Abstract
Background and Objectives
QRS complex fragmentations are frequently seen on routine electrocardiograms with narrow or wide QRS complex. Fragmented QRS complex (fQRS) is associated with increased morbidity and mortality, sudden cardiac death and recurrent cardiovascular events. In this study, we aimed to interrogate the relationship of systemic inflammation with the presence of fQRS in patients with acute coronary syndromes (ACS).
Subjects and Methods
Two-hundred and twenty eligible patients with ACS that underwent coronary angiography were enrolled consecutively in this study. Patients with significant organic valve disease and those with any QRS morphology that had a QRS duration ≥120 ms as well as patients with permanent pacemakers were excluded from this study.
Results
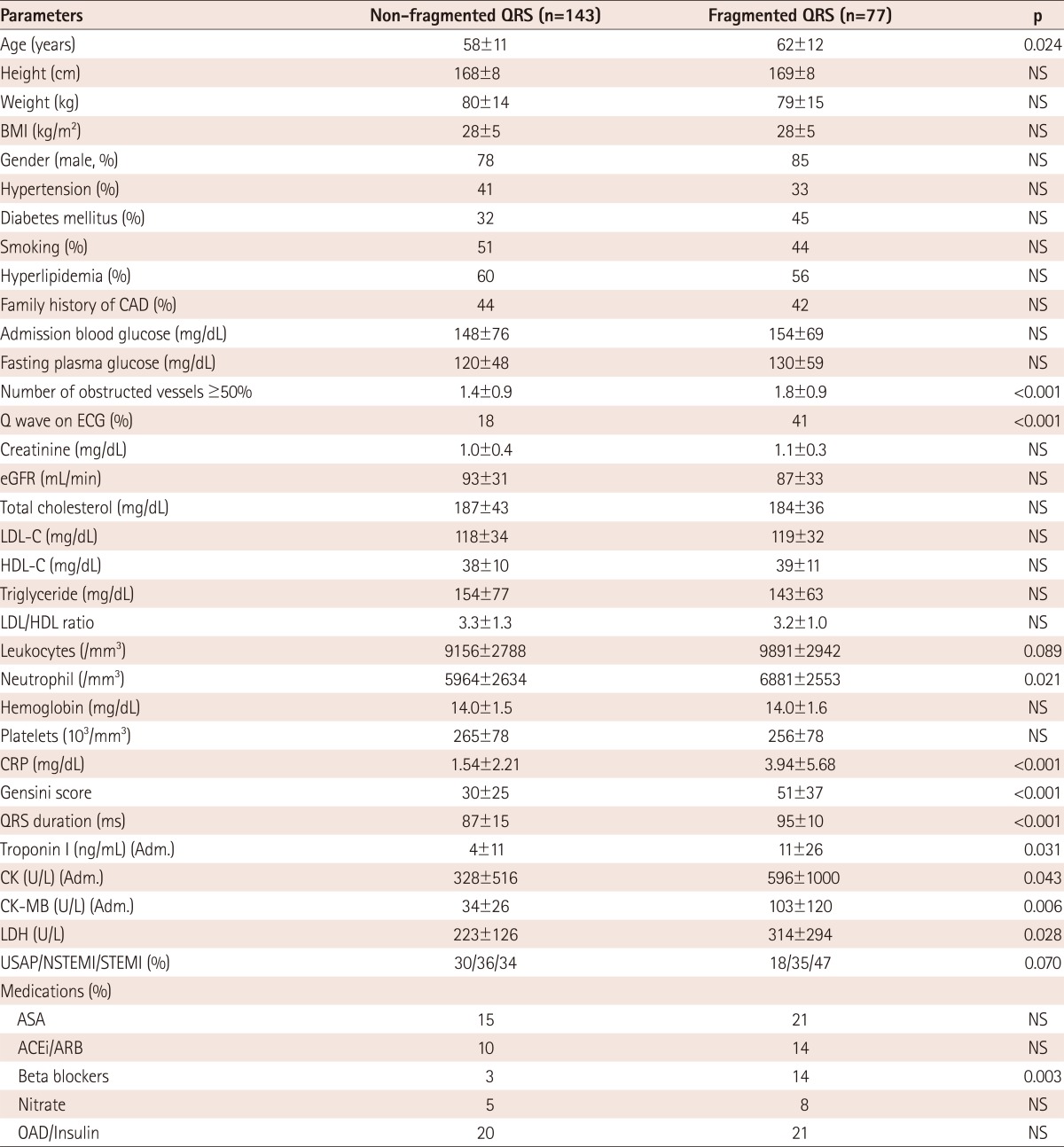
Patients with fQRS were of a higher age (p=0.02), had increased C-reactive protein (CRP) levels (p<0.001), prolonged QRS time (p<0.001), extent of coronary artery disease (CAD) (p<0.001), creatine kinase-MB (CK-MB) levels (p=0.006) and Q wave on admission electrocardiography (p<0.001) in comparison to patients with non-fragmented QRS. When we performed multiple logistic regression analysis, fQRS was found to be related to increased CRP levels {odds ratio (OR): 1.2, 95% confidence interval (CI): 1.045-1.316, p=0.007}, QRS duration (OR: 1.1, 95% CI: 1.033-1.098, p<0.001), extent of CAD (OR: 1.5, 95% CI: 1.023-2.144, p=0.037), Q wave (OR: 2.2, 95% CI: 1.084-4.598, p=0.03) and CK-MB levels (OR: 1.0, 95% CI: 1.001-1.037, p=0.04) independently.
Conclusion
In our study, we found that fQRS was independently related to increased CRP. Fragmented QRS that may result as an end effect of inflammation at cellular level can represent increased cardiac risk by different causative mechanisms in patients with ACS.
Keywords: Electrocardiography, Coronary artery disease, Inflammation, Acute coronary syndrome, Risk assessment
Introduction
QRS complex fragmentations are frequently seen on routine surface electrocardiograms with narrow or wide QRS complex that include paced rhythm, bundle branch block or ventricular premature beats.1) These fragmentations on surface electrocardiography (ECG) have been associated with increased adverse cardiac events in previous studies.2-5)
Fragmented QRS complexes (fQRS) on a 12-lead resting ECG are defined as various RSR' patterns with or without Q waves, without a typical bundle-branch block in 2 contiguous leads corresponding to a major coronary artery territory.6) Based on their duration, they are sub-classified into two subgroups of fQRS complexes with QRS duration <120 ms or ≥120 ms (fragmented wide-QRS complexes), and can be found on ECG with different QRS morphologies. Sometimes, fQRS may be the only electrocardiographic marker of myocardial damage in patients with non-Q myocardial infarction and in patients with resolved Q wave.6)
The association between fQRS and increased morbidity and mortality, sudden cardiac death and recurrent cardiac events have been studied previously.4),5),7-10) In these studies, cardiac fibrosis has been shown to be the main causative mechanism.11),12) Additionally, fQRS may represent altered ventricular depolarization, which may result in different mechanisms in addition to fibrosis. Therefore, in patients with unstable coronary artery disease (CAD), there may be a possible causative association of fQRS with some factors including systemic inflammation.
Systemic inflammation has been shown to play a significant role in cardiac arrhythmias and conduction disturbances. The possible reason of cardiac arrhythmias and conduction disturbances seems to be related to myocardial inflammation, focal fibrosis, and ischemia within the conduction system.13) In a recent study, Kadi et al.14) showed that fQRS increased even in patients with rheumatoid arthritis without cardiovascular disease, in which it is speculated that inflammatory processes may play a pivotal role to produce fragmentations on ECG. Therefore, we hypothesized that the development of fQRS may be related to systemic inflammation as well as myocardial ischemia and necrosis at the early acute coronary syndrome (ACS) stage.
In this study, we interrogated the relationship between fQRS and systemic inflammation in patients with ACS.
Subjects and Methods
Patient population and study protocol
The current study had a cross-sectional observational design. The study was conducted in the cardiology clinic at the Rize Education and Research Hospital in Rize, Turkey. Two-hundred and twenty eligible patients with ACS that underwent coronary angiography at our institution between January 2009 and December 2009 were enrolled in this study consecutively. All patients were examined by an experienced cardiologist immediately after hospitalization. Of the 220 patients included in the study, the diagnosis was ST-segment elevation myocardial infarction (STEMI) in 38% of patients, non-ST elevation myocardial infarction (NSTEMI) in 36% of patients and unstable angina pectoris (USAP) in 26% of patients.
Clinical characteristics, which consisted of multiple descriptors from each patient's history and physical examination, were collected by physicians from the cardiology laboratory for each patient prior to cardiac catheterization and were stored in the database at the coronary angiography laboratory at our institution. We recorded baseline characteristics, which included hypertension, diabetes mellitus, smoking history, family history for CAD, and lipid parameters.
Patients with significant organic valvular heart disease and bundle branch block (BBB) (left BBB, incomplete or complete right BBB or duration QRS ≥120 ms) and patients with permanent pacemakers were excluded from the study.
Because our patients had ACS, we did not use a C-reactive protein (CRP) cutoff value of ≥10 mg/dL, which could be helpful for distinguishing vascular inflammation from inflammation of the infective diseases in stable CAD. ACS itself is an inflammatory process and it is difficult to distinguish these inflammatory processes by this well-known cut-off value. Therefore, we clinically excluded the patients with any acute or chronicle infection or inflammatory disease which could be a confounding factor for our study.
The patients were first divided into two groups according to the presence or absence of fQRS. Afterwards, additional categories determined by the subtype of ACS and number of fQRS were used for presentation of data. Lastly, logistic regression analysis was used to determine independent predictors of fQRS.
Laboratory measurements
Cardiac biomarker levels including CK, creatine kinase-MB (CK-MB), Troponin-I, and inflammatory markers including leukocytes and CRP were measured at our emergency department. Plasma blood glucose levels were measured as both admission blood glucose and fasting plasma glucose. The lipid samples were drawn by venipuncture to perform routine blood chemistry after fasting for at least 8 hours before coronary angiography. Fasting blood glucose, total cholesterol, high density lipoprotein-cholesterol (HDL-C), low density lipoprotein-cholesterol (LDL-C), triglyceride levels and other parameters were recorded in our hospital database. Glucose, creatinine, and lipid profiles were determined by standard methods. White blood cell (leukocyte) counts were obtained from an automated cell counter (Coulter Gen-S, COULTER Corp., Miami, FL, USA). Serum CRP levels were determined by the nephelometric method.
Electrocardiography
A 12-lead admission ECG was obtained from all patients. All ECGs (filter range 0.5 Hz to 150 Hz, AC filter 60 Hz, 25 mm/s, 10 mm/mV) were analyzed by two independent clinicians who were blinded to the study design and the data collected.
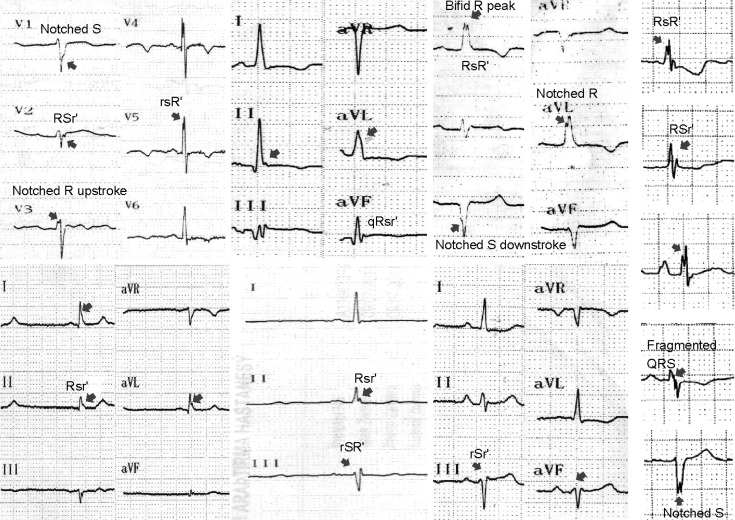
The fQRS was defined by the presence of various RSR' patterns (QRS duration <120 ms) with or without Q wave, which included an additional R wave (R' prime) or notching of the R wave or S wave, or the presence of more than one R prime (fragmentation) without typical bundle branch block in two contiguous leads corresponding to a major lead set for major coronary artery territory (Fig. 1). A notch on an R or S wave was defined as a definite but transient reversal of direction of the main deflection. The presence of fQRS was detected by inspection of tracings with the naked eye. Analysis of the standard 12-lead ECG was performed without using any magnification. The inter-observer concordance rate with regards to detecting the presence of fQRS was 97.8% between the two readers. In case of disagreement, the final diagnosis was achieved by mutual agreement. The intra-observer concordance rate was 98%. Fragmentations were considered to be present if a visually identifiable signal was demonstrated in all complexes of a particular lead. For statistical analysis, fQRS was defined to be present if found in ≥2 contiguous leads in anterior, lateral or inferior derivations. We also used the concept of "number of fQRS" which represents number of fQRS ≥2 because "one fQRS complex" was alone not accepted for the presence of fQRS. The QRS time was measured by manual and digitalized methods and a significant difference was not found between both methods. It was determined by the longest QRS in any lead.
Fig. 1.
The various types of notched and fragmented QRS complexes used to select patients in our study. Different fQRS patterns are shown by arrows including rSr', rSR', RSr', notched R up-stroke and notched S down-stroke, bifid R peak and bifid R nadir.
Diagnosis of an acute STEMI was made by the presence of new or presumed new ST-segment elevations at the J point in ≥2 contiguous leads of ≥0.2 mV in leads V 1, V 2, or V 3 and ≥0.1 mV in other leads.15) Marked ST depression, which was maximal in leads V 1 through V 3, without ST-segment elevation in other leads, was designated as posterior wall MI and included in the STEMI group.
Pathologic Q wave: any Q wave in lead V 2 or V 3 ≥0.02 seconds or QS complex in leads V 2 and V 3 Q wave ≥0.03 seconds and ≥0.1 mV deep or QS complex in lead I, II, aVL, aVF, or V 4 to V 6 in any 2 leads of a contiguous lead grouping (I, aVL, and V 6; V 4 to V 6; and II, III, and aVF). R wave ≥0.04 seconds in lead V 1 or V 2 and R/S ratio ≥1 with a concordant positive T wave in the absence of a conduction defect.15) The diagnosis of USAP and NSTEMI was performed according to the American American College of Cardiology/American Heart Association 2007 guidelines for the management of patients that were unstable.16)
Evaluation of the extent and severity of the coronary lesions at angiography
Standard selective coronary angiography with at least 4 views of the left coronary system and 2 views of the right coronary artery was performed in all patients using Judkins technique. Coronary angiograms were recorded on compact discs in DICOM format. Atherosclerotic coronary involvement was assessed by the number of vessels involved (vessel score) and by a severity score. Significant stenosis was determined visually and defined as ≥50% reduction in lumen diameter in any view compared with the nearest normal segment. Vessel score ranged from 0 to 4, depending on the vessels involved (0: normal, 1: <50% luminal narrowing, 2, 3 and 4: ≥50% luminal narrowing for 1, 2 and 3 vessels). Coronary atherosclerotic burden was assessed using the Gensini score.
Gensini score considers both the extent and the severity of the lesions at coronary angiography and was calculated for each patient.17) This scoring system grades the stenosis in the epicardial coronary arteries (1 for 1-25% stenosis, 2 for 26-50% stenosis, 4 for 51-75% stenosis, 8 for 76-90% stenosis, 16 for 91-99% stenosis, and 32 for total occlusion) and multiplies this number by a constant number determined according to the anatomical position of the lesion.
Statistical analysis
Continuous variables are shown as mean±standard deviation, and categorical variables are defined as percentages. Continuous variables were compared by the Student's t-test and the χ2 test was used for analyzing categorical variables between two groups. Mean values were compared by analysis of variance among different groups. Logistic regression analyses were used for the multivariate analysis of independent variables which were included if they were significantly different in the univariate analyses (age, number of obstructed vessels ≥50%, Q wave on ECG, creatinine levels, leukocytes, CRP, Gensini score, QRS duration, CK-MB and Troponin I). All tests of significance were two-tailed. Statistical significance was defined as p<0.05. The Statistical Package for the Social Sciences (SPSS) statistical software (SPSS 15.0 for windows, Inc., Chicago, IL, USA) was used for all statistical calculations.
Results
The baseline clinical characteristics are detailed in Table 1. Patients with fQRS were older (p=0.02), with higher CRP (p<0.001), CK-MB levels (p=0.006), prolonged QRS time (p<0.001), extended CAD (p<0.001), and Q wave on admission ECG (p<0.001) in comparison to patients with non-fragmented QRS.
Table 1.
Baseline characteristics of the study subjects
ASA: acetyl salicylic acid, ACEI: angiotensin converting enzyme inhibitor, ARB: angiotensin II receptor blocker, CAD: coronary artery disease, BMI: body mass index, HDL-C: high density lipoprotein-cholesterol, LDL-C: low density lipoprotein-cholesterol, OAD: oral anti-diabetic drugs, CRP: C-reactive protein, NS: not significant, Adm.: admission value, eGFR: estimated glomerular filtration rate, CK-MB: creatinine kinase muscle-brain, LDH: lactate dehydrogenase, USAP: unstable angina pectoris, NSTEMI: non-ST elevated myocardial infarction, STEMI: ST elevated myocardial infarction, ECG: electrocardiography
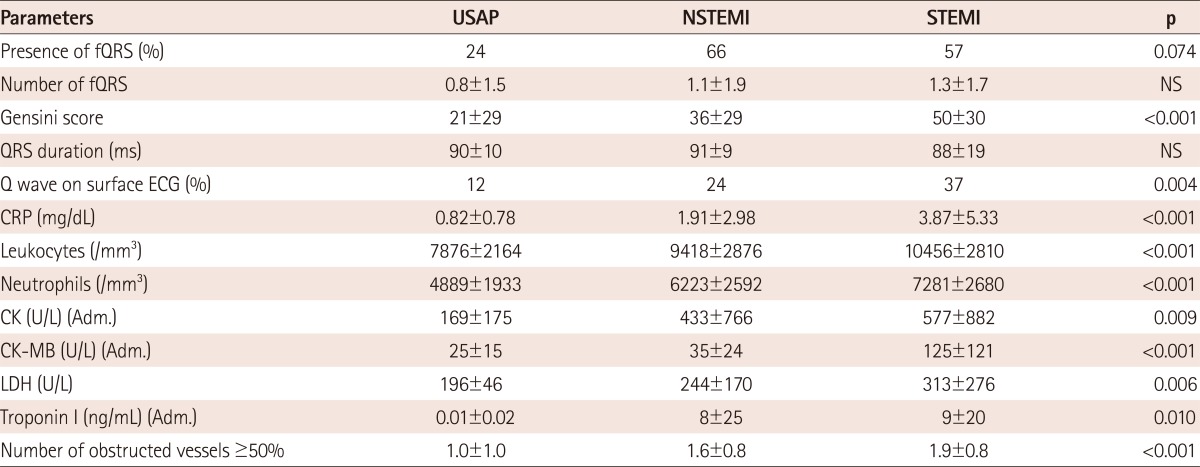
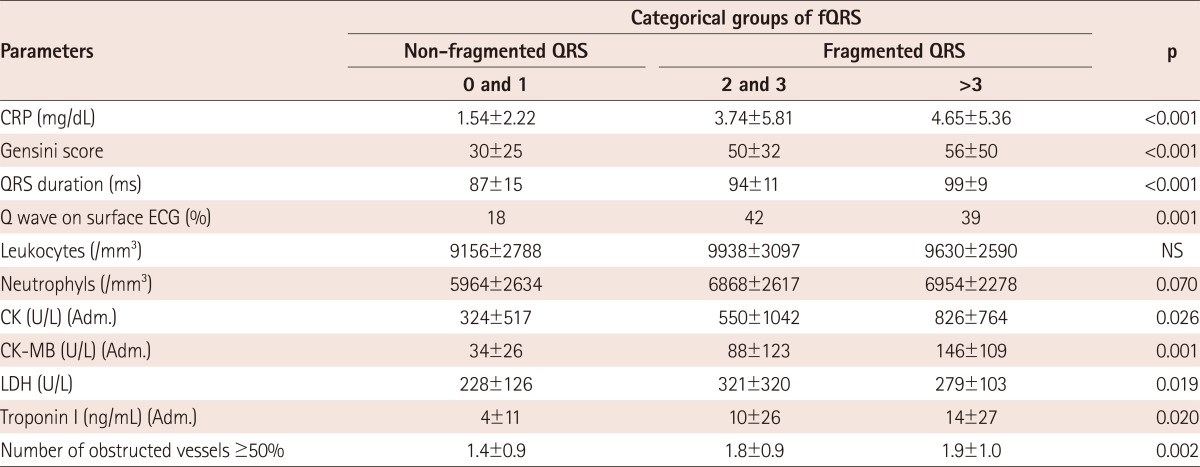
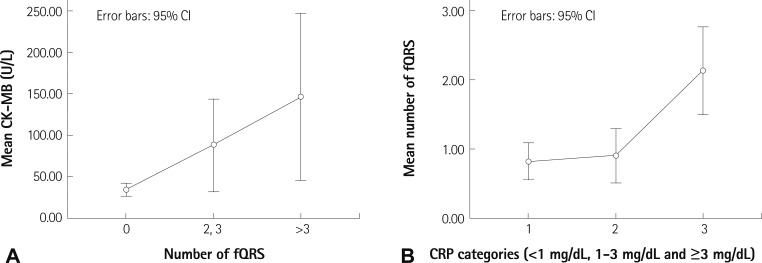
In Table 2, study parameters are presented by group determined according to the subtype of ACS. Presence and number of fQRS, and the other study parameters were significantly different in these groups. The fQRS complexes were seen in more than one coronary territory in some patients. Additionally, the study parameters were presented in the groups and determined according to number of fQRS (0-1, 2-3 and >3), as shown in Table 3. Gensini score (p<0.001), Q wave on surface ECG (p=0.001), admission CK (p=0.026), CK-MB (p=0.001) (Fig. 2A), Troponin I levels (p=0.020), CRP (p<0.001), lactate dehydrogenase (p=0.019), Gensini score (p<0.001), number of obstructed vessels ≥50% (p=0.002) and QRS duration (p<0.001) were found to be increased with significantly raised numbers of fQRS.
Table 2.
Distribution of fQRS and the prognostic factors in subgroups determined by the clinical type of acute coronary syndromes
fQRS: fragmented QRS complex, USAP: unstable angina pectoris, NSTEMI: non-ST elevated myocardial infarction, STEMI: ST elevated myocardial infarction, NS: not significant, ECG: electrocardiography, CRP: C-reactive protein, CK-MB: creatinine kinase muscle-brain, Adm.: admission value, LDH: lactate dehydrogenase
Table 3.
Distribution of study parameters in the subgroups determined according to the fQRS
fQRS: fragmented QRS complex, CRP: C-reactive protein, ECG: electrocardiography, NS: not significant, CK-MB: creatinine kinase muscle-brain, Adm.: admission value, LDH: lactate dehydrogenase
Fig. 2.
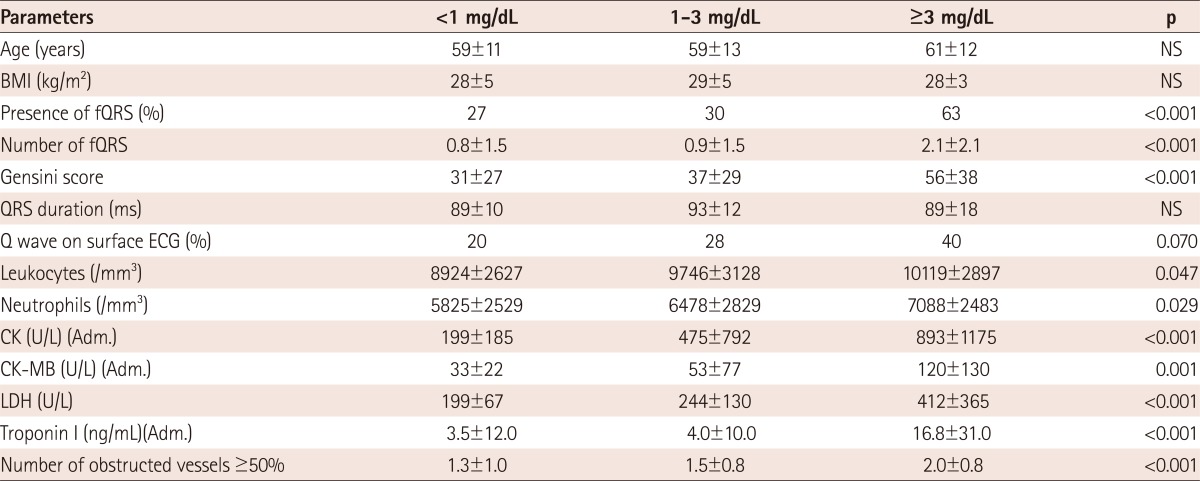
Relationship of fragmented QRS and CK-MB or CRP. A: the increased CK-MB levels in subgroups determined by raised numbers of fQRS. B: the increased number of fQRS in subgroups determined by raised CRP levels. CK-MB: creatine kinase-MB, fQRS: fragmented QRS complex, CRP: C-reactive protein, CI: confidence interval.
Distribution of study parameters in subgroups determined according to CRP levels (<1, 1-3 and ≥3 mg/dL) is shown in Table 4. CRP was significantly related to presence and number of fQRS (Fig. 2B), Gensini score (p<0.001), inflammatory cells, CK-MB (p<0.001), troponin levels (p<0.001) and number of obstructed vessels ≥50% (p<0.001).
Table 4.
Distribution of study parameters in subgroups determined by raised CRP levels
CRP: C-reactive protein, NS: not significant, Adm.: admission value, CK-MB: creatinine kinase muscle-brain, LDH: lactate dehydrogenase, BMI: Body Mass Index, fQRS: fragmented QRS complex, ECG: electrocardiography
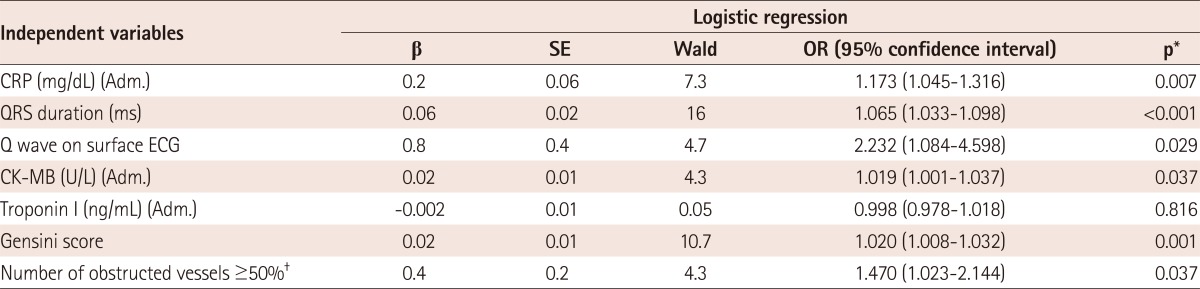
When we performed multiple logistic regression analysis, fQRS was found to be related to increased CRP levels {odds ratio (OR): 1.2, 95% confidence interval (CI): 1.045-1.316, p=0.007}, QRS duration (OR:1.1, 95% CI: 1.033-1.098, p<0.001), extent of CAD (OR: 1.5, 95% CI: 1.023-2.144, p=0.037), Q wave (OR: 2.2, 95% CI: 1.084-4.598, p=0.03) and CK-MB levels (OR: 1.0, 95% CI: 1.001-1.037, p=0.04) independently (Table 5).
Table 5.
The relationship between the presence of fQRS on admission ECG and independent predictors
*Logistic regression with enter method was used for multivariate analysis of independent variables. The variables which had significant p (p<0.05) in univariate analyses were included in the multivariate analysis, †When included in the analysis. OR: odds ratio, CRP: C-reactive protein, Adm.: admission value, β: βeta coefficient, SE: standard error, ECG: electrocardiography, CK-MB: creatinine kinase muscle-brain, fQRS: fragmented QRS complex
Discussion
In this study, we interrogated the possible independent relationship of systemic inflammation with presence of fQRS in patients with ACS. Our findings suggested that higher levels of CRP are related to the presence and number of fQRS. To our knowledge, this is the first report demonstrating the potential role of systemic inflammation for the development of fQRS in patients with ACS.
Although fQRS is defined by unexpected deviations in QRS morphology, the specific cause of fractionation on surface ECG is not fully known yet. fQRS has been shown to predict cardiac events in several populations. Pathophysiologically, fQRS is generally accepted to derive from regional myocardial fibrosis/scar and ischemia, which causes nonhomogeneous myocardial electrical activation.18-22) In patients with ischemic or non-ischemic left ventricular dysfunction, fQRS has been shown to be related to myocardial fibrosis.23) In previous studies in which Gadolinium delayed enhancement on cardiac magnetic resonance imaging was used to determine myocardial structure, fQRS has been found to be related with extensive myocardial scar.11),12) fQRS was also found to be a marker of a prior MI, demonstrated by regional perfusion abnormalities with scintigraphic evaluation, which has a substantially higher sensitivity and negative predictive value compared with the Q wave.6),24) Regional fQRS patterns denote to the presence of a greater corresponding focal regional myocardial scar on stress myocardial perfusion imaging.25) Our study findings also supported this reverse relationship between fQRS and LV systolic functions. Additionally, the fact that chronic ischemia could cause myocardial patchy fibrosis without prior MI.26) Similarly, in our study coronary atherosclerotic burden and extent of CAD were related to fQRS.
Today, it is well known that myocardial ischemia may cause heart failure and ventricular arrhythmias due to stimulation of the development of scar tissue, which is related with increased mortality and morbidity.6),18),25),27) In a setting of ACS, non-homogenous depolarization of myocardium caused by ischemia and infarct may be the main determinant for increased arrhythmic events in a hospital course. In our study, the extent of infarcted myocardium on admission was assessed by measuring the cardiac biomarkers and fQRS was found to be related to the extent of infarcted myocardium at admission independently. In particular, this relationship was significant for CK and CK-MB, but not for Troponin I. This may possibly be related to a late increase in Troponin I levels in the setting of ACS. Similarly, we also found that fQRS was related with the extent and severity of CAD. This is possibly derived from jeopardized ischemic myocardium which can also cause non-homogenous conduction on myocardium.2),21)
Elevated admission CRP levels have been related with increased short and long-term prognosis and complications in patients with ACS.28) In our study, we also found that fQRS was independently related to CRP. Its exact mechanism is beyond the scope of the current study. However, the presence of an independent relationship between fQRS and CRP may rule out a secondary interaction of infarct induced non-specific inflammation. In a previous study, it has been shown that CRP is able to directly induce cardiac fibrosis and inflammation on cardiac fibroblasts and also promotes Ang II-mediated cardiac remodeling in vivo and in vitro by up-regulating the AT1 receptor and by enhanced activation of the transforming growth factor β/Smad and NF-kB signaling pathways.29) Similarly, tumor necorsis factor-α, interleukin-1b, and TWEAK could induce myocardial fibrosis by the NF-kB pathway. Otherwise, we think that CRP may also have possible effects on myocardium and the conductive system in patients with ACS. In the literature, these effects have been supported by some studies.30) Systemic inflammation may play a significant role in cardiac arrhythmias and conduction disturbances. The possible reason behind cardiac arrhythmias and conduction disturbances seems to be related to myocardial inflammation, focal fibrosis, and ischemia within the conduction system.13) In a recent study, Kadi et al.14) showed that fQRS increased even in patients with rheumatoid arthritis without cardiovascular disease, in which it is speculated that inflammatory processes may play a pivotal role to produce fragmentations on ECG.
In patients with ACS, it has been demonstrated that admission prolonged QRS time is related with increased long-term mortality by possibly increasing heart failure, arrhythmia and ischemia. In our study, prolonged QRS time was related to fQRS even relatively in the normal range of QRS (<120 ms). This relationship may have two possible explanations. Either fragmentation on QRS complex is induced by prolongation in QRS time or fragmentation on QRS causes an increase of the the duration of QRS complex. However, by our study design, we can only speculate which one is the cause and which one is the result or response for fragmentation. This interaction should be searched to clarify cause-result relationship in an electrophysiological based study.
Study limitations
In our study, we found a relationship between CRP and fQRS independent of other study parameters, but increased CRP may only be a side result of undetermined inducers, which may induce both production of fQRS and CRP. Possible dose-response relationships between CRP and development of fQRS may be evaluated in such a study in which dynamic changes of fQRS with different CRP levels are shown. In clinical practice, we use the nephelometric method to measure CRP; we did not have high-sensitive CRP (HsCRP) values in our study. HsCRP as a scanning test is superior to detect low-grade inflammation, especially in patients with stable CAD compared to other CRP measurements. Since we applied the same method to determine the degree of systemic inflammation in both groups, this problem should not change the interpretation of the results.
We only included patients with a QRS duration <120 ms in our study. We excluded patients with complete bundle branch block, intra-ventricular conduction delay, and patients with permanent pacemakers in our study. Therefore, our results do not apply to patients with wide QRS complexes.
In conclusion, in our study, we found that fQRS is related to increased CRP. Additionally, QRS time, Q-wave and the extent of infarct and CAD were also independent predictors for fQRS. The development of fQRS may also be related to systemic inflammation as well as myocardial ischemia and necrosis at early ACS stage. fQRS, which may result as an end effect of inflammation at the cellular level can represent increased cardiac risk in patients with ACS. fQRS is a simple, cheap and non-invasive modality that can be a valuable tool for predicting cardiac status and risk.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Das MK, Suradi H, Maskoun W, et al. Fragmented wide QRS on a 12-lead ECG: a sign of myocardial scar and poor prognosis. Circ Arrhythm Electrophysiol. 2008;1:258–268. doi: 10.1161/CIRCEP.107.763284. [DOI] [PubMed] [Google Scholar]
- 2.Das MK, Michael MA, Suradi H, et al. Usefulness of fragmented QRS on a 12-lead electrocardiogram in acute coronary syndrome for predicting mortality. Am J Cardiol. 2009;104:1631–1637. doi: 10.1016/j.amjcard.2009.07.046. [DOI] [PubMed] [Google Scholar]
- 3.Korhonen P, Husa T, Konttila T, et al. Fragmented QRS in prediction of cardiac deaths and heart failure hospitalizations after myocardial infarction. Ann Noninvasive Electrocardiol. 2010;15:130–137. doi: 10.1111/j.1542-474X.2010.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Das MK, Saha C, El Masry H, et al. Fragmented QRS on a 12-lead ECG: a predictor of mortality and cardiac events in patients with coronary artery disease. Heart Rhythm. 2007;4:1385–1392. doi: 10.1016/j.hrthm.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 5.Pietrasik G, Goldenberg I, Zdzienicka J, Moss AJ, Zareba W. Prognostic significance of fragmented QRS complex for predicting the risk of recurrent cardiac events in patients with Q-wave myocardial infarction. Am J Cardiol. 2007;100:583–586. doi: 10.1016/j.amjcard.2007.03.063. [DOI] [PubMed] [Google Scholar]
- 6.Das MK, Khan B, Jacob S, Kumar A, Mahenthiran J. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation. 2006;113:2495–2501. doi: 10.1161/CIRCULATIONAHA.105.595892. [DOI] [PubMed] [Google Scholar]
- 7.Das MK, Zipes DP. Fragmented QRS: a predictor of mortality and sudden cardiac death. Heart Rhythm. 2009;6(3 Suppl):S8–S14. doi: 10.1016/j.hrthm.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 8.Cheema A, Khalid A, Wimmer A, et al. Fragmented QRS and mortality risk in patients with left ventricular dysfunction. Circ Arrhythm Electrophysiol. 2010;3:339–344. doi: 10.1161/CIRCEP.110.940478. [DOI] [PubMed] [Google Scholar]
- 9.Das MK, El Masry H. Fragmented QRS and other depolarization abnormalities as a predictor of mortality and sudden cardiac death. Curr Opin Cardiol. 2010;25:59–64. doi: 10.1097/HCO.0b013e328333d35d. [DOI] [PubMed] [Google Scholar]
- 10.Das MK, Maskoun W, Shen C, et al. Fragmented QRS on twelve-lead electrocardiogram predicts arrhythmic events in patients with ischemic and nonischemic cardiomyopathy. Heart Rhythm. 2010;7:74–80. doi: 10.1016/j.hrthm.2009.09.065. [DOI] [PubMed] [Google Scholar]
- 11.Gardner PI, Ursell PC, Fenoglio JJ, Jr, Wit AL. Electrophysiologic and anatomic basis for fractionated electrograms recorded from healed myocardial infarcts. Circulation. 1985;72:596–611. doi: 10.1161/01.cir.72.3.596. [DOI] [PubMed] [Google Scholar]
- 12.Chatterjee S, Changawala N. Fragmented QRS complex: a novel marker of cardiovascular disease. Clin Cardiol. 2010;33:68–71. doi: 10.1002/clc.20709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisen A, Arnson Y, Dovrish Z, Hadary R, Amital H. Arrhythmias and conduction defects in rheumatological diseases: a comprehensive review. Semin Arthritis Rheum. 2009;39:145–156. doi: 10.1016/j.semarthrit.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Kadi H, Inanir A, Habiboglu A, et al. Frequency of fragmented QRS on ECG is increased in patients with rheumatoid arthritis without cardiovascular disease: a pilot study. Mod Rheumatol. 2012;22:238–242. doi: 10.1007/s10165-011-0493-9. [DOI] [PubMed] [Google Scholar]
- 15.Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 16.Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients with Unstable Angina/Non ST-Elevation Myocardial Infarction): developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation. 2007;116:e148–e304. doi: 10.1161/CIRCULATIONAHA.107.181940. [DOI] [PubMed] [Google Scholar]
- 17.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51:606. doi: 10.1016/s0002-9149(83)80105-2. [DOI] [PubMed] [Google Scholar]
- 18.Flowers NC, Horan LG, Thomas JR, Tolleson WJ. The anatomic basis for high-frequency components in the electrocardiogram. Circulation. 1969;39:531–539. doi: 10.1161/01.cir.39.4.531. [DOI] [PubMed] [Google Scholar]
- 19.Lesh MD, Spear JF, Simson MB. A computer model of the electrogram: what causes fractionation? J Electrocardiol. 1988;21(Suppl):S69–S73. doi: 10.1016/0022-0736(88)90061-1. [DOI] [PubMed] [Google Scholar]
- 20.Friedman PL, Fenoglio JJ, Wit AL. Time course for reversal of electrophysiological and ultrastructural abnormalities in subendocardial Purkinje fibers surviving extensive myocardial infarction in dogs. Circ Res. 1975;36:127–144. doi: 10.1161/01.res.36.1.127. [DOI] [PubMed] [Google Scholar]
- 21.Wiener I, Mindich B, Pitchon R. Fragmented endocardial electrical activity in patients with ventricular tachycardia: a new guide to surgical therapy. Am Heart J. 1984;107:86–90. doi: 10.1016/0002-8703(84)90138-8. [DOI] [PubMed] [Google Scholar]
- 22.Basaran Y, Tigen K, Karaahmet T, et al. Fragmented QRS complexes are associated with cardiac fibrosis and significant intraventricular systolic dyssynchrony in nonischemic dilated cardiomyopathy patients with a narrow QRS interval. Echocardiography. 2011;28:62–68. doi: 10.1111/j.1540-8175.2010.01242.x. [DOI] [PubMed] [Google Scholar]
- 23.Calore C, Cacciavillani L, Boffa GM, et al. Contrast-enhanced cardiovascular magnetic resonance in primary and ischemic dilated cardiomyopathy. J Cardiovasc Med (Hagerstown) 2007;8:821–829. doi: 10.2459/JCM.0b013e3280101e3c. [DOI] [PubMed] [Google Scholar]
- 24.Reddy CV, Cheriparambill K, Saul B, et al. Fragmented left sided QRS in absence of bundle branch block: sign of left ventricular aneurysm. Ann Noninvasive Electrocardiol. 2006;11:132–138. doi: 10.1111/j.1542-474X.2006.00094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahenthiran J, Khan BR, Sawada SG, Das MK. Fragmented QRS complexes not typical of a bundle branch block: a marker of greater myocardial perfusion tomography abnormalities in coronary artery disease. J Nucl Cardiol. 2007;14:347–353. doi: 10.1016/j.nuclcard.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Weinberg SL, Reynolds RW, Rosenman RH, Katz LN. Electrocardiographic changes associated with patchy myocardial fibrosis in the absence of confluent myocardial infarction; an anatomic correlative study. Am Heart J. 1950;40:745–759. doi: 10.1016/0002-8703(50)90203-1. [DOI] [PubMed] [Google Scholar]
- 27.Varriale P, Chryssos BE. The RSR' complex not related to right bundle branch block: diagnostic value as a sign of myocardial infarction scar. Am Heart J. 1992;123:369–376. doi: 10.1016/0002-8703(92)90648-f. [DOI] [PubMed] [Google Scholar]
- 28.Bursi F, Weston SA, Killian JM, Gabriel SE, Jacobsen SJ, Roger VL. C-reactive protein and heart failure after myocardial infarction in the community. Am J Med. 2007;120:616–622. doi: 10.1016/j.amjmed.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 29.Zhang R, Zhang YY, Huang XR, et al. C-reactive protein promotes cardiac fibrosis and inflammation in angiotensin II-induced hypertensive cardiac disease. Hypertension. 2010;55:953–960. doi: 10.1161/HYPERTENSIONAHA.109.140608. [DOI] [PubMed] [Google Scholar]
- 30.Kazumi T, Kawaguchi A, Hirano T, Yoshino G. C-reactive protein in young, apparently healthy men: associations with serum leptin, QTc interval, and high-density lipoprotein-cholesterol. Metabolism. 2003;52:1113–1116. doi: 10.1016/s0026-0495(03)00184-7. [DOI] [PubMed] [Google Scholar]