Highlights
► Interleukin-6 is a multifunctional cytokine which regulates growth of prostate cancer. ► Stimulation of androgen receptor activity by interleukin-6 may enhance or inhibit proliferation. ► Interleukin-6 inhibits apoptosis in several prostate cancer cell lines. ► Suppressor of cytokine signalling-3 is expressed in prostate cancer. ► Antibody CNTO 328 (Anti IL-6) was tested in preclinical research and clinical trials.
Keywords: Interleukin-6, Prostate cancer, Androgen receptor, Apoptosis, Therapy, SOCS
Abstract
Several cytokines are involved in regulation of cellular events in prostate cancer. Interleukin-6 (IL-6) was frequently investigated in prostate cancer models because of its increased expression in cancer tissue at early stages of the disease. In patients with metastatic prostate cancer, it is well-known that IL-6 levels increase in serum. High levels of IL-6 were measured in the supernatants of cells which do not respond to androgenic stimulation. IL-6 expression in prostate cancer increases due to enhanced expression of transforming growth factor-beta, and members of the activating protein-1 complex, and loss of the retinoblastoma tumour suppressor. IL-6 activation of androgen receptor (AR) may contribute to progression of a subgroup of prostate cancers. Results obtained with two prostate cancer cell lines, LNCaP and MDA PCa 2b, indicate that IL-6 activation of AR may cause either stimulatory or inhibitory responses on proliferation. Interestingly, prolonged treatment with IL-6 led to establishment of an IL-6 autocrine loop, suppressed signal transducer and activator of transcription (STAT)3 activation, and increased mitogen-activated protein kinase phosphorylation. In several cell lines IL-6 acts as a survival molecule through activation of the signalling pathway of phosphotidylinositol 3-kinase. Expression of suppressors of cytokine signalling (SOCS) has been studied in prostate cancer. SOCS-3 prevents phosphorylation of STAT3 and is an important anti-apoptotic factor in AR-negative prostate cancer cells. Experimental therapy against IL-6 in prostate cancer is based on the use of the monoclonal antibody siltuximab which may be used for personalised therapy coming in the future.
1. Multiple effects of interleukin-6 (IL-6) in human prostate cancer
Expression and function of pro-inflammatory cytokines in prostate cancer has been extensively investigated because of their role in regulation of proliferation, apoptosis, migration, invasion, and angiogenesis. In this manuscript, we will pay attention to the role of IL-6. Although investigations on IL-6 in prostate carcinogenesis were mostly carried out in models representing advanced tumours, it is anticipated that the cytokine has a major role in early stages of carcinogenesis (Spiotto and Chung, 2000; Hobisch et al., 2001). This is an important issue which will surely get more attention in the next years because of a need to better understand the events in very small locally-confined prostate cancers. As a consequence of improved diagnostic and screening, it became possible to detect a larger number of small tumours which will most probably not become clinically significant during patients’ life time. The subject of long-term development of pre-malignant lesions and cancer has been studied in a small number of reliable models. We briefly mention induction of inflammatory pre-malignant lesions in Noble and Fisher rats. For the first time, inflammatory-like changes could be induced in prostates of Noble rats by co-administration of testosterone and 17 beta oestradiol (Tam et al., 2007). In addition, treatment with the chemical carcinogen PhIP which is present in red meat may induce morphological changes such as chronic inflammation, proliferative inflammatory atrophy, and prostate intraepithelial neoplasia (Borowsky et al., 2006; Nakai et al., 2007). The role of pro-inflammatory cytokines in these pre-malignant lesions has not been clarified so far but may represent an interesting area of investigation in order to delineate their specific functions during early prostate tumour development.
IL-6 is known as a multifunctional cytokine which is a major activator of the signalling pathway of Janus kinases (JAK)/signal transducer and activator of transcription (STAT)3 (Masuda et al., 2010). In addition to JAK/STAT, IL-6 may phosphorylate mitogen-activated protein kinases (MAPK) and Akt. Different pathways can be activated in response to IL-6 in a cell line at the same time. STAT3 is also phosphorylated by epidermal growth factor. STAT3 has been regarded as an oncogene in many cancers and its ability to cause malignant cellular transformation has been demonstrated in multiple models. In prostate cancers, the situation appears to be more complex (Degeorges et al., 1996; Giri et al., 2001). It should be pointed out that the treatment of LNCaP xenografts with IL-6 resulted in a reduction of tumour volume (Wang et al., 2004). The variable effects of IL-6 on proliferation of cancer cells, positive or negative, could be explained by differences in sera used in various laboratories or in autocrine loops’ activation (Komyod et al., 2007). These experimental data have been obtained in melanoma but may be also relevant in prostate cancer. If cellular density is high, autocrine growth factors may prevent a growth-inhibitory effect of IL-6/STAT3. In some of the experiments performed with LNCaP cells, it was shown that IL-6-caused growth arrest is associated with neuroendocrine differentiation, as manifested with cellular morphological changes such as elongation (Spiotto and Chung, 2000). In clinics, the presence of neuroendocrine cells is frequently correlated with less favourable prognosis because of their ability to secrete proteins which affect proliferation, migration or invasion in a paracrine manner (Yuan et al., 2007). Some of these neuropeptides, such as bombesin, induce AR activity in a ligand-independent manner (Desai et al., 2006).
2. IL-6 and androgen receptor (AR) activation
AR has been identified as a target for therapy in human prostate cancer since it is overexpressed in many therapy-resistant tumours and its inhibition may contribute to delayed tumour progression (Zegarra-Moro et al., 2002; Chen et al., 2004; Andersen et al., 2010; Siddiqui et al., 2011). AR activation by non-steroidal compounds may be of importance for cancer progression, for example in case of the oncogene HER2 or related growth factors. However, this type of receptor activation may also contribute to a pro-differentiation therapeutic effect of compounds such as phenylbutyrate or phenylacetate (Sadar and Gleave, 2000; Mellinghoff et al., 2004). In our earlier studies, we demonstrated that IL-6 causes activation of the AR in DU-145 cells transfected with AR cDNA and in LNCaP cells which express the endogenous receptor (Hobisch et al., 1998). The treatment of LNCaP cells with IL-6 has resulted in inhibition of proliferation and in increased expression of the differentiation marker prostate-specific antigen (PSA) in the absence of androgen. This effect was blocked by the non-steroidal anti-androgen bicalutamide. Thus, in case of LNCaP cells, the effect of IL-6 on the AR supports cellular differentiation (Hobisch et al., 1998). Interestingly, in the experiments in which another cell line, MDA PCa 2b, was used, it has been demonstrated that IL-6 causes a stimulatory effect on proliferation. Those data were supported by results of an in vivo experiment in which it was shown that IL-6-induced activation of the AR leads to an enhanced growth. This effect could be antagonized by application of bicalutamide (Malinowska et al., 2009). Although the effect of IL-6 in comparison to that of androgen was relatively low, AR activity was sufficiently induced to enhance tumour growth in vivo. In the MDA PCa 2b cell line, the p44/p42 MAPK PD 98059 inhibitor blocked the effects of IL-6. The reasons for a different outcome of IL-6/AR activation in prostate cancer cells have not been clarified yet. Cell line dependent differences in recruitment of specific coactivators of the AR, one possible explanation of this discrepancy has to be investigated in future studies. In this context, it was demonstrated that the transcriptional integrator p300 which is overexpressed in prostate cancer and correlates with poor prognosis is required for ligand-independent activation of AR (Debes et al., 2002). Similar findings were also reported for SRC-1 (Ueda et al., 2002). MAPK phosphorylation of SRC-1 was required for the induction of AR activity by IL-6. It is understood that IL-6 causes phosphorylation of Etk kinase which can be additionally activated by the kinase Pim1 if coexpressed in prostate cancer cells (Kim et al., 2004). These kinases synergize in ligand-independent activation of AR by IL-6 (Kim et al., 2004).
3. Prolonged treatment of prostate cancer cells with IL-6 and its clinical relevance
In order to improve understanding of cellular events which may be relevant to patients with prostate cancer who are exposed to IL-6 during a prolonged period of time, we have treated LNCaP cells with IL-6 over several months (Hobisch et al., 2001). Prostate cancers do not lack the IL-6 receptor (IL-6R) and therefore continuous stimulation with a cytokine in an in vitro model is relevant (Hobisch et al., 2000). The findings obtained on primary tumour material regarding IL-6 and IL-6 receptor correspond with those seen in cultured cell lines (Hobisch et al., 2001). IL-6 immunoreactivity was additionally confirmed in tumour tissue. The newly developed cell line, LNCaP-IL-6+, showed a growth advantage and different activation of signalling pathways in comparison to parental cells (Steiner et al., 2003). Instead of phosphorylation of STAT3, an increased expression and phosphorylation of MAPK was observed in association with higher expression of cyclin-dependent kinases and loss of the tumour suppressors retinoblastoma and p27. LNCaP-IL-6+ cells also show a high expression of vascular-endothelial growth factor, a peptide which could be also induced after short-time treatment with IL-6 (Steiner et al., 2004). As a consequence, it is not surprising that LNCaP-IL-6+ cells grow faster in vivo in comparison to control cells. A growth advantage of LNCaP-IL-6+ was confirmed by the Gao’s laboratory. Those researchers demonstrated that cells generated after prolonged treatment with the cytokine grow faster (Lee et al., 2007). In concordance with the development of this model in which the IL-6 autocrine loop was demonstrated, it was shown that the transfection of LNCaP cells with IL-6 cDNA also results in enhanced growth due to activation of STAT and MAPK signalling (Lee et al., 2003). Moreover, those authors showed that overexpression of IL-6 in prostate cancer leads to an androgen-independent growth of tumour cells. LNCaP-IL-6+ cells are a good model for studies of function of AR co-activators in prostate cancer. The AR coactivator p300 was found to increase expression of endogenous genes which are AR-regulated even in conditions where the receptor is strongly inhibited (Debes et al., 2005). Those findings may be of special interest because there is a subgroup of prostate cancer cells in which the AR is silenced as a result of epigenetic changes (Jarrard et al., 1998). Even in this situation some of the AR target genes may be expressed, thus contributing to prostate cancer progression.
Importantly, it was also demonstrated that IL-6 may promote intracellular synthesis of androgens in prostate (Chun et al., 2009). This mechanism is clearly relevant to cancer progression because androgens synthesised in conditions in which AR is hypersensitive may enhance tumour growth after prolonged androgen ablation.
4. Molecular mechanisms leading to increase in IL-6 expression
Up-regulation of IL-6 in prostate cancer is a result of several cellular regulatory processes some of which are interconnected. It is not surprising that transforming growth factor (TGF)-beta, whose expression is also elevated in sera of prostate cancer patients (Adler et al., 1999), acts as a molecule which causes elevation of expression of IL-6 (Park et al., 2003). TGF-beta is a potent growth-inhibitory factor in prostate cancer in vitro. In contrast, it acts in vivo through activation of the angiogenetic cascade, suppression of immune responses, and induction of expression of matrix metalloproteinases thus causing enhanced tumour growth. One of the mechanisms which may contribute to this effect is thus increased IL-6 production which could in turn stimulate angiogenesis. Increased IL-6 production by LNCaP-IL-6+ cells may be also a consequence of a reduced expression of the retinoblastoma protein, which prevents up-regulation of IL-6. It is also important to mention that the presence of AR inhibits expression of IL-6. This finding is in concordance with the previously observed inhibition of nuclear factor kappa B activity by dihydrotestosterone (Keller et al., 1996). AR-negative PC-3 and DU-145 cells secrete high levels of IL-6 (Twillie et al., 1995). Finally, the expression of IL-6 is up-regulated by elements of the activating protein-1 complex Fra-1 and JunD (Zerbini et al., 2003) and a protein kinase C lambda/iota (Ishiguro et al., 2009).
5. The role of IL-6 in inhibition of apoptosis
In many cancers, IL-6 has been associated with an anti-apoptotic effect. In PC-3 cells, it was shown that IL-6 leads to activation of the phosphotidylinositol 3-kinase (PI3K) pathway (Chung et al., 2000). Thus, IL-6 contributes to enhanced activity of Akt which was frequently reported in prostate tumours (Ghosh et al., 2005). Etk/Bmx is another kinase which is an effector of the PI3K pathway and is induced by IL-6 in prostate cancer (Qiu et al., 1998). Further studies were performed in LNCaP-IL-6+ and DU-145 cells, in which it was demonstrated that Mcl-1, a member of the Bcl-2 family is up-regulated. These cells also have a high expression of IL-6. The effect of IL-6 on Mcl-1 up-regulation is mediated through the MAPK pathway. Mcl-1 was previously shown to be up-regulated in prostate cancer in comparison to the benign tissue (Krajewska et al., 1996). We demonstrated that LNCaP-IL-6+ cells are more resistant to induction of apoptosis due to increase of IL-6 expression (Cavarretta et al., 2007). Mcl-1 expression was down-regulated by siRNA which in consequence resulted in an increased sensitivity to apoptosis. In addition, Mcl-1 was also down-regulated after addition of the anti-IL-6 antibody siltuximab. In cells in which expression of Mcl-1 was inhibited by siRNA prior to siltuximab treatment, the anti-IL-6 antibody did not achieve further induction of apoptosis. In those experiments, we confirmed that, in cells with an established IL-6 autocrine loop, Mcl-1 is a critical factor being responsible for reduced sensitivity to apoptosis. IL-6, in addition to apoptosis, regulates migration and invasion of prostate cancer cells (Santer et al., 2010). These pro-metastatic events are regulated through the soluble IL-6 receptor which is expressed in most prostate cancer cells (Santer et al., 2010).
6. The role of endogenous inhibitors of IL-6 signalling in prostate cancer
Binding of IL-6 to the IL-6 receptor leads to an activation of intracellular signalling mostly through the JAK/STAT signal transduction pathway. At least three different classes of negative feedback regulators of cytokine signalling are known; (1) protein inhibitors of activated STATs (PIAS), (2) the Src-homology 2 (SH2) containing protein tyrosine phosphatases (SHPs), and (3) suppressors of cytokine signalling (SOCS) (Larsen and Ropke, 2002).
Proteins of the PIAS family prevent STAT–DNA interaction either directly or indirectly by inhibiting STAT dimerisation. These proteins act as a buffer thus minimising the amount of activated STAT that is available within the cell following stimulation by the cytokine. The SHPs are recruited to the phosphorylated tyrosine residues following JAK phosphorylation. They reduce their activity by enhancing dephosphorylation of tyrosine residues critical for the activation of the kinases (Hilton, 1999).
The SOCS family consists of eight members, cytokine inducible SH2 protein (CIS) and SOCS-1 to 7. The genomic structure suggests that the pairs of these proteins are more closely related to each other than to other SOCS proteins; CIS and SOCS-2, SOCS-1 and SOCS-3, SOCS-4 and SOCS-5, and SOCS-6 and SOCS-7, respectively (Hilton, 1999). All proteins contain a N-terminal region of variable length, a central SH2 domain, and a conserved C-terminal region called SOCS box. The SOCS box is not required for inhibitory actions of SOCS proteins. Despite this, the conservation of this domain in all SOCS proteins suggests an important role in their physiological action. This region interacts with Elongin-B, Elongin-C, Cullin-5, and RING-box-2 (Garrett et al., 1995). The complex can recruit E2 ubiquitin transferases (Kamura et al., 2004). Thus, the proteins can act as E3 ubiquitin ligases and mediate the proteasomal degradation of associated proteins. In addition, SOCS-1 and SOCS-3 have a kinase-inhibiting region within the N-terminal region. At present, there seems to be no clear evidence that SOCS-4, 5, 6, 7 mRNA or protein are induced after cytokine stimulation and not much is known about their function. CIS, SOCS-1, SOCS-2 and SOCS-3 are induced by various cytokines. SOCS-1 and SOCS-3 are especially induced by IL-6. Upon IL-6 stimulation, transcription of SOCS-1 and SOCS-3 genes is rapidly increased in vitro and in vivo (Starr et al., 1997). SOCS-1 and -3 can inhibit IL-6-mediated JAK activation directly through their kinase inhibitory region (Sasaki et al., 1999). Especially SOCS-3 can additionally bind with high affinity to phosphothyrosine 757 within the IL-6 receptor subunit glycoprotein 130 (gp130) and inhibits activation of the IL-6 signalling cascade (Nicholson et al., 2000) (Fig. 1). It is generally accepted that SOCS-1 and especially SOCS-3 act as important negative feedback regulators of the IL-6-JAK-STAT signalling pathway (Alexander and Hilton, 2004).
Fig. 1.
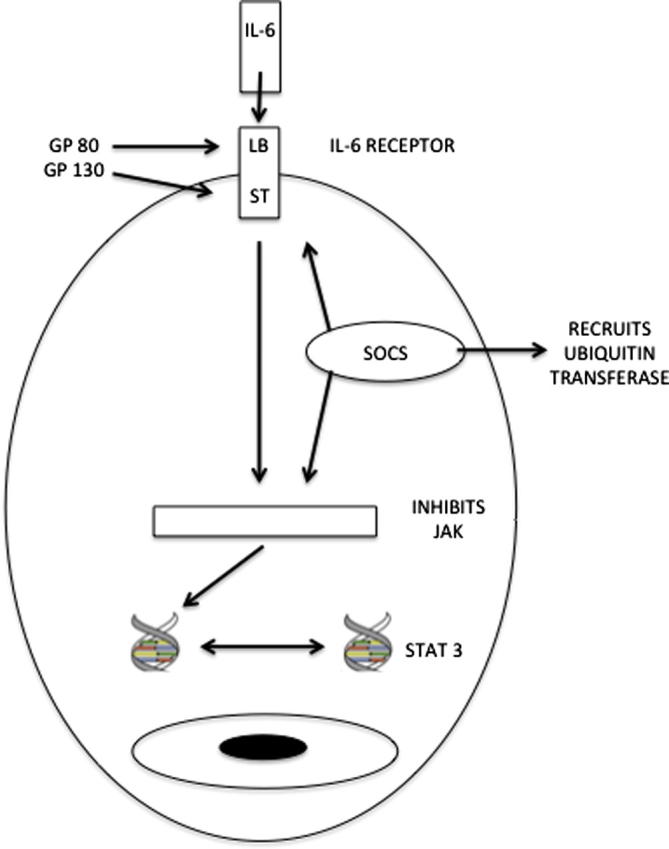
The mechanisms by which SOCS proteins inhibit IL-6-mediated signal transduction. SOCS may prevent IL-6 signalling through binding to the receptor, inhibiting JAK, or enhancing ubiquitination (LB = ligand binding, ST = signal transduction).
As dysfunction of this pathway has been implicated in various malignant diseases, it was suggested that losses of SOCS-1 and SOCS-3 are relevant to cancer initiation and progression (Yoshimura et al., 2007). In many types of carcinomas, the oncogenic role of STAT factors, especially of STAT3 is clearly documented (Burke et al., 2001; Hodge et al., 2005). Loss of the inhibitory effect of SOCS-1 and SOCS-3 leads to hyperactivity of the JAK/STAT pathway, thus promoting uncontrolled cellular proliferation. Therefore, it was believed that SOCS-1 and SOCS-3 generally act as tumour suppressors. Silencing of SOCS-1 and SOCS-3 due to promoter hypermethylation has been reported in several types of tumours including human lung (He et al., 2003), hepatocellular (Niwa et al., 2005), and head and neck cancer (Weber et al., 2005) This causes a growth advantage for cancer cells in vitro and in vivo. In particular, silencing of SOCS-3 enhanced cellular proliferation and its re-expression after treatment with de-methylating agents caused growth inhibition of treated cancer cells.
However, there are increasing indications that SOCS-3 has different functions depending on the origin of the tumour. In breast cancer, SOCS-3 is not silenced and SOCS-3 protein expression does not cause an inhibition of cellular proliferation (Evans et al., 2007). In previous studies, our group proved that SOCS-1 and SOCS-3 are also expressed in prostate cancer. Moreover, expression of SOCS-3 significantly increases in malignant versus benign prostate cells and persists in castration therapy refractory prostate cancer patients. Its expression is inversely correlated with STAT3 phosphorylation, suggesting an inactivation of the JAK-STAT pathway (Bellezza et al., 2006; Puhr et al., 2009). Our results could be partly confirmed by Pierconti and colleagues. They also observed an increased expression of SOCS-3 protein in prostate cancer patients. However, those researchers also identified a patient subgroup with methylated SOCS-3 status and correlated absence of SOCS-3 with a more aggressive cancer phenotype and poor prognosis (Pierconti et al., 2010). In contrast, we observed a significant increase in apoptosis after SOCS-3 down-regulation in different prostate cancer cell lines concluding that SOCS-3 expression is a critical factor for cell survival. Our findings are in line with observations of Masuhiro and associates. They demonstrated that SOCS-3 is an important key regulator in cell cycle progression. It is influenced by mutual interaction with the transcriptional factor DP-1 and down-regulation of SOCS-3 resulted in an increase of apoptotic cells (Masuhiro et al., 2008). Therefore, it seems that SOCS-3 is implicated in cell cycle regulation and programmed cell death.
Recent studies have indicated that SOCS-3 effects are not limited solely to IL-6 signalling. The increased expression of SOCS-3 also differentially affects proliferation of prostate cancer cells, depending on androgen sensitivity. Our group was able to demonstrate that SOCS-3 is increasingly expressed in response to androgen treatment and inhibits androgen-mediated proliferation in vitro (Neuwirt et al., 2007). Ben-Zvi and associates reported an interaction between SOCS-3 and the fibroblast growth factor receptor 3 (FGFR3) (Ben-Zvi et al., 2006). In line with these results our group has provided for the first time evidence that SOCS-3 is a major key regulator of the FGF pathway. We proved that SOCS-3 interferes with FGF-2 signalling by influencing the phosphorylation of the MAPK p44 and p42. By modulating SOCS-3 expression levels we could clearly demonstrate that SOCS-3 has an inhibitory effect on activation of the MAPK signal transduction in prostate cancer (Puhr et al., 2010). Taken together, SOCS-3 is a multifunctional protein and the specific role of SOCS-3 in cancer is tumour type-dependent and both tumour suppressive and promoting roles of SOCS-3 have been described so far.
We were also able to demonstrate that SOCS-1 is expressed in prostate cancer cells and tissue specimens (Neuwirt et al., 2009). An inhibitory role for SOCS-1 in prostate cancer could be demonstrated in cell lines. Decreased SOCS-1 expression leads to an up-regulation of cyclin-dependent kinases and cyclins which govern the G1/S cell cycle progression. Thus, SOCS-1 acts as a tumour suppressor in prostate cancer cell lines although it is expressed at a higher level in tumours.
7. Therapy approaches aimed to target IL-6
Different possibilities to target IL-6 are available. For example, in haematological malignancies it was demonstrated that IL-6 super-antagonists have a therapeutic potential. In prostate cancer, the monoclonal antibody siltuximab (CNTO 328) was used in experimental and clinical studies (Fig. 2). Siltuximab strongly inhibited growth of the PC-3 xenograft through induction of apoptosis (Smith and Keller, 2001). The LuCaP xenograft which represents an androgen-sensitive tumour was also inhibited by siltuximab and the progression towards androgen-independence was delayed (Wallner et al., 2006). Siltuximab showed a less potent effect on the LNCaP-IL-6+ cell line in vitro and in vivo. However, in those studies it was observed that siltuximab-induced growth inhibition is associated with re-activation of the JAK-STAT signalling pathway (Steiner et al., 2006). Since activation of AR in prostate cancer cells may lead to both proliferative and inhibitory responses, it is important to understand that a more personalized approach in the attempt to improve anti IL-6 therapy needs to be developed.
Fig. 2.
Anti-IL-6 antibody siltuximab was used in prostate cancer cell lines, studies with xenografts, and in a clinical phase II study.
In clinical settings, it was recently shown that the administration of siltuximab in a collective of patients who have already received docetaxel therapy has a biological but not clinical efficacy (Dorff et al., 2010). There is an evidence indicating that IL-6 predicts docetaxel resistance in prostate cancer (Domingo-Domenech et al., 2006). The development of the resistance to docetaxel seems to be very complex and it could not be excluded that these tumours are very heterogenous. It was demonstrated that another member of the STAT family of transcription factors, STAT1, is increasingly expressed in docetaxel-resistant prostate cancer cells (Patterson et al., 2006).
8. Outlook and future perspectives
Our understanding of the role of IL-6 in prostate cancer has improved over years. This cytokine is a good candidate for the development of targeted therapies in prostate cancer. In cells representing advanced stage of the disease, it is obvious that IL-6 acts as a survival factor and stimulates migration and apoptosis. From laboratory studies, it is important to design rational clinical approaches with aim to identify candidate patients subgroups in which therapy will have most chances for success.
Recently, we have also improved our understanding of action of important inhibitors of cytokine signalling SOCS-3 and -1 in prostate cancer. Although these proteins block IL-6-induced signal transduction, they have also cell type-dependent effects which differ between AR-positive and -negative cells. Targeting SOCS-3 in prostate cells which do not express AR may be an attractive therapy option in the future.
Acknowledgements
The work in the authors’ laboratory was supported by Austrian Science Fund FWF (projects P19933 and W1101 to Z.C.). The authors acknowledge the expert editorial assistance of Robert Schober.
References
- Adler H.L., McCurdy M.A., Kattan M.W., TImme T.L., Scardino P.T., Thompson T.C. Elevated levels of circulating interleukin-6 and transforming growth factor-beta1 in patients with metastatic prostatic carcinoma. J. Urol. 1999;161:182–187. [PubMed] [Google Scholar]
- Alexander W.S., Hilton D.J. The role of suppressors of cytokine signalling (SOCS) proteins in regulation of the immune response. Annu. Rev. Immunol. 2004;22:503–529. doi: 10.1146/annurev.immunol.22.091003.090312. [DOI] [PubMed] [Google Scholar]
- Andersen R.J., Mawji N.R., Wang J., Wang G., Haile S., Myung J.K., Watt K., Tam T., Yang Y.C., Banuelos C.A., Williams D.E., McEvan I.J., Wang Y., Sadar M.D. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell. 2010;17:535–536. doi: 10.1016/j.ccr.2010.04.027. [DOI] [PubMed] [Google Scholar]
- Bellezza I., Neuwirt H., Nemes C., Cavarretta I.T., Puhr M., Steiner H., Minelli A., Bartsch G., Offner F., Hobisch A., Doppler W., Culig Z. Suppressor of cytokine signalling-3 antagonizes camp effects on proliferation and apoptosis and is expressed in human prostate cancer. Am. J. Pathol. 2006;169:2199–2208. doi: 10.2353/ajpath.2006.060171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zvi T., Yayon A., Gertler A., Monsonego-Ornan E. Suppressors of cytokine siignaling (SOCS)1 and SOCS3 interact with and modulate fibroblast growth factor receptor signalling. J. Cell Sci. 2006;119:380–387. doi: 10.1242/jcs.02740. [DOI] [PubMed] [Google Scholar]
- Borowsky A.D., Dingley K.H., Ubick E., Turteltaub K.W., Cardiff R.D., Devere-White R. Inflammation and atrophy precede prostate neoplasia in PhIP-induced rat model. Neoplasia. 2006;8:708–715. doi: 10.1593/neo.06373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke W.M., Jin X., Lin H.J., Huang M., Liu R., Reynolds R.K., Lin J. Inhibition of constitutively active Stat3 suppresses growth of human ovarian and breast cancer cells. Oncogene. 2001;20:7925–7934. doi: 10.1038/sj.onc.1204990. [DOI] [PubMed] [Google Scholar]
- Cavarretta I.T., Neuwirt H., Untergasser G., Moser P.L., Zaki M.H., Steiner H., Rumpold H., Fuchs D., Hobisch A., Nemeth J.A., Culig Z. The antiapoptotic effect of IL-6 autocrine loop in a cellular model of advanced prostate cancer is mediated by Mcl-1. Oncogene. 2007;26:2822–2832. doi: 10.1038/sj.onc.1210097. [DOI] [PubMed] [Google Scholar]
- Chen C.D., Welsbie D.S., Tran C., Baek S.H., Chen R., Vessella R., Rosenfeld M.G., Sawyers C. Molecular determinants of resistance to antiandrogen therapy. Nat. Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- Chun J.Y., Nadiminty N., Dutt S., Lou W., Yang J.C., Kung H.J., Evans C.P., Gao A.C. Interleukin-6 regulates androgen synthesis in prostate cancer cells. Clin. Cancer Res. 2009;15:4815–4822. doi: 10.1158/1078-0432.CCR-09-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung T.D., Yu J.J., Kong T.A., Spiotto M.T., Lin J.M. Interleukin-6 activates phosphatidylinositol-3 kinase, which inhibits apoptosis in human prostate cancer cell lines. Prostate. 2000;42:1–7. doi: 10.1002/(sici)1097-0045(20000101)42:1<1::aid-pros1>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Debes J.D., Schmidt L.J., Huang H., Tindall D.J. P300 mediates androgen-independent transactivation of the androgen receptor by interleukin 6. Cancer Res. 2002;62:5632–5636. [PubMed] [Google Scholar]
- Debes J.D., Comuzzi B., Schmidt L.J., Dehm S.M., Culig Z., Tindall D.J. P300 regulates androgen receptor-independent expression of prostate-specific antigen in prostate cancer cells treated chronically with interleukin-6. Cancer Res. 2005;65:5965–5973. doi: 10.1158/0008-5472.CAN-04-2837. [DOI] [PubMed] [Google Scholar]
- Degeorges A., Tatoud R., Fauvel-Lafeve F., Podgornik M.P., Millot G., de Cremous P., Calvo F. Stromal cells from human benign prostate hyperplasia produce a growth-inhibitory factor for LNCaP prostate cancer cells, identified as interleukin-6. Int. J. Cancer. 1996;68:207–214. doi: 10.1002/(SICI)1097-0215(19961009)68:2<207::AID-IJC12>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Desai S.J., Ma A.H., Tepper C.G., Chen H.W., Kung H.J. Inappropriate activation of the androgen receptor by nonsteroids: involvement of the Src kinase pathway and its therapeutic implications. Cancer Res. 2006;66:10449–10459. doi: 10.1158/0008-5472.CAN-06-2582. [DOI] [PubMed] [Google Scholar]
- Domingo-Domenech J., Oliva C., Rovira A., Codony-Servat J., Bosch M., Filella X., Montagut C., Tapia M., Campas C., Dang L., Rolfe M., Ross J.S., Gascon P., Albanell J., Mellado B. Interleukin 6, a nuclear factor-kappa B target, predicts resistance to docetaxel in hormone-independent prostate cancer and nuclear factor kappa B inhibition by PS-1145 enhances docetaxel antitumor activity. Clin. Cancer Res. 2006;12:5578–5586. doi: 10.1158/1078-0432.CCR-05-2767. [DOI] [PubMed] [Google Scholar]
- Dorff T.B., Goldman B., Pinski J.K., Mack P.C., Lara P.N., Jr., Van Veldhuizen P.J., Jr., Quinn D.I., Vogelzang N.J., Thompson I.M., Jr., Hussain M.H. Clinical and correlative results of SWOG S0354: a phase II trial of CNTO328 Isiltuximab, a monoclonal antibody against IL-6, in patients with castration-resistant prostate cancer. Clin. Cancer Res. 2010;16:3028–3034. doi: 10.1158/1078-0432.CCR-09-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M.K., Yu C.R., Lohani A., Mahdi R.M., Liu X., Trzeciak A.R., Egwuagu C.E. Expression of SOCS1 and SOCS3 genes is differentially regulated in breast cancer cells in response to proinflammatory cytokine and growth factor signals. Oncogene. 2007;26:1941–1948. doi: 10.1038/sj.onc.1209993. [DOI] [PubMed] [Google Scholar]
- Garrett K.P., Aso T., Bradsher J.N., Foundling S.I., Lane W.S., Conaway R.C., Conaway J.W. Positive regulation of general transcription factor SIII by a taiedubiquitin homolog. Proc. Natl. Acad. Sci. USA. 1995;92:7172–7176. doi: 10.1073/pnas.92.16.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P.M., Malik S.N., Bedolla R.G., Wang Y., Mikhailova M., Prihoda T.J., Troyer D.A., Kreisberg J.I. Signal transduction pathways in androgen-dependent and -independent prostate cancer cell proliferation. Endocr. Relat. Cancer. 2005;12:119–134. doi: 10.1677/erc.1.00835. [DOI] [PubMed] [Google Scholar]
- Giri D., Ozen M., Ittmann M. Interleukin-6 is an autocrine growth factor in human prostate cancer. Am. J. Pathol. 2001;159:2159–2165. doi: 10.1016/S0002-9440(10)63067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B., You L., Uematsu K., Zang K., Xu Z., Lee A.Y., Costello J.F., McCormick F., Jablons D.M. SOCS-3 is frequently silenced by hypermethylation and suppresses cell growth in human lung cancer. Proc. Natl. Acad. Sci. USA. 2003;100:14133–14138. doi: 10.1073/pnas.2232790100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton D.J. Negative regulators of cytokine signal transduction. Cell. Mol. Life Sci. 1999;55:1568–1577. doi: 10.1007/s000180050396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobisch A., Eder I.E., Putz T., Horninger W., Bartsch G., Klocker H., Culig Z. Interleukin-6 regulates prostate-specific protein expression in prostate carcinoma cells by activation of the androgen receptor. Cancer Res. 1998;58:4640–4645. [PubMed] [Google Scholar]
- Hobisch A., Rogatsch H., Hittmair A., Fuchs D., Bartsch G., Jr., Klocker H., Bartsch G., Culig Z. Immunohistochemical localization of interleukin-6 and its receptor in benign, premalignant and malignant prostate tissue. J. Pathol. 2000;191:239–244. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH633>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Hobisch A., Ramoner R., Fuchs D., Godoy-Tundidor S., Bartsch G., Klocker H., Culig Z. Prostate cancer cells (LNCaP) generated after long-term interleukin 6 (IL-6) treatment express IL-6 and acquire an IL-6 partially resistant phenotype. Clin. Cancer Res. 2001;7:2941–2948. [PubMed] [Google Scholar]
- Hodge D.R., Hurt E.M., Farrar W.L. The role of IL-6 and STAT3 in inflammation and cancer. Eur. J. Cancer. 2005;41:2502–2512. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Ishiguro H., Akimoto K., Nagashima Y., Kojima Y., Sasaki T., Ishiguro-Imagawa Y., Nakaigawa N., Ohno S., Kubota Y., Uemura H. aPKClambda/iota promotes growth of prostate cancer cells in an autocrine manner through transcriptional activation of interleukin-6. Proc. Natl. Acad. Sci. USA. 2009;106:16369–16374. doi: 10.1073/pnas.0907044106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrard D.F., Kinoshita H., Shi Y., Sandefur C., Hoff D., Meisner L.F., Chang C., Herman J.G., Isaacs W.B., Nassif N. Methylation of the androgen receptor promoter CpG island is associated with loss of androgen receptor expression in prostate cancer cells. Cancer Res. 1998;58:5310–5314. [PubMed] [Google Scholar]
- Kamura T., Maenaka K., Kotoshiba S., Matsumoto M., Kohda D., Conaway R.C., Conaway J.W., Nakayama K.I. VHL-box and SOCS-box domains determine binding specificity for Cul2-Rbx1 and Cul5-Rbx2 modules of ubiquitin ligases. Genes Dev. 2004;18:3055–3065. doi: 10.1101/gad.1252404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller E.T., Chang C., Ershler W.B. Inhibition of NFkappaB activity through maintenance of IkapaBalpha levels contributes to dihydrotestosterone-mediated repression of the interleukin-6 promoter. J. Biol. Chem. 1996;27:26267–26275. doi: 10.1074/jbc.271.42.26267. [DOI] [PubMed] [Google Scholar]
- Kim O., Jiang T., Xie Y., Guo Z., Chen H., Qiu Y. Synergism of cytoplasmic kinases in IL6-induced ligand-independent activation of androgen receptor in prostate cancer cells. Oncogene. 2004;23:1838–1844. doi: 10.1038/sj.onc.1207304. [DOI] [PubMed] [Google Scholar]
- Komyod W., Böhm M., Metze D., Heinrich P.C., Behrmann I. Constitutive suppressor of cytokine signalling 3 expression confers a growth advantage to a human melanoma cell line. Mol. Cancer Res. 2007;5:271–281. doi: 10.1158/1541-7786.MCR-06-0274. [DOI] [PubMed] [Google Scholar]
- Krajewska M., Krajewski S., Epsein J.I., Shabaik A., Sauvageot J., Song K., Kitada S., Reed J.C. Immunohistochemical analysis of bcl-2, bax, bcl-x, and mcl-1 expression in prostate cancers. Am. J. Pathol. 1996;148:1567–1576. [PMC free article] [PubMed] [Google Scholar]
- Larsen L., Ropke C. Suppressors of cytokine signalling: SOCS. Apmis. 2002;110:833–844. doi: 10.1034/j.1600-0463.2002.1101201.x. [DOI] [PubMed] [Google Scholar]
- Lee S.O., Lou W., Hou M., de Miguel F., Gerber L., Gao A.C. Interleukin-6 promotes androgen-independent growth in LNCaP human prostate cancer cells. Clin. Cancer Res. 2003;9:370–376. [PubMed] [Google Scholar]
- Lee S.O., Chung J.Y., Nadiminty N., Lou W., Gao A.C. Interleukin-6 undergoes transition from growth inhibitor associated with neuroendocrine differentiation to stimulator accompanied by androgen receptor activation during LNCaP prostate cancer cell progression. Prostate. 2007;67:764–773. doi: 10.1002/pros.20553. [DOI] [PubMed] [Google Scholar]
- Malinowska K., Neuwirt H., Cavarretta I.T., Bektic J., Steiner H., Dietrich H., Moser P.L., Fuchs D., Hobisch A., Culig Z. Interleukin-6 stimulation of growth of prostate cancer in vitro and in vivo through activation of the androgen receptor. Endocr. Relat. Cancer. 2009;16:155–169. doi: 10.1677/ERC-08-0174. [DOI] [PubMed] [Google Scholar]
- Masuda M., Wakasaki T., Suzuki M., Toh S., Joe A.K., Weinstein I.B. Stat3 orchestrates tumor development and progression: the Achilles´ heel of head and neck cancers? Curr. Cancer Drug Targets. 2010;10:117–126. doi: 10.2174/156800910790980197. [DOI] [PubMed] [Google Scholar]
- Masuhiro Y., Kayama K., Fukushima A., Baba K., Soutsu M., Kamiya Y., Gotoh M., Yamaguchi N., Hanazawa S. SOCS-3 inhibits E2F/DP-1 transcriptional activity and cell cycle progression via interaction with DP-1. J. Biol. Chem. 2008;283:31575–31583. doi: 10.1074/jbc.M800328200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellinghoff I.K., Vivanco I., Kwon A., Tran C., Wongvipat J., Sawyers C.L. HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell. 2004;6:517–527. doi: 10.1016/j.ccr.2004.09.031. [DOI] [PubMed] [Google Scholar]
- Nakai Y., Nelson W.G., De Marzo A.W. The dietary charred meat carcinogen 2-amino-1-methyl-6-phenylimidazo(4,5-b)pyridine acts as both a tumor initiator and promoter in the rat ventral prostate. Cancer Res. 2007;67:1378–1384. doi: 10.1158/0008-5472.CAN-06-1336. [DOI] [PubMed] [Google Scholar]
- Neuwirt H., Puhr M., Cavarretta I.T., Mitterberger M., Hobisch A., Culig Z. Suppressor of cytokine signalling-3 is up-regulated by androgen in prostate cancer cell lines and inhibits androgen-mediated proliferation and secretion. Endocr. Relat. Cancer. 2007;14:1007–1019. doi: 10.1677/ERC-07-0172. [DOI] [PubMed] [Google Scholar]
- Neuwirt H., Puhr M., Santer F.R., Susani M., Doppler W., Marcias G., Rauch V., Brugger M., Hobisch A., Kenner L., Culig Z. Suppressor of cytokine signalling (SOCS)-1 is expressed in human prostate cancer and exerts growth-inhibitory function through down-regulation of cyclins and cyclin-dependent kinases. Am. J. Pathol. 2009;174:1921–1930. doi: 10.2353/ajpath.2009.080751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson S.E., De Souza D., Fabri L.J., Corbin J., Wislon T.A., Zhang J.G., Silva A., Asimakis M., Farley A., Nash A.D., Metcalf D., Hilton D.J., Nicola N., Baca M. Suppressor of cytokine signalling-3 preferentially binds to the SHP-2 binding site on the shared cytokine receptor subunit gp130. Proc. Natl. Acad. Sci. USA. 2000;97:6493–6498. doi: 10.1073/pnas.100135197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa Y., Kanda H., Shikauchi Y., Saiura A., Matsubara K., Kitagawa T., Yamamoto J., Kubo T., Yoshikawa H. Methylation silencing of SOCS-3 promotes cell growth and migration by enhancing JAK/STAT and FAK signalings in human hepatocellular carcinoma. Oncogene. 2005;24:6406–6417. doi: 10.1038/sj.onc.1208788. [DOI] [PubMed] [Google Scholar]
- Park J.I., Lee M.G., Cho K., Park B.J., Chae K.S., Byun D.S., Ryu B.K., Park Y.K., Chi S.G. Transforming growth factor-beta1 activates interleukin-6 expression in prostate cancer cells through the synergistic collaboration of the Smad2, p38-NF-kappa B, JNK, and Ras signalling pathways. Oncogene. 2003;22:4314–4332. doi: 10.1038/sj.onc.1206478. [DOI] [PubMed] [Google Scholar]
- Patterson S.G., Wei S., Chen X., Sallman D.A., Gilvary D.L., Zhong B., Pow-Sang J., Yeatman T., Djeu J.Y. Novel role of Stat1 in the development of docetaxel resistance in prostate tumor cells. Oncogene. 2006;25:6113–6122. doi: 10.1038/sj.onc.1209632. [DOI] [PubMed] [Google Scholar]
- Pierconti F., Martini M., Pinto F., Cenci T., Capodimonti S., Calarco A., Bassi P.F., Larocca L.M. Epigenetic silencing of SOCS3 identifies a subset of prostate cancers with an aggressive behavior. Prostate. 2010;71:318–325. doi: 10.1002/pros.21245. [DOI] [PubMed] [Google Scholar]
- Puhr M., Santer F.R., Neuwirt H., Susani M., Nemeth J.A., Hobisch A., Kenner L., Culig Z. Down-regulation of suppressor of cytokine signalling-3 causes prostate cancer cell death through activation of the extrinsic and intrinsic apoptosis pathways. Cancer Res. 2009;69:7375–7384. doi: 10.1158/0008-5472.CAN-09-0806. [DOI] [PubMed] [Google Scholar]
- Puhr M., Saner F.R., Neuwirt H., Marcias G., Hobisch A., Culig Z. SOCS-3 antagonises the proliferative and migratory effects of fibroblast growth factor-2 in prostate cancer by inhibition of p44/p42 MAPK signalling. Endocr. Relat. Cancer. 2010;18:525–538. doi: 10.1677/ERC-10-0007. [DOI] [PubMed] [Google Scholar]
- Qiu Y., Robinson D., Pretlow T.G., Kung H.J. Etk/Bmx, a tyrosine kinase with a pleckstrin-homology domain, is an effector of phosphatidylinositol 3′kinase and is involved in interleukin-6-induced neuroendocrine differentiation of prostate cancer cells. Proc. Natl. Acad. Sci. USA. 1998;95:3644–3649. doi: 10.1073/pnas.95.7.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadar M.D., Gleave M.E. Ligand-independent activation of the androgen receptor by the differentiation agent butyrate in human prostate cancer cells. Cancer Res. 2000;60:5825–5831. [PubMed] [Google Scholar]
- Santer F., Malinowska K., Culig Z., Cavarretta I.T. Interleukin-6 trans-signalling differentially regulates proliferation, migration, adhesion and maspin expression in human prostate cancer cells. Endocr. Relat. Cancer. 2010;17:241–253. doi: 10.1677/ERC-09-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A., Yasukawa H., Suzuki A., Kamizono S., Syoda T., Kinjyo I., Sasaki M., Johnston J.A., Yoshimura A. Cytokine-inducible SH2 protein-3 (CIS/SOCS3) inhibits Janus tyrosine kinase by binding through the N-terminal kinase inhibitory regions well as SH2 domain. Genes Cells. 1999;4:339–351. doi: 10.1046/j.1365-2443.1999.00263.x. [DOI] [PubMed] [Google Scholar]
- Siddiqui I.A., Asim M., Hafeez B.B., Adhami V.M., Tarapore R.S., Mukhtar H. Green tea polyphonol EGCG blunts androgen receptor function in prostate cancer. FASEB J. 2011;25:1198–1207. doi: 10.1096/fj.10-167924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P.C., Keller E.T. Anti-interleukin-6 monoclonal antibody induces regression of human prostate cancer xenografts in nude mice. Prostate. 2001;48:47–53. doi: 10.1002/pros.1080. [DOI] [PubMed] [Google Scholar]
- Spiotto M.T., Chung T.D. STAT3 mediates IL-6-induced neuroendocrine differentiation in prostate cancer cells. Prostate. 2000;42:186–195. doi: 10.1002/(sici)1097-0045(20000215)42:3<186::aid-pros4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Starr R., Wilson T.A., Viney E.M., Murray L.J., Rayner J.R., Jenkins B.J., Gonda T.J., Alexander W.S., Metcalf D., Nicola N.A., Hilton D.J. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- Steiner H., Godoy-Tundidor S., Rogatsch H., Berger A.P., Fuchs D., Comuzzi B., Bartsch G., Hobisch A., Culig Z. Accelerated in vivo growth of prostate tumors that up-regulate interleukin-6 is associated with reduced retinoblastoma protein expression and activation of the mitogen-activated protein kinase pathway. Am. J. Pathol. 2003;162:655–663. doi: 10.1016/S0002-9440(10)63859-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H., Berger A.P., Godoy-Tundidor S., Bjartell A., Lilja H., Bartsch G., Hobisch A., Culig Z. An autocrine loop for vascular endothelial growth factor is established in prostate cancer cells generated after prolonged treatment with interleukin 6. Eur. J. Cancer. 2004;40:1066–1072. doi: 10.1016/j.ejca.2003.11.033. [DOI] [PubMed] [Google Scholar]
- Steiner H., Cavarretta I.T., Moser P.L., Berger A.P., Bektic J., Dietrich H., Zaki M.H., Nakada M., Hobisch A., Nemeth J.A., Culig Z. Regulation of growth of prostate cancer cells selected in the presence of interleukin-6 by the anti-interleukin-6 antibody CNTO 328. Prostate. 2006;66:1744–1752. doi: 10.1002/pros.20492. [DOI] [PubMed] [Google Scholar]
- Tam N.N., Leav I., Ho S.M. Sex hormones induce direct epithelial and inflammation-mediated oxidative/nitrosative stress that favors prostatic carcinogenesis in the noble rat. Am. J. Pathol. 2007;171:1334–1341. doi: 10.2353/ajpath.2007.070199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twillie D.A., Eisenberger M.A., Carducci M.A., Hseih W.S., Kim W.Y., Simons J.W. Interleukin-6: a candidate mediator of human prostate cancer morbidity. Urology. 1995;45:542–549. doi: 10.1016/S0090-4295(99)80034-X. [DOI] [PubMed] [Google Scholar]
- Ueda T., Mawji N.R., Bruchovsky N., Sadar M.D. Ligand-independent activation of the androgen receptor by interleukin-6 and the role of steroid receptor coactivator-1 in prostate cancer cells. J. Biol. Chem. 2002;277:38087–38094. doi: 10.1074/jbc.M203313200. [DOI] [PubMed] [Google Scholar]
- Wallner L., Dai J., Escara-Wilke J., Zhang J., Yao Z., Lu Y., Trikha M., Nemeth J.A., Zaki M.H., Keller E.T. Inhibition of interleukin-6 with CNTO328, an anti-interleukin-6 monoclonal antibody, inhibits conversion of androgen-dependent to an androgen-independent phenotype in orchiectomized mice. Cancer Res. 2006;66:3087–3095. doi: 10.1158/0008-5472.CAN-05-3447. [DOI] [PubMed] [Google Scholar]
- Wang Q., Horiatis D., Pinski J. Interleukin-6 inhibits the growth of prostate cancer xenografts in mice by the process of neuroendocrine differentiation. Int. J. Cancer. 2004;111:508–513. doi: 10.1002/ijc.20286. [DOI] [PubMed] [Google Scholar]
- Weber A., Hengge U.R., Bardenheuer W., Tischoff I., Sommerer F., Markwarth A., Dietz A., Wittekind C., Tannapfel A. SOCS-3 is frequently methylated in head and neck squamous cell carcinoma and its precursor lesions and causes growth inhibition. Oncogene. 2005;24:6699–6708. doi: 10.1038/sj.onc.1208818. [DOI] [PubMed] [Google Scholar]
- Yoshimura A., Naka T., Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat. Rev. Immunol. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- Yuan T.C., Veeramani S., Lin M.F. Neuroendocrine-like prostate cancer cells: neuroendocrine transdifferentiation of prostate adenocarcinoma cells. Endocr. Relat. Cancer. 2007;14:531–547. doi: 10.1677/ERC-07-0061. [DOI] [PubMed] [Google Scholar]
- Zegarra-Moro O.L., Schmidt L.J., Huang H., Tindall D.J. Disruption of androgen receptor function inhibits proliferation of androgen-refractory prostate cancer cells. Cancer Res. 2002;62:1008–1013. [PubMed] [Google Scholar]
- Zerbini L.F., Wang Y., Cho J.Y., Liebermann T.A. Constitutive activation of nuclear factor kappaB p50/p65 and Fra-1 and Jund D is essential for deregulated interleukin 6 expression in prostate cancer. Cancer Res. 2003;63:2206–2215. [PubMed] [Google Scholar]