Abstract
Harknessiaceae is introduced as a new family in the ascomycete order Diaporthales to accommodate species of Harknessia with their Wuestneia-like teleomorphs. The family is distinguished by having pycnidial conidiomata with brown, furfuraceous margins, brown conidia with hyaline, tube-like basal appendages, longitudinal striations, and rhexolytic secession. Six species occurring on Eucalyptus are newly introduced, namely H. australiensis, H. ellipsoidea, H. pseudohawaiiensis, and H. ravenstreetina from Australia, H. kleinzeeina from South Africa, and H. viterboensis from Italy. Epitypes are designated for H. spermatoidea and H. weresubiae, both also occurring on Eucalyptus. Members of Harknessia are commonly associated with leaf spots, but also occur as saprobes and endophytes in leaves and twigs of various angiosperm hosts.
Keywords: biodiversity, fungal pathogens, Harknessiaceae, ITS, LSU, phylogeny, systematics
INTRODUCTION
Members of the genus Harknessia have a worldwide distribution, and are commonly associated with leaves and branches (twigs) of a wide range of hosts (Nag Raj 1993, Sankaran et al. 1995, Farr & Rossman 2001). Although some species have been reported as being associated with leaf spots (Crous et al. 1989, 1993), many have been isolated from leaf and twig litter (Sutton & Pascoe 1989, Swart et al. 1998, Crous & Rogers 2001, Lee et al. 2004, Marincowitz et al. 2008), or from leaves with symptoms of tip dieback or leaf scorch (Fig. 1). Conidiomata readily develop in moist chambers, and species appear to be endophytic (Bettuci & Saravay 1993), often fruiting on leaf spots of more aggressive foliar pathogens. Although several Harknessia species may be pathogenic, not much is known about their pathogenicity, and in general they are regarded of little economic importance (Park et al. 2000). Species of Harknessia occur on diverse gymnosperm and dicotyledonous hosts, with the genus Eucalyptus (Myrtaceae) harbouring up to 21 of the 53 species recognised. Several major treatments have focused on revising the genus (Sutton 1971, 1980, Nag Raj & DiCosmo 1981, Nag Raj 1993), although only a few studies have employed an integrated approach with molecular data to resolve species boundaries and host specificity (Castlebury et al. 2002, Lee et al. 2004, Summerell et al. 2006, Crous et al. 2007).
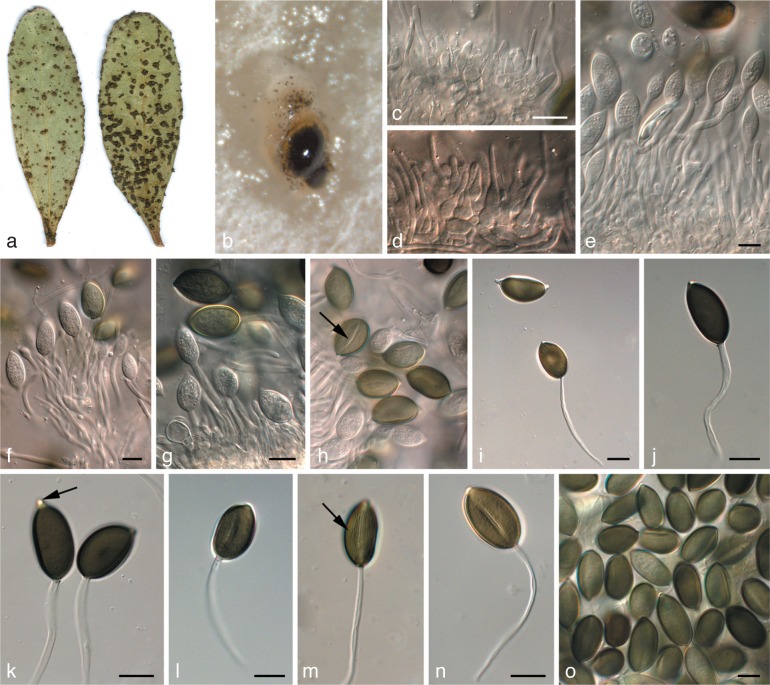
Fig. 1.

Leaf spot disease symptoms associated with Harknessia spp. on different Eucalyptus hosts. a. H. fusiformis (CPC 13649); b. H. hawaiiensis (15003); c, d. H. rhabdosphaera (CPC 13593 and CPC 12847); e. H. globispora (CPC 14924); f. H. eucalyptorum (CPC 12697).
The genus Harknessia is characterised by having stromatic to pycnidial conidiomata, and dark brown conidia with tube-shaped basal appendages, longitudinal striations, and rhexolytic secession. Taxa with hyaline conidia and apical appendages were placed in Mastigosporella (von Höhnel 1914), while Apoharknessia was introduced for species with brown conidia and apical as well as basal appendages (Lee et al. 2004), and Dwiroopa for species with very thick conidial walls and longitudinal slits (Farr & Rossman 2003). Several genera were also seen as synonyms, namely Caudosporella, Mastigonetron, and Cymbothyrium (Nag Raj & DiCosmo 1981).
Teleomorphs of Harknessia were initially described in Cryptosporella (Nag Raj & DiCosmo 1981) (Cryptosporellaceae; von Arx & Müller 1954), which Reid & Booth (1989) reduced to synonymy with the older Wuestneia (Diaporthales) (Barr 1978, Castlebury et al. 2002, Lee et al. 2004). Seven of the 13 Wuestneia species known to date have been linked to Harknessia anamorphs (Reid & Booth 1989, Sutton & Pascoe 1989, Crous et al. 1993, Yuan & Mohammed 1997, Crous & Rogers 2001) (Fig. 2, 3).
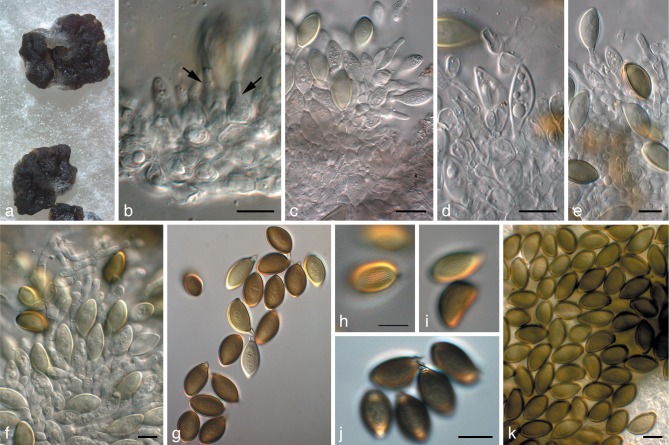
Fig. 2.
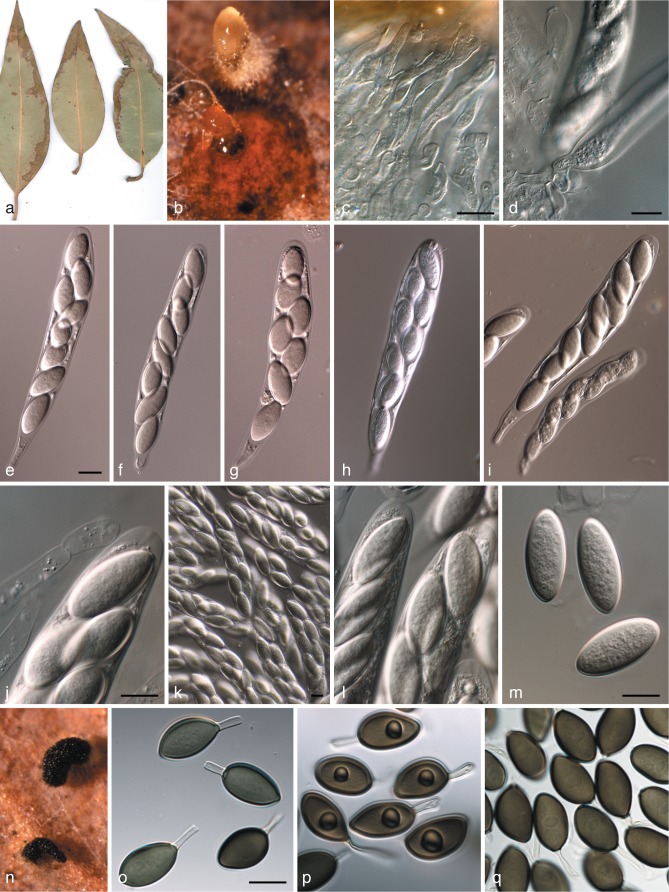
Harknessia eucalyptorum and its teleomorph (CPC 12697). a. Leaf spot symptoms on Eucalyptus sp.; b. ascomatum with short neck, oozing ascospores; c, d. paraphyses and asci; e–i. asci; j. paraphyse and ascal tip; k, l. asci; m. ascospores; n. conidiomata oozing conidia; o–q. conidia with basal appendages and central guttules. — Scale bars = 10 μm.
Fig. 3.
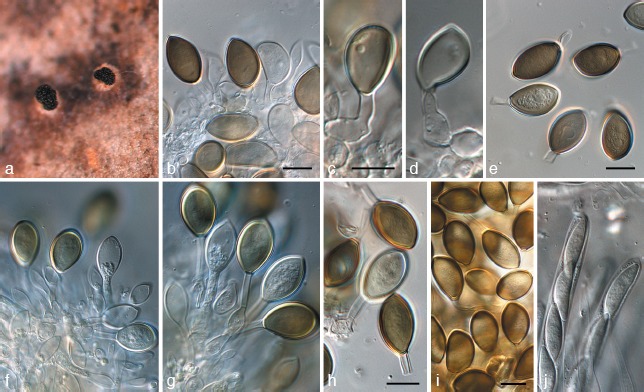
Harknessia gibbosa (CPC 12473). a. Conidiomata sporulating on leaf tissue; b–d, f, g. conidiogenous cells giving rise to conidia; e, h, i. conidia; j. asci of teleomorph. — Scale bars = 10 μm.
Castlebury et al. (2002) provided an overview of Diaporthales, recognising six major lineages, of which Melanconidaceae had an affinity with Gnomoniaceae, highlighting unresolved complexes such as the Wuestneia/Harknessia complex, Cryphonectria/Endothia complex, and the Schizoparme/Pilidiella complex. Subsequent studies have resolved the latter two complexes to represent the Cryphonectriaceae (Gryzenhout et al. 2006) and Schizoparmaceae (Rossman et al. 2007), respectively. The Wuestneia/Harknessia complex has still remained unresolved within Diaporthales. The aims of the present study were to introduce a family for the Wuestneia/Harknessia complex, and to name several newly collected species.
MATERIALS AND METHODS
Isolates
Symptomatic or dead leaves and twigs were collected in different countries from a wide range of hosts (Table 1). Samples were incubated in damp chambers for 2–3 d before examination. Single-spore isolation was carried out and cultures were established on malt extract agar (MEA) as described by Crous et al. (1991). Colonies were subcultured onto 2 % potato-dextrose agar (PDA), MEA, and oatmeal agar (OA) (Crous et al. 2009b), and incubated under continuous near-ultraviolet light at 25 °C to promote sporulation. Reference strains are maintained in the CBS-KNAW Fungal Biodiversity Centre (CBS) Utrecht, The Netherlands (Table 1). Nomenclatural novelties and descriptions were deposited in MycoBank (Crous et al. 2004).
Table 1.
Harknessia and Harknessia-like isolates included in the morphological and/or phylogenetic analyses.
| Species | Culture accession numbers1,2 | Substrate | Country | Collector | GenBank accession numbers3 |
|||
|---|---|---|---|---|---|---|---|---|
| ITS | TUB | CAL | LSU | |||||
| Apoharknessia insueta | CPC 10947; CBS 114575 | Leaf spots on Eucalyptus sp. | Colombia | M.J. Wingfield | – | – | – | AY720813 |
| CPC 11775 | Yucca elephantipes | Costa Rica | A. Igram | JQ706082 | – | – | JQ706209 | |
| CPC 1451; CBS 111377ET | Leaves of Eucalyptus pellita | Brazil | P.W. Crous | JQ706083 | – | – | AY720814 | |
| Foliocryphia eucalypti | CPC 12494; CBS 124779ET | Eucalyptus coccifera | Australia: Tasmania | C. Mohammed | GQ303276 | JQ706128 | – | GQ303307 |
| Harknessia australiensis | CPC 13596; CBS 132120 | Leaves of Eucalyptus sclerophylla | Australia: New South Wales | B.A. Summerell | JQ706084 | JQ706129 | JQ706170 | JQ706210 |
| CPC 15029ET; CBS 132119 | Leaves of Eucalyptus dissita | Australia: New South Wales | B.A. Summerell | JQ706085 | JQ706130 | JQ706171 | JQ706211 | |
| Harknessia capensis | CPC 10867; CBS 115061 | Eucalyptus leaves | South Africa: Western Cape Province | P.W. Crous | AY720718 | AY720750 | AY720781 | AY720815 |
| CPC 5468; CBS 111829ET | Dead twigs and leaf litter of Brabejum stellatifolium | South Africa: Western Cape Province | S. Lee | AY720719 | AY720751 | AY720782 | AY720816 | |
| Harknessia ellipsoidea | CPC 13077; CBS 132122 | Leaves of Eucalyptus propinque | Australia: New South Wales | B.A. Summerell | JQ706086 | JQ706131 | JQ706172 | JQ706212 |
| CPC 17111ET; CBS 132121 | Leaves of Eucalyptus sp. | Australia: Queensland | P.W. Crous & R.G. Shivas | JQ706087 | JQ706132 | JQ706173 | JQ706213 | |
| CPC 17113ET | Leaves of Eucalyptus sp. | Australia: Queensland | P.W. Crous & R.G. Shivas | JQ706088 | JQ706133 | JQ706174 | JQ706214 | |
| Harknessia eucalypti | CBS 342.97 | Eucalyptus regnans | Australia: Tasmania | Z.-Q. Yuan | AY720745 | AY720777 | AY720808 | AF408363 |
| CPC 13643 | Eucalyptus regnans | Australia: Tasmania | B.A. Summerell | JQ706089 | JQ706134 | JQ706175 | JQ706215 | |
| Harknessia eucalyptorum | CBS 113620 | Leaves of Eucalyptus sp. | Spain | P.W. Crous & G. Bills | AY720746 | AY720778 | AY720809 | AY720839 |
| CPC 85; CBS 111115ET | Leaves of Eucalyptus andrewsii | South Africa: Western Cape Province | P.W. Crous | AY720747 | AY720779 | AY720810 | AY720840 | |
| CPC 11302 | Eucalyptus sp. | Italy | W. Gams | JQ706090 | JQ706135 | JQ706176 | – | |
| CPC 12697 | Leaf litter of Eucalyptus sp. | South Africa: Western Cape Province | P.W. Crous | JQ706091 | JQ706136 | JQ706177 | JQ706216 | |
| CPC 13074 | – | Italy | W. Gams | JQ706092 | JQ706137 | – | JQ706217 | |
| CPC 14951 | Eucalyptus sp. | Portugal | P.W. Crous | JQ706093 | JQ706138 | JQ706178 | JQ706218 | |
| CPC 14954 | Eucalyptus sp. | Portugal | P.W. Crous | JQ706094 | – | – | JQ706219 | |
| CPC 19659 | Eucalyptus cypellocarpa | Australia: Northern Territory | P.W. Crous | JQ706095 | – | – | – | |
| Harknessia fusiformis | CPC 295; CBS 110785ET | Leaf litter of Eucalyptus sp. | South Africa: Orange Free State | P.W. Crous | AY720721 | AY720753 | AY720784 | AY720818 |
| CPC 10488; CBS 115649 | Leaves of Eucalyptus sp. | South Africa: Orange Free State | P.W. Crous | AY720720 | AY720752 | AY720783 | AY720817 | |
| CPC 11124 | Eucalyptus sp. | New Zealand | J. Stalpers | JQ706096 | JQ706139 | JQ706179 | – | |
| CPC 13649 | Eucalyptus globulus | Australia: Tasmania | B.A. Summerell | JQ706097 | JQ706140 | JQ706180 | JQ706220 | |
| CPC 16550 | Eucalyptus dives | Australia: Southern Highlands | B.A. Summerell | JQ706098 | JQ706141 | JQ706181 | JQ706221 | |
| Harknessia gibbosa | CPC 12473; CBS 120033ET | Eucalyptus delegatensis | Australia: Tasmania | C. Mohammed | EF110615 | JQ706142 | JQ706182 | EF110615 |
| CPC 13646 | Eucalyptus delegatensis | Australia: Tasmania | B.A. Summerell | JQ706099 | JQ706143 | JQ706183 | JQ706222 | |
| CPC 17626 | Acacia pycnantha | Australia: Victoria | P.W. Crous | JQ706100 | JQ706144 | JQ706184 | JQ706223 | |
| CPC 17627 | Acacia pycnantha | Australia: Victoria | P.W. Crous | JQ706101 | JQ706145 | JQ706185 | JQ706224 | |
| CPC 17642 | Eucalyptus sp. | Australia: Victoria | P.W. Crous | JQ706102 | JQ706146 | JQ706186 | JQ706225 | |
| CPC 17676 | Eucalyptus sp. | Australia: Victoria | P.W. Crous | JQ706103 | JQ706147 | JQ706187 | JQ706226 | |
| Harknessia globispora | CPC 12799 | Eucalyptus globulus | Portugal | A.L. Phillips | JQ706104 | – | JQ706188 | JQ706227 |
| CPC 14924 | Eucalyptus sp. | Portugal | P.W. Crous | JQ706105 | JQ706148 | JQ706189 | JQ706228 | |
| CPC 3710; CBS 111578ET | Leaf litter of Eucalyptus globulus | Portugal | S. Denman | AY720722 | AY720754 | AY720785 | AY720819 | |
| Harknessia hawaiiensis | CPC 10957; CBS 114811 | Leaf litter of Eucalyptus sp. | Colombia | M.J. Wingfield | AY720723 | AY720755 | AY720786 | AY720820 |
| CPC 10960; CBS 115650 | Leaf litter of Eucalyptus sp. | Colombia | M.J. Wingfield | AY720724 | AY720756 | AY720787 | AY720821 | |
| CPC 11013 | Eucalyptus sp. | Indonesia | M.J. Wingfield | JQ706106 | JQ706149 | JQ706190 | JQ706229 | |
| CPC 113; CBS 110728 | Leaves of Eucalyptus viminalis | South Africa: Western Cape Province | P.W. Crous | AY720725 | AY720757 | AY720788 | AY720822 | |
| CPC 15003 | Eucalyptus sp. | Ecuador | A.C. Alfenas | JQ706107 | JQ706150 | JQ706191 | JQ706230 | |
| CPC 180; CBS 111122 | Leaves of Eucalyptus grandis | South Africa: Mpumalanga | P.W. Crous | AY720726 | AY720758 | AY720789 | AY720823 | |
| Harknessia ipereniae | CPC 12480; CBS 120030ET | Eucalyptus leaf litter | Australia: Western Australia | A. van Iperen | EF110614 | JQ706151 | JQ706192 | EF110614 |
| Harknessia karwarrae | CPC 10928; CBS 115648 | Leaves of Eucalyptus botryoides | New Zealand | M. Dick | AY720748 | AY720780 | AY720811 | AY720841 |
| Harknessia kleinzeeina | CPC 108; CBS 110729 | Eucalyptus leaf litter | South Africa: Western Cape Province | P.W. Crous | AY720739 | AY720771 | AY720802 | – |
| CPC 16277ET | Leaves of Eucalyptus sp. | South Africa: Northern Cape Province | Z.A. Pretorius | JQ706108 | JQ706152 | JQ706193 | JQ706231 | |
| Harknessia leucospermi | CPC 1373; CBS 775.97ET | Leaf litter of Leucospermum sp. | South Africa: Western Cape Province | P.W. Crous | AY720727 | AY720759 | AY720790 | AY720824 |
| CPC 2849; CBS 114150 | Seedling of Leucospermum sp. | South Africa: Western Cape Province | J.E. Taylor | AY720728 | AY720760 | AY720791 | AY720825 | |
| CPC 5400; CBS 113526 | Dead twigs of Leucospermum praecox | South Africa: Western Cape Province | S. Lee | AY720729 | AY720761 | AY720792 | AY720826 | |
| CPC 5403; CBS 112620 | Dead twigs of unidentified tree (Proteaceae) | South Africa: Western Cape Province | S. Lee | AY720730 | AY720762 | AY720793 | AY720827 | |
| CPC 5404; CBS 112619 | Dead twigs of Protea laurifolia | South Africa: Western Cape Province | S. Lee | AY720731 | AY720763 | AY720794 | – | |
| Harknessia protearum | CPC 5405; CBS 112618ET | Leaf litter of Leucospermum oleaefolium | South Africa: Western Cape Province | S. Lee | AY720732 | AY720764 | AY720795 | AY720828 |
| CPC 5406; CBS 112617 | Leaf litter of Leucospermum sp. | South Africa: Western Cape Province | S. Lee | AY720733 | AY720765 | AY720796 | AY720829 | |
| CPC 5407; CBS 112616 | Dead twig of Leucadendron sp. | South Africa: Western Cape Province | S. Lee | AY720734 | AY720766 | AY720797 | AY720830 | |
| CPC 5469; CBS 111830 | Dead twigs of Leucospermum sp. | South Africa: Western Cape Province | S. Lee | AY720735 | AY720767 | AY720798 | AY720831 | |
| CPC 5470; CBS 111831 | Dead twigs of Leucadendron conocarpodendron | South Africa: Western Cape Province | S. Lee | AY720736 | AY720768 | AY720799 | AY720832 | |
| Harknessia pseudohawaiiensis | CPC 13001 | Leaves of Eucalyptus tereticornis | Australia: New South Wales | A. Carnegie | JQ706109 | JQ706153 | JQ706194 | JQ706232 |
| CPC 17300 | Leaves of Eucalyptus sp. | Australia: Queensland | P.W. Crous | JQ706110 | JQ706154 | JQ706195 | JQ706233 | |
| CPC 17379ET; CBS 132124 | Leaves of Eucalyptus dunnii | Australia: New South Wales | A. Carnegie | JQ706111 | JQ706155 | JQ706196 | JQ706234 | |
| Harknessia ravenstreetina | CPC 17095ET; CBS 132125 | Leaf litter of Eucalyptus sp. | Australia: Queensland | P.W. Crous & R.G. Shivas | JQ706112 | JQ706156 | JQ706197 | JQ706235 |
| CPC 17209; CBS 132126 | Twigs of thin-leaved Acacia sp. | Australia: Queensland | P.W. Crous & R.G. Shivas | JQ706113 | JQ706157 | JQ706198 | JQ706236 | |
| Harknessia renispora | CBS 153.71EI | Dead leaf of Melaleuca pubescens | Australia: Victoria | H.J. Swart | AY720737 | AY720769 | AY720800 | AY720833 |
| CPC 17163 | Callistemon pinifolius | Australia: Queensland | P.W. Crous | JQ706114 | JQ706158 | JQ706199 | JQ706237 | |
| Harknessia rhabdosphaera | CPC 12455; CBS 122372 | Eucalyptus nitida | Australia: Tasmania | M. Glen | JQ706115 | JQ706159 | – | JQ706238 |
| CPC 12922; CBS 120082ET | Leaves of Corymbia henryi | Australia: New South Wales | B.A. Summerell | DQ923532 | – | – | DQ923532 | |
| CPC 13593 | Eucalyptus michaeliana | Australia | B.A. Summerell | JQ706116 | JQ706160 | JQ706200 | JQ706239 | |
| CPC 13594 | Eucalyptus michaeliana | Australia | B.A. Summerell | JQ706117 | – | – | – | |
| CPC 12847; CBS 122373 | Eucalyptus baxteri | Australia: South Australia | B.A. Summerell | JQ706118 | JQ706161 | JQ706201 | JQ706240 | |
| Harknessia sp. | CPC 11153 | Leaf litter of Eucalyptus sp. | India | W. Gams | JQ706119 | JQ706162 | JQ706202 | – |
| Harknessia spermatoidea | CPC 13937ET; CBS 132127 | Leaf litter of Eucalyptus sp. | Cyprus | A. van Iperen | JQ706120 | JQ706163 | JQ706203 | JQ706241 |
| Harknessia syzygii | CPC 184; CBS 111124ET | Syzygium cordatum | South Africa: Limpopo | M.J. Wingfield | AY720738 | AY720770 | AY720801 | AY720834 |
| Harknessia viterboensis | CPC 10843; CBS 115647ET | Leaves of Eucalyptus sp. | Italy | W. Gams | AY720740 | AY720772 | AY720803 | JQ706242 |
| Harknessia weresubiae | CPC 12718; CBS 132129 | Eucalyptus sp. | South Africa: Western Cape Province | P.W. Crous | JQ706121 | JQ706164 | JQ706204 | JQ706243 |
| CPC 17670EE; CBS 132128 | Eucalyptus leaf litter | Australia: Victoria | P.W. Crous, J. Edwards, | |||||
| I.J. Porter & I.G. Pascoe | JQ706122 | JQ706165 | JQ706205 | JQ706244 | ||||
| CPC 5106; CBS 113075 | Leaf litter of Eucalyptus sp. | South Africa: Western Cape Province | P.W. Crous & J. Stone | AY720741 | AY720773 | AY720804 | AY720835 | |
| CPC 5107; CBS 113074 | Leaf litter of Eucalyptus sp. | South Africa: Western Cape Province | P.W. Crous & J. Stone | AY720742 | AY720774 | AY720805 | AY720836 | |
| CPC 5108; CBS 113073 | Leaf litter of Eucalyptus sp. | South Africa: Western Cape Province | P.W. Crous & J. Stone | AY720743 | AY720775 | AY720806 | AY720837 | |
| CPC 5109 | Leaf litter of Eucalyptus sp. | South Africa: Western Cape Province | P.W. Crous & J. Stone | AY720744 | AY720776 | AY720807 | AY720838 | |
| Wuestneia molokaiensis | CPC 11127 | Eucalyptus globulus | Spain | M.J. Wingfield | JQ706123 | JQ706166 | JQ706206 | – |
| CPC 12373 | Eucalyptus globulus | Australia: Victoria | I. Smith | JQ706124 | JQ706167 | – | JQ706245 | |
| CPC 12995 | Eucalyptus mannifera | Australia | B.A. Summerell | JQ706125 | JQ706168 | JQ706207 | JQ706246 | |
| CPC 13859 | Eucalyptus sp. | South Africa | P.W. Crous | JQ706126 | JQ706169 | JQ706208 | JQ706247 | |
| CPC 19269 | Eucalyptus cypellocarpa | Australia: Northern Territory | P.W. Crous | JQ706127 | – | – | JQ706248 | |
| CPC 3797; CBS 114877ET | Eucalyptus robusta | USA: Hawaii | J.D. Rogers | AY720749 | AY579335 | AY720812 | AY720842 | |
1 CBS: CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands; CPC: Culture collection of Pedro Crous, housed at CBS.
2 ET: ex-type strain; EE: ex-epitype strain; EI: ex-isotype strain.
3 LSU: partial 28S nrRNA gene; ITS: internal transcribed spacer regions 1 & 2 including 5.8S nrRNA gene; TUB: partial beta-tubulin gene; CAL: partial calmodulin gene.
DNA phylogeny
Genomic DNA was extracted from fungal colonies growing on MEA using the UltraCleanTM Microbial DNA Isolation Kit (MoBio Laboratories, Inc., Solana Beach, CA, USA) according to the manufacturer’s protocol. The primers V9G (de Hoog & Gerrits van den Ende 1998) and LR5 (Vilgalys & Hester 1990) were used to amplify part (ITS) of the nuclear rDNA operon spanning the 3′ end of the 18S rRNA gene, the first internal transcribed spacer (ITS1), the 5.8S rRNA gene, the second ITS region and the 5’ end of the 28S rRNA gene. The primers ITS4 (White et al. 1990) and LSU1Fd (Crous et al. 2009a) were used as internal sequence primers to ensure good quality sequences over the entire length of the amplicon.
For species delimitation, ITS was supplemented with the partial gene sequences for calmodulin (CAL), determined using the primers CAL-228F (Carbone & Kohn 1999) and CAL-737R (Carbone & Kohn 1999) or CAL2Rd (Quaedvlieg et al. 2011) and beta-tubulin (TUB), amplified and sequenced using the primers T1 (O’Donnell & Cigelnik 1997) and Bt-2b (Glass & Donaldson 1995). Amplification conditions followed Lee et al. (2004). The sequence alignment and subsequent phylogenetic analyses for all the above were carried out using methods described by Crous et al. (2006). Gaps longer than 10 bases were coded as single events for the phylogenetic analyses (see TreeBASE); the remaining gaps were treated as ‘fifth state’ data. Sequence data were deposited in GenBank (Table 1) and the alignments and trees in TreeBASE (http://www.treebase.org).
Taxonomy
Culture characteristics were determined in triplicate from MEA plates after 1 mo of incubation at 25 °C in the dark, and colours determined according to Rayner (1970). Measurements and photographs were made from structures mounted in clear lactic acid. The 95 % confidence intervals were derived from 30 observations (×1 000 magnification), with the extremes given in parentheses. Ranges of the dimensions of other characters are given. Observations were made with a Zeiss V20 Discovery stereo microscope, and with a Zeiss Axio Imager 2 light microscope using differential interference contrast (DIC) illumination and an AxioCam MRc5 camera and software.
RESULTS
DNA phylogeny
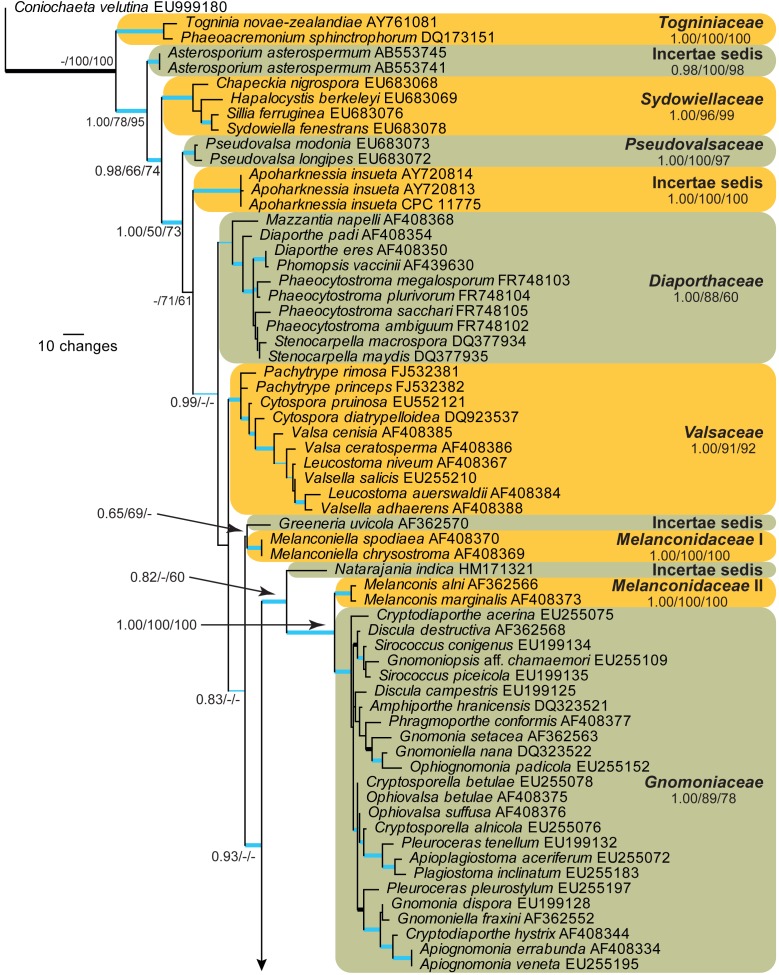
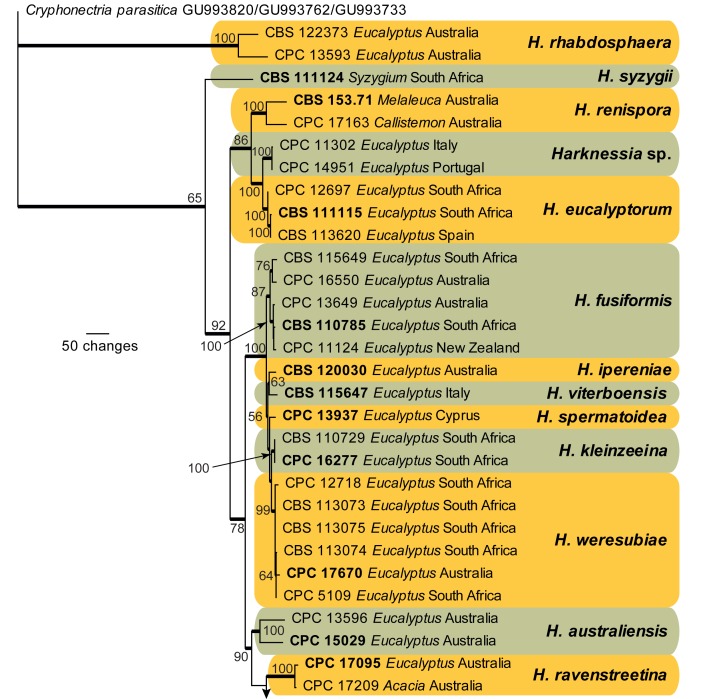
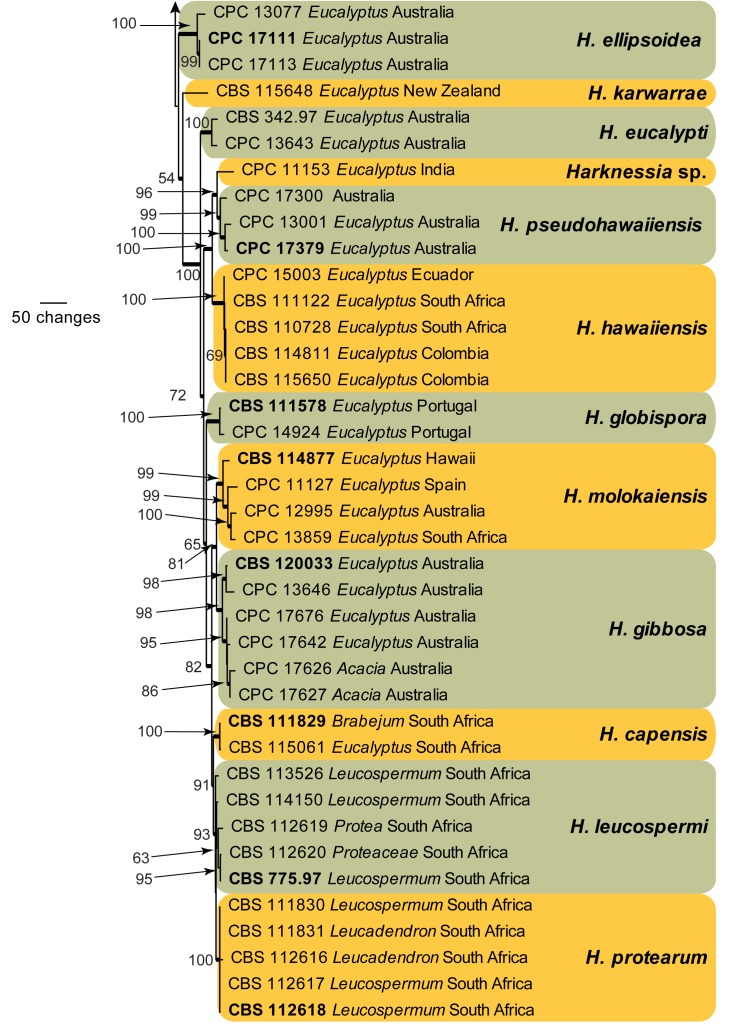
The LSU sequences were used to obtain additional sequences from NCBI’s GenBank nucleotide database, which were added to the alignment (Fig. 4) and the combined ITS, CAL, and TUB alignment to determine species identification (Fig. 5).
Fig. 4.
The first of 1 000 equally most parsimonious trees obtained from a heuristic search with 100 random taxon additions of the LSU sequence alignment. The scale bar shows 10 changes, and posterior probability (PP), distance (NJBS), and maximum parsimony (MPBS) bootstrap support values from 1 000 replicates are shown (PP/NJBS/MPBS) for simplicity only for the families and backbone of the phylogenetic tree. Families are indicated to the right of the tree. Branches present in the parsimony strict consensus tree are thickened and those present in both the parsimony consensus and Bayesian tree are drawn in blue. The tree was rooted to a sequence of Coniochaeta velutina (GenBank accession EU999180).
Fig. 5.
The first of 1 000 equally most parsimonious trees obtained from a heuristic search with 100 random taxon additions of the combined ITS, CAL, and TUB sequence alignment. The scale bar shows 50 changes, and bootstrap support values from 1 000 replicates are shown at the nodes. Ex-type strains are printed in bold. Branches present in the strict consensus tree are thickened and the tree was rooted to sequences of Cryphonectria parasitica (GenBank ITS: GU993820, CAL: GU993762, TUB: GU993733).
28S nrDNA generic overview
Amplicons of approximately 1 600 bases were obtained for ITS (including the first approx. 900 bp of LSU) of the isolates listed in Table 1. The manually adjusted LSU alignment contained 106 sequences (including the outgroup sequence) and 763 characters including alignment gaps (available in TreeBASE) were used in the phylogenetic analysis; 164 of these were parsimony-informative, 44 were variable and parsimony-uninformative, and 555 were constant. Neighbour-joining analyses using three substitution models on the sequence alignment yielded tree topologies delimiting similar terminal clades to those of the parsimony analysis (Fig. 4). Only the first 1 000 equally most parsimonious trees were saved (TL = 692 steps; CI = 0.400; RI = 0.842; RC = 0.337).
Bayesian analysis was conducted on the same aligned LSU dataset using a general time-reversible (GTR) substitution model with inverse gamma rates and dirichlet base frequencies. The Markov Chain Monte Carlo (MCMC) analysis of two sets of 4 chains started from a random tree topology and lasted 6 450 000 generations, after which the split frequency reached less than 0.01. Trees were saved each 1 000 generations, resulting in 12 902 saved trees. Burn-in was set at 25 %, leaving 9 678 trees from which the consensus tree (Fig. 5) and posterior probabilities (PP’s) were calculated.
A comparison between the tree topologies obtained through the Bayesian, parsimony, and distance analyses yielded mostly the same terminal clades, corresponding to the families as they are delimited in Fig. 4. Some rearrangements are present in the backbone of the tree, for example Apoharknessia is intermediate between Pseudovalsaceae and Diaporthaceae (parsimony, Fig. 4), an unresolved sister clade of Natarajania indica basal to Melanconidaceae I (distance) or a sister clade to Pseudovalsaceae (MrBayes). Similarly, the Diaporthaceae and Valsaceae are not sister clades (parsimony, Fig. 4), are sister clades with a common node (distance) or are sister clades from a polytomy (MrBayes). The position of Natarajania indica also changes with the algorithm used; in parsimony it is a basal sister to Melanconidaceae II and Gnomoniaceae (Fig. 4), a basal polytomy sister of Apoharknessia (distance) or sister to Gnomoniaceae (MrBayes). Schizoparmaceae is either a direct sister of Harknessia (parsimony, Fig. 4), separated from Harknessia by Cryphonectriaceae (distance) or nestled as a clear lineage in a polytomy of Harknessia species (MrBayes). Cryphonectriaceae is either a sister clade to Schizoparmaceae and Harknessia (parsimony, Fig. 4), an intermediate clade between Schizoparmaceae and Harknessia (distance) or a clade in an unresolved polytomy together with Natarajania indica, Melanconidaceae II, Gnomoniaceae, Schizoparmaceae, and Harknessia (MrBayes). From the analyses, it is evident that Cryphonectriaceae, Schizoparmaceae and Harknessia are highly similar based on their LSU sequences and that the delimitation of the three clades are sensitive to the algorithm used for the phylogenetic analysis. In all three analyses, Cryphonectriaceae is a distinct, well-supported lineage, whereas Schizoparmaceae and Harknessia form separate clades in the parsimony and distance analyses, albeit without support or poorly supported. In the distance analysis, the bootstrap support values are 62 % for Harknessia, 85 % for Cryphonectriaceae, and 98 % for Schizoparmaceae, 54 % for the association of Harknessia and Cryphonectriaceae, and 57 % for the branch linking all three clades. The parsimony bootstrap analysis yielded little support for the overall backbone of the tree, although the main families are supported (Fig. 4). However, even more so than in the Bayesian analysis, the Harknessia clade collapses to a polytomy with the other families, and Cryphonectriaceae and Schizoparmaceae receive some to good support (58 % and 98 %, respectively).
Species delimitation with combined ITS, CAL, and TUB loci
Amplicons of approximately 700, 700, and 900 bases were obtained for ITS, CAL, and TUB, respectively, of the isolates listed in Table 1. The manually adjusted combined alignment contained 70 sequences (including the outgroup sequence) and 1 829 characters (614, 505, and 710 characters, respectively) including alignment gaps (available in TreeBASE) which were used in the phylogenetic analysis; 463 of these were parsimony-informative, 393 were variable and parsimony-uninformative, and 973 were constant. Neighbour-joining analyses using three substitution models on the sequence alignment yielded trees with similar topologies to those of the parsimony analysis (Fig. 5). Only the first 1 000 equally most parsimonious trees were saved (TL = 1 813 steps; CI = 0.673; RI = 0.850; RC = 0.572). While many species clades are well-defined, the intraspecific variation for some species such as H. australiensis, H. fusiformis, H. renispora, and H. rhapdosphaera appear to be larger than the interspecific variation in the genus (Fig. 5) and these species probably represent species complexes which require the collection of more strains and further study. Other results are discussed under the species notes below, where applicable.
Taxonomy
Harknessiaceae Crous, fam. nov. — MycoBank MB564740
Typus. Harknessia Cooke, Grevillea 9: 85. 1881.
Mycelium internal, branched, septate, hyaline to pale brown. Conidiomata eustromatic to pycnidial, immersed, globose, unilocular to convoluted and multilocular, brown; walls composed of thin-walled, pale brown to brown textura angularis. Ostiolar opening central, circular, wide, surrounded by brown furfuraceous cells. Conidiophores lining the inner cavity, or limited to a basal layer in some species; usually reduced to conidiogenous cells, rarely septate and branched; commonly invested in mucus. Conidiogenous cells discrete, ampulliform, lageniform, subcylindrical to cylindrical, hyaline, smooth, giving rise to macroconidia, and in some cases also microconidia in the same conidioma, proliferating one to several times percurrently; secession rhexolytic. Macroconidia consisting of a conidium body and a basal appendage, delimited by a septum; conidium body unicellular, of various shapes, thick-walled, smooth, brown, with or without light and dark coloured longitudinal bands, occasionally longitudinally striate, guttulate; basal appendage cellular, cylindrical to subcylindrical, hyaline, flexuous, thin-walled and devoid of contents; apical appendage mostly lacking, when present elongated, attenuated; in some species the conidium body and basal appendage are invested in a thin layer of mucus. Microconidia oval to ellipsoid, aseptate, hyaline, smooth. Ascomata perithecial, single or aggregated, immersed, disc furfuraceous brown, neck emergent to depressed; wall of 3–5 layers of brown textura angularis. Asci unitunicate, cylindrical to clavate, hyaline, smooth, 8-spored, with apical apparatus. Paraphyses hyaline, septate, interspersed among asci. Ascospores aseptate, uni- to biseriate, ellipsoid to fusoid, hyaline, thick-walled, guttulate, smooth.
Notes — The Cryptosporellaceae, erected for Cryptosporella, is based on C. hypodermia, a species having a Disculina anamorph (Reid & Booth 1989), thereby making Cryptosporella (= Winterella) unavailable for Harknessia teleomorphs. The genus Wuestneia, based on W. aurea (= Wuestneia xanthostroma), seems an unlikely home for the Wuestneia/Harknessia complex, as Reid & Booth (1989) found it was associated with a coelomycete anamorph having hyaline conidia. Given the confusion that exists over the genus most suitable for Harknessia teleomorphs, the best option is to use a single generic name Harknessia (Hawksworth et al. 2011, Wingfield et al. 2012), based on H. eucalypti, and introduce Harknessiaceae (Diaporthales) as a family for these taxa.
Harknessia australiensis Crous & Summerell, sp. nov. — MycoBank MB564741; Fig. 6
Fig. 6.
Harknessia australiensis (CPC 15029). a. Sporulating colony on OA; b–f. conidiogenous cells giving rise to conidia (arrows in b denote conidiogenous cells); g–k. conidia with short basal appendages and restricted zones of longitudinal striations. — Scale bars = 10 μm.
Etymology. Named after the country where it was collected, Australia.
Foliicolous, isolated from leaves incubated in moist chambers (presumed endophyte). Conidiomata pycnidioid, stromatic, amphigenous, scattered, subepidermal, becoming erumpent, globose, up to 300 μm diam; with irregular opening and border of yellowish, furfuraceous cells; wall of textura angularis. Conidiophores reduced to conidiogenous cells lining the inner conidiomatal cavity. Conidiogenous cells 5–10 × 4–6 μm, ampulliform to lageniform, hyaline, smooth, invested in mucilage, proliferating once or twice percurrently near apex. Conidia (16−)18–20(−22) × (9−)10–11(−12) μm (av. 19 × 11 μm) in vitro, ellipsoid to broadly ventricose, aseptate, golden brown to olivaceous brown, with acutely rounded apex, non-apiculate, thick-walled, smooth, with longitudinal striations along the whole length of the body, granular to multi-guttulate. Basal appendage (1.5−)2–3(−4) × 2.5–3 μm in vitro, hyaline, tubular, smooth, thin-walled, devoid of cytoplasm. Microconidia not seen.
Culture characteristics — Colonies spreading, fluffy, with abundant aerial mycelium; surface dirty white to cream; cream in reverse; covering the dish in 1 mo.
Specimens examined. Australia, New South Wales, Gibraltar Range National Park, S29°32′22″ E152°17′43″, 980 m, on leaves of Eucalyptus dissita, 19 Mar. 2008, B.A. Summerell (CBS H-20911 holotype, cultures ex-type CPC 15029 = CBS 132119); New South Wales, Woodford, S33°43′30″ E150°29′25″, on leaves of Eucalyptus sclerophylla (NSW616452), 26 June 2007, B.A. Summerell, CPC 13596–13598 = CBS 132120.
Notes — Morphologically there is little to separate between H. ravenstreetina (which appears to occur on a wide host range) and H. australiensis (occurs on different Eucalyptus spp.). The main distinguishing features are its conidial shape, with conidia of H. ravenstreetina being broadly ventricose, and absence of striations, while those of H. australiensis are ellipsoid to broadly ventricose, and have prominent striations. These two species were also phylogenetically distinct (Fig. 2).
Harknessia ellipsoidea Crous, R.G. Shivas & Summerell, sp. nov. — MycoBank MB564742; Fig. 7
Fig. 7.
Harknessia ellipsoidea (CPC 17111). a. Sporulating colony on OA; b–d. conidiogenous cells giving rise to conidia; e–h. conidia with short basal appendages. — Scale bars = 10 μm.
Etymology. Named after its conidial shape, which is broadly ellipsoid.
Foliicolous, isolated from leaves incubated in moist chambers (presumed endophyte). Conidiomata pycnidioid, stromatic, amphigenous, scattered, subepidermal, erumpent, globose, up to 400 μm diam; glabrous with wide ruptured opening and border of yellowish, furfuraceous cells; wall of textura angularis. Conidiophores reduced to conidiogenous cells lining the inner conidiomatal cavity. Conidiogenous cells 5–10 × 4–6 μm, ampulliform to lageniform, hyaline, smooth, invested in mucilage, proliferating several times percurrently near apex. Conidia (9−)11–12(−13) × 7(−8) μm (av. 11.5 × 7 μm) in vitro, broadly ellipsoid to subglobose, aseptate, brown to dark brown, non-apiculate, thick-walled, smooth, granular to multi-guttulate or with large central guttule, non-striate. Basal appendage 1–2(−4) × 2 μm in vitro, hyaline, tubular, smooth, thin-walled, devoid of cytoplasm. Microconidia not seen.
Culture characteristics — Colonies spreading, fluffy, with moderate to abundant aerial mycelium; surface dirty white to cream to pale luteous; covering the dish in 1 mo.
Specimens examined. Australia, Queensland, Brisbane, Bardon Trail, on leaves of Eucalyptus sp., 12 July 2009, P.W. Crous & R.G. Shivas (CBS H-20912 holotype, cultures ex-type CPC 17111 = CBS 132121, CPC 17112, 17113); New South Wales, Kew, S31°42′38″ E152°42′20″, on leaves of Eucalyptus propinqua, 26 Apr. 2006, B.A. Summerell, CPC 13077–13079 = CBS 132122.
Notes — This species is phylogenetically distinct from any of the other Harknessia species known from sequence data (Fig. 2). Conidia are similar in size to those of H. pseudohawaiiensis but differ by being broadly ellipsoidal in shape.
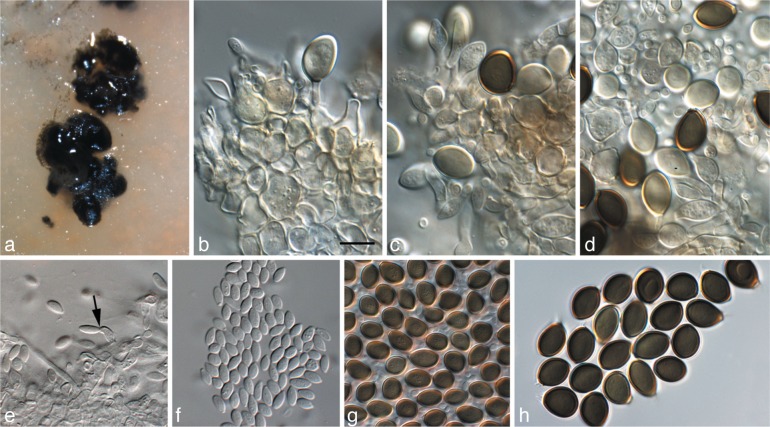
Harknessia kleinzeeina Crous, sp. nov. — MycoBank MB564743; Fig. 8
Fig. 8.
Harknessia kleinzeeina (CPC 16277). a. Insect damage on leaves, creating lesions from which H. kleinzeeina was isolated; b. sporulating colony on OA; c–g. conidiogenous cells giving rise to conidia; h–o. conidia with long basal appendages (arrow in k denotes apiculus, and in h and m longitudinal striations). — Scale bars = 10 μm.
Etymology. Named after the locality where it was collected in South Africa, Kleinzee.
Foliicolous, associated with irregular leaf spots induced by insect damage, pale brown, but appearing to be secondary infections, probably saprobic. Description on PNA. Conidiomata pycnidioid, subepidermal, becoming erumpent, ovoid, black, up to 350 μm diam; dehiscence irregular with wide opening, border with pale yellow furfuraceous cells; wall of brown textura angularis. Conidiophores reduced to conidiogenous cells lining the base of conidiomatal cavity. Conidiogenous cells lageniform to subcylindrical, hyaline, smooth, proliferating 1–3 times percurrently near apex, 5–10 × 3–4 μm. Macroconidia (20−)22–24(−27) × (11−)12–13 μm (av. 23 × 12 μm) in vitro, composed of a body with basal appendage; body brown, smooth, ellipsoid to oblong-ellipsoid, rarely ventricose, apiculate, aseptate, with longitudinal band of lighter pigment, at times bordered by longitudinal striations covering the length of the conidium body, granular to guttulate, at times with central guttule. Basal appendage (30−)45–65(−80) × 2–3 μm in vitro, hyaline, tubular, smooth, thin-walled, flexuous, devoid of cytoplasm, at times walls collapsing, covered in mucilaginous layer when immature. Microconidia not seen.
Culture characteristics — Colonies fluffy, spreading with abundant aerial mycelium; surface dirty white to cream or pale luteous; covering the dish in 1 mo; sporulating with black conidiomata, oozing black spore masses.
Specimens examined. South Africa, Northern Cape Province, Kleinzee, on leaves of Eucalyptus sp., 27 Feb. 2009, Z.A. Pretorius (CBS H-20913 holotype, cultures ex-type CPC 16277 = CBS 132123); Western Cape Province, Stellenbosch Mountain, on Eucalyptus leaf litter, 8 Dec. 1988, P.W. Crous, PREM 50834, culture CBS 110729 = STE-U 108.
Notes — Harknessia kleinzeeina is similar to the type of H. uromycoides (basal appendages 57–130 × 2–2.5 μm; Nag Raj 1993), but has shorter basal appendages (30–80 × 2–3 μm). Although originally reported from South Africa as H. uromycoides (Crous et al. 1993), Lee et al. (2004) stated that South African strains might well represent a different species within the H. uromycoides complex. The collection of a second specimen, which is phylogenetically identical (Fig. 2), supports this hypothesis. Although phylogenetically close to H. ipereniae, H. spermatoidea and H. viterboensis, these species can be distinguished by their CAL and TUB sequences, and less so by their ITS sequences.
Harknessia pseudohawaiiensis Crous & Carnegie, sp. nov. — MycoBank MB564744; Fig. 9
Fig. 9.
Harknessia pseudohawaiiensis (CPC 17380). a. Sporulating colony on OA; b–d. conidiogenous cells giving rise to conidia; e. microconidiogenous cell giving rise to microconidium (arrow); f. microconidia; g, h. macroconidia. — Scale bars = 10 μm.
Etymology. Named after its morphological similarity to H. hawaiiensis.
Foliicolous, isolated from leaves incubated in moist chambers (presumed endophyte). Conidiomata pycnidioid, stromatic, amphigenous, scattered, subepidermal, becoming erumpent, globose, up to 400 μm diam; glabrous with wide opening and border of yellowish, furfuraceous cells; wall of textura angularis. Conidiophores reduced to conidiogenous cells lining the inner conidiomatal cavity. Macroconidiogenous cells 5–9 × 4–6 μm, ampulliform to lageniform, hyaline, smooth, invested in mucilage, proliferating several times percurrently near apex. Macroconidia (9−)10–12(−13) × (8−)9(−10) μm (av. 12 × 9 μm) in vitro, subglobose to broadly ellipsoid, aseptate, golden brown to brown, non-apiculate, thick-walled, smooth, granular, with or without longitudinal striations along the length of the body. Basal appendage 1–2(−5) × 2 μm in vitro, hyaline, tubular, smooth, thin-walled, devoid of cytoplasm. Microconidiogenous cells 4–8 × 4–6 μm, ampulliform to lageniform, hyaline, smooth, with visible apical periclinal thickening. Microconidia 4–7 × 2.5–3 μm, hyaline, smooth, fusoid with obtuse apex and tapering to a truncate base.
Culture characteristics — Colonies spreading, fluffy, with moderate to abundant aerial mycelium; surface dirty white to cream to pale luteous; covering the dish in 1 mo.
Specimens examined. Australia, New South Wales, Dundurabbin, Neaves plantation, S30°10′15″ E152°30′33″, on leaves of Eucalyptus dunnii, 22 Sept. 2009, A.J. Carnegie (CBS H-20914 holotype, cultures ex-type CPC 17380, 17379 = CBS 132124); Queensland, Cairns Road to Atherton Gillies Highway, on leaves of Eucalyptus sp., 16 Aug. 2009, P.W. Crous, CPC 17300–17301; New South Wales, Bonalbo, Morpeth Park plantation, S28°46′3″ E152°36′47″, on leaves of E. tereticornis, 30 Mar. 2006, A.J. Carnegie, CPC 13001–13003.
Notes — Harknessia pseudohawaiiensis is similar to H. hawaiiensis in macroconidial shape, the presence of longitudinal striations, and the abundance of microconidia. It differs in having smaller macroconidia than H. hawaiiensis (macroconidia 11–15 × 6.5–8 μm, appendages 2–3 × 2.5 μm), and shorter appendages. These two species are also phylogenetically distinct (Fig. 2). An isolate obtained from Eucalyptus in India (on leaf litter of Eucalyptus sp., 3 Jan. 2004, W. Gams, CPC 11153–11154) appears to represent a closely allied species.
Harknessia ravenstreetina Crous & R.G. Shivas, sp. nov. — MycoBank MB564745; Fig. 10
Fig. 10.
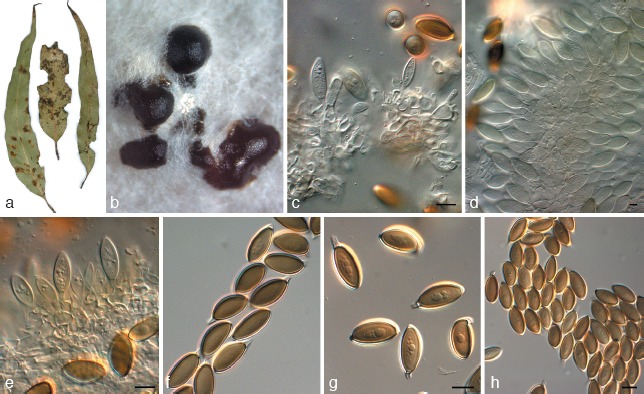
Harknessia ravenstreetina (CPC 17095). a. Leaf spot symptoms on Eucalyptus; b. sporulating colony on OA; c–e. conidiogenous cells giving rise to conidia; f–h. conidia. — Scale bars = 10 μm.
Etymology. Named after the location where it was collected, Raven Street Reserve, Brisbane, Australia.
Caulicolous and foliicolous, isolated from leaves and twigs incubated in moist chambers (presumed endophyte). Conidiomata pycnidioid, separate to gregarious, subepidermal, becoming erumpent, stromatic, amphigenous, depressed globose, up to 250 μm diam; with irregular opening and border of yellowish, furfuraceous cells; wall of textura angularis. Conidiophores reduced to conidiogenous cells lining the inner conidiomatal cavity. Conidiogenous cells 6–10 × 4–6 μm, ampulliform to subcylindrical, hyaline, smooth, invested in mucilage, percurrently proliferating once or twice near apex. Conidia (14−)16–18(−20) × (7−)8(−9) μm (av. 17 × 9 μm) in vitro, broadly ventricose, apex subobtusely rounded, aseptate, non-apiculate, pale yellow-brown, thick-walled, smooth, lacking striations, multi-guttulate. Basal appendage (1.5−)2–3(−5) × 2–2.5 μm in vitro, hyaline, tubular, smooth, thin-walled, devoid of cytoplasm. Microconidia not seen.
Culture characteristics — Colonies spreading, fluffy, with moderate to abundant aerial mycelium; surface dirty white to cream; cream in reverse; covering the dish in 1 mo.
Specimens examined. Australia, Queensland, Brisbane, Raven Street Reserve, S27°23′22.8″ E153°00′16.9″ on leaf litter of Eucalyptus sp., 12 July 2009, P.W. Crous & R.G. Shivas (CBS H-20915 holotype, cultures ex-type CPC 17095 = CBS 132125); Raven Street Reserve, S27°23′22.8″ E153°00′16.9″ on twigs of thin-leaved Acacia sp., 12 July 2009, P.W. Crous & R.G. Shivas, cultures CPC 17209 = CBS 132126.
Notes — Harknessia ravenstreetina is similar to H. antarctica in conidium shape (conidia 20–24 × 10–12 μm, basal appendages 11–28 × 2–3 μm; Nag Raj 1993), although it has smaller conidia, and shorter basal appendages. Unfortunately, a culture of H. antarctica was not available for inclusion in the phylogenetic study. Harknessia ravenstreetina is phylogenetically distinct from other Harknessia species known from sequence data (Fig. 2).
Harknessia spermatoidea R. Galán, G. Moreno & B. Sutton, Trans. Brit. Mycol. Soc. 87: 636. 1986. — Fig. 11
Fig. 11.
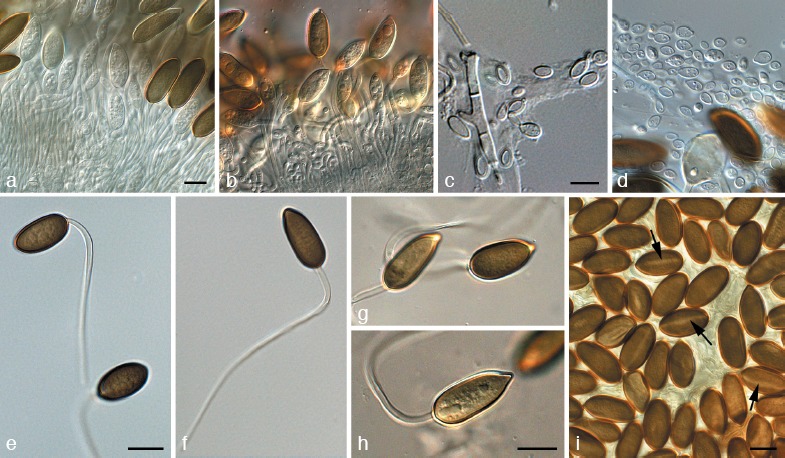
Harknessia spermatoidea (CPC 13937). a, b. Conidiogenous cells giving rise to conidia; c, d. microconidia; e–i. macroconidia with long basal appendages. — Scale bars = 10 μm.
Specimens examined. Cyprus, on leaf litter of Eucalyptus sp., salt lake, near airport and Sultan Moskee, 28 Mar. 2007, A. van Iperen, CBS H-20924 epitype designated here, culture ex-epitype CPC 13937 = CBS 132127. – Spain, Pontaverda, La Toja, on leaf litter of Eucalyptus globulus, 4 Oct. 1985, N. Manzano, GM-RG 9320 (holotype), IMI 295508 (isotype).
Notes — Harknessia spermatoidea was originally described from Spain, but the specimen collected on Eucalyptus from Cyprus closely matches the morphology observed in the holotype, enabling us to designate an epitype for this taxon. Although phylogenetically closely related to H. ipereniae, H. kleinzeeina, and H. viterboensis, these species can be distinguished by their CAL and TUB sequences, and less easily by their ITS sequences.
Harknessia viterboensis Crous, sp. nov. — MycoBank MB564746; Fig. 12
Fig. 12.
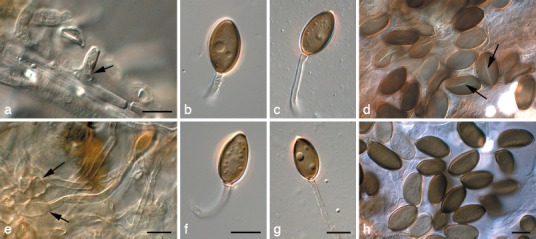
Harknessia viterboensis (CBS 115647). a, e. Conidiogenous cells (arrows); b–d, f–h. conidia with appendages (arrows in d denote apparent germ slit). — Scale bars = 10 μm.
Etymology. Named after the location where it was collected in Italy, Viterbo.
Foliicolous, amphigenous, developing on brown leaf spots after incubation in moist chambers (presumed endophyte). Description on OA, as cultures remained sterile on PNA. Conidiomata pycnidioid, erumpent, globose, black, solitary, up to 250 μm diam; dehiscence irregular with wide opening, but generally not exuding excessive amounts of conidia; wall of brown textura angularis. Conidiophores reduced to conidiogenous cells lining the base of conidiomatal cavity, but also forming separately on superficial mycelium. Conidiogenous cells ampulliform to lageniform, hyaline, smooth, covered in a mucilaginous layer, holoblastic, rarely proliferating percurrently near apex, 12–20 × 4–6 μm, becoming pale brown with age. Macroconidia (17−)20–23(−25) × (9−)10–13(−15) μm (av. 23 × 12 μm) in vitro, composed of a body with basal appendage; body brown to dark brown, smooth, broadly ellipsoid, aseptate, apiculate or apex acutely rounded, aseptate, with longitudinal band of lighter pigment, which can appear like a germ slit in older conidia, at times bordered by longitudinal striations covering the length of the conidium body, multi-guttulate or at times with central guttule. Basal appendage (25−)35–60 × (2−)3(−4) μm in vitro, hyaline, tubular, smooth, thin-walled, flexuous, devoid of cytoplasm, at times walls collapsing, covered in mucilaginous layer when immature; characteristically wide, at times becoming pale brown with age. Microconidia not seen.
Culture characteristics — Colonies spreading, somewhat fluffy, with moderate aerial mycelium; surface dirty white to cream; cream in reverse; covering the dish in 1 mo; sporulating poorly, with small, globose olivaceous black conidiomata forming on OA.
Specimen examined. Italy, Viterbo, Vulci, on leaves of Eucalyptus sp., Dec. 2003, W. Gams (CBS H-9904 holotype, cultures ex-type CPC 10843 = CBS 115647).
Notes — Lee et al. (2004) reported the Italian collection to represent a different species within the H. uromycoides complex, but did not formally describe it. It is primarily distinguished from H. uromycoides by its shorter and wider appendages, and its prominent longitudinal band of lighter pigment, almost resembling a germ slit. Although phylogenetically related to H. spermatiodea, H. ipereniae and H. kleinzeeina, these species can be distinguished by their CAL and TUB sequences, and less easily by their ITS sequences.
Harknessia weresubiae Nag Raj, DiCosmo & W.B. Kendr., Biblioth. Mycol. 80: 53. 1981.
Specimens examined. Australia, Saddleworth, on Eucalyptus leaf litter, 22 Sept. 1979, B. Kendrick, DAOM 173902 (holotype); Victoria, Melbourne, on Eucalyptus leaf litter, 21 Oct. 2009, P.W. Crous, J. Edwards, I.J. Porter & I.G. Pascoe (CBS H-20925 epitype designated here, cultures ex-epitype CPC 17670 = CBS 132128). – South Africa, Western Cape Province, Tulbach, on leaf litter of Eucalyptus sp., 13 Mar. 2002, P.W. Crous & J. Stone, CBS H-9903, cultures CBS 113075 = CPC 5106, CBS 113074 = CPC 5107, CBS 113073 = CPC 5108; Western Cape Province, Malmesbury, on leaf litter of Eucalyptus sp., 9 Feb. 2006, P.W. Crous, CBS 132129 = CPC 12718–12720.
Notes — Harknessia weresubiae occurs on eucalypts in Australia and South Africa (Lee et al. 2004). The species was originally described from Australia, and the fresh Australian collection obtained in the present study enabled us to designate an epitype, and fix the application of the name.
DISCUSSION
The Diaporthales is a distinct order within Sordariomycetes, a class including perithecial ascomycetous fungi (Zhang & Blackwell 2001, Castlebury et al. 2003). In a recent overview of the order, Rossman et al. (2007) recognised nine families, namely Sydowiellaceae (Sydowiella and aggregates), Schizoparmeaceae (Schizoparme/Pilidiella and Coniella; van Niekerk et al. 2004), Gnomoniaceae (more than 10 sexual genera; Mejía et al. 2011), Cryphonectriaceae (Cryphonectria generic complex; Gryzenhout et al. 2004, 2006), Valsaceae (Valsa and aggregates; Castlebury et al. 2002, Adams et al. 2005), Diaporthaceae (Diaporthe/Phomopsis and aggregates; Mostert et al. 2001, Castlebury et al. 2002, van Rensburg et al. 2006), Melanconidaceae (Melanconis/Melanconium), Pseudovalsaceae (Pseudovalsa; Castlebury et al. 2002), and Togniniaceae (Togninia/Phaeoacremonium and Jobellisia; Réblová et al. 2004, Mostert et al. 2003, 2006).
Phylogenetic analysis of the LSU sequence data generated in this study resolved a new family in the Diaporthales, introduced here as the Harknessiaceae (Fig. 4). Morphologically the Harknessiaceae is distinct within the order by having Wuestneia-like teleomorphs, and pycnidial conidiomata with brown, furfuraceous margins, brown conidia with hyaline, tube-like basal appendages, longitudinal striations, and rhexolytic secession. Furthermore, in addition to previous studies, a multi-gene analysis (ITS, CAL, and TUB), supplemented by morphological criteria, provided additional support to distinguish a further six novel species of Harknessia on Eucalyptus (Fig. 5), occurring in diverse countries such as Australia, Italy, and South Africa. Although some of these species were clearly associated with leaf spots and are suspected pathogens, many isolates were obtained from asymptomatic leaf tissue, and are presumed to be saprobic.
Although the genus Harknessia (type species H. eucalypti, teleomorph unknown) was recognised as a separate group in the Diaporthales (Castlebury et al. 2002), its family relationships remained unresolved. The main reason for this was that its teleomorph states were placed in Wuestneia (Crous et al. 1993, Crous & Rogers 2001). The latter genus is based on W. xanthostroma, which has affinities to Cryphonectriaceae (Rossman et al. 2007). By establishing the Harknessiaceae the correct placement of Wuestneia is essentially avoided, as the family is based on the anamorphic genus Harknessia, which has Wuestneia-like teleomorphs.
Nag Raj (1993) listed several synonyms of Harknessia, such as Caudosporella (based on H. antarctica), Mastigonetron (based on M. fuscum; having an apical conidial appendage and Wuestneia-like teleomorph), and Cymbothyrium (based on M. sudans; conidiomata with clypeus). Of these, the synonymy of Mastigonetron and Cymbothyrium are questionable, but fresh material needs to be collected to facilitate molecular studies to resolve this issue. Other genera that have since been split from Harknessia include Apoharknessia (with blunt apical appendage; Lee et al. 2004) and Dwiroopia (with longitudinal conidial germ slits; Farr & Rossman 2003).
More than 40 species of Harknessia have thus far been described, mainly from stems and leaves of angiosperms. Although they are highly variable in morphology and culture characteristics (Fig. 13, 14), they all have brown conidia with basal, cellular appendages. The present study adds an additional six species, and designates epitype specimens for a further two. In spite of extensive collections, the Harknessiaceae does not appear to be as species-rich as other families in Diaporthales. The addition of fresh collections, and molecular studies conducted on these cultures, will help resolve the uncertainties that remain in Harknessiaceae, especially with regards to the host range and distribution of taxa, and the proposed generic synonyms of Harknessia.
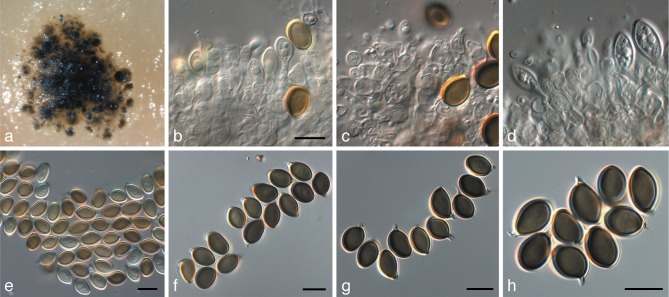
Fig. 13.
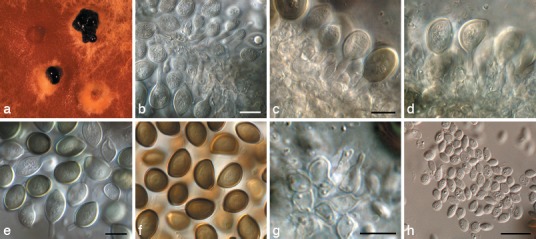
Harknessia molokaiensis (CPC 3797). a. Sporulating colony on MEA; b–d. conidiogenous cells giving rise to macroconidia; e, f. macroconidia; g. microconidiogenous cells giving rise to microconidia; h. microconidia. — Scale bars = 10 μm.
Fig. 14.
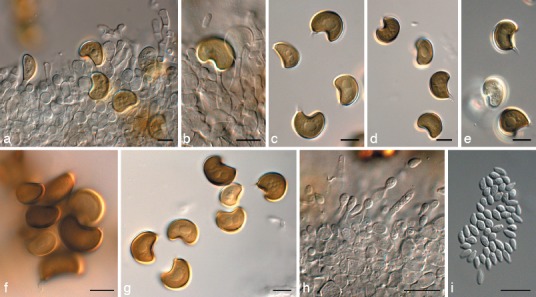
Harknessia renispora (CPC 17163). a, b. Conidiogenous cells giving rise to macroconidia; c–g. macroconidia (not striations in f, and central guttules in g); h. microconidiogenous cells giving rise to microconidia; i. microconidia. — Scale bars = 10 μm.
Acknowledgments
Prof. dr U. Braun (Martin-Luther-Univ., Halle, Germany) is thanked for commenting on the nomenclature of the species treated. We thank the technical staff, Arien van Iperen (cultures), Marjan Vermaas (photographic plates), and Mieke Starink-Willemse (DNA isolation, amplification, and sequencing) for their invaluable assistance.
REFERENCES
- Adams GC, Wingfield MJ, Common R, Roux J. 2005. Phylogenetic relationships and morphology of Cytospora species and related teleomorphs (Ascomycota, Diaporthales, Valsaceae) from Eucalyptus. Studies in Mycology 52: 1–142 [Google Scholar]
- Arx JA von, Müller E. 1954. Die Gattungen der amerosporen Pyrenomyceten. Beiträge zur Kryptogamenflora der Schweiz 11, 1: 1–434 [Google Scholar]
- Barr ME. 1978. Diaporthales in North America with emphasis on Gnomonia and its segregates. Mycologia Memoir 7: 1–232 [Google Scholar]
- Bettucci L, Saravay M. 1993. Endophytic fungi of Eucalyptus globulus: a preliminary study. Mycological Research 97: 679–682 [Google Scholar]
- Carbone I, Kohn LM. 1999. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556 [Google Scholar]
- Castlebury LA, Farr DF, Rossman AY, Jaklitsch WJ. 2003. Diaporthe angelicae comb. nov., a modern description and placement of Diaporthopsis in Diaporthe. Mycoscience 44: 203–208 [Google Scholar]
- Castlebury LA, Rossman AY, Jaklitsch WJ, Vasileva LN. 2002. A preliminary overview of the Diaporthales based on large subunit nuclear ribosomal DNA sequences. Mycologia 94: 1017–1031 [PubMed] [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G. 2004. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22 [Google Scholar]
- Crous PW, Knox-Davies PS, Wingfield MJ. 1989. Newly-recorded foliage fungi of Eucalyptus spp. in South Africa. Phytophylactica 21: 85–88 [Google Scholar]
- Crous PW, Mohammed C, Glen M, Verkley GJM, Groenewald JZ. 2007. Eucalyptus microfungi known from culture. 3. Eucasphaeria and Sympoventuria genera nova, and new species of Furcaspora, Harknessia, Heteroconium and Phacidiella. Fungal Diversity 25: 19–36 [Google Scholar]
- Crous PW, Rogers JD. 2001. Wuestneia molokaiensis and its anamorph Harknessia molokaiensis spp. nov. from Eucalyptus. Sydowia 53: 74–80 [Google Scholar]
- Crous PW, Schoch CL, Hyde KD, Wood AR, Gueidan C , et al. 2009a. Phylogenetic lineages in the Capnodiales. Studies in Mycology 64: 17–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO , et al. 2006. Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology 55: 235–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ, Samson RA. (eds). 2009b. Fungal Biodiversity. CBS Laboratory Manual Series 1: 1–269. CBS-KNAW Fungal Biodiversity Centre, Utrecht, Netherlands [Google Scholar]
- Crous PW, Wingfield MJ, Nag Raj TR. 1993. Harknessia species occurring in South Africa. Mycologia 85: 275–280 [Google Scholar]
- Crous PW, Wingfield MJ, Park RF. 1991. Mycosphaerella nubilosa a synonym of M. molleriana. Mycological Research 95: 628–632 [Google Scholar]
- Farr DF, Rossman AY. 2001. Harknessia lythri, a new species on purple loosestrife. Mycologia 93: 997–1001 [Google Scholar]
- Farr DF, Rossman AY. 2003. Dwiroopa, a coelomycetous genus with two species. Mycoscience 44: 443–446 [Google Scholar]
- Glass NL, Donaldson G. 1995. Development of primer sets designed for use with PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryzenhout M, Myburg H, Merwe NA van der, Wingfield BD, Wingfield MJ. 2004. Crysoporthe, a new genus to accommodate Cryphonectria cubensis. Studies in Mycology 50: 119–141 [Google Scholar]
- Gryzenhout M, Myburg H, Wingfield BD, Wingfield MJ. 2006. Cryphonectriaceae (Diaporthales) a new family including Cryphonectria, Chrysoporthe, Endothia, and allied genera. Mycologia 98: 239–249 [DOI] [PubMed] [Google Scholar]
- Hawksworth DL, Crous PW, Redhead SA, Reynolds DR, Samson RA, Seifert KA, Taylor JW, Wingfield MJ , et al. 2011. The Amsterdam Declaration on Fungal Nomenclature. IMA Fungus 2: 105–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höhnel F von. 1914. Fragmente zur Mycologie 864. Über die Gattung Harknessia Cooke. Sitzungsberichte der Akademie der Wissenschaften in Wien, Mathematisch – Naturwissenschaftliche Klasse. Abteilung 1, 123: 86–87 [Google Scholar]
- Hoog GS de, Gerrits van den Ende AHG. 1998. Molecular diagnostics of clinical strains of filamentous Basidiomycetes. Mycoses 41: 183–189 [DOI] [PubMed] [Google Scholar]
- Lee S, Groenewald JZ, Crous PW. 2004. Phylogenetic reassessment of the coelomycete genus Harknessia and its teleomorph Wuestneia (Diaporthales), and the introduction of Apoharknessia gen. nov. Studies in Mycology 50: 235–252 [Google Scholar]
- Marincowitz S, Crous PW, Groenewald JZ, Wingfield MJ. 2008. Microfungi occurring on Proteaceae in the fynbos. CBS Biodiversity Series 7: 1–166 CBS-KNAW Fungal Biodiversity Centre, Utrecht, Netherlands [Google Scholar]
- Mejía LC, Castlebury LA, Rossman AY, Sogonov MV, White JF., Jr.2011. A systematic account of the genus Plagiostoma (Gnomoniaceae, Diaporthales) based on morphology, host-associations, and a four-gene phylogeny. Studies in Mycology 68: 211–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostert L, Crous PW, Groenewald JZ, Gams W, Summerbell RC. 2003. Togninia (Calosphaeriales) is confirmed as teleomorph of Phaeoacremonium by means of morphology, sexual compatibility, and DNA phylogeny. Mycologia 95: 646–659 [DOI] [PubMed] [Google Scholar]
- Mostert L, Crous PW, Kang J-C, Phillips AJL. 2001. Species of Phomopsis and a Libertella sp. occurring on grapevines with specific reference to South Africa: morphological, cultural, molecular and pathological characterization. Mycologia 93: 145–166 [Google Scholar]
- Mostert L, Groenewald JZ, Summerbell RC, Gams W, Crous PW. 2006. Taxonomy and pathology of Togninia (Diaporthales) and its Phaeoacremonium anamorphs. Studies in Mycology 54: 1–115 [Google Scholar]
- Nag Raj TR. 1993. Coelomycetous anamorphs with appendage-bearing conidia. Mycologue Publications, Waterloo, Ontario [Google Scholar]
- Nag Raj TR, DiCosmo F. 1981. A monograph of Harknessia and Mastigosporella with notes on associated teleomorphs. Bibliotheca Mycologica 80: 1–62 [Google Scholar]
- Niekerk JM van, Groenewald JZ, Verkley GJM, Fourie PH, Wingfield MJ, Crous PW. 2004. Systematic reappraisal of Coniella and Pilidiella, with specific reference to species occurring on Eucalyptus and Vitis in South Africa. Mycological Research 108: 283–303 [DOI] [PubMed] [Google Scholar]
- O’Donnell K, Cigelnik E. 1997. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution 7: 103–116 [DOI] [PubMed] [Google Scholar]
- Park RF, Keane PJ, Wingfield MJ, Crous PW. 2000. Fungal diseases of eucalypt foliage. In: Keane PJ, Kile GA, Podger FD, Brown BN. (eds), Diseases and pathogens of eucalypts: 153–239. CSIRO publishing, Australia [Google Scholar]
- Quaedvlieg W, Kema GHJ, Groenewald JZ, Verkley GJM, Seifbarghi S, Razavi M, Mirzadi Gohari A, Mehrabi R, Crous PW. 2011. Zymoseptoria gen. nov.: a new genus to accommodate Septoria-like species occurring on graminicolous hosts. Persoonia 26: 57–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner RW. 1970. A mycological colour chart. CMI and British Mycological Society. Kew, Surrey, England [Google Scholar]
- Réblová M, Mostert L, Gams W, Crous PW. 2004. New genera in the Calosphaeriales: Togniniella and its anamorph Phaeocrella, and Calosphaeriophora as anamorph of Calosphaeria. Studies in Mycology 50: 533–550 [Google Scholar]
- Reid J, Booth C. 1989. On Cryptosporella and Wuestneia. Canadian Journal of Botany 67: 879–908 [Google Scholar]
- Rensburg JCJ van, Lamprecht SC, Groenewald JZ, Castlebury LA, Crous PW. 2006. Characterisation of Phomopsis spp. associated with die-back of rooibos (Aspalathus linearis) in South Africa. Studies in Mycology 55: 65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman AY, Farr DF, Castlebury LA. 2007. A review of the phylogeny and biology of the Diaporthales. Mycoscience 48: 135–144 [Google Scholar]
- Sankaran KV, Sutton BC, Minter DW. 1995. A checklist of fungi recorded on Eucalyptus. Mycological Papers 170: 1–376 [Google Scholar]
- Summerell BA, Groenewald JZ, Carnegie AJ, Summerbell RC, Crous PW. 2006. Eucalyptus microfungi known from culture. 2. Alysidiella, Fusculina and Phlogicylindrium genera nova, with notes on some other poorly known taxa. Fungal Diversity 23: 323–350 [Google Scholar]
- Sutton BC. 1971. Coelomycetes. IV. The genus Harknessia, and similar fungi on Eucalyptus. Mycological Papers 123: 1–46 [Google Scholar]
- Sutton BC. 1980. The coelomycetes: Fungi imperfecti with pycnidia, acervuli, and stromata. Commonwealth Mycological Institute, Kew, Surrey [Google Scholar]
- Sutton BC, Pascoe I. 1989. Addenda to Harknessia (Coelomycetes). Mycological Research 92: 431–439 [Google Scholar]
- Swart L, Crous PW, Denman S, Palm ME. 1998. Fungi occurring on Proteaceae I. South African Journal of Botany 64: 137–145 [Google Scholar]
- Vilgalys R, Hester M. 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee J, Taylor SB. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds), PCR protocols: a guide to methods and applications: 315–322. Academic Press, San Diego, California, USA [Google Scholar]
- Wingfield MJ, Beer ZW de, Slippers B, Wingfield BD, Groenewald JZ, Lombard L, Crous PW. 2012. One fungus, one name promotes progressive plant pathology. Molecular Plant Pathology doi: 10.1111/J.1364-3703.2011.00768.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan ZQ, Mohammed C. 1997. Wuestneia epispora sp. nov. on stems of eucalypts from Australia. Mycological Research 101: 195–200 [Google Scholar]
- Zhang N, Blackwell M. 2001. Molecular phylogeny of dogwood anthracnose fungus (Discula destructiva) and the Diaporthales. Mycologia 93: 355–365 [Google Scholar]