Abstract
Functions of adrenal medulla, particularly synthesis of catecholamine, are under the control of glucocorticoids. To further investigate whether development/differentiation of the adrenal medulla is associated with proper organization of the adrenal cortex, we examined development of the medulla in four different mouse models with various defects in the adrenal cortex. By using the Sf1/Cre mouse line that inactivates/activates genes in Steroidogenic factor 1 (SF1)-positive cells of the fetal adrenal cortex, we produced mice that exhibit either 1) cortex hypoplasia, 2) progressive degeneration of fetal adrenal cortex, 3) cortex dysgenesis, or 4) cortex-medulla disorganization. The formation of phenylethanolamine N-methyltransferase (PNMT)-positive medulla in all models indicating that differentiation of adrenal medulla is independent of the growth of adrenal cortex. However, the misplaced/dysgenic medulla which is outside of adrenal proper, indicating that the beta-catenin pathway in the adrenal cortical cells plays an indirect role in controlling proper organization of the adrenal medulla.
Keywords: adrenal cortex, adrenal medulla, beta-catenin, Sonic hedgehog, Dicer1
1. Introduction
The adrenal cortex and medulla develop from two distinct origins: the coelomic epithelium for the adrenal cortex and the neural crest for the medulla. Cells in the coelomic epithelium proliferate and give rise to the adrenogonadal primordium, the primitive organs for adrenal and gonads. In the mouse embryos, the adrenogonadal primordium starts to express SF1 at approximately 9.0 days post coitum or dpc in the mouse embryos (Ikeda et al., 1994). SF1 marks the steroidogenic precursors, which later become adrenal cortex and somatic cells in the gonads. The adrenogonadal primordium separates into adrenal and gonads between 11.5–12.5 dpc and immediately after the separation, neural crest cells migrate into the growing adrenal and form the medulla. Around 14.5 dpc, the adrenal is encapsulated by a layer of mesenchyme-derived tissue and distinct cortex and medulla are established at 16.5 dpc (Keegan and Hammer, 2002). By the time of birth, establishment of three cortical zones (zona glomerulosa, zona fesciculata and zona reticularis) and medulla are nearly completed. Defects in prenatal adrenal development often lead to adrenal hypoplasia or aplasia, which is life threatening. Mutations of genes such as Sf1, Pbxl, CBP/p300-interacting transactivator with ED-rich tail 2 (Cited2) or Wilms tumorl (Wtl) have been linked to adrenal agenesis (Bamforth et al., 2001; Beuschlein et al., 2002; Else and Hammer, 2005; Kreidberg et al., 1993; Luo et al., 1994; Schnabel et al., 2003). Other signaling pathway including Sonic hedgehog (SHH) and Wingless (WNT) are also implicated in adrenal hypoplasia or aplasia (Ching and Vilain, 2009; Huang et al., 2010a; Huang et al., 2010b; Kim et al., 2008; King et al., 2009).
The major endocrine functions of adrenal gland are to produce catecholamines and steroids. Catecholamine, synthesized by the adrenal medulla, is responsible for blood pressure and blood flow regulation whereas steroids produced by the cortex controls energy and water homeostasis and immune responses. Glucocorticoids, a major group of adrenal steroids, have a stimulatory effect on catecholamine synthesis in the medulla. PNMT, the enzyme that converts norepinephrine to epinephrine, is activated by glucocorticoid in vitro and in vivo (Evinger et al., 1992; Wong et al., 1992). In hypophysectomized rats, the expression of PNMT in the adrenal medulla was significantly reduced (Wurtman, 1966; Wurtman and Axelrod, 1966). The reduced PNMT expression was restored by adrenocorticotropic hormone (ACTH) replacement or high dose of dexamethasone treatment, indicating glucocorticoids from the adjacent cortex are a major stimulator of PNMT expression (Wurtman, 1966). Based on the histological arrangement of medulla and cortex, steroids from the cortex possibly regulates PNMT production in the medulla by either a paracrine mechanism or through the local vascular system (Einer-Jensen and Carter, 1995). Despite of this particular aspect of the medulla differentiation, the role of adrenal cortex on adrenal medulla development and function remains unclear.
In this study, we examined whether formation and differentiation of the adrenal medulla is affected in the adrenal in four mouse models that developed different types of defects in the cortex. Defects in the adrenal cortex were induced by inactivating or activating of Shh, Dicer1, or β-catenin in the SF1-positive cortical cells.
2. Materials and methods
2.1 Animals
Detailed information of generating Sf1/Cre; Shhf/- (Shh KO), Sf1/Cre; Dicer1f/f (Dicer1 KO) and Sf1/Cre; Ctnnb1f/- (β-catenin KO) mice has been published in our previous studies (Huang et al., 2010a; Huang et al., 2010b; Huang and Yao, 2010). Mice carrying floxed β-catenin activation allele were generated by Dr. T. Uomoto (University of Tokyo, Japan). Ctnnb1flox(exon3)/flox(exon3) mice were mated with Sf1/Cre transgenic mice to obtain the Sf1/Cre-mediated β-catenin activation mice. Female and male mice were paired together and checked for the presence of a vaginal plug the next morning. The day when the vaginal plug was detected was considered 0.5 day post coitum or dpc. The day when new born mice were found was considered P0. All procedures described were reviewed and approved by the Institutional Animal Care and Use Committee at University of Illinois and were performed in accordance with the Guiding Principles for the Care and Use of Laboratory Animals. All experiments were performed on at least three animals for each genotype.
2.2 Immunohistochemistry
Detailed method was indicated in our previous study (Bland et al., 2000). In short, the specimens were fixed in 4% paraformaldehyde/phosphate-buffered saline (PBS) at 4°C overnight, and embedded in paraffin following standard procedures for sectioning. Paraffin embedded sections were dewaxed and rehydrated in a series of alcohol/PBS gradient. The endogenous peroxidase activity was blocked by 3% H2O2 in methanol for 8 minutes and rinsed with PBS 3 times for 5 minutes each. Slides were pretreated in 0.1mM citrate acid for 20 minutes in the microwave. Tissue sections were treated with primary antibodies followed by appropriate secondary antibodies. For TH and PNMT double immunohistochemistry, TH was detected by biotinylated secondary antibody followed by fluorescence TSA kit (PerkinElmer, Waltham, MA); PNMT was detected by Cy3-conjugated secondary antibody. The primary antibodies used were anti-CYP11b1 (Dr. C. Gomez-Sanchez, University of Mississippi Medical Center, MS), anti-HSD3b (1:1000, kindly provided by Dr. K. Morohashi, National Institutes of Natural Sciences, Japan), anti-TH and anti-PNMT (1:1000, Millipore, Billerica, MA, USA).
2.3 Chromaffin reaction
Adrenals were fixed in Muller’s Fluid (0.1M K2Cr2O7, 30mM Na2SO4·10H2O) in 4% PFA for 48 hours and washed in running water overnight. For sections, stained tissues were embedded in paraffin following standard procedures. 15µm sections were dewaxed and mounted without counter stain.
3. Results
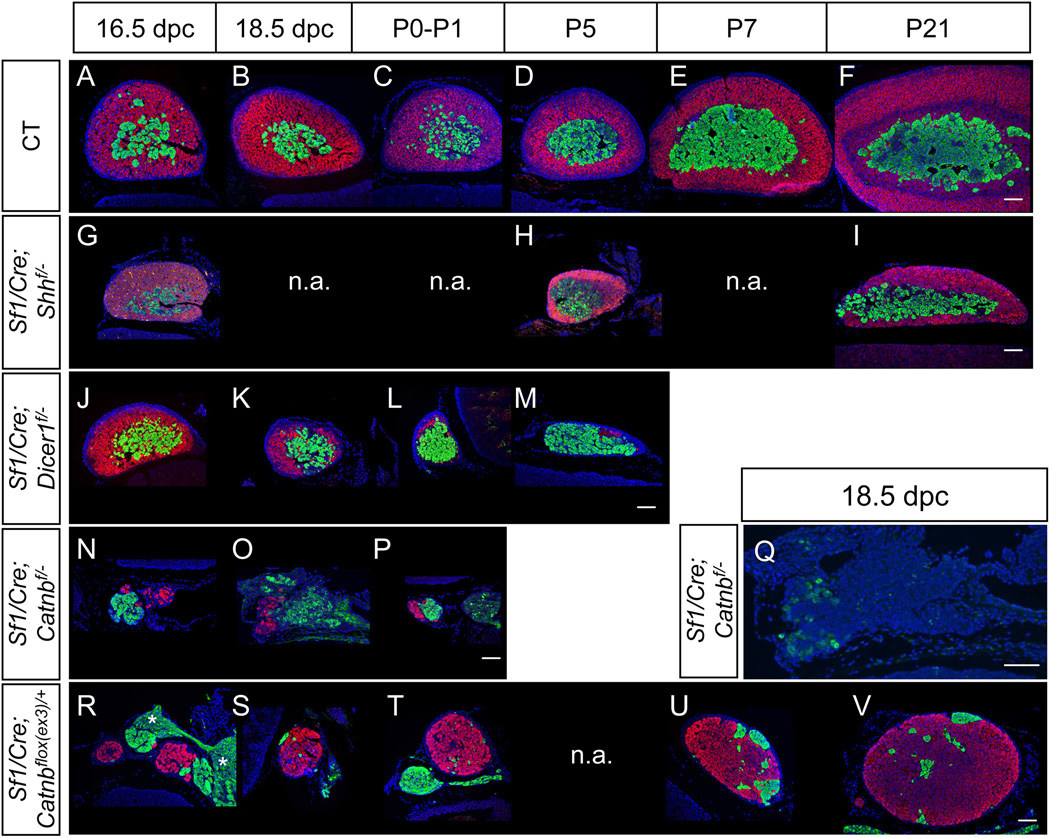
3.1 Model 1: Development of the medulla in the hypoplastic cortex in the Sf1/Cre-mediated Shh knockout adrenal
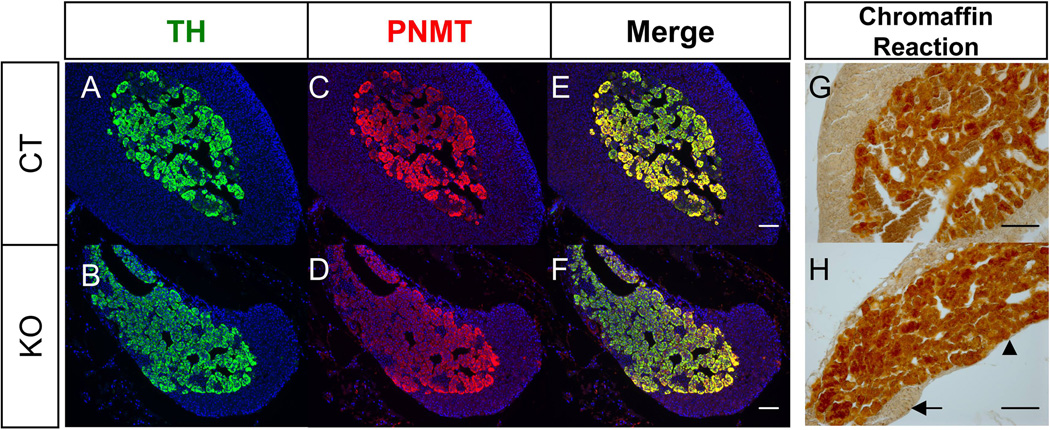
We and others have found that loss of Shh in the SF1-positive cells in the cortex resulted in a hypoplastic cortex due to decreased proliferation of cortical progenitor cells in the capsule (Fig. 1G, H, & I) (Ching and Vilain, 2009; Huang et al., 2010a; King et al., 2009). Despite previous cortical hypoplasia, the medulla was present in the adrenal of the Shh knockout mice (Huang et al., 2010a). In control adrenals from 16.5 dpc to 21 days after birth (P21), the 3 beta-hydroxysteroid dehydrogenase (HSD3b) positive adrenal cortex and the tyrosine hydroxylase (TH; a marker for the medulla)-positive medulla expanded over time (Fig. 1A-F). However in the Shh knockout mice, only the TH-positive medulla population increased over time but the growth of adrenal cortex was reduced (Fig. 1G-I). When the knockout animals reached adulthood (30 weeks), a part of the adrenal medulla was directly covered by the adrenal capsule rather than the cortex as a result of underdevelopment of the cortex (Fig. 2A-F). To further study whether the medulla cells in the hypoplastic adrenal are fully differentiated and functional, we performed double immunohistochemistry for PNMT and TH, markers for neural crest origin cells (Le Douarin and Dupin, 1993). Similar to the control adrenal, most of the TH-positive cells produced PNMT in the knockout adrenal at all stages we have examined (Fig. 2A-F). To test whether the under-developed cortex has a long-term impact on medulla functions, we performed the chromaffin reaction, which detects the presence of catecholamines, in the adrenals of aging knockout mice (98 weeks). The positive chromaffin reaction in the 98-weeks old adrenal indicates that the medulla in the hypoplastic cortex in the Shh knockout adrenal still had active catecholamine synthesis (Fig. 2G-H).
Figure 1. Models of adrenal cortex abnormality.
Adrenal glands from wild type (CT, A-F) or Sf1-Cre mediated Shh KO (G-I) or Dicer1 KO (J-M) or β-catenin KO (N-P) or β-catenin activation (R-V) were stained for immunofluorescent for HSD3b (magenta) and TH (green) or CYP11B1 (Q, in green). Asterisk symbols: ganglia. Blue: DAPI. Scale bars represent 100 µm. n.a., not analyzed.
Figure 2. Effects of cortical hypoplasia (Sf1/Cre-mediated Shh knockout) on medulla cell differentiation in the adrenal.
Fluorescent immunohistochemistry for TH (A & B) and PNMT (C & D) was performed on sections of 30 weeks old control (CT) and Shh conditional KO adrenals. (E) and (F) are merged images of TH and PNMT. Chromaffin reaction was performed on 98 weeks old adrenals (G & H). Arrow: cortex. Arrowhead: medulla. Blue: DAPI. Scale bars represent 100 µm.
3.2 Model 2: Progressive degeneration of adrenal cortex in Dicer1 knockout mice does not affect medulla cell differentiation
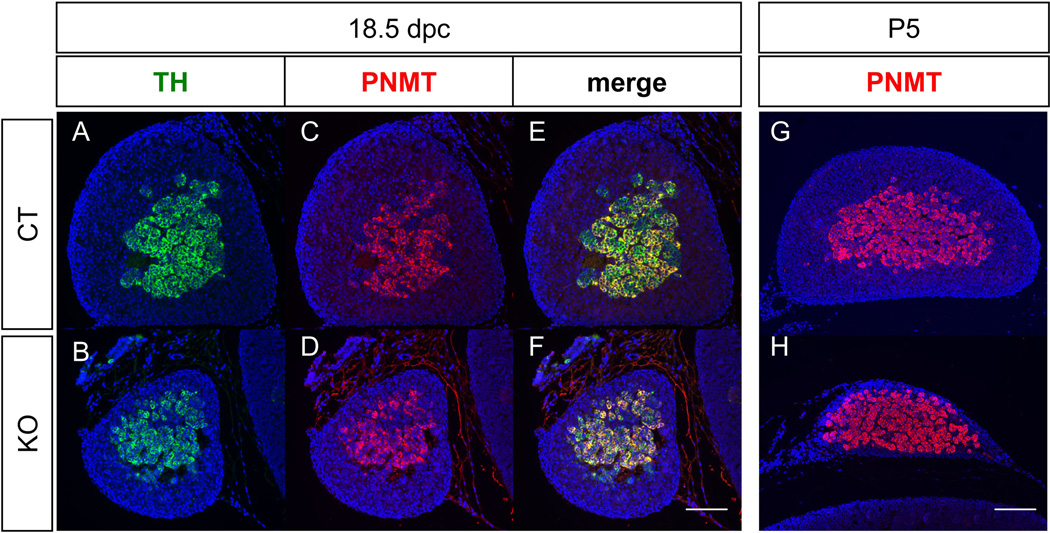
In the Sf1/Cre-mediated Dicer1 knockout mice, the increased apoptosis in the cortex resulted in progressive degeneration of adrenal cortex (Huang and Yao, 2010). We therefore used this model to test whether progressive degeneration of adrenal cortex has any impacts on medulla differentiation. Immunohistochemistry results showed that Dicer1 knockout mice developed and maintained their medulla (TH-positive cells) from 16.5dpc to P5 in spite of a degenerating cortex (HSD3b-positive in Fig. 1J-M). To further study whether the degeneration of adrenal cortex affects medullar cells differentiation, double immunohistochemistry was used to examine the expression of PNMT and TH in the Dicer1 knockout adrenal. At 18.5 dpc, similar to the control adrenal, most of the medulla cells in the knockout adrenal were positive for both TH and PNMT (Fig. 3A-F). Five days after birth, PNMT-positive medulla remained in the adrenal underneath the capsule (Fig. 3G-H). Due to the loss of functional cortex, Dicer1 knockout mice were not able to survive beyond D5 of age. The expression of PNMT in D5 Dicer1 knockout adrenal suggests that the progressive loss of adrenal cortex does not result in acute adrenal medulla defect.
Figure 3. Effects of progressive degeneration of cortex (Sf1/Cre-mediated Dicer1 knockout) on medulla cell differentiation in the adrenal.
Fluorescent immunohistochemistry for TH (A & B) and PNMT (C & D) was performed on sections of control and Dicer1 conditional KO adrenals at 18.5 dpc. (G) and (H) are merged images of TH and PNMT. Fluorescent immunohistochemistry for PNMT on P5 adrenals was also performed (G & H). Blue: DAPI. Scale bars represent 100 µm.
3.3 Model 3 and 4: Depletion of β-catenin in the SF1-positive cells results in cortex dysgenesis whereas ectopic activation of β-catenin in the SF1-positive cells causes cortex-medulla disorganization
Both Shh knockout and Dicer1 knockout mice formed normal adrenal cortex at early stages and the cortical defects did not became apparent at or after 16.5 dpc. Therefore these two models are not suitable to study the potential interaction between cortical cells and medulla cells at early developmental stages before 16.5 dpc. We and others had found that deletion of β-catenin in the cortical layer led to severe adrenal cortical dysgenesis before or at 16.5 dpc (Fig. 1N-P) (Huang et al., 2010b; Kim et al., 2008). The size of adrenals in the β-catenin knockout animals was reduced to 5–10% of the size of the control littermates at 16.5 dpc. By using immunohistochemistry for HSD3b to detect the adrenal cortical cells, we found that the number of cortical cells was dramatically reduced (Fig. 1N-P). Not only the adrenal cortex was affected in the β-catenin knockout adrenal, the population of TH-positive cells was also significantly reduced. The remaining cortex was positive for CYP11B1, indicating that the mutant adrenal cortex is capable to synthesize corticosterone (Fig. 1Q).
To activate β-catenin, we used the Sf1/Cre, which is active as early as at 10 dpc, to induce ectopic activation of the β-catenin pathway. In this model, the critical phosphorylation sites of β-catenin in the third exon are removed by the Cre-recombinase, preventing β-catenin from degradation and leading to constitutively active β-catenin signaling (Harada et al., 1999). Activation of β-catenin by Sf1/Cre did not result in adrenal hyperplasia at early stages. Instead, the size of adrenal in the Sf1-mediated β-catenin activation animals was reduced in fetal life (Fig. 1R & S). Despite the defects in the cortex, the medulla still formed. However, from 16.5 dpc to D1, the TH-positive cells were located outside of, rather than enclosed by, the cortical cells (Fig. 1R-T). Although the mutant adrenal was small at embryonic stages, the adrenal cortex progressively grew during development. At P21, the size of the β-catenin activation adrenal was 50% of the control littermates (Fig. 1U & V). Medulla cells were scattered in the cortex and did not cluster in the center of the cortex (Fig. 1U & V). At the age of 3 months, medulla cells were still scattered in the cortex and most of them were located in the periphery of the adrenal (Supplementary Figure 1). At the age of 10 months, the size of the β-catenin activation adrenal was still smaller than the control littermates (Supplementary Figure 1). No tumor formation was found at this stage.
3.4 Depletion or activation of β-catenin does not affect medulla differentiation
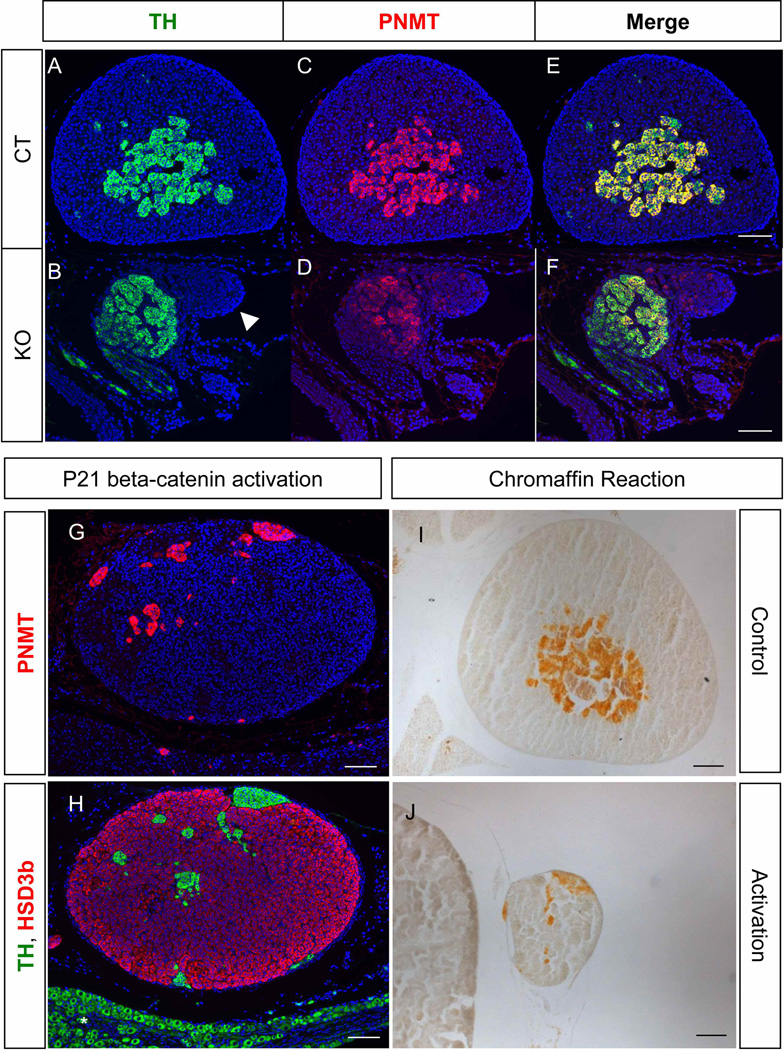
The striking defects of the adrenal cortex and disorganization of the medulla in the β-catenin mutants prompted us to examine whether the medulla differentiates properly. In the control adrenal, PNMT and TH double-positive medulla cells accumulated centripetally in the adrenal and were surrounded by adrenal cortical cells (Fig. 4A, C, & E). In the β-catenin knockout adrenal, clusters of TH and PNMT double positive chromaffin cells were present and attached to the remaining cortical cells (Fig. 4B, D, & F). On the other hand in the adrenal with ectopic activation of β-catenin in the cortex, TH-positive cells were not located in the center of the adrenal; instead, the medulla cells were separated into clusters and scattered throughout the adrenal cortex (Fig. 4G). The scattered TH-positive medulla cells were also PNMT-positive (Fig. 4H) and positive for chromaffin reaction (Fig. 4I & J), indicating that medulla cells were able to differentiate even though a medulla proper is not properly formed.
Figure 4. Effects of cortex dysgenesis (Sf1/Cre-mediated β-catenin knockout) or cortex disorganization (Sf1/Cre-mediated β-catenin activation) on medulla functions in the adrenal.
Fluorescent immunohistochemistry for TH (A & B) and PNMT (C & D) was performed on sections of β-catenin WT and KO adrenals at P0-P1. (E) and (F) are merged images of TH and PNMT. Fluorescent immunohistochemistry for PNMT (G, magenta) and TH (H, green) and HSD3b (H, magenta) was performed on sections of β-catenin activation adrenals at P21. Chromaffin reaction was performed on P21 adrenals (I & J). Asterisk symbols: ganglia. Blue: DAPI. Scale bars represent 100 µm.
4. Discussion
In this study, we used Sf1/Cre line to manipulate gene inactivation and activation in the SF1-positive cells in the fetal adrenal cortex. These various models developed unique adrenal cortex phenotypes (Table 1): Disruption of Shh or Dicer1 resulted in hypoplastic or degenerated adrenal cortex. β-catenin knockout caused adrenal cortex dysgenesis. Adrenals with ectopic activation of β-catenin developed disorganized cortex-medulla structure. In all these models, although the organization of the medulla was affected in various degrees, the initial formation and final differentiation of the medulla remained grossly intact with proper expression of TH and PNMT (Table 1). We therefore conclude that adrenal cortex is dispensable for most aspects of the initial formation and chromaffin cell differentiation of adrenal medulla. However, adrenocortical cells and/or signals from adrenal cortex are responsible for the growth of medulla cells for proper cortex-medulla organization.
Table 1.
Medulla Phenotypes in Mouse Models with Various Defects in Adrenal Cortex
| Model | Mutation (mediated by Sf1/Cre) |
Defects in the adrenal cortex | Medulla phenotypes | Medulla differentiation |
|---|---|---|---|---|
| 1 | Shh knockout | Hypoplasia (unable to surround the medulla) | Part of the medulla is directly covered by the capsule in adult mice | Positive |
| 2 | Dicer1 knockout | Progressive degeneration (resulting in almost no cortical cells at P5) | Medulla is directly covered by the capsule at P5 | Positive* |
| 3 | beta-catenin knockout | Dysgenesis (only few cortical cells remain) | Hypoplasia; Misplaced medulla; medulla is outside the adrenal proper | Positive* |
| 4 | beta-catenin activation | Hypoplasia (prenatal stages) Disorganization (postnatal stages) |
Hypoplasia; misplaced medulla; part of the medulla is directly covered by the capsule | Positive |
Medulla differentiation was accessed neonatally as the knockout animals did not survive beyond first week after birth.
Results from this study and by others indicate that migration of neural crest cells into the adrenal is probably not dependent upon the presence of adrenal cortex. The existence of neural crest cells within/around the adrenal proper in Sf1 knockout, Cited2 knockout or conditional GR knockout mice, demonstrating that the initial migration of neural crest cells still occurs despite a complete degeneration of the cortex or a loss of glucocorticoids signaling of chromaffin cells (Bland et al., 2004; Gut et al., 2005; Parlato et al., 2009; Val et al., 2007). However, the reduced medulla portion in these knockout models with adrenal cortex defects suggest that the adrenal cortex is essential for assembling the chromaffin cells to the correct site (Huber, 2006) and/or the growth/survival of chromaffin cells in the medulla (Parlato et al., 2009). In our dysgenic cortex model (Sf1/Cre-mediated β-catenin knockout mice) and cortical disorganization model (Sf1/Cre-mediated β-catenin activation mice), the reduced number of neural crest cells at embryonic stages further supports the idea that normal growth and/or survival of medullar cells depend on proper number of adrenocortical cells. It is possible that (1) adrenocortical cells may provide structural support for medulla development and/or (2) factors from the cortex that are under the control of signaling pathways such as beta-catenin may also involve in this developmental process. However, the unaffected medulla size in neonatal Sf1/Cre-mediated Dicer1 knockout adrenal suggest that signals/supports from the cortex is more critical at early developmental stages rather than at later stages. To further test whether adrenocortical cells are critical for medulla cells survival at later developmental stages, Sf1-mediated Dicer1 knockout mice were rescued by steroids administration up to 21 days old (data not shown). The Dicer1 knockout mice without steroids treatment did not survive after day 5 and the adrenal medulla was surrounded by capsule without adrenocortical portion at this stage. Steroids treatment was able to prolong the survival of Dicer1 knockout mice to 21 days after birth and the medulla of the adrenal was significantly reduced (data not shown). The result of this preliminary test suggests and supports a possible role of adrenal cortex on adrenal medulla survival (Kim et al., 2008).
The loss of β-catenin resulted in reduced proliferation and cortex dysgenesis and further caused medulla apoptosis at aged adrenals (Kim et al., 2008). In contrast, ectopic activation of β-catenin in the mouse adrenal cortex at later stages induced adrenal hyperplasia and promotes adrenal cancer development (Berthon et al., 2010). In this particular study (Berthon et al., 2010), β-catenin was activated at 14.5 dpc, which is 4 days later than the Sf1/Cre mediated one. This stage difference of β-catenin activation probably explains why their adrenals were normal at early stages and hyperplastic adrenals were found at 5 months after birth, rather than a hypoplastic adrenal with scattered medulla at early stages. Interestingly, in their tumorized adrenal at the age of 10 month old, TH positive cells became scarce in the adrenal. All these data suggests that loss or gain of function of β-catenin, no matter a dysgenic or tumorized cortex, all results in medulla loss. The spatial relationship of cortical cells with medullary cells and the role of cortical β-catenin in medullary cells are still unclear.
Differentiation of the adrenal medulla, specifically the production of PNMT, requires a steroidogenic cortex. PNMT apparently is under the control of glucocorticoids from the cortex based on the observation that mice lacking ability to produce or respond to glucocorticoids such as Cyp21 knockout, CRHR1 (corticotropin-releasing hormone receptor) knockout, Sf1 heterozygous and GR (glucocorticoids receptor) knockout, had decreased or absent PNMT expression in the medulla (Bland et al., 2000; Bornstein et al., 1999; Cole et al., 1995; Yoshida-Hiroi et al., 2002). Glucocorticoid responsive element is found upstream of Pnmt gene and is required for glucocorticoid-induced PNMT expression (Ross et al., 1990). All these data suggest that PNMT expression in medulla requires a relatively high level of glucocorticoid, which comes from the surrounding cortex. The Sf1/Cre-mediated β-catenin knockout mice had a hypoplastic adrenal cortical population with a hypoplastic medulla attached. The hypoplastic adrenal cortex that could not synthesize and secrete sufficient steroids for life maintenance may be one of the causes that lead to neonatal death of the β-catenin knockout mice. However, the affected cortex apparently produces enough of glucocorticoid locally to support medulla differentiation and function. In the Sf1/Cre-mediated Shh knockout mice, despite a significant reduction of the serum corticosterone level (Huang et al., 2010a), the medulla is still catecholaminergic and positive for TH and PNMT. All these findings are consistent with the hypothesis that locally produced glucocorticoid, rather than the circulating glucocorticoid, is responsible for PNMT expression in the medulla. Interestingly, even though PNMT is expressed in the medulla of these models, the population of medullar cells is still dramatically reduced.
In the adrenal, the blood supply enters from the cortex and flows inward. Cortical capillaries run through all three zones of cortex and then drain into the medulla vein (Murakami et al., 1989; Tokunaga, 1996). It is proposed that the glucocorticoid from the cortex were collected in the blood and go through the medulla. The glucocorticoid reaches medulla before entering the general circulation. The high concentration of glucocorticoid in the local blood supply therefore induces PNMT expression in the medulla. The histological structure of blood vessels in the adrenal explains how could endocrine hormones such as glucocorticoid elicit their function locally. However, results from the four models in our study challenge this “endocrine” theory of the stimulation of PNMT expression. In the β-catenin knockout adrenal, PNMT-positive medulla sits beside the cortex and was not covered by the cortex. In the aged Shh knockout adrenal, part of the PNMT-positive medulla was found directly underneath the capsule. In the β-catenin activation adrenal, medulla cells were not formed centripetally. Although the direction of the blood flow in the adrenal of these models has not been analyzed, the finding of PNMT-positive cells located directly underneath the capsule indicates that the de novo synthesized glucocorticoid could elicit its function on PNMT expression without a well organized cortex-medulla structure, further suggesting a possible paracrine signaling of glucocorticoid on medullary PNMT expression.
Other than medulla development, one unique phenotype was observed in two of the models that we generated. Knockout or ectopic activation of β-catenin both result in adrenal cortex dysgenesis at 16.5 dpc. The similar phenotype of loss- or gain-of-function of β-catenin suggests a properly balanced β-catenin activity in the SF1-positive cortical cells is critical for early adrenal development. β-catenin is essential for formation of the adrenal cortex development during early stages. Inactivation of β-catenin leads to loss of adrenal at birth, which is associated with decrease proliferation at early stages (Kim et al., 2008). Sf1 haploinsufficiency also impaired adrenal development and caused blunted compensatory cortex proliferation, indicating the importance of dosage of Sf1 genes in adrenal cortical cell proliferation and survival (Beuschlein et al., 2002; Bland et al., 2004). The synergic effect of β-catenin and SF1 on activation of α-inhibin promoter suggests that β-catenin and SF1 may regulate crucial target genes controlling cell proliferation through similar signaling cascade (Gummow et al., 2003). It is also possible that β-catenin and SF1 may have direct correlation in terms of expression.
In summary, we used four mouse models with various adrenocortical defects to study the role of adrenal cortex on medulla development. The reduced medulla portion or cortex-medulla disorganization in models with hypoplastic or dysgenic adrenal cortex suggests that signals from adrenal cortex may involve in controlling proper organization of the adrenal medulla. However, the presence of functional medulla in all cortex defected models suggests that a well-organized cortex-medulla structure is not crucial for differentiation of the medulla.
Highlights.
> We examine formation and differentiation of adrenal medulla in four mouse models with various defects in the cortex. > Defects in adrenal cortex do not significantly impact medulla differentiation. > We discover that properly tuned β-catenin pathway in the cortex is essential for organization of the medulla.
Supplementary Material
Acknowledgements
We thank Dr. T. Uomoto (University of Tokyo, Japan) for the Ctnnb1flox(exon3)/flox(exon3) mice, Dr. C. Gomez-Sanchez (University of Mississippi Medical Center, Jackson, MS) for the CYP11B1 antibody and Dr. K. Morohashi (National Institutes of Natural Sciences, Okazaki, Japan) for the HSD3b antibody. We also thank all the Yao laboratory members for their assistance and support.
Abbreviations
- ACTH
adrenocorticotropic hormone
- CYP
Cytochrome P450
- DAPI
4’,6-diamidino-2-phenylindole
- Dpc
days post coitum
- Hh
Hedgehog
- HSD
hydroxysteroid dehydrogenase
- KO
Knockout
- PNMT
Phenylethanolamine N-methyltransferase
- SF1
steroidogenic factor 1
- SHH
sonic hedgehog
- TH
tyrosine hydroxylase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors state no conflict of interest.
References
- Bamforth SD, et al. Cardiac malformations, adrenal agenesis, neural crest defects and exencephaly in mice lacking Cited2, a new Tfap2 co-activator. Nat Genet. 2001;29:469–474. doi: 10.1038/ng768. [DOI] [PubMed] [Google Scholar]
- Berthon A, et al. Constitutive beta-catenin activation induces adrenal hyperplasia and promotes adrenal cancer development. Hum Mol Genet. 2010;19:1561–1576. doi: 10.1093/hmg/ddq029. [DOI] [PubMed] [Google Scholar]
- Beuschlein F, et al. Steroidogenic factor-1 is essential for compensatory adrenal growth following unilateral adrenalectomy. Endocrinology. 2002;143:3122–3135. doi: 10.1210/endo.143.8.8944. [DOI] [PubMed] [Google Scholar]
- Bland ML, et al. Haploinsufficiency of steroidogenic factor-1 in mice disrupts adrenal development leading to an impaired stress response. Proc Natl Acad Sci USA. 2000;97:14488–14493. doi: 10.1073/pnas.97.26.14488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland ML, et al. Differential requirement for steroidogenic factor-1 gene dosage in adrenal development versus endocrine function. Mol Endocrinol. 2004;18:941–952. doi: 10.1210/me.2003-0333. [DOI] [PubMed] [Google Scholar]
- Bornstein SR, et al. Adrenomedullary function is severely impaired in 21-hydroxylase-deficient mice. Faseb J. 1999;13:1185–1194. doi: 10.1096/fasebj.13.10.1185. [DOI] [PubMed] [Google Scholar]
- Ching S, Vilain E. Targeted disruption of Sonic Hedgehog in the mouse adrenal leads to adrenocortical hypoplasia. Genesis. 2009;47:628–637. doi: 10.1002/dvg.20532. [DOI] [PubMed] [Google Scholar]
- Cole TJ, et al. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 1995;9:1608–1621. doi: 10.1101/gad.9.13.1608. [DOI] [PubMed] [Google Scholar]
- Einer-Jensen N, Carter AM. Local transfer of hormones between blood vessels within the adrenal gland may explain the functional interaction between the adrenal cortex and medulla. Med Hypotheses. 1995;44:471–474. doi: 10.1016/0306-9877(95)90508-1. [DOI] [PubMed] [Google Scholar]
- Else T, Hammer GD. Genetic analysis of adrenal absence: agenesis and aplasia. Trends Endocrinol Metab. 2005;16:458–468. doi: 10.1016/j.tem.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Evinger MJ, et al. Glucocorticoids stimulate transcription of the rat phenylethanolamine N-methyltransferase (PNMT) gene in vivo and in vitro. Cell Mol Neurobiol. 1992;12:193–215. doi: 10.1007/BF00712926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gummow BM, et al. Convergence of Wnt signaling and steroidogenic factor-1 (SF-1) on transcription of the rat inhibin alpha gene. J Biol Chem. 2003;278:26572–26579. doi: 10.1074/jbc.M212677200. [DOI] [PubMed] [Google Scholar]
- Gut P, et al. Lack of an adrenal cortex in Sfl mutant mice is compatible with the generation and differentiation of chromaffin cells. Development. 2005;132:4611–4619. doi: 10.1242/dev.02052. [DOI] [PubMed] [Google Scholar]
- Harada N, et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, et al. Progenitor cell expansion and organ size of mouse adrenal is regulated by sonic hedgehog. Endocrinology. 2010a;151:1119–1128. doi: 10.1210/en.2009-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, et al. Loss of beta-catenin in adrenal cortical cells leads to congenital adrenal hypoplasia without affecting adrenal chromaffin cells differentiation. Adaptive Medicine. 2010b;2:42–46. [Google Scholar]
- Huang CC, Yao HH. Inactivation of Dicerl in Steroidogenic factor 1-positive cells reveals tissue-specific requirement for Dicerl in adrenal, testis, and ovary. BMC Dev Biol. 2010;10:66. doi: 10.1186/1471-213X-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber K. The sympathoadrenal cell lineage: specification, diversification, and new perspectives. Dev Biol. 2006;298:335–343. doi: 10.1016/j.ydbio.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, et al. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol Endocrinol. 1994;8:654–662. doi: 10.1210/mend.8.5.8058073. [DOI] [PubMed] [Google Scholar]
- Keegan CE, Hammer GD. Recent insights into organogenesis of the adrenal cortex. Trends Endocrinol Metab. 2002;13:200–208. doi: 10.1016/s1043-2760(02)00602-1. [DOI] [PubMed] [Google Scholar]
- Kim AC, et al. Targeted disruption of beta-catenin in Sfl-expressing cells impairs development and maintenance of the adrenal cortex. Development. 2008;135:2593–2602. doi: 10.1242/dev.021493. [DOI] [PubMed] [Google Scholar]
- King P, et al. Shh signaling regulates adrenocortical development and identifies progenitors of steroidogenic lineages. Proc Natl Acad Sci USA. 2009;106:21185–21190. doi: 10.1073/pnas.0909471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreidberg JA, et al. WT-1 is required for early kidney development. Cell. 1993;74:679–691. doi: 10.1016/0092-8674(93)90515-r. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Dupin E. Cell lineage analysis in neural crest ontogeny. J Neurobiol. 1993;24:146–161. doi: 10.1002/neu.480240203. [DOI] [PubMed] [Google Scholar]
- Luo X, et al. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77:481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Murakami T, et al. Blood vascular beds of rat adrenal and accessory adrenal glands, with special reference to the corticomedullary portal system: a further scanning electron microscopic study of corrosion casts and tissue specimens. Arch Histol Cytol. 1989;52:461–476. doi: 10.1679/aohc.52.461. [DOI] [PubMed] [Google Scholar]
- Parlato R, et al. Conditional inactivation of glucocorticoid receptor gene in dopamine-beta- hydroxylase cells impairs chromaffin cell survival. Endocrinology. 2009;150:1775–1781. doi: 10.1210/en.2008-1107. [DOI] [PubMed] [Google Scholar]
- Ross ME, et al. Identification of a functional glucocorticoid response element in the phenylethanolamine N-methyltransferase promoter using fusion genes introduced into chromaffin cells in primary culture. J Neurosci. 1990;10:520–530. doi: 10.1523/JNEUROSCI.10-02-00520.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel CA, et al. Pbxl is essential for adrenal development and urogenital differentiation. Genesis. 2003;37:123–130. doi: 10.1002/gene.10235. [DOI] [PubMed] [Google Scholar]
- Tokunaga H. Postnatal development of the blood vasculature in the rat adrenal gland: a scanning electron microscope study of microcorrosion casts. Arch Histol Cytol. 1996;59:305–315. doi: 10.1679/aohc.59.305. [DOI] [PubMed] [Google Scholar]
- Val P, et al. Adrenal development is initiated by Cited2 and Wtl through modulation of Sf-1 dosage. Development. 2007;134:2349–2358. doi: 10.1242/dev.004390. [DOI] [PubMed] [Google Scholar]
- Wong DL, et al. Glucocorticoid regulation of phenylethanolamine N-methyltransferase in vivo. Faseb J. 1992;6:3310–3315. doi: 10.1096/fasebj.6.14.1426768. [DOI] [PubMed] [Google Scholar]
- Wurtman RJ. Control of epinephrine synthesis in the adrenal medulla by the adrenal cortex: hormonal specificity and dose-response characteristics. Endocrinology. 1966;79:608–614. doi: 10.1210/endo-79-3-608. [DOI] [PubMed] [Google Scholar]
- Wurtman RJ, Axelrod J. Control of enzymatic synthesis of adrenaline in the adrenal medulla by adrenal cortical steroids. J Biol Chem. 1966;241:2301–2305. [PubMed] [Google Scholar]
- Yoshida-Hiroi M, et al. Chromaffin cell function and structure is impaired in corticotropin- releasing hormone receptor type 1-null mice. Mol Psychiatry. 2002;7:967–974. doi: 10.1038/sj.mp.4001143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.