Abstract
We examined the role of T1r3 and Trpm5 taste signaling proteins in carbohydrate-induced overeating and obesity. T1r3, encoded by Tas1r3, is part of the T1r2+T1r3 sugar taste receptor, while Trpm5 mediates signaling for G protein-coupled receptors in taste cells. It is known that C57BL/6 wild-type (WT) and Tas1r3 knock-out (KO) mice are attracted to the taste of Polycose (a glucose polymer), but not sucrose. In contrast, Trpm5 KO mice are not attracted to the taste of sucrose or Polycose. In Experiment 1, we maintained the WT, Tas1r3 KO and Trpm5 KO mice on one of three diets for 38 days: lab chow plus water (Control diet); chow, water and 34% Polycose solution (Polycose diet); or chow, water and 34% sucrose solution (Sucrose diet). The WT and Tas1r3 KO mice overconsumed the Polycose diet and became obese. The WT and Tas1r3 KO mice also overconsumed the Sucrose diet, but only the WT mice became obese. The Trpm5 KO mice, in contrast, showed little or no overeating on the Sucrose and Polycose diets, and gained slightly or significantly less weight than WT mice on these diets. In Experiment 2, we asked whether the Tas1r3 KO mice exhibited impaired weight gain on the Sucrose diet because it was insipid. To test this hypothesis, we maintained the WT and Tas1r3 KO mice on one of two diets for 38 days: chow, water and a dilute (1%) but highly palatable Intralipid emulsion (Control diet); or chow, water and a 34% sucrose + 1% Intralipid solution (Suc+IL diet). The WT and Tas1r3 KO mice both gained weight and became obese on the Suc+IL diet. Our results suggest that nutritive solutions must be highly palatable to cause carbohydrate-induced obesity in mice, and that palatability produces this effect in part by enhancing nutrient utilization.
Keywords: palatability, taste, carbohydrate, diet-induced obesity, nutrient utilization
1. Introduction
There is widespread concern that the superabundance of sugar- and/or fat-rich foods is contributing to the current obesity epidemic in the United States [4,21,28,52]. While the evidence linking the intake of nutrient-rich foods to obesity in humans is largely circumstantial [1,23,44], the evidence in rats [31,39,40,43,54,53] and mice [12,30,65] is incontrovertible. The rodent studies report that unlimited access to foods or solutions rich in sugars, fats or both leads to diet-induced obesity, although there are some exceptions (e.g., see [25]). Several factors contribute to diet-induced obesity, including oral (sweet taste, fatty taste and texture) and post-oral (nutritive) stimulation [37], increased nutrient utilization [72] and reduced energy expenditure [2,27].
Rodents are attracted to both the oral and post-oral effects of sugar and fat, but the relative contribution of each factor in promoting diet-induced obesity is unclear [56]. One way to separate oral from post-oral factors involves a chronic nutrient self-infusion procedure. In one study, separate groups of rats were permitted to self-infuse a high-fat or isocaloric high-carbohydrate liquid diet by licking a saccharin solution [71]. The high-fat group self-infused more liquid diet into their stomach and gained more weight than did the high-carbohydrate group. This demonstrated that with flavor held constant, a high-fat diet promoted more obesity than did a high-carbohydrate diet. A subsequent study demonstrated the importance of flavor palatability to diet-induced obesity [55]. In this case, intragastric infusion of a concentrated maltodextrin solution was paired with the consumption of either a highly palatable (saccharin + maltodextrin) or mildly unpalatable (bitter-tasting sucrose octaacetate) solution. The rats with the palatable solution self-infused more of the concentrated maltodextrin solution and gained more weight than did the rats with the unpalatable solution.
The present study further investigated the contribution of oral and post-oral factors to diet-induced obesity, using genetically modified mice. In mammals, the oral attraction to sugars is mediated by the heterodimeric T1r2+T1r3 sweet taste receptor [17,47] as well as downstream signaling elements including Trpm5, a transient Ca2+-activated cation channel [51,76]. Tas1r3, the gene that encodes T1r3, knockout (KO) mice show little or no attraction to the taste of sucrose [68,77,78], but learn to prefer sucrose solutions based on their post-oral nutritional actions [68,78]. Yet, even after acquiring a strong sucrose preference, Tas1r3 KO mice consume less sucrose at high concentrations than do C57BL/6 (B6) wild-type (WT) mice [78]. In contrast, Tas1r3 KO (and Tas1r2 KO) mice are strongly attracted to the taste of Polycose [68,78], a starch-derived maltodextrin that is rapidly absorbed as glucose, indicating that Polycose taste is not mediated by the sweet receptor. Despite this strong oral attraction, Tas1r3 KO mice ingest fewer calories from concentrated (16–32%) Polycose solutions than do WT controls in 24-hr tests [78]. This may be because, in addition to serving as a sweet taste receptor in the mouth, Tas1r3 is expressed in enteroendocrine and pancreatic beta cells [29,42,45]. The absorption of glucose from the gut of Tas1r3 KO mice may be compromised because T1r3 mediates the up-regulation of the glucose transporter, SGLT1, on high-carbohydrates diets [36,42]. Because T1r3 also contributes to the release of incretin hormones [33,64] and insulin [32,34,45], Tas1r3 KO mice may also have impaired post-absorptive processing of carbohydrates.
Like Tas1r3, Trpm5 is necessary for the taste response to sugars and other sweeteners [19,58,75]. Trpm5 KO mice are also impaired in their taste response to Polycose [58], which indicates that Trpm5 serves as a downstream signaling element in Polycose taste detection. Trpm5 KO mice can learn to prefer sugar and Polycose solutions based on post-oral nutritive feedback, but they still underconsume these solutions compared with WT mice [20,60]. The attenuated intake of carbohydrate solution by Trpm5 KO mice may reflect their impaired taste response to these carbohydrates, but post-oral metabolic deficits may also contribute. Like Tas1r3, Trpm5 is expressed in both enteroendocrine [8] and pancreatic beta [15] cells of mice. The function of Trpm5 in intestinal cells is uncertain, but Trpm5 KO mice display impaired insulin release in response to circulating glucose and fructose [10,15,34].
While the taste deficits of Tas1r3 and Trpm5 KO mice are well-documented, little is known about their long-term ingestive and weight gain responses to carbohydrate solutions. Given the differential involvement of Tas1r3 in sucrose and Polycose taste, we predicted that Tas1r3 KO mice would consume fewer calories and gain less weight on a sucrose-supplemented diet than on a Polycose-supplemented diet. The Polycose-fed Tas1r3 KO mice, in turn, should gain less weight than WT mice because their post-oral absorption and metabolism of carbohydrates are impaired. The importance of SGLT1-mediated glucose absorption to carbohydrate-induced obesity is indicated by the recent finding that the addition of gum arabic to a glucose solution reduced SGLT1 expression and weight gain, but not energy intake in mice, relative to that observed in control mice [46]. These findings indicate that alterations in SGLT1-mediated glucose absorption attenuate weight gain by influencing carbohydrate utilization rather than intake. In contrast, we predicted that Trpm5 KO mice would show similar reductions in carbohydrate intake and weight gain on the sucrose- and Polycose-supplemented diets because of their attenuated taste response to and post-oral processing of both carbohydrates.
In Experiment 1, we tested the aforementioned predictions by supplementing the diet of Tas1r3 KO, Trpm5 KO and WT mice with a carbohydrate solution (34.2% sucrose or 34.2% Polycose) for 38 days, and measuring caloric intake, weight gain, and adiposity. In Experiment 2, we asked whether enhancing the palatability of the sucrose solution to Tas1r3 KO mice would increase daily caloric intake, weight gain and adiposity. This was accomplished by adding a calorically insignificant concentration (i.e., 1%) of Intralipid, a soybean oil emulsion, to the 34.2% sucrose solution. Both the Tas1r3 KO and WT mice are attracted to the orosensory attributes of 1% Intralipid [24,59]. In addition to enhancing the palatability of the sucrose solution, the oral and post-oral effects of the Intralipid could alter post-oral processing of the sucrose by stimulating GLP-1 [3] and insulin [14] release and increasing insulin sensitivity [70]. Trpm5 KO mice were excluded from Experiment 2 because they are not attracted to the taste of dilute Intralipid [58].
2. Methods
2.1 Animals and housing conditions
Tas1r3 KO and Trpm5 KO mice were derived from parental stock produced by homologous recombination in C57BL/6J embryonic stem cells and maintained on this background [19,18]. The C57BL/6 WT mice were derived from parental stock obtained from the Jackson Laboratories (Bar Harbor, ME). Because mice from all three strains had virtually identical genetic backgrounds, we treated the responses of the WT strain as “normal” and inferred how deletion of Tas1r3 or Trpm5 altered these responses. None of the mice had prior exposure to the sapid solutions. All mice were housed and tested individually in standard polycarbonate cages (27.5 × 17 × 12.5 cm) with Bed-O'Cobs™ bedding (Andersons; Maumee, OH) and Nestlets™ cotton pads (Ancare; Bellmore, NY). Mice obtained water and test solutions through sipper spouts (with a 1.5 mm hole; Ancare, Belmore, NY) attached to bottles that were placed on the wire cage-top. The housing facilities had automatically controlled temperature, humidity and lighting (12 h:12 h light:dark cycle). All mice were offered laboratory chow (Rat Diet 5012; PMI Nutrition, Brentwood, MO) and tap water ad libitum throughout the experiments. According to the manufacturer, the chow had a physiological fuel value of 3.43 kcal/g. All experimental protocols were approved by the Institutional Animal Care and Use Committees at Columbia University and Brooklyn College, and were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Approximately equal numbers of adult males and females from each strain were assigned randomly to each treatment group, beginning at 7–9 weeks of age (see Tables S1 and S4 for sample sizes). To confirm that the mice did not differ systematically in initial body weight across strain or dietary treatment, we ran a two-way ANOVA, separately for each experiment. For Experiment 1, there was no significant main effect of strain (F2,115 = 1.8, P > 0.05) or diet treatment (F2,115 = 1.5, P > 0.05); further the interaction of strain × diet treatment was non-significant (F4,115 = 1.4, P > 0.05). The mean (± S.E.) initial weights (in g) on the control, sucrose and Polycose diets were, in respective order: 21.0 ± 0.6, 21.9 ± 0.8 and 21.9 ± 1.0 for the WT mice; 21.5 ± 0.5, 22.6 ± 1.0 and 21.7 ± 0.8 for the Tas1r3 KO mice; and 20.1 ± 1.5, 19.3 ± 0.7 and 22.5 ± 0.9 for the Trpm5 KO mice. Likewise, for Experiment 2, there was no significant main effect of strain (F1,36 = 0.6, P > 0.05) or diet treatment (F1,36 = 0.4, P > 0.05); further the interaction of strain × diet treatment was also non-significant (F1,36 = 0.7, P > 0.05). The mean (± S.E.) initial weights (in g) on the Suc+IL and IL diets were, in respective order: 21.9 ± 0.7 and 20.8 ± 0.9 for the WT mice; and 21.8 ± 0.8 and 21.8 ± 0.8 for the Tas1r3 KO mice.
2.2. Test solutions
The test solutions were prepared by dissolving sucrose (Domino Foods, Inc., Yonkers, NY), Polycose (Ross Laboratories, Columbus, OH), soybean oil emulsion (20% Intralipid™; Baxter; Dearfield, IL) or sodium saccharin (Sigma-Aldrich, St. Louis, MO) in deionized water. All solutions were presented at room temperature, and were replaced with fresh solutions every two days.
For the phenotypic screening, the test solution was 10 mM saccharin. We selected the 10 mM concentration because WT mice, but not Tas1r3 KO or Trpm5 KO mice, strongly prefer it over water [19,18,58,78]. For Experiment 1, we used 34.2% (1.0 M) sucrose and 34.2% (0.34 M) Polycose; both solutions had a caloric density of 1.19 kcal/g. We chose these solutions because (a) ad libitum access to 32–34.2% sucrose or Polycose causes diet-induced obesity in rodents [25,54]; (b) 32–34.2% sucrose solution elicits vigorous taste-mediated licking responses in WT, but not Tas1r3 KO or Trpm5 KO mice [19,68,78]; and (c) 32% Polycose solutions cause vigorous taste-mediated licking responses in WT and Tas1r3 KO mice [68,78]. For Experiment 2, we used 1% Intralipid (IL, 0.105 kcal/g) and a mixture of 34.2% sucrose plus 1% IL (Suc+IL, 1.295 kcal/g). The 1% IL emulsion was prepared by diluting the 20% IL with deionized water. The caloric density of the 1% IL solution was 0.1 kcal/g, while that of the sucrose + IL solution was 1.295 kcal/g. We selected these solutions because the Tas1r3 KO and WT mice avidly consume IL [24,59].
2.3. Phenotypic screen for saccharin preference
We subjected mice from the two experiments to a 24-h two-bottle test with 10 mM saccharin vs. water; the test was conducted for two days with the position of the bottles alternated each day. Saccharin solution percent preferences were calculated as follows: 100 × [saccharin intake / (saccharin + water intake)]. A preference approaching 100% indicates that the mice strongly preferred the saccharin solution; a preference approaching 50% indicates that they were indifferent to it; and a preference approaching 0% indicates that they strongly avoided it.
We analyzed saccharin preferences (following arcsine transformation) in two ways. First, we compared preference scores across strains, using one-way ANOVA and Tukey post hoc tests. In this and all subsequent statistical tests, we set the alpha level at 0.05. Second, we asked whether any strain was attracted to (i.e., exhibited a preference significantly > 50%) or repelled by (i.e., exhibited a preference significantly < 50%) the saccharin solution, using one-sample t-tests.
2.4. Experiment 1: Do carbohydrates induce dietary obesity in WT, Tas1r3 KO and Trpm5 KO mice?
This experiment asked how deletion of Tas1r3 or Trpm5 influenced caloric intake, weight gain and adiposity on chow diets supplemented with a 34.2% sucrose or Polycose solution. To this end, male and female mice from the WT, Tas1r3 KO and Trpm5 KO strains were each assigned randomly to one of three treatment groups. All mice were given ad libitum access to lab chow and two sipper tubes. For the Control diet, both sipper tubes contained water. For the Sucrose diet, one sipper contained water and the other 34.2% sucrose. For the Polycose diet, one sipper contained water and the other 34.2% Polycose. The sipper tubes were positioned approximately 5 cm apart. To control for side preferences, the left-right position of each sipper tubes was alternated each day.
In this and the next experiment, body weight was measured on alternate days (to the nearest 0.1 g). Daily fluid intakes were measured (to the nearest 0.1 g) by recording the change in weight of the drinking bottles, using an electronic balance interfaced to a computer. Daily fluid spillage was estimated by recording the change in weight of bottles representing each fluid treatment in an empty cage. To correct for fluid spillage, we subtracted the estimated spill from the quantity consumed over the 24 hr test.
Testing was conducted at two sites: Barnard College and Brooklyn College. While we standardized the testing conditions at both sites in most respects (e.g., diet, caging, bedding, temperature and day-length), we used different systems for presenting lab chow and measuring its consumption. At Brooklyn College, we presented chow in a customized food hopper, which captured the vast majority of spilled diet in a trough. To estimate chow consumption, we weighed the food hoppers every two days, and assumed that the loss in weight reflected intake. At Barnard College, we presented chow in the wire cage-top. Chow consumption was measured one day a week (i.e., 5 times over the 38-day experiment). On each measurement day, a mouse was given approximately 7 g of chow pellets, and the change in weight of the pellets over the subsequent 24 hr was measured. To recover any diet that fell into the cage as the mice ate, we sifted the cage bedding each day by passing it through two sieves; the first had a grid size of 2.3 × 2.3 mm and the second a grid size of 1.5 × 1.5 mm. The diet recovered from the sifting was subtracted from the daily intake for each mouse (range = 0.1 to 1.6 g/day). To determine whether the two systems for presenting and measuring intake of chow influenced the results, we ran redundant experiments with control and some experimental treatments at both sites. Because the mean weight gains and chow intakes of analogous control and experimental groups at the two sites were statistically indistinguishable (P > 0.05), we pooled results across both sites.
At the end of this and the next experiment, we euthanized each mouse, measured its weight and body length (nose-anus; to nearest 1 mm), excised the gonadal, retroperitoneal, omental and mesenteric fat depots, and measured the wet weight of each (to the nearest 0.001 g). We focused on these fat depots because they together constitute a large proportion of total body fat, can be removed easily, and respond to high-calorie diets in mice [72]. The retroperitoneal depot (in males and females) consisted of fat associated with the perirenal capsule and the dorsal body wall near the kidneys. In cases where an adrenal gland was embedded in the fat tissue, it was removed. The gonadal depot consisted of fat associated with the epididymis and vesicular gland in males, and the ovary, fallopian tubes and uterus in females. The mesenteric depot consisted of fat associated with the omental membrane and the mesentery along the duodenum, jejunum, and ileum. The mesentery was carefully stripped off the small intestine as a continuous piece; no attempt was made to dissociate fat from the mesenteric tissue. We combined the weights of the omental and mesenteric fat depots, and henceforth refer to them collectively as the “mesenteric” fat depot. We standardized the fat depot extractions across mice by having the same person remove the fat depots from all mice, under a blind protocol.
2.4.1. Data Analyses
We used ANOVAs to examine effect of diet, strain and sex on each of three dependent measures: daily caloric intake averaged over 38 days, weight gain over 38 days, and terminal adiposity. We examined adiposity by measuring the final wet weight of each of three depots: gonadal, retroperitoneal and mesenteric. Finally, we examined percent preference for each carbohydrate solution over water during the initial four days of the experiment. To this end, we tested for an effect of strain and time, using a mixed-model ANOVA and one-sample t-tests; all preference scores were subjected to arcsine transformation. All statistics in this and the next experiment were conducted with IBM SPSS Statistics (version 20). We set the alpha level for all tests at 0.05.
2.5. Experiment 2: Does increasing the palatability of the Sucrose diet cause obesity in Tas1r3 KO mice?
In Experiment 1, the Tas1r3 KO mice did not develop diet-induced obesity on the Sucrose diet. Here, we asked whether the addition of a highly palatable (but dilute) concentration of Intralipid to the 34.2% sucrose solution would cause the Tas1r3 KO mice to develop diet-induced obesity.
We tested both Tas1r3 KO and WT mice. Trpm5 KO mice were not tested because they are indifferent to dilute oil emulsions [58]. Mice from both strains were assigned randomly to one of two treatment diets. All mice were given daily access to lab chow and two sipper tubes. For the Control diet (i.e., IL diet), one sipper tube contained water and the other contained 1% IL. For the experimental diet (i.e., Suc+IL diet), one sipper tube contained water and the other contained a mixture of 34.2% sucrose plus 1% IL. Caloric intake, body weight gain, and adiposity were recorded as in Experiment 1.
3. Results
3.1. Saccharin phenotypic screen
The mean saccharin preferences differed significantly across strains (F5,327 = 611.9, P < 0.05), with the following relative magnitudes: WT (93.2%) > Trpm5 KO (45.3%) > Tas1r3 KO (33.5%). A one-sample t-test revealed that the saccharin solution was preferred by WT mice (t = 42.4, df = 74, P < 0.05), avoided by sweet-insensitive Tas1r3 KO mice (t = 8.9, df = 59, P < 0.05), and treated indifferently by sweet- and bitter-insensitive Trpm5 KO mice (t = 1.6, df = 28, P > 0.05). These results establish that the deletion of Tas1r3 and Trpm5 eliminated the preference for saccharin; and that the two KO strains had divergent responses to the taste of 10 mM saccharin.
3.2. Experiment 1: Impact of deleting Tas1r3 or Trpm5 on responses to the carbohydrate diets
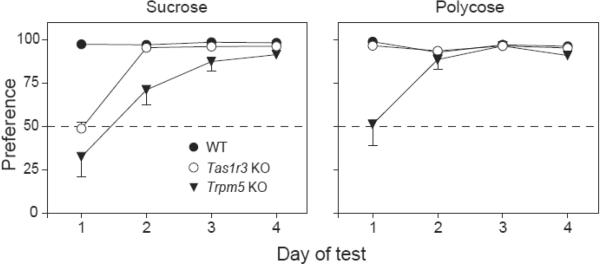
There were significant strain differences in sucrose and Polycose preferences across the initial four days of the experiment (Figure 1). With respect to sucrose, there was a strain × day interaction (F6,105 = 24.6, P < 0.05). Individual one-sample t-tests indicated that Tas1r3 KO and Trpm5 KO mice were indifferent to the sucrose solution on day 1 (i.e., they did not prefer the sucrose solution over water) and displayed lower (P < 0.05) preferences than did WT mice. On days 2–4, sucrose preference in Tas1r3 KO mice was comparable to that in WT mice and approached 100%; in contrast, Trpm5 KO mice displayed a reduced (P < 0.05) sucrose preference, relative to WT mice, on days 2 and 3, but not day 4. The strains also differed in their Polycose preference (strain × days interaction: F6,78 =13,2, P < 0.05), although in this case Trpm5 KO mice were indifferent to Polycose exclusively on day 1. All strains displayed Polycose preferences approaching 100% on days 2–4. On days 5–38 of the experiment, the KO and WT mice all continued to exhibit strong preferences for the sucrose and Polycose solutions.
Figure 1.
Preferences of WT, Tas1r3 KO and Trpm5 KO mice for (A) 34.2% sucrose or (B) 34.2% Polycose over water during the initial 4 days of Experiment 1. The dashed line in each panel indicates 50% preference (i.e., when the mouse consumed equal quantities of the carbohydrate solution and water). We present mean ± S.E. See text for statistical analyses of these data.
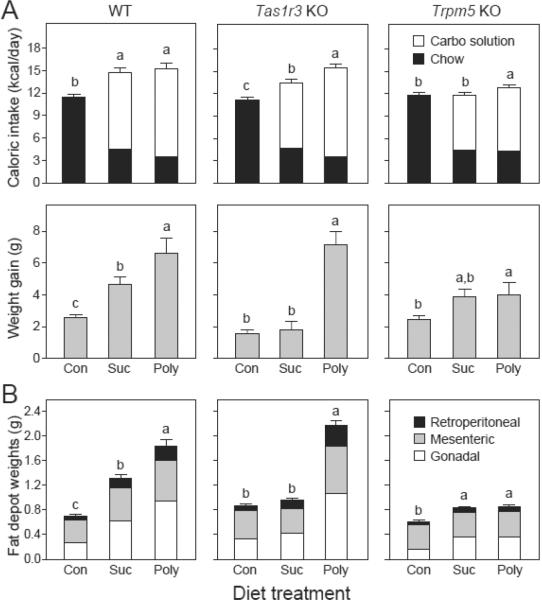
We used 3-way ANOVAs to examine the effects of diet, mouse strain and sex on daily caloric intake and weight gain. For daily caloric intake, there was a significant main effect of diet (F2,123 = 50.5, P < 0.001) and strain (F2,123 = 17.0, P < 0.001), but not of sex (F1,123 = 2.9, P > 0.05); the only significant interaction was diet × strain (F4,123 = 7.1, P < 0.001). These results reflect three notable findings. First, when offered the Sucrose or Polycose diet, all strains obtained the majority of their calories from the carbohydrate solution (Figure 2, top row of panels). Second, the WT and Tas1r3 KO strains obtained significantly more calories overall from the Sucrose and Polycose diets than from the Control diet. The Trpm5 KO mice, on the other hand, obtained significantly more calories from the Polycose than the Control diet, but the same number of calories from the Sucrose and Control diets. Third, across-strain analyses indicated that the three strains responded similarly to the Control diet (i.e., consumed similar quantities of chow), but differently to the carbohydrate diets (Table S1). On the Sucrose diet, WT mice consumed more sucrose solution than did Tas1r3 KO mice, which, in turn, consumed more than Trpm5 KO mice; chow intakes did not differ across strains. The total daily caloric intake of WT and Tas1r3 KO mice did not differ, but exceeded that of Trpm5 KO mice. On the Polycose diet, intakes of the Polycose solution by WT and Tas1r3 KO mice were similar, and exceeded those of Trpm5 KO mice; chow intakes did not differ across strains. The daily caloric intake of WT and Tas1r3 KO mice on the Polycose diet was the same, but exceeded that of Trpm5 KO mice. Taken together, these findings indicate that genetic deletion of Trpm5, but not Tas1r3 diminished daily caloric intake from the Sucrose and Polycose diets.
Figure 2.
Comparison of how the WT (left column of panels), Tas1r3 KO (middle column of panels) and Trpm5 KO (right column of panels) mice responded to the Control (Con), Sucrose (Suc) and Polycose (Poly) diets in Experiment 1. The composition of each diet was as follows: Con (chow and water), Suc (chow, water and a 34.2% sucrose solution) and Poly (i.e., chow, water and a 34.2% Polycose solution). In A, we show daily caloric intake and weight gain, both of which were calculated across the 38-day experiment. For daily caloric intake, we distinguish between calories obtained from the carbohydrate solution and chow. In each panel, we compare responses across diets with a Tukey HSD post hoc test; bars with different letters atop differ significantly from one another (P < 0.05). In B, we show wet weights of the gonadal, mesenteric and retroperitoneal fat depots at the end of Experiment 1; the weight weights of each depot are stacked upon each other for a given strain and diet. In each panel, we compare the total weight of all three fat depots diets with a Tukey HSD post hoc test; bars with different letters atop differ significantly from one another (P < 0.05). For the Control, Sucrose and Polycose diets, we tested (in respective order) 28, 18 and 9 WT mice; 20, 10 and 10 Tas1r3 KO mice; and 9, 10 and 10 Trpm5 KO mice. We present mean ± S.E.
For weight gain, there were significant main effects of diet (F2,123 > 50.5, P < 0.001), strain (F2,123 > 17.0, P < 0.001) and sex (F1,123 > 8.0, P > 0.05); furthermore, the two-way interactions were all significant (P < 0.05), but the three-way interaction was not. In WT mice, the Sucrose diet induced greater weight gain than did the Control diet, and the Polycose diet, in turn, induced greater weight gain than did the Sucrose diet (Figure 2, middle row of panels). In Tas1r3 KO mice, the Sucrose diet failed to induce greater weight gain than the Control diet, even though it stimulated greater caloric intake. However, the Polycose diet induced dramatically higher weight gain than did both the Control and Sucrose diets. In Trpm5 KO mice, there was a trend for the Sucrose diet to induce greater weight gain than the Control diet, but the difference was not significant. The Polycose diet, however, induced significantly more weight gain than did the Control diet. Between strain analyses indicated that WT and Trpm5 KO mice gained more weight than the Tas1r3 KO mice on the Control and Sucrose diets (Table S1). In contrast, the WT and Tas1r3 KO mice gained substantially more weight on the Polycose diet than did the Trpm5 KO mice.
The carbohydrate-induced weight gains covaried with the final fat depot weights across the three diets (Figure 2, bottom row of panels), indicating that the weight gains were associated with increased fat accumulation. Between-strain comparisons, however, revealed a dissociation between weight gain and fat depot weight. In particular, the Trpm5 KO mice gained more weight on the Control and Sucrose diets than did the Tas1r3 KO mice, but did not have heavier fat depots (Table S1). There were no significant effects of diet treatment on body length in any of the mouse strains (in all one-way ANOVAs, P > 0.05).
Although the three fat depots were generally larger in the mice on the carbohydrate diets, there were some notable strain differences (Table S2). For WT mice, the relative size of the gonadal fat depot across diets (Polycose diet > Sucrose diet > Control diet) differed from that of the mesenteric and retroperitoneal fat depots (Polycose diet = Sucrose diet > Control diet). For Tas1r3 KO mice, the relative size of all three fat depots across diets was uniform (i.e., Polycose diet > Sucrose diet = Control diet). For Trpm5 KO mice, consumption of the carbohydrate diets increased the relative size of the gonadal and retroperitoneal fat depots, but not of the mesenteric fat depot.
To explore the specific contribution of sex to daily caloric intake and weight gain, we compared male and females, separately for each strain and diet (Table S3). However, we observed only two significant sex differences: (a) WT males exhibited higher weight gain than WT females on the Sucrose and Polycose diets; and (b) Tas1r3 KO males displayed higher weight gains than Tas1r3 KO females on the Polycose diet. Because sex differences were the exception rather than the rule, we pooled the sexes for the statistical analyses described previously.
Taken together, these results indicate that genetic deletion of Tas1r3 impaired weight gain on the Sucrose but not the Polycose diet; and that genetic deletion of Trpm5 impaired weight gain on both the Sucrose and Polycose diets.
3.3. Experiment 2: Does increasing the palatability of the Sucrose diet cause obesity in Tas1r3 KO mice?
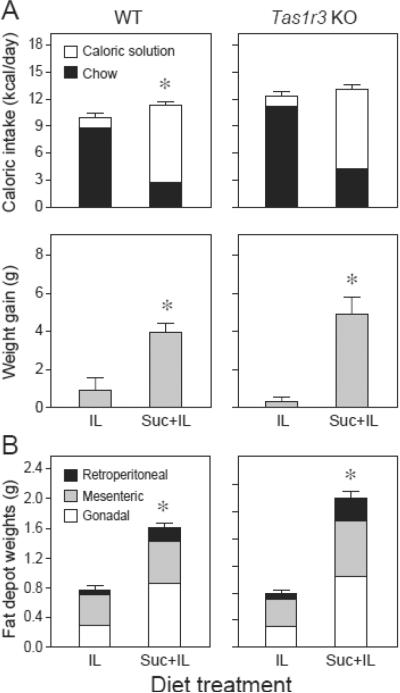
The WT and Tas1r3 KO mice both preferred the Suc+IL solution over water (> 80%) from the first day of the experiment (data not shown). Likewise, the WT and Tas1r3 KO mice also preferred the IL solution over water (> 95%) from the first experimental day (data not shown). These results establish that the addition of 1% IL to the sucrose solution or water alone rendered the solutions highly palatable to Tas1r3 KO and WT mice.
We used 3-way ANOVAs to examine the effects of diet, mouse strain and sex on each of the dependent measures. For daily caloric intake, there was a significant main effect of diet (F1,39 = 6.8, P < 0.02) and strain (F1,39= 32.4, P < 0.001), but not of sex (F1,39 = 3.3, P > 0.05); none of the interactions were significant (in all cases, P > 0.05). For weight gain, there was a significant main effect of diet (in both cases, F1,39 > 57.4, P < 0.001) and sex (in both cases, F1,39 > 8.1, P < 0.001), but not of strain (in both cases, F1,39 < 0.5, P > 0.05); the only three-way interactions was significant (in both cases, F1,39 > 8.3, P < 0.01), but the 2-way interactions were not (in all cases, P > 0.05).
There were three notable findings for daily caloric intake (Figure 3, top row of panels). First, when offered the Suc+IL diet, both strains obtained the majority of their calories from the caloric solution (Figure 3, top row of panels). Second, the WT (but not the Tas1r3 KO) mice ingested significantly more calories overall from the Suc+IL diet than from the IL diet. Third, the Tas1r3 KO mice consumed significantly more calories from both the IL and Suc+IL diets than did the WT mice from the corresponding diets (in both t-test comparisons, P < 0.05, Tukey HSD test). The higher caloric intake of the Tas1r3 KO mice reflected higher intake from the chow (not the caloric solution) in both diets (Table S4).
Figure 3.
Comparison of how the WT (left column of panels) and Tas1r3 KO (right column of panels) mice responded to the IL (chow, water and a 1% IL solution) and Suc+IL (chow, water, and a 1% IL + 34.2% sucrose solution) diets in Experiment 2. In A, we show daily caloric intake and weight gain, both of which were calculated across the 38-day experiment. For daily caloric intake, we distinguish between calories obtained from the caloric solution and chow. In each panel, we compare responses across diets with an unpaired t-test (* P < 0.05). In B, we show wet weights of the gonadal, mesenteric and retroperitoneal fat depots at the end of Experiment 2; the weight weights of each depot are stacked upon each other for a given strain and diet. In each panel, we compare the total weight of all three fat depots diets with an unpaired t-test (* P < 0.05). N = 10 per strain and diet treatment. We show mean ± S.E.
Both strains exhibited similarly robust weight gains (i.e., 4–5 g) on the Suc+IL diet (Figure 3, middle row of panels). In contrast, neither strain gained weight on the IL diet (i.e., weight gains were not significantly greater than 0). In WT mice, the lack of weight gain could be due in part to the fact that they consumed fewer calories from the IL diet than did the WT mice from the Control diet in Experiment 1 (10.8 vs. 11.7 kcal/day; t-value = 3.82, df = 36, P < 0.001). In Tas1r3 KO mice, the lack of weight gain cannot be attributed to low caloric intake. This is because they consumed more calories from the IL diet than from the Control diet in Experiment 1 (12.5 vs. 11.3 kcal/day, respectively, t-value = 2.62, df = 28, P < 0.02), but nevertheless gained less weight. Both the WT and Tas1r3 KO and mice also consumed more total fluid (IL + water) from the IL diet than did either strain from the Control diet in Experiment 1 (for WT mice, 11.1 vs. 4.5 g/day; t-value = 6.6, df = 36, P < 0.001; for Tas1r3 KO mice: 11.3 vs. 4.2 g/day; t-value = 5.9, df = 28, P < 0.001). Taken together, these results reveal that (a) weight gain (in both strains) was impaired by the IL diet, but not by the Suc+IL diet; and (b) that addition of 1% IL to the 34.2% sucrose solution not only rendered it highly palatable to the Tas1r3 KO mice, but it also permitted them to experience normal diet-induced obesity relative to WT mice.
The weight of the fat depots in Tas1r3 KO mice did not differ significantly from those of the WT mice on the Suc+IL or IL diets (Figure 3, bottom row of panels), even though they consumed more calories from both diets. Further, there were no significant strain differences in total weight gain or final fat mass (Table S4).
In Figure 3 (bottom row of panels) we distinguish the final wet weights of three fat depots (gonadal, mesenteric and retroperitoneal). We ran a three-way mixed-model ANOVA on these data, using fat depot as a within factor and strain and diet as between factors. There was a significant main effect of fat depot (F2,36 = 62.5, P < 0.001), diet (F1,36 = 25.0, P < 0.001), but not of strain (F1,36 = 0.7, P > 0.05); further the interaction of diet × strain was non-significant (F1,36 = 1.1, P > 0.05). These ANOVA results, together with the results in Figure 3, reveal that all three fat depots were heavier in mice offered the Suc+IL than the IL diet, and that both strains had statistically indistinguishable amounts of fat on each of the corresponding diets.
To explore the specific effect of sex on daily caloric intake and weight gain, we compared male and females, separately for each strain and diet (Table S5). We observed only one significant sex difference: Tas1r3 KO males accumulated more weight than Tas1r3 KO females on the Suc+IL diet. As in Experiment 1, because sex differences were the exception rather than the rule, we pooled the sexes for the statistical analyses described above.
4. Discussion
4.1. Preferences for saccharin, carbohydrate and Intralipid in Tas1r3 KO and Trpm5 KO mice
Previous studies have established that genetic deletion of Tas1r3 or Trpm5 eliminates the preference for saccharin in mice [19,18,58,77] and our data corroborate this finding. In fact, the Tas1r3 KO mice avoided 10 mM saccharin. This observation is consistent with prior results and is attributed to their aversion to the bitter taste component of saccharin [9,79]. The Trpm5 KO mice, on the other hand, were indifferent to saccharin, which is explained by the fact that Trpm5 deletion impairs both sweet and bitter taste signaling [19,75],
Although Trpm5 KO mice were initially indifferent to the 34.2% sucrose and Polycose solutions on day 1 of testing, they rapidly developed a strong preference for them in Experiment 1. This agrees with earlier findings, showing that Trpm5 KO mice have an impaired taste response to sucrose and Polycose, but learn to prefer these carbohydrates based on post-oral nutritional feedback [19,20,60,58,75]. In contrast, Tas1r3 KO mice were indifferent to sucrose, but strongly preferred Polycose on day 1, and then preferred both carbohydrates thereafter. The initial Polycose preference of Tas1r3 KO mice is consistent with recent findings that the taste of Polycose is not mediated by the T1r3 (or T1r2) receptor [68,69,78]. It is notable, however, that even after the Tas1r3 and Trpm5 KO mice acquired a preference for sucrose, and the Trpm5 KO mice acquired a preference for Polycose, the feeding and weight gain responses of these mice to the these carbohydrates remained substantially impaired as discussed below.
We also found that the WT and Tas1r3 KO mice strongly preferred both the IL and the Suc+IL solutions over water on day 1 and across all subsequent days of Experiment 2. This confirms earlier reports that the taste of 1% IL is not attenuated in mice missing the T1r3 component of the sweet taste receptor [59].
4.2. How did knocking out Tas1r3 impact overeating and obesity?
Tas1r3 KO mice exhibited reduced weight gain and adiposity (relative to WT mice) on both the Control and Sucrose diets. Yet, Tas1r3 KO strongly preferred sucrose to water (after day 1), and obtained more calories from the Sucrose diet than from the Control diet. Because the Tas1r3 KO mice did not differ from WT mice in their intake of the Control and Sucrose diets, the most parsimonious explanation for their reduced weight gain on these diets is reduced nutrient utilization. This could have stemmed from the reduced expression of SGLT1 protein in Tas1r3 KO mice [42]. For instance, a recent study found that dietary gum arabic diminished SGLT1 expression, glucose absorption and glucose-induced weight gain, but not glucose solution intake in B6 mice [46]. Likewise, cats experience reduced glucose absorption because they do not express T1r3 [11,38]. However, the fact that Tas1r3 KO mice gained weight normally on the Polycose diet argues against an important contribution of glucose malabsorption to their low weight gain on the Sucrose diet. This is because Polycose is digested and absorbed as free glucose.
The observation that the Tas1r3 KO mice did not differ from WT mice in their obesity response to the Polycose diet was unexpected for two reasons. First, in an earlier study, Tas1r3 KO mice consumed fewer calories from a 32% Polycose solution than did WT mice during a two-day test [78]. However, while the 38-day Polycose intake of the Tas1r3 KO mice was similar to that of the WT mice, during the first two study days they consumed significantly less Polycose calories than did the WT mice (7.2 vs. 8.1 kcal/day, respectively; t = 2.7, df = 17, P < 0.05). This establishes that, with sufficient time, the Tas1r3 KO mice were able to overcome their initial Polycose intake deficit. The second reason we expected Tas1r3 KO mice to underconsume the Polycose diet (relative to WT mice) is that T1r3 is necessary for (i) sugar-activated up-regulation of SGLT1 glucose transporters in the small intestine [41,42,62], (ii) GLP-1 release from duodenal L cells [33], and (iii) normal glucose tolerance [45]. To explain the apparent contradiction between carbohydrate processing by Tas1r3 KO mice in the present study and prior studies, it is important to keep in mind the significant methodological differences. In particular, prior studies were based on acute experiments with either intact mice or extracted tissue samples. In contrast, the mice in the present study were allowed to ingest the carbohydrates ad libitum over a 38-day period. This long-time period may have allowed the animals to adapt to any post-oral deficit in carbohydrate processing.
We can propose three non-mutually exclusive explanations for why Tas1r3 KO mice became obese on the Polycose diet, but not on the Sucrose diet. One is based on the fact that Polycose is a glucose polymer while sucrose is a disaccharide of glucose and fructose. There is evidence that glucose-stimulated insulin release in beta cells is potentiated by fructose, and that this potentiation is mediated by the T1r sweet taste receptor [34]. Accordingly, the absence of T1r3 protein may have disrupted post-absorptive processing of sucrose.
A second explanation stems from the observation that Tas1r3 KO mice show impaired taste responses to sucrose, but not to Polycose [18,68,77,78]. This was confirmed by their indifference to sucrose and strong preference for Polycose on the first day of Experiment 1. Yet, Tas1r3 KO mice quickly overcame their sweet taste deficit and by day 2 strongly preferred sucrose to water. This latter observation can be attributed to a learned preference for Tas1r3-independent flavor components (e.g., odor or texture) of the sucrose solution, reinforced by its post-oral nutritive effects [59,77–79]. While the post-oral effects of sugars can condition preferences for hedonically neutral or even aversive solutions [55], they stimulate more robust consumption when the flavored solution is highly palatable [57]. This may explain why, despite conditioning a preference for the sucrose solution, the Tas1r3 KO mice nevertheless consumed significantly fewer calories from the sucrose diet than the Polycose diet. Further, the percentage of total daily calories obtained from the sucrose solution (65%) was less than that obtained form the Polycose solution (76%).
The third explanation for why Tas1r3 KO mice became obese on the Polycose diet is based on the findings that oral simulation with a variety of palatable sweeteners—fructose [5,67], glucose [6,26,61,67], sucrose [67], maltose [5] and saccharin [6,66]—elicits cephalic phase insulin release (CPIR) in rats, and that an increase in the magnitude of the CPIR has been reported to promote diet-induced obesity in rats [7]. Accordingly, the highly palatable Polycose solution could have induced a CPIR in Tas1r3 KO mice, and thereby promoted post-absorptive processing of the ingested carbohydrates and fats [50]. In contrast, because severely attenuating the peripheral taste response to sugars prevents a CPIR [61], the post-absorptive processing of sucrose may have been impaired in Tas1r3 KO mice, leading to impaired nutrient utilization. This CPIR hypothesis could explain the higher weight gain of Tas1r3 KO mice on the Polycose diet, and the lower weight gain on the Sucrose diet. We should also not that, in addition to insulin, oral exposure to palatable stimuli can trigger the release of saliva, gastric acid, pancreatic exocrine enzymes, and glucagon [63], all of which might have contributed to the observed findings.
In Experiment 2, we tested a logical prediction of the sweet aguesia hypothesis. If Tas1r3 KO mice consumed less sucrose solution and gained less weight than WT mice on the Sucrose diet because of their sweet ageusia, then adding a calorically insignificant but highly palatable concentration of Intralipid to the sucrose solution should increase the efficiency of utilization of ingested sucrose, and promote obesity. We found strong support for this prediction. When offered the Suc+IL diet, the Tas1r3 KO mice consumed the same amount of Suc+IL solution and accumulated the same amounts of weight (and fat) as the WT mice (Table S4). We believe that this effect was mediated by an interaction between IL and sucrose because Tas1r3 KO mice on the IL or Sucrose diets gained almost no weight. Given that fats bind to taste receptors other than T1r3 [13,35], the addition of 1% IL to the sucrose solution could have activated cephalic-phase responses and thereby enhanced utilization of the sucrose calories. In support of this possibility, oral stimulation with fatty acids has been reported to elicit insulin release in humans ([14]; but see [16]), and a cephalic-phase increase in the protein content of pancreatobiliary secretions in B6 mice [35].
There are two CPIR-independent mechanisms by which the Intralipid in the Suc+IL diet could have augmented sucrose utilization in the Tas1r3 mice. One stems from the observation that Tas1r3 KO mice have an attenuated incretin response to sugars [33]. Given that both oral and post-oral stimulation by fats or free fatty acids can stimulate incretin release [3,22,49,74], the Intralipid in the Suc+IL diet could have elicited incretin release. Another mechanism is based on the observation that small quantities of ingested fats can increase hepatic insulin sensitivity in rats [70]. If the Intralipid in the Suc+IL diet enhanced insulin sensitivity in Tas1r3 KO mice, it may have improved their utilization of the sucrose. More work is needed to assess the relative contribution of these mechanisms to the Suc+IL-induced dietary obesity in Tas1r3 KO mice.
Unexpectedly, the WT and Tas1r3 KO mice on the IL diet in Experiment 2 gained less weight than did the same strains of mice on the Control diet in Experiment 1. Conceivably, the dilute soybean oil emulsion may have activated negative feedback mechanisms that inhibited caloric intake and thereby retarded weight gain [56]. Indeed, WT mice on the IL diet consumed fewer calories than did WT mice on the Control diet in Experiment 1. This explanation is contradicted, however, by the observation that the Tas1r3 KO mice consumed more energy from the IL diet than from the Control diet in Experiment 1, but nevertheless failed to gain weight. A more likely explanation stems from the fact that the Tas1r3 KO and WT mice on the IL diet consumed more than twice as much total fluid (IL + water) as the same strains of mice on the Control diet in Experiment. This excess fluid intake may have interfered with nutrient absorption.
4.3. How did knocking out Trpm5 impact overeating and obesity?
The effect of knocking out Trpm5 on diet-induced obesity was quite distinct from that of knocking out Tas1r3. Indeed, the Trpm5 KO mice gained less weight and/or fat than did WT mice on both the Polycose and Sucrose diets. The lower adiposity of Trpm5 KO mice stems in part from a loss of responsiveness of the mesenteric fat depot to carbohydrate intake (Table S3). It also stems from the fact that Trpm5 KO mice consumed significantly less of the carbohydrate solutions than WT mice (Table S1), which is consistent with their impaired taste response to sucrose and Polycose [19,58,75]. The attenuated growth of Trpm5 KO mice on the carbohydrate diets (relative to WT mice) may also reflect inefficient utilization. Indeed, there are two reports of impaired glucose tolerance and insulin release in Trpm5 KO mice [10,15]. However, two observations point to a limited role of Trpm5 in post-oral processing of carbohydrates: (a) caloric intake and weight (and fat) gain on the Control diet were all statistically indistinguishable between Trpm5 KO and WT mice (Table S1); and (b) Trpm5 KO mice show a normal conditioning response to the post-oral nutritive actions of sugar [20,60].
4.4. Sex differences in carbohydrate-induced obesity
In a study of fat-induced obesity in B6 mice, Nishikawa et al. [48] reported that males become more obese than females. Here, we obtained analogous findings with WT mice on high carbohydrate diets—i.e., male mice gained significantly more weight than female mice on the Sucrose and Polycose diets (Table S3). However, the sex difference in diet-induced obesity was not observed on all diets and in all strains. For instance, when WT mice were offered the Suc+IL diet, both sexes became similarly obese (Table S5). This observation indicates that the diet composition contributes to the sexually dimorphic response. Furthermore, Tas1r3 KO and Trpm5 KO mice differed in expression of the sexually dimorphic obesity response. For example, male Tas1r3 KO mice gained significantly more weight than female Tas1r3 KO mice on the two diets that caused extensive weight gain (i.e., the Polycose and Suc+IL diets). In contrast, male and female Trpm5 KO mice experienced a similar degree of diet-induced obesity on the Polycose and Sucrose diets.
Sex differences in diet-induced obesity are thought to stem from differential sensitivity of the brain to adiposity hormones (e.g., insulin and leptin) [73]. Additional work is needed to provide a hormonal explanation for why male WT mice became disproportionately obese on the Polycose and Sucrose diets, but not on the Suc+IL diet. Likewise, it would be valuable to explain why Trpm5 KO mice failed to exhibit any sex differences in diet-induced obesity.
4.5. Conclusion
The present findings indicate that T1r3 and Trpm5 play critical roles in carbohydrate-induced dietary obesity. Tas1r3 KO and Trpm5 KO mice, which are not inherently attracted to sucrose, showed little or no excess weight gain when their diet was supplemented with a sucrose solution. Even though the KO mice rapidly learned to prefer the sucrose solution based on its post-oral actions, this learned preference was not sufficient to promote obesity. In contrast, when Tas1r3 KO mice were offered diets supplemented with carbohydrate solutions to which they were inherently attracted (i.e., the Polycose and Suc+IL solutions), they became obese.
Unexpectedly, the diet-induced obesity appeared to be due in large part to increases in carbohydrate utilization not caloric intake. This is illustrated most clearly in Tas1r3 KO mice, which exhibited elevated caloric intake but no excess weight gain on the Sucrose diet compared to WT and Trpm5 KO mice. However, when offered the highly palatable Suc+IL solution, the Tas1r3 KO gained a significant amount of weight although their caloric intake was not elevated.
The fact that Tas1r3 KO mice exhibited high weight gain on the Polycose and Suc+IL diets indicates that T1r3 signaling is not necessary for efficient carbohydrate metabolism. Conceivably, palatability-driven cephalic responses to Polycose or IL in the mouth may promote carbohydrate processing via Tas1r3-independent chemosensory pathways. In addition, gut sensors of Polycose or IL may affect carbohydrate absorption via regulation of SGLT1. Development of tissue-specific knock-out mice would greatly enhance our ability to assess the relative contribution of taste signaling proteins in the mouth versus gut to carbohydrate-induced obesity.
Highlights
T1r3 and Trpm5 play critical roles in carbohydrate-induced dietary obesity.
T1r3 knock-out (KO) and Trpm5 KO mice, which are not inherently attracted to sucrose, showed little or no excess weight when their diet was supplemented with a sucrose solution.
When T1r3 KO mice were offered diets supplemented with carbohydrate solutions to which they were inherently attracted (i.e., the Polycose or sucrose+dilute Intralipid solutions), they became obese.
The diet-induced obesity appeared to be due largely to increases in carbohydrate utilization, not caloric intake.
The fact that T1r3 KO mice exhibited high weight gain on the Polycose and sucrose+dilute Intralipid diets demonstrates that T1r3 signaling is not necessary for efficient carbohydrate metabolism.
Acknowledgments
We thank Chaya Goodman and Mohammed Riad for their technical assistance, and Gary Schwartz for sage advice. This research was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK-31135) to AS, the National Institute on Deafness and Other Communication Disorders (DC03055 and DC03155) to RFM, and the Amgen Foundation to Barnard College and Columbia University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allison DB, Mattes RD. Nutritively sweetened beverage consumption and obesity: the need for solid evidence on a fluid issue. JAMA. 2009;301:318–320. doi: 10.1001/jama.2008.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almind K, Manieri M, Sivitz WI, Cinti S, Kahn CR. Ectopic brown adipose tissue in muscle provides a mechanism for differences in risk of metabolic syndrome in mice. PNAS. 2007;104:2366–2371. doi: 10.1073/pnas.0610416104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroentrol. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 4.Bermudez OI, Gao X. Greater consumption of sweetened beverages and added sugars is associated with obesity among US young adults. Ann Nutr Metab. 2010;57:211–218. doi: 10.1159/000321542. [DOI] [PubMed] [Google Scholar]
- 5.Berridge K, Grill HJ, Norgren R. Relation of consummatory responses and preabsorptive insulin release to palatability and learned taste aversions. J Comp Physiol Psychol. 1981;95:363–382. doi: 10.1037/h0077782. [DOI] [PubMed] [Google Scholar]
- 6.Berthoud H-R, Trimble ER, Siegel EG, Bereiter DA, Jeanrenaud B. Cephalic-phase insulin secretion in normal and pancreatic islet-transplanted rats. Am J Physiol. 1980;238:E336–E340. doi: 10.1152/ajpendo.1980.238.4.E336. [DOI] [PubMed] [Google Scholar]
- 7.Berthoud H-R, Bereiter DA, Trimble ER, Siegel EG, Jeanrenaud B. Cephalic phase, reflex insulin secretion: neuroanatomical and physiological characterization. Diabetol. 1981;20:393–401. [PubMed] [Google Scholar]
- 8.Bezençon C, le Coutre J, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses. 2007;32:41–49. doi: 10.1093/chemse/bjl034. [DOI] [PubMed] [Google Scholar]
- 9.Blednov YA, Walker D, Martinez M, Levine M, Damak S, Margolskee RF. Perception of sweet taste is important for voluntary alcohol consumption in mice. Genes Brain Behav. 2008:1–13. doi: 10.1111/j.1601-183X.2007.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brixel LR, Monteilh-Zoller MK, Ingenbrandt CS, Fleig A, Penner R, Enklaar T, Zabel BU, Prawitt D. TRPM5 regulates glucose-stimulated insulin secretion. Pflugers Arch - Eur J Physiol. 2010;460:69–76. doi: 10.1007/s00424-010-0835-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buddington RK, Chen JW, Diamond JM. Dietary regulation of intestinal brush-border sugar and amino acid transport in carnivores. Am J Physiol. 1991;261:R793–R801. doi: 10.1152/ajpregu.1991.261.4.R793. [DOI] [PubMed] [Google Scholar]
- 12.Cao D, Lu H, Lewis TL, Li L. Intake of sucrose-sweetened water induces insulin resistance and exacerbates memory deficits and amyloidosis in a transgenic mouse model of Alzheimer disease. J Biol Chem. 2007;282:36275–36282. doi: 10.1074/jbc.M703561200. [DOI] [PubMed] [Google Scholar]
- 13.Cartoni C, Yasumatsu K, Ohkuri T, Shigemura N, Yoshida R, Godinot N, le Coutre J, Ninomiya Y, Damak S. Taste preference for fatty acids is mediated by GPR40 and GPR120. J Neurosci. 2010;30:8376–8382. doi: 10.1523/JNEUROSCI.0496-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chavez-Jauregui RN, Mattes RD, Parks EJ. Dynamics of fat absorption and effect of sham feeding on postprandial lipema. Gastroenterol. 2010;139:1538–1548. doi: 10.1053/j.gastro.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colsoul B, Schraenenb A, Lemaireb K, Quintensb R, Van Lommelb L, Segala A, Owsianika G, Talaveraa K, Voetsa T, Margolskeec RF, Kokrashvilic Z, Gilond P, Niliusa B, Schuitb FC, Vennekensa R. Loss of high-frequency glucose-induced Ca2+ oscillations in pancreatic islets correlates with impaired glucose tolerance in Trpm5−/− mice. PNAS. 2010;107:5208–5213. doi: 10.1073/pnas.0913107107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crystal SR, Teff KL. Tasting fat: cephalic phase hormonal responses and food intake in restrained and unrestrained eaters. Physiol Behav. 2006;89:213–220. doi: 10.1016/j.physbeh.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 17.Cui M, Jiang P, Maillet E, Max M, Margolskee RF, Osman R. The heterodimeric sweet taste receptor has multiple potential ligand binding sites. Curr Pharm Design. 2006;12:4591–4600. doi: 10.2174/138161206779010350. [DOI] [PubMed] [Google Scholar]
- 18.Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1R3. Science. 2003;301:850–853. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- 19.Damak S, Rong M, Yasumatsu K, Kokrashvili K, Pérez CA, Shigemura N, Yoshida R, Mosinger B, Jr., Glendinning JI, Ninomiya Y, Margolskee RF. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses. 2006;31:253–264. doi: 10.1093/chemse/bjj027. [DOI] [PubMed] [Google Scholar]
- 20.de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MAL, Simon SA. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 21.Drewnowski A. Obesity and the food environment: dietary energy density and diet costs. Am J Prev Med. 2004;27(3S):154–162. doi: 10.1016/j.amepre.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes. 2008;57:2280–2287. doi: 10.2337/db08-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forshee RA, Anderson PA, Storey ML. Sugar-sweetened beverages and body mass index in children and adolescents: a metaanalysis. Am J Clin Nutr. 2008;87:1662–1671. doi: 10.1093/ajcn/87.6.1662. [DOI] [PubMed] [Google Scholar]
- 24.Glendinning JI, Feld N, Goodman L, Bayor R. Contribution of orosensory stimulation to strain differences in oil intake by mice. Physiol Behav. 2008;95:476–483. doi: 10.1016/j.physbeh.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 25.Glendinning JI, Breinager L, Kyrillou E, Lacuna K, Rocha R, Sclafani A. Differential effects of sucrose and fructose on dietary obesity in four mouse strains. Physiol Behav. 2010;101:331–343. doi: 10.1016/j.physbeh.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grill HJ, Berridge KC, Ganster DJ. Oral glucose is the prime elicitor of preabsorptive insulin secretion. Am J Physiol. 1984;246:R88–R95. doi: 10.1152/ajpregu.1984.246.1.R88. [DOI] [PubMed] [Google Scholar]
- 27.Hesse D, Dunn M, Heldmaier G, Klingenspor M, Rozman J. Behavioural mechanisms affecting energy regulation in mice prone or resistant to diet- induced obesity. Physiol Behav. 2010;99:370–380. doi: 10.1016/j.physbeh.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Hu FB, Malik VS. Sugar-sweetened beverages and risk of obesity and type 2 diabetes: epidemiologic evidence. Physiol Behav. 2010;100:47–54. doi: 10.1016/j.physbeh.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jang H-J, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim B-J, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, Bernier M, Mosinger B, Margolskee RF, Egan JM. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. PNAS. 2007;104:15069–15074. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jürgens H, Haass W, Castaneda TR, Schürmann A, Koebnick C, Dombrowski F, Otto B, Nawrocki AR, Scherer PE, Spranger J, Ristow M, Joost HG, Havel PJ, Tschöp MH. Consuming fructose-sweetened beverages increases body adiposity in mice. Obes Res. 2005;13:1146–1156. doi: 10.1038/oby.2005.136. [DOI] [PubMed] [Google Scholar]
- 31.Kanarek RB, Orthen-Gambill N. Differential effects of sucrose, fructose and glucose on carbohydrate-induced obesity in rats. J Nutr. 1982;112:1546–1554. doi: 10.1093/jn/112.8.1546. [DOI] [PubMed] [Google Scholar]
- 32.Kojima I, Nakagawa Y. The role of the sweet taste receptor in enteroendocrine cells and pancreatic β-cells. Diabetes Metab J. 2011;35:451–457. doi: 10.4093/dmj.2011.35.5.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kokrashvili Z, Mosinger B, Margolskee RF. T1r3 and α-gustducin in gut regulate secretion of glucagon-like peptide-1. Ann NY Acad Sci. 2009;1170:91–94. doi: 10.1111/j.1749-6632.2009.04485.x. [DOI] [PubMed] [Google Scholar]
- 34.Kyriazis GA, Soundarapandian MM, Tyrberg B. PNAS. 2012. Sweet taste receptor signaling in beta cells mediates fructose-induced potentiation of glucose-stimulated insulin secretion. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, Montmayeur J-P, Besnard P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest. 2005;115:3177–3184. doi: 10.1172/JCI25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Gall M, Tobin V, Stolarczyk E, Dalet V, Leturque A, Brot-Laroche E. Sugar sensing by enterocytes combines polarity, membrane bound detectors and sugar metabolism. J Cell Physiol. 2007;213:834–843. doi: 10.1002/jcp.21245. [DOI] [PubMed] [Google Scholar]
- 37.Lenard NR, Berthoud H-R. Central and peripheral regulation of food intake and physical activity: pathways and genes. Obesity. 2008;16(Supplement 3):S11–S22. doi: 10.1038/oby.2008.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X, Li W, Wang H, Cao J, Maehashi K, Huang L, Bachmanov AA, Reed DR, Legrand-Defretin V, Beauchamp GK, Brand JG. Pseudogenization of a sweet-receptor gene accounts for cats' indifference toward sugar. PLoS Genet. 2005;1:e3. doi: 10.1371/journal.pgen.0010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Light HR, Tsanzi E, Gigliotti J, Morgan K, Tou JC. The type of caloric sweetener added to water influences weight gain, fat mass, and reproduction in growing Sprague-Dawley female rats. Exp Biol Med. 2009;234:651–661. doi: 10.3181/0812-RM-368. [DOI] [PubMed] [Google Scholar]
- 40.Lindqvist A, Baelemans A, Erlanson-Albertsson C. Effects of sucrose, glucose and fructose on peripheral and central appetite signals. Regul Peptides. 2008;150:26–32. doi: 10.1016/j.regpep.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 41.Mace OJ, Affleck J, Nick Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol. 2007;582:379–392. doi: 10.1113/jphysiol.2007.130906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Margolskee RF, Dyer J, Kokrashvili Z, Salmon KSH, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter. PNAS. 2007;104:15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marks-Kaufman R, Hamm MW, Barbato GF. The effects of dietary sucrose on opiate receptor binding in genetically obese (ob/ob) and lean mice. J Am College Nutr. 1989;8:9–14. doi: 10.1080/07315724.1989.10720272. [DOI] [PubMed] [Google Scholar]
- 44.Mucci L, Santilli F, Cuccurullo C, Davi G. Cardiovascular risk and dietary sugar intake: is the link so sweet? Intern Emerg Med. 2012 doi: 10.1007/s11739-011-0606-7. In press. [DOI] [PubMed] [Google Scholar]
- 45.Nakagawa Y, Nagasawa M, Yamada S, Hara A, Mogami H, Nikolaev VO, Lohse MJ, Shigemura N, Ninomiya Y, Kojima I. Sweet taste receptor expressed in pancreatic b-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLoS ONE. 2009;4:e5106. doi: 10.1371/journal.pone.0005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nasir O, Artunc F, Wang K, Rexhepaj R, Föller M, Ebrahim A, Kempe DS, Biswas R, Madhuri Bhandaru M, Michael Walter M, Mohebbi N, Wagner CA, Saeed AM, Lang F. Downregulation of mouse intestinal Na+-coupled glucose transporter SGLT1 by gum arabic (Acacia senegal) Cell Physiol Biochem. 2010;25:203–210. doi: 10.1159/000276554. [DOI] [PubMed] [Google Scholar]
- 47.Nelson G, Hoon MA, Chadrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 48.Nishikawa S, Yasoshima A, Doi K, Nakayama H, Uetsuka K. Involvement of sex, strain and age factors in high fat diet-induced obesity in C57BL/6J and BALB/cA mice. Exp Anim. 2007;56:263–272. doi: 10.1538/expanim.56.263. [DOI] [PubMed] [Google Scholar]
- 49.Parker HE, Habib AM, Rogers GJ, Gribble FM, Reimann F. Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetol. 2009;52:289–298. doi: 10.1007/s00125-008-1202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Picard F, Naïmi N, Richard D, Deshaies Y. Response of adipose tissue lipoprotein lipase to the cephalic phase of insulin secretion. Diabetes. 1999;48:452–459. doi: 10.2337/diabetes.48.3.452. [DOI] [PubMed] [Google Scholar]
- 51.Prawitt D, Monteilh-Zoller MK, Brixel L, Spangenberg C, Zabel B, Fleig A, Penner R. TRPM5 is a transient Ca2+-activated cation channel responding to rapid changes in [Ca2+]i. Proc Natl Acad Sci USA. 2003;100:15166–15171. doi: 10.1073/pnas.2334624100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruxton CHS, Garnder EJ, McNulty HM. Is sugar consumption detrimental to health? A review of the evidence 1995–2006. Crit Rev Food Sci Nutr. 2010;50:1–19. doi: 10.1080/10408390802248569. [DOI] [PubMed] [Google Scholar]
- 53.Sclafani A, Springer D. Dietary obesity in adult rats: similarities to hypothalamic and human obesity syndromes. Physiol Behav. 1974;17:461–471. doi: 10.1016/0031-9384(76)90109-8. [DOI] [PubMed] [Google Scholar]
- 54.Sclafani A. Carbohydrate-induced hyperphagia and obesity in the rat: effects of saccharide type, form, and taste. Neurosci Biobehav Rev. 1987;11:155–162. doi: 10.1016/s0149-7634(87)80020-9. [DOI] [PubMed] [Google Scholar]
- 55.Sclafani A, Lucas F, Ackroff K. The importance of taste and palatability in carbohydrate-induced overeating in rats. Am J Physiol. 1996;270:R1197–R1202. doi: 10.1152/ajpregu.1996.270.6.R1197. [DOI] [PubMed] [Google Scholar]
- 56.Sclafani A. Oral and postoral determinants of food reward. Physiol Behav. 2004;81:773–779. doi: 10.1016/j.physbeh.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 57.Sclafani A, Glendinning JI. Sugar and fat conditioned flavor preferences in C57BL/6J and 129 mice: oral and postoral interactions. Am J Physiol. 2005;289:R712–R720. doi: 10.1152/ajpregu.00176.2005. [DOI] [PubMed] [Google Scholar]
- 58.Sclafani A, Zukerman S, Glendinning JI, Margolskee RF. Fat and carbohydrate preferences in mice: the contribution of alpha-gustducin and Trpm5 taste-signaling proteins. Am J Physiol. 2007;293:R1504–R1513. doi: 10.1152/ajpregu.00364.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sclafani A, Glass DS, Margolskee RF, Glendinning JI. Gut T1R3 sweet taste receptors do not mediate sucrose-conditioned flavor preferences in mice. Am J Physiol. 2010;299:R1643–R1650. doi: 10.1152/ajpregu.00495.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sclafani A, Ackroff K. The role of gut nutrient sensing in stimulating appetite and conditioning food preferences. Am J Physiol. 2012;302:R1119–R1133. doi: 10.1152/ajpregu.00038.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shinozaki K, Shimizu Y, Shiina T, Morita H, Takewaki T. Relationship between taste-induced physiological reflexes and temperature of sweet taste. Physiol Behav. 2008;93:1000–1004. doi: 10.1016/j.physbeh.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 62.Shirazi-Beechey SP, Moran AW, Batchelor DJ, Daly K, Al-Rammahi M. Session 3: Influences of food constituents on gut health. Glucose sensing and signalling; regulation of intestinal glucose transport. Proc Nutr Soc. 2011;70:185–193. doi: 10.1017/S0029665111000103. [DOI] [PubMed] [Google Scholar]
- 63.Smeets PAM, Erkner A, de Graaf C. Cephalic phase responses and appetite. Nutri Rev. 2010;68:643–655. doi: 10.1111/j.1753-4887.2010.00334.x. [DOI] [PubMed] [Google Scholar]
- 64.Steinert RE, Gerspach AC, Gutmann H, Asarian L, Drewe J, Beglinger C. The functional involvement of gut-expressed sweet taste receptors in glucose-stimulated secretion of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) Clin Nutr. 2011;30:524–532. doi: 10.1016/j.clnu.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 65.Takeda M, Imaizumi M, Sawano S, Manabe Y, Fushiki T. Long-term optional ingestion of corn oil induces excessive caloric intake and obesity in mice. Nutrition. 2001;17:117–120. doi: 10.1016/s0899-9007(00)00513-x. [DOI] [PubMed] [Google Scholar]
- 66.Tonosaki K, Hori Y, Shimizu Y, Tonosaki K. Relationship between insulin release and taste. Biomed Res. 2007;28:79–83. doi: 10.2220/biomedres.28.79. [DOI] [PubMed] [Google Scholar]
- 67.Tonosaki K. Relationships between cephalic phase insulin release and taste modalities. In: Zafra MA, Molina F, Puerto A, editors. The cephalic/neural phase in nutrition. Research Signpost; Kerala, India: 2009. pp. 83–92. [Google Scholar]
- 68.Treesukosol Y, Blonde GD, Spector AC. T1R2 and T1R3 subunits are individually unnecessary for normal affective licking responses to Polycose: implications for saccharide taste receptors in mice. Am J Physiol. 2009;296:R855–R865. doi: 10.1152/ajpregu.90869.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Treesukosol Y, Smith KR, Spector AC. Behavioral evidence for a glucose polymer taste receptor that is independent of the T1R2+3 heterodimer in a mouse model. J Neuroscience. 2011;31:13527–13534. doi: 10.1523/JNEUROSCI.2179-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang PYT, Caspi L, Lam CKL, Chari M, Li X, Light PE, Gutierrez-Juarez R, Ang M, Schwartz GJ, Lam TKT. Upper intestinal lipids trigger a gut–brain–liver axis to regulate glucose production. Nature. 2008;452:1012–1016. doi: 10.1038/nature06852. [DOI] [PubMed] [Google Scholar]
- 71.Warwick ZS, Weingarten HP. Determinants of high-fat diet hyperphagia: experimental dissection of orosensory and postingestive effects. Am J Physiol. 1995;269:R30–R37. doi: 10.1152/ajpregu.1995.269.1.R30. [DOI] [PubMed] [Google Scholar]
- 72.West DB, Boozer CN, Moody DL, Atkinson RL. Dietary obesity in nine inbred mouse strains. Am J Physiol. 1992;262:R1025–R1032. doi: 10.1152/ajpregu.1992.262.6.R1025. [DOI] [PubMed] [Google Scholar]
- 73.Woods SC, Gotoh K, Clegg DJ. Gender differences in the control of energy homeostasis. Exp Biol Med. 2003;228:1175–1180. doi: 10.1177/153537020322801012. [DOI] [PubMed] [Google Scholar]
- 74.Yoder SM, Yang Q, L. Kindel TL, Tso P. Stimulation of incretin secretion by dietary lipid: is it dose dependent? Am J Physiol. 2009;297:G299–G305. doi: 10.1152/ajpgi.90601.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 76.Zhang Z, Zhao Z, Margolskee RF, Liman E. The transduction channel TRPM5 is gated by intracellular calcium in taste cells. J Neurosci. 2007;27:5777–5786. doi: 10.1523/JNEUROSCI.4973-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJP, Zuker CS. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- 78.Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. T1R3 taste receptor is critical for sucrose but not Polycose taste. Am J Physiol. 2009;296:R866–R876. doi: 10.1152/ajpregu.90870.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zukerman S, Touzani K, Margolskee RF, Sclafani A. Role of olfaction in the conditioned sucrose preference of sweet-ageusic T1R3 knockout mice. Chem Senses. 2009;34:685–694. doi: 10.1093/chemse/bjp055. [DOI] [PMC free article] [PubMed] [Google Scholar]