Abstract
AIM: To study the effect of regular aerobic exercise on insulin resistance, serum aminotransferase and liver histology in nonalcoholic fatty liver disease (NAFLD) patients.
METHODS: Sixty (mean age 40.0 ± 8.5 years, 75% male) NAFLD patients were included in the study. After baseline anthropometric measurement i.e., body mass index (BMI), waist circumference (WC); all patients were advised regular aerobic exercise for 30 min/d, for at least 5 d/wk and trained to achieve around 70% of his maximal heart rate. In addition, moderately energy restricted diet was advised to patients with high BMI (> 25 kg/m2). Monthly follow up was done by measuring BMI, WC, aspartate aminotransferase, and alanine aminotransferase (ALT). Insulin resistance was calculated using homeostasis model assessment (HOMA) of insulin resistance (HOMA-IR) model, at baseline and after 6 mo. Insulin resistance was arbitrarily considered altered when it was ≥ 2. Liver biopsy was done in a section of patients at baseline and after 6 mo.
RESULTS: Seventy percent (42/60) patients were overweight or obese; 95% (57/60) had central obesity (WC > 90 cm in men, > 80 cm in women). In the 45 exercise compliant patients insulin resistance decreased from 6.4 ± 6.1 to 1.3 ± 1.0, BMI from 26.7 ± 3.3 kg/m2 to 25.0 ± 3.3 kg/m2, WC from 95.7 ± 8.9 cm to 90.8 ± 7.3 cm and ALT from 84.8 ± 43.5 U/L to 41.3 ± 18.2 U/L (P < 0.01). In 15 exercise noncompliant patient’s insulin resistance, BMI, WC and ALT did not show significant change at 6 mo follow up. Six of 8 patients in compliant group on repeat liver biopsy showed significant change in steatosis and necroinflammation. Nonalcoholic steatohepatitis scores improved form 5.3 ± 1.5 to 3.35 ± 1.5. The decline in insulin resistance correlated with decline in ALT (P = 0.01, rs = 0.90) and liver histology (P = 0.03, rs = 0.73).
CONCLUSION: Life style modification improves insulin resistance resulting in improvement in ALT and liver histology in NAFLD patients.
Keywords: Lifestyle changes, Insulin resistance, Metabolic syndrome, Nonalcoholic steatohepatitis, Liver histology
INTRODUCTION
Obesity and insulin resistance related health hazards (metabolic syndrome) has become major health burden in the present century. In the United Stated, around 30% of population is obese and three fourth of them have fatty liver disease[1]. Nonalcoholic fatty liver disease (NAFLD) is a spectrum of clinicopathologic liver disease ranging from simple steatosis to nonalcoholic steatohepatitis (NASH) and is being increasingly recognized as a major cause of liver related morbidity and mortality[2,3]. With emerging clinical and epidemiological evidence, NAFLD is considered as hepatic component of metabolic syndrome[4].
At present, there is no established means for treating or preventing NASH. Clinical trials of potential treatments have mostly been conducted in uncontrolled settings on small number of subjects and extrapolation of these observations to large populations of patients with NAFLD is unreasonable. Available clinical and epidemiological data indicates that obesity, hyperlipidemia and diabetes are associated with NASH[5-8].
As insulin resistance has been recognized as major mechanism for development of NAFLD (proposed to be the “first hit” leading to hepatic steatosis), improving insulin-sensitivity has been considered as a strategy in the treatment of NAFLD[9-11]. Oxidative stress with subsequent lipid peroxidation and generation of reactive oxygen species seems to be prominent in NASH (“second hit”) and has been identified as therapeutic target for antioxidants[9,12]. As NASH is often categorized as part of metabolic syndrome, drug treatment carries risk of significant side effects and probably requires life long therapy resulting in poor compliance.
Lifestyle modifications with dietary restriction of calorie and exercise have shown to improve insulin sensitivity and help in reducing the risk of chronic illnesses like coronary artery disease and maturity-onset diabetes[13,14]. In the United States, at any given time, it has been estimated that around 30% men and 45% women are on lifestyle changes to lose weight. Short term weight loss resulting from dietary modification and exercise have been shown to improve obesity and aminotransferase, however its ultimate effect on liver histology and the natural course of NASH is not well documented[15,16,17]. We performed a prospective study aimed to asses the effect of regular aerobic exercise and dietary modification on insulin resistance liver histology in patients with NASH.
MATERIALS AND METHODS
Patients and methods
Sixty patients diagnosed as NAFLD attending Liver clinic were included in the study. Diagnosis of NAFLD was based on the presence of fatty liver on ultrasonography and an elevated serum alanine aminotransferase (ALT) > 1.5 times the upper limit of normal for a minimum period of 3 mo. Patients with other causes of chronic hepatitis including viral hepatitis, autoimmune hepatitis, cholestatic liver disease, hemochromotosis, Wilson’s disease and alcoholic liver disease (alcohol use > 20 g/d) were excluded from study.
This study was carried out in accordance with the principles of the Helsinki declaration and was formally approved by institutional ethical committee. All patients gave a written, informed consent before participation in the study.
Clinical and anthropometric data
Detailed history including use of drugs, particularly oral contraceptives, corticosteroids and antituberculosis, antidiabetics, insulin sensitizers was obtained and clinical examination to look for any evidence of chronic liver disease was done at initial screening. Bodyweight was measured using self-zeroing weight scale with light clothing without shoes to the nearest half-kilogram. Height was measured to the nearest 2 mm with patient standing on bare feet closely apposed to each other and against the wall with patient looking straight. Body mass index (BMI) was calculated as follows: body weight (kg/m2)[18]. Waist circumference (WC) in centimeters was measured at a level midway between the lower rib margin and iliac crest and hip circumference at the widest portion of buttocks. Waist-hip ratio (WHR) was calculated by dividing waist circumference by hip circumference. Increased WHR was defined as ≥ 0.90 in men and ≥ 0.85 in women[19].
Metabolic syndrome
Metabolic syndrome was defined according to National Cholesterol Education Program (NCEP) adult treatment panel III (ATP III) guidelines as the presence of 3 or more of the following 5 risk factors: (1) Waist circumference > 102 cm (men) and > 88 cm (women); (2) Fasting triglycerides ≥ 150 mg/dL; (3) High density lipoprotein cholesterol < 40 mg/dL (men) and < 50 mg/dL (women); (4) blood pressure ≥ 130/≥ 85 mmHg; (5) fasting glucose > 110 mg/dL[20]. The cut off for normal waist circumference in adult Indians has been found to be lower than Caucasians; hence we used a cut off of > 90 cm (men) and > 80 cm (women) in our study[21].
Laboratory tests
Overnight fasting blood sample were obtained for measurement of plasma glucose and serum lipids. Plasma glucose 2 h after 50 gm of glucose load was also done in all patients. Plasma glucose was measured with an automated analyzer using glucose oxidase and peroxidase method. Fasting lipid profile for total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, very low-density lipoprotein and triglycerides was obtained (RA-XT random access clinical chemistry analyzer, Bayer Diagnostics, Tarrytown, NY, USA). Serum iron studies, albumin, aspartate aminotransferase (AST) and ALT were done on all patients at baseline.
To exclude hepatitis B and hepatitis C, HBsAg (Hepanostika, Biomerieux bv, Boxtel, NL, US) and anti-hepatitis C virus (UBI, United Biochemicals Inc, Houppauge, NY, US) and to exclude autoimmune hepatitis, anti nuclear antibody, anti smooth muscle antibody, anti mitochondrial antibody and anti liver kidney microsomal antibody were done in all patients. Patients less than 40 years of age also underwent slit lamp examination to rule out Wilson’s disease.
Insulin resistance
Fasting samples of serum obtained after centrifugation were stored at -70 °C until assayed. Fasting insulin levels (mU/L) were measured using radioimmunoassay (Diagnostic Products Corporation, Los Angeles, CA). The hinsulin resistance was calculated on the basis of fasting values of plasma glucose and insulin according to homeostasis model assessment (HOMA) of insulin resistance (HOMA-IR) model formula,
HOMA-IR = fasting insulin (mU/L) × fasting glucose (mmol/L) ÷ 22.5[22].
As previously recommended, insulin resistance was arbitrarily considered altered when it was > 2[23].
Ultrasonography
Ultrasonography of liver was performed in all participants in fasting state by trained radiologist blinded for laboratory and anthropometric data. Brightness and posterior attenuation were considered indices of the extent of fatty infiltration. The diagnosis of fatty liver was made based on findings of echogenicity: graded as: grade 0: normal echogenicity; grade 1: slight, diffuse increase in fine echoes in liver parenchyma with normal visualization of diaphragm and intrahepatic vessel borders; grade 2: moderate, diffuse increase in fine echoes with slightly impaired visualization of intrahepatic vessels and diaphragm; and grade 3: marked increase in fine echoes with poor or non-visualization of the intrahepatic vessel borders, diaphragm, and posterior right lobe of the liver[24].
Liver biopsy
In patients who gave consent, liver biopsy was done as indoor procedure using Menghini’s needle. All biopsies were reported by one pathologist blinded for clinical data using the Brunt’s scoring system for non-alcoholic steatohepatitis[25]. Steatosis was graded according to percentage of cells with fatty droplets (grade I, 0%-33%; grade II, 34%-66%; grade III > 66%). Necroinflammation was graded 0-3 (0, absent; 1, occasional ballooning and no or minimal inflammation; 2, ballooning of hepatocytes with mild to moderate portal inflammation; 3, intra-acinar inflammation and portal inflammation). Fibrosis was graded as 0-4: (0, no fibrosis; 1, perisinusoidal/ pericellular fibrosis; 2, periportal fibrosis; 3, bridging fibrosis; 4, cirrhosis.
Study design
All patients were counseled to an aerobic exercise regimen. Moderately energy-restricted diet was advised by a qualified dietician to those with high BMI (> 25 kg/m2).
Exercise program and diet regimen
All patients were given training by professionally qualified physical instructor from “Health Zone” Lucknow about exercise programme at beginning and at regular interval. During workshop two physical trainers assessed and individualized the exercise program for each patient, and supervised their performance during workshops and were trained to achieve target heart rate. The exercise program included brisk walking; jogging or rhythmic aerobic exercises set to beat music, for a minimum period of 45 min/d, for at least 5 d/wk. They were counseled to achieve approximately 70% of their maximal heart rate for minimum period of 20 min. Maximum heart rate was estimated from the formula 220 - age (years) with a standard deviation of 10-12 beats/min[26]. All patients were asked to maintain records about exercise programme and MHR in the provided Performa to assess their compliance. Those patients who exercised on less than 4 d/wk (16 d/mo) were considered as exercise non-compliant.
All patients received standardized nutritional counseling by registered Dietician, who supervised them regularly. Moderate energy restricted diet containing 60% carbohydrate, 20% fat, 20% protein and 200 mg cholesterol (National cholesterol education program step I diet, (25 kcal/kg ideal body weight) was advised to patients with high BMI[20]. Ideal body weights were calculated in kg using the formula: Ht (cm) - 100 × 0.9[27].
Follow-up
All patients were followed up monthly by measuring anthropometric data and laboratory assessment of serum albumin, ALT and AST levels. Fasting insulin levels were taken at baseline and after 6 mo of exercise program. Care was taken so that none of them took any other pharmacological intervention except for coexisting condition like hypertension.
Statistical analysis
Statistical analysis was performed using SPSS 10.0.1 software (SPSS Inc., Chicago, IL, US). All data was expressed as mean ± SD. Baseline parameters were compared between groups using Mann-Whitney U test and for comparing the variables before and after therapy, Wilcoxon signed ranks test was used. The degree of association between the decline in insulin resistance, ALT and liver histology; before and after exercise was done using Spearman’s correlation coefficient (rs). A significance level of P < 0.05 was considered as statistical significance.
RESULTS
Seventy-five patients were initially screened for study of which 60 agreed to participate. Forty five of 60 complied with the exercise program (compliant group) while 15 did not (non-compliant group). Majority of our patients had non-specific minor symptoms like dyspepsia, right upper quadrant heaviness or had been referred for incidental detection of fatty liver on ultrasonography(USG) and elevated transaminases on routine blood tests.
Baseline parameters
Majority of study population were male (70%, 42/70) with mean age being 40.0 ± 8.9 years. While 70% (42/60) patients were overweight, 95% (57/60) had central obesity (WC ≥ 0.90 in men and ≥ 0.80 in women). Two of our patient had essential hypertension and none of our patients were diabetic. All of our patients were nonalcoholic and had negative viral markers. On USG examination 19 (32%) patients had grade 1, 30 (50%) had grade 2 and 11 (18%) had grade 3 fatty liver.
Histopathology
Thirty-two (53%) patients gave consent for liver biopsy and findings were given in Table 1. All liver biopsies showed fatty infiltration predominantly macro vesicular. Grade 1 fatty infiltration was present in 12 patients, while grade 2 in 15 and grade 3 in 5. Ballooning degeneration was present in 8 (25%) and glycogenated nuclei in 11 (34.3%). Majority of the biopsies had mild to moderate necroinflammatory activity (grade 1 in 10, grade 2 in 17). Only 5 biopsies showed severe (grade 3) necroinflammatory activity. None of the biopsies showed cirrhotic changes, while grade 1 fibrosis was seen in 14 patients, grade 2 in 4 and grade 3 in 3 patients. Eleven patients had no fibrosis on liver biopsy.
Table 1.
Histological characteristics of nonalcoholic steatohepatitis on liver biopsy
| Histological finding | n = 32 |
| Steatosis | |
| Grade 1 | 12 |
| Grade 2 | 15 |
| Grade 3 | 5 |
| Ballooning degeneration | 8 |
| Glycogenated nuclei | 11 |
| Necroinflammatory activity | |
| Grade 1 | 10 |
| Grade 2 | 17 |
| Grade 3 | 5 |
| Staging fibrosis | |
| Stage 0 | 11 |
| Stage 1 | 14 |
| Stage 2 | 4 |
| Stage 3 | 3 |
Insulin resistance and metabolic syndrome
Mean insulin resistance level in our patients was 7.1 ± 5.1. Fifty-six (93%) patients had HOMA-IR > 2. The details of the presence of the components of metabolic syndrome according World Health Organization (WHO) criteria and according to modified Indian criteria are given in Table 2. 17% (10) patients had metabolic syndrome according to WHO criteria, but when the modified Indian criterion for WC was used 37% (22) fulfilled the criteria. Only one patient in our study population did not have any component of metabolic syndrome when Indian criteria were used. The most common feature observed was high WC (modified) in 57 (95%) followed by low HDL in 38 (63%).
Table 2.
Components of metabolic syndrome seen in our patients n = 60 (%) according to National Cholesterol Education Program adult treatment panel III and waist modified by Indian criteria
| Components present | NCEP ATP III | Indian criteria |
| None | 7 (12) | 1 (2) |
| 1 | 20 (34) | 13 (22) |
| 2 | 23 (38) | 24 (40) |
| 3 | 8 (13) | 18 (30) |
| 4 | 2 (4) | 4 (7) |
NCEP ATP III: National Cholesterol Education Program adult treatment panel III.
Baseline comparison of compliant and non-compliant groups
Exercise compliant and non-compliant groups had no significant difference in baseline characteristics as shown in Table 3. Demographic profile, anthropometric parameters and baseline biochemical results were not different in two groups.
Table 3.
Baseline demographic, anthropometric and biochemical characteristics of patients
| Characteristic | Non-compliant (n = 15) | Compliant group (n = 45) | P value1 |
| Age (yr) | 39.6 ± 8.9 | 40.1 ± 9.0 | NS |
| Gender (Males) | 9 | 37 | |
| BMI (kg/m2) | 27.6 ± 3.8 | 26.7 ± 3.3 | NS |
| < 25 | 6 | 15 | |
| 25-30 | 6 | 20 | |
| > 30 | 3 | 10 | |
| WC | 98.0 ± 8.6 | 95.7 ± 8.9 | NS |
| Fasting glucose (mg/dL) | 86.4 ± 11.3 | 86.8 ± 14.3 | NS |
| 2 h glucose (mg/dL) | 128.2 ± 43.5 | 123.5 ± 41.8 | NS |
| Serum albumin (g/dL) | 3.9 ± 0.4 | 4.0 ± 0.4 | NS |
| AST (U/L) | 78.3 ± 460.2 | 76.2 ± 46.2 | NS |
| ALT (U/L) | 82.0 ± 45.0 | 84.8 ± 43.5 | NS |
| Total cholesterol (mg/dL) | 202.2 ± 45.0 | 194.6 ± 62.0 | NS |
| Triglycerides (mg/dL) | 178.5 ± 102.6 | 186.4 ± 112.5 | NS |
| LDL (mg/dL) | 114.5 ± 43.5 | 120.4 ± 56.4 | NS |
| HDL (mg/dL) | 38.5 ± 18.5 | 37.4 ± 8.6 | NS |
Mann Whitney U test. BMI: Body mass index; WC: Waist circumference; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; LDL: Low-density lipoprotein; HDL: High-density lipoprotein. NS: Not significant.
Effect of exercise on BMI, WC, aminotransferases
Comparison of anthropometric measurements before after 6 mo of intervention is given in Table 4. Compliant group showed significant decrease in BMI [26.7 ± 3.3 kg/m2 vs 25.0 ± 3.3 kg/m2 (P < 0.001)] and WC [95.7 ± 8.9 cm to 90.8 ± 7.3 cm (P < 0. 001)]. ALT also showed significant improvement from 84.8 ± 43.5 U/L to 41.3± 18.2 U/L, (P < 0.001) respectively.
Table 4.
Sub-group analysis of the compliant group - exercise alone and both exercise and diet modification and comparison with non-compliant group
| Variable | Non-compliant (n = 15) | Exercise (n = 12) | Exercise and diet (n = 33) | ||||||
| Pre | Post | P value1 | Pre | Post | P value1 | Pre | Post | P value1 | |
| BMI (kg/m2) | 27.6 ± 3.8 | 27.7 ± 3.5 | NS | 23.0 ± 1.4 | 21.7 ± 1.6 | NS | 28.5 ± 2.4 | 26.6 ± 2.7 | < 0.001 |
| WC (cm) | 99.2 ± 7.00 | 98.7 ± 8.4 | NS | 88.0 ± 7.0 | 85.4 ± 7.2 | 0.001 | 99.5 ± 7.2 | 93.6 ± 5.5 | < 0.001 |
| WHR | 0.9 ± 0.3 | 0.9 ± 0.1 | NS | 0.9 ± 0.3 | 0.9 ± 0.1 | 0.006 | 0.9 ± 0.6 | 0.9 ± 0.1 | 0.001 |
| ALT (U/L) | 82.0 ± 45.0 | 78.2 ± 18.6 | NS | 69.8 ± 32.3 | 34.6 ± 13.1 | 0.001 | 90.3 ± 46.1 | 43.8 ± 19.4 | 0.001 |
| HOMA-IR | 8.7 ± 4.1 | 10.5 ± 9.8 | NS | 6.2 ± 4.6 | 1.2 ± 0.8 | 0.002 | 6.5 ± 4.4 | 1.4 ± 1.1 | < 0.001 |
Wilcoxon signed rank test. BMI: Body mass index; WC: Waist circumference; WHR: Waist-hip ratio; ALT: Alanine aminotransferase; HOMA-IR: Homeostasis model assessment-estimated insulin resistance. NS: Not significant.
Among compliant group, there was 2.9 kg mean weight loss in patients who were advised both exercise and dietary restriction, while 0.3 kg decrease in weight was seen in patients who were advised only exercise (normal BMI group). Noncompliant group had increase of 0.4kg weight at the end of 6 mo. Patients who were advised exercise only showed no significant change in BMI [23.0 ± 1.4 kg/m2 vs 21.7 ± 1.6 kg/m2; P = not significant (NS)], but they had significant improvement in waist circumference (88.0 ± 7.0 cm vs 85.4 ± 7.2 cm, P = 0.001). Patients who were advised exercise and diet restriction (high BMI) showed significant improvement in BMI, waist circumference (28.5 ± 2.4 kg/m2 vs 26.6 ± 2.7 kg/m2, P < 0.001 and 99.5 ± 7.2 cm vs 93.6 ± 5.5 cm, P < 0.001, respectively).
Effect of exercise on insulin resistance and correlation with ALT
Insulin resistance showed a significant decline at the end of exercise program in the compliant group. Insulin resistance decreased significantly in both combined group and exercise alone group (6.5 ± 4.8 to 1.4 ± 1.1 and 6.2 ± 4.6 to 1.2 ± 0.8 respectively) (Figure 1). In the non-compliant group there was no significant change in insulin resistance level before and after exercise (Table 4). Using Spearman’s correlation, the decline in ALT correlated with decline in insulin resistance levels in the compliant group (P = 0.01, rs = 0.903), but there was no correlation in noncompliant group (P = NS), rs = 0.321), (Figure 2).
Figure 1.
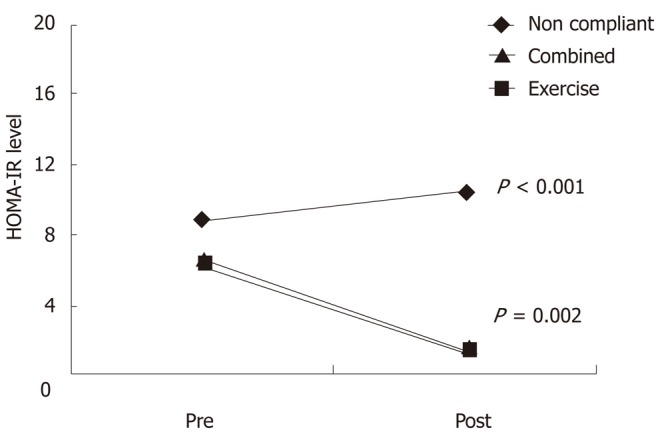
Diagrammatic representation of homeostasis model assessment-estimated insulin resistance levels of all the patients in the 3 groups before and after exercise. HOMA-IR: Homeostasis model assessment-estimated insulin resistance.
Figure 2.
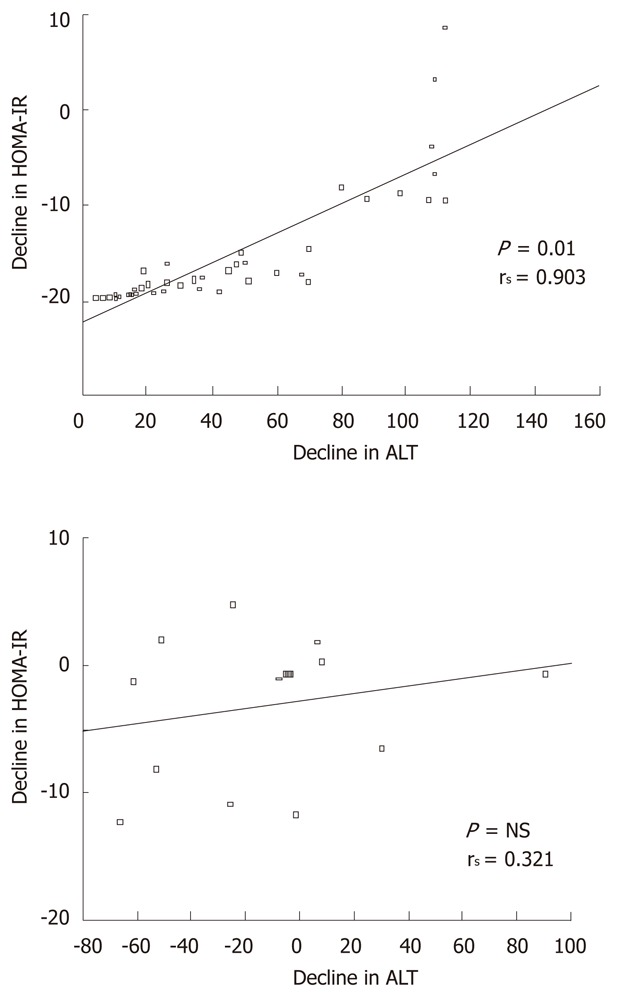
Correlation of decline in homeostasis model assessment-estimated insulin resistance levels to serum alanine aminotransferase in the compliant and non-compliant group using Spearman’s test. NS: Not significant.
At the end of follow-up, 24 compliant patients had normal ALT levels while in none of the noncompliant patients ALT normalized.
Effect of exercise on liver histology and correlation with insulin resistance and anthropometry
Eight patients had repeat liver biopsy in compliant group, of which six showed improvement in steatosis, necroinflammatroy score with no change in fibrosis score and two had no change of the NASH score (Table 5 and Figures 3 and 4). Total NASH score in eight patients decreased from 5.3 ± 1.5 to 3.3 ± 1.5 (P = 0.02).
Table 5.
Anthropometric, biochemical and histological characteristics of patients with paired liver biopsy (n = 8)
| Variable | Pre-intervention | Post-intervention | P value1 |
| BMI (kg/m2) | 27.6 ± 3.8 | 26.0 ± 2.4 | 0.012 |
| WC (cm) | 96.3 ± 7.6 | 91.0 ± 6.3 | 0.01 |
| ALT (U/L) | 99.8 ± 37.2 | 34.0 ± 16.8 | 0.01 |
| HOMA-IR | 6.1 ± 3.8 | 1.04 ± 0.5 | 0.01 |
| NASH score | 5.3 ± 1.5 | 3.3 ± 1.5 | 0.02 |
Wilcoxon sign rank test. BMI: Body mass index; WC: Waist circumference; ALT: Alanine aminotransferase; HOMA-IR: Homeostasis model assessment-estimated insulin resistance; NASH: Nonalcoholic steatohepatitis.
Figure 3.
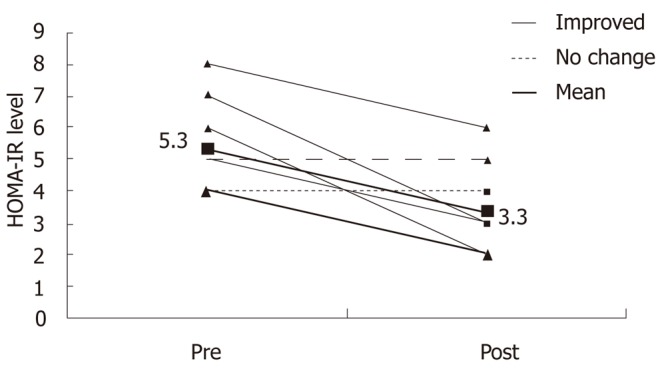
Change in nonalcoholic steatohepatitis score pre and post intervention (n = 8).
Figure 4.
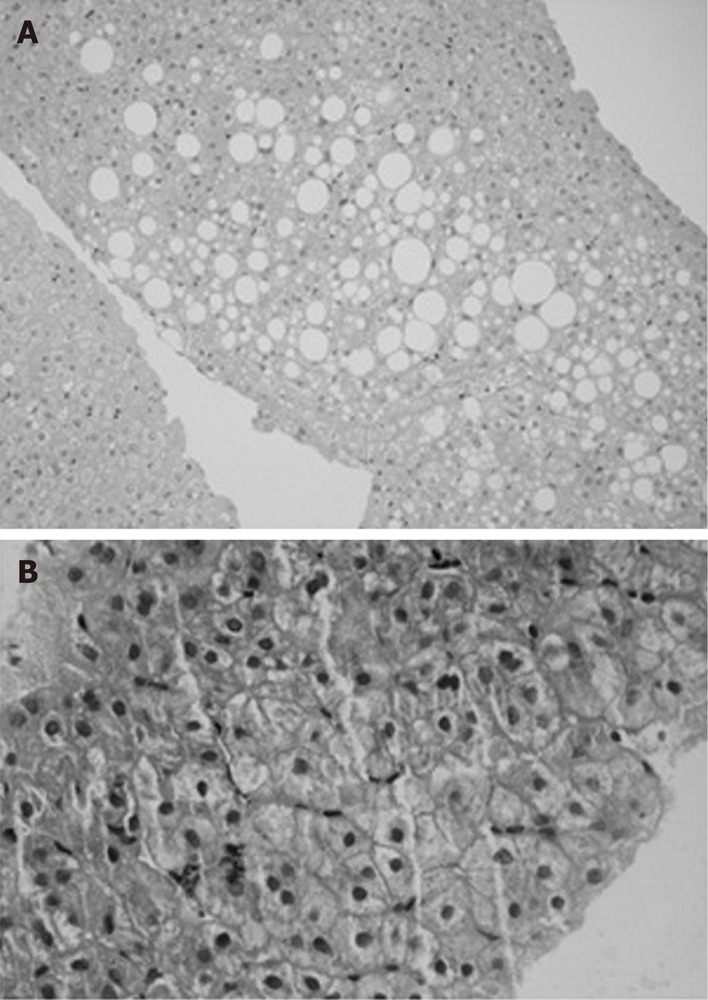
Liver histology pre (A) and post (B) intervention in compliant group.
Using spearman’s correlation decline in HOMA-IR correlated with decline in NASH score in these 8 patient with repeat histology (P = 0.03, rs = 0.73). Improvement in NASH score also correlated with decline in WC (P = 0.04, rs = 0.65), BMI (P = 0.05, rs = 0.62) and ALT (P = 0.05, rs = 0.54) (Table 6.)
Table 6.
Correlation between improvement in nonalcoholic steatohepatitis score and anthropometry, biochemical characteristics
| Variable | rs | P value1 |
| BMI (kg/m2) | 0.62 | 0.05 |
| WC (cm) | 0.68 | 0.04 |
| ALT (U/L) | 0.54 | 0.05 |
| HOMA-IR | 0.73 | 0.03 |
Spearman correlation. BMI: Body mass index; WC: Waist circumference; ALT: Alanine aminotransferase; HOMA-IR: Homeostasis model assessment-estimated insulin resistance.
DISCUSSION
Our study showed that regular aerobic exercise by promoting weight loss resulted in improvement in insulin resistance, aminotransferase level and liver histology after 6 mo. All these patients had insulin resistance at baseline and persistent elevation of serum aminotransferase before entering into the study. Previous studies have shown that weight reduction results in normalization of alanine aminotransferase[16,17]. In the present study, improvement in insulin resistance and aminotransferase was seen in all exercise compliant subjects.
In an earlier study, we have shown that lifestyle modification adapted by previously sedentary patients resulted in improvement aminotransferase levels after 3 mo[17]. In the present study we demonstrated that decline in aminotransferase correlated with improvement in insulin resistance and in a small proportion of exercise compliant patient we documented histological improvement with paired liver biopsy.
There was significant correlation in the decline of ALT to improvement insulin resistance which shows the causal relation of insulin resistance in NAFLD. This proves that exercise improves insulin sensitivity resulting to decline in ALT level. Insulin resistance, with the other features of the metabolic syndrome, is now regarded major mechanism for development of NAFLD, even in the absence of obesity and diabetes mellitus[10]. Regular aerobic exercise has been shown to improve insulin sensitivity and alter substrate use in skeletal muscle. This effect is by up -regulation of insulin receptor substrate (IRS-1) which promotes GLUT4 transporter protein necessary for the uptake of glucose by muscle[28]. Exercise has also been shown to increase oxidative capacity of muscle cells and utilization of fatty acids for oxidation. This decreases fatty acids and triglyceride accumulation in the myocytes and thereby improves insulin sensitivity[29].
In this study exercise compliant patients had an average 2.9 kg weight reduction in high BMI group compared to 0.4 kg weight in noncompliant group. Previous studies have shown benefit of weight reduction in improving serum aminotransferases, but optimum rate and amount of weight loss needed to achieve this benefit are still unclear. In obese children reduction of around 0.5 kg/wk with dietary modification had been shown to improve serum aminotransferase level[30]. We found around 5% decline in weight and BMI is sufficient to achieve significant decline in insulin resistance. Earlier studies have shown that around 10 % weight reduction results in significant improvement in clinical condition[31].
Majority patients in our study had visceral adiposity. While 30% patients had normal BMI (< 23 kg/m2), visceral adiposity was present in 95%. Earlier studies have shown that Asian people have higher visceral fat compared to western population for a given BMI[32]. The normal BMI and WHR were also found to be lower in Asian Indians. The key finding in our study was significant improvement in IR with decline observed in waist circumference and WHR in the normal BMI group without significant reduction in their BMI. This decrease in waist circumference in these groups is likely to be due to decrease in abdominal fat stores. Regular aerobic exercise seems to redistribute the fat stores in the body, which ultimately leads to decrease in visceral obesity and heightens the insulin responsiveness in adipose tissue[33]. Data from other studies suggest that regular physical exercise reduces the risk of developing non-insulin dependent diabetes mellitus and improves blood cholesterol (LDL and HDL) level, both of which are significant risk factors for NASH[33,34]. The use of life style modification has shown more encouraging results than metformin in prevention of diabetes (58% vs 31%) in Diabetes Prevention Program[35]. As type 2 diabetes is the ultimate outcome of severe insulin resistance and NAFLD is considered as another consequence of insulin resistance, should also preventable by lifestyle modification. Obesity and insulin resistance with all known health consequences is now considered high priority, it is reasonable to think widespread community adoption of lifestyle modification will help to overcome present century epidemic of obesity related health hazard including NAFLD.
There is enough data to suggest reduction in visceral adiposity is an important step in improving insulin resistance. In a previous study with 32 obese patients, improvement in insulin resistance correlated with change in regional adiposity[33]. Previous data suggest exercise induced reduction in total and regional adiposity is positively associated with intensity of exercise[36]. These data corroborate our finding of improvement in IR correlates with improvement in visceral adiposity.
There is very limited data available on the efficacy of non-pharmacological and pharmacological interventions on liver histology, particularly necroinflammation with NAFLD. Two previous studies with paired liver biopsies showed improvement or stable liver histology (both necro-inflammation and fibrosis score)[37,38]. In our study eight patients had repeat liver biopsy, six had significant improvement in necroinflammatory score, 2 had stable score and none had worsening of NASH score over 6 mo period.
Improvement in NASH score correlated with improvement in insulin resistance, waist circumference, BMI and ALT. Our study highlights the fact serum aminotrasferase levels correlates with histological improvement in NAFLD patients. In a study by Gomez et al[38], addition of antioxidants to lifestyle modification intensifies improvement in insulin resistance and liver histology. This gives scope for future research about addition of pharmacological treatment (antioxidants and insulin sensitizers) to life style changes and their synergistic benefit on liver histology.
As this study was short-term, the greatest improvement in liver histology was seen in steatosis, and necroinflammatory score. Decrease in necroinflammatory score by ≥ 2 was observed in 2 patients and by 1 point in 4 patients indicating that life style changes was effective in decreasing hepatocyte inflammation. There was no change in fibrosis score, which is likely related to the short duration of the study.
Pharmacological treatment of NAFLD has shown variable results [11,12,39]. At present, very limited data available on impact of pharmacological agent on histological progression in NAFLD. Most promising among them are insulin sensitizer (thiazolidendiones and metformin) and antioxidants. However weight gain is known side effect of thiazolidendiones and the long-term effects in patients with NAFLD of these drugs are currently unknown.
We agree that our study was short term study, and none of noncompliant subjects had second liver biopsy as these patients were less motivated about life style changes and reluctant for repeat procedure. Despite these limitations we feel that aerobic exercise and dietary modifications improves insulin resistance resulting in histological improvement.
To summarize, based on these results, lifestyle modification improves insulin resistance and liver histology. All patients with NAFLD should be encouraged to continue moderate intensity aerobic exercise; improvement in serum ALT and insulin resistance can be used as laboratory parameters for effective treatment. We acknowledge that our study was short term and involved highly motivated patients, and confirming these results requires larger randomized controlled trials. Despite these limitations, we feel it is important to recommend lifestyle changes as the first line therapy in the treatment of NAFLD.
COMMENTS
Background
With the global epidemic of obesity, the problem of Nonalcoholic fatty liver disease (NAFLD) is going to be increasingly encounterd by clinicians. Most clinicians with emerging clinical epidemiological evidence regard NAFLD as part of metabolic syndrome. At present there is no established mode for treatment or prevention of NAFLD and metabolic syndrome. Short term weight losing measures has shown to improve obesity and aminotransferase levels, but its effect on insulin resistance and liver histology has not been fully established.
Research frontiers
Insulin resistance has been recognized as major mechanism (first hit) for the development of NAFLD. In this study author demonstrated improvement in insulin sensitivity with life style modification and its benefit on liver histology.
Innovations and breakthroughs
Recent studies have shown that life style modification shows improvement in liver histology. In this study, authors showed improvement in insulin sensitivity correlates with improvement in liver histology. Improvement in aminotransferase level and insulin resistance with life style modification can be used as a laboratory parameter for effective treatment.
Applications
Future studies needed to evaluate the effect of life style modification with pharmacological treatment (insulin sensitizer) on insulin sensitivity and liver histology.
Terminology
Inulin resistances play key role in the pathogenesis of NAFLD. homeostasis model assessment-estimated insulin resistance method, even though not so accurate, can be used as simple method to calculate insulin resistance in clinical practice.
Peer review
In this paper, the authors found that in exercise compliant individuals decline in insulin resistance correlated with decline in alanine aminotransferase levels and liver histology. An increase in serum ferritin level is feature of NAFLD and authors have to discuss effect on ferritin levels. An intake of fish is associated with insulin resistance and ALT levels, authors’ needs to investigate the change in dietary habits. Inflammation is associated with insulin resistance and authors needs to demonstrate data for inflammation including C - reactive protein and interleukin-6. One of the key issues is how to treat NAFLD patient with poor compliance for lifestyle modification. The authors have to discuss the point by referring the new exercise device.
Footnotes
Peer reviewers: Manuel Vázquez-Carrera, PhD, Department of Pharmacology and Therapeutics, Faculty of Pharmacy, Diagonal 643, Barcelona E-08028, Spain; Dr. Takumi Kawaguchi, Kurume University School of Medicine, 67 Asahi-machi, Kurume 830-0011, Japan
S- Editor Wu X L- Editor A E- Editor Wu X
References
- 1.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 2.Abdelmalek MF, Diehl AM. Nonalcoholic fatty liver disease as a complication of insulin resistance. Med Clin North Am. 2007;91:1125–149, ix. doi: 10.1016/j.mcna.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 4.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 5.Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40 Suppl 1:S5–10. doi: 10.1097/01.mcg.0000168638.84840.ff. [DOI] [PubMed] [Google Scholar]
- 6.Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679–689. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, Omatsu T, Nakajima T, Sarui H, Shimazaki M, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722–728. doi: 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]
- 8.Gupte P, Amarapurkar D, Agal S, Baijal R, Kulshrestha P, Pramanik S, Patel N, Madan A, Amarapurkar A. Non-alcoholic steatohepatitis in type 2 diabetes mellitus. J Gastroenterol Hepatol. 2004;19:854–858. doi: 10.1111/j.1440-1746.2004.03312.x. [DOI] [PubMed] [Google Scholar]
- 9.Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 10.Utzschneider KM, Kahn SE. Review: The role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006;91:4753–4761. doi: 10.1210/jc.2006-0587. [DOI] [PubMed] [Google Scholar]
- 11.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang CY, Argo CK, Al-Osaimi AM, Caldwell SH. Therapy of NAFLD: antioxidants and cytoprotective agents. J Clin Gastroenterol. 2006;40 Suppl 1:S51–S60. doi: 10.1097/01.mcg.0000168648.79034.67. [DOI] [PubMed] [Google Scholar]
- 13.Kantartzis K, Thamer C, Peter A, Machann J, Schick F, Schraml C, Königsrainer A, Königsrainer I, Kröber S, Niess A, et al. High cardiorespiratory fitness is an independent predictor of the reduction in liver fat during a lifestyle intervention in non-alcoholic fatty liver disease. Gut. 2009;58:1281–1288. doi: 10.1136/gut.2008.151977. [DOI] [PubMed] [Google Scholar]
- 14.Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol. 2012;56:255–266. doi: 10.1016/j.jhep.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Ueno T, Sugawara H, Sujaku K, Hashimoto O, Tsuji R, Tamaki S, Torimura T, Inuzuka S, Sata M, Tanikawa K. Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol. 1997;27:103–107. doi: 10.1016/s0168-8278(97)80287-5. [DOI] [PubMed] [Google Scholar]
- 16.Hickman IJ, Jonsson JR, Prins JB, Ash S, Purdie DM, Clouston AD, Powell EE. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut. 2004;53:413–419. doi: 10.1136/gut.2003.027581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sreenivasa Baba C, Alexander G, Kalyani B, Pandey R, Rastogi S, Pandey A, Choudhuri G. Effect of exercise and dietary modification on serum aminotransferase levels in patients with nonalcoholic steatohepatitis. J Gastroenterol Hepatol. 2006;21:191–198. doi: 10.1111/j.1440-1746.2005.04233.x. [DOI] [PubMed] [Google Scholar]
- 18.Willett WC, Dietz WH, Colditz GA. Guidelines for healthy weight. N Engl J Med. 1999;341:427–434. doi: 10.1056/NEJM199908053410607. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications - Part 1: Diagnosis and classification of diabetes mellitus. Geneva: World Health Organization;; 1999. pp. 20–21. [Google Scholar]
- 20.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 21.Misra A, Vikram NK, Gupta R, Pandey RM, Wasir JS, Gupta VP. Waist circumference cutoff points and action levels for Asian Indians for identification of abdominal obesity. Int J Obes (Lond) 2006;30:106–111. doi: 10.1038/sj.ijo.0803111. [DOI] [PubMed] [Google Scholar]
- 22.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, Monauni T, Muggeo M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 23.Romero-Gómez M, Del Mar Viloria M, Andrade RJ, Salmerón J, Diago M, Fernández-Rodríguez CM, Corpas R, Cruz M, Grande L, Vázquez L, et al. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology. 2005;128:636–641. doi: 10.1053/j.gastro.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 24.Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705–1725. doi: 10.1053/gast.2002.36572. [DOI] [PubMed] [Google Scholar]
- 25.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 26.Chaitman BR. Exercise stress testing. In: Heart disease: a textbook of cardiovascular medicine., editor. 9th ed. Braunwald E, editor. Philadelphia: WB Saunders; 2008. pp. 168–197. [Google Scholar]
- 27.Yamamoto R, Inoue S, Saito M, Okamoto M, Okamura A, Takamura Y. Very-low-calorie-diet therapy in severe obesity. Am J Clin Nutr. 1992;56:299S–302S. doi: 10.1093/ajcn/56.1.299S. [DOI] [PubMed] [Google Scholar]
- 28.Short KR, Vittone JL, Bigelow ML, Proctor DN, Rizza RA, Coenen-Schimke JM, Nair KS. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes. 2003;52:1888–1896. doi: 10.2337/diabetes.52.8.1888. [DOI] [PubMed] [Google Scholar]
- 29.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. 2000;49:677–683. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- 30.Vajro P, Fontanella A, Perna C, Orso G, Tedesco M, De Vincenzo A. Persistent hyperaminotransferasemia resolving after weight reduction in obese children. J Pediatr. 1994;125:239–241. doi: 10.1016/s0022-3476(94)70202-0. [DOI] [PubMed] [Google Scholar]
- 31.Palmer M, Schaffner F. Effect of weight reduction on hepatic abnormalities in overweight patients. Gastroenterology. 1990;99:1408–1413. doi: 10.1016/0016-5085(90)91169-7. [DOI] [PubMed] [Google Scholar]
- 32.Bergmann J, Oehme P, Bienert M, Niedrich H. [Studies on the mechanism of action of peptide attacking smooth muscle. II. Differentiation of biologic activity of tachykinins in affinity and intrinsic efficacy] Acta Biol Med Ger. 1975;34:475–481. [PubMed] [Google Scholar]
- 33.Goodpaster BH, Kelley DE, Wing RR, Meier A, Thaete FL. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes. 1999;48:839–847. doi: 10.2337/diabetes.48.4.839. [DOI] [PubMed] [Google Scholar]
- 34.St George A, Bauman A, Johnston A, Farrell G, Chey T, George J. Independent effects of physical activity in patients with nonalcoholic fatty liver disease. Hepatology. 2009;50:68–76. doi: 10.1002/hep.22940. [DOI] [PubMed] [Google Scholar]
- 35.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ross R, Janssen I. Physical activity, total and regional obesity: dose-response considerations. Med Sci Sports Exerc. 2001;33:S521–S57; discussion S521-S57. doi: 10.1097/00005768-200106001-00023. [DOI] [PubMed] [Google Scholar]
- 37.Huang MA, Greenson JK, Chao C, Anderson L, Peterman D, Jacobson J, Emick D, Lok AS, Conjeevaram HS. One-year intense nutritional counseling results in histological improvement in patients with non-alcoholic steatohepatitis: a pilot study. Am J Gastroenterol. 2005;100:1072–1081. doi: 10.1111/j.1572-0241.2005.41334.x. [DOI] [PubMed] [Google Scholar]
- 38.Vilar Gomez E, Rodriguez De Miranda A, Gra Oramas B, Arus Soler E, Llanio Navarro R, Calzadilla Bertot L, Yasells Garcia A, Del Rosario Abreu Vazquez M. Clinical trial: a nutritional supplement Viusid, in combination with diet and exercise, in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2009;30:999–1009. doi: 10.1111/j.1365-2036.2009.04122.x. [DOI] [PubMed] [Google Scholar]
- 39.Van Wagner LB, Rinella ME. The role of insulin-sensitizing agents in the treatment of nonalcoholic steatohepatitis. Therap Adv Gastroenterol. 2011;4:249–263. doi: 10.1177/1756283X11403809. [DOI] [PMC free article] [PubMed] [Google Scholar]


