Abstract
The medial preoptic area (MPOA) is an integral site for male sexual behavior. Dopamine is released in the MPOA before and during copulation and facilitates male rat sexual behavior. Repeated sexual experience and noncopulatory exposures to an estrous female facilitate subsequent copulation. However, the neurobiological mechanisms that mediate such enhancement remain unclear. Here, we examined the role of dopamine D1 receptors in the MPOA in experience-induced enhancement of male sexual behavior in rats. In Experiment 1, microinjections of the D1 antagonist SCH-23390 into the MPOA before each of 7 daily 30-min noncopulatory exposures to a receptive female impaired copulation on a drug-free test on day 8, compared to vehicle-treated female-exposed animals. Copulatory performance in drug-treated animals was similar to vehicle-treated males that had not been pre-exposed to females. This effect was site specific. There were no group differences in locomotor activity in an open field on the copulation test day. In Experiment 2, a separate cohort of animals was used to examine phosphorylation of dopamine-and cAMP-regulated phosphoprotein (DARPP-32) in the MPOA of animals with acute and/or chronic sexual experience. DARPP-32 is a downstream marker of D1 receptor signaling and substrate of cAMP-dependent protein kinase (PKA). Western immunoblot analysis revealed that p-DARPP-32 expression was greatest in the MPOA of males that received both acute and chronic sexual experience, compared to all other mated conditions and naïve controls. These data suggest that D1 receptors in the MPOA contribute to experience-induced enhancement of male sexual behavior, perhaps through a PKA regulated mechanism.
Keywords: sexual behavior, medial preoptic area, D1 receptor, SCH-23390, DARPP-32
The role of dopamine (DA) is well-established in the regulation of learning, movement, and motivation (reviewed in Wise, 2004). DA acting on D1-like receptors facilitates male and female sexual behavior (Graham & Pfaus, 2010; Markowski, Eaton, Lumley, Moses, & Hull, 1994), maternal behavior (Miller & Lonstein, 2005; Parada, King, Li, & Fleming, 2008; Stolzenberg et al., 2010), associative learning (El-Ghundi, O'Dowd, & George, 2007), synaptic plasticity (Yao, Spealman, & Zhang, 2008), intracranial self-stimulation (Cheer et al., 2007), and drug addiction (Anderson & Pierce, 2005; Dietz, Dietz, Nestler, & Russo, 2009; Self, 2004).
D1 receptor actions may also facilitate the acquisition of sexual experience in male rodents (Bialy, Kalata, Nikolaev-Diak, & Nikolaev, 2010). Previous sexual experience improves the efficiency of copulation, with experienced males exhibiting shorter latencies to mount, intromit, and ejaculate, and having fewer mounts and intromissions preceding ejaculation (Dewsbury, 1969; Larsson, 1978). Repeated noncopulatory exposures to an estrous female can also enhance copulation on the first sexual experience in sexually naïve males (Lagoda, Muschamp, Vigdorchik, & Hull, 2004; Powell, Dominguez, & Hull, 2003; Vigdorchik, Parrish, Lagoda, McHenry, & Hull, 2012). However, the neural alterations that mediate these enhancements remain unclear.
One candidate region in which sexual experience or female exposure could induce neural changes is the medial preoptic area (MPOA) of the anterior hypothalamus, a highly integrative and critical site for the regulation of male sexual behavior in all vertebrate species studied. Expression of a paternally imprinted gene, Peg3, is necessary for male mice to show improved mating or preference for the odor of a receptive female as a result of sexual experience (Swaney, Curley, Champagne, & Keverne, 2007, 2008). Additionally, knock out of Peg3 in male mice impairs female odor-induced c-Fos expression in the MPOA, as well as other sex-related brain regions (Swaney et al., 2007). DA is released in the MPOA of male rats in the presence of an estrous female and throughout copulation (Hull, Du, Lorrain, & Matuszewich, 1995; Sato et al., 1995) and acts on D1 and D2 receptors to facilitate sexual behavior (Hull et al., 1995; Hull et al., 1992; Markowski et al., 1994). Ejaculation-induced Fos-immunoreactivity (ir) is also higher in the medial preoptic nucleus (MPN) after repeated sexual experiences, compared to the first sexual experience (Kollack-Walker & Newman, 1997; Lumley & Hull, 1999). Further, administration of a D1 receptor antagonist prior to the first sexual experience, but not prior to the eighth experience, decreases the amount of ejaculation-induced Fos-like-ir in the MPN (Lumley & Hull, 1999). However, the contributions of D1 receptors in the MPOA to experience-induced enhancement of male sexual behavior have not been tested.
The present experiments tested the effects of the D1 receptor antagonist SCH-23390, microinjected into the MPOA, on female exposure-induced enhancement of mating and also examined the effects of copulatory experience on a downstream marker of D1 receptor signaling. Activation of D1 receptors results in increased cAMP, which activates protein kinase A (PKA), which in turn can phosphorylate an intracellular protein, dopamine-and cAMP-regulated phosphoprotein (DARPP-32). Phosphorylated DARPP-32 (p-DARPP-32) is an inhibitor of protein-phosphatase-1 (PP1), which dephosphorylates and inactivates certain enzymes (Gould & Manji, 2005; Nairn et al., 2004). Since D1 receptors, acting through p-DARPP-32, can enhance certain intracellular signaling pathways, we also tested whether sexual experience influences levels of p-DARPP-32 in the MPOA.
Methods
Subjects
Adult male Long Evans/Blue Spruce rats (250-300 g Harlan, Indianapolis, IN) were individually housed in large plastic cages in climate-controlled rooms on a 14:10-hr light:dark cycle, with lights off at 1100 and on at 2100. Food and water were available ad libitum. All procedures were in accordance with the National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee of the Florida State University.
Copulation Testing
Stimulus females were injected with 10 μg estradiol benzoate 48 hr before testing and with 500 μg progesterone 4 hr before testing. Receptivity was confirmed with a stud male before females were used for testing. Copulation testing took place in the male's home cage under dim red light and lasted a total of 30 min from the first intromission or for 30 min total if the male failed to intromit.
The following behavioral measures were recorded and calculated: mount and intromission latencies (ML and IL), time from the introduction of the female to the first mount or intromission; ejaculation latency (EL), time from the first intromission to the first ejaculation; post-ejaculatory interval (PEI), time from the first ejaculation to the subsequent intromission; total frequencies of mounts (MFT), intromissions (IFT), and ejaculations (EFT); first ejaculatory series mount (MF1) and intromission (IF1) frequencies; intromission ratio 1 (IR1), number of intromissions divided by the number of mounts plus intromissions prior to the first ejaculation; inter-intromission interval 1 (III1), the latency to the first ejaculation divided by the number of intromissions preceding ejaculation; and total intromission ratio (IRT), total intromissions divided by mounts plus intromissions for the entire test.
Experiment 1 Design
The protocol and methods for this experiment were adapted from Lagoda et al. (2004), Powell et al. (2003), and Vigdorchik et al. (2012). Forty-eight sexually naïve male rats were separated into 4 groups, 3 of which received either vehicle (n=10), 1μg SCH-23390 (n=15), or 3μg of SCH-23390 (n=14) into the MPOA preceding exposure to an estrous female; the fourth group received vehicle but were not exposed to a female (n=9). For males in the first 3 groups, a sexually receptive but inaccessible female was presented in a wire mesh cage (12.5 cm × 26.0 cm × 15.0 cm) above the test subject's home cage for a 30 min noncopulatory exposure. Controls were presented with an empty box placed over their home cage for 30 min. These procedures were repeated for 7 consecutive days for all treatment groups. On Day 8, all subjects were tested drug-free for copulatory behavior in their home cage, followed by a locomotor activity test in an open field apparatus.
Surgeries
Sexually naïve male rats weighing between 300-350 g were anesthetized with ketamine hydrochloride (80 mg/kg) and xylazine hydrochloride (10 mg/kg), and a 23-gauge thin-wall stainless steel guide cannula was unilaterally implanted, aimed to end 1 mm above the anterior MPOA. The stereotaxic coordinates with respect to bregma were AP, +2.3 mm, ML, +0.4 mm, and DV, -6.3 mm (Pellegrino, Pellegrino, & Cushman, 1979). Cannulae were secured to the skull with three anchoring screws and dental acrylic. A stainless steel stylet was inserted into the guide cannula to protect the brain from infection and was removed only during microinjections.
Females of the same strain were anesthetized with ketamine hydrochloride (50mg/kg) and xylazine hydrochloride (4mg/kg) and ovariectomized using bilateral flank incisions. Buprenex (1.5 mg/0.1 ml) was administered subcutaneously following all surgical procedures to alleviate post-operative discomfort. All animals received ground rat chow (Purina LabDiet) mixed with water for 1 day post-surgery and were allowed 1 week for recovery before testing.
Microinjections
The dopamine D1-antagonist SCH-23390 (Sigma-Aldrich, St. Louis, MO) was dissolved in 10% dimethylsulfoxide (DMSO) in sterile water and administered at a concentration of either 1 or 3 μg/μl for a total of 1.0 μ1 into the MPOA. For the vehicle condition, 1.0 μ1 of 10% DMSO was administered. A stainless steel injection cannula was inserted into the brain ending 1 mm below the end of the guide cannula, and microinjections were administered using a Harvard infusion pump at a rate of 0.5 μl/min. Following the injection, the injection cannula was left in place for 2 min to facilitate diffusion into the tissue while the animal moved freely in his home cage. Fifteen minutes elapsed between microinjection and the female exposure to ensure full drug diffusion.
Preliminary Testing of Drug Doses
These drug doses were chosen based on preliminary testing, which revealed that administration of the drug at concentrations of 1, 3, 5, or 10 μg/μl into the MPOA before noncopulatory female exposure dose-dependently decreased the amount of time spent investigating the female, compared to vehicle-treated controls. It was previously reported that even the highest dose (10 μg/μl) administered in the MPOA decreased female choice preference testing but did not impair motoric activity (Moses, Loucks, Watson, Matuszewich, & Hull, 1995). Others have also used doses in this range in the MPOA for maternal behavior testing of female rats (Miller & Lonstein, 2005; Numan et al., 2005). The 1 μg/μl and 3 μg/μl doses of SCH-23990 were chosen for the present study because they were the lowest doses that resulted in a decrease in the amount of time spent investigating the female.
Open Field Testing
The open field apparatus was an opaque gray Plexiglas square (89 × 89 cm) with 46 cm walls. A clear Plexiglas floor was divided into 5 × 5 squares; each square was 17.78 × 17.78 cm. Locomotor activity was measured as the number of times the animal crossed into another square with all 4 feet during a 10 min test.
Histology
After behavioral testing was complete, rats were euthanized with an overdose of sodium pentobarbital (100 mg/kg, i.p.),, and 1 μl of green dye was microinjected in the same manner as during drug injection, for later visualization of cannula placement. Rats were then decapitated and their brains collected and frozen on dry ice. Frozen coronal brain sections were cut at 40 μm on a cryostat and mounted for verification of correct cannula placement. Animals with misplaced cannulae outside the MPOA were analyzed separately as described below.
Experiment 2 Design
Twenty-four animals were randomly divided equally into the following four groups: naïve no sex (NNS) animals did not receive any sexual experience; naïve sex (NS) animals copulated once through ejaculation 1-2 hours prior to sacrifice; experienced no sex (ENS) animals copulated to ejaculation thrice weekly for a total of 14 sessions, but did not copulate on the day of sacrifice; and experienced sex (ES) animals received 15 similar copulation sessions, with the final ejaculation occurring 1-2 hours prior to sacrifice. Rats were anesthetized with CO2 and rapidly decapitated; brains were extracted and flash-frozen in 2-methylbutane.
Western Immunoblot
Total protein was extracted from 1.0 mm tissue punches of total MPOA, and tissue from two animals was pooled into each tube. Tissue samples were homogenized in RIPA buffer 10X (Cell Signaling), and supernatant was extracted; protein estimates were run on this supernatant using spectrophotometry (PowerWaveX; BioTek, MD). These protein estimates were used as a standard for loading volume (50 μg of protein each) onto 10% polyacrylamide gels for separation by SDS electrophoresis.
The fractionated protein from electrophoresis was transferred to a polyvinylidene difluoride (PVDF) membrane. Between incubations, membranes were washed extensively with 0.1M phosphate buffered saline (PBS, pH=7.35). All washing and incubations were performed at room temperature unless otherwise noted. Membranes were incubated in a blocking solution consisting of 5% non-fat dry milk in PBS + 0.1% Tween for 1 hr. After blocking, the membrane was incubated overnight (18-20 hours) at 4°C in blocking solution and DARPP-32, phosphoThr34 rabbit polyclonal antibody (1:1k, Millipore, CA). The following day membranes were incubated for 1 hr in horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG secondary antibody (1:2k, Pierce Biotechnology, IL). The membrane was then assessed for HRP-conjugated chemiluminescence (ECL; Amersham Biosciences, NJ) by exposing the membrane to Kodak BioMax film. The membrane was then stripped and labeled against mouse anti-ß-actin monoclonal antibody (1:1k, Sigma-Aldrich, MO) as a loading control. The developed films were scanned into a computer and band density was quantified using the NIH ImageJ program (Version 1.33U, NIH).
Statistical Analysis
All statistical analyses were performed with SPSS (Version 18.0).
Experiment 1
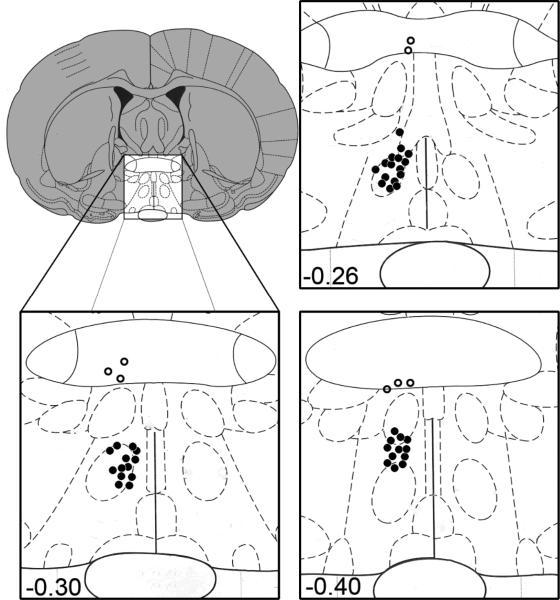
Six drug-treated animals had cannulae misplaced outside the MPOA (Figure 1); a Student's t test was used to assess any behavioral differences between these animals and vehicle-treated exposed controls. This analysis revealed no significant differences in copulatory behavior between these two groups. Therefore, subsequent analyses reported below combined drug-treated animals with misplaced cannulae together with vehicle-treated exposed controls; this grouping is referred to as “combined controls with exposure.” Thus, the final groups used for analyses were as follows: combined controls with exposure (n=16), 1μg SCH-23390 with exposure (n=13), 3μg SCH-23390 with exposure (n=10), and vehicle-treated unexposed controls (n=9).
Figure 1.
Diagram illustrating the site of injection at the level of the MPOA (bregma, -0.26,-0.30,-0.40). Filled circles indicate correct placements and open circles represent missed placements outside the MPOA. From The Rat Brain in Stereotaxic Coordinates (4th ed.), figs. 18-20, by G. Paxinos & C. Watson, 1998, San Diego: Academic Press. Copyright 1998 by Elsevier Academic Press. Adapted with permission.
One-way between-subjects ANOVAs were used to assess differences between groups for all copulatory and motoric activity measures described above, and post-hoc analyses (Tukey's Honestly Significant Difference, HSD) were subsequently performed to reveal differences between specific groups. In cases where fewer than 3 subjects per group performed a behavior, that group was excluded from statistical analysis. For all statistical analyses, p < 0.05 was considered statistically significant and p < 0.10 was considered a trend.
Experiment 2
One-way analyses of variance (ANOVA) were used in conjunction with Tukey's HSD post-hoc analyses to probe for differences in relative band densities between groups.
Results
Experiment 1
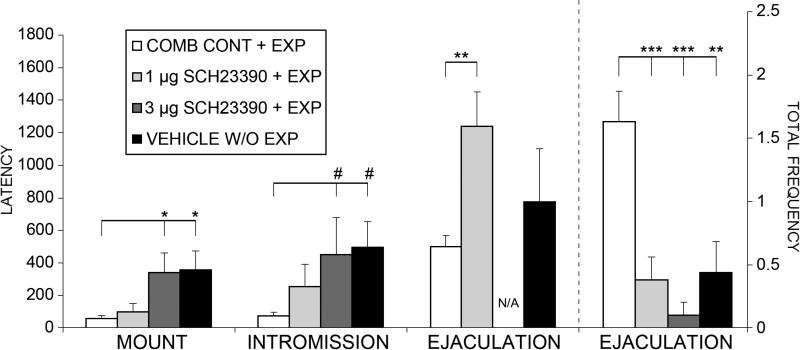
Drug-treated exposed males (SCH-23390 1μg and 3μg) were impaired on several copulatory measures and displayed copulatory behavior more similar to that of vehicle-treated, unexposed controls. One-way between subjects ANOVAs revealed significant main effects for mount latency (F(3,40) = 4.911, p = 0.006), intromission latency (F(3,29) = 3.201, p = 0.040), ejaculation latency (F(2,18) = 7.394, p = 0.005), and ejaculation frequency (F(3,47) = 9.997, p < 0.001; see Figure 2). Post-hoc analyses revealed that the combined controls with exposure displayed significantly shorter latencies to mount (p < .05) and a trend for shorter latencies to intromit (p < .10), compared to vehicle-treated unexposed controls and those treated with 3μg SCH-23390. Further, combined controls with exposure had significantly shorter latencies to ejaculate, compared to those treated with 1μg SCH-23390 (p < .01). Lastly, combined controls with exposure had significantly more ejaculations, compared to unexposed controls (p =.005), and those treated with 1μg (p =.001) or 3μg (p < .0001) SCH-23390.
Figure 2.
Mean (± SE) mount, intromission, and ejaculation latencies in sexually naive males treated with SCH-23390 (1 or 3 μg) or vehicle before each of 7 noncopulatory exposures to a receptive female and tested drug-free on Day 8, and in vehicle-treated males without female exposures. COMB. CONTROLS + EXP: males that were exposed to females following either vehicle or misplaced drug injections; 1 μg SCH-23390 + EXP: males that received 1 μg SCH-23390 before female exposures; 3 μg SCH-23390 + EXP: males that received 3 μg SCH-23390 before female exposures; VEH W/O EXP: males that received vehicle but were not exposed to females. #p<.10, *p < .05, **p < .01, ***p<.001.
One-way between subjects ANOVAs also revealed trends for main effects of drug-treated animals and vehicle-treated unexposed controls having lower total intromission ratios (F(3,47) = 2.531, p = 0.069), lower total intromission frequencies (F(3,47) = 2.326, p = 0.088), lower mount frequencies before the first ejaculation (F(2,18) = 3.219, p = 0.071), and higher inter-intromission intervals before the first ejaculation (F(2,18) = 3.096, p = 0.073), compared to combined controls with exposure. One-way between subjects ANOVAs revealed no significant main effects for locomotor activity in the drug-free open field test (F(3,26) = 0.817, p = 0.528), indicating that the drug-induced copulatory impairments observed on the day 8 test were not due to differences in motor activity.
Experiment 2
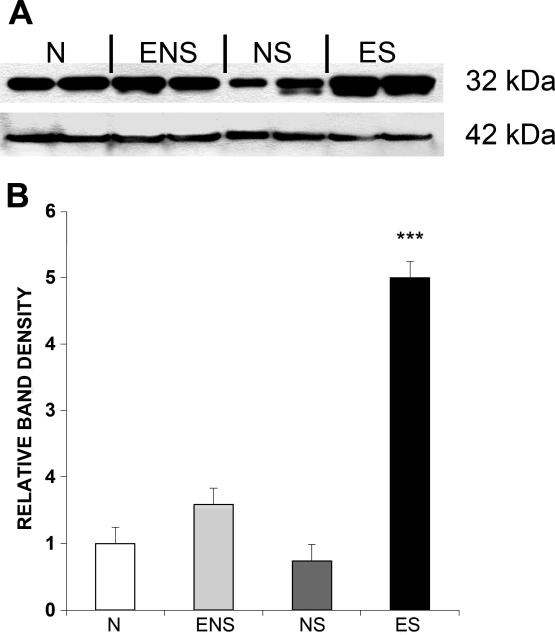
A one-way between subjects ANOVA revealed a significant main effect for relative band density of p-DARPP-32 expression in the MPOA (F(3,15) = 142.946, p < 0.001) (see Figure 3). Post-hoc analysis revealed that sexually experienced males that had sex the day of euthanasia (ES) had significantly more p-DARPP-32 expression in the MPOA, compared to all other groups (p <.0001).
Figure 3.
Protein concentration for phosphorylated dopamine- and cAMP-regulated phosphoprotein (pThr34-DARPP-32) in the medial preoptic area (MPOA) of male rats. A: Western immunoblot of samples collected from the MPOA of male rats in the following groups: sexually naive rats that did (NS) or did not (N) mate on the day of testing and sexually experienced rats that did (ES) or did not (ENS) mate on day of testing. Pictured on top are bands for pThr34-DARPP-32 (32 kDa); below are bands for beta-actin (42 kDa). B: Comparison of relative band densities from Western immunoblots. Values are expressed as M (± SE). ***p<.001
Discussion
The present experiments demonstrate that administration of a D1 antagonist into the MPOA before each of 7 daily noncopulatory female exposures resulted in impaired sexual function on the subsequent drug-free copulation test on day 8, compared to combined controls with exposure. Additionally, drug-treated animals with misplaced cannulae outside the MPOA displayed no differences in copulatory behavior, compared to vehicle-treated female-exposed animals, indicating that this is a site-specific action. We also replicated our previous findings, demonstrating that repeated noncopulatory female exposures facilitate subsequent copulatory performance in vehicle-treated animals (Lagoda et al., 2004; Powell et al., 2003; Vigdorchik et al., 2012). Furthermore, no differences in locomotor activity were found between groups on the copulation test day; thus, it is unlikely that the copulatory impairments resulted from deficits in motor activity. Lastly, there was more p-DARPP-32 in the MPOA of experienced males that copulated on the day of euthanasia (ES), compared to all other groups. Therefore, chronic sexual experience imparts changes in mating-induced phosphorylation of DARPP-32 in the MPOA that may be mediated by D1 receptors, as discussed below.
Contributions of DA D1 receptors
The MPOA is a highly integrated region with indirect reciprocal connections to and from every sensory modality (Simerly & Swanson, 1986, 1988); thus, it can modify and bias the processing of sex-related sensory information. Moreover, copulation as well as chemosensory stimuli from estrous females is sufficient to elicit Fos-ir in the MPOA as well as in other sex-related areas (reviewed in Hull & Rodriguez-Manzo, 2009). Previous data demonstrated that DA D1 receptors are involved in induction of copulation-induced Fos-ir in the MPOA (Lumley & Hull, 1999). The present findings expand upon these data and suggest that D1 receptor actions in the MPOA may also increase responsiveness to sex-related input and facilitate experience-induced enhancements of male sexual behavior.
The neural mechanisms in the MPOA that facilitate sexual experience may be similar to those in other regions of the brain. DA is released in the MPOA as soon as a male rat detects the presence of an estrous female and increases further during copulation (Hull et al., 1995; Sato et al., 1995). Similarly, DA is also released in the NAc in the presence of an estrous female and throughout copulation (reviewed in Hull & Rodriguez-Manzo, 2009). As in the MPOA, female-stimulated Fos-ir is also higher in the NAc in sexually experienced males, compared to naïve controls (Lopez and Ettenberg, 2002). Furthermore, D1 receptors in the NAc contribute to the experience-induced enhancement of male sexual behavior (Bialy et al., 2010). These data, along with the present findings, suggest that DA release in multiple brain areas in response to copulation or sex-related stimuli may act through similar processes at D1 receptors, which collectively contribute to enhancement of sexual behavior.
D1 receptors act synergistically with NMDA glutamate receptors in other brain regions to facilitate learning and synaptic plasticity (Lovinger, 2010; Sarantis, Matsokis, & Angelatou, 2009; Scott & Aperia, 2009; Smith-Roe & Kelley, 2000). For example, NMDA receptor activation can recruit D1 receptors to the membrane, which facilitates synaptic activity and surface targeting of NMDA receptors, acting as a positive feedback loop (Cepeda & Levine, 2006). Given that sexual input increases DA and glutamate release in the MPOA (Dominguez, Gil, & Hull, 2006; Hull et al., 1995), and stimulates phosphorylation of glutamate NMDA receptors (Dominguez et al., 2007), they may also work together to promote sexual learning. Indeed, systemic administration of a D1 or NMDA receptor antagonist impairs the acquisition of sexual experience (Bialy et al., 2010; Bialy, Rydz, & Kaczmarek, 2000) and blocking NMDA receptors in the MPOA can impair the enhancement that would otherwise have resulted from repeated noncopulatory exposures to an estrous female (Vigdorchik et al., 2012). However, it is not known whether D1 and NMDA receptors also interact in the MPOA to mediate sexual learning, although these and previous data demonstrate that, when tested separately, both play a role in the enhancement of copulation following repeated female exposures.
Phosphorylation of DARPP-32
Phosphorylation of DARPP-32 influences various intracellular actions, which can alter ion channel excitability, trigger gene transcription, and induce histone modifications (Stipanovich et al., 2008; Svenningsson et al., 2004). Stimulation of D1 receptors leads to activation of adenylyl cyclase, cAMP, and PKA, which phosphorylates DARPP-32 at Thr34 (Beaulieu & Gainetdinov, 2011); this in turn amplifies PKA-mediated signaling by inhibiting protein phosphatase-1 (PP-1) (reviewed in Svenningsson, Nairn, & Greengard, 2005).
Here, we report that acute copulation increased pThr34-DARPP-32 in the MPOA only in males with previous sexual experience. pThr34-DARPP-32 was also up-regulated in the MPOA of ovariectomized, estradiol-treated female rats by artificial vagino-cervical stimulation (Meredith et al., 1998). The increased pThr34–DARPP-32 in female rats occurred after the first vagino-cervical stimulation, indicating a potential sex difference in this phosphorylation, although that study did not test after chronic stimulation. It is not clear whether natural copulation to ejaculation, as opposed to artificial vaginal stimulation, or perhaps the length of time of stimulation or the precise MPOA area studied, can account for the male/female difference in phosphorylation in the MPOA. Future studies are necessary to clarify the role of pThr34–DARPP-32 in male and female sexual behavior.
In summary, microinjection of a D1 antagonist into the MPOA of male rats before each of 7 noncopulatory exposures to an estrous female blocked the facilitative effects of such exposures in combined controls with female exposure, compared to vehicle-treated males that were not exposed to a female. Thus, D1 receptors in the MPOA contribute to sensitization to sexual stimuli, a form of sexual learning. One possible mediator of these effects is phosphorylation of DARPP-32, which is activated by D1, as well as by other types of receptors, and also contributes various forms of learning. Experiment 2 revealed that previous plus acute sexual experience increases copulation-induced pThr34-DARPP-32 in the MPOA. Therefore, copulation initiates D1 receptor-mediated actions in the MPOA that may produce long-lasting intracellular changes that alter the processing of sexual stimuli.
Acknowledgements
This research was supported by the National Institute of Mental Health Grant R01 MH040826 to EMH. Parts of these data from Experiment 1 were presented at the Annual Meetings for the Society for Neuroscience (2009) and Society for Behavioral Neuroendocrinology (2010). The authors thank Christopher Robison for his graphical assistance with figures, Dr. Teresa Aubele for her editorial assistance, and Dr. Renu Bhatt for her assistance with the western immunoblot protocol. We also acknowledge Text and Academic Authors Association for a publication grant (http://www.taaonline.net/publication_grants/index.html).
References
- Anderson SM, Pierce RC. Cocaine-induced alterations in dopamine receptor signaling: implications for reinforcement and reinstatement. Pharmacology & Therapeutics. 2005;106(3):389–403. doi: 10.1016/j.pharmthera.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacological Reviews. 2011;63(1):182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Bialy M, Kalata U, Nikolaev-Diak A, Nikolaev E. D1 receptors involved in the acquisition of sexual experience in male rats. Behavioural Brain Research. 2010;206(2):166–176. doi: 10.1016/j.bbr.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Bialy M, Rydz M, Kaczmarek L. Precontact 50-kHz vocalizations in male rats during acquisition of sexual experience. Behavioral Neuroscience. 2000;114(5):983–990. doi: 10.1037//0735-7044.114.5.983. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Levine MS. Where do you think you are going? The NMDA-D1 receptor trap. Science Signaling: Signal Transduction Knowledge Envrionment. 2006;2006(333):e20. doi: 10.1126/stke.3332006pe20. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Aragona BJ, Heien ML, Seipel AT, Carelli RM, Wightman RM. Coordinated accumbal dopamine release and neural activity drive goal-directed behavior. Neuron. 2007;54(2):237–244. doi: 10.1016/j.neuron.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Dewsbury DA. Copulatory behaviour of rats (Rattus norvegicus) as a function of prior copulatory experience. Animal Behaviour. 1969;17(2):217–223. doi: 10.1016/0003-3472(69)90004-9. [DOI] [PubMed] [Google Scholar]
- Dietz DM, Dietz KC, Nestler EJ, Russo SJ. Molecular mechanisms of psychostimulant-induced structural plasticity. Pharmacopsychiatry. 2009;42(Suppl 1):S69–78. doi: 10.1055/s-0029-1202847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez JM, Balfour ME, Lee HS, Brown JL, Davis BA, Coolen LM. Mating activates NMDA receptors in the medial preoptic area of male rats. Behavioral Neuroscience. 2007;121(5):1023–1031. doi: 10.1037/0735-7044.121.5.1023. [DOI] [PubMed] [Google Scholar]
- Dominguez JM, Gil M, Hull EM. Preoptic glutamate facilitates male sexual behavior. Journal of Neuroscience. 2006;26(6):1699–1703. doi: 10.1523/JNEUROSCI.4176-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ghundi M, O'Dowd BF, George SR. Insights into the role of dopamine receptor systems in learning and memory. Reviews in the Neurosciences. 2007;18(1):37–66. doi: 10.1515/revneuro.2007.18.1.37. [DOI] [PubMed] [Google Scholar]
- Gould TD, Manji HK. DARPP-32: A molecular switch at the nexus of reward pathway plasticity. Procedings of the National Academy of Sciences U S A. 2005;102(2):253–254. doi: 10.1073/pnas.0408700102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham MD, Pfaus JG. Differential regulation of female sexual behaviour by dopamine agonists in the medial preoptic area. Pharmacology Biochemistry and Behavior. 2010;97(2):284–292. doi: 10.1016/j.pbb.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Hull EM, Du J, Lorrain DS, Matuszewich L. Extracellular dopamine in the medial preoptic area: implications for sexual motivation and hormonal control of copulation. Journal of Neuroscience. 1995;15(11):7465–7471. doi: 10.1523/JNEUROSCI.15-11-07465.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull EM, Eaton RC, Markowski VP, Moses J, Lumley LA, Loucks JA. Opposite influence of medial preoptic D1 and D2 receptors on genital reflexes: implications for copulation. Life Sciences. 1992;51(22):1705–1713. doi: 10.1016/0024-3205(92)90299-5. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Newman SW. Mating-induced expression of c-fos in the male Syrian hamster brain: role of experience, pheromones, and ejaculations. Journal of Neurobiology. 1997;32(5):481–501. doi: 10.1002/(sici)1097-4695(199705)32:5<481::aid-neu4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Lagoda G, Muschamp JW, Vigdorchik A, Hull EM. A nitric oxide synthesis inhibitor in the medial preoptic area inhibits copulation and stimulus sensitization in male rats. Behaviroal Neuroscience. 2004;118(6):1317–1323. doi: 10.1037/0735-7044.118.6.1317. [DOI] [PubMed] [Google Scholar]
- Larsson K. Experiential factors in the development of sexual behavior. In: Hutchison JB, editor. Biological determinants of sexual behaviour. Wiley; New York: 1978. pp. 55–86. [Google Scholar]
- Lovinger DM. Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology. 2010;58(7):951–961. doi: 10.1016/j.neuropharm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley LA, Hull EM. Effects of a D1 antagonist and of sexual experience on copulation-induced Fos-like immunoreactivity in the medial preoptic nucleus. Brain Research. 1999;829(1-2):55–68. doi: 10.1016/s0006-8993(99)01338-4. [DOI] [PubMed] [Google Scholar]
- Markowski VP, Eaton RC, Lumley LA, Moses J, Hull EM. A D1 agonist in the MPOA facilitates copulation in male rats. Pharmacology Biochemistry and Behavior. 1994;47(3):483–486. doi: 10.1016/0091-3057(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Meredith JM, Moffatt CA, Auger AP, Snyder GL, Greengard P, Blaustein JD. Mating-related stimulation induces phosphorylation of dopamine- and cyclic AMP-regulated phosphoprotein-32 in progestin receptor-containing areas in the female rat brain. Journal of Neuroscience. 1998;18(23):10189–10195. doi: 10.1523/JNEUROSCI.18-23-10189.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SM, Lonstein JS. Dopamine d1 and d2 receptor antagonism in the preoptic area produces different effects on maternal behavior in lactating rats. Behavioral Neuroscience. 2005;119(4):1072–1083. doi: 10.1037/0735-7044.119.4.1072. [DOI] [PubMed] [Google Scholar]
- Moses J, Loucks JA, Watson HL, Matuszewich L, Hull EM. Dopaminergic drugs in the medial preoptic area and nucleus accumbens: effects on motor activity, sexual motivation, and sexual performance. Pharmacology Biochemistry and Behavior. 1995;51(4):681–686. doi: 10.1016/0091-3057(94)00437-n. [DOI] [PubMed] [Google Scholar]
- Nairn AC, Svenningsson P, Nishi A, Fisone G, Girault JA, Greengard P. The role of DARPP-32 in the actions of drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):14–23. doi: 10.1016/j.neuropharm.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, Pliakou N, Stolzenberg DS, Mullins OJ, Murphy JM, Smith CD. The effects of D1 or D2 dopamine receptor antagonism in the medial preoptic area, ventral pallidum, or nucleus accumbens on the maternal retrieval response and other aspects of maternal behavior in rats. Behavioral Neuroscience. 2005;119(6):1588–1604. doi: 10.1037/0735-7044.119.6.1588. [DOI] [PubMed] [Google Scholar]
- Parada M, King S, Li M, Fleming AS. The roles of accumbal dopamine D1 and D2 receptors in maternal memory in rats. Behavioral Neuroscience. 2008;122(2):368–376. doi: 10.1037/0735-7044.122.2.368. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed. Academic Press; San Diego: 1998. [Google Scholar]
- Pellegrino LJ, Pellegrino AS, Cushman AJ. A stereotaxic atlas of the rat brain. 2nd edition 1979.
- Powell WS, Dominguez JM, Hull EM. An NMDA antagonist impairs copulation and the experience-induced enhancement of male sexual behavior in the rat. Behavioral Neuroscience. 2003;117(1):69–75. doi: 10.1037//0735-7044.117.1.69. [DOI] [PubMed] [Google Scholar]
- Sarantis K, Matsokis N, Angelatou F. Synergistic interactions of dopamine D1 and glutamate NMDA receptors in rat hippocampus and prefrontal cortex: involvement of ERK1/2 signaling. Neuroscience. 2009;163(4):1135–1145. doi: 10.1016/j.neuroscience.2009.07.056. [DOI] [PubMed] [Google Scholar]
- Sato Y, Wada H, Horita H, Suzuki N, Shibuya A, Adachi H, Kato R, Tsukamoto T, Kumamoto Y. Dopamine release in the medial preoptic area during male copulatory behavior in rats. Brain Research. 1995;692(1-2):66–70. doi: 10.1016/0006-8993(95)00656-b. [DOI] [PubMed] [Google Scholar]
- Scott L, Aperia A. Interaction between N-methyl-D-aspartic acid receptors and D1 dopamine receptors: an important mechanism for brain plasticity. Neuroscience. 2009;158(1):62–66. doi: 10.1016/j.neuroscience.2008.10.020. [DOI] [PubMed] [Google Scholar]
- Self DW. Regulation of drug-taking and -seeking behaviors by neuroadaptations in the mesolimbic dopamine system. Neuropharmacology. 2004;47(Suppl 1):242–255. doi: 10.1016/j.neuropharm.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. The organization of neural inputs to the medial preoptic nucleus of the rat. Journal of Comparative Neurology. 1986;246(3):312–342. doi: 10.1002/cne.902460304. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. Projections of the medial preoptic nucleus: a Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. Journal of Comparative Neurology. 1988;270(2):209–242. doi: 10.1002/cne.902700205. [DOI] [PubMed] [Google Scholar]
- Smith-Roe SL, Kelley AE. Coincident activation of NMDA and dopamine D1 receptors within the nucleus accumbens core is required for appetitive instrumental learning. Journal of Neuroscience. 2000;20(20):7737–7742. doi: 10.1523/JNEUROSCI.20-20-07737.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipanovich A, Valjent E, Matamales M, Nishi A, Ahn JH, Maroteaux M, Bertran-Gonzalez J, Brami-Cherrier K, Enslen H, Corbille AG, Filhol O, Nairn AC, Greengard P, Herve D, Girault JA. A phosphatase cascade by which rewarding stimuli control nucleosomal response. Nature. 2008;453(7197):879–884. doi: 10.1038/nature06994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzenberg DS, Zhang KY, Luskin K, Ranker L, Bress J, Numan M. Dopamine D(1) receptor activation of adenylyl cyclase, not phospholipase C, in the nucleus accumbens promotes maternal behavior onset in rats. Hormones and Behavior. 2010;57(1):96–104. doi: 10.1016/j.yhbeh.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Nairn AC, Greengard P. DARPP-32 mediates the actions of multiple drugs of abuse. American Association of Pharmaceutical Scientists. 2005;7(2):E353–360. doi: 10.1208/aapsj070235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: an integrator of neurotransmission. Annual Reviews of Pharmacology and Toxicology. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- Swaney WT, Curley JP, Champagne FA, Keverne EB. Genomic imprinting mediates sexual experience-dependent olfactory learning in male mice. Proc Natl Acad Sci U S A. 2007;104(14):6084–6089. doi: 10.1073/pnas.0609471104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaney WT, Curley JP, Champagne FA, Keverne EB. The paternally expressed gene Peg3 regulates sexual experience-dependent preferences for estrous odors. Behavioral Neuroscience. 2008;122(5):963–973. doi: 10.1037/a0012706. [DOI] [PubMed] [Google Scholar]
- Vigdorchik AV, Parrish BP, Lagoda GA, McHenry JA, Hull EM. An NMDA antagonist in the MPOA impairs copulation and stimulus sensitization in male rats. Behavioral Neuroscience. 2012;126(1):186–195. doi: 10.1037/a0026460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5(6):483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Yao WD, Spealman RD, Zhang J. Dopaminergic signaling in dendritic spines. Biochemical Pharmacology. 2008;75(11):2055–2069. doi: 10.1016/j.bcp.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]