Abstract
Murine CCR5−/− recipients produce high titers of antibody to complete MHC-mismatched heart and renal allografts. To study mechanisms of class I MHC antibody-mediated allograft injury, we tested the rejection of heart allografts transgenically expressing a single class I MHC disparity in wild-type C57BL/6 (H-2b) and B6.CCR5−/− recipients. Donor-specific antibody titers in CCR5−/− recipients were 30-fold higher than in wild-type recipients. B6.Kd allografts survived longer than 60 days in wild-type recipients whereas CCR5−/− recipients rejected all allografts within 14 days. Rejection was accompanied by infiltration of CD8 T cells, neutrophils, and macrophages and C4d deposition in the graft capillaries. B6.Kd allografts were rejected by CD8−/−/CCR5−/−, but not μMT−/−/CCR5−/−, recipients indicating the need for antibody but not CD8 T cells. Grafts retrieved at day 10 from CCR5−/− and CD8−/−/CCR5−/− recipients and from RAG-1−/− allograft recipients injected with anti-Kd antibodies expressed high levels of perforin, myeloperoxidase and CCL5 mRNA. These studies indicate that the continual production of anti-donor class I MHC antibody can mediate allograft rejection, that donor-reactive CD8 T cells synergize with the antibody to contribute to rejection, and that expression of three biomarkers during rejection can occur in the absence of this CD8 T cell activity.
Keywords: antibody-mediated rejection, animal models, basic science, biomarker, cardiac transplant, graft rejection, leukocyte infiltration, neutrophils
Introduction
The use of current immunosuppressive strategies has markedly decreased the incidence of T cell mediated acute rejection in transplant patients. In contrast, the detected incidence of antibody-mediated graft rejection in solid organ recipients is increasing. Acute humoral rejection (AHR) occurs in almost 7% of renal transplant patients and is also prevalent in cardiac and lung graft recipients (1–4). The presence of donor-specific antibodies can directly mediate injury and contribute to the rejection of grafts as well as promote the development of graft tissue fibrosis and vasculopathy. Studies of serum from heart and renal transplant patients experiencing acute humoral rejection indicate that antibodies to both donor class I and class II HLA antigens can cause graft injury but that injury mediated by class II MHC specific antibodies is characterized by more intense infiltration of neutrophils and other inflammatory components in the allograft (4–9). Recent recognition of the high incidence of AHR has generated considerable interest in defining mechanisms by which donor-reactive anti-class I MHC and anti-class II MHC antibodies mediate graft tissue injury.
Graft endothelial cells are the primary target of donor-reactive antibodies during AHR (10–12). Activation of complement is an important effector function contributing to antibody-mediated tissue injury of allografts. In a murine transplant model, complement activation is required for passively transferred donor-reactive monoclonal IgG antibody on day 10 post-transplant to provoke rejection of heart allografts in μMT−/− recipients unable to produce antibodies (13, 14). Formation of the membrane attack complex (MAC) as the terminal stage of the classical complement pathway may result in lysis of endothelial cells although this is not frequently observed in the graft endothelium as endothelial cells express a battery of complement regulatory proteins (2, 15). Complement activation also stimulates endothelial cells to produce many inflammatory molecules including cytokines, chemokines, adhesion molecules, and growth f actors (16–19). Antibody-mediated rejection is characterized by neutrophil and macrophage infiltration that is directed in response to these inflammatory mediators (1, 4, 10).
We recently observed marked increases in serum levels of donor-reactive antibody induced to complete MHC-disparate heart and kidney allografts in CCR5−/− recipients (20, 21). These dysregulated antibody responses in CCR5−/− recipients appear more quickly and have titers 15–50 fold higher than those observed in wild-type C57BL/6 recipients. The consequence of these antibody responses is acute humoral rejection of the grafts that is accompanied by intense C4d/C3d deposition in the capillaries and large vessels of the allograft. Since our previous studies were restricted to the AHR of complete MHC-mismatched grafts, the goal of this study was to investigate mechanisms mediating AHR of single class I MHC disparate allografts in CCR5−/− recipients and to identify the expression of effector molecules induced by anti-class I MHC antibodies during rejection.
Materials and Methods
Mice
C57BL/6 (B6, H-2b) mice were obtained from Charles River Laboratories (Wilmington, MA). The generation of C57BL/6 mice transgenically expressing Kd, B6.Kd, has been previously detailed (22). Colonies of B6.CCR5−/−, B6.CCR5−/−/B6.μMT−/−, B6.RAG-1−/−, B6.CD8α−/− and B6.Kd mice were established and maintained at our facility. B6.CCR5−/− and B6.CD8α−/− mice were crossed to generate the B6.CD8−/−/CCR5−/− double-knockout mice. All experiments used 8- to 12-week-old male mice and all animal procedures were approved by the Institutional Animal Care and Use Committee at the Cleveland Clinic.
Cardiac transplantation and harvest
Heterotopic intra-abdominal cardiac transplantation was performed using previously reported microsurgical techniques (23, 24). Total operative times averaged 45 min and the graft resumed spontaneous contraction immediately upon reperfusion. Graft survival was monitored daily by abdominal palpation and graft rejection and confirmed visually by laparotomy. At the time of cardiac graft harvest, 10 mL of Ringer's solution was first flushed into the recipient circulatory system. The graft was then harvested and cut into pieces that were either snap-frozen in liquid nitrogen or placed in media for digestion and analysis of graft-infiltrating cells.
In vivo antibody treatment
Allograft recipients were treated with control rat IgG (Sigma, St. Louis, MO) or anti-CD25 mAb PC 61.5 (purified in-house from spent hybridoma supernatants), 0.5 mg on day −1 followed by 0.25 mg every other day on days 1–9 post-transplant. B6.RAG-1−/− recipients were treated with control rat IgG or anti-Kd mAb SF1-1.1.10, 200 μg on days 8 and 9 post-transplant. The serum from groups of B6.Kd heart transplanted CCR5−/− recipients was collected at the time of rejection (from day 10–14 posttransplant), pooled, and 400 μl of the pooled serum was injected i.v. to RAG-1−/− recipients of a B6.Kd heart graft on days 8 and 9 posttransplant.
Quantitation of cytokine-producing T cells
Priming of graft donor-reactive T cells to IFN-γ and IL-4 producing cells was quantified by ELISPOT assays as previously described (24, 25). Briefly, splenic responder cells and mitomycin C-treated self, donor and third-party stimulator cell populations were cocultured for 24 h at 37°C in serum-free HL-1 media in 96 well Multiscreen-IP (Millipore) plates coated with anti-IFN-γ or anti-IL-4 capture mAb. To compare alloreactive CD4 and CD8 T cell priming, each cell population was enriched from recipient spleen cell suspensions using negative selection columns (R&D Systems, Minneapolis, MN) to remove CD4 or CD8 T cells from the suspension and the enriched CD4 or CD8 responder T cells were stimulated with T cell depleted splenocytes from graft donor, third-party allogeneic, or recipient strain mice. After 24 h culture, all cells were washed from the plate and biotinylated anti-IFN-γ or anti-IL-4 detecting mAb (BD Biosciences, San Jose, CA) was added, followed by anti-biotin alkaline phosphatase (Vector Laboratories, Burlingame, CA). After development with nitroblue tetrazolium/5-bromo-4-chloro-30-indolyl substrate (Bio-Rad Laboratories, Hercules, CA), the total number of spots per well was quantified using an ImmunoSpot Series 3 Analyzer (Cellular Technology Ltd., Shaker Heights, OH).
RNA purification and qRT-PCR
Snap-frozen grafts were crushed, homogenized, and RNA was isolated using fibrous tissue kits (QIAGEN, Valencia, CA) and reverse transcribed using the High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). Reverse transcription and Real-Time PCR were performed using commercially available reagents and probes on a 7500 Fast Real-Time Thermocycler, all from Applied Biosystems (Foster City, CA). For quantification of mRNA expression, target gene expression was normalized to Mrpl32 gene expression. Individual graft samples were plated in duplicate and results expressed as mean (± SEM) fold increase in gene expression when compared with controls for groups of 4–5 grafts.
Flow cytometry
Absolute numbers of graft infiltrating neutrophils, macrophages, CD4 T cells and CD8 T cells were determined in heart grafts using a modification of the methodology published by Afanasyev and coworkers (25, 26). Harvested heart grafts were weighed and incubated at 37°C in RPMI plus type II collagenase (Sigma, St. Louis, MO) for 1 h and then pushed through a 40 mm cell strainer and the cells collected. Following RBC lysis with ACK Lysing Buffer (GIBCO, Carlsbad, CA), the leukocytes were counted using a hemocytometer. Cell aliquots were preincubated with anti-CD16/CD32 Fc receptor mAb for 5 min to block nonspecific antibody binding and then each sample was incubated for 30 min at 4°C with fluorochrome-labeled anti-mouse CD45 mAb and antibodies to detect specific leukocyte (neutrophils, macrophages and CD4 and CD8 T cells) populations. Cells were analyzed by three-color immunofluorescence on a FACSCalibur (BD Biosciences, San Jose, CA) and FlowJo analysis software (Tree Star Inc., Ashland, OR). Total numbers of each leukocyte population were determined by: (the total number of leukocytes counted) × (% of the leukocyte population counted in the CD45+ cells)/100. The data are reported as number of each leukocyte population/mg graft tissue.
Immunohistochemistry
Heart grafts were harvested at the designed day post-transplant and paraffin-embedded methanol and acetic acid fixed sections or frozen sections were prepared for histology and immnunohistochemistry analyses. Cross-sections of the center of cardiac grafts were embedded in OCT compound (Sakura Finetek, Torrance, CA) and immediately frozen in liquid nitrogen and 5 μm sections transferred to glass microscope slides and allowed to air dry for 30 min before immersing in ice-cold acetone for fixation for 10 min. Endogenous peroxidase activity was blocked using 0.3% hydrogen peroxide in PBS for 10 min at RT. Sections were washed with PBS and non-specific binding sites blocked for 30 min at RT with Protein Block (DAKO, Carpinteria, CA). Endogenous biotin activity was blocked with the Biotin Blocking System (DAKO). Purified rat-anti-mouse CD4 or purified rat-anti-mouse CD8 antibody (BD Biosciences, San Jose, CA) diluted 1:50 or anti-Ly-6G mAb (RB6-8C5) diluted 1:100 in Block Solution was added and slides were kept in a humidified chamber for 1 h. After washing, biotinylated polyclonal anti-rat Ig 1:100 in PBS (DAKO) was added for 1 h then incubated with Vectastain ABC Elite (Vector Labs, Burlingame, CA) for 30 min followed by incubation with DAB (3,3'-Diaminobenzidine) substrate-chromagen solution (Vector Laboratories).
For some slides, methanol-fixed sections were stained with hematoxylin and eosin (H&E) or for the complement split product C4d. The sections were boiled with Trilogy retrieval buffer (Cell Marque Cooperation, Austin, TX) for 60 min, followed by treatment with 0.3% hydrogen peroxide in PBS. After washing and protein blocking, purified rabbit-anti-mouse C4d (27) diluted 1:500 was added and slides were kept in a humidified chamber for 1 h. After washing, biotinylated polyclonal anti-rabbit Ig (Jackson ImmunoResearch, West Grove, PA) 1:1000 in PBS was added for 1 h then incubated with Vectastain ABC Elite for 30 min followed by incubation with DAB. `Sections were counter stained with hematoxylin (Richard Allan Scientific, MI), dehydrated, cleared in Clear Rite3 (Richard Allan Scientific) and mounted. Representative images were captured and analyzed with NIS-Elements BR (Nikon, Melville, NY).
Donor-specific antibody detection and quantitation by flow cytometry
Flow cytometry to detect and quantitate donor-specific antibodies in non-recipient and cardiac graft recipient serum was performed using a previously reported method (20, 21). Briefly, aliquots of donor or recipient strain thymocyte suspensions were incubated with serial dilutions of recipient sera taken pre-transplant and at several time points between transplantation and rejection. After 30 min, the cells were washed and suspended in staining buffer (Dulbecco's PBS with 2% FCS/0.2% NaN3) containing FITC-conjugated goat anti-mouse IgG serum (Jackson ImmunoResearch, West Grove, PA). To test for titers of donor-reactive IgG isotypes in naïve and cardiac allograft recipient serum, rat anti-mouse IgG1, rat anti-mouse IgG2a, rat anti-mouse IgG2b, rat anti-mouse IgG3 mAb (BD Biosciences) were used. The mean channel fluorescence (MCF) of each dilution of each serum sample was determined and the dilution that returned the MCF to the level observed when Kd thymocytes were stained with a 1:4 dilution of normal wild-type serum was divided by two and reported as the titer.
Statistical analysis
All data were analyzed using GraphPad Prism Pro (GraphPad Software Inc, San Diego CA). Kaplan-Meier analysis was performed for graft survival. Log-rank testing was performed to determine differences in survival data and Mann-Whitney nonparametric test was used to determine significance throughout as indicated with p < 0.05 being considered a significant difference.
Results
Absence of recipient CCR5 promotes rejection of single class I MHC-mismatched cardiac allografts
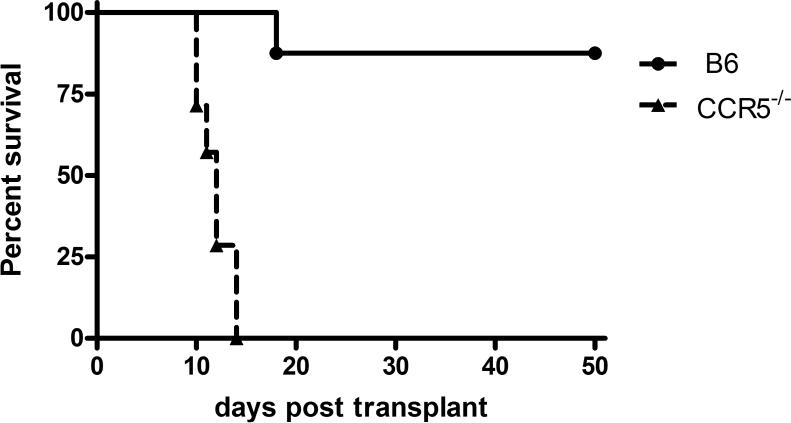
Since previous studies from this laboratory had indicated the antibody-mediated rejection of complete MHC-mismatched cardiac allografts in CCR5−/− recipients (21), the rejection of grafts expressing a single class I MHC disparity in CCR5−/− recipients was tested. Groups of C57BL/6 wild-type or B6.CCR5−/− mice received B6.Kd cardiac allografts and graft survival was monitored daily. Wild-type recipients only rejected 1 of 8 of the B6.Kd allografts and this did not occur until day 18 post-transplant (Figure 1A). In contrast, CCR5−/− recipients rejected all B6.Kd allografts within 14 days (mean survival 11.8 ± 1.6 days).
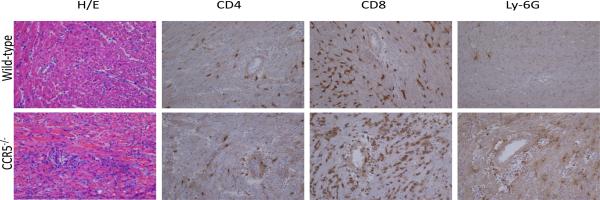
Figure 1. CCR5-deficient recipients acutely reject single class I MHC-disparate heart grafts.
Groups of wild-type C57BL/6 (— n = 8) and B6.CCR5−/− (--- n = 7) mice received cardiac allografts from B6.Kd donors. (A) Graft survival was monitored daily by abdominal palpation and rejection confirmed by laparotomy. (B) On day 10 post-transplant, B6.Kd allografts were harvested from randomly selected wild-type and B6.CCR5−/− recipients. Methanol-fixed sections were prepared and stained with hematoxylin and eosin. Prepared frozen sections were stained to detect the presence of CD4 and CD8 T cells and Ly-6G+ cells that are primarily neutrophils. Sections shown are representative of 3–4 grafts from each group. Magnification, 200×.
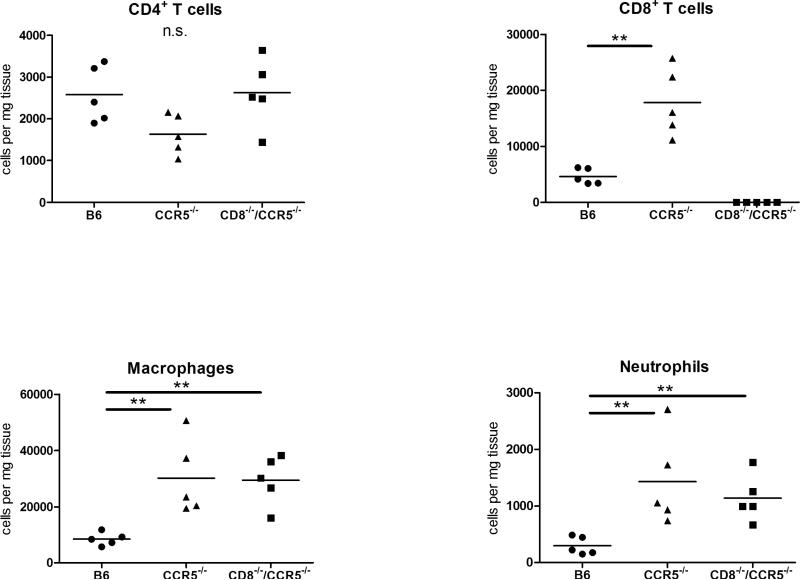
Grafts were harvested from recipients on day 10 post-transplant and sections prepared for immunohistochemical analysis. While allografts from wild-type recipients had mild leukocyte infiltration at day 10 post-transplant, grafts from CCR5−/− recipients had intense leukocytic infiltration accompanied by hemorrhage and tissue necrosis (Figure 1B). Infiltration of CD8 T cells and neutrophils was markedly higher in grafts from CCR5−/− recipients when compared to grafts from wild-type recipients (Figure 1B). Similar levels of CD4 T cell graft infiltration were observed in allografts from CCR5−/− and wild-type recipients and infiltration of these T cells was markedly lower than CD8 T cells (Figure 1B). To complement the histological analyses, numbers of allograft infiltrating CD4 T cells, CD8 T cells, macrophages and neutrophils on day 10 post-transplant was directly quantified using digestion of the graft followed by flow cytometry analysis. Consistent with the histology analyses, the numbers of allograft infiltrating CD4 T cells was similar in B6.Kd allografts in wild-type and CCR5−/− recipients whereas the numbers of macrophages, neutrophils and CD8 T cells were 3-fold higher in allografts in CCR5−/− recipients versus wild-type recipients (Figure 2).
Figure 2. Intense cell infiltration in B6.Kd cardiac allografts retrieved from B6.CCR5−/− recipients.
B6.Kd cardiac allografts were excised from wild-type C57BL/6, B6.CCR5−/− and CD8−/−/CCR5−/− recipients on day 10 post-transplant (5 individual grafts per group), were digested and stained with leukocyte-specific antibodies. Flow cytometry analyses of anti-CD45 and anti-CD4, anti-CD8, anti-F4/80 and anti-Ly-6G mAb stained cells from digested grafts were performed to determine numbers of CD4 T cells, CD8 T cells, macrophages and neutrophils infiltrating the allografts. Data indicate mean number of cells/mg graft tissue ± SEM and are representative of two individual experiments. (**p<0.01; n.s.= p>0.05).
Increased donor-reactive IgG antibody production and CD8 T cell responses in CCR5−/− recipients
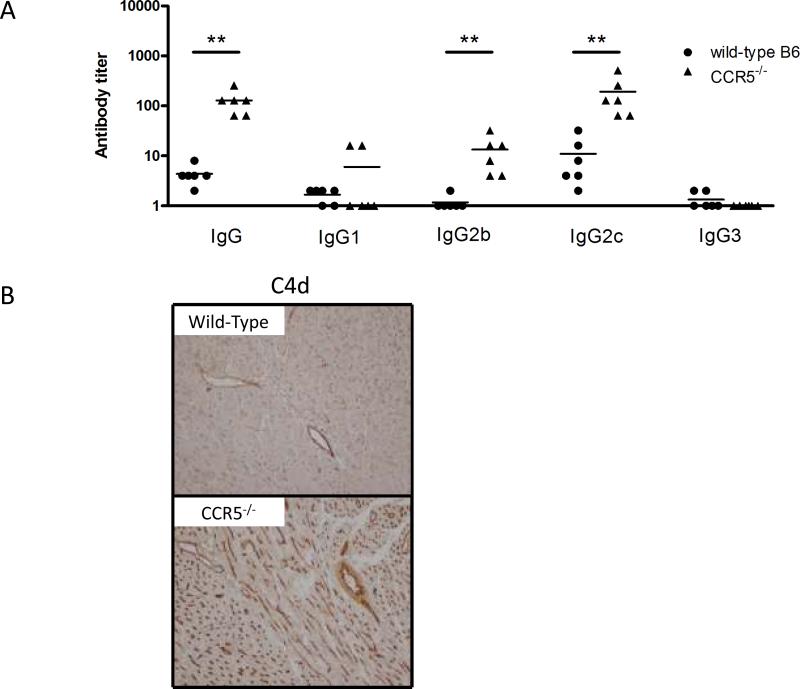
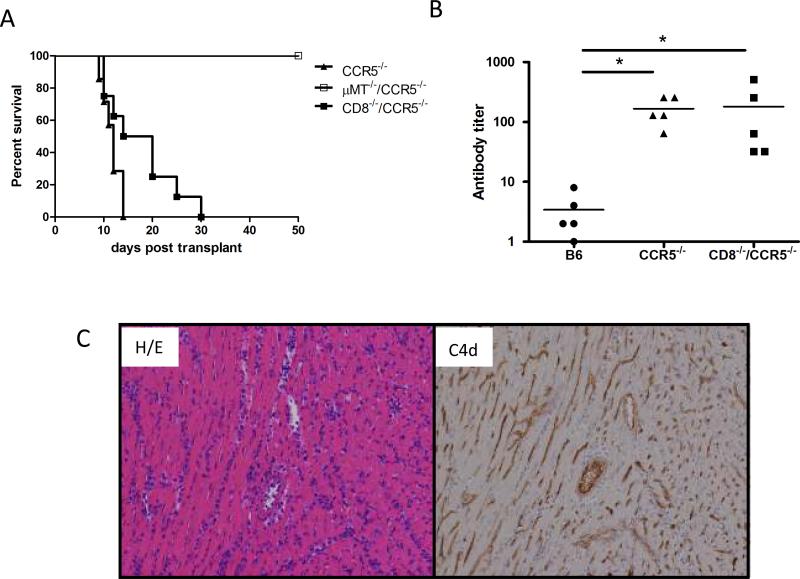
Previous studies had indicated the induction of high levels of donor-reactive antibody to complete MHC-mismatched cardiac and renal allografts in CCR5−/− recipients (20, 21). To evaluate the induction of Kd-specific antibody in recipients of the B6.Kd allografts, serum was collected on day 10 post-transplant from wild-type and CCR5−/− allograft recipients and donor-reactive IgG antibody titers compared using a flow cytometric based analysis (Figure 3A). In contrast to the low donor-reactive titers observed in wild-type recipients, high titers of donor-reactive IgG were observed in CCR5−/− recipients (p = 0.0015). In particular, donor-specific IgG2c and IgG2b antibodies were significantly elevated in the CCR5−/− recipients when compared with wild type recipients whereas titers of donor-reactive IgG1 and IgG3 antibodies were low/undetectable in both wild-type and CCR5−/− allograft recipients. Reflecting the donor-reactive IgG titers, allografts from CCR5−/− recipients had intense and diffuse deposition of the split complement product C4d in the large vessels and myocardial capillaries whereas grafts from wild-type recipients had weak C4d deposition in the large vessels of the allograft and no detectable deposition in the myocardial capillaries of the graft (Figure 3B).
Figure 3. Increased donor-reactive antibody production in B6.CCR5−/− recipients of B6.Kd cardiac allografts.
(A) Serum from wild-type C57BL/6 and B6.CCR5−/− allograft recipients was collected on day 10 post-transplant and tested for reactivity to B6.Kd thymocytes using flow cytometric based analyses to determine donor-reactive antibody titers. Data indicate mean titer ± SEM for n = 6/group and are representative of two independent experiments. **p<0.01. (B) On day 10 post-transplant, cardiac allografts were harvested from wild-type C57BL/6 recipients and from B6.CCR5−/− recipients. Methanol-fixed sections were stained with C4d specific antibody for immunohistochemical analysis. Sections shown are representative of 4 grafts analyzed in each group. Magnification, 200×.
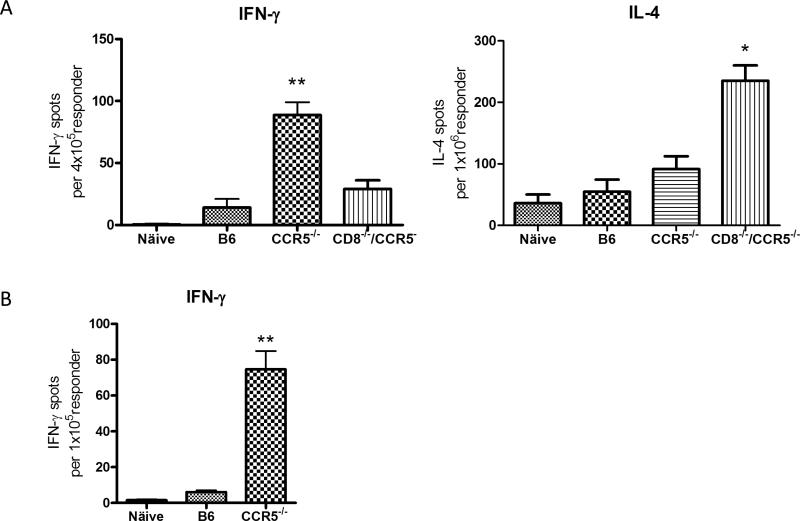
To assess the priming of donor-reactive T cells in CCR5−/− recipients of B6.Kd cardiac allografts, recipient spleen cell suspensions were prepared on day 10 post-transplant and numbers of donor-reactive T cells producing IFN-γ and IL-4 were enumerated by ELISPOT assay. Whereas low numbers of donor-reactive T cells producing IFN-γ were observed in the spleens of wild-type recipients of Kd allografts, the numbers in CCR5−/− recipients was markedly higher (Figure 4A; p = 0.004). Numbers of T cells producing IL-4 were low and not significantly different from the numbers in spleens from naïve mice. To extend these observations, CD8 T cell populations were enriched from the spleens of wild-type and CCR5−/− recipients on day 10 post-transplant and again numbers of donor-reactive CD8 T cells producing IFN-γ were low in wild-type recipients and substantially higher in CCR5 recipients (Figure 4B; p = 0.003).
Figure 4. Increased donor-reactive T cell response in B6.CCR5−/− recipients and CD8−/−/CCR5−/− recipients.
Groups of wild-type C57BL/6, B6.CCR5−/− and B6.CD8−/−/CCR5−/− mice (n = 3/each group) received B6.Kd heart allografts. On day 10 post-transplant (A) whole spleen cell suspensions or (B) CD8 T cells enriched from recipient spleens by negative selection were tested by ELISPOT assay to enumerate cells producing IFN-γ and IL-4. Data are representative of two independent experiments and the data indicate mean number of donor-reactive T cells or CD8 T cells producing the test cytokine ± SEM. (A) **p =0.004 vs. wild-type C57BL/6 recipients and p = 0.009 vs. B6.CD8−/−/CCR5−/− recipients; *p = 0.012 vs. CCR5−/− recipients; (B) **p = 0.003 vs. wild-type C57BL/6 recipients.
CD25+ CD4+ T cells do not restrict wild-type alloreactive T cell responses to Kd-disparate cardiac allografts
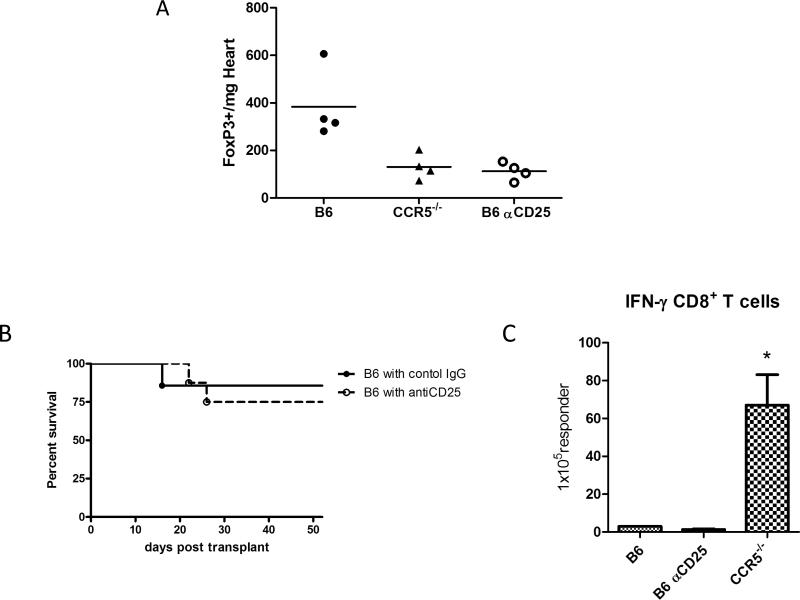
Previous studies from this laboratory had indicated that CD4+CD25+ regulatory T cells restrict the magnitude and duration of alloreactive CD4 T cell responses to single class II MHC disparate heart allografts and that the absence of CCR5 expression results in dysregulated donor-reactive T cell responses and rapid rejection of the allografts (28, 29). The potential effects of regulatory T cells in restricting the donor-specific responses to single class I MHC disparate allografts in wild-type C57BL/6 recipients were tested by administering regulatory T cell depleting anti-CD25 mAb or control rat IgG to wild-type recipients of the B6.Kd cardiac allografts. Consistent with observations in single class II MHC disparate allografts in CCR5−/− recipients (28), single class I MHC disparate B6.Kd allografts in CCR5−/− recipients had marked reductions in Foxp3 expressing cells when compared to the allografts in wild-type C57BL/6 recipients (Figure 5A; p = 0.019). However, treatment with anti-CD25 mAb decreased the presence of CD25+Foxp3+ T cells in the graft of wild-type recipients to the same level as in grafts from CCR5−/− recipients (Figure 5A) but did not promote acute rejection of the B6.Kd cardiac allografts in the wild-type recipients (Figure 5B). In addition, anti-CD25 mAb did not alter the number of donor-reactive T cells producing IFN-γ in the spleens of the wild-type recipients of the B6.Kd allografts (Figure 5C) or the low titers of donor-reactive antibodies in the serum of wild-type recipients when tested on days 10 and 50 post-transplant (data not shown).
Figure 5. The alloreactive T cell response to single class I MHC mismatched cardiac allografts in wild-type C57BL/6 recipients is not restricted by CD25+ T cells.
(A) Groups of wild-type C57BL/6 and CCR5−/− mice received B6.Kd cardiac allografts. The wild-type mice were treated with control rat IgG or anti-CD25 mAb, 0.5 mg on day −1 and 0.25 mg of Ab on days 1, 3, 5, 7, and 9 post-transplant. On day 10 post-transplant, grafts were harvested, weighed, digested, and single cell suspensions stained with anti-CD4, anti-CD25 and anti-Foxp3 mAb and analyzed by flow cytometry. The data shown indicate numbers of graft infiltrating CD4+FoxP3+ cells/mg graft tissue for five mice per group and are representative of two separate experiments. (B) Groups of wild-type C57BL/6 mice were treated with control rat IgG (—●— n = 7) or anti-CD25 mAb i.p. (—◯— n = 7) and received B6.Kd cardiac allografts. Cardiac allograft survival was monitored daily by abdominal palpation and rejection was confirmed visually by laparotomy. (C) On day 10 post-transplant, spleen cell suspensions from wild-type C57BL/6 recipients treated with control rat IgG or anti-CD25 mAb and B6.CCR5−/− recipients of B6.Kd cardiac allografts were depleted of CD4 T cells and ELISPOT assays were performed on day 10 post-transplant to enumerate donor-reactive IFN-γ producing CD8 T cells. Data indicate mean numbers of donor-reactive CD8 T cells producing IFN-γ ± SEM and are representative of two independent experiments. *p < 0.05.
Kd-disparate allograft rejection in CCR5−/− recipients requires antibody but not CD8 T cells
The induction of high numbers of donor-reactive CD8 T cells induced in CCR5−/− recipients of B6.Kd heart allografts added an unanticipated component to potential mechanisms underlying rejection of the allografts in these recipients. To directly investigate the role of donor-reactive antibody in the rejection of cardiac allografts in CCR5−/− recipients, B6.Kd cardiac allografts were transplanted into antibody-deficient μMT−/−/CCR5−/− recipients. All B6.Kd allografts survived for >100 days in μMT−/−/CCR5−/− recipients in contrast to the rapid rejection in CCR5−/− recipients (Figure 6A).
Figure 6. Kd-disparate allograft rejection in CCR5−/− recipients requires antibody but not CD8 T cells.
(A) Groups of B6.CCR5−/− (—▴— n = 7), CD8−/−/CCR5−/− (—∎— n = 7) and B6.μMT−/−/CCR5−/− (—◻— n = 6) mice received B6.Kd cardiac allografts. Cardiac allograft survival was monitored daily by palpation and rejection was confirmed visually by laparotomy. (B) Serum from the groups of allograft recipients was collected on day 10 post-transplant and tested for reactivity to B6.Kd thymocytes using flow cytometric based analyses to determine donor-reactive antibody titers. Data indicate mean titer ± SEM for n = 5/group and are representative of two independent experiments. *p < 0.05 (C) B6.Kd allografts were retrieved from B6.CD8−/−/CCR5−/− recipients on day 10 post-transplant. Prepared sections were stained with hematoxylin and eosin or C4d-specific antibodies for immunohistochemical analyses. Sections shown are representative of 4 grafts analyzed in each group. Magnification, 200×.
To test rejection of the Kd allografts in the absence of the increased donor-reactive CD8 T cell response in CCR5−/− recipients, CCR5−/− mice were crossed with CD8α−/− mice to generate CD8−/−/CCR5−/− mice. Graft survival in the CD8−/−/CCR5−/− recipients was extended with a third of the grafts surviving 20–30 days (mean graft survival 17.6 ± 6.9 days, Figure 6A), but was not significantly different from the graft survival observed in CCR5−/− recipients. The donor-reactive antibody titers in the serum from CCR5−/− and CD8−/−/CCR5−/− allograft recipients were equivalent at day 10 post-transplant (Figure 6B). At the time of rejection, allografts from CD8−/−/CCR5−/− recipients had intense mononuclear cell infiltration and diffuse C4d deposition in the myocardial capillaries (Figure 6C). In addition, infiltration of CD4 T cells into the B6.Kd allografts in CD8−/−/CCR5−/− recipients was increased to the levels observed in grafts from wild-type recipients and the infiltration of macrophages and neutrophils into the allografts was similar to that observed in B6.Kd allografts in CCR5−/− recipients (Figure 2). Priming of donor-reactive T cells to IFN-γ producing cells in CD8−/−/CCR5−/− recipients was significantly decreased compared to the levels observed in the CCR5−/− recipients and priming to IL-4 producing cells was markedly increased (Figure 4 A and B; p = 0.009 and p = 0.012).
Intragraft expression of myeloperoxidase, perforin, and CCL5 mRNA is associated with Kd allograft rejection in CCR5−/−/CD8−/− recipients
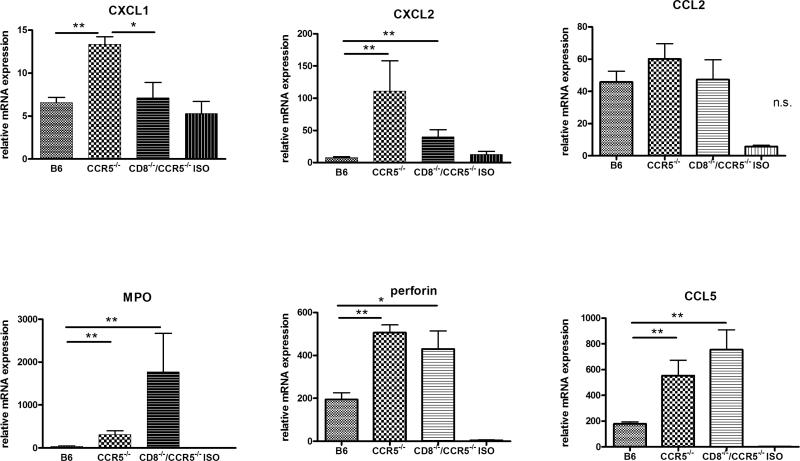
To investigate potential factors underlying antibody-mediated injury of B6.Kd allografts, grafts were retrieved from wild-type C57BL/6, CCR5−/−, and CD8−/−/CCR5−/− allograft recipients and from wild-type C57BL/6 isograft recipients at day 10 post-transplant and expression levels of mRNA encoding inflammatory mediators were compared (Figure 7). The expression level of the macrophage and T cell chemoattractant CCL2 was low in isografts and markedly increased in the B6.Kd allografts but was not significantly different in allografts placed in any of the three test recipients. The expression of the neutrophil chemoattractants CXCL1 and CXCL2 was markedly higher in B6.Kd allografts in the CCR5−/− recipients than in isografts or in allografts from either the wild-type or CD8−/−/CCR5−/− recipients suggesting that this increased expression was promoted by the allograft-infiltrating CD8 T cells. The expression levels of perforin and CCL5 mRNA were significantly elevated in allografts placed in both CCR5−/− and CD8−/−/CCR5−/− recipients versus the iso- or allografts placed in wild-type recipients suggesting the induced expression of these genes was mediated by donor-reactive antibody and/or CD8 T cells induced in the CCR5−/− recipients. Surprisingly, the expression level of myeloperoxidase (MPO) mRNA was significantly higher in the Kd allografts in CD8−/−/CCR5−/− recipients when compared to allografts in the CCR5−/− or wild-type recipients.
Figure 7. Intragraft cytokine and chemokine mRNA expression in B6.Kd cardiac allografts retrieved from wild-type C57BL/6, B6.CCR5−/− and CD8−/−/CCR5−/− recipients.
B6.Kd cardiac allografts were harvested from groups of 5 wild-type C57BL/6, B6.CCR5−/− and CD8−/−/CCR5−/− recipients and C57BL/6 isografts from C57BL/6 recipients on day 10 post-transplant. Whole cell mRNA was isolated from graft tissue homogenates, and the levels of mRNA encoding each test gene were determined by quantitative RT-PCR. Data represent mean mRNA expression level ± SEM. *p < 0.05, **p < 0.01, n.s.= p>0.05.
Graft-reactive antibody induces neutrophil recruitment and high expression levels of MPO, perforin and CCL5 mRNA in Kd allografts placed in RAG-1−/− recipients
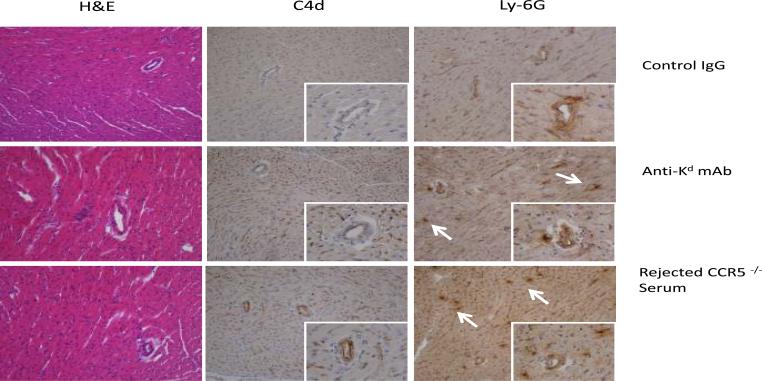
To extend investigation into the effect of donor specific antibody to the single class I MHC disparate allograft injury, RAG1−/− mice received B6.Kd cardiac allografts and were treated with 200 μg aliquots of anti-Kd mAb or control rat IgG on days 8 and 9 post-transplant. This treatment did not induce rejection of the grafts but did promote C4d deposition in the myocardial capillaries and neutrophil graft infiltration when analyzed on day 10 post-transplant (Figure 8A). Similarly, aliquots of pooled serum from CCR5−/− mice that had rejected B6.Kd allografts were injected into RAG1−/− recipients of B6.Kd allografts and also induced C4d deposition in the myocardial capillaries and neutrophil infiltration at day 10 post-transplant (Figure 8A).
Figure 8. Transferred Kd-reactive antibodies mediate Kd cardiac graft injury in RAG-1−/−recipients.
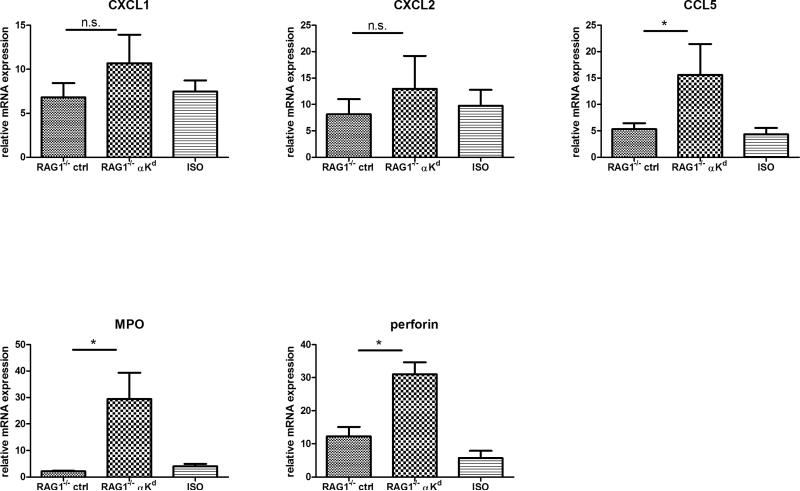
Groups of 4–5 RAG-1−/− recipients of B6.Kd cardiac allografts were treated on days 8 and 9 post-transplant with 200 μg of control rat IgG or anti-Kd mAb or 400 μl pooled serum from CCR5−/− mice that had rejected B6.Kd cardiac allografts. The allografts were harvested from the RAG-1−/− recipients on day 10 post-transplant. (A) Prepared sections were stained with hematoxylin and eosin or with C4d specific antibody for immunohistochemical analysis. Sections shown are representative of 4 grafts analyzed in each group. Magnification, 200x. (B) B6.Kd cardiac allografts were retrieved from RAG-1−/− recipients treated with control rat IgG or with anti-Kd mAb. Whole cell mRNA was isolated from graft tissue homogenates and the levels of mRNA encoding CXCL1, CXCL2, myeloperoxidase, perforin and CCL5 were determined by quantitative RT-PCR. Data indicate mean mRNA expression levels ± SEM for 4–5 grafts per group. *p < 0.05, n.s.= P>0.05.
To further investigate the mechanism of graft inflammation mediated by allograft-reactive antibody, intragraft mRNA expression levels of MPO, perforin and CCL5 were determined in B6.Kd cardiac allografts in RAG-1−/− recipients given control IgG or anti-Kd mAb (Figure 8B). Similar to the induction of these inflammatory mediators during rejection of the B6.Kd allografts in CD8−/−/CCR5−/− recipients, high levels of MPO, perforin, and CCL5 mRNA expression were induced in the allografts by injection of the anti-Kd mAb.
Discussion
It has long been recognized that the presence of circulating alloantibodies portends poorer graft function and survival. Acute rejection of renal and cardiac allografts is more severe in recipients with serum anti-donor class I HLA antibodies (30–32). Anti-HLA antibodies are also associated with late loss of solid organ grafts and with the development of chronic graft pathology (31, 33, 34). In many instances diagnosis of antibody-mediated allograft rejection is accompanied by T cell infiltration into the graft, but the incidence of AHR without an accompanying cellular rejection in cardiac transplant patients may be as high as 15% (35–37). Antibody binding to allogeneic MHC molecules on graft endothelium and other cells could induce direct injury to the cells through complement mediated effects or could induce injury indirectly by stimulating endothelial cells to produce acute phase cytokines and chemoattractants that increase recipient leukocyte infiltration and activation in the graft. Recent clinical studies have reported more intense renal and heart graft inflammation associated with the presence of recipient serum antibodies with reactivity to donor class II MHC when compared to antibodies with reactivity to donor class I MHC (4–9). These studies have instigated efforts in our laboratory to investigate mechanisms of allograft injury mediated by donor class I vs. class II MHC reactive antibodies and to identify biomarkers associated with antibody-mediated rejection of the grafts. The goal of the current study was to develop a model of anti-class I MHC antibody mediated allograft rejection using a CCR5-deficient recipient model. We have previously reported that antibody responses to complete MHC-mismatched heart and kidney allografts are dysregulated in CCR5−/− recipients, resulting in the production of high serum titers of antibody that mediate rejection of the allografts (20, 21).
The results of the current study indicate the ability of CCR5−/− but not wild-type recipients to reject cardiac allografts with a single, transgenically expressed class I MHC disparity. CCR5−/− recipient rejection of the B6.Kd allografts was accompanied by a potent CD8 T cell response to the donor alloantigen and intense infiltration of CD8 T cells and neutrophils into the rejecting grafts. This raised the possibility of a required or substantial contribution by donor-reactive CD8 T cells in the rejection of the single class I MHC disparate allografts. Antibody-deficient μMT−/−/CCR5−/− recipients could not reject the B6.Kd allografts indicating the need for antibody in this rejection. Conversely, CCR5−/− recipients did reject the B6.Kd allografts in the absence of CD8 T cells with a slight extension in allograft survival. At the time of rejection in CD8−/−/CCR5−/− recipients, the level of CD4 T cell infiltration into the Kd allografts was similar to that observed in grafts from CCR5−/− recipients suggesting little contribution to rejection by the infiltrating CD4 T cells. Previous studies by one of us (WMB III) reported the ability of passively transferred anti-donor class I MHC mAb to provoke rejection of complete MHC-mismatched heart allografts in μMT−/− recipients (13, 14). However, this antibody transfer was most effective when administered 10 days after the transplant and is consistent with the current results that the presence of donor antigen-primed CD8 T cells can synergize with the anti-donor antibody to provoke rejection. Although these and the current studies indicate synergy between antibody and CD8 T cells in mediating rejection of the allograft, the ability of the anti-donor antibody to enhance the infiltration and/or function of graft infiltrating CD8 T cells should also be considered and is not yet known.
We also noticed infiltration of NK cells into the B6.Kd allografts in the CD8−/−/CCR5−/− recipients at the time of rejection (Y. Hattori, data not shown). Recent studies by Hidalgo and colleagues (38) have documented the expression of NK cell related transcripts in renal graft biopsies during antibody-mediated rejection. These and other studies have proposed a role for NK cells in mediating graft injury (38–40). However, depletion of NK cells through administration of anti-NK1.1 mAb did not alter the rejection of the B6.Kd allografts in the CD8−/−/CCR5−/− recipients (Y. Hattori, data not shown). We interpret these results to indicate that anti-donor class I MHC antibody binding to allografts does promote the localization and infiltration of NK cells that would account for the presence of NK cell associated transcripts in grafts during antibody-mediated rejection as observed by other investigators. The activation of NK cells may contribute to graft injury but the infiltration and activation of NK cells does not appear to be a requirement for the antibody-mediated rejection of the Kd allografts.
The presence of CD8 T cells and NK cells that contribute to, but are not required for, anti-donor MHC-reactive antibody allograft injury raises further questions about the antibody-mediated mechanisms that directly cause graft tissue injury and eventually lead to graft failure. Antibody binding to the endothelium could potentially stimulate the formation of the MAC and mediate lysis of donor-derived endothelial cells but the endothelial cells also express complement regulatory proteins that are likely to diminish the formation of the MAC and this may account for the infrequent observation of graft endothelial cell lysis during antibody-mediated rejection (2, 41–43). Complement activation also stimulates endothelial cells to produce many inflammatory mediators including neutrophil and macrophage chemoattractants CXCL1, CXCL2 and CCL2 as well as upregulated expression of adhesion molecules (16–19). Antibody-mediated rejection is typically characterized by neutrophil and macrophage infiltration that is directed in response to these chemoattractants (1, 4, 10). Induction of high levels of neutrophil chemoattractants was observed in B6.Kd allografts in CCR5−/− and CD8−/−/CCR5−/− recipients producing anti-Kd antibody and was accompanied by intense neutrophil infiltration into the allografts. The impact of neutrophil infiltration and activation in graft tissue injury remains poorly understood. Certainly CXCL1/CXCL2 binding to CXCR2 would stimulate neutrophils to release primary and secondary granules containing many different proteases and oxygen radicals capable of mediating injury of the allograft tissue (44, 45). We observed the extended survival of B6.Kd allografts in CCR5−/−/CXCR2−/− recipients with 35% surviving beyond 50 days despite high titers of donor-reactive serum antibody in the serum (Y Hattori, unpublished results). These results support a role for neutrophils as cellular mediators of antibody-mediated allograft injury and graft-infiltrating macrophages may also contribute to this injury.
Antibody-mediated rejection of the single class I MHC disparate allografts was accompanied by increased mRNA expression of perforin, myeloperoxidase, and CCL5/RANTES in the allografts. Two of these markers, perforin and CCL5, were observed at equivalent levels in B6.Kd allografts from CCR5−/− and CD8−/−/CCR5−/− recipients whereas the expression of MPO was markedly increased in rejecting allografts in CD8−/−/CCR5−/− recipients. Neutrophils are the major source of MPO and it was expected that the levels of this mRNA expression in the B6.Kd allografts would correlate with the number of graft infiltrating neutrophils. Although the numbers of neutrophils in rejecting B6.Kd allografts from CCR5−/− and CD8−/−/CCR5−/− recipients was equivalent, the marked increase in myeloperoxidase expression in the allografts in CD8−/−/CCR5−/− recipients suggests that graft infiltrating CD8 T cells temper the transcriptional activity of the infiltrating neutrophils. In the absence of graft-infiltrating CD8 T cells the source of perforin and CCL5 during antibody-mediated rejection of the B6.Kd allografts is unknown. While NK cells are an obvious source of each, neutrophils also produce CCL5 and our recent results have indicated the expression of perforin in a neutrophil population during elicitation of CD8 T cell mediated responses in the skin (R. Fairchild, unpublished results). Transfer of Kd-reactive antibody to RAG-1−/− mice bearing B6.Kd transplants induced obvious graft inflammation one day after the transfer. This included complement activation in the myocardial capillaries and large vessels and neutrophil infiltration into the allograft that was accompanied by expression of perforin, MPO and CCL5. It was noteworthy that administration of two doses of this antibody did not lead to rejection of the allografts, defined as cessation of heart allograft beating. We interpret the absence of rejection in RAG-1−/− recipients injected with the donor-reactive antibody as indicating that continuous high titers of the antibody are needed to achieve sufficient injury to reject the graft as is observed in CCR5−/− and CD8−/−/CCR5−/− recipients. It is worth considering that the induction of neutrophil chemoattractants in the allograft by passively transferred donor-reactive antibody is likely to be short-lived when compared to the continual production of high titers of the antibody and that the infiltration and activation of neutrophils and macrophages to mediate graft tissue injury are therefore likely to also be of short duration.
The current studies indicate that high titers of antibody reactive to single class I MHC disparate heart allografts mediate graft rejection as observed in CCR5−/− and CD8−/−/CCR5−/− recipients. These antibodies promote neutrophil graft infiltration and activation that are likely to contribute to the graft tissue injury. This antibody-mediated rejection is accompanied by the expression of 3 markers in the allograft but we were unable to detect any in the serum of recipients with rejecting grafts. Nevertheless, serial endomyocardial biopsies are used to monitor the status of cardiac grafts by histopathologic examination and could be used to test expression of these markers to provide further confirmatory evidence of antibody-mediated rejection.
Acknowledgements
We thank the staff of the Cleveland Clinic Biological Resources Unit for excellent care of the animals used in this study. The authors thank Drs. Anna Valujskikh for helpful comments during the course of this work and preparation of the manuscript.
This work was supported by grants from the NIH (RO1 AI40459 and PO1 AI087586) to RLF.
Abbreviations
- AHR
acute humoral rejection
- DAB
3,3'-Diaminobenzidine
- MAC
membrane attack complex
- MPO
myeloperoxidase
Footnotes
Disclosure The authors have no financial conflict of interest to declare.
References
- 1.Colvin RB, Smith RN. Antibody-mediated organ-allograft rejection. Nat. Rev. Immunol. 2005;5:807–817. doi: 10.1038/nri1702. [DOI] [PubMed] [Google Scholar]
- 2.Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, Cecka MJ, Cosyns J-P, Demetris AJ, Fishbein MC, Fogo A, Furness P, Gibson IW, Glotz D, Hayry P, Hunsickern L, Kashgarian M, Kerman R, Magil AJ, Montgomery R, Morozumi K, Nickeleit V, Randhawa P, Regele H, Seron D, Seshan S, Sund S, Trpkov K. Antibody-mediated rejection criteria-an addition ot the Banff '97 classification of renal allograft rejection. Am. J. Transplant. 2003;3:708–714. doi: 10.1034/j.1600-6143.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 3.Takemoto SK, Zeevi A, Feng S, Colvin RB, Jordan S, Kobashigawa J, Kupiec-Weglinski J, Matas A, Montgomery RA, Nickerson P, Platt JL, Rabb H, Thistlethwaite R, Tyan D, Delmonico FL. National conference to assess antibody-mediated rejection in solid organ transplantation. Am. J. Transplant. 2004;4:1033–1041. doi: 10.1111/j.1600-6143.2004.00500.x. [DOI] [PubMed] [Google Scholar]
- 4.Uber WE, Self SE, van Bakel AB, Pereira NL. Acute antibody-mediated rejection following heart trasnplantation. Am. J. Transplant. 2007;7:2064–2074. doi: 10.1111/j.1600-6143.2007.01900.x. [DOI] [PubMed] [Google Scholar]
- 5.Crespo M, Pascual M, Tolkoff-Rubin N, Mauiyyedi S, Collins AB, Fitzpatrick D, Farrell ML, Williams WW, Delmonico FL, Cosimi AB, Colvin RB, Saidman SL. Acute humoral rejection in renal allograft recipients: I. Incidence, serology and clinical characteristics. Transplantation. 2001;71:652–658. doi: 10.1097/00007890-200103150-00013. [DOI] [PubMed] [Google Scholar]
- 6.Pollinger HS, Stegall MD, Gloor JM, Moore SB, Degoey SR, Ploeger NA, Park WD. Kidney transplantation in patients with antibodies against donor HLA class II. Am. J. Transplant. 2007;7:857–863. doi: 10.1111/j.1600-6143.2006.01699.x. [DOI] [PubMed] [Google Scholar]
- 7.Gloor JM, Sethi S, Stegall MD, Park WD, Moore SB, DeGoey S, Griffin MD, Larson TS, Cosio FG. Transplant glomerulopathy: subclinical incidence and association with alloantibody. Am. J. Transplant. 2007;7:2124–2132. doi: 10.1111/j.1600-6143.2007.01895.x. [DOI] [PubMed] [Google Scholar]
- 8.Langan LL, Park LP, Hughes TL, Irish A, Luxton G, Witt CS, Christiansen FT. Post-tranplant HLA class II antibodies and high soluble CD30 levels are independently associated with poor kidney graft survival. Am. J. Transplant. 2007;7:847–856. doi: 10.1111/j.1600-6143.2006.01691.x. [DOI] [PubMed] [Google Scholar]
- 9.Campos EF, Tedesco-Silva H, Machado PG, Franco M, Medina-Pestana JO, Gerbase-DeLima M. Post-transplant anti-HLA class II antibodies as risk factor for late kidney allograft failure. Am. J. Transplant. 2006;6:2316–2320. doi: 10.1111/j.1600-6143.2006.01503.x. [DOI] [PubMed] [Google Scholar]
- 10.Gloor J, Cosio F, Lager DJ, Stegall MD. The spectrum of antibody-mediated renal allograft injury: implications for treatment. Am. J. Transplant. 2008;8:1367–1373. doi: 10.1111/j.1600-6143.2008.02262.x. [DOI] [PubMed] [Google Scholar]
- 11.Taflin C, Charron D, Glotz D, Mooney N. Immunological function of the endothelial cell within the setting of organ transplantation. Immunol. Lett. 2011;139:1–6. doi: 10.1016/j.imlet.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Reed EF. Effect of antibodies on endothelium. Am. J. Transplant. 2009;9:2459–2465. doi: 10.1111/j.1600-6143.2009.02819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahimi S, Qian Z, Layton J, Fox-Talbot K, Baldwin WMI, Wasowska BA. Non-complement- and complement-activating antibodies synergize to cause rejection of cardiac allografts. Am. J. Transplant. 2004;4:326–334. doi: 10.1111/j.1600-6143.2004.00334.x. [DOI] [PubMed] [Google Scholar]
- 14.Wasowska BA, Qian Z, Cangellow DLB, van Tran E,K, Layton J, Sanfilippo F, Baldwin WMI. Passive transfer of alloantibodies restores acute cardiac rejection in IgKO mice. Transplantation. 2001;71:727–736. doi: 10.1097/00007890-200103270-00007. [DOI] [PubMed] [Google Scholar]
- 15.Nakashima S, Qian Z, Rahimi S, Wasowska BA, Baldwin WM. Membrane attack complex contributes to destruction of vascular integrity in acute lung allograft rejection. J. Immunol. 2002;169:4620–4627. doi: 10.4049/jimmunol.169.8.4620. [DOI] [PubMed] [Google Scholar]
- 16.Albrecht EA, Chinnaiyan AM, Varambally S, Kumar-Sinha C, Barrette TR, Sarma JV, Ward PA. C5a-induced gene expression in human umbilical vein endothelial cells. Am. J. Pathol. 2004;164:849–859. doi: 10.1016/S0002-9440(10)63173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monsinjon T, Gasque P, Chan P, Ischenko A, Brady JJ, Fontaine MC. Regulation of complement C3a and C5a anaphylatoxins of cytokine production in human umbilical vein endothelial cells. FASEB J. 2003;17:1003–1014. doi: 10.1096/fj.02-0737com. [DOI] [PubMed] [Google Scholar]
- 18.Saadl S, Holzknecht RA, Patte CP, Platt JL. Endothelial cell activation by pore-forming structures: pivotal role for interleukin-1a. Circulation. 2000;101:1867–1873. doi: 10.1161/01.cir.101.15.1867. [DOI] [PubMed] [Google Scholar]
- 19.Saadl S, Platt JL. Humoral rejection and endothelial cell activation. Xenotransplantation. 2002;9:239–241. doi: 10.1034/j.1399-3089.2002.02042.x. [DOI] [PubMed] [Google Scholar]
- 20.Bickerstaff A, Nozaki T, Wang J-J, Pelletier R, Hadley G, Nadasdy G, Nadasdy T, Fairchild RL. Acute humoral rejection of renal allografts in CCR5−/− recipients. Am. J. Transplant. 2008;8:557–566. doi: 10.1111/j.1600-6143.2007.02125.x. [DOI] [PubMed] [Google Scholar]
- 21.Nozaki T, Amano H, Bickerstaff A, Orosz CG, Novick AC, Tanabe K, Fairchild RL. Antibody-mediated rejection of cardiac allografts in CCR5-deficient recipients. J. Immunol. 2007;179:5238–5245. doi: 10.4049/jimmunol.179.8.5238. [DOI] [PubMed] [Google Scholar]
- 22.Honjo K, Xu XY, Kapp JA, Bucy RP. Evidence for cooperativity in the rejection of cardiac grafts mediated by CD4+ TCR Tg T cells specific for a defined allopeptide. Am. J. Transplant. 2004;4:1762–1768. doi: 10.1046/j.1600-6143.2004.00596.x. [DOI] [PubMed] [Google Scholar]
- 23.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. Transplantation. 1973;1973:343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Setoguchi K, Schenk AD, Ishi D, Hattori Y, Baldwin WM, III, Tanabe K, Fairchild RL. LFA-1 antagonism inhibits early infiltration of endogenous memory CD8 T cells into cardiac allografts and donor-reactive T cell priming. Am. J. Transplant. 2011;11:923–935. doi: 10.1111/j.1600-6143.2011.03492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schenk AD, Nozaki T, Rabant M, Valujskikh A, Fairchild RL. Donor-reactive CD8 memory T cells infiltrate cardiac allografts within 24-h posttransplant in naive recipients. Am. J. Transplant. 2008;8:1652–1661. doi: 10.1111/j.1600-6143.2008.02302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Afanasyeva M, Georgakopoulos D, Belardi DF, Ramsundar AC, Barin JG, Kass DA, Rose NR. Quantitative analysis of myocardial inflammation by flow cytometry in murine autoimmune myocarditis: correlation with cardiac function. Am. J. Pathol. 2004;164:807–815. doi: 10.1016/S0002-9440(10)63169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murata K, Fox-Talbot K, Qian Z, Takahashi K, Stahl GL, Baldwin WM, 3rd, Wasowska BA. Synergistic depostiion of C4d by complement-activating and non-activaing antibodies in cardiac transplants. Am. J. Trasnplant. 2007;7:2605–2614. doi: 10.1111/j.1600-6143.2007.01971.x. [DOI] [PubMed] [Google Scholar]
- 28.Nozaki T, Rosenblum JM, Schenk AD, Ishii D, Fairchild RL. CCR5 is required for regulation of alloreactive T-cell responses to single class II MHC-mismatched murine cardiac grafts. Am. J. Transplant. 2009;9:2251–2261. doi: 10.1111/j.1600-6143.2009.02786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schenk S, Kish DD, He C, El-Sawy T, Chiffoleau E, Chen C, Wu Z, Sandner S, Gorbachev AV, Fukamachi K, Heeger PS, Sayegh MH, Turka LA, Fairchild RL. Alloreactive T cell responses and acute rejection of single class II MHC disparate heart allografts is under strict regulation by CD4+CD25+ T cells. J. Immunol. 2005;174:3741–3748. doi: 10.4049/jimmunol.174.6.3741. [DOI] [PubMed] [Google Scholar]
- 30.Halloran PF, Schlaut J, Solez K, Srinivasa NS. The significance of the anti-class I antibody response. II. Clinical and pathologic features of renal transpalntas with anti-class I-like antibody. Transplantation. 1992;53:550–555. [PubMed] [Google Scholar]
- 31.McKenna RM, Takemoto SK, Terasaki PI. Anti-HLA antibodies after solid organ transplantation. Transplantation. 2000;69:319–326. doi: 10.1097/00007890-200002150-00001. [DOI] [PubMed] [Google Scholar]
- 32.Tambur AR, Pamboukian SV, Costanzo MR, Herrerra ND, Dunlap S, Montpetit M, Herouz A. The presence of HLA-directed antibodies after heart transplantation is associated with poor allograft outcome. Transplantation. 2005;80:1019–1025. doi: 10.1097/01.tp.0000180564.14050.49. [DOI] [PubMed] [Google Scholar]
- 33.Terasaki PI. Humoral theory of transplantation. Am. J. Transplant. 2003;3:665–673. doi: 10.1034/j.1600-6143.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- 34.Lee P-C, Terasaki PI, Takemoto SK, Lee P-H, Hung C-J, Chen Y-L, Tsai A, Lei H-Y. All chronic rejection failures of kidney transplants were preceded by the development of HLA antibodies. Transplantation. 2002;74:1192–1194. doi: 10.1097/00007890-200210270-00025. [DOI] [PubMed] [Google Scholar]
- 35.Reed EF, Demetris AJ, Hammond E, Itescu S, Kobashigawa JA, Reinsmoen NL, Rodriguez RR, Rose M, Stewart S, Sucia-Foca N, Zeevi A, Fishbein MC. Acute antibody-mediated rejection of cardiac transplants. J. Heart Lung Transplant. 2006;25:153–159. doi: 10.1016/j.healun.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Mauiyyedi S, Crespo M, Collins AB, Schneeberger EE, Pascual MA, Saidman SL, Tolkoff-Rubin NE, Williams WW, Delmonico FL, Cosimi AB, Colvin RB. Acute humoral rejection in kidney transplantation: II. Morphology, immunopathology, and pathologic classification. J. Am. Soc. Nephrol. 2002;13:779–787. doi: 10.1681/ASN.V133779. [DOI] [PubMed] [Google Scholar]
- 37.Moll SPM. Humoral rejection of organ allografts. Am. J. Transplant. 2005;5:2611–2618. doi: 10.1111/j.1600-6143.2005.01086.x. [DOI] [PubMed] [Google Scholar]
- 38.Hidalgo LG, Sis B, Sellares J, Campbell PM, Mengel M, Einecke G, Chang J, Halloran PF. NK cell transcripts and NK cells in kidney biopsies from patients with donor-specific antibodies: evidence for NK cell involvment in antibody-mediated rejection. Am. J. Transplant. 2010;10:1812–1822. doi: 10.1111/j.1600-6143.2010.03201.x. [DOI] [PubMed] [Google Scholar]
- 39.Hirohashi t., Chase CM, Della Pelle P, Sebastian D, Alessandrini A, Madsen JC, Russell PS, Colvin RB. A novel pathway of chronic allograft rejection mediaed by NK cells and alloantibody. Transplalntation Am. J. Transplant. 2012 doi: 10.1111/j.1600-6143.2011.03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uehara S, Chase CM, Kitchens WH, Rose HS, Colvin RB, Russell PS, Madsen JC. NK cells can trigger allograft vasculopathy: the role of hybrid resistance in solid organ allografts. J. Immunol. 2005;175:3424–3230. doi: 10.4049/jimmunol.175.5.3424. [DOI] [PubMed] [Google Scholar]
- 41.Asgari E, Zhou W, Sacks S. Complement in ogan transplantation. Curr. Opin. Organ Transplant. 2010;15:486–491. doi: 10.1097/MOT.0b013e32833b9cb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brodsky SV, Nadasdy GM, Pelletier R, Satoskar A, Birmingham DJ, Hadley GA, Obeidat K, Nadasdy T. Expression of the decay-accelerating factor (CD55) in renal transplants-a possible preduction marker of allograft survival. Transplantation. 2009;88:457–464. doi: 10.1097/TP.0b013e3181b0517d. [DOI] [PubMed] [Google Scholar]
- 43.Tan CD, Sokos GG, Pidwell DJ, Smedira NG, Gonzalez-Stawinki GV, Taylor DO, Starling RC, Rodriguez ER. Correlation of donor-specific antibodies, complement and its regulators with gaft dysfunction in cardiac antibody-mediated rejection. Am. J. Transplant. 2009;9:2075–2084. doi: 10.1111/j.1600-6143.2009.02748.x. [DOI] [PubMed] [Google Scholar]
- 44.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines-CXC and CC chemokines. Adv. Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 45.Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am. J. Physol. Regul. Integr. Comp. Physiol. 2002;283:R7–R28. doi: 10.1152/ajpregu.00738.2001. [DOI] [PubMed] [Google Scholar]