Abstract
We hypothesized that Nox2, the classical phagocytic NADPH oxidase, plays an important role in calcineurin inhibitor (CNI)-induced renal fibrosis. We tested this hypothesis in vitro, in animal and in human studies. Cyclosporine A (CsA) and tacrolimus (TAC) were associated with greater levels of Nox2 mRNA and epithelial to mesenchymal transition (EMT) in NRK52E cells. CsA increased Nox2, α-SMA and phosphorylated-p38MAPK, Smad3, and NFκB proteins. Nox2 upregulation and EMT were inhibited in TGF-β1 knockout cells suggesting that TGF-β1 is required for Nox2 activation. Fisher344 rats treated with high dose CsA showed increased Nox2 in the tubulointerstitium and greater Nox2, α-SMA, phosphorylated Smad3 and nitrotyrosine by immunoblot analyses. Inhibition of Nox2 by coadministration of apocynin or diphenyleneiodonium was associated with reduced fibrogenesis. We validated these findings by treating wild type and Nox2 null (B6.129S-CybbTm1Din/J) mice with high dose CsA. Western blot analyses confirmed the absence of Nox2 and significantly lower levels of α-SMA and 4-hydroxynonenal in CsA-treated knockout mice. These findings were clinically relevant since Nox2 and α-SMA were increased in the tubulointerstitium of kidneys from 15 liver transplant recipients with biopsy-confirmed chronic CsA or TAC nephrotoxicity. In conclusion, specific Nox2 inhibition strategies may improve chronic CNI nephrotoxicity in solid organ transplantation.
Keywords: CsA, Nox2, Fibrosis, EMT, Oxidative Stress
Introduction
Calcineurin inhibitors (CNIs) including cyclosporine (CsA) and tacrolimus (TAC) are the cornerstone of maintenance immunosuppression in solid organ transplantation (1, 2). These drugs are so effective that newer generation CNIs such as Voclosporin (Isotechnika Pharma, Inc, Edmonton, Alberta) are in development (3). They play their immunosuppressive role by inhibiting the activity of calcineurin, a serine phosphatase that normally dephosphorylates nuclear factor of activated T cells (NFAT). Dephosphorylated NFAT translocates into the nucleus and induces the transcription of interleukin-2, an important cytokine for the activation and proliferation of T-lymphocytes (1). Despite their beneficial immunosuppressive actions in transplantation and many autoimmune disorders, the clinical use of CNIs is compromised by their chronic nephrotoxicity (4–10). As an example, the 10-year incidence of chronic CNI nephrotoxicity was reported to be 100% in kidney-pancreas transplant recipients (11). Although recent advances in the diagnosis of antibody-mediated renal injury suggest that acute and chronic antibody-mediated rejection (AMR) play a more important role than CNI nephrotoxicity in late kidney allograft loss (12, 13), CNI nephrotoxicity remains the dominant causative factor for kidney failure in non-kidney organ transplant recipients (6–10). In this group, the 5-year risk of end stage renal disease ranges from 7 to 21% and is associated with a 4-fold greater risk of death (14).
The pathological features of chronic CNI nephrotoxicity include progressive and irreversible interstitial fibrosis, tubular atrophy and arteriolar hyaline changes (6–9). Typically, fibrosis follows a “striped pattern” from the medulla to the cortex and is associated with vacuolization of the cytoplasm in tubular epithelial cells. At cellular and molecular levels, CNI nephrotoxicity results in matrix accumulation and epithelial dedifferentiation via TGF-β1 dependent and independent pathways (5, 9, 15–18). A better understanding of the molecular mechanisms that regulate CNI-induced fibrosis and epithelial dedifferentiation will pave the way for the development of antifibrotic strategies (19).
Originally named gp91phox, Nox2 is the classical phagocytic NADPH oxidase (20, 21), an enzyme that is naturally involved in the innate immune response including the “oxidative burst” (20, 21). Nox2 was the first identified example of a family of NADPH oxidases that generate reactive oxygen species (ROS) as their primary function rather than as a byproduct of oxidative metabolism as in the mitochondria or peroxisomes. It is one of the seven currently known Nox isoforms, is constitutively associated with the transmembrane stabilizing protein p22phox and requires the recruitment of cytosolic components p47phox, p67phox, and p40phox for function (20). Nox2-null transgenic mice (B6.129S-CybbTm1Din/J) are viable but exhibit increased susceptibility to infection by Staphylococcus aureus and Aspergillus fumigatus, and an altered inflammatory response to thioglycollate peritonitis, as well as altered renal function (22, 23).
The working hypothesis of our study was that Nox2 activation by CsA results in the activation of ROS-sensitive pathways involved in renal fibrogenesis. To test this hypothesis, we first examined the effects of CsA on Nox2 expression, epithelial cell phenotype and fibrosis in vitro. Next we assessed the role of specific Nox2-inhibition on CNI-induced renal fibrosis in rodent models. Lastly, we evaluated the clinical validity of our studies in liver transplant recipients with chronic CNI nephrotoxicity.
Materials and Methods
Animals
Adult (9–11 week old) male Fisher 344 rats were purchased from Harlan (Madison, WI). Four to six week old wild type C57BL/6J (cat # 000664) and B6.129S-CybbTm1Din/J (cat # 2365) mice were purchased from Jackson Labs (Bar Harbor, Maine). Animals were housed in the animal care facility at the William S. Middleton VA Hospital in Madison, WI and all procedures were performed in accordance with the Animal Care Policies at the VA Hospital and the UW. All animals were placed on a 0.1% low salt diet (Teklad TD.94268) 1 week prior to treatment and for the duration of the experiments.
The rats were randomized into five treatment groups: Olive oil vehicle, Cyclosporine (15mg/kg/day), Cyclosporine (15mg/kg/day) + diphenyleneiodonium (DPI, 0.5mg/kg/day), Cyclosporine (15mg/kg/day) + DPI (1.0mg/kg/day) or Cyclosporine (15mg/kg/day) + Apocynin (16mg/kg/day). Cyclosporine and DPI were administered IP. Apocynin was administered PO in the drinking water. Treatments were given for 1 month. After 1 month, animals were euthanized by exsanguination under general anesthesia. Six to eight rats were included in each experimental group.
Cyclosporine (30mg/kg/day) was administered IP to six C57BL/6J mice and six B6.129S-CybbTm1Din/J mice for 2 months. After 2 months, animals were euthanized by exsanguination under general anesthesia.
Patients
We identified subjects and controls from patients who had undergone a native kidney biopsy or nephrectomy as a standard of care procedure between 1995 and 2009. Briefly, a query of the UW Transplant Data System (TDS) was done for retrospective data on liver transplant recipients who had biopsy-proven chronic CNI nephrotoxicity between January 1, 1995 and June 1, 2009. The list was provided to the Director of UWHC Surgical Pathology (JT), who is an expert in renal pathology and who re-examined the slides to confirm the diagnosis. CNI nephrotoxicity was defined according to the Banff 2009 report and the new arteriolar hyaline thickening-scoring scheme suggested by Sis et al (24, 25). Baseline characteristics and demographic data on these patients were then collected using the TDS. A second slide from each of the same subjects was provided and stained for Nox2 and α-SMA. Tissue staining was compared to healthy samples recovered from nephrectomy in patients with renal cell carcinoma, and to experimental samples from rats and mice with chronic CNI nephrotoxicity. These studies were performed in adherence to the Declaration of Helsinki and were approved by the Institutional Review Board at the University of Wisconsin School of Medicine and Public Health.
Antibodies and compounds
We used the following antibodies: Nox2/gp91phox (1:100-250), BD Biosciences, San Diego, CA, 611414, Phospho p38 (1:500), BD 612288, Phospho NFκB (1:250), Abcam, Cambridge, MA, ab28849, α-SMA (1:2000), Sigma, St. Louis, MO, A2547, β-Actin (1:7500), US Biological, Marblehead, MA, A0760-40, Fibronectin (1:400), Abcam, ab23750, HNE (mouse, 15ug/mL), Abcam, ab48506, GAPDH (1:5000), Abcam, ab8245, Phospho Smad3 (1:500) Cell Signaling, Danvers, MA, 9520, HNE (rabbit, 1:1000), Abcam, ab4654. Nitrotyrosine (1:750) Millipore. Billerica, MA, Ab5411.
The following compounds were used in our studies: TGF-β1, Sigma, St. Louis, MO, T7039, Cyclosporine A, Bedford Labs, Bedford, OH, Prograf (tacrolimus), Astellas Pharma US, Deerfield, IL, Chemical Inhibitors of NOX, Calbiochem, Gibbstown, NJ, Apocynin 178385, Diphenyleneiodonium Chloride (DPI) 300260.
In vitro Studies
NRK52E cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 5% heat inactivated Fetal Bovine Serum (FBS), 5000 IU Penicillin/Streptomycin, 5000 μg/ml L-glutamine and 44 mM NaHCO3. Cells were passaged when 95% confluent as assessed by visual observation. For experiments, NRK52E were seeded in 5% FBS DMEM at 2.5×105 cells per well, in 6-well cell culture plates. After 24 hours, culture medium was replaced with 0% FBS, DMEM supplemented with 0.1% Bovine Serum Albumin to arrest cell growth and synchronize the cells. After 12 hours in serum free media, cells were treated with TGF-β1 (10 or 20ng/mL), CsA 1 or 10μM, or TAC 1 or 10μM for 4 or 48 hours. After treatment, cells were harvested for RNA and protein analysis. Early passage (passage 2) TGF-β1 knockout murine renal tubular epithelial cells (mTEC-KO) and early passage (passage 5) murine renal tubular epithelial cells (mTECWT) were generously provided to us by Dr. Jeffrey Kopp (National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD). The cells were grown until passage 20. They were maintained in Renal Epithelial Cell Growth Medium supplemented with 0.25% FBS, a Bullet Kit that contained epidermal growth factor, insulin, hydrocortisone, GA-1000, epinephrine, T3, and transferrin (Cambrex, NJ), and penicillin and streptomycin (Gibco-Invitrogen, CA). After 12 hours in serum free media, cells were treated with Cyclosporine (10μM) for 24 hours.
RNA extraction, purification and real-time PCR analysis
We have previously published these methods in detail (26). Briefly, total RNA extraction was done using Trizol (Gibco Life Technologies). RNA was purified using the RNAeasy kit (Qiagen). A First Strand cDNA synthesis kit (Roche) was used to generate cDNA. Nox2 mRNA was quantified using TaqMan Gene Expression Assays (Applied Biosystems, NOX2: cat # Rn00576710_m1, internal control S26: cat# Rn00820743_g1) run on a 7500 Real Time PCR System from Applied Biosystems. Mean Ct, standard deviation (SD) and delta Ct (compared to S26 internal control) for Nox2 were evaluated.
Immunoblotting
Western blotting was performed as described previously (26, 27). Briefly, proteins were separated by SDS-PAGE (7.5% Tris-HCl gel, Biorad, Hercules, CA) transferred to nitrocellulose membrane (Bio-Rad) by ‘wet’ electrophoretic transfer (80V, 45 min) and then blocked overnight at 4°C in a solution containing 1% nonfat dry milk, 1% BSA, 0.05% Tween in PBS. Membranes were incubated the next day with primary antibodies either at 4°C overnight or at room temperature for 1 hour. HRP-conjugated goat anti-mouse (BD Biosciences, CA, cat no. M15345) and goat anti-rabbit (eBiosciences, CA, cat. no. 18-8816-31) secondary antibodies were diluted in 10% non-fat dry milk, 0.1%BSA, 0.05% Tween in PBS at dilutions of 1:4500 and 1:10,000, respectively. HRP signals were visualized by enhanced chemiluminescence using a West Femto kit (Pierce, IL, cat. no. 34095) and imaged with a Fotodyne bench top CCD camera system. Densitometric quantification of immunoblot signals was done using Image J (NIH, rsbweb.nih.gov/ij/).
Histology
Formalin-fixed, paraffin-embedded kidneys were cut into 5μm sections. Slides were deparaffinized and rehydrated from xylene through a graded ethanol series to dH2O. For specific staining, slides were subsequently treated as described below. Following staining, cover slips were secured using Cytoseal 60 mounting medium. Slides were viewed on a Nikon Eclipse E600 microscope and analyzed with an Olympus DP70 camera and software.
One Step Trichrome Staining (American MasterTech cat # STOSTB)
Slides were placed in Bouin’s Fixative and allowed to mordant for 1 hour at 56°C, washed in tap water for 5 minutes followed by a 7 minute stain in Wiegert’s Hematoxylin and washed for 3 minutes. They were then placed in Trichrome plus aniline blue stain for 8 minutes followed by a brief tap water wash. Slides were dehydrated through absolute alcohol and cleared in xylene.
Picrosirius Staining
Picrosirius Red solution was prepared using 0.5g Sirius red (Sigma-Aldrich Cat# 365548) in 500mL saturated aqueous solution of picric acid (Sigma-Aldrich Cat# P6744-1GA). Slides were placed in Wiegert’s Hematoxylin and stained for 8 minutes, then washed for 10 minutes in running tap water. The slides were then placed in picrosirius red for 60 minutes, followed by two changes of 0.5% acetic acid. Slides were dehydrated through absolute alcohol and cleared in xylene.
Digital Image Analysis
Slides were quantitatively analyzed using the Nuance multispectral imaging system (28, 29). Spectral libraries were defined individually for each staining series as follows: picrosirius (red), trichrome (red and blue), Nox2/α-SMA (brown and purple). Pixel counts and optical densities were determined after color separation was achieved using the defined spectral libraries. 5 non-overlapping fields were scanned and analyzed for each kidney (28, 29).
Immunostaining (Nox2 - αSMA double staining)
Heat-induced antigen retrieval was performed using a 10 mM Citrate solution (pH = 6.0) at 25 psi for 2 min. Nonspecific staining was blocked using Sniper (Biocare Medical) for 9 min. Slides were incubated overnight at 4°C with anti-Nox2 primary antibody (1:50), then washed and incubated with 3% hydrogen peroxide for 15 min. The MACH 2 HRP polymer detection system (Biocare Medical) and DAB substrate were used to detect the Nox2 primary antibody as brown pigment. Slides were then incubated with anti-α-SMA primary antibody (1:50,000) at room temp for 1 h. The MACH 2 HRP polymer detection system and VIP substrate (Vector Laboratories, Burlingame, CA) were used to detect α-SMA primary antibody as purple pigment. Tissue sections were washed in distilled water, counter-stained with hematoxylin and dehydrated through an ethanol series.
Statistical Analysis
Student’s T test and Mann-Whitney rank sum test were utilized to compare parametric and non-parametric continuous data when appropriate. Similarly, Chi-square or Fisher’s exact tests were utilized when appropriate to compare categorical data between groups. P values ≤ 0.05 were considered significant.
Results
Calcineurin Inhibitors increased Nox2 mRNA levels in rat tubular epithelial cells (Figure 1)
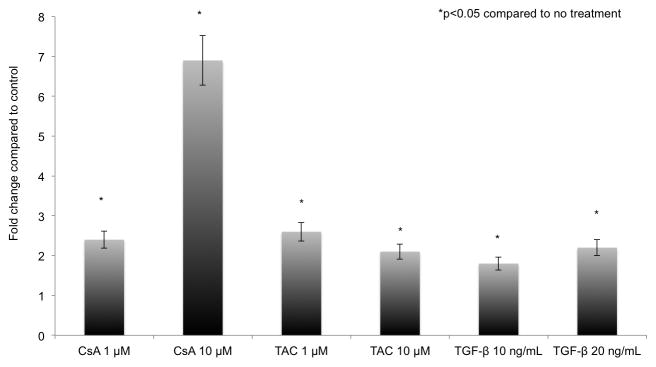
Figure 1. Calcineurin inhibitors increased Nox2 mRNA in NRK52E cells.
NRK52E cells (rat proximal tubular epithelial cell line) were cultured in the presence of Vehicle (media), TGF-β1, CsA or TAC. After four hours of treatment, Nox2 mRNA levels increased by 2.2 (CsA 1 μM), 6.8 (CsA 10 μM), 2.3 (TAC 1μM), 2.1 (TAC 10 μM), 1.8 (TGF-β1 10 ng/ml) and 2 (TGF-β1 20 ng/ml) fold compared to control. All studies were performed in triplicate.
Nox2 was originally described as the phagocytic Nox. To determine whether tubular epithelial cells can upregulate Nox2 mRNA in profibrotic conditions, we examined the response of NRK52E cells (rat proximal tubular epithelial cell line) to TGF-β1, CsA and TAC. We demonstrated that after four hours of treatment, Nox2 mRNA levels increased by 2.2 (CsA 1 μM), 6.8 (CsA 10 μM), 2.3 (TAC 1μM), 2.1 (TAC 10 μM), 1.8 (TGF-β1 10 ng/ml) and 2 (TGF-β1 20 ng/ml) fold compared to control (Figure 1).
CsA increased Nox2 and TGF-β1-related proteins in rat tubular epithelial cells (Figure 2)
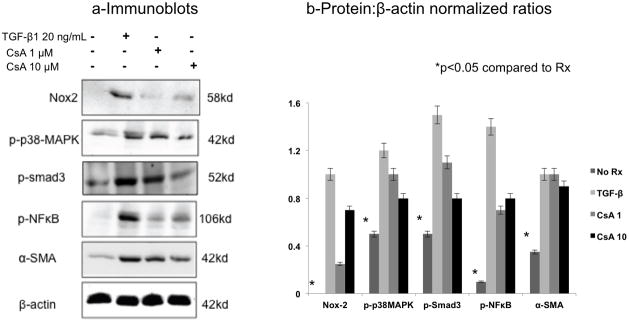
Figure 2. CsA increased Nox2 and TGF-β1-related proteins in NRK52E cells.
NRK52E cells were cultured in the presence of CsA (1μM and 10μM) or TGF-β1 (20 ng/ml) for 48 hours. Immunoblot analyses demonstrated a significant increase in phosphorylated (activated) p38MAPK, Smad3, and NFκB signaling molecules, as well as Nox2 and α-SMA protein levels in response to CsA and TGF-β1. The blot shown is a representative sample of three replicate studies, summarized in the bar graph.
Similarly, we examined fibrogenesis and Nox2 protein levels in response to CsA (1μM and 10μM) and TGF-β1 (20 ng/ml) in NRK52E cells treated for 48 hours (Figure 2). These studies demonstrated a significant increase in phosphorylated (activated) p38MAPK, Smad3, and NFκB signaling molecules as well as Nox2 and α-SMA suggesting that Nox2 may be involved in CsA-induced fibrogenesis.
Calcineurin inhibitors dedifferentiated epithelial NRK52E cells (Figure 3)
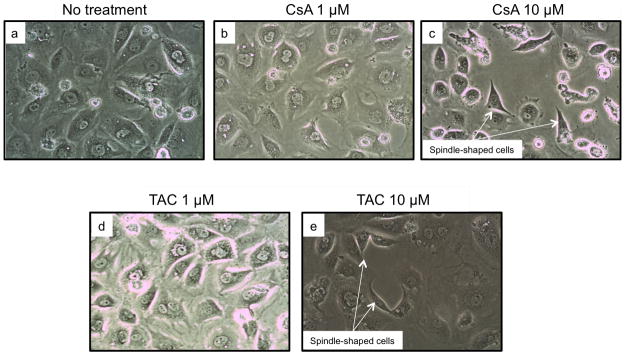
Figure 3. Calcineurin inhibitors dedifferentiated epithelial NRK52E cells.
We examined the effects of CsA and TAC treatment on tubular epithelial cell phenotype in vitro. CsA and TAC (10 μM for 48 hours) were associated with the loss of cobblestone morphology. Compared to controls and lower concentrations of CNIs, tubular cells treated with higher dose CsA and TAC displayed a spindle shape and loss of cell-cell contact suggestive of epithelial to mesenchymal transition (EMT).
We next examined the effects of CsA and TAC (both at 1μM and 10μM for 48 hours) on tubular epithelial cell morphology in vitro. Compared to controls, tubular cells treated with higher dose calcineurin inhibitors lost their cobblestone pattern and displayed the spindle shape characteristic of cells undergoing epithelial to mesenchymal transition (EMT), a process associated with fibrogenesis (30, 31).
CsA-induced EMT and Nox2 protein expression were dependent on TGF-β1 (Figure 4)
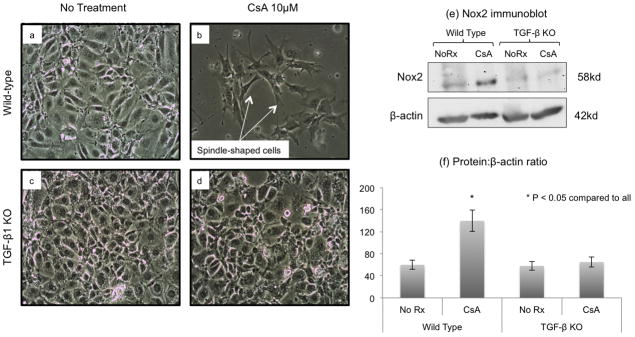
Figure 4. CsA-induced EMT and Nox2 protein expression were TGF-β1 dependent.
To determine the role of TGF-β1 in CNI-induced EMT and Nox2 expression, we treated wild type and TGF-β1-knockout tubular epithelial cells with CsA (10 μM) and evaluated cell morphology and Nox2 protein levels. CsA treatment was associated with EMT in wild type, but not in knockout cells (Panels b and d). Similarly, CsA did not increase Nox2 protein levels in knockout cells (Panels e and f), indicating that TGF-β1 is required for EMT and Nox2 synthesis in these experimental settings.
TGF-β1 is the cytokine most commonly associated with EMT (32). To determine the role of this molecule in CNI-induced EMT and Nox2 expression, we treated wild type and TGF-β1-knockout tubular epithelial cells with CsA and evaluated cell morphology and Nox2 protein levels (Figure 4). As shown in Panels b and d, CsA treatment was associated with EMT in wild type, but not in knockout cells, suggesting that TGF-β1 is an important mediator of CsA-induced EMT. Furthermore, CsA treatment did not increase Nox2 protein in knockout cells (Panels e and f), indicating that TGF-β1 is required for Nox2 synthesis in these experimental settings.
Chemical inhibition of Nox2 was associated with reduced CsA nephrotoxicity in vivo (Figures 5–7)
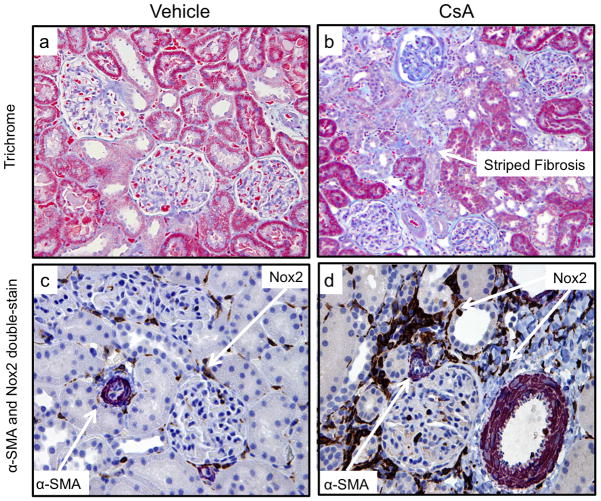
Figure 5. Fibrosis and Nox2 in the Rat Model of CsA Nephrotoxicity.
Adult male Fisher344 rats were randomized into five groups (n=6–8 in each group): Olive oil, Cyclosporine (15mg/kg/day), Cyclosporine + DPI (15mg/kg/day CsA + 0.5mg/kg/day), Cyclosporine + DPI (15mg/kg/day CsA + 1.0mg/kg/day) or Cyclosporine + Apocynin (15mg/kg/day + 16mg/kg/day). They received treatment for 30 days. Trichrome (a,b) studies demonstrated the typical striped fibrosis and cytoplasmic vacuolization that characterize chronic CNI toxicity. Immunoperoxidase studies showed that CsA nephrotoxicity was associated with upregulation of Nox2 (brown) in tubular and interstitial cells, and interstitial upregulation of α-SMA (purple) (c, d).
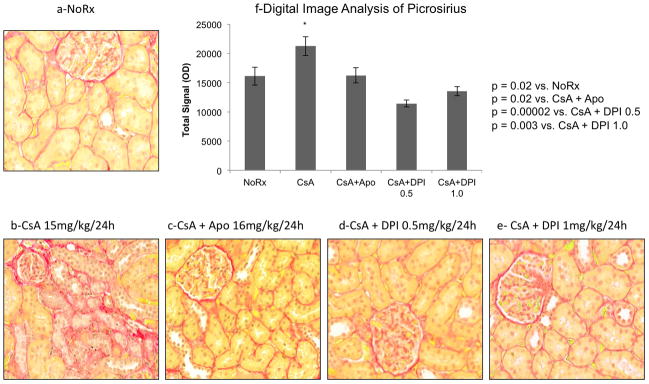
Figure 7. Inhibition of Nox2 was associated with reduced picrosirius staining.
We evaluated renal fibrosis using digital image analysis of picrosirius, a biomarker of collagen deposition. These studies confirmed the antifibrotic effects of DPI and Apocynin since both drugs were associated with a significant reduction in picrosirius staining intensity.
To confirm our in vitro data we devised experimental studies that reproduced chronic CsA nephrotoxicity in rats and examined whether non-specific inhibition of Nox2 prevented kidney fibrosis (Table 1 and Figures 5–7). Both apocynin and diphenyleneiodonium (DPI) are considered classical inhibitors of Nox2 activity, though they also inhibit other Nox isoforms and have other metabolic effects (26, 33). Adult male Fisher344 rats were treated with vehicle (olive oil), CsA, or CsA plus the Nox2 inhibitors, apocynin and DPI (Table 1, groups 1 to 5).
Table 1.
Rat and Mouse Models of Chronic CsA-induced Nephropathy
| Group | Species | Strain | Treatment |
|---|---|---|---|
| 1 | Rat | F344 | Vehicle |
| 2 | Rat | F344 | CsA(15 mg/kg/24h) × 30 days |
| 3 | Rat | F344 | CsA(15 mg/kg/24h) + Apocynin (16 mg/kg/24h) × 30 days |
| 4 | Rat | F344 | CsA(15 mg/kg/24h) + DPI (0.5 mg/kg/24h) × 30 days |
| 5 | Rat | F344 | CsA(15 mg/kg/24h) + DPI (1 mg/kg/24h) × 30 days |
| 6 | Mouse | C57Bl/6 | CsA(30 mg/kg/24h) × 60 days |
| 7 | Mouse | C57Bl/6 (Nox2−/−) | CsA(30 mg/kg/24h) × 60 days |
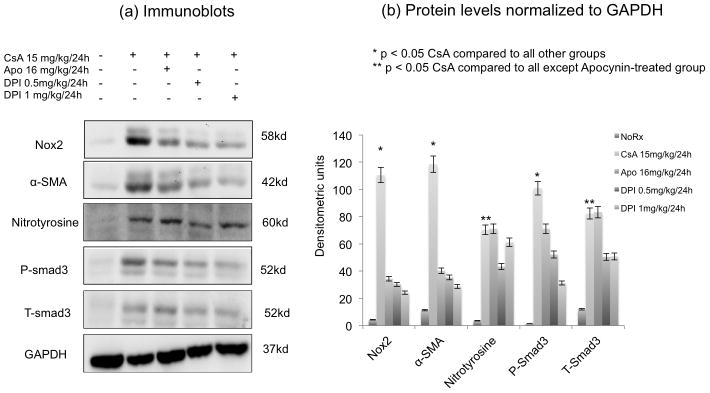
We first confirmed the characteristic lesions of chronic CsA nephrotoxicity in our animal model. Trichrome (Figure 5a,b) and H&E (not shown) studies demonstrated the typical striped fibrosis, cytoplasmic vacuolization and arteriolar hyalinosis that are observed in chronic CNI toxicity. Next, we demonstrated that CsA nephrotoxicity was associated with tubulointerstitial upregulation of Nox2 and α-SMA (Figure 5c,d), confirming our in vitro results. To quantitate these changes and to examine the effects of CsA on fibrogenesis in vivo, we performed immunoblot analyses for Nox2, fibrosis (α-SMA), oxidative stress (nitrotyrosine) and the profibrotic signaling molecule Smad3 (both total and phosphorylated) (Figure 6). These studies showed that CsA increased fibrogenesis (Nox2, α-SMA, nitrotyrosine, P-smad3 and T-smad3) compared to no treatment (Figure 6a,b). Nox2 inhibitors (DPI more than Apocynin) were associated with reduced fibrogenesis suggesting that Nox2 is involved in the pathogenesis of chronic CsA-induced nephrotoxicity (Figure 6a,b). Last, we evaluated renal fibrosis using digital image analysis of picrosirius, a biomarker of collagen deposition (Figure 7). These studies confirmed the antifibrotic effects of DPI and Apocynin since both drugs were associated with a significant reduction in picrosirius staining intensity.
Figure 6. Inhibition of Nox2 was associated with reduced CsA-induced Fibrogenesis.
Immunoblot analyses examining the expression of Nox2, fibrosis (α-SMA), oxidative stress (nitrotyrosine), and the profibrotic signaling molecule, phosphorylated Smad3 and total Smad3) in rats treated with high dose CsA (15 mg/kg/24h for 30 days), CsA + Apocynin (16 mg/kg/24h) and CsA + DPI (0.5 or 1 mg/kg/24h). Panel a shows representative blots while Panel b represents normalized ratios of proteins to GAPDH. The studies showed that CsA increased fibrogenesis (Nox2, α-SMA, nitrotyrosine, P-smad3 and T-smad3) compared to no treatment. Nox2 inhibitors were associated with reduced fibrogenesis, which was more pronounced with DPI than apocynin.
Genetic inhibition of Nox2 was associated with reduced CsA nephrotoxicity in vivo (Figure 8)
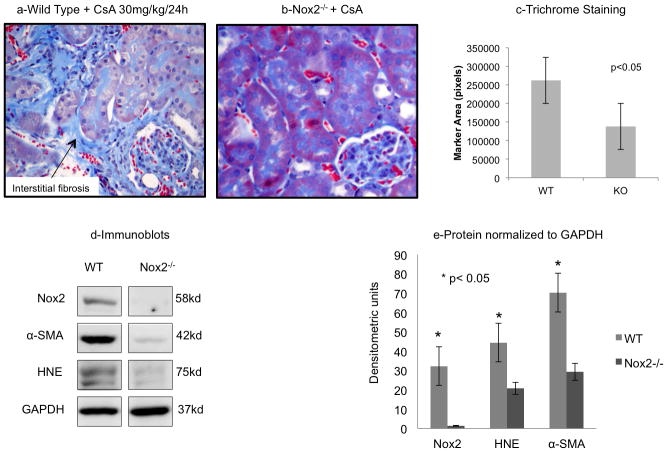
Figure 8. CsA Nephrotoxicity was reduced in Nox2−/− mice.
To specifically examine the role of Nox2 in the pathogenesis of CsA-induced nephropathy, we tested our hypothesis in wild type (C57Bl6/J) and Nox2 null (B6.129S-CybbTm1Din/J) mice (n=6 in each group). Trichrome staining demonstrated a significant reduction in collagen tubulointerstial staining in Nox2 null animals (a,b,c). Immunoblot analyses were consistent with this finding as the Nox2 knockout mice had significantly lower quantities of Nox2, α-SMA, and HNE following CsA treatment than the wild type mice (d,e).
Despite their classical description as Nox2 inhibitors, apocynin and DPI can have other non-specific effects (33). To specifically examine the role of Nox2 in the pathogenesis of CsA-induced nephropthay, we tested our hypothesis in wild type (C57Bl6/J) and Nox2 null (B6.129S-CybbTm1Din/J) mice (groups 6,7 in Table 1 and Figure 8). Trichrome staining and digital image analyses of trichrome blue demonstrated a significant reduction in collagen deposition in Nox2 null animals compared to wild type mice (Figure 8a,b,c). Immunoblot analyses were consistent with this finding as CsA-treated knockout animals showed the expected absence of Nox2, and had significantly lower quantities ofα-SMA and HNE compared to wild type mice (Figure 8d,e). In aggregate, these findings indicate that Nox2 is a mediator of chronic CsA nephrotoxicity in experimental settings.
Nox2 was upregulated in the kidney of liver transplant recipients with chronic CNI nephrotoxicity (Table 2, Figure 9)
Table 2.
Liver Transplant Recipients with Biopsy-Proven Chronic CNI Toxicity
| Baseline Characteristics | ||
|---|---|---|
| TAC | CsA | |
| Number of Patients | 9 | 6 |
| Year of Transplant | 1995–2005 | 1995–2005 |
| Age at transplant (years) | 55.8±2.6 | 53.5±3.6 |
| White Race | 8 | 5 |
| Male Gender | 5 | 3 |
| Time to Biopsy (years) | 4.3±0.6 | 6.4±0.8 |
| coexisting Diabetes | 4 | 4 |
| coexisting HTN | 9 | 5 |
| Serum creatinine at biopsy (mg/dL) | 1.5±0.9 | 1.6±0.08 |
| Proteinuria on urinalysis > 1 + | 3 | 2 |
| Follow-up after biopsy (years) | 2.9±0.2 | 2.1±0.3 |
| Last serum creatinine (mg/dL) | 1.9±0.7 | 1.8±0.7 |
CNI: Calcineurin inhibitor, CsA: Cyclosporine A, TAC: Tacrolimus, HTN: Hypertension
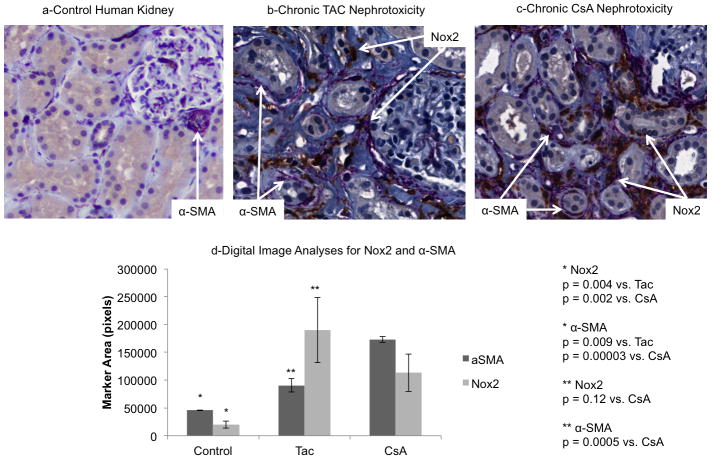
Figure 9. Nox2 was increased in human kidneys with CNI-induced fibrosis.
Immunohistochemical analyses of kidney biopsy samples from 15 liver transplant recipients with chronic CNI nephrotoxicity demonstrated a significant increase in both α-SMA and Nox2 compared to controls (a–d). Nox2 was upregulated in the tubules and interstitial cells of human kidneys undergoing CNI-induced fibrosis associated with either TAC or CsA (b–d). TAC was associated with less α-SMA staining than CsA by digital image analysis (d).
To determine the clinical relevance of our studies we examined the expression of Nox2 and α-SMA in kidneys from liver transplant recipients with biopsy-confirmed chronic CNI nephrotoxicity. We identified 15 consecutive patients with this diagnosis between 1995 and 2005. Table 2 displays the baseline characteristics of patients on either TAC or CsA at the time of biopsy. There were no significant clinical differences between the two groups. Mean age in the entire cohort was 54.5 years and the majority were male and white. Mean time to biopsy was 5.4±0.4 years after liver transplantation. At the time of biopsy, most patients had diabetes (53%) and hypertension (93%), mean serum creatinine was 1.50±08mg/dL, and five patients (33%) had more than 1+ proteinuria. Patients were followed up for 2.5±0.3 years. There was no kidney failure or patient mortality during follow-up, but serum creatinine levels increased significantly (1.8±0.1 mg/dL, p = 0.04) suggestive of disease progression.
Immunohistochemical analyses demonstrated a significant increase of both α-SMA and Nox2 staining in patients with chronic TAC and CsA nephrotoxicity compared to controls (Figure 9a-d). Similar to our findings in mice and rats, Nox2 was upregulated in the interstitium and tubular epithelial cells of kidneys undergoing CNI-induced fibrosis. When TAC and CsA groups were compared, we found less α-SMA staining in the TAC group. However, Nox2 immunostaining was not significantly different between the two cohorts.
Discussion
CNI-induced kidney fibrosis is an important obstacle to successful long-term outcomes in organ transplantation and autoimmune diseases. Our studies demonstrate that Nox2 is a mediator of chronic CsA nephrotoxicity and that its synthesis requires TGF-β1. Our findings also indicate that CsA and TAC have similar mechanisms of injury, including EMT and induction of Nox2, although TAC may have a less profibrotic profile. If confirmed, these results could lead to the development of Nox2 inhibition strategies to improve long-term outcomes in organ transplantation and autoimmune diseases.
Experimental studies suggesting that vitamin E inhibits CsA-induced lipid peroxidation and renal damage (34, 35) support identification of CsA as a pro-oxidant molecule. Similarly, catalase, an enzyme that specifically breaks down the reactive hydrogen peroxide (H2O2) into H2O and O2 and acts as a scavenger of ROS, reduced CsA-associated renal tubular epithelial cell senescence (36). In addition, rat proximal tubular epithelial cells exposed to CsA accumulate intracellular ROS and lipid peroxidation products, along with an altered glutathione redox state (37). However, the molecular mechanisms that regulate CNI-induced generation of ROS remain unclear.
Originally named gp91phox, Nox2 is the classical phagocytic NADPH oxidase (20, 21), an enzyme that is naturally involved in the innate immune response including the “oxidative burst” (20, 21). We elected to study this isoform rather than the other Nox isoforms for three reasons. First, apocynin and DPI, the most commonly used inhibitors of Nox are not specific to Nox or its isoforms and have global antioxidant characteristics as well as other metabolic effects (33, 38). The availability of Nox2 null mice (compared to other isoforms) enabled us to rigorously test our hypotheses in vivo. Furthermore, use of siRNA to specifically reduce Nox2 in vivo is limited by the off target effects and short half-life of the molecule. Second, we have been unable to detect measurable levels of other Nox isoforms in renal tubular epithelial cells undergoing fibrogenesis in vitro, while we have repeatedly demonstrated that Nox2 protein is induced in tubular cells under similar conditions (26, 39). Third, the functions of the Nox isoforms may be different and cell/tissue specific (40–42).
The current clinical paradigm is to minimize or discontinue CNIs in long-term organ transplant recipients to prevent renal damage (2). However, this strategy is limited by the onset of acute rejection, which is a significant safety concern, especially in nonkidney organ transplant recipients. It is therefore unlikely that we will be able to eliminate CNIs from the armamentarium of immunosuppressive therapies in organ transplantation. We also propose a novel role for Nox2, the classical phagocytic Nox isoform. Nox2 is thought to be essentially involved in the generation of superoxide to eliminate pathogens. Our data demonstrate that this molecule is also inducible in renal tubular epithelial cells (26, 39) and that it mediates CsA-induced renal fibrosis in animals. Development of Nox2 inhibition strategies thus provides an attractive approach to prevent renal injury during therapy with CNIs. Bolanowski et al demonstrated successful outcomes in three kidney transplant recipients with chronic granulomatous disease or CGD (43), a genetic disease caused by structural mutations in the enzyme NADPH oxidase that results in immunodeficiency. Of the three patients, one was transplanted concurrent with CGD, another was transplanted after being cured of CGD with bone marrow transplantation and the third received a kidney transplant prior to being cured of CGD via a subsequent peripheral blood stem cell transplant. All three recipients received either tacrolimus or CsA immunosuppressive therapy and enjoyed excellent outcomes. There is no long-term data reported on chronic CNI nephrotoxicity in these patients.
Nox2 is not specific to CNI nephrotoxicity. For example, we have demonstrated that Nox2 is increased in the tubulointerstitium of human and rat kidney allografts undergoing acute or chronic rejection (26, 39). Similarly, Vaziri et al have found greater levels of Nox2 in kidneys of rats with CKD and hypertension, other models of chronic renal injury (44, 45). It is therefore likely that Nox2 is a nonspecific, proinflammatory mediator of fibrosis. As indicated in recent in depth reviews, Nox2 activation by insults such as rejection, infections, drugs or metabolic disorders could result in overproduction of superoxide anion and reactive oxygen species which then activate new injury pathways (30, 46). Although we provide new insights regarding the signaling pathways involved in Nox2 activation and fibrogenesis in this manuscript, more studies are needed to determine the sequence of events, including whether Nox2 activation precedes or follows smad/NFκB activation. Another limitation of our study concerns its clinical component. More precisely, this study does not exclude the possibility that Nox2 effects are a general reaction of kidneys to stress factors, and not necessarily specific for CNI nephrotoxicity. Although the definition of CNI nephrotoxicity was based on the Banff criteria and the new arteriolar hyalinosis scoring scheme, it is impossible to completely rule out the role of concomitant stressors.
In summary, CNIs, the backbone of anti-rejection therapies are also profibrotic molecules, making it difficult to effectively manage immunosuppression and long-term outcomes. Our studies rigorously and specifically indicate a role for Nox2 in mediating CNI-induced renal fibrosis. If confirmed in humans, the results of our studies will provide a significant step forward in the design of new diagnostic, monitoring and treatment options aimed to offset the deleterious effects of immunosuppressant therapy and improve long-term kidney outcomes in organ transplantation.
Acknowledgments
Supported by the American Society of Nephrology-American Society of Transplantation John Merrill Award (AD) and National Institutes of Health, NIDDK grant R01 DK092454-01 (AD).
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Samaniego M, Becker BN, Djamali A. Drug insight: maintenance immunosuppression in kidney transplant recipients. NatClinPractNephrol. 2006;2(12):688–699. doi: 10.1038/ncpneph0343. [DOI] [PubMed] [Google Scholar]
- 2.Flechner SM, Kobashigawa J, Klintmalm G. Calcineurin inhibitor-sparing regimens in solid organ transplantation: focus on improving renal function and nephrotoxicity. Clin Transplant. 2008;22(1):1–15. doi: 10.1111/j.1399-0012.2007.00739.x. [DOI] [PubMed] [Google Scholar]
- 3.Cooper JE, Wiseman AC. Novel immunosuppressive agents in kidney transplantation. Clin Nephrol. 2010;73(5):333–343. doi: 10.5414/cnp73333. [DOI] [PubMed] [Google Scholar]
- 4.Copeland JW, Beaumont BW, Merrilees MJ, Pilmore HL. Epithelial-to-mesenchymal transition of human proximal tubular epithelial cells: effects of rapamycin, mycophenolate, cyclosporin, azathioprine, and methylprednisolone. Transplantation. 2007;83(6):809–814. doi: 10.1097/01.tp.0000255680.71816.aa. [DOI] [PubMed] [Google Scholar]
- 5.Slattery C, Campbell E, McMorrow T, Ryan MP. Cyclosporine A-induced renal fibrosis: a role for epithelial-mesenchymal transition. Am J Pathol. 2005;167(2):395–407. doi: 10.1016/S0002-9440(10)62984-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burdmann EA, Andoh TF, Yu L, Bennett WM. Cyclosporine nephrotoxicity. SeminNephrol. 2003;23(5):465–476. doi: 10.1016/s0270-9295(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 7.Liptak P, Ivanyi B. Primer: Histopathology of calcineurin-inhibitor toxicity in renal allografts. Nat Clin Pract Nephrol. 2006;2(7):398–404. doi: 10.1038/ncpneph0225. quiz following 404. [DOI] [PubMed] [Google Scholar]
- 8.Olyaei AJ, de Mattos AM, Bennett WM. Nephrotoxicity of immunosuppressive drugs: new insight and preventive strategies. Curr Opin Crit Care. 2001;7(6):384–389. doi: 10.1097/00075198-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4(2):481–508. doi: 10.2215/CJN.04800908. [DOI] [PubMed] [Google Scholar]
- 10.Ojo AO. Renal disease in recipients of nonrenal solid organ transplantation. Semin Nephrol. 2007;27(4):498–507. doi: 10.1016/j.semnephrol.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. NEnglJMed. 2003;349(24):2326–2333. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 12.Gaston RS, Cecka JM, Kasiske BL, Fieberg AM, Leduc R, Cosio FC, et al. Evidence for antibody-mediated injury as a major determinant of late kidney allograft failure. Transplantation. 2010;90(1):68–74. doi: 10.1097/TP.0b013e3181e065de. [DOI] [PubMed] [Google Scholar]
- 13.Gaston RS. Our evolving understanding of late kidney allograft failure. Current opinion in organ transplantation. 2011;16(6):594–599. doi: 10.1097/MOT.0b013e32834c23a7. [DOI] [PubMed] [Google Scholar]
- 14.Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, et al. Chronic renal failure after transplantation of a nonrenal organ. NEnglJMed. 2003;349(10):931–940. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 15.McMorrow T, Gaffney MM, Slattery C, Campbell E, Ryan MP. Cyclosporine A induced epithelial-mesenchymal transition in human renal proximal tubular epithelial cells. NephrolDialTransplant. 2005;19 doi: 10.1093/ndt/gfh967. [DOI] [PubMed] [Google Scholar]
- 16.Khanna AK, Cairns VR, Becker CG, Hosenpud JD. Transforming growth factor (TGF)-beta mimics and anti-TGF-beta antibody abrogates the in vivo effects of cyclosporine: demonstration of a direct role of TGF-beta in immunosuppression and nephrotoxicity of cyclosporine. Transplantation. 1999;67(6):882–889. doi: 10.1097/00007890-199903270-00016. [DOI] [PubMed] [Google Scholar]
- 17.Akool el S, Doller A, Babelova A, Tsalastra W, Moreth K, Schaefer L, et al. Molecular mechanisms of TGF beta receptor-triggered signaling cascades rapidly induced by the calcineurin inhibitors cyclosporin A and FK506. J Immunol. 2008;181(4):2831–2845. doi: 10.4049/jimmunol.181.4.2831. [DOI] [PubMed] [Google Scholar]
- 18.Krauskopf A, Lhote P, Petermann O, Ruegg UT, Buetler TM. Cyclosporin A generates superoxide in smooth muscle cells. Free Radic Res. 2005;39(9):913–919. doi: 10.1080/10715760500104009. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol. 21(2):212–222. doi: 10.1681/ASN.2008121226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 21.Gill PS, Wilcox CS. NADPH oxidases in the kidney. AntioxidRedoxSignal. 2006;8(9–10):1597–1607. doi: 10.1089/ars.2006.8.1597. [DOI] [PubMed] [Google Scholar]
- 22.Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, et al. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9(2):202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 23.Haque MZ, Majid DS. Assessment of renal functional phenotype in mice lacking gp91PHOX subunit of NAD(P)H oxidase. Hypertension. 2004;43(2):335–340. doi: 10.1161/01.HYP.0000111137.15873.4a. [DOI] [PubMed] [Google Scholar]
- 24.Sis B, Dadras F, Khoshjou F, Cockfield S, Mihatsch MJ, Solez K. Reproducibility studies on arteriolar hyaline thickening scoring in calcineurin inhibitor-treated renal allograft recipients. Am J Transplant. 2006;6(6):1444–1450. doi: 10.1111/j.1600-6143.2006.01302.x. [DOI] [PubMed] [Google Scholar]
- 25.Sis B, Mengel M, Haas M, Colvin RB, Halloran PF, Racusen LC, et al. Banff ‘09 Meeting Report: Antibody Mediated Graft Deterioration and Implementation of Banff Working Groups. Am J Transplant. 2010 doi: 10.1111/j.1600-6143.2009.02987.x. [DOI] [PubMed] [Google Scholar]
- 26.Djamali A, Vidyasagar A, Adulla M, Hullett D, Reese S. Nox-2 Is a Modulator of Fibrogenesis in Kidney Allografts. Am J Transplant. 2009;9(1):74–82. doi: 10.1111/j.1600-6143.2008.02463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vidyasagar A, Reese S, Acun Z, Hullett D, Djamali A. HSP27 is involved in the pathogenesis of kidney tubulointerstitial fibrosis. Am J Physiol Renal Physiol. 2008;295(3):F707–716. doi: 10.1152/ajprenal.90240.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levenson RM. Spectral imaging perspective on cytomics. Cytometry A. 2006;69(7):592–600. doi: 10.1002/cyto.a.20292. [DOI] [PubMed] [Google Scholar]
- 29.Rojo MG, Bueno G, Slodkowska J. Review of imaging solutions for integrated quantitative immunohistochemistry in the Pathology daily practice, Folia histochemica et cytobiologica/Polish Academy of Sciences. Polish Histochemical and Cytochemical Society. 2009;47(3):349–354. doi: 10.2478/v10042-008-0114-4. [DOI] [PubMed] [Google Scholar]
- 30.Djamali A, Samaniego M. Fibrogenesis in kidney transplantation: potential targets for prevention and therapy. Transplantation. 2009;88(10):1149–1156. doi: 10.1097/TP.0b013e3181bcccea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeisberg M, Neilson EG. Mechanisms of Tubulointerstitial Fibrosis. J Am Soc Nephrol. 2010 doi: 10.1681/ASN.2010080793. [DOI] [PubMed] [Google Scholar]
- 32.Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24(37):5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 33.Aldieri E, Riganti C, Polimeni M, Gazzano E, Lussiana C, Campia I, et al. Classical Inhibitors of NOX NAD(P)H Oxidases Are Not Specific. Curr Drug Metab. 2008;9(8):686–696. doi: 10.2174/138920008786049285. [DOI] [PubMed] [Google Scholar]
- 34.Wang C, Salahudeen AK. Lipid peroxidation accompanies cyclosporine nephrotoxicity: effects of vitamin E. Kidney Int. 1995;47(3):927–934. doi: 10.1038/ki.1995.138. [DOI] [PubMed] [Google Scholar]
- 35.Wolf A, Clemann N, Frieauff W, Ryffel B, Cordier A. Role of reactive oxygen formation in the cyclosporin-A-mediated impairment of renal functions. Transplant Proc. 1994;26(5):2902–2907. [PubMed] [Google Scholar]
- 36.Jennings P, Koppelstaetter C, Aydin S, Abberger T, Wolf AM, Mayer G, et al. Cyclosporine A induces senescence in renal tubular epithelial cells. Am J Physiol Renal Physiol. 2007;293(3):F831–838. doi: 10.1152/ajprenal.00005.2007. [DOI] [PubMed] [Google Scholar]
- 37.Galletti P, Di Gennaro CI, Migliardi V, Indaco S, Della RF, Manna C, et al. Diverse effects of natural antioxidants on cyclosporin cytotoxicity in rat renal tubular cells. Nephrol DialTransplant. 2005;20(8):1551–1558. doi: 10.1093/ndt/gfh846. [DOI] [PubMed] [Google Scholar]
- 38.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, et al. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51(2):211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 39.Djamali A, Vidyasagar A, Yagci G, Huang LJ, Reese S. Mycophenolic Acid May Delay Allograft Fibrosis by Inhibiting Transforming Growth Factor-beta1-Induced Activation of Nox-2 Through the Nuclear Factor-kappaB Pathway. Transplantation. 2010 doi: 10.1097/TP.0b013e3181e6ae0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, et al. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol. 2004;24(5):1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Q, Harraz MM, Zhou W, Zhang LN, Ding W, Zhang Y, et al. Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol Cell Biol. 2006;26(1):140–154. doi: 10.1128/MCB.26.1.140-154.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.von Lohneysen K, Noack D, Wood MR, Friedman JS, Knaus UG. Structural insights into Nox4 and Nox2: motifs involved in function and cellular localization. Mol Cell Biol. 30(4):961–975. doi: 10.1128/MCB.01393-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bolanowski A, Mannon RB, Holland SM, Malech HL, Aschan J, Palmblad J, et al. Successful renal transplantation in patients with chronic granulomatous disease. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6(3):636–639. doi: 10.1111/j.1600-6143.2006.01232.x. [DOI] [PubMed] [Google Scholar]
- 44.Zhan CD, Sindhu RK, Vaziri ND. Up-regulation of kidney NAD(P)H oxidase and calcineurin in SHR: reversal by lifelong antioxidant supplementation. Kidney Int. 2004;65(1):219–227. doi: 10.1111/j.1523-1755.2004.00372.x. [DOI] [PubMed] [Google Scholar]
- 45.Vaziri ND, Dicus M, Ho ND, Boroujerdi-Rad L, Sindhu RK. Oxidative stress and dysregulation of superoxide dismutase and NADPH oxidase in renal insufficiency. Kidney Int. 2003;63(1):179–185. doi: 10.1046/j.1523-1755.2003.00702.x. [DOI] [PubMed] [Google Scholar]
- 46.Djamali A. Oxidative Stress as a Common Pathway to Chronic Tubulointerstitial Injury in Kidney Allografts. Am J Physiol Renal Physiol. 2007;293(2):F445–455. doi: 10.1152/ajprenal.00037.2007. [DOI] [PubMed] [Google Scholar]