [Google Scholar]
- 29.Weber PA, Chang HC, Spaeth KE, Nitsche JM, Nicholson BJ. The permeability of gap junction channels to probes of different size is dependent on connexin composition and permeant-pore affinities. Biophysical Journal. 2004;87:958–973. doi: 10.1529/biophysj.103.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J-H, Chung TDY, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 31.Bilodeau-Goeseels S. Effects of phosphodiesterase inhibitors on spontaneous nuclear maturation and cAMP concentration in bovine oocytes. Theriogenology. 2003;60:1679–1690. doi: 10.1016/s0093-691x(03)00173-0. [DOI] [PubMed] [Google Scholar]
- 32.Shitsukawa K, Anderson CB, Richard FJ, Horner AK, Wiersma A, van Duin M, et al. Cloning and characterization of the cyclic guanosine monophosphate-inhibited phosphodiesterase PDE3A expressed in mouse oocyte. Biol Reprod. 2001;65:188–196. doi: 10.1095/biolreprod65.1.188. [DOI] [PubMed] [Google Scholar]
- 33.Kawamura K, Cheng Y, Kawamura N, Takae S, Okada A, Kawagoe Y, et al. Pre-ovulatory LH/hCG surge decreases C-type natriuretic peptide secretion by ovarian granulosa cells to promote meiotic resumption of pre-ovulatory oocytes. Human Reprod. 2011;26:3094–3101. doi: 10.1093/humrep/der282. [DOI] [PubMed] [Google Scholar]
- 34.Norris RP, Freudzon M, Mehlmann LM, Cowan AE, Simon AM, Paul DL, et al. Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption. Development. 2008;135:3229–3238. doi: 10.1242/dev.025494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sela-Abramovich S, Edry I, Galiani D, Nevo N, Dekel N. Disruption of gap junctional communication within the ovarian follicle induces oocyte maturation. Endocrinology. 2006;147:2280–2286. doi: 10.1210/en.2005-1011. [DOI] [PubMed] [Google Scholar]
- 36.Kocabas AM, Crosby J, Ross PJ, Otu HH, Beyhan Z, Can H, et al. The transcriptome of human oocytes. Proc Natl Acad Sci U S A. 2006;103:14027–14038. doi: 10.1073/pnas.0603227103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fisher DA, Smith JF, Pillar JS, St. Denis SH, Cheng JB. Isolation and Characterization of PDE9A, a Novel Human cGMP-specific Phosphodiesterase. J Biol Chem. 1998;273:15559–15564. doi: 10.1074/jbc.273.25.15559. [DOI] [PubMed] [Google Scholar]
- 38.Beltman J, Becker DE, Butt E, Jensen GS, Rybalkin SD, Jastorff B, et al. Characterization of cyclic nucleotide phosphodiesterases with cyclic GMP analogs: Topology of the catalytic domains. Mol Pharmacol. 1995;47:330–339. [PubMed] [Google Scholar]
- 39.Downs SM. Mouse versus rat: Profound differences in meiotic regulation at the level of the isolated oocyte. Mol Reprod Dev. 2011;78:778–794. doi: 10.1002/mrd.21377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petr J, Rajmon R, Chmelikova E, Tomanek M, Lanska V, Pribanova M, et al. Nitric-oxide-dependent activation of pig oocytes: the role of the cGMP-signalling pathway. Zygote. 2006;14:9–16. doi: 10.1017/S0967199406003546. [DOI] [PubMed] [Google Scholar]
- 41.Bu S, Xie H, Tao Y, Wang J, Xia G. Nitric oxide influences the maturation of cumulus cell-enclosed mouse oocytes cultured in spontaneous maturation medium and hypoxanthine-supplemented medium through different signaling pathways. Mol Cell Endrocrinol. 2004;223:85–93. doi: 10.1016/j.mce.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 42.Hubbard CJ, Terranova PF. Inhibitory action of cyclic guanosine 5'-phosphoric acid (GMP) on oocyte maturation: dependence on an intact cumulus. Biol Reprod. 1982;26:628–632. doi: 10.1095/biolreprod26.4.628. [DOI] [PubMed] [Google Scholar]
- 43.Zhang M, Tao Y, Zhou B, Xie H, Wang F, Lei L, et al. Atrial natriuretic peptide inhibits the actions of FSH and forskolin in meiotic maturation of pig oocytes via different signaling pathways. J Mol Endocrinol. 2005;34:459–472. doi: 10.1677/jme.1.01673. [DOI] [PubMed] [Google Scholar]
- 44.Washington SL, Shindel AW. A once-daily dose of tadalafil for erectile dysfunction: compliance and efficacy. Drug Des Devel Ther. 2010;4:159–171. doi: 10.2147/dddt.s9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Furger C, Pouchelet M, Zorn JR, Ferre F. Cell shape change reveals the cyclic AMP-mediated action of follicle stimulating hormone, human chorionic gonadotrophin and vasoactive intestinal peptide in primary cultured human granulosa-lutein cells. Mol Hum Reprod. 1996;2:251–257. doi: 10.1093/molehr/2.4.251. [DOI] [PubMed] [Google Scholar]
- 46.Thomas RE, Armstrong DT, Gilchrist RB. Differential effects of specific phosphodiesterase isoenzyme inhibitors on bovine oocyte meiotic maturation. Dev Biol. 2002;244:215–225. doi: 10.1006/dbio.2002.0609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.